Maternal Thymus Adaptations and Hormone Regulation During Pregnancy
Abstract
1. Introduction
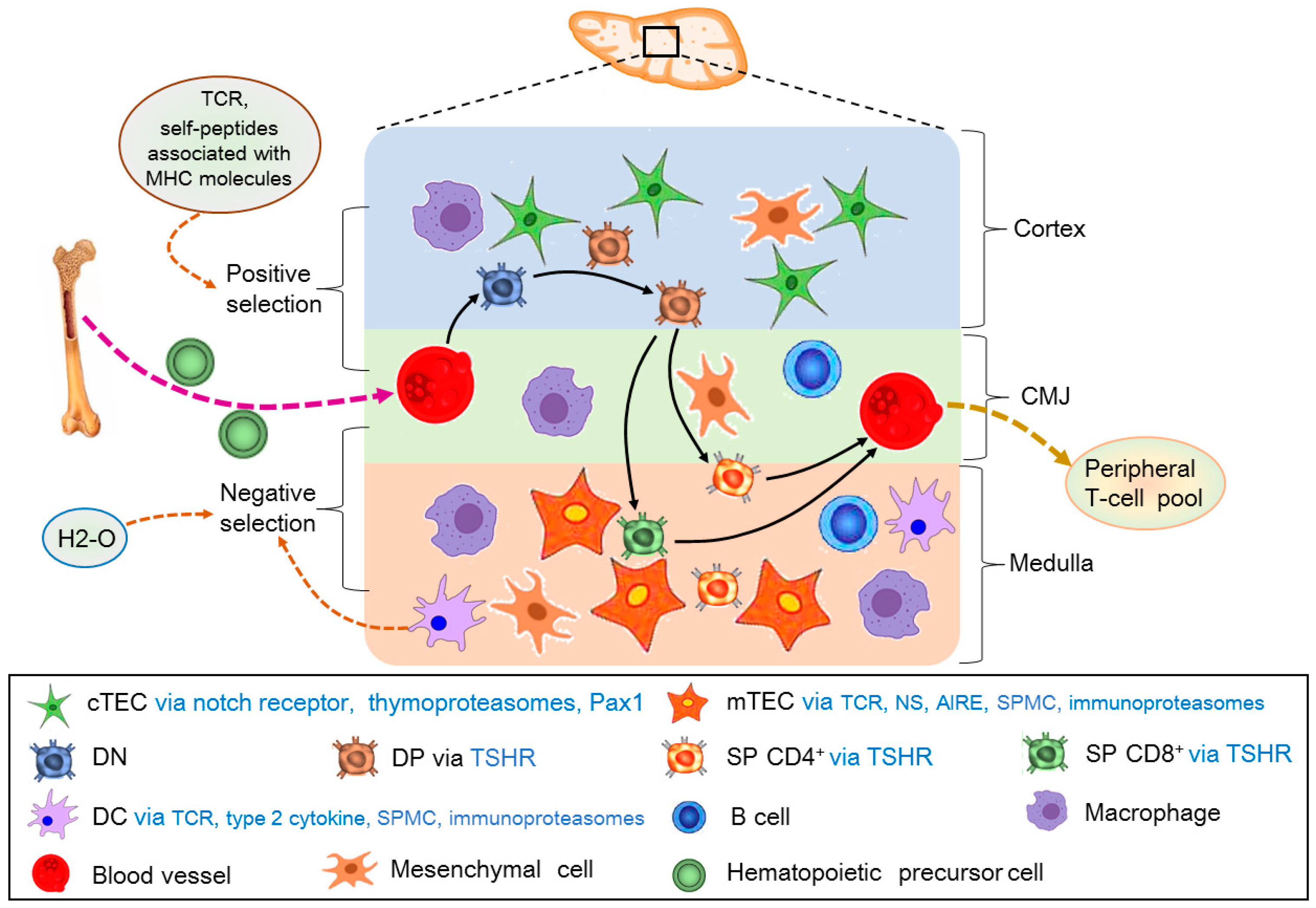
2. Adult Thymic Cellular Anatomy
3. T-Cell Development in the Thymus
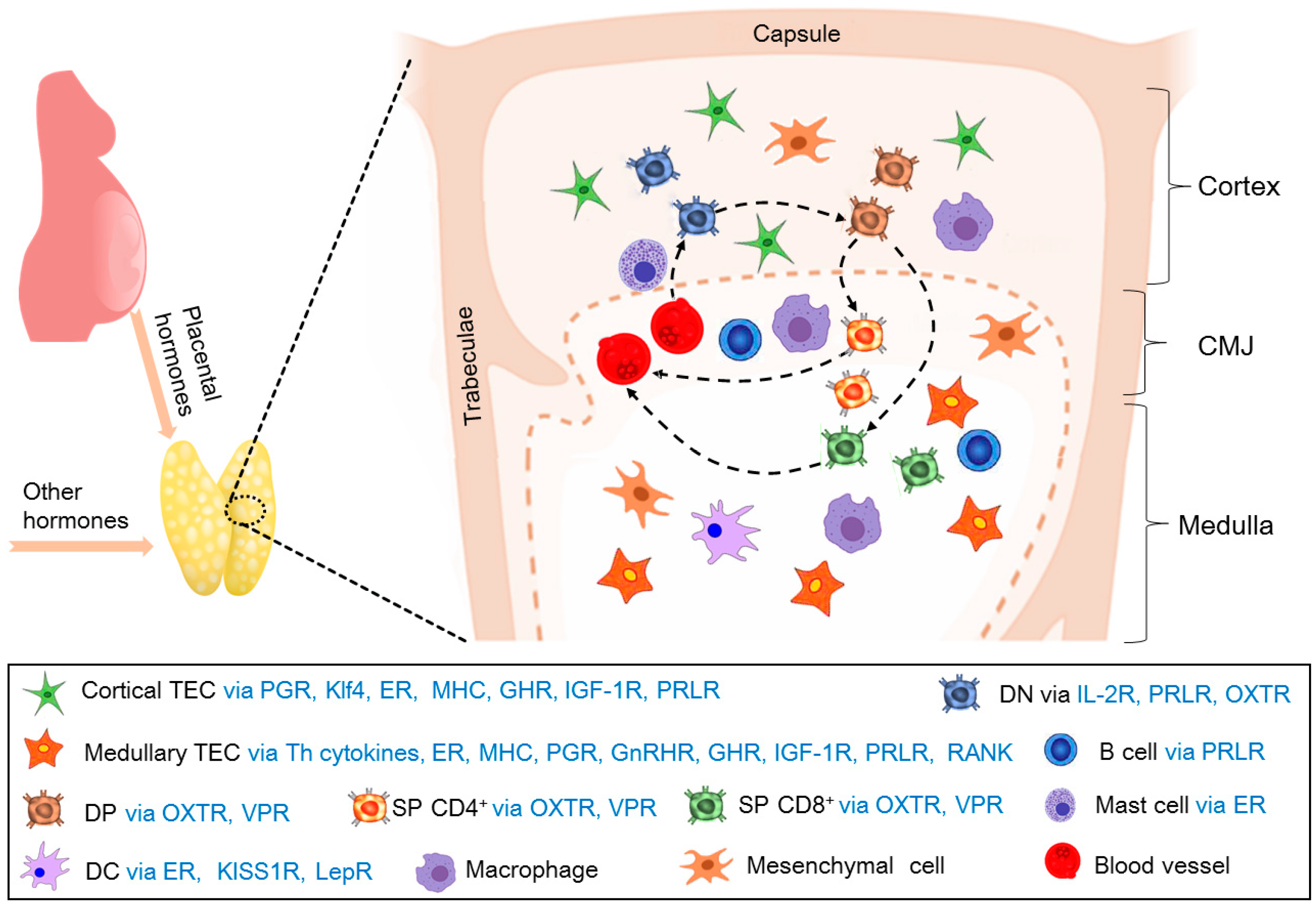
4. Maternal Thymic Adaptations During Pregnancy
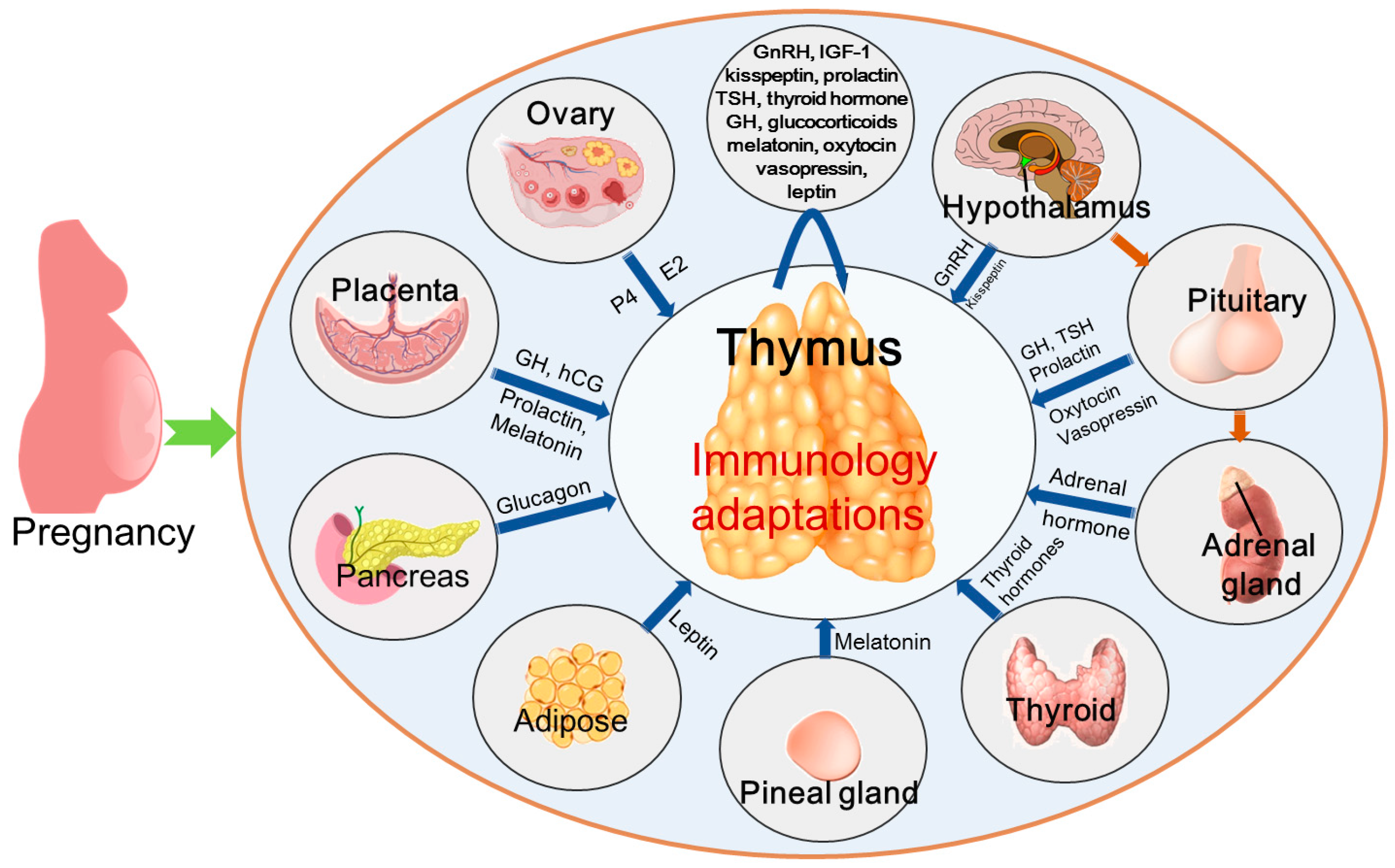
5. Hormones That Regulate Thymic Immunology During Pregnancy
5.1. Human Chorionic Gonadotropin
5.2. Estrogen
5.3. Progesterone
5.4. GnRH
5.5. Growth Hormone/Insulin-Like Growth Factor 1
5.6. Kisspeptin
5.7. Prolactin
5.8. Thyroid-Stimulating Hormone and Thyroid Hormone
5.9. Glucocorticoids
5.10. Melatonin
5.11. Oxytocin and Vasopressin
5.12. Leptin
5.13. Insulin
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIRE | Autoimmune regulator |
| CG | Chorionic gonadotrophin |
| cTECs | Cortical thymic epithelial cells |
| DCs | Dendritic cells |
| DN | Double negative |
| DNTregs | Double negative T regulatory cells |
| DP | Double positive |
| ERα | Estrogen receptor-α |
| GH | Growth hormone |
| GnRH | Gonadotropin-releasing hormone |
| GnRHR | Gonadotropin-releasing hormone receptor |
| GRs | Glucocorticoid receptors |
| HCG | Human chorionic gonadotropin |
| IGF-1 | Insulin-like growth factor 1 |
| IL-2 | Interleukin-2 |
| MHC | Major histocompatibility complex |
| mTECs | Medullary thymic epithelial cells |
| NES | Non-epithelial stromal cells |
| PGR | Progesterone receptor |
| PRL | Prolactin |
| RTE-Treg | Recent thymic emigrant-regulatory T-cell |
| SP | Single-positive |
| TCRs | T-cell receptors |
| TECs | Thymic epithelial cells |
| Th | Helper T |
| Treg | Regulatory T |
| TSH | Thyroid-stimulating hormone |
| TSHR | Thyroid-stimulating hormone receptor |
| tTRegs | TRegs derived from the thymus |
References
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.A.P. The function of the thymus and its impact on modern medicine. Science 2020, 369, eaba2429. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Botting, R.A.; Domínguez Conde, C.; Popescu, D.M.; Lavaert, M.; Kunz, D.J.; Goh, I.; Stephenson, E.; Ragazzini, R.; Tuck, E.; et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 2020, 367, eaay3224. [Google Scholar] [CrossRef]
- James, K.D.; Jenkinson, W.E.; Anderson, G. Non-epithelial stromal cells in thymus development and function. Front. Immunol. 2021, 12, 634367. [Google Scholar] [CrossRef]
- Paolino, M.; Koglgruber, R.; Cronin, S.J.F.; Uribesalgo, I.; Rauscher, E.; Harreiter, J.; Schuster, M.; Bancher-Todesca, D.; Pranjic, B.; Novatchkova, M.; et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature 2021, 589, 442–447. [Google Scholar] [CrossRef]
- Ahn, S.H.; Nguyen, S.L.; Kim, T.H.; Jeong, J.W.; Arora, R.; Lydon, J.P.; Petroff, M.G. Nuclear progesterone receptor expressed by the cortical thymic epithelial cells dictates thymus involution in murine pregnancy. Front. Endocrinol. 2022, 13, 846226. [Google Scholar] [CrossRef]
- Zoller, A.L.; Schnell, F.J.; Kersh, G.J. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology 2007, 121, 207–215. [Google Scholar] [CrossRef]
- Laan, M.; Haljasorg, U.; Kisand, K.; Salumets, A.; Peterson, P. Pregnancy-induced thymic involution is associated with suppression of chemokines essential for T-lymphoid progenitor homing. Eur. J. Immunol. 2016, 46, 2008–2017. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Cao, N.; Zhang, L.; Han, X.; Yang, L. Early pregnancy affects the expression of toll-like receptor pathway in ovine thymus. Reprod. Biol. 2020, 20, 547–554. [Google Scholar] [CrossRef]
- Yang, L.; Cai, C.; Fang, S.; Hao, S.; Zhang, T.; Zhang, L. Changes in expression of nuclear factor kappa B subunits in the ovine thymus during early pregnancy. Sci. Rep. 2022, 12, 17683. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhao, Z.; Cai, J.; Zhao, S.; Yang, L. Modulation of nod-like receptor expression in the thymus during early pregnancy in ewes. Vaccines 2022, 10, 2128. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Wang, H.; Feng, P.; Yang, G.; Yang, L. Effects of early pregnancy on the complement system in the ovine thymus. Vet. Res. Commun. 2022, 46, 137–145. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, Z.; Quan, Y.; Zhao, S.; Zhang, L.; Yang, L. Regulation of ikappaB protein expression by early gestation in the thymus of ewes. Vet. Sci. 2023, 10, 462. [Google Scholar] [CrossRef]
- Yang, L.; Lv, W.; Liu, Y.; Chen, K.; Xue, J.; Wang, Q.; Wang, B.; Zhang, L. Effect of early pregnancy on the expression of prostaglandin synthases in the ovine thymus. Theriogenology 2019, 136, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Jenkinson, E.J. Lymphostromal interactions in thymic development and function. Nat. Rev. Immunol. 2001, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Manley, N.R.; Richie, E.R.; Blackburn, C.C.; Condie, B.G.; Sage, J. Structure and function of the thymic microenvironment. Front. Biosci. 2011, 16, 2461–2477. [Google Scholar] [CrossRef]
- Sharma, H.; Moroni, L. Recent advancements in regenerative approaches for thymus rejuvenation. Adv. Sci. 2021, 8, 2100543. [Google Scholar] [CrossRef]
- Bosticardo, M.; Notarangelo, L.D. Human thymus in health and disease: Recent advances in diagnosis and biology. Semin. Immunol. 2023, 66, 101732. [Google Scholar] [CrossRef]
- Petrie, H.T. Role of thymic organ structure and stromal composition in steady-state postnatal T-cell production. Immunol. Rev. 2002, 189, 8–19. [Google Scholar] [CrossRef]
- Zúñiga-Pflücker, J.C. T-cell development made simple. Nat. Rev. Immunol. 2004, 4, 67–72. [Google Scholar] [CrossRef]
- Figueiredo, M.; Zilhão, R.; Neves, H. Thymus inception: Molecular network in the early stages of thymus organogenesis. Int. J. Mol. Sci. 2020, 21, 5765. [Google Scholar] [CrossRef]
- Breed, E.R.; Vobořil, M.; Ashby, K.M.; Martinez, R.J.; Qian, L.; Wang, H.; Salgado, O.C.; O’Connor, C.H.; Hogquist, K.A. Type 2 cytokines in the thymus activate Sirpα+ dendritic cells to promote clonal deletion. Nat. Immunol. 2022, 23, 1042–1051. [Google Scholar] [CrossRef]
- Akagbosu, B.; Tayyebi, Z.; Shibu, G.; Paucar Iza, Y.A.; Deep, D.; Parisotto, Y.F.; Fisher, L.; Pasolli, H.A.; Thevin, V.; Elmentaite, R.; et al. Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature 2022, 610, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Frantzeskakis, M.; Takahama, Y.; Ohigashi, I. The role of proteasomes in the thymus. Front. Immunol. 2021, 12, 646209. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.A.; Song, N.; Park, C.S.; Peske, J.D.; Sadegh-Nasseri, S. H2-O deficiency promotes regulatory T cell differentiation and CD4 T cell hyperactivity. Front. Immunol. 2024, 14, 1304798. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Kondo, K.; Takahama, Y. Generation of peptides that promote positive selection in the thymus. J. Immunol. 2017, 198, 2215–2222. [Google Scholar] [CrossRef]
- Parker, M.E.; Ciofani, M. Regulation of γδ T cell effector diversification in the thymus. Front. Immunol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Březina, J.; Vobořil, M.; Filipp, D. Mechanisms of direct and indirect presentation of self-antigens in the thymus. Front. Immunol. 2022, 13, 926625. [Google Scholar] [CrossRef]
- Castañeda, J.; Hidalgo, Y.; Sauma, D.; Rosemblatt, M.; Bono, M.R.; Núñez, S. The multifaceted roles of B cells in the thymus: From immune tolerance to autoimmunity. Front. Immunol. 2021, 12, 766698. [Google Scholar] [CrossRef]
- Wallin, J.; Eibel, H.; Neubüser, A.; Wilting, J.; Koseki, H.; Balling, R. Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development 1996, 122, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.G.; Kendall, M.D. The thymus in pregnancy: The interplay of neural, endocrine and immune influences. Immunol. Today 1994, 15, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Leeming, G.; McLean, J.; Gibbs, A.C. Thymic and body weight during first syngeneic and allogeneic pregnancy in the rat, and the effects of strain difference. Thymus 1984, 6, 153–165. [Google Scholar] [PubMed]
- Kendall, M.D.; Clarke, A.G. The thymus in the mouse changes its activity during pregnancy: A study of the microenvironment. J. Anat 2000, 197 Pt 3, 393–411. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Chen, X.; Chen, Y.; Wang, F.; Wang, S.; Liao, Y.; Li, L. The role of thymus- and extrathymus-derived regulatory T cells in maternal-fetal tolerance. Front. Immunol. 2023, 14, 1109352. [Google Scholar] [CrossRef]
- Gambel, P.I.; Cleland, A.W.; Ferguson, F.G. Alterations in thymus and spleen cell populations and immune reactivity during syngeneic pregnancy and lactation. J. Clin. Lab. Immunol. 1980, 3, 115–119. [Google Scholar]
- Dixit, V.D.; Sridaran, R.; Edmonsond, M.A.; Taub, D.; Thompson, W.E. Gonadotropin-releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology 2003, 144, 1496–1505. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Xu, X.; Zhang, Z.W.; Gao, J.L. Mechanism of pregnancy-induced thymus involution and regeneration and medication rules of postpartum prescriptions. Zhongguo Zhong Yao Za Zhi 2023, 48, 4275–4284. [Google Scholar] [CrossRef]
- Ahn, S.H.; Nguyen, S.L.; Petroff, M.G. Exploring the origin and antigenic specificity of maternal regulatory T cells in pregnancy. Front. Immunol. 2020, 11, 1302. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, L.; Zhao, Z.; Li, N.; Wang, B.; Yang, L. Expression of melatonin receptors and CD4 in the ovine thymus, lymph node, spleen and liver during early pregnancy. Immunology 2020, 160, 52–63. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Mi, H.; Liu, B.; Wang, B.; Yang, L. Modulation of helper t cytokines in thymus during early pregnancy in ewes. Animals 2019, 9, 245. [Google Scholar] [CrossRef]
- Depoërs, L.; Dumont-Lagacé, M.; Trinh, V.Q.; Houques, C.; Côté, C.; Larouche, J.D.; Brochu, S.; Perreault, C. Klf4 protects thymus integrity during late pregnancy. Front. Immunol. 2023, 14, 1016378. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.C.; Chapman, F.M.; Michael, S.D. The production of alpha/beta and gamma/delta double negative (DN) T-cells and their role in the maintenance of pregnancy. Reprod. Biol. Endocrinol. 2015, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Bafor, E.E.; Valencia, J.C.; Young, H.A. Double negative T regulatory cells: An emerging paradigm shift in reproductive immune tolerance? Front. Immunol. 2022, 13, 886645. [Google Scholar] [CrossRef] [PubMed]
- Green, E.S.; Moldenhauer, L.M.; Groome, H.M.; Sharkey, D.J.; Chin, P.Y.; Care, A.S.; Robker, R.L.; McColl, S.R.; Robertson, S.A. Regulatory T cells are paramount effectors in progesterone regulation of embryo implantation and fetal growth. JCI Insight 2023, 8, e162995. [Google Scholar] [CrossRef]
- Sun, I.H.; Gillis-Buck, E.; Mackenzie, T.C.; Gardner, J.M. Thymic and extrathymic Aire-expressing cells in maternal-fetal tolerance. Immunol. Rev. 2022, 308, 93–104. [Google Scholar] [CrossRef]
- Savino, W.; Mendes-da-Cruz, D.A.; Lepletier, A.; Dardenne, M. Hormonal control of T-cell development in health and disease. Nat. Rev. Endocrinol. 2016, 12, 77–89. [Google Scholar] [CrossRef]
- Paulesu, L.; Rao, C.V.; Ietta, F.; Pietropolli, A.; Ticconi, C. hCG and its disruption by environmental contaminants during human pregnancy. Int. J. Mol. Sci. 2018, 19, 914. [Google Scholar] [CrossRef]
- Yoon, J.; Sun, S.; Moon, S.; Yang, H. Repeated gonadotropin administration suppresses T cell development in the mouse thymus. Dev. Reprod. 2025, 29, 1–11. [Google Scholar] [CrossRef]
- Labunets, I.F. Effect of chorionic gonadotropin on the structure and endocrine function of the thymus gland in mice. Fiziol. Zh 1991, 37, 75–80. [Google Scholar] [PubMed]
- Kuklina, E.M.; Shirshev, S.V.; Sharova, N.I.; Iarilin, A.A. Effect of chorionic gonadotropin on thymocyte differentiation in the presence of thymus epithelial cells. Ontogenez 2003, 34, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kuklina, E.M.; Shirshev, S.V.; Sharova, N.I.; Iarilin, A.A. Rol’ khorionicheskogo gonadotropina v differentsirovke timotsitov [Role of chorionic gonadotropin in the differentiation of thymocytes]. Ontogenez 1999, 30, 341–345. [Google Scholar] [PubMed]
- Lentz, L.S.; Stutz, A.J.; Meyer, N.; Schubert, K.; Karkossa, I.; von Bergen, M.; Zenclussen, A.C.; Schumacher, A. Human chorionic gonadotropin promotes murine Treg cells and restricts pregnancy-harmful proinflammatory Th17 responses. Front. Immunol. 2022, 13, 989247. [Google Scholar] [CrossRef]
- Staples, J.E.; Gasiewicz, T.A.; Fiore, N.C.; Lubahn, D.B.; Korach, K.S.; Silverstone, A.E. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J. Immunol. 1999, 163, 4168–4174. [Google Scholar] [CrossRef]
- Seiki, K.; Sakabe, K. Sex hormones and the thymus in relation to thymocyte proliferation and maturation. Arch. Histol. Cytol. 1997, 60, 29–38. [Google Scholar] [CrossRef]
- Merrheim, J.; Villegas, J.; Van Wassenhove, J.; Khansa, R.; Berrih-Aknin, S.; le Panse, R.; Dragin, N. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun. Rev. 2020, 19, 102468. [Google Scholar] [CrossRef]
- Shirshev, S.V.; Orlova, E.G.; Loginova, O.A.; Nekrasova, I.V.; Gorbunova, O.L.; Maslennikova, I.L. Hormonal regulation of dendritic cell differentiation in the thymus. Bull. Exp. Biol. Med. 2018, 165, 230–234. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex hormones in acquired immunity and autoimmune disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Rijhsinghani, A.G.; Thompson, K.; Bhatia, S.K.; Waldschmidt, T.J. Estrogen blocks early T cell development in the thymus. Am. J. Reprod. Immunol. 1996, 36, 269–277. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Chung, Y.; Kim, J.; Yang, H. Thymocyte differentiation is regulated by a change in estradiol levels during the estrous cycle in mouse. Dev. Reprod. 2013, 17, 441–449. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Effects of sex steroids on thymic epithelium and thymocyte development. Front. Immunol. 2022, 13, 975858. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Cao, Z.; Li, Z.; Zhang, L.; Yang, L. Expression of estrogen receptors in main immune organs in sheep during early pregnancy. Int. J. Mol. Sci. 2025, 26, 3528. [Google Scholar] [CrossRef]
- Conneely, O.M.; Mulac-Jericevic, B.; Lydon, J.P. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 2003, 68, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Head, G.M.; Downing, J.E.; Brucker, C.; Mentlein, R.; Kendall, M.D. Rapid progesterone actions on thymulin-secreting epithelial cells cultured from rat thymus. Neuroimmunomodulation 1999, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, T.A.; DeMayo, F.; Rich, S.; Conneely, O.M.; O’Malley, B.W. Progesterone receptors in the thymus are required for thymic involution during pregnancy and for normal fertility. Proc. Natl. Acad. Sci. USA 1999, 96, 12021–12026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, J.; Wang, Q.; Lv, W.; Mi, H.; Liu, Y.; Yang, L. Changes in expression of ISG15, progesterone receptor and progesterone-induced blocking factor in ovine thymus during early pregnancy. Theriogenology 2018, 121, 153–159. [Google Scholar] [CrossRef]
- Zakharova, L.; Sharova, V.; Izvolskaia, M. Mechanisms of reciprocal regulation of gonadotropin-releasing hormone (GnRH)-producing and immune systems: The role of GnRH, cytokines and their receptors in early ontogenesis in normal and pathological conditions. Int. J. Mol. Sci. 2020, 22, 114. [Google Scholar] [CrossRef]
- Golsteyn, E.J.; Fritzler, M.J. The role of the thymus-hypothalamus-pituitary-gonadal axis in normal immune processes and autoimmunity. J. Rheumatol. 1987, 14, 982–990. [Google Scholar]
- Jacobson, J.D.; Crofford, L.J.; Sun, L.; Wilder, R.L. Cyclical expression of GnRH and GnRH receptor mRNA in lymphoid organs. Neuroendocrinology 1998, 67, 117–125. [Google Scholar] [CrossRef]
- Ullewar, M.P.; Umathe, S.N. Gonadotropin-releasing hormone agonist prevents l-arginine induced immune dysfunction independent of gonadal steroids: Relates with a decline in elevated thymus and brain nitric oxide levels. Nitric. Oxide 2016, 57, 40–47. [Google Scholar] [CrossRef]
- Blacker, C.M.; Ataya, K.M.; Savoy-Moore, R.T.; Subramanian, M.G.; Mutchnick, M.G.; Dunbar, J.C. The gonadotropin-releasing hormone agonist leuprolide affects the thymus and other non-reproductive systems of female rats. Acta Endocrinol. 1991, 125, 581–589. [Google Scholar] [CrossRef]
- Cao, N.; Cao, L.; Gao, M.; Wang, H.; Zhang, L.; Yang, L. Changes in mRNA and protein levels of gonadotropin releasing hormone and receptor in ovine thymus, lymph node, spleen, and liver during early pregnancy. Domest. Anim. Endocrinol. 2021, 76, 106607. [Google Scholar] [CrossRef]
- Reis, M.D.D.S.; Veneziani, L.P.; Porto, F.L.; Lins, M.P.; Mendes-da-Cruz, D.A.; Savino, W. Intrathymic somatotropic circuitry: Consequences upon thymus involution. Front. Immunol. 2023, 14, 1108630. [Google Scholar] [CrossRef] [PubMed]
- Savino, W.; de Mello-Coelho, V.; Dardenne, M. Control of the thymic microenvironment by growth hormone/insulin-like growth factor-I-mediated circuits. Neuroimmunomodulation 1995, 2, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, P.; Varma, S. Growth hormone synthesized and secreted by human thymocytes acts via insulin-like growth factor I as an autocrine and paracrine growth factor. J. Clin. Endocrinol. Metab. 1996, 81, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- de Mello Coelho, V.; Villa-Verde, D.M.; Farias-de-Oliveira, D.A.; de Brito, J.M.; Dardenne, M.; Savino, W. Functional insulin-like growth factor-1/insulin-like growth factor-1 receptor-mediated circuit in human and murine thymic epithelial cells. Neuroendocrinology 2002, 75, 139–150. [Google Scholar] [CrossRef]
- de Mello Coelho, V.; Savino, W.; Postel-Vinay, M.C.; Dardenne, M. Role of prolactin and growth hormone on thymus physiology. Dev. Immunol. 1998, 6, 317–323. [Google Scholar] [CrossRef]
- Chu, Y.W.; Schmitz, S.; Choudhury, B.; Telford, W.; Kapoor, V.; Garfield, S.; Howe, D.; Gress, R.E. Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood 2008, 112, 2836–2846. [Google Scholar] [CrossRef]
- Tasaki, M.; Villani, V.; Shimizu, A.; Sekijima, M.; Yamada, R.; Hanekamp, I.M.; Hanekamp, J.S.; Cormack, T.A.; Moran, S.G.; Kawai, A.; et al. Role of bone marrow maturity, insulin-like growth factor 1 receptor, and forkhead box protein N1 in thymic involution and rejuvenation. Am. J. Transpl. 2016, 16, 2877–2891. [Google Scholar] [CrossRef]
- Vila, G.; Luger, A. Growth hormone deficiency and pregnancy: Any role for substitution? Minerva Endocrinol. 2018, 43, 451–457. [Google Scholar] [CrossRef]
- Li, Z.; Ma, X.; Du, Z.; Li, J.; Zhang, L.; Yang, L. Gestation regulates growth hormone and its receptor expression in sheep immune organs. Biology 2025, 14, 1318. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The role of kisspeptin in the control of the hypothalamic-pituitary-gonadal axis and reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Tng, E.L. Kisspeptin signalling and its roles in humans. Singap. Med. J. 2015, 56, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, J.; Yang, G.; Guo, Y.; Huang, L. Characterization of the porcine Kisspeptins receptor gene and evaluation as candidate for timing of puberty in sows. J. Anim. Breed. Genet. 2008, 125, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Liu, F.; Yang, Y.; Cui, X.; Wang, T.; Xie, L.; Zhao, Y.; Fang, L.; Yi, T.; Zheng, B.; et al. GPR54 deficiency reduces the Treg population and aggravates experimental autoimmune encephalomyelitis in mice. Sci. China Life Sci. 2018, 61, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsouki, J.; Patel, B.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Kisspeptin in the prediction of pregnancy complications. Front. Endocrinol. 2022, 13, 942664. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Binart, N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef]
- Carreño, P.C.; Sacedón, R.; Jiménez, E.; Vicente, A.; Zapata, A.G. Prolactin affects both survival and differentiation of T-cell progenitors. J. Neuroimmunol. 2005, 160, 135–145. [Google Scholar] [CrossRef]
- Russell, D.H.; Mills, K.T.; Talamantes, F.J.; Bern, H.A. Neonatal administration of prolactin antiserum alters the developmental pattern of T- and B-lymphocytes in the thymus and spleen of BALB/c female mice. Proc. Natl. Acad. Sci. USA 1988, 85, 7404–7407. [Google Scholar] [CrossRef]
- Legorreta-Haquet, M.V.; Santana-Sánchez, P.; Chávez-Sánchez, L.; Chávez-Rueda, A.K. The effect of prolactin on immune cell subsets involved in SLE pathogenesis. Front. Immunol. 2022, 13, 1016427. [Google Scholar] [CrossRef]
- Medeiros, N.V.C.; Porto, F.L.; De Menezes, C.A.; Santos Reis, M.D.D.; Smaniotto, S.; Lins, M.P. CXCL12-driven thymocyte migration is increased by thymic epithelial cells treated with prolactin in vitro. J. Biosci. 2021, 46, 103. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Wu, J.; Ren, Y.; Zhang, L.; Cao, J.; Yang, L. Early pregnancy regulates the expression of prolactin and its receptor in the thymus, the liver, the spleen and lymph nodes in sheep. Domest. Anim. Endocrinol. 2022, 81, 106731. [Google Scholar] [CrossRef] [PubMed]
- van der Weerd, K.; van Hagen, P.M.; Schrijver, B.; Heuvelmans, S.J.; Hofland, L.J.; Swagemakers, S.M.; Bogers, A.J.; Dik, W.A.; Visser, T.J.; van Dongen, J.J.; et al. Thyrotropin acts as a T-cell developmental factor in mice and humans. Thyroid 2014, 24, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Batanero, E.; de Leeuw, F.E.; Jansen, G.H.; van Wichen, D.F.; Huber, J.; Schuurman, H.J. The neural and neuro-endocrine component of the human thymus. II. Hormone immunoreactivity. Brain Behav. Immun. 1992, 6, 249–264. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, M.; Ma, C.; Zhang, H.; Liu, X.; Zhou, L.; Zhao, J.; Gao, L.; Wang, D. Thyrotropin alters T cell development in the thymus in subclinical hypothyroidism mouse model. Scand. J. Immunol. 2017, 85, 35–42. [Google Scholar] [CrossRef]
- Villa-Verde, D.M.; de Mello-Coelho, V.; Farias-de-Oliveira, D.A.; Dardenne, M.; Savino, W. Pleiotropic influence of triiodothyronine on thymus physiology. Endocrinology 1993, 133, 867–875. [Google Scholar] [CrossRef]
- Dardenne, M.; Savino, W.; Bach, J.F. Modulation of thymic endocrine function by thyroid and steroid hormones. Int. J. Neurosci. 1988, 39, 325–334. [Google Scholar] [CrossRef]
- Moleti, M.; Trimarchi, F.; Vermiglio, F. Thyroid physiology in pregnancy. Endocr. Pract. 2014, 20, 589–596. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Salehzadeh, M.; Hamden, J.E.; Li, M.X.; Bajaj, H.; Wu, R.S.; Soma, K.K. Glucocorticoid production in lymphoid organs: Acute effects of lipopolysaccharide in neonatal and adult mice. Endocrinology 2022, 163, bqab244. [Google Scholar] [CrossRef]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef]
- Mittelstadt, P.R.; Monteiro, J.P.; Ashwell, J.D. Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J. Clin. Investig. 2012, 122, 2384–2394. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.P.; Khuat, L.T.; Sckisel, G.D.; Vick, L.V.; Minnar, C.M.; Dunai, C.; Le, C.T.; Curti, B.D.; Crittenden, M.; Merleev, A.; et al. Systemic immunostimulation induces glucocorticoid-mediated thymic involution succeeded by rebound hyperplasia which is impaired in aged recipients. Front. Immunol. 2024, 15, 1429912. [Google Scholar] [CrossRef] [PubMed]
- Ugor, E.; Prenek, L.; Pap, R.; Berta, G.; Ernszt, D.; Najbauer, J.; Németh, P.; Boldizsár, F.; Berki, T. Glucocorticoid hormone treatment enhances the cytokine production of regulatory T cells by upregulation of Foxp3 expression. Immunobiology 2018, 223, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Solano, M.E.; Arck, P.C. Steroids, pregnancy and fetal development. Front. Immunol. 2020, 10, 3017. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and health: Insights of melatonin action, biological functions, and associated disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Hardeland, R.; Rodella, L.F. Thymus-pineal gland axis: Revisiting its role in human life and ageing. Int. J. Mol. Sci. 2020, 21, 8806. [Google Scholar] [CrossRef]
- Michurina, S.V.; Miroshnichenko, S.M.; Ishchenko, I.Y.; Serykh, A.E.; Rachkovskaya, L.N. Effect of melatonin on the content of CD3low and CD3hi T cells in the thymus of mice with functional pinealectomy. Bull. Exp. Biol. Med. 2023, 174, 754–757. [Google Scholar] [CrossRef]
- Ozkanlar, S.; Kara, A.; Sengul, E.; Simsek, N.; Karadeniz, A.; Kurt, N. Melatonin modulates the immune system response and inflammation in diabetic rats experimentally-induced by alloxan. Horm. Metab. Res. 2016, 48, 137–144. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Assunção Salustiano, E.M.; Yen, P.W.; Soliman, A.; Vaillancourt, C. Melatonin in pregnancy: Effects on brain development and CNS programming disorders. Curr. Pharm. Des. 2016, 22, 978–986. [Google Scholar] [CrossRef]
- Hansenne, I.; Rasier, G.; Péqueux, C.; Brilot, F.; Renard, C.; Breton, C.; Greimers, R.; Legros, J.J.; Geenen, V.; Martens, H.J. Ontogenesis and functional aspects of oxytocin and vasopressin gene expression in the thymus network. J. Neuroimmunol. 2005, 158, 67–75. [Google Scholar] [CrossRef]
- Rae, M.; Lemos Duarte, M.; Gomes, I. Oxytocin and vasopressin: Signalling, behavioural modulation and potential therapeutic effects. Br. J. Pharmacol. 2022, 179, 1544–1564. [Google Scholar] [CrossRef]
- Francelin, C.; Veneziani, L.P.; Farias, A.D.S. Neurotransmitters modulate intrathymic T-cell development. Front. Cell. Dev. Biol. 2021, 9, 668067. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Wang, S.C.; Wang, Y.F. Approaches mediating oxytocin regulation of the immune system. Front. Immunol. 2017, 7, 693. [Google Scholar] [CrossRef] [PubMed]
- van der Post, J.A.; van Buul, B.J.; Hart, A.A.; van Heerikhuize, J.J.; Pesman, G.; Legros, J.J.; Steegers, E.A.; Swaab, D.F.; Boer, K. Vasopressin and oxytocin levels during normal pregnancy: Effects of chronic dietary sodium restriction. J. Endocrinol. 1997, 152, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Cameron, N.M. The role of oxytocin in mating and pregnancy. Horm. Behav. 2012, 61, 266–276. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Gruver, A.L.; Ventevogel, M.S.; Sempowski, G.D. Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J. Endocrinol. 2009, 203, 75–85. [Google Scholar] [CrossRef]
- Orlova, E.; Loginova, O.; Shirshev, S. Leptin regulates thymic plasmacytoid dendritic cell ability to influence the thymocyte distribution in vitro. Int. Immunopharmacol. 2023, 117, 109912. [Google Scholar] [CrossRef]
- Khant Aung, Z.; Grattan, D.R.; Ladyman, S.R. Pregnancy-induced adaptation of central sensitivity to leptin and insulin. Mol. Cell. Endocrinol. 2020, 516, 110933. [Google Scholar] [CrossRef]
- Veselský, L.; Holán, V.; Dostál, J.; Zelezná, B. Boar seminal immunosuppressive fraction attenuates the leptin concentration and restores the thymus mass during pregnancy in mice. Reproduction 2004, 127, 581–585. [Google Scholar] [CrossRef]
- Lamas, A.; Lopez, E.; Carrio, R.; Lopez, D.M. Adipocyte and leptin accumulation in tumor-induced thymic involution. Int. J. Mol. Med. 2016, 37, 133–138. [Google Scholar] [CrossRef]
- van Niekerk, G.; Christowitz, C.; Conradie, D.; Engelbrecht, A.M. Insulin as an immunomodulatory hormone. Cytokine Growth Factor. Rev. 2020, 52, 34–44. [Google Scholar] [CrossRef]
- Vafiadis, P.; Bennett, S.T.; Todd, J.A.; Nadeau, J.; Grabs, R.; Goodyer, C.G.; Wickramasinghe, S.; Colle, E.; Polychronakos, C. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 1997, 15, 289–292. [Google Scholar] [CrossRef]
- Mendes-da-Cruz, D.A.; Lemos, J.P.; Passos, G.A.; Savino, W. Abnormal T-cell development in the thymus of non-obese diabetic mice: Possible relationship with the pathogenesis of type 1 autoimmune diabetes. Front. Endocrinol. 2018, 9, 381. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Polychronakos, C. Insulin expression levels in the thymus modulate insulin-specific autoreactive T-cell tolerance: The mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes 2002, 51, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Yuen, B.T.K.; Purtha, W.; Balolong, J.M.; Phipps, J.D.; Crawford, F.; Bluestone, J.A.; Kappler, J.W.; Anderson, M.S. Aire mediates tolerance to insulin through thymic trimming of high-affinity T cell clones. Proc. Natl. Acad. Sci. USA 2024, 121, e2320268121. [Google Scholar] [CrossRef]
- Sabater, L.; Ferrer-Francesch, X.; Sospedra, M.; Caro, P.; Juan, M.; Pujol-Borrell, R. Insulin alleles and autoimmune regulator (AIRE) gene expression both influence insulin expression in the thymus. J. Autoimmun. 2005, 25, 312–318. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Brooks, V.L. Central actions of insulin during pregnancy and lactation. J. Neuroendocr. 2021, 33, e12946. [Google Scholar] [CrossRef]

| Factors and Cells | Effects on Thymic Function | Effects of Pregnancy | Species | Ref. |
|---|---|---|---|---|
| Osteoclast differentiation receptor RANK | Development of thymic regulatory T-cells | Fetal loss and gestational diabetes | Mice | [6] |
| Nuclear progesterone receptor | Regulation of thymus involution | Increases in expression of progesterone receptor | Mice | [7] |
| Estrogen and thymocyte | Regulation of thymus involution | Elevating levels of hormones | Mice | [8] |
| Thymic nonlymphoid cells, CCL25, CXCL12, CCL21, and CCL19 | Thymic involution | Maternal–fetal tolerance | Mice | [9] |
| Toll-like receptor | Thymic immune | Maternal immune tolerance | Sheep | [10] |
| NF-κB subunits | Thymic immune | Maternal central immune tolerance | Sheep | [11] |
| Nod-like receptor | Thymic immune responses | Maternal immunomodulation | Sheep | [12] |
| Complement components | Thymic immune | Maternal immune regulation | Sheep | [13] |
| IkappaB protein | Thymic immune regulation | Maternal immunologic tolerance | Sheep | [14] |
| Interferon-stimulated genes | Thymic immune | Maternal immunologic tolerance | Sheep | [67] |
| Prostaglandin synthases | Thymic immune regulation | Maternal immunologic tolerance | Sheep | [15] |
| Sex steroids | Cortical involution of the thymus | Immune suppression of the mother to paternal and fetal antigens | Humans and mice | [32] |
| Strain difference | Thymic weight | Number of progeny | Rats | [33] |
| Epithelial cells of the subcapsular cortex, mTECs | Thymus shrinks in size and the cortex | Maternal tolerance to fetal antigens | Mice | [34] |
| Estrogen and progesterone | Thymic involution | Maternal immune system maintains tolerance towards the allogeneic fetus | Humans | [35] |
| Treg cells | Immunosuppressive role of the thymus | Maternal–fetal tolerance | Mice | [36] |
| Cell populations | Thymic immune | Maternal immune reactivity | Mice | [37] |
| Gonadotropin-releasing hormone | Thymic involution | Maturation of T lymphocytes during pregnancy | Rats | [38] |
| Progesterone | Thymic involution | Adaptations to the semi-allogeneic fetus | Humans | [39] |
| Thymus regulatory T-cells, T-cell receptor, autoimmune regulator, and mTECs | Thymocyte development and differentiation in the thymus | Maternal–fetal tolerance to the fetus | Humans | [40] |
| CD4, MT1, and MT2 | Thymic immune regulation | Immune regulation of the maternal immune system | Sheep | [41] |
| Helper T cytokines | Thymic immune regulation | Immune tolerance in maternal immune system | Sheep | [42] |
| Klf4, thymic epithelial cells | Thymic involution | Maintaining cTEC numbers during pregnancy | Mice | [43] |
| α/β and γ/δ double negative T-cells | Thymocyte loss and thymic involution | Maintenance of pregnancy | Humans and mice | [44] |
| Double negative T regulatory cells | Thymic development | Implantation failure, and pregnancy loss | Humans and mice | [45] |
| Progesterone, CD4+Foxp3+ TReg cells | Thymic involution | Embryo implantation and fetal growth | Mice | [46] |
| Aire and mTECs | Thymic selection | Maintaining maternal–fetal tolerance | Humans and mice | [47] |
| Human chorionic gonadotropin | Antigen-independent differentiation of T-lymphocytes | Production of autocrine growth factors during pregnancy | Humans | [53] |
| Estriol and kisspeptin | Myeloid DC maturation in the thymus | Maintaining systemic tolerance of the mother | Humans | [58] |
| Estrogen | T-cell development | Immune suppression for a potential pregnancy | Humans and mice | [59] |
| Estrogen | Inhibit cortical double negative development | Pregnancy-driven involution | Humans and mice | [62] |
| Estrogen receptor α and β | Thymic immune regulation | Regulation of maternal immune function | [63] | |
| Progesterone | Thymic involution | Normal fertility | Humans, rats, and mice | [64] |
| Progesterone | Thymic involution and T-cell development | T-cell lymphopoiesis during pregnancy | Mice | [66] |
| Progesterone receptor and PIBF | Thymic immunoregulatory functions | Maternal immune tolerance | Sheep | [67] |
| GnRH and GnRHR | Modulation of thymus function | Blockade of lymphocyte development in maternal thymus | Sheep | [73] |
| Prolactin and PRLR | Thymic innate immune | Pregnancy increases expression of prolactin and PRLR | Sheep | [93] |
| Glucocorticoid | Thymic immune tolerance | Pregnancy increases expression of glucocorticoid receptors | Humans and mice | [106] |
| Oxytocins | Thymic immune regulation | Initiation of pregnancy | Mice and rats | [116] |
| Leptin | Thymus involution | Loss of thymus mass during pregnancy | Mice | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wang, X.; Zhang, L. Maternal Thymus Adaptations and Hormone Regulation During Pregnancy. Cells 2025, 14, 1534. https://doi.org/10.3390/cells14191534
Yang L, Wang X, Zhang L. Maternal Thymus Adaptations and Hormone Regulation During Pregnancy. Cells. 2025; 14(19):1534. https://doi.org/10.3390/cells14191534
Chicago/Turabian StyleYang, Ling, Xinxin Wang, and Leying Zhang. 2025. "Maternal Thymus Adaptations and Hormone Regulation During Pregnancy" Cells 14, no. 19: 1534. https://doi.org/10.3390/cells14191534
APA StyleYang, L., Wang, X., & Zhang, L. (2025). Maternal Thymus Adaptations and Hormone Regulation During Pregnancy. Cells, 14(19), 1534. https://doi.org/10.3390/cells14191534








