Arabinogalactan Proteins Mark the Generative Cell–Vegetative Cell Interface in Monocotyledonous Pollen Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Histological and Immunochemical Analysis
2.3. Transmission Electron Microscopy
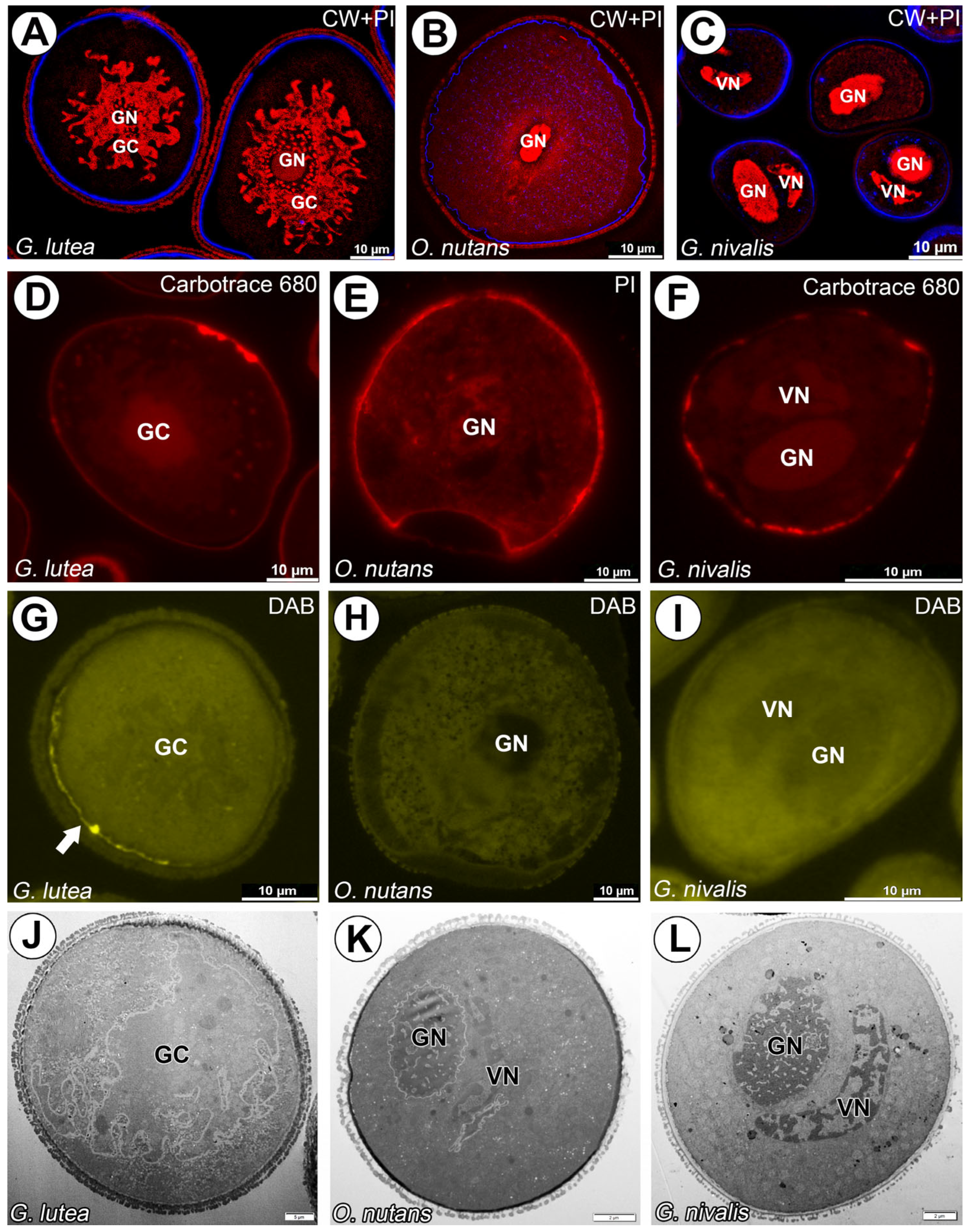
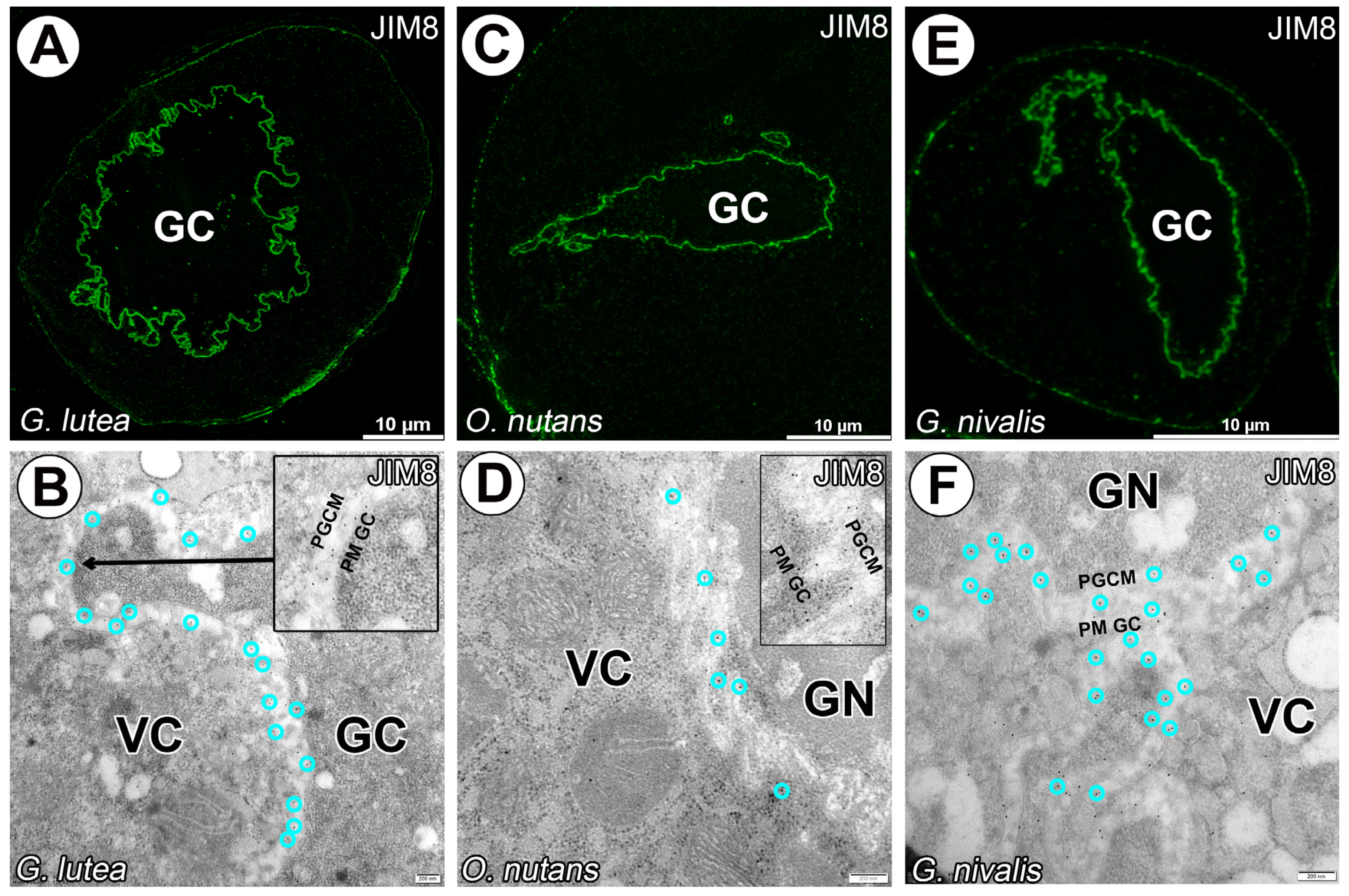
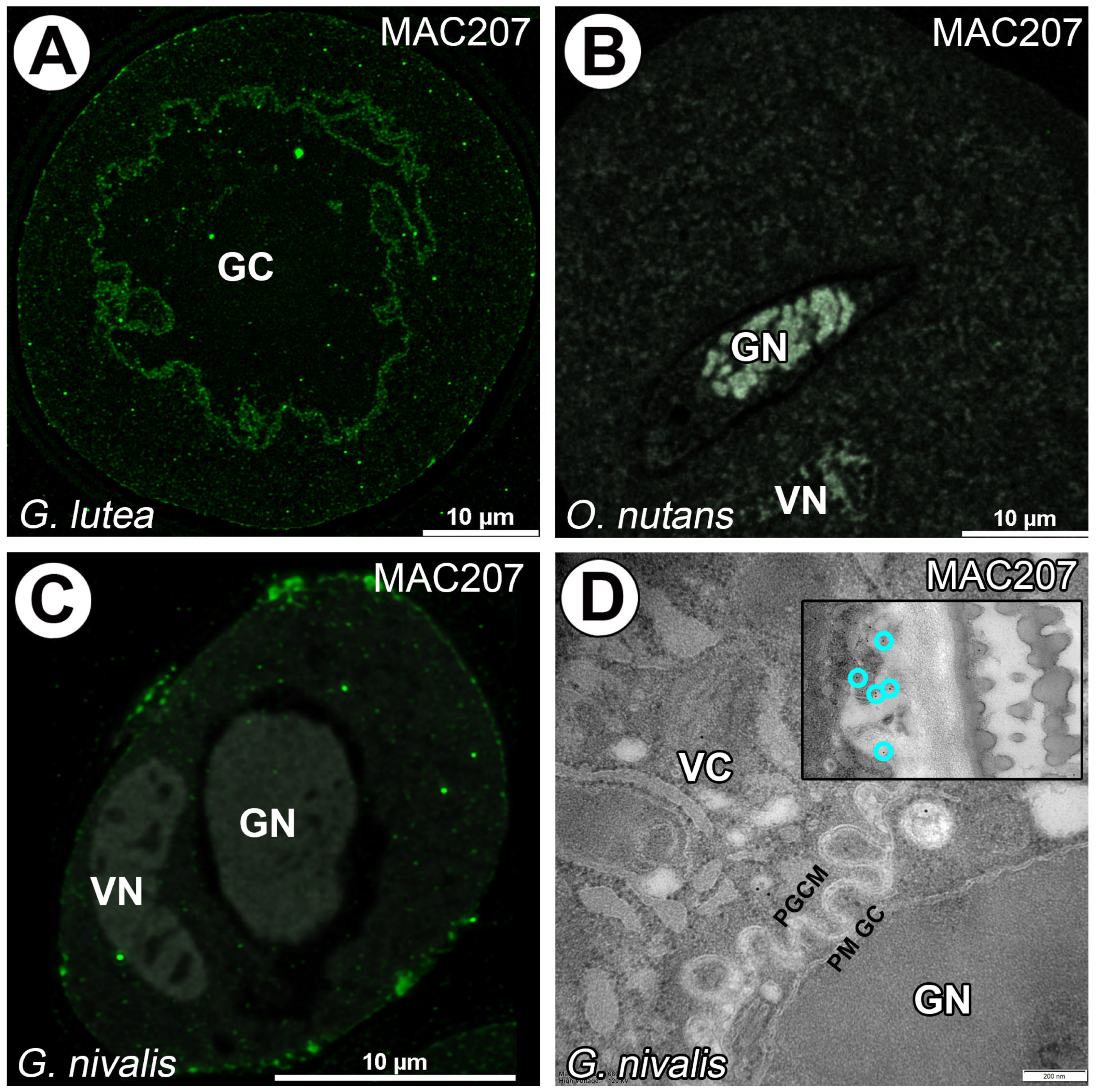
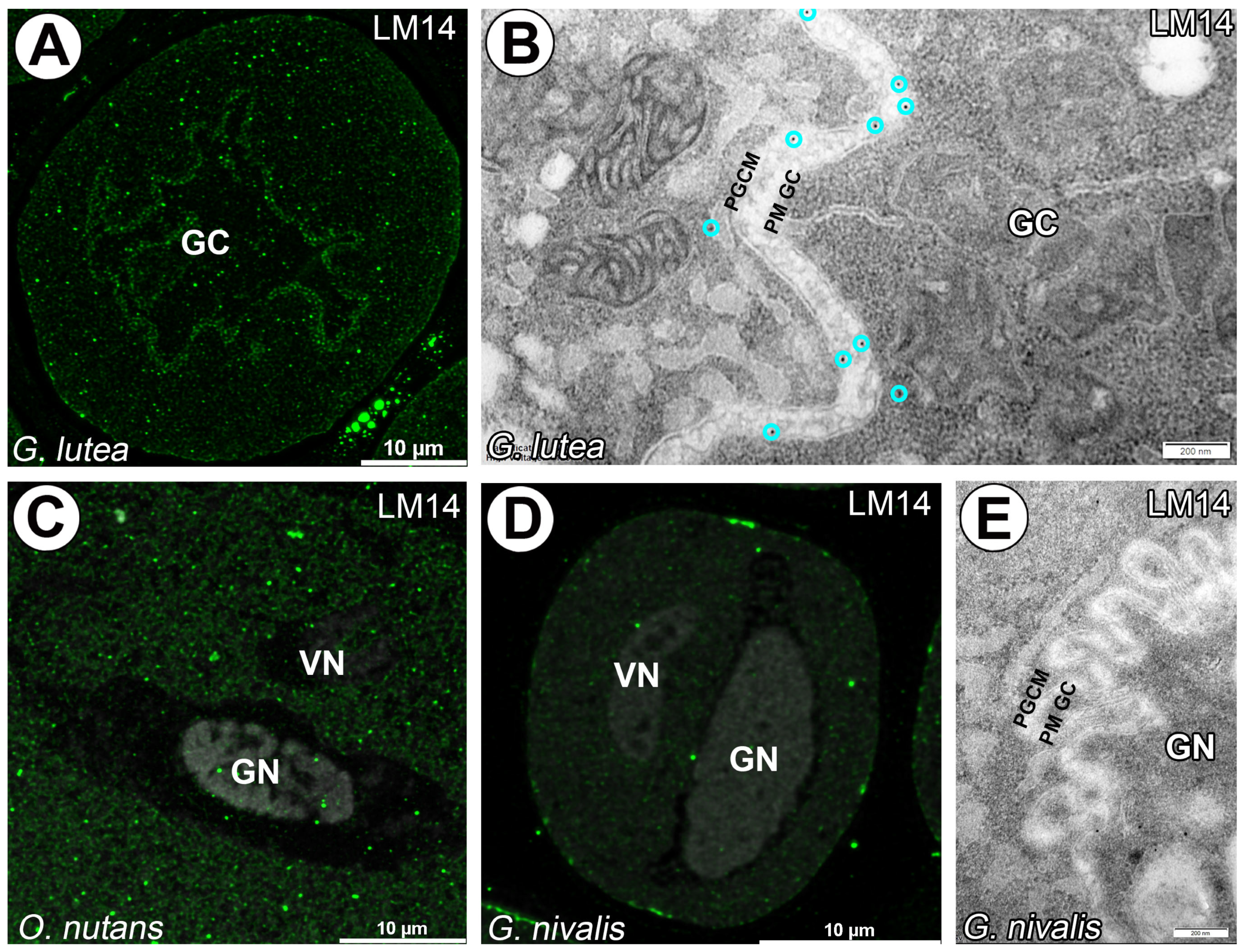
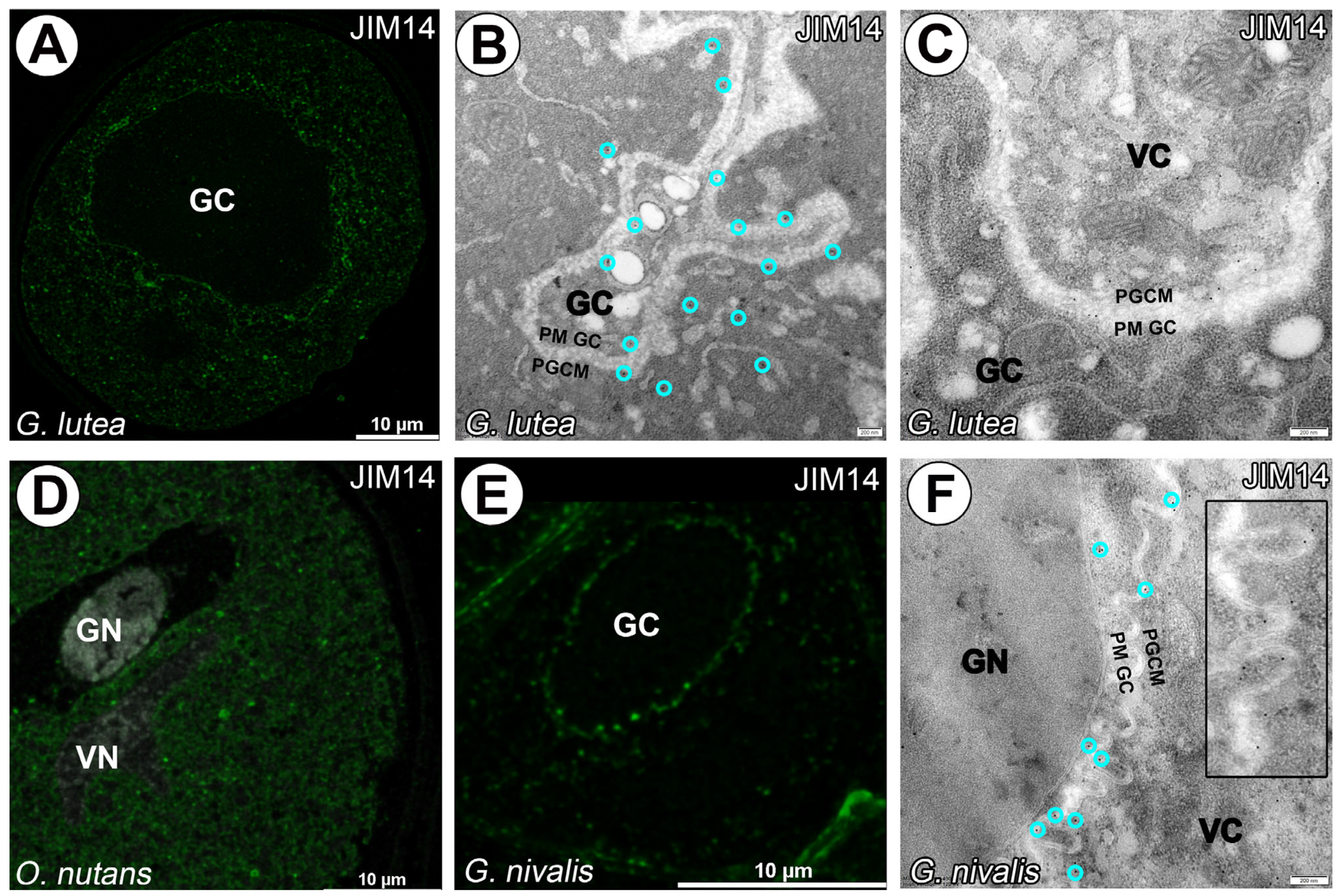
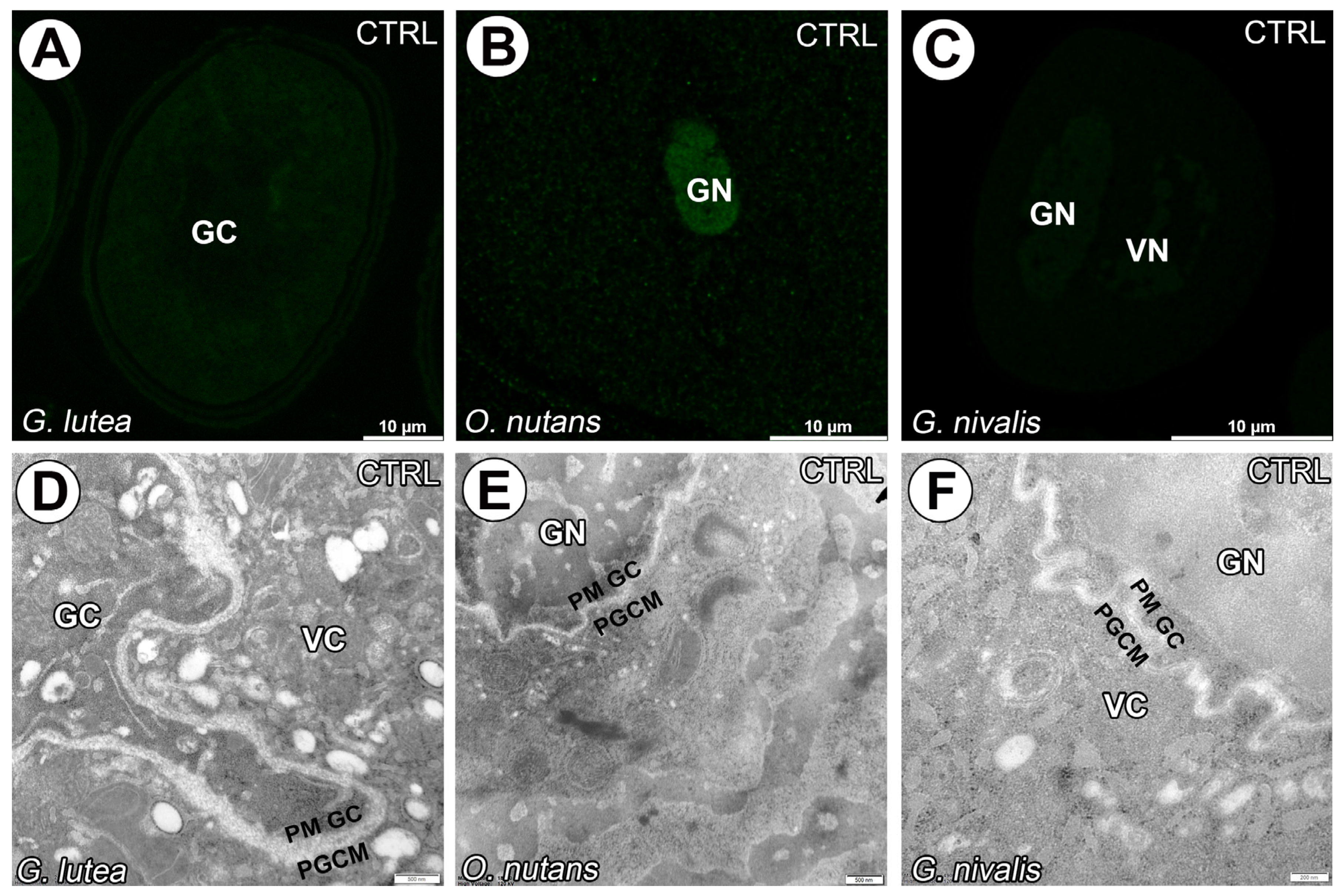
3. Results
4. Discussion
4.1. Cell–Cell Junction or Cell Wall Surrounding Generative Cell?
4.2. Presence of Β-Glucans in Generative Cell
4.3. AGPs in Generative Cell of Angiosperms
4.4. Evolutionary Perspective of AGPs in Generative Cell
5. Conclusions
- Our work is the first to demonstrate the presence of AGPs between the generative and vegetative cells in pollen of monocotyledonous species. These findings suggest that certain AGPs detected with JIM8 and JIM13 may serve as a marker of the generative cell not only in dicotyledons but also in monocotyledons. Further ultrastructural studies are needed to determine the exact localization of AGPs in the species studied; they are either located in the intercellular matrix or sub-membranously in the generative or vegetative cell.
- We found differences between LM2, LM14, JIM14 and MAC207 in the studied species. These results may suggest interspecific variations and require further investigations among monocot species.
- We did not detect any signal with JIM4 and JIM15 antibodies, which may be due to interspecific variations or AGPs not specific to these species or even to the mature pollen grains of monocots.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dresselhaus, T.; Franklin-Tong, N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant 2013, 6, 1018–1036. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Sprunck, S.; Wessel, G.M. Fertilization mechanisms in flowering plants. Curr. Biol. 2016, 26, R125–R139. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Yang, W.C. Gametophytic Pollen Tube Guidance: Attractant Peptides, Gametic Controls, and Receptors. Plant Physiol. 2017, 173, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P. An Introduction to the Embryology of Angiosperms; McGraw-Hill: New York, NY, USA, 1950. [Google Scholar]
- Dumas, C.; Knox, R.; Gaude, T. The spatial association of the sperm cells and vegetative nucleus in the pollen grain of Brassica. Protoplasma 1985, 124, 168–174. [Google Scholar] [CrossRef]
- Mogensen, H. Juxtaposition of the generative cell and vegetative nucleus in the mature pollen grain of amaryllis (Hippeastrum vitatum). Protoplasma 1986, 134, 67–72. [Google Scholar] [CrossRef]
- Mogensen, H.L. The male germ unit: Concept, composition, and significance. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1992; Volume 140, pp. 129–147. [Google Scholar]
- Mogensen, H.; Rusche, M.L. Quantitative ultrastructural analysis of barley sperm. Protoplasma 1985, 128, 1–13. [Google Scholar] [CrossRef]
- Mogensen, H.; Wagner, V. Associations among components of the male germ unit following in vivo pollination in barley. Protoplasma 1987, 138, 161–172. [Google Scholar] [CrossRef]
- Russell, S.D. Attraction and transport of male gametes for fertilization. Sex. Plant Reprod. 1996, 6, 337–342. [Google Scholar] [CrossRef]
- Batygina, T.B. (Ed.) Embryology of Flowering Plants: Terminology and Concepts, Vol. 1: Generative Organs of Flower, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Williams, J.H.; Taylor, M.L.; O’Meara, B.C. Repeated evolution of tricellular (and bicellular) pollen. Am. J. Bot. 2014, 101, 559–571. [Google Scholar] [CrossRef]
- Brewbaker, J.L. The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in the angiosperms. Am. J. Bot. 1967, 54, 1069–1083. [Google Scholar] [CrossRef]
- Liu, B.; Palevitz, B.A. Anaphase chromosome separation in dividing generative cells of Tradescantia: Changes in microtubule organization and kinetochore distribution visualized by antitubulin and CREST immunocytochemistry. Protoplasma 1992, 166, 122–133. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef]
- Sugi, N.; Calhau, A.R.M.; Jacquier, N.M.A.; Millan-Blanquez, M.; Becker, J.D.; Begcy, K.; Berger, F.; Bousquet-Antonelli, C.; Bouyer, D.; Cai, G.; et al. The Peri-Germ Cell Membrane: Poorly Characterized but Key Interface for Plant Reproduction. Nat. Plants 2024, 10, 1607–1609. [Google Scholar] [CrossRef] [PubMed]
- Southworth, D.; Russell, S.D. Male gametogenesis-development and structure of sperm. In Current Trends in the Embryology of Angiosperm; Bhojwani, S.S., Soh, W.Y., Eds.; Pod red; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 1–16. [Google Scholar]
- Delmer, D.; Dixon, R.A.; Keegstra, K.; Mohnen, D. The plant cell wall-dynamic, strong, and adaptable-is a natural shapeshifter. Plant Cell 2024, 36, 1257–1311. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.A.; York, W.S. The composition and structure of plant primary cell walls. Ann. Plant Rev. 2018, 8, 1–54. [Google Scholar]
- Dauphin, B.G.; Ranocha, P.; Dunand, C.; Burlat, V. Cell-wall microdomain remodeling controls crucial developmental processes. Trends Plant Sci. 2022, 27, 1033–1048. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Wang, B.; Andargie, M.; Fang, R. The function and biosynthesis of callose in high plants. Heliyon 2022, 8, e09248. [Google Scholar] [CrossRef]
- Chase, M.W. Monocot relationships: An overview. Am. J. Bot. 2004, 91, 1645–1655. [Google Scholar] [CrossRef]
- Haberer, G.; Mayer, K.F.; Spannagl, M. The big five of the monocot genomes. Curr. Opin. Plant Biol. 2016, 30, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.T.; Peaucelle, A. From monocots to dicots: The multifold aspect of cell wall expansion. J. Exp. Bot. 2021, 72, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, S.; Wortley, A.H.; Skvarla, J.J.; Rowley, J.R. Pollen wall development in flowering plants. N. Phytol. 2007, 174, 483–498. [Google Scholar] [CrossRef]
- Furness, C.A.; Rudall, P.J.; Sampson, F.B. Evolution of microsporogenesis in Angiosperms. Int. J. Plant Sci. 2002, 163, 235–260. [Google Scholar] [CrossRef]
- Furness, C.A.; Rudall, P.J. Microsporogenesis in Monocotyledons. Ann. Bot. 1999, 84, 475–499. [Google Scholar] [CrossRef]
- Coimbra, S.; Almeida, J.; Junqueira, V.; Costa, M.L.; Pereira, L.G. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J. Exp. Bot. 2007, 58, 4027–4035. [Google Scholar] [CrossRef]
- Leszczuk, A.; Szczuka, E.; Zdunek, A. Arabinogalactan proteins: Distribution during the development of male and female gametophytes. Plant Physiol. Biochem. 2019, 135, 9–18. [Google Scholar] [CrossRef]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. CMLS 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Annu. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef]
- Coimbra, S.; Costa, M.; Jones, B.; Mendes, M.A.; Pereira, L.G. Pollen grain development is compromised in Arabidopsis agp6 agp11 null mutants. J. Exp. Bot. 2009, 60, 3133–3142. [Google Scholar] [CrossRef]
- Costa, M.; Pereira, A.M.; Rudall, P.J.; Coimbra, S. Immunolocalization of arabinogalactan proteins (AGPs) in reproductive structures of an early-divergent angiosperm, Trithuria (Hydatellaceae). Ann. Bot. 2013, 111, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Lopes, A.L.; Coimbra, S. JAGGER, an AGP essential for persistent synergid degeneration and polytubey block in Arabidopsis. Plant Signal. Behav. 2016, 11, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, B.; Szczuka, E.; Zarzyka, B.; Leszczuk, A. Distribution of arabinogalactan proteins during microsporogenesis in the anther of Bellis perennis L. (Asteraceae). Acta Biol. Cracov. Ser. Bot. 2014, 56, 49–60. [Google Scholar] [CrossRef]
- Costa, M.L.; Sobral, R.; Costa, M.M.R.; Amorim, M.I.; Coimbra, S. Evaluation of the presence of arabinogalactan proteins and pectins during Quercus suber male gametogenesis. Ann. Bot. 2015, 115, 81–92. [Google Scholar] [CrossRef]
- Li, Y.Q.; Bruun, L.; Pierson, E.S.; Cresti, M. Periodic deposition of arabinogalactan epitopes in the cell wall of pollen tubes of Nicotiana tabacum L. Planta 1992, 188, 532–538. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, D.; Zhao, J. Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma 2007, 231, 43–53. [Google Scholar] [CrossRef]
- Southworth, D.; Kwiatkowski, S. Arabinogalactan proteins at the cell surface of Brassica sperm and Lilium sperm and generative cells. Sex. Plant Reprod. 1996, 9, 269–272. [Google Scholar] [CrossRef]
- Mogami, N.; Nakamura, S.; Nakamura, N. Immunolocalization of the cell wall components in Pinus densiflora pollen. Protoplasma 1999, 206, 1–10. [Google Scholar] [CrossRef]
- Yatomi, R.; Nakamura, S.; Nakamura, N. Immunochemical and cytochemical detection of wall components of germinated pollen of gymnosperms. Grana 2002, 41, 21–28. [Google Scholar] [CrossRef]
- Dardelle, F.; Lehner, A.; Ramdani, Y.; Bardor, M.; Lerouge, P.; Driouich, A.; Mollet, J.C. Biochemical and immunocytological characterizations of Arabidopsis pollen tube cell wall. Plant Physiol. 2010, 153, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Nguema-Ona, E.; Coimbra, S.; Vicré-Gibouin, M.; Mollet, J.C.; Driouich, A. Arabinogalactan proteins in root and pollen-tube cells: Distribution and functional aspects. Ann. Bot. 2012, 110, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Suárez, C.; Zienkiewicz, K.; Alché Jde, D.; Zienkiewicz, A.; Rodríguez-García, M.I. Electrophoretic profiling and immunocytochemical detection of pectins and arabinogalactan proteins in olive pollen during germination and pollen tube growth. Ann. Bot. 2013, 112, 503–513. [Google Scholar]
- Moreira, D.; Kaur, D.; Pereira, A.M.; Held, M.A.; Showalter, A.M.; Coimbra, S. Type II arabinogalactans initiated by hydroxyproline-O-galactosyltransferases play important roles in pollen–pistil interactions. Plant J. 2023, 114, 371–389. [Google Scholar] [CrossRef]
- Pereira, A.M.; Masiero, S.; Nobre, M.S.; Costa, M.L.; Solís, M.T.; Testillano, P.S.; Coimbra, S. Differential expression patterns of arabinogalactan proteins in Arabidopsis thaliana reproductive tissues. J. Exp. Bot. 2014, 65, 5459–5471. [Google Scholar] [CrossRef]
- Leszczuk, A.; Kalaitzis, P.; Kulik, J.; Zdunek, A. Review: Structure and modifications of arabinogalactan proteins (AGPs). BMC Plant Biol. 2023, 23, 45. [Google Scholar] [CrossRef]
- Ščepánková, I.; Hudák, J. Leaf and tepal anatomy, plastid ultrastructure and chlorophyll content in Galanthus nivalis L. and Leucojum aestivum L. Plant. Syst. Evol. 2004, 243, 211–219. [Google Scholar] [CrossRef]
- Gargano, D.; Peruzzi, L. Comparing flower biology in five species of Gagea (Liliaceae) from southern Italy. Flora Mediterr. 2021, 31, 131–144. [Google Scholar] [CrossRef]
- Chudzik, B.; Zarzyka, B.; Śnieżko, R. Immunodetection of arabinogalactan proteins in different types of plant ovules. Acta Biol. Cracov. Ser. Bot. 2005, 47, 139–146. [Google Scholar]
- Weryszko-Chmielewska, E.; Chwil, M. Flowering biology and structure of floral nectaries in Galanthus nivalis L. Acta Societat. Bot. Pol. 2016, 85, 3486. [Google Scholar]
- Erdelská, O.G. Reproduction of snowdrop (Galanthus nivalis L.) in West Slovakia. Thaiszia J. Bot. 2018, 28, 49–57. [Google Scholar]
- Kozieradzka-Kiszkurno, M.; Świerczyńska, J.; Bohdanowicz, J. Endoreduplication in the suspensor of Gagea lutea (L.) Ker Gawl. (Liliaceae). Acta Biol. Cracov. Ser. Bot. 2007, 49, 33–37. [Google Scholar]
- Newton, R.J.; Hay, F.R.; Ellis, R.H. Seed development and maturation in early spring-flowering Galanthus nivalis and Narcissus pseudonarcissus continues post-shedding with little evidence of maturation in planta. Ann. Bot. 2013, 111, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Swierczynska, J.; Bohdanowicz, J. Suspensor development in Gagea lutea (L.) Ker Gawl., with emphasis on the cytoskeleton. Acta Biol. Cracov. Ser. Bot. 2014, 56, 79–90. [Google Scholar]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Some permeability properties of angiosperm pollen grains, pollen tubes and generative cells. Sex. Plant Reprod. 1988, 1, 65–73. [Google Scholar] [CrossRef]
- Bohdanowicz, J.; Lewandowska, B. Participation of endoplasmic reticulum in the unequal distribution of plastids during generative cell formation in Gagea lutea (L.) Ker-Gaw. (Liliaceae). Acta Biol. Cracov. Ser. Bot. 1999, 41, 77–183. [Google Scholar]
- Penet, L.; Nadot, S.; Ressayre, A.; Forchioni, A.; Dreyer, L.; Gouyon, P.H. Multiple developmental pathways leading to a single morph: Monosulcate pollen (examples from the Asparagales). Ann. Bot. 2005, 95, 331–343. [Google Scholar] [CrossRef][Green Version]
- Szczuka, E.; Bohdanowicz, J.; Swierczynska, J.; Sobieska, J.; Pietrusiewicz, J. Zaburzenia w mikrosporogenezie i rozwoju ziarna pyłku Gagea lutea (L.) Ker.-Gaw. Acta Agrobot. 2006, 59, 71–82. [Google Scholar] [CrossRef][Green Version]
- Ekici, N.; Dane, F. Some histochemical features of anther wall of Leucojum aestivum (Amaryllidaceae) during pollen development. Biologia 2012, 67, 857–866. [Google Scholar] [CrossRef]
- Ekici, N. Microsporogenesis, pollen mitosis and pollen tube growth in Gagea villosa (Liliaceae). Biologia 2014, 69, 1323–1330. [Google Scholar] [CrossRef]
- Foubert-Mendes, S.; Silva, J.; Ferreira, M.J.; Pereira, L.G.; Coimbra, S. A Review on the Function of Arabinogalactan-Proteins during Pollen Grain Development. Plant Reprod. 2025, 38, 8. [Google Scholar] [CrossRef]
- Read, S.M.; Clarke, A.E.; Bacic, A. Stimulation of growth of cultured Nicotiana tabacum W38 pollen tubes by poly(ethylene glycol) and Cu(II) salts. Protoplasma 1993, 177, 1–14. [Google Scholar] [CrossRef]
- Brewbaker, J.L.; Kwack, B.H. The Essential Role of Calcium Ion in Pollen Germination and Pollen Tube Growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Bohdanowicz, J.; Ciampolini, F.; Cresti, M. Striped projections of the outer membrane of the generative cell in Convallaria majalis pollen. Sex. Plant Reprod. 1995, 8, 223–227. [Google Scholar] [CrossRef]
- Paul Knox, PhD, University of Leeds. Available online: https://www.kerafast.com/cat/799/paul-knox-phd (accessed on 13 November 2023).
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Knox, P.J.; Scofield, G.N.; Selvendran, R.R.; Roberts, K. A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J. Cell Biol. 1989, 108, 1967–1977. [Google Scholar] [CrossRef]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef]
- Rojek, J.; Kapusta, M.; Kozieradzka-Kiszkurno, M.; Majcher, D.; Górniak, M.; Śliwinska, E.; Sharbel, T.F.; Bohdanowicz, J. Establishing the cell biology of apomictic reproduction in diploid Boechera stricta (Brassicaceae). Ann. Bot. 2018, 122, 513–539. [Google Scholar] [CrossRef]
- Chebli, Y.; Kaneda, M.; Zerzour, R.; Geitmann, A. The cell wall of the Arabidopsis pollen tube--spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol. 2012, 160, 1940–1955. [Google Scholar] [CrossRef]
- Ascari, L.; Novara, C.; Dusio, V.; Oddi, L.; Siniscalco, C. Quantitative methods in microscopy to assess pollen viability in different plant taxa. Plant Reprod. 2020, 33, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrast. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Górska-Brylass, A. The “callose stage” of the generative cells in pollen grains. Grana 1970, 10, 21–30. [Google Scholar] [CrossRef]
- Southworth, D. Freeze fracture of male reproductive cells. Intl. Rev. Cytol. 1992, 140, 187–204. [Google Scholar]
- Raghavan, V. Pollen development and maturation. In Molecular Embryology of Flowering Plants; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Lancelle, S.A.; Hepler, P.K. Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma 1992, 167, 215–230. [Google Scholar] [CrossRef]
- Kaul, V.; Theunis, C.H.; Palser, B.F.; Knox, R.B.; Williams, E.G. Association of the generative cell and vegetative nucleus in pollen tubes of Rhododendron. Ann. Bot. 1987, 59, 227–235. [Google Scholar] [CrossRef]
- Cresti, M.; Lancelle, S.A.; Hepler, P.K. Structure of the generative cell wall complex after freeze substitution in pollen tubes of Nicotiana and Impatiens. J. Cell Sci. 1987, 88, 373–388. [Google Scholar] [CrossRef]
- Southworth, D. Sperm and Generative Cell. In Current Trends in the Embryology of Angiosperms; Bhojwani, S.S., Soh, W.Y., Eds.; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Southworth, D.; Platt-Aloia, K.A.; DeMason, D.A.; Thomson, W.W. Freezefracture of the generative cell of Phoenix dactylifera (Arecaceae). Sex. Plant Reprod. 1989, 2, 270–276. [Google Scholar] [CrossRef]
- Browning, A.J.; Gunning, B.E.S. An ultrastructural and cytochemical study of the wall-membrane apparatus of transfer cells using freeze-substitution. Protoplasma 1977, 93, 7–26. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Giddings, T.H.; Staehelin, L.A.; Sack, F.D. Comparison of the ultrastructure of conventionally fixed and high pressure frozen/freeze substituted root tips of Nicotiana and Arabidopsis. Protoplasma 1990, 157, 64–74. [Google Scholar] [CrossRef]
- Matias, V.R.; Beveridge, T.J. Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J. Bacteriol. 2006, 188, 1011–1121. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.P.; Lancelle, S.; Świątek, P.; Hepler, P.K.; Weidinger, M.; Lichtscheidl, I. Cyto-architecture of Byblis glands and leaf cells based on freeze-substitution and conventional TEM. Ann. Bot. 2025, 135, 463–482. [Google Scholar] [CrossRef]
- Park, S.K.; Twell, D. Novel patterns of ectopic cell plate growth and lipid body distribution in the Arabidopsis gemini pollen1 mutant. Plant Physiol. 2001, 126, 899–909. [Google Scholar] [CrossRef]
- Heslop-Harrison, J. Pollen wall development. The succession of events in the growth of intricately patterned pollen walls is described and discussed. Science 1968, 161, 230–237. [Google Scholar] [CrossRef]
- Tanaka, I. Isolation of generative cells and their pro top lasts from pollen of Lilium longiflorum. Protoplasma 1988, 142, 68–73. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Wang, Y.; Wang, Y.C.; Wang, N.N.; Lu, R.; Wu, Y.W.; Li, X.B. Pollen-Specific Protein PSP231 Activates Callose Synthesis to Govern Male Gametogenesis and Pollen Germination. Plant Physiol. 2020, 184, 1024–1041. [Google Scholar] [CrossRef]
- Nepi, M.; Franchi, G.G. Cytochemistry of mature angiosperm pollen. In Pollen and Pollination; Dafni, A., Hesse, M., Pacini, E., Eds.; Springer: Vienna, Austria, 2000. [Google Scholar]
- Wiśniewska, E.; Majewska-Sawka, A. Cell wall polysaccharides in differentiating anthers and pistils of Lolium perenne. Protoplasma 2006, 228, 65–71. [Google Scholar] [CrossRef]
- Nothnagel, E.A.; Munster, A.P.; Majewska-Sawka, A.; Bohanec, B. Arabinogalactan proteins in differentiation of gametes and somatic cells. In Biotechnological Approaches for Utilisation of Gametic Cells. COST 824: Final Meeting, Bled, Slovenia, 1–5 July 2000; Publications Office of the European Union: Luxembourg, 2001. [Google Scholar]
- Coimbra, S.; Pereira, G.L. Arabinogalactan Proteins in Arabidopsis thaliana Pollen Development. In Transgenic Plants-Advances and Limitations; InTech: New South Wales, Australia, 2012. [Google Scholar]
- El-Tantawy, A.A.; Solís, M.T.; Da Costa, M.L.; Coimbra, S.; Risueño, M.C.; Testillano, P.S. Arabinogalactan protein profiles and distribution patterns during microspore embryogenesis and pollen development in Brassica napus. Plant Reprod. 2013, 26, 231–243. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-L-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Sakata, Y.; Mau, S.L.; Pettolino, F.; Bacic, A.; Quatrano, R.S.; Knight, C.D.; Knox, J.P. Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patens. Plant Cell 2005, 17, 3051–3065. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, Y.; Carneros, E.; Berenguer, E.; Solís, M.T.; Bárány, I.; Pintos, B.; Gómez-Garay, A.; Risueño, M.C.; Testillano, P.S. Pectin De-methylesterification and AGP Increase Promote Cell Wall Remodeling and Are Required During Somatic Embryogenesis of Quercus suber. Front. Plant Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Nakamura, S.; Miki-Hiroshige, H. Fine-structural study on the formation of the generative cell wall and intine-3 layer in a growing pollen grain of Lilium longiflorum. Am. J. Bot. 1985, 72, 365–375. [Google Scholar] [CrossRef]
- Maruyama, K.; Gay, H.; Kaufmann, B.P. The nature of the wall between generative and vegetative nuclei in the pollen grain of Tradescantia paludosa. Am. J. Bot. 1965, 52, 605–610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terasaka, O.; Niitsu, T. The mitotic apparatus during unequal microspore division observed by a confocal laser scanning microscope. Protoplasma 1995, 189, 187–193. [Google Scholar] [CrossRef]
- Tanaka, I. Development of male gametes in flowering plants. J. Plant Res. 1993, 106, 55–63. [Google Scholar] [CrossRef]
- Fernando, D.D.; Quinn, C.R.; Brenner, E.D.; Owens, J.N. Male gametophyte development and evolution in extant gymnosperms. Int. J. Plant Dev. Biol. 2010, 4, 47–63. [Google Scholar]
- Fang, K.; Wang, Y.; Yu, T.; Zhang, L.; Baluška, F.; Šamaj, J.; Lin, J. Isolation of de-exined pollen and cytological studies of the pollen intines of Pinus bungeana Zucc. Ex Endl. and Picea wilsonii Mast. Flora-Morphology, Distribution, Funct. Ecol. Plants 2008, 203, 332–340. [Google Scholar] [CrossRef]
- Rafińska, K.; Niedojadło, K.; Świdziński, M.; Niedojadło, J.; Bednarska-Kozakiewicz, E. Spatial and temporal distribution of arabinogalactan proteins during Larix decidua Mill. male gametophyte and ovule interaction. Int. J. Mol. Sci. 2021, 22, 4298. [Google Scholar] [CrossRef]
- Lopez, R.A.; Renzeglia, K.S. Multiflagellated sperm cells of Ceratopteris richardii are bathed in arabinogalactan proteins throughout development. Am. J. Bot. 2014, 101, 2052–2061. [Google Scholar] [CrossRef]
- Lopez, R.A.; Renzeglia, K.S. The Ceratopteris (fern) developing motile gamete walls contain diverse polysaccharides, but not pectin. Planta 2018, 247, 393–404. [Google Scholar] [CrossRef]
- Lopez-Swalls, R. The Special Walls Around Gametes in Ceratopteris richardii and Aulacomnium palustre: Using Immunocytochemistry to Expose Structure, Function, and Development. Ph.D. Thesis, Southern Illinois University Carbondale, Carbondale, IL, USA, 2016. [Google Scholar]
- Henry, J. Studies on Cell Wall Composition in Bryophytes Across Taxa, Tissue, and Time. Ph.D. Thesis, Southern Illinois University, Carbondale, IL, USA, 2021. [Google Scholar]
- Johnson, E.E.; Taylor-Lapole, A.; Bavuso, M. Downregulation of arabinogalactan proteins during spermatogenesis in the moss Physcomitrium patens. J. Plant Develop. 2023, 30, 3–15. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusta, M.; Narajczyk, M.; Płachno, B.J. Arabinogalactan Proteins Mark the Generative Cell–Vegetative Cell Interface in Monocotyledonous Pollen Grains. Cells 2025, 14, 1549. https://doi.org/10.3390/cells14191549
Kapusta M, Narajczyk M, Płachno BJ. Arabinogalactan Proteins Mark the Generative Cell–Vegetative Cell Interface in Monocotyledonous Pollen Grains. Cells. 2025; 14(19):1549. https://doi.org/10.3390/cells14191549
Chicago/Turabian StyleKapusta, Małgorzata, Magdalena Narajczyk, and Bartosz J. Płachno. 2025. "Arabinogalactan Proteins Mark the Generative Cell–Vegetative Cell Interface in Monocotyledonous Pollen Grains" Cells 14, no. 19: 1549. https://doi.org/10.3390/cells14191549
APA StyleKapusta, M., Narajczyk, M., & Płachno, B. J. (2025). Arabinogalactan Proteins Mark the Generative Cell–Vegetative Cell Interface in Monocotyledonous Pollen Grains. Cells, 14(19), 1549. https://doi.org/10.3390/cells14191549






