Improved Murine Model for the Intravital Microscopic Examination of Manifest Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Carrier
2.2. Animals
2.3. Anesthesia
2.4. Tumor Induction, Dorsal Skinfold Chamber
2.5. Intravital Fluorescence Microscopy
2.6. Analysis of Microcirculatory Parameters
2.7. Histology
2.8. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Microhemodynamics
3.3. Inflammatory Response
3.4. Angiogenesis
3.5. Histology and Immunohistology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSG | NOD scid gamma |
| FITC | fluorescein isothiocyanate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SEM | standard error of mean |
| SD | standard deviation |
| ANOVA | analysis of variance |
References
- Mast, G.; Pigorsch, S.U.; Ihrler, S.; Kolk, A.; Betz, C. Kopf-Hals-Malignome: Empfehlungen zur Diagnostik, Therapie und Nachsorge, 5th ed.; W. Zuckschwerdt Verlag: München, Germany, 2014; ISBN 9783863711337. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Wiseman, S.M.; Swede, H.; Stoler, D.L.; Anderson, G.R.; Rigual, N.R.; Hicks, W.L.; Douglas, W.G.; Tan, D.; Loree, T.R. Squamous cell carcinoma of the head and neck in nonsmokers and nondrinkers: An analysis of clinicopathologic characteristics and treatment outcomes. Ann. Surg. Oncol. 2003, 10, 551–557. [Google Scholar] [CrossRef]
- Koch, W.M.; Patel, H.; Brennan, J.; Boyle, J.O.; Sidransky, D. Squamous cell carcinoma of the head and neck in the elderly. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 262–265. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Pandya, N.M.; Dhalla, N.S.; Santani, D.D. Angiogenesis—A new target for future therapy. Vascul. Pharmacol. 2006, 44, 265–274. [Google Scholar] [CrossRef]
- Shojaei, F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Lett. 2012, 320, 130–137. [Google Scholar] [CrossRef]
- Alexander, S.; Koehl, G.E.; Hirschberg, M.; Geissler, E.K.; Friedl, P. Dynamic imaging of cancer growth and invasion: A modified skin-fold chamber model. Histochem. Cell Biol. 2008, 130, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Biel, N.M.; Lee, J.A.; Sorg, B.S.; Siemann, D.W. Limitations of the dorsal skinfold window chamber model in evaluating anti-angiogenic therapy during early phase of angiogenesis. Vasc Cell 2014, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; DaCosta, R.S. Optimization of the dorsal skinfold window chamber model and multi-parametric characterization of tumor-associated vasculature. Intravital 2014, 3, e27935. [Google Scholar] [CrossRef]
- Ludwig, N.; Szczepanski, M.J.; Gluszko, A.; Szafarowski, T.; Azambuja, J.H.; Dolg, L.; Gellrich, N.-C.; Kampmann, A.; Whiteside, T.L.; Zimmerer, R.M. CD44(+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019, 467, 85–95. [Google Scholar] [CrossRef]
- Turk, M.; Naumenko, V.; Mahoney, D.J.; Jenne, C.N. Tracking Cell Recruitment and Behavior within the Tumor Microenvironment Using Advanced Intravital Imaging Approaches. Cells 2018, 7, 69. [Google Scholar] [CrossRef]
- Pittet, M.J.; Weissleder, R. Intravital imaging. Cell 2011, 147, 983–991. [Google Scholar] [CrossRef]
- Baron, V.T.; Welsh, J.; Abedinpour, P.; Borgström, P. Intravital microscopy in the mouse dorsal chamber model for the study of solid tumors. Am. J. Cancer Res. 2011, 1, 674–686. [Google Scholar]
- Lunt, S.J.; Gray, C.; Reyes-Aldasoro, C.C.; Matcher, S.J.; Tozer, G.M. Application of intravital microscopy in studies of tumor microcirculation. J. Biomed. Opt. 2010, 15, 11113. [Google Scholar] [CrossRef]
- Hansen-Algenstaedt, N.; Schaefer, C.; Wolfram, L.; Joscheck, C.; Schroeder, M.; Algenstaedt, P.; Rüther, W. Femur window—A new approach to microcirculation of living bone in situ. J. Orthop. Res. 2005, 23, 1073–1082. [Google Scholar] [CrossRef]
- Stuehmer, C.; Schumann, P.; Bormann, K.-H.; Laschke, M.W.; Menger, M.D.; Gellrich, N.-C.; Rücker, M. A new model for chronic in vivo analysis of the periosteal microcirculation. Microvasc. Res. 2009, 77, 104–108. [Google Scholar] [CrossRef]
- Capurso, G.; Fendrich, V.; Rinzivillo, M.; Panzuto, F.; Bartsch, D.K.; Delle Fave, G. Novel molecular targets for the treatment of gastroenteropancreatic endocrine tumors: Answers and unsolved problems. Int. J. Mol. Sci. 2012, 14, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.L.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Ludwig, N.; et al. Vascularization and biocompatibility of poly(ε-caprolactone) fiber mats for rotator cuff tear repair. PLoS ONE 2020, 15, e0227563. [Google Scholar] [CrossRef] [PubMed]
- Klyscz, T.; Jünger, M.; Jung, F.; Zeintl, H. Cap Image--ein neuartiges computerunterstütztes Videobildanalysesystem für die dynamische Kapillarmikroskopie. Biomed. Tech. 1997, 42, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.; Wayland, H. On-line volume flow rate and velocity profile measurement for blood in microvessels. Microvasc. Res. 1974, 7, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Chrysos, E.; Athanasakis, E.; Saridaki, Z.; Kafetzakis, A.; Dimitriadou, D.; Koutsoumpas, V.; Chalkiadakis, G.; Xynos, E.; Zoras, O. Surgical Repair of Incisional Ventral Hernias: Tension-Free Technique Using Prosthetic Materials (Expanded Polytetrafluoroethylene Gore-Tex Dual Mesh). Am. Surg. 2000, 66, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Bezon, E.; Maguid, Y.A.; Gueret, G.; Choplain, J.N.; Aziz, A.A.; Barra, J.A. Wrapping of the left internal thoracic artery with an expanded polytetrafluoroethylene membrane. Ann. Thorac. Surg. 2006, 81, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Vayvada, H.; Aydin, E.; Menderes, A.; Atabey, A. Repair of fractures of the orbital floor with porous polyethylene implants. Br. J. Oral Maxillofac. Surg. 2007, 45, 640–644. [Google Scholar] [CrossRef]
- Bugyik, E.; Renyi-Vamos, F.; Szabo, V.; Dezso, K.; Ecker, N.; Rokusz, A.; Nagy, P.; Dome, B.; Paku, S. Mechanisms of vascularization in murine models of primary and metastatic tumor growth. Chin. J. Cancer 2016, 35, 19. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Weng, X.; Zhang, C.; Wang, S.; Wu, X.; Cheng, B. Inhibition of SUV39H1 reduces tumor angiogenesis via Notch1 in oral squamous cell carcinoma. PeerJ 2024, 12, e17222. [Google Scholar] [CrossRef]
- Katori, H.; Baba, Y.; Imagawa, Y.; Nishimura, G.; Kagesato, Y.; Takagi, E.; Ishii, A.; Yanoma, S.; Maekawa, R.; Yoshioka, T.; et al. Reduction of in vivo tumor growth by MMI-166, a selective matrix metalloproteinase inhibitor, through inhibition of tumor angiogenesis in squamous cell carcinoma cell lines of head and neck. Cancer Lett. 2002, 178, 151–159. [Google Scholar] [CrossRef]
- Cao, C.; Chen, Y.; Masood, R.; Sinha, U.K.; Kobielak, A. α-Catulin marks the invasion front of squamous cell carcinoma and is important for tumor cell metastasis. Mol. Cancer Res. 2012, 10, 892–903. [Google Scholar] [CrossRef]
- Kalle, F.; Stadler, V.P.; Brach, J.K.; Grote, V.F.; Pohl, C.; Schulz, K.; Seidenstuecker, M.; Jonitz-Heincke, A.; Bader, R.; Mlynski, R.; et al. High hydrostatic pressure treatment for advanced tissue grafts in reconstructive head and neck surgery. J. Biomed. Mater. Res. A 2025, 113, e37791. [Google Scholar] [CrossRef]
- Laschke, M.W.; Witt, K.; Pohlemann, T.; Menger, M.D. Injectable nanocrystalline hydroxyapatite paste for bone substitution: In vivo analysis of biocompatibility and vascularization. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 494–505. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Gray, J.W.; Howley, P.M.; Israel, M.; Thompson, C. (Eds.) The Molecular Basis of Cancer, 4th ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2015; ISBN 9781455740666. [Google Scholar]
- Onizuka, S.; Kawakami, S.; Taniguchi, K.; Fujioka, H.; Miyashita, K. Pancreatic carcinogenesis: Apoptosis and angiogenesis. Pancreas 2004, 28, 317–319. [Google Scholar] [CrossRef]
- Feng, X.; Tian, L.; Zhang, Z.; Yu, Y.; Cheng, J.; Gong, Y.; Li, C.-Y.; Huang, Q. Caspase 3 in dying tumor cells mediates post-irradiation angiogenesis. Oncotarget 2015, 6, 32353–32367. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J. 2017, 36, 2187–2203. [Google Scholar] [CrossRef] [PubMed]
- Abebayehu, D.; Spence, A.J.; McClure, M.J.; Haque, T.T.; Rivera, K.O.; Ryan, J.J. Polymer scaffold architecture is a key determinant in mast cell inflammatory and angiogenic responses. J. Biomed. Mater. Res. A 2019, 107, 884–892. [Google Scholar] [CrossRef]
- Park, J.-H.; Song, H.-Y.; Shin, J.H.; Kim, J.H.; Jun, E.J.; Cho, Y.C.; Kim, S.H.; Park, J. Polydioxanone biodegradable stent placement in a canine urethral model: Analysis of inflammatory reaction and biodegradation. J. Vasc. Interv. Radiol. 2014, 25, 1257–1264.e1. [Google Scholar] [CrossRef] [PubMed]
- Lingen, M.W. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch. Pathol. Lab. Med. 2001, 125, 67–71. [Google Scholar] [CrossRef]
- Schumann, P.; von See, C.; Kampmann, A.; Lindhorst, D.; Tavassol, F.; Kokemüller, H.; Bormann, K.-H.; Gellrich, N.-C.; Rücker, M. Comparably accelerated vascularization by preincorporation of aortic fragments and mesenchymal stem cells in implanted tissue engineering constructs. J. Biomed. Mater. Res. A 2011, 97, 383–394. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. The dorsal skinfold chamber: A versatile tool for preclinical research in tissue engineering and regenerative medicine. eCM 2016, 32, 202–215. [Google Scholar] [CrossRef]
- Sinikovic, B.; Schumann, P.; Winkler, M.; Kuestermeyer, J.; Tavassol, F.; von See, C.; Carvalho, C.; Mülhaupt, R.; Bormann, K.-H.; Kokemueller, H.; et al. Calvaria bone chamber—A new model for intravital assessment of osseous angiogenesis. J. Biomed. Mater. Res. A 2011, 99, 151–157. [Google Scholar] [CrossRef]
- Tavassol, F.; Kampmann, A.; Schumann, P.; Lindhorst, D.; Kokemüller, H.; Essig, H.; Meemken, J.-H.; Rücker, M.; Gellrich, N.-C. A novel approach for studying microcirculation in bone defects by intravital fluorescence microscopy. Tissue Eng. Part C Methods 2011, 17, 1151–1159. [Google Scholar] [CrossRef]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.-L.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Wellmann, M.; et al. In vivo analysis of vascularization and biocompatibility of electrospun polycaprolactone fibre mats in the rat femur chamber. J. Tissue Eng. Regen. Med. 2019, 13, 1190–1202. [Google Scholar] [CrossRef]
- Bakker, G.-J.; Weischer, S.; Ferrer Ortas, J.; Heidelin, J.; Andresen, V.; Beutler, M.; Beaurepaire, E.; Friedl, P. Intravital deep-tumor single-beam 3-photon, 4-photon, and harmonic microscopy. Elife 2022, 11, 63776. [Google Scholar] [CrossRef]
- Weigelin, B.; Bakker, G.-J.; Friedl, P. Intravital third harmonic generation microscopy of collective melanoma cell invasion: Principles of interface guidance and microvesicle dynamics. Intravital 2012, 1, 32–43. [Google Scholar] [CrossRef]
- Weigelin, B.; Bolaños, E.; Teijeira, A.; Martinez-Forero, I.; Labiano, S.; Azpilikueta, A.; Morales-Kastresana, A.; Quetglas, J.I.; Wagena, E.; Sánchez-Paulete, A.R.; et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc. Natl. Acad. Sci. USA 2015, 112, 7551–7556. [Google Scholar] [CrossRef] [PubMed]
- Heinz, M.C.; Peters, N.A.; Oost, K.C.; Lindeboom, R.G.H.; van Voorthuijsen, L.; Fumagalli, A.; van der Net, M.C.; de Medeiros, G.; Hageman, J.H.; Verlaan-Klink, I.; et al. Liver Colonization by Colorectal Cancer Metastases Requires YAP-Controlled Plasticity at the Micrometastatic Stage. Cancer Res. 2022, 82, 1953–1968. [Google Scholar] [CrossRef]
- Beerling, E.; Oosterom, I.; Voest, E.; Lolkema, M.; van Rheenen, J. Intravital characterization of tumor cell migration in pancreatic cancer. Intravital 2016, 5, e1261773. [Google Scholar] [CrossRef] [PubMed]
- Margarido, A.S.; Uceda-Castro, R.; Hahn, K.; de Bruijn, R.; Kester, L.; Hofland, I.; Lohuis, J.; Seinstra, D.; Broeks, A.; Jonkers, J.; et al. Epithelial-to-Mesenchymal Transition Drives Invasiveness of Breast Cancer Brain Metastases. Cancers 2022, 14, 3115. [Google Scholar] [CrossRef] [PubMed]
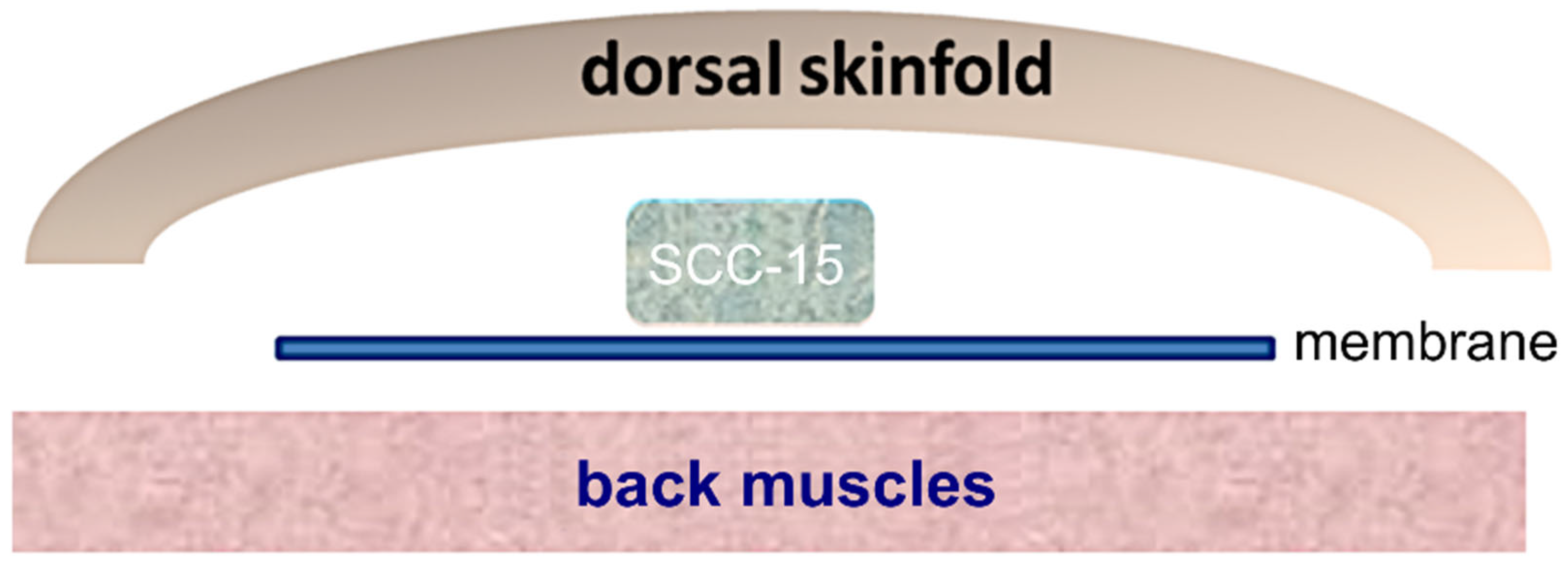
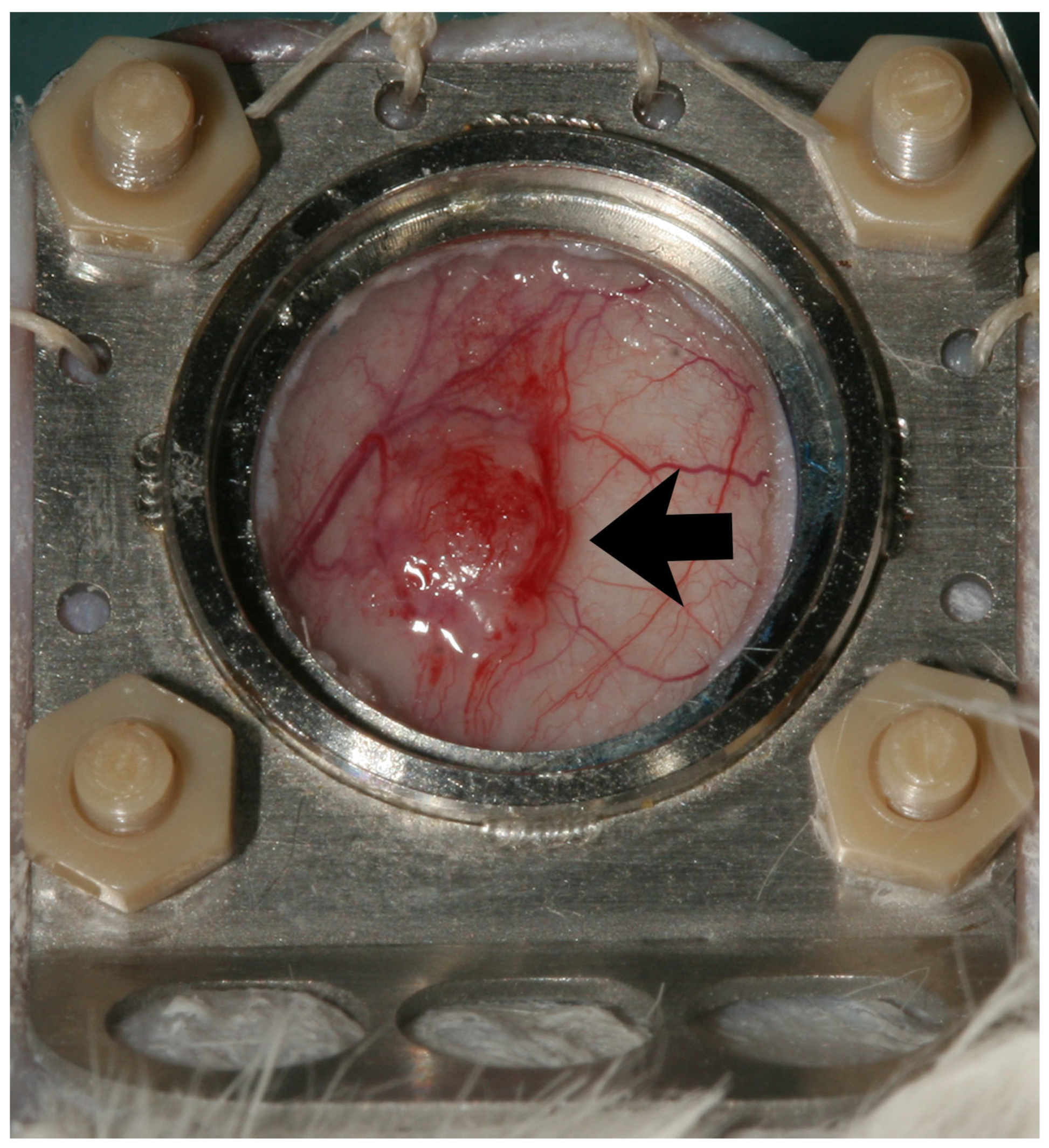
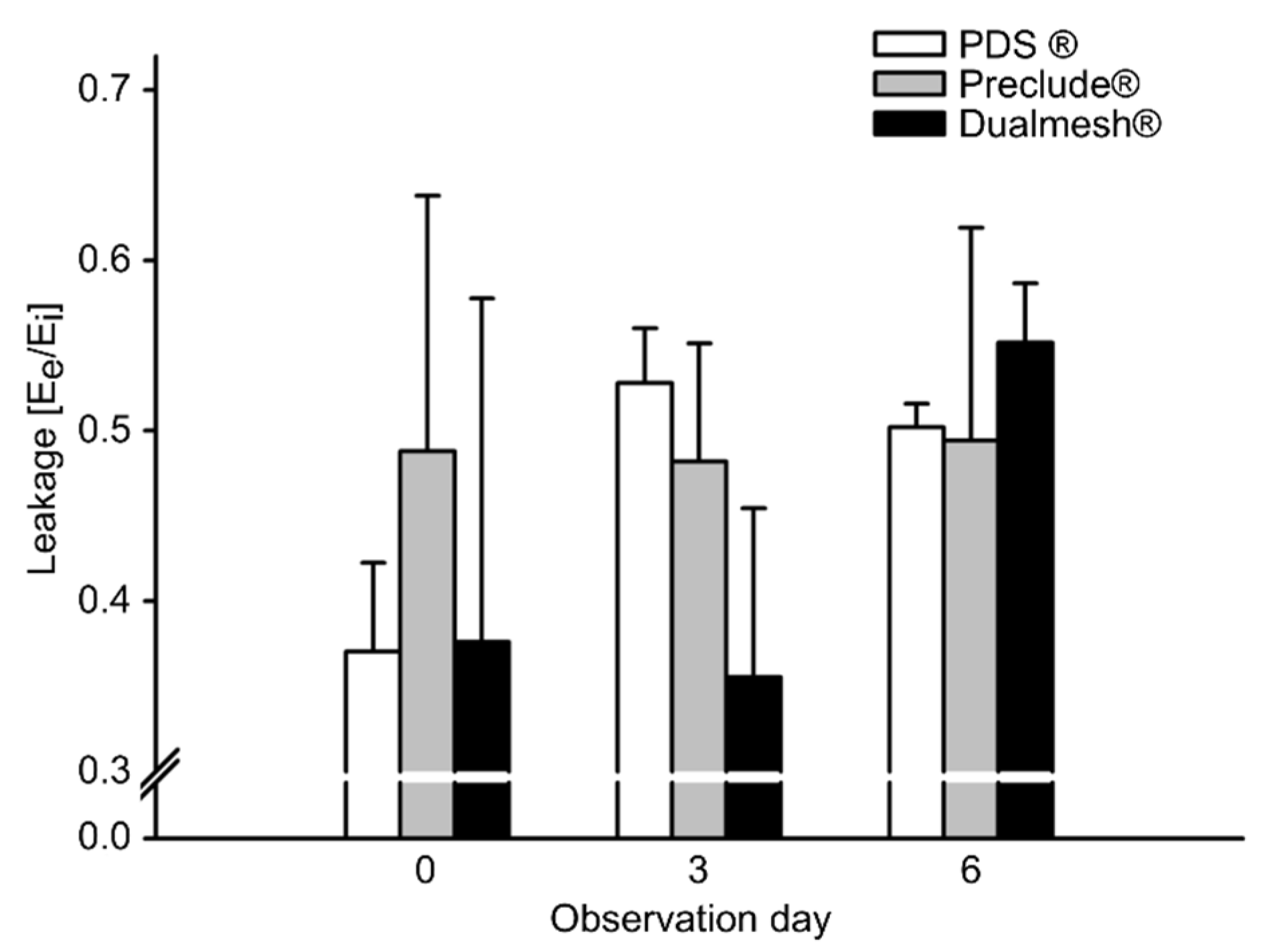
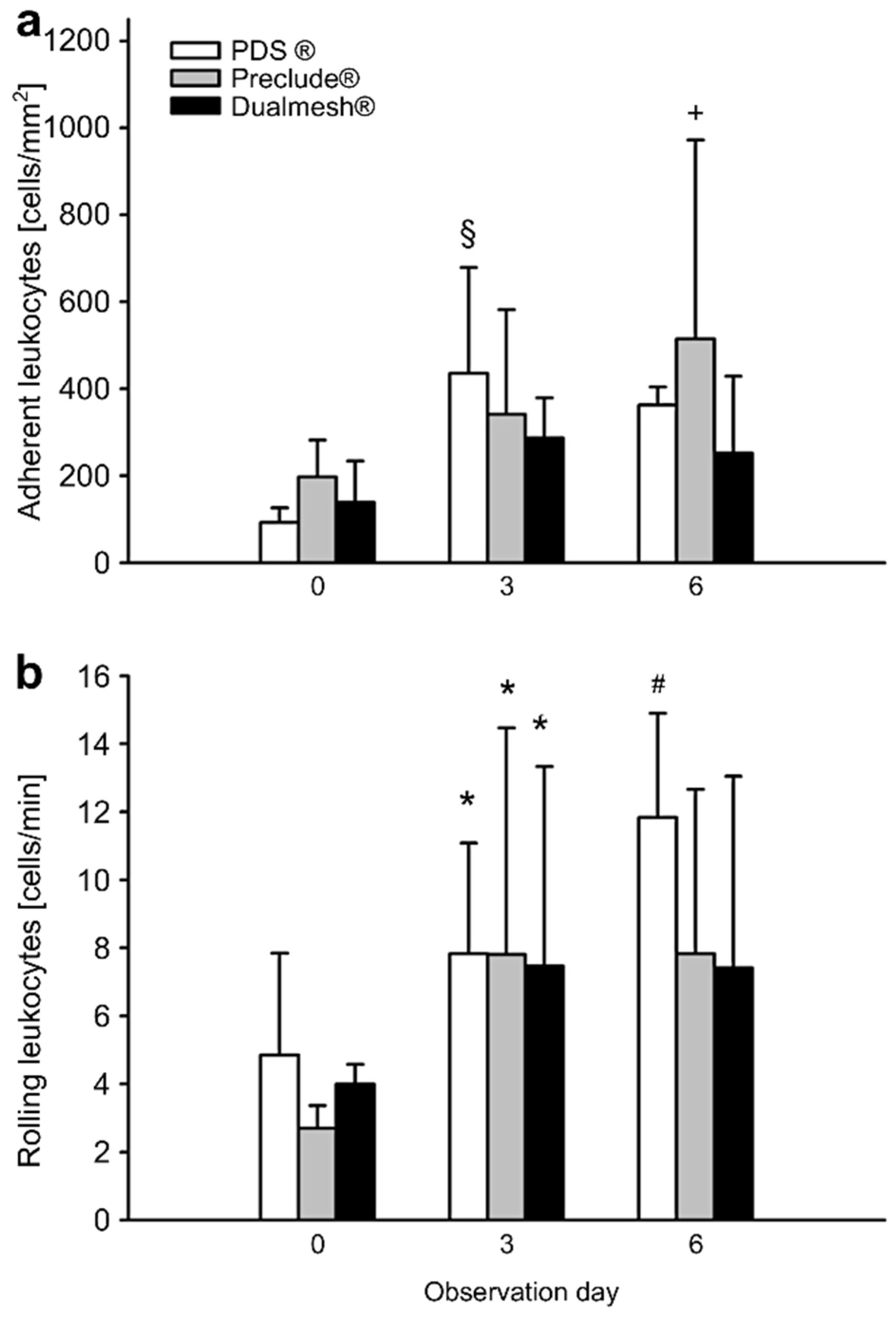
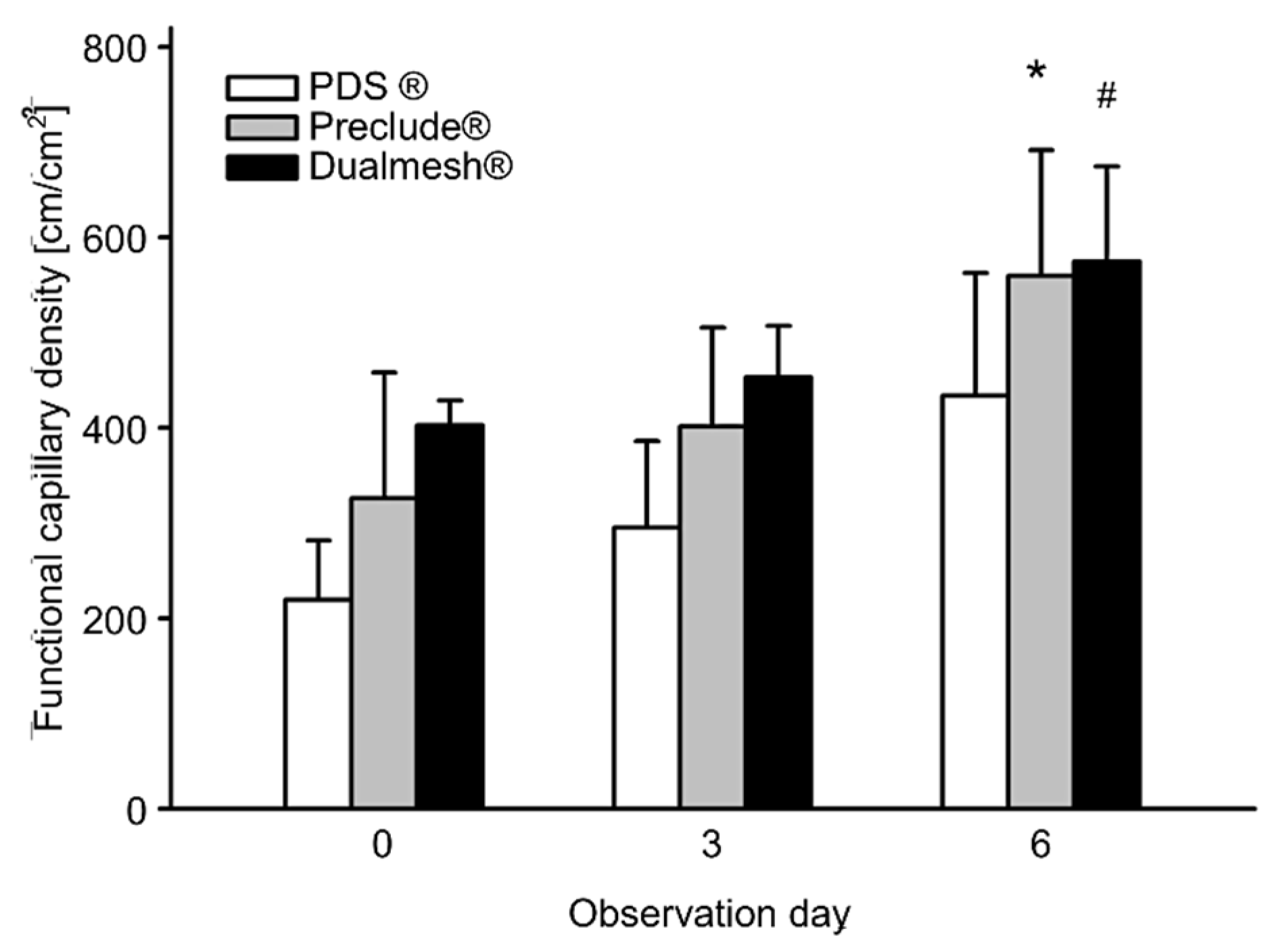

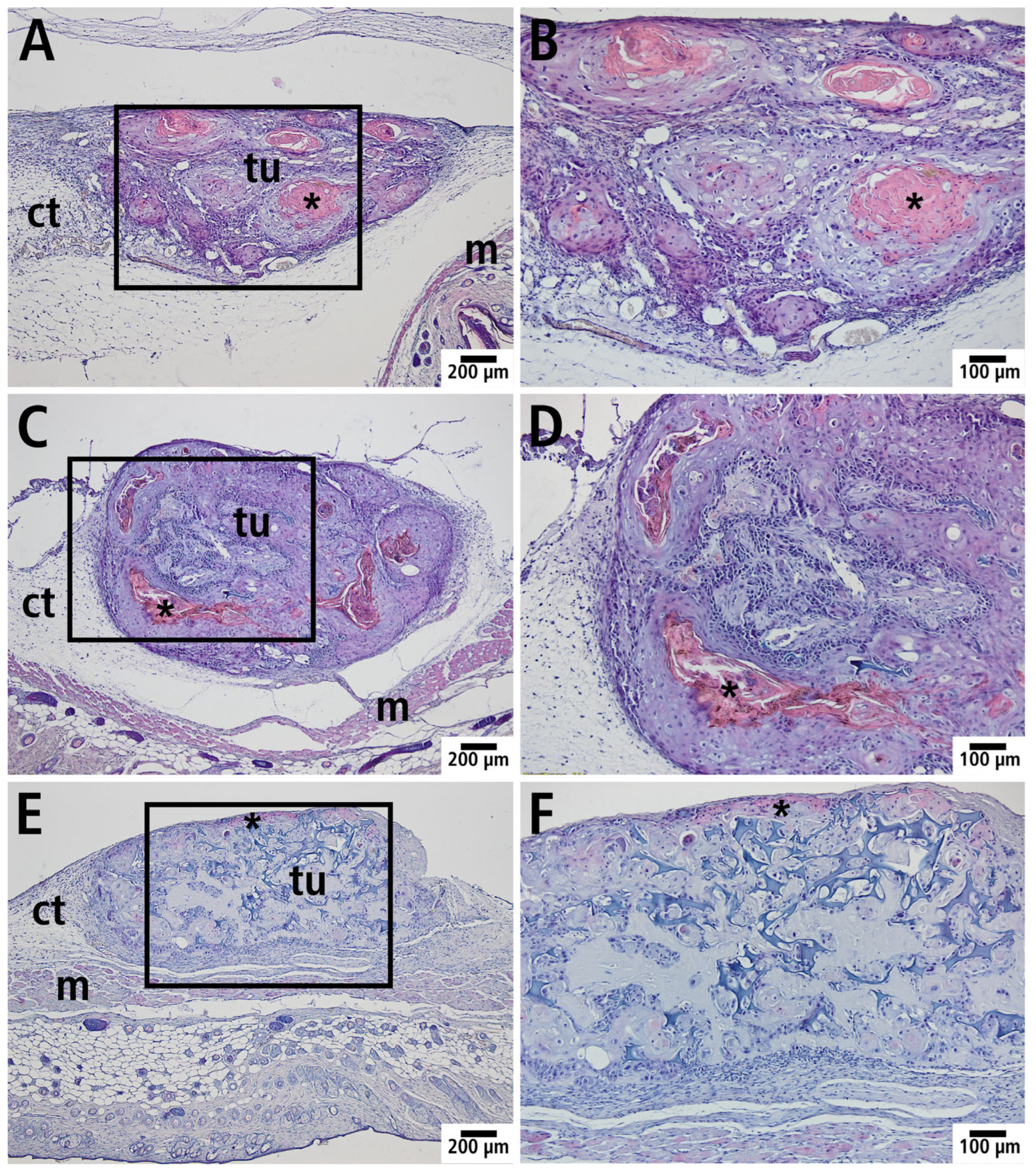

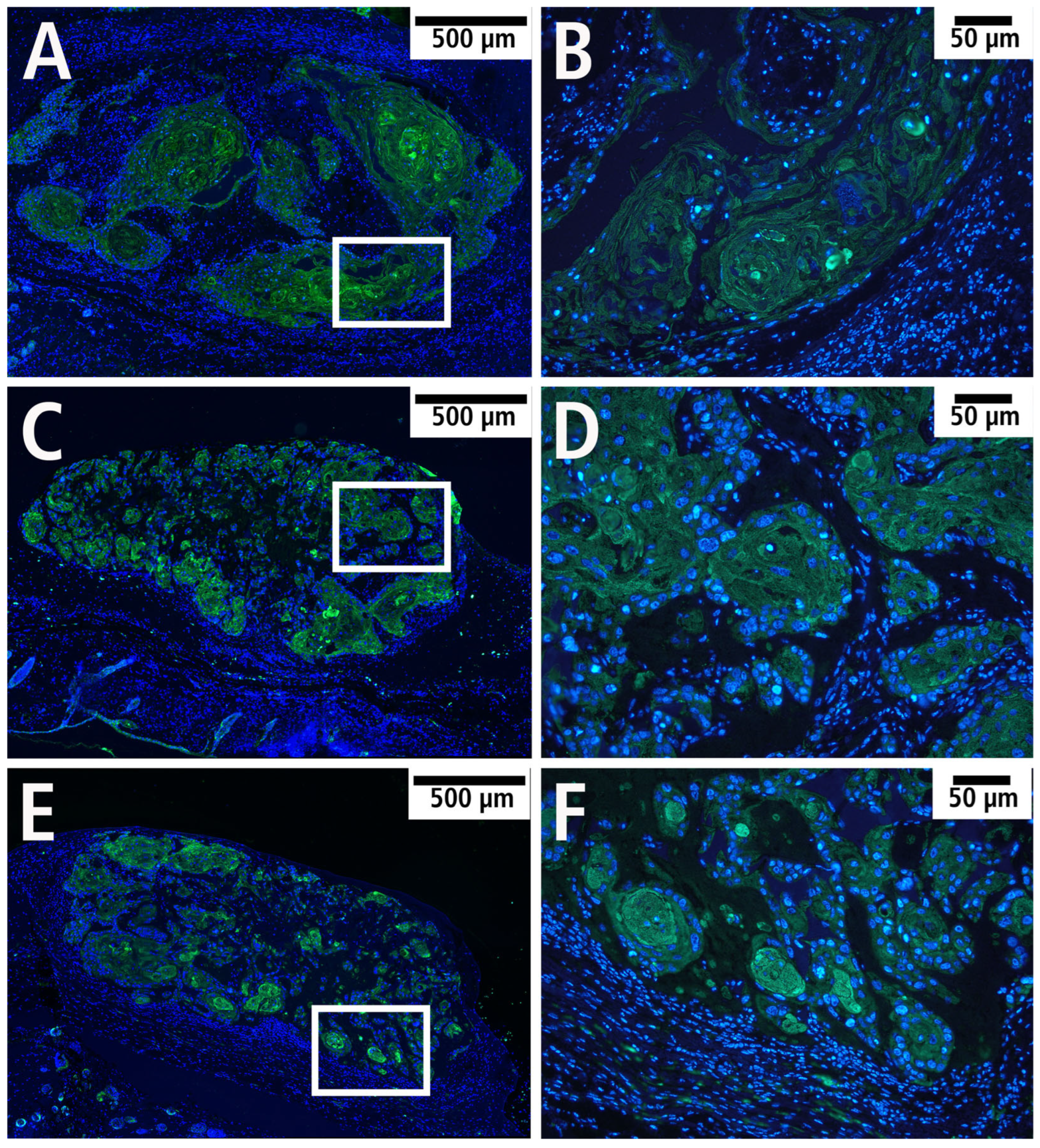
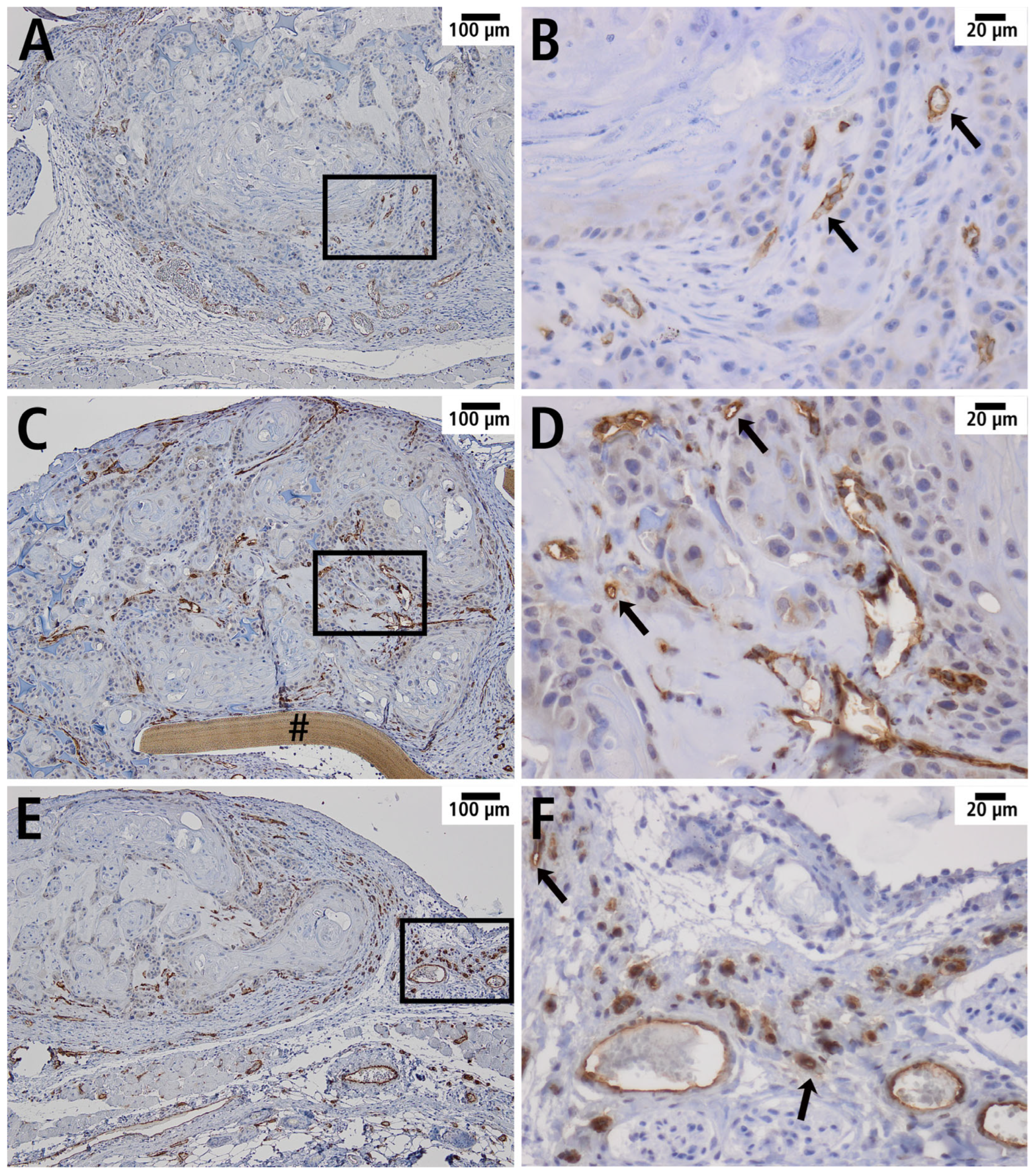
| Day 0 | Day 3 | Day 6 | |
|---|---|---|---|
| Blood cell velocity [µm/s] | |||
| PDS® | 377.0 ± 63.4 | 446.9 ± 72.2 | 435.1 ± 74.7 |
| Preclude® | 346.4 ± 123.9 | 469.3 ± 58.1 | 486.6 ± 133.0 |
| Dualmesh® | 341.8 ± 15.3 | 465.5 ± 45.6 | 553.7 ± 128.1 |
| Vessel diameter [µm] | |||
| PDS® | 31.4 ± 4.1 | 26.5 ± 4.2 | 31.5 ± 6.1 |
| Preclude® | 26.7 ± 3.5 | 33.3 ± 5.5 | 34.9 ± 2.6 |
| Dualmesh® | 28.0 ± 5.7 | 29.4 ± 3.1 | 32.3 ± 3.9 |
| Day 0 | Day 3 | Day 6 | |
|---|---|---|---|
| Volumetric blood flow [pL/s] | |||
| PDS® | 189.4 ± 63.4 | 156.0 ± 53.5 | 208.3 ± 45.5 |
| Preclude® | 123.6 ± 64.4 | * 258.4 ± 85.2 | * 291.7 ± 81.7 |
| Dualmesh® | 134.0 ± 48.2 | 198.4 ± 41.5 | # 289.8 ± 117.6 |
| Wall shear rate [s−1] | |||
| PDS® | 96.1 ± 0.9 | 137.8 ± 3.7 | 114.7 ± 4.1 |
| Preclude® | 105.5 ± 3.8 | 116.1 ± 2.7 | 112.3 ± 3.4 |
| Dualmesh® | 100.9 ± 2.5 | 127.7 ± 2.1 | 137.9 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavassol, F.; Winterboer, J.; Jehn, P.; Kappler, M.; Tilsen, F.; Kampmann, A. Improved Murine Model for the Intravital Microscopic Examination of Manifest Tumors. Cells 2025, 14, 1556. https://doi.org/10.3390/cells14191556
Tavassol F, Winterboer J, Jehn P, Kappler M, Tilsen F, Kampmann A. Improved Murine Model for the Intravital Microscopic Examination of Manifest Tumors. Cells. 2025; 14(19):1556. https://doi.org/10.3390/cells14191556
Chicago/Turabian StyleTavassol, Frank, Jan Winterboer, Philipp Jehn, Matthias Kappler, Felix Tilsen, and Andreas Kampmann. 2025. "Improved Murine Model for the Intravital Microscopic Examination of Manifest Tumors" Cells 14, no. 19: 1556. https://doi.org/10.3390/cells14191556
APA StyleTavassol, F., Winterboer, J., Jehn, P., Kappler, M., Tilsen, F., & Kampmann, A. (2025). Improved Murine Model for the Intravital Microscopic Examination of Manifest Tumors. Cells, 14(19), 1556. https://doi.org/10.3390/cells14191556






