Is TREM2 a Stretch: Implications of TREM2 Along Spinal Cord Circuits in Health, Aging, Injury, and Disease
Abstract
1. Introduction
2. Triggering Receptor Expressed on Myeloid Cells 2 (TREM2)
2.1. What and Where Is TREM2?
2.2. TREM2 Ligands and Signaling Cascade
2.3. sTREM2
2.4. TREM2/DAP12 Mutations in Disease
| Mutation | Region | Associated Pathology | Reference |
|---|---|---|---|
| E14X | Signal Peptide | PLOSL | [81] |
| Q33X | Ig Domain | AD, PLOSL, FTD | [84,85,96,97,98,99,100,101] |
| Y38C | Ig Domain | AD, PLOSL, FTD | [83,84,97,101] |
| W44X | Ig Domain | PLOSL | [102] |
| R47H | Ig Domain | AD, ALS, FTD, PD | [84,85,93,94,103] |
| W50C | Ig Domain | PLOSL | [104] |
| R62H | Ig Domain | AD, ALS | [84,95,96,105] |
| T66M | Ig Domain | AD, PLOSL, FTD | [83,84,85,101,106,107] |
| N68K | Ig Domain | AD | [84] |
| W78X | Ig Domain | PLOSL | [80] |
| D86V | Ig Domain | FTD | [108] |
| D87N | Ig Domain | AD, ALS | [84,95] |
| T96K | Ig Domain | AD, FTD | [84,103,109] |
| R98W | Ig Domain | AD | [84] |
| S116C | Ig Domain | FTD | [85] |
| V126G | Ig Domain | PLOSL | [83,99] |
| D134G | Ig Domain | PLOSL | [80] |
| R136Q | Stalk | AD | [84] |
| H157Y | Stalk | AD, FTD | [84,86,110] |
| K186N | Helix | PLOSL | [80] |
| W198X | Tail | FTD | [107] |
| L211P | Tail | AD, FTD | [84,109,111] |
2.5. Different Mouse Models May Contribute to Conflicting TREM2 Results
3. The Dorsal Root Ganglion
3.1. Anatomical Overview of the Dorsal Root Ganglion
3.2. TREM2 in the Dorsal Root Ganglion
4. TREM2 in the Spinal Cord
Anatomical Overview of the Spinal Cord
5. TREM2 in Microglia
5.1. Disease-Associated Microglia
5.2. Synaptic Plasticity
5.3. Neuronal Bioenergetic Support
6. TREM2 in the Dorsal Horn
Neuropathic Pain
7. TREM2 in the Ventral Horn
7.1. Peripheral Nerve Injury
7.2. Aging
7.3. Amyotrophic Lateral Sclerosis
8. Spinal Cord Injury
9. TREM2 Along Peripheral Axons
9.1. Anatomical Overview of Peripheral Nerves
9.2. TREM2 in Schwann Cells
9.3. TREM2 in Peripheral Macrophages
10. TREM2 in Muscle
10.1. Anatomical Overview of Skeletal Muscle
10.2. Overview of Neuromuscular Junction Denervation/Reinnervation
10.3. TREM2 and the Neuromuscular Junction
11. TREM2 as a Therapeutic Target
12. Conclusions
| Pathology | Species | Modulation | Effect | Reference |
|---|---|---|---|---|
| Peripheral Nerve Injury | Mouse | TREM2 knockout | Reduced phagocytosis in DAM-like and female microglia, motoneuron chromatolytic reaction, muscle reinnervation | [142,146] |
| DAP12 knockout | Reduced allodynia, pro-inflammatory cytokines, motoneuron cell death | [60,186,209] | ||
| Intrathecal TREM2 agonist | Increased allodynia and DAP12 | [186] | ||
| Rat | Intrathecal TREM2 lentivirus overexpression | Increased allodynia | [187] | |
| Diabetes | Mouse | Intrathecal TREM2 lentivirus overexpression | Increased microglia number, phagocytosis, pro-inflammatory cytokines | [193] |
| Intrathecal TREM2 neutralizing antibody | Reduced microglia number, phagocytosis, pro-inflammatory cytokines | [193] | ||
| Bone fracture | Mouse | TREM2 Inhibition via Artesunate | Reduced allodynia | [196] |
| Chemotherapy | Mouse | Intrathecal TREM2 neutralizing antibody | Reduced pro-inflammatory cytokines, allodynia, and peripheral small nerve fiber loss | [199] |
| Amyotrophic Lateral Sclerosis | Mouse (SOD1 mutant) | TREM2 knockout | Reduced disease-associated microglia, increased homeostatic microglia | [91,146] |
| Mouse (AAV- overexpression of hTDP-43) | TREM2 knockout | Increased protein aggregation/reduced TDP-43 clearance, more rapid disease progression, increased loss of motor function | [53] | |
| Spinal Cord Injury | Mouse | TREM2 knockout | Improved microglia autophagy and lysosomal pathways at seven days post injury, reduced microglia phagocytosis, reduced glial scar formation, reduced lesion size, improved locomotion | [79,250] |
| BV2 Cells | TREM2 siRNA | Improved microglia autophagy and mitochondrial metabolism | [250] | |
| Health | Primary mouse Schwann Cells | Lentiviral TREM2 knockdown | In Schwann cells: impaired PI3K-AKT-mTOR signaling, activated AMPK and caspases, mitochondrial damage, impaired mitochondrial metabolism, increased apoptosis | [25] |
| Mouse | Mutant TREM2 | Altered skeletal muscle composition and strength | [307] | |
| Acute Motor Axonal Neuropathy | Mouse | TREM2 knockout | Decreased nerve conduction, myelin debris clearance, axon regeneration, and increased Schwann cell apoptosis | [25] |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5XFAD | 5 familial Alzheimer’s disease mutations |
| aa | Amino acids |
| AD | Alzheimer’s disease |
| ADAM10 | A Disintegrin and Metalloproteinase domain-containing protein 10 |
| ADAM17 | A Disintegrin and Metalloproteinase domain-containing protein 17 |
| ADI-R | Autism Diagnostic Interview-Revised |
| AKT | Protein kinase B |
| ALS | Amyotrophic Lateral Sclerosis |
| ALSP | Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia |
| AMAN | Acute Motor Axonal Neuropathy |
| AMPK | AMP-activated protein kinase |
| APOE | Apolipoprotein E |
| app1 | Antiphagocytic Protein 1 |
| arg1 | Arginase 1 |
| BBB | Blood–Brain Barrier |
| C1q | Complement Component 1q |
| CCL21 | CC chemokine ligand 21/6Ckine |
| ccr2 | Chemokine (C-C motif) Receptor 2 |
| CKO | Conditional Knockout |
| Clec7a | C-type lectin domain family 7, member A. |
| CNS | Central Nervous System |
| CSF1 | Colony Stimulating Factor 1 |
| CSF1-R | Colony Stimulating Factor 1 Receptor |
| CTSD | Cathepsin D |
| CX3CL1 | Fractalkine |
| CX3CR1 | C-X3-C motif chemokine receptor 1/Fractalkine receptor |
| DAM | Disease Associate Microglia |
| DAP10/Hcst | DNAX activating protein of 10 KD |
| DAP12/tryobp | DNAX activating protein of 12 KD |
| DRG | Dorsal Root Ganglion |
| ePtdSer | Externalized Phosphatidylserine |
| ERK | Extracellular Signal-Regulated Kinase |
| GKO | Global Knockout |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1 β |
| IL-34 | Interleukin-34 |
| IL-6 | Interleukin-6 |
| ITAM | Immunoreceptor Tyrosine-based Activation Motif. |
| KV2.1 | Potassium Voltage-Gated Channel, Shab-Related Subfamily, Member 1. |
| LAMP1 | Lysosome Associated Membrane Protein 1 |
| LPL | Lipoprotein Lipase |
| LPS | Lysophosphatidylcholine |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-activated protein kinase kinase |
| MND | Motor Neuron Disease |
| mTOR | Mechanistic target of rapamycin/mammalian target of rapamycin |
| NF-κB | Nuclear Factor kappa-light-chain enhancer of activated B cells |
| NF2 | Merline |
| NMJ | Neuromuscular Junction |
| olfml3 | Olfactomedin-like 3 |
| P2RX4 | P2X purinoceptor 4 |
| P2RY12 | Purinergic Receptor P2Y, G Protein Coupled, 12 |
| PD | Parkinson’s disease |
| PI3K | Phosphatidylinositol 3-kinase. |
| PLCγ | Phospholipase C |
| PNS | Peripheral Nervous System |
| PtdSer | Phosphatidylserine |
| PYK2 | Proline-rich tyrosine kinase 2 |
| Raf | Rapidly Accelerated Fibrosarcoma |
| RAS | Reticular Activating System |
| RNA | Ribonucleic acid |
| SHIP1 | Src homology 2 (SH2) domain-containing inositol polyphosphate 5-phosphatase 1. |
| SMA | Spinal Muscular Atrophy |
| SOD1 | Superoxide Dismutase 1. |
| spp1 | Secreted Phosphoprotein 1. |
| sTREM2 | Soluble Triggering Receptor Expressed on Myeloid Cells 2 |
| STZ | Streptozotocin |
| SYK | Spleen Tyrosine Kinase |
| TAMS | Tumor-Associated Macrophages |
| TDP-43 | TAR DNA-binding protein 43 |
| TGF-β | Transforming Growth Factor beta |
| Tmem119 | Transmembrane protein 119 |
| TNFα | Tumor Necrosis Factor-alpha |
| TREM2 | Triggering Receptor Expressed on Myeloid Cells 2 |
| VAV2/3 | Vav family of guanine nucleotide exchange factors 2/3 |
| VEGFA | Vascular Endothelial Growth Factor A |
References
- Philips, T.; Robberecht, W. Neuroinflammation in amyotrophic lateral sclerosis: Role of glial activation in motor neuron disease. Lancet Neurol. 2011, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, F. Role of neuroinflammation in amyotrophic lateral sclerosis: Cellular mechanisms and therapeutic implications. Front. Immunol. 2017, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Hooten, K.G.; Beers, D.R.; Zhao, W.; Appel, S.H. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics 2015, 12, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.H.; Swarts, E.A.; Kigerl, K.A.; Mifflin, K.A.; Guan, Z.; Noble, B.T.; Wang, Y.; Witcher, K.G.; Godbout, J.P.; Popovich, P.G. Microglia promote maladaptive plasticity in autonomic circuitry after spinal cord injury in mice. Sci. Transl. Med. 2024, 16, eadi3259. [Google Scholar] [CrossRef]
- Thompson, A.K.; Sinkjær, T. Chapter 30—Features and physiology of spinal stretch reflexes in people with chronic spinal cord injury. In Cellular, Molecular, Physiological, and Behavioral Aspects of Spinal Cord Injury; Rajendram, R., Preedy, V.R., Martin, C.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2022; pp. 365–375. [Google Scholar]
- Li, C.; Xiong, W.; Wan, B.; Kong, G.; Wang, S.; Wang, Y.; Fan, J. Role of peripheral immune cells in spinal cord injury. Cell. Mol. Life Sci. 2022, 80, 2. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Marion, C.M.; Mifflin, K.A.; Alfredo, A.N.; Rodgers, K.A.; Kigerl, K.A.; Popovich, P.G.; McTigue, D.M. System failure: Systemic inflammation following spinal cord injury. Eur. J. Immunol. 2024, 54, 2250274. [Google Scholar] [CrossRef]
- Schwab, J.M.; Zhang, Y.; Kopp, M.A.; Brommer, B.; Popovich, P.G. The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury. Exp. Neurol. 2014, 258, 121–129. [Google Scholar] [CrossRef]
- Pottorf, T.S.; Rotterman, T.M.; McCallum, W.M.; Haley-Johnson, Z.A.; Alvarez, F.J. The Role of Microglia in Neuroinflammation of the Spinal Cord after Peripheral Nerve Injury. Cells 2022, 11, 2083. [Google Scholar] [CrossRef]
- Konishi, H.; Kiyama, H. Microglial TREM2/DAP12 Signaling: A Double-Edged Sword in Neural Diseases. Front. Cell. Neurosci. 2018, 12, 206. [Google Scholar] [CrossRef]
- Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Colonna, M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 2003, 3, 445–453. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Z.; Zhang, X.; Xiong, M.; Meng, L.; Zhang, Z. TREM2 ectodomain and its soluble form in Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 204. [Google Scholar] [CrossRef]
- Lanier, L.L. DAP10-and DAP12-associated receptors in innate immunity. Immunol. Rev. 2009, 227, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Allcock, R.J.; Barrow, A.D.; Forbes, S.; Beck, S.; Trowsdale, J. The human TREM gene cluster at 6p21. 1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur. J. Immunol. 2003, 33, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, J.; Deng, M.; Azuma, M.; Funami, K.; Shime, H.; Oshiumi, H.; Matsumoto, M.; Kasahara, M.; Seya, T. Double-stranded RNA analog and type I interferon regulate expression of Trem paired receptors in murine myeloid cells. BMC Immunol. 2016, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Stet, R.J.; Hermsen, T.; Westphal, A.H.; Jukes, J.; Engelsma, M.; Lidy Verburg-van Kemenade, B.; Dortmans, J.; Aveiro, J.; Savelkoul, H.F. Novel immunoglobulin-like transcripts in teleost fish encode polymorphic receptors with cytoplasmic ITAM or ITIM and a new structural Ig domain similar to the natural cytotoxicity receptor NKp44. Immunogenetics 2005, 57, 77–89. [Google Scholar] [CrossRef]
- Viertlboeck, B.C.; Schmitt, R.; Göbel, T.W. The chicken immunoregulatory receptor families SIRP, TREM, and CMRF35/CD300L. Immunogenetics 2006, 58, 180–190. [Google Scholar] [CrossRef]
- Jin, S.C.; Benitez, B.A.; Karch, C.M.; Cooper, B.; Skorupa, T.; Carrell, D.; Norton, J.B.; Hsu, S.; Harari, O.; Cai, Y. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum. Mol. Genet. 2014, 23, 5838–5846. [Google Scholar] [CrossRef]
- Jay, T.R.; von Saucken, V.E.; Landreth, G.E. TREM2 in neurodegenerative diseases. Mol. Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef]
- Shaw, B.C.; Snider, H.C.; Turner, A.K.; Zajac, D.J.; Simpson, J.F.; Estus, S.; Combs, C. An Alternatively Spliced TREM2 Isoform Lacking the Ligand Binding Domain is Expressed in Human Brain. J. Alzheimer’s Dis. 2022, 87, 1647–1657. [Google Scholar] [CrossRef]
- Peng, Q.; Malhotra, S.; Torchia, J.A.; Kerr, W.G.; Coggeshall, K.M.; Humphrey, M.B. TREM2-and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci. Signal. 2010, 3, ra38. [Google Scholar] [CrossRef]
- Wunderlich, P.; Glebov, K.; Kemmerling, N.; Tien, N.T.; Neumann, H.; Walter, J. Sequential proteolytic processing of the triggering receptor expressed on myeloid cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J. Biol. Chem. 2013, 288, 33027–33036. [Google Scholar] [CrossRef]
- Lin, M.; Yu, J.X.; Zhang, W.X.; Lao, F.X.; Huang, H.C. Roles of TREM2 in the Pathological Mechanism and the Therapeutic Strategies of Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2024, 11, 1682–1695. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, Q.; Chen, Y.; Wen, X.; Shan, F. TREM2 deficiency impairs the energy metabolism of Schwann cells and exacerbates peripheral neurological deficits. Cell Death Dis. 2024, 15, 193. [Google Scholar] [CrossRef]
- Sun, H.; Feng, J.; Tang, L. Function of TREM1 and TREM2 in liver-related diseases. Cells 2020, 9, 2626. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, I.H.; Iimura, T.; Kong, S.W. Two macrophages, osteoclasts and microglia: From development to pleiotropy. Bone Res. 2021, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Yang, L.; Zhang, Y.; Yang, L. TREM2+ macrophages: A key role in disease development. Front. Immunol. 2025, 16, 1550893. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Colonna, M. TREM2—A key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Wang, Y. TREM2 variants: New keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 2016, 17, 201–207. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Painter, M.M.; Atagi, Y.; Liu, C.C.; Rademakers, R.; Xu, H.; Fryer, J.D.; Bu, G. TREM2 in CNS homeostasis and neurodegenerative disease. Mol. Neurodegener. 2015, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Brantley-Sieders, D.M.; Zhuang, G.; Vaught, D.; Freeman, T.; Hwang, Y.; Hicks, D.; Chen, J. Host deficiency in Vav2/3 guanine nucleotide exchange factors impairs tumor growth, survival, and angiogenesis in vivo. Mol. Cancer Res. 2009, 7, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Götz, J. Pyk2 is a Novel Tau Tyrosine Kinase that is Regulated by the Tyrosine Kinase Fyn. J. Alzheimer’s Dis. 2018, 64, 205–221. [Google Scholar] [CrossRef]
- Martelli, M.P.; Lin, H.; Zhang, W.; Samelson, L.E.; Bierer, B.E. Signaling via LAT (linker for T-cell activation) and Syk/ZAP70 is required for ERK activation and NFAT transcriptional activation following CD2 stimulation. Blood 2000, 96, 2181–2190. [Google Scholar] [CrossRef]
- Tan, M.S.; Yu, J.T.; Tan, L. Bridging integrator 1 (BIN1): Form, function, and Alzheimer’s disease. Trends Mol. Med. 2013, 19, 594–603. [Google Scholar] [CrossRef]
- Griciuc, A.; Patel, S.; Federico, A.N.; Choi, S.H.; Innes, B.J.; Oram, M.K.; Cereghetti, G.; McGinty, D.; Anselmo, A.; Sadreyev, R.I.; et al. TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron 2019, 103, 820–835.e827. [Google Scholar] [CrossRef]
- Cheng, B.; Li, X.; Dai, K.; Duan, S.; Rong, Z.; Chen, Y.; Lü, L.; Liu, Z.; Huang, X.; Xu, H. Triggering receptor expressed on myeloid cells-2 (TREM2) interacts with colony-stimulating factor 1 receptor (CSF1R) but is not necessary for CSF1/CSF1R-mediated microglial survival. Front. Immunol. 2021, 12, 633796. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, X.; Li, Y.; Bu, G.; Chen, X.F. TREM2 and sTREM2 in Alzheimer’s disease: From mechanisms to therapies. Mol. Neurodegener. 2025, 20, 43. [Google Scholar] [CrossRef]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Shirotani, K.; Hori, Y.; Yoshizaki, R.; Higuchi, E.; Colonna, M.; Saito, T.; Hashimoto, S.; Saito, T.; Saido, T.C.; Iwata, N. Aminophospholipids are signal-transducing TREM2 ligands on apoptotic cells. Sci. Rep. 2019, 9, 7508. [Google Scholar] [CrossRef] [PubMed]
- Kober, D.L.; Stuchell-Brereton, M.D.; Kluender, C.E.; Dean, H.B.; Strickland, M.R.; Steinberg, D.F.; Nelson, S.S.; Baban, B.; Holtzman, D.M.; Frieden, C. Functional insights from biophysical study of TREM2 interactions with apoE and Aβ1-42. Alzheimer’s Dement. 2021, 17, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.P.; O’Driscoll, M.; Litman, G.W. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics 2012, 64, 39–47. [Google Scholar] [CrossRef]
- Song, W.; Hooli, B.; Mullin, K.; Jin, S.C.; Cella, M.; Ulland, T.K.; Wang, Y.; Tanzi, R.E.; Colonna, M. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimer’s Dement. 2017, 13, 381–387. [Google Scholar] [CrossRef]
- Atagi, Y.; Liu, C.C.; Painter, M.M.; Chen, X.F.; Verbeeck, C.; Zheng, H.; Li, X.; Rademakers, R.; Kang, S.S.; Xu, H.; et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2). J. Biol. Chem. 2015, 290, 26043–26050. [Google Scholar] [CrossRef]
- Bailey, C.C.; DeVaux, L.B.; Farzan, M. The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J. Biol. Chem. 2015, 290, 26033–26042. [Google Scholar] [CrossRef]
- Kawabori, M.; Kacimi, R.; Kauppinen, T.; Calosing, C.; Kim, J.Y.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015, 35, 3384–3396. [Google Scholar] [CrossRef]
- Daws, M.R.; Sullam, P.M.; Niemi, E.C.; Chen, T.T.; Tchao, N.K.; Seaman, W.E. Pattern recognition by TREM-2: Binding of anionic ligands. J. Immunol. 2003, 171, 594–599. [Google Scholar] [CrossRef]
- Iizasa, E.i.; Chuma, Y.; Uematsu, T.; Kubota, M.; Kawaguchi, H.; Umemura, M.; Toyonaga, K.; Kiyohara, H.; Yano, I.; Colonna, M.; et al. TREM2 is a receptor for non-glycosylated mycolic acids of mycobacteria that limits anti-mycobacterial macrophage activation. Nat. Commun. 2021, 12, 2299. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Piña-Crespo, J.C.; Zhang, M. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron 2018, 97, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, Y.U.; Zhao, S.; Zhang, L.; Bosco, D.B.; Pang, Y.P.; Zhong, J.; Sheth, U.; Martens, Y.A.; Zhao, N. TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration. Nat. Neurosci. 2022, 25, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Mills III, W.A.; Eyo, U.B. TREMble before TREM2: The mighty microglial receptor conferring neuroprotective properties in TDP-43 mediated neurodegeneration. Neurosci. Bull. 2023, 39, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Willette-Brown, J.; Taylor, L.S.; McVicar, D.W. Regulation of Ly49D/DAP12 Signal Transduction by Src-Family Kinases and CD4512. J. Immunol. 2006, 176, 6615–6623. [Google Scholar] [CrossRef]
- Alonso, G.; Koegl, M.; Mazurenko, N.; Courtneidge, S.A. Sequence Requirements for Binding of Src Family Tyrosine Kinases to Activated Growth Factor Receptors. J. Biol. Chem. 1995, 270, 9840–9848. [Google Scholar] [CrossRef]
- Luo, J.; Elwood, F.; Britschgi, M.; Villeda, S.; Zhang, H.; Ding, Z.; Zhu, L.; Alabsi, H.; Getachew, R.; Narasimhan, R.; et al. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J. Exp. Med. 2013, 210, 157–172. [Google Scholar] [CrossRef]
- Hamilton, J.A. CSF-1 signal transduction. J. Leukoc. Biol. 1997, 62, 145–155. [Google Scholar] [CrossRef]
- Pixley, F.J.; Stanley, E.R. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol. 2004, 14, 628–638. [Google Scholar] [CrossRef]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M. Injured sensory neuron–derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef]
- Otero, K.; Turnbull, I.R.; Poliani, P.L.; Vermi, W.; Cerutti, E.; Aoshi, T.; Tassi, I.; Takai, T.; Stanley, S.L.; Miller, M. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. Nat. Immunol. 2009, 10, 734–743. [Google Scholar] [CrossRef]
- Thornton, P.; Sevalle, J.; Deery, M.J.; Fraser, G.; Zhou, Y.; Ståhl, S.; Franssen, E.H.; Dodd, R.B.; Qamar, S.; Gomez Perez-Nievas, B.; et al. TREM2 shedding by cleavage at the H157-S158 bond is accelerated for the Alzheimer’s disease-associated H157Y variant. EMBO Mol. Med. 2017, 9, 1366–1378. [Google Scholar] [CrossRef]
- Schlepckow, K.; Kleinberger, G.; Fukumori, A.; Feederle, R.; Lichtenthaler, S.F.; Steiner, H.; Haass, C. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol. Med. 2017, 9, 1356–1365. [Google Scholar] [CrossRef]
- Glebov, K.; Wunderlich, P.; Karaca, I.; Walter, J. Functional involvement of γ-secretase in signaling of the triggering receptor expressed on myeloid cells-2 (TREM2). J. Neuroinflamm. 2016, 13, 17. [Google Scholar] [CrossRef]
- Feuerbach, D.; Schindler, P.; Barske, C.; Joller, S.; Beng-Louka, E.; Worringer, K.A.; Kommineni, S.; Kaykas, A.; Ho, D.J.; Ye, C.; et al. ADAM17 is the main sheddase for the generation of human triggering receptor expressed in myeloid cells (hTREM2) ectodomain and cleaves TREM2 after Histidine 157. Neurosci. Lett. 2017, 660, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Filipello, F.; Goldsbury, C.; You, S.F.; Locca, A.; Karch, C.M.; Piccio, L. Soluble TREM2: Innocent bystander or active player in neurological diseases? Neurobiol. Dis. 2022, 165, 105630. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, M.; Coronel, I.; Tsai, A.P.; Di Prisco, G.V.; Pennington, T.; Atwood, B.K.; Puntambekar, S.S.; Smith, D.C.; Martinez, P.; Han, S.; et al. TREM2 splice isoforms generate soluble TREM2 species that disrupt long-term potentiation. Genome Med. 2023, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, M.; Landreth, G.E. TREM2 splicing emerges as crucial aspect to understand TREM2 biology. J. Leukoc. Biol. 2021, 110, 827–828. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J., II; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain J. Neurol. 2008, 131, 3081–3091. [Google Scholar] [CrossRef]
- Jericó, I.; Vicuña-Urriza, J.; Blanco-Luquin, I.; Macias, M.; Martinez-Merino, L.; Roldán, M.; Rojas-Garcia, R.; Pagola-Lorz, I.; Carbayo, A.; De Luna, N.; et al. Profiling TREM2 expression in amyotrophic lateral sclerosis. Brain Behav. Immun. 2023, 109, 117–126. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, X.F.; Wang, T.; Wang, Z.; Liao, C.; Wang, Z.; Huang, R.; Wang, D.; Li, X.; Wu, L.; et al. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 2017, 214, 597–607. [Google Scholar] [CrossRef]
- Song, W.M.; Joshita, S.; Zhou, Y.; Ulland, T.K.; Gilfillan, S.; Colonna, M. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med. 2018, 215, 745–760. [Google Scholar] [CrossRef]
- Wu, K.; Byers, D.E.; Jin, X.; Agapov, E.; Alexander-Brett, J.; Patel, A.C.; Cella, M.; Gilfilan, S.; Colonna, M.; Kober, D.L.; et al. TREM-2 promotes macrophage survival and lung disease after respiratory viral infection. J. Exp. Med. 2015, 212, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, E.H.; Kim, H.J.; Jo, S.; Lee, S.; Seo, S.W.; Park, H.H.; Koh, S.H.; Lee, J.H. The relationship of soluble TREM2 to other biomarkers of sporadic Alzheimer’s disease. Sci. Rep. 2021, 11, 13050. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; Seyedmirzaei, H.; Karami, S. Neuroimaging biomarkers and CSF sTREM2 levels in Alzheimer’s disease: A longitudinal study. Sci. Rep. 2024, 14, 15318. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhan, Y.; Zhu, W.; Yang, Q.; Pei, J. Association of soluble TREM2 with Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Front. Aging Neurosci. 2024, 16, 1407980. [Google Scholar] [CrossRef]
- Jiao, L.; Yang, J.; Wang, W.; Liu, X.; Fu, Y.; Fan, D. sTREM2 cerebrospinal fluid levels are a potential biomarker in amyotrophic lateral sclerosis and associate with UMN burden. Front. Neurol. 2024, 15, 1515252. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Green, C.; Altschuler, G.; Wei, W.; Bury, J.J.; Heath, P.R.; Wyles, M.; Gelsthorpe, C.; Highley, J.R.; Lorente-Pons, A. A data-driven approach links microglia to pathology and prognosis in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2017, 5, 23. [Google Scholar] [CrossRef]
- Gao, H.; Di, J.; Clausen, B.H.; Wang, N.; Zhu, X.; Zhao, T.; Chang, Y.; Pang, M.; Yang, Y.; He, R. Distinct myeloid population phenotypes dependent on TREM2 expression levels shape the pathology of traumatic versus demyelinating CNS disorders. Cell Rep. 2023, 42, 112629. [Google Scholar] [CrossRef]
- Paloneva, J.; Manninen, T.; Christman, G.; Hovanes, K.; Mandelin, J.; Adolfsson, R.; Bianchin, M.; Bird, T.; Miranda, R.; Salmaggi, A.; et al. Mutations in Two Genes Encoding Different Subunits of a Receptor Signaling Complex Result in an Identical Disease Phenotype. Am. J. Hum. Genet. 2002, 71, 656–662. [Google Scholar] [CrossRef]
- Paloneva, J.; Mandelin, J.; Kiialainen, A.; Böhling, T.; Prudlo, J.; Hakola, P.; Haltia, M.; Konttinen, Y.T.; Peltonen, L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med. 2003, 198, 669–675. [Google Scholar] [CrossRef]
- Zhou, Y.; Tada, M.; Cai, Z.; Andhey, P.S.; Swain, A.; Miller, K.R.; Gilfillan, S.; Artyomov, M.N.; Takao, M.; Kakita, A.; et al. Human early-onset dementia caused by DAP12 deficiency reveals a unique signature of dysregulated microglia. Nat. Immunol. 2023, 24, 545–557. [Google Scholar] [CrossRef]
- Kober, D.L.; Alexander-Brett, J.M.; Karch, C.M.; Cruchaga, C.; Colonna, M.; Holtzman, M.J.; Brett, T.J. Neurodegenerative disease mutations in TREM2 reveal a functional surface and distinct loss-of-function mechanisms. eLife 2016, 5, e20391. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Borroni, B.; Ferrari, F.; Galimberti, D.; Nacmias, B.; Barone, C.; Bagnoli, S.; Fenoglio, C.; Piaceri, I.; Archetti, S.; Bonvicini, C.; et al. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol. Aging 2014, 35, 934.e7–934.e10. [Google Scholar] [CrossRef] [PubMed]
- Ogonowski, N.; Santamaria-Garcia, H.; Baez, S.; Lopez, A.; Laserna, A.; Garcia-Cifuentes, E.; Ayala-Ramirez, P.; Zarante, I.; Suarez-Obando, F.; Reyes, P. Frontotemporal dementia presentation in patients with heterozygous p. H157Y variant of TREM2. J. Med. Genet. 2023, 60, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Y.; Hwang, S.; Archuleta, K.; Huang, H.; Campos, A.; Murad, R.; Piña-Crespo, J.; Xu, H.; Huang, T.Y. Trem2 deletion enhances tau dispersion and pathology through microglia exosomes. Mol. Neurodegener. 2022, 17, 58. [Google Scholar] [CrossRef]
- Popescu, A.S.; Butler, C.A.; Allendorf, D.H.; Piers, T.M.; Mallach, A.; Roewe, J.; Reinhardt, P.; Cinti, A.; Redaelli, L.; Boudesco, C.; et al. Alzheimer’s disease-associated R47H TREM2 increases, but wild-type TREM2 decreases, microglial phagocytosis of synaptosomes and neuronal loss. Glia 2023, 71, 974–990. [Google Scholar] [CrossRef]
- Zhou, S.L.; Tan, C.C.; Hou, X.H.; Cao, X.P.; Tan, L.; Yu, J.T. TREM2 Variants and Neurodegenerative Diseases: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 68, 1171–1184. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e569. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, S.; Mullen, B.; Baker, M.; Lynch, T.; Finger, E.; Seeley, W.W.; Hatanpaa, K.J.; Lomen-Hoerth, C.; Kertesz, A.; Bigio, E.H. TREM2 in neurodegeneration: Evidence for association of the p. R47H variant with frontotemporal dementia and Parkinson’s disease. Mol. Neurodegener. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Cady, J.; Koval, E.D.; Benitez, B.A.; Zaidman, C.; Jockel-Balsarotti, J.; Allred, P.; Baloh, R.H.; Ravits, J.; Simpson, E.; Appel, S.H. TREM2 variant p. R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014, 71, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Peplonska, B.; Berdynski, M.; Mandecka, M.; Barczak, A.; Kuzma-Kozakiewicz, M.; Barcikowska, M.; Zekanowski, C. TREM2 variants in neurodegenerative disorders in the Polish population. Homozygosity and compound heterozygosity in FTD patients. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 407–412. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, W.; Wang, X. TREM2 variants and risk of Alzheimer’s disease: A meta-analysis. Neurol. Sci. 2015, 36, 1881–1888. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Lohmann, E.; Brás, J.M.; Gibbs, J.R.; Rohrer, J.D.; Gurunlian, N.; Dursun, B.; Bilgic, B.; Hanagasi, H.; Gurvit, H. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia–like syndrome without bone involvement. JAMA Neurol. 2013, 70, 78–84. [Google Scholar] [CrossRef]
- Bock, V.; Botturi, A.; Gaviani, P.; Lamperti, E.; Maccagnano, C.; Piccio, L.; Silvani, A.; Salmaggi, A. Polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL): A new report of an Italian woman and review of the literature. J. Neurol. Sci. 2013, 326, 115–119. [Google Scholar] [CrossRef]
- Klunemann, H.; Ridha, B.; Magy, L.; Wherrett, J.; Hemelsoet, D.; Keen, R.; De Bleecker, J.; Rossor, M.; Marienhagen, J.; Klein, H. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology 2005, 64, 1502–1507. [Google Scholar] [CrossRef]
- Soragna, D.; Tupler, R.; Ratti, M.; Montalbetti, L.; Papi, L.; Sestini, R. An Italian family affected by Nasu-Hakola disease with a novel genetic mutation in the TREM2 gene. J. Neurol. Neurosurg. Psychiatry 2003, 74, 825–826. [Google Scholar] [CrossRef]
- Samanci, B.; Bilgiç, B.; Gelişin, Ö.; Tepgeç, F.; Guven, G.; Tüfekçioğlu, Z.; Alaylıoğlu, M.; Hanagasi, H.A.; Gürvit, H.; Guerreiro, R. TREM2 variants as a possible cause of frontotemporal dementia with distinct neuroimaging features. Eur. J. Neurol. 2021, 28, 2603–2613. [Google Scholar] [CrossRef]
- Numasawa, Y.; Yamaura, C.; Ishihara, S.; Shintani, S.; Yamazaki, M.; Tabunoki, H.; Satoh, J.I. Nasu–Hakola disease with a splicing mutation of TREM2 in a Japanese family. Eur. J. Neurol. 2011, 18, 1179–1183. [Google Scholar] [CrossRef]
- Dijkstra, J.I.R.; Vermunt, L.; Venkatraghavan, V.; Ozhegov, G.; Coomans, E.M.; Ossenkoppele, R.; van de Giessen, E.; Hulsman, M.; de Geus, C.M.; van der Flier, W.M.; et al. TREM2 risk variants and associated endophenotypes in alzheimer’s disease. Alzheimer’s Res. Ther. 2025, 17, 57. [Google Scholar] [CrossRef]
- Dardiotis, E.; Siokas, V.; Pantazi, E.; Dardioti, M.; Rikos, D.; Xiromerisiou, G.; Markou, A.; Papadimitriou, D.; Speletas, M.; Hadjigeorgiou, G.M. A novel mutation in TREM2 gene causing Nasu-Hakola disease and review of the literature. Neurobiol. Aging 2017, 53, 194.e113–194.e122. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.; Katsel, P.; Fam, P.; Tan, W.; Purohit, D.P.; Haroutunian, V. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer’s dementia. Alzheimer’s Dement. 2015, 11, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Le Ber, I.; De Septenville, A.; Guerreiro, R.; Bras, J.; Camuzat, A.; Caroppo, P.; Lattante, S.; Couarch, P.; Kabashi, E.; Bouya-Ahmed, K. Homozygous TREM2 mutation in a family with atypical frontotemporal dementia. Neurobiol. Aging 2014, 35, 2419.e23–2419.e25. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.; Lopera, F.; Siniard, A.L.; Corneveaux, J.J.; Schrauwen, I.; Carvajal, J.; Muñoz, C.; Ramirez-Restrepo, M.; Gaiteri, C.; Myers, A.J. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2077.e2011–2077.e2018. [Google Scholar] [CrossRef]
- Guerreiro, R.; Bilgic, B.; Guven, G.; Brás, J.; Rohrer, J.; Lohmann, E.; Hanagasi, H.; Gurvit, H.; Emre, M. A novel compound heterozygous mutation in TREM2 found in a Turkish frontotemporal dementia-like family. Neurobiol. Aging 2013, 34, 2890.e2891–2890.e2895. [Google Scholar] [CrossRef]
- Thelen, M.; Razquin, C.; Hernández, I.; Gorostidi, A.; Sanchez-Valle, R.; Ortega-Cubero, S.; Wolfsgruber, S.; Drichel, D.; Fliessbach, K.; Duenkel, T. Investigation of the role of rare TREM2 variants in frontotemporal dementia subtypes. Neurobiol. Aging 2014, 35, 2657.e2613–2657.e2619. [Google Scholar] [CrossRef]
- Jiang, T.; Tan, L.; Chen, Q.; Tan, M.S.; Zhou, J.S.; Zhu, X.C.; Lu, H.; Wang, H.F.; Zhang, Y.D.; Yu, J.T. A rare coding variant in TREM2 increases risk for Alzheimer’s disease in Han Chinese. Neurobiol. Aging 2016, 42, 217.e211–217.e213. [Google Scholar] [CrossRef]
- Jin, S.C.; Carrasquillo, M.M.; Benitez, B.A.; Skorupa, T.; Carrell, D.; Patel, D.; Lincoln, S.; Krishnan, S.; Kachadoorian, M.; Reitz, C. TREM2 is associated with increased risk for Alzheimer’s disease in African Americans. Mol. Neurodegener. 2015, 10, 19. [Google Scholar] [CrossRef]
- Kang, S.S.; Kurti, A.; Baker, K.E.; Liu, C.C.; Colonna, M.; Ulrich, J.D.; Holtzman, D.M.; Bu, G.; Fryer, J.D. Behavioral and transcriptomic analysis of Trem2-null mice: Not all knockout mice are created equal. Hum. Mol. Genet. 2018, 27, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Filipello, F.; Morini, R.; Corradini, I.; Zerbi, V.; Canzi, A.; Michalski, B.; Erreni, M.; Markicevic, M.; Starvaggi-Cucuzza, C.; Otero, K. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 2018, 48, 979–991.e978. [Google Scholar] [CrossRef] [PubMed]
- Tagliatti, E.; Desiato, G.; Mancinelli, S.; Bizzotto, M.; Gagliani, M.C.; Faggiani, E.; Hernández-Soto, R.; Cugurra, A.; Poliseno, P.; Miotto, M.; et al. Trem2 expression in microglia is required to maintain normal neuronal bioenergetics during development. Immunity 2024, 57, 86–105.e109. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.D.; Sautkulis, L.N.; Danielson, P.E.; Cooper, J.; Hasel, K.W.; Hilbush, B.S.; Sutcliffe, J.G.; Carson, M.J. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 2002, 83, 1309–1320. [Google Scholar] [CrossRef]
- Forabosco, P.; Ramasamy, A.; Trabzuni, D.; Walker, R.; Smith, C.; Bras, J.; Levine, A.P.; Hardy, J.; Pocock, J.M.; Guerreiro, R.; et al. Insights into TREM2 biology by network analysis of human brain gene expression data. Neurobiol. Aging 2013, 34, 2699–2714. [Google Scholar] [CrossRef]
- Leyns, C.E.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Remolina Serrano, J.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529. [Google Scholar] [CrossRef]
- Leyns, C.E.; Gratuze, M.; Narasimhan, S.; Jain, N.; Koscal, L.J.; Jiang, H.; Manis, M.; Colonna, M.; Lee, V.M.; Ulrich, J.D. TREM2 function impedes tau seeding in neuritic plaques. Nat. Neurosci. 2019, 22, 1217–1222. [Google Scholar] [CrossRef]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef]
- Brierley, J.B. The penetration of particulate matter from the cerebrospinal fluid into the spinal ganglia, peripheral nerves, and perivascular spaces of the central nervous system. J. Neurol. Neurosurg. Psychiatry 1950, 13, 203–215. [Google Scholar] [CrossRef]
- Devor, M. Unexplained peculiarities of the dorsal root ganglion. PAIN 1999, 82, S27–S35. [Google Scholar] [CrossRef]
- Nagashima, K.; Oota, K. A Histopathological Study of the Human Spinal Ganglia. Normal Variations in Aging. Acta Patholigica Jpn. 1974, 24, 333–344. [Google Scholar]
- Tandrup, T. Unbiased estimates of number and size of rat dorsal root ganglion cells in studies of structure and cell survival. J. Neurocytol. 2004, 33, 173–192. [Google Scholar] [CrossRef] [PubMed]
- West, C.A.; McKay Hart, A.; Terenghi, G.; Wiberg, M. Sensory neurons of the human brachial plexus: A quantitative study employing optical fractionation and in vivo volumetric magnetic resonance imaging. Neurosurgery 2012, 70, 1183–1194, discussion 1194. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.V.; Kuramatilake, J.; Barry, C.M.; Matusica, D. Ultrastructure of dorsal root ganglia. Cell Tissue Res. 2023, 393, 17–36. [Google Scholar] [CrossRef]
- Avraham, O.; Feng, R.; Ewan, E.E.; Rustenhoven, J.; Zhao, G.; Cavalli, V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. eLife 2021, 10, e68457. [Google Scholar] [CrossRef]
- Feringa, E.R.; Lee, G.W.; Vahlsing, H.L.; Gilbertie, W.J. Cell death in the adult rat dorsal root ganglion after hind limb amputation, spinal cord transection, or both operations. Exp. Neurol. 1985, 87, 349–357. [Google Scholar] [CrossRef]
- Lu, S. Single-Cell Transcriptome Analyses of Dorsal Root Ganglia in Aged Hyperlipidemic Mice. Ph.D. Thesis, Faculty of Medicine, LMU München, München, Germany, 2023. [Google Scholar]
- Li, X.; Zhuang, Z.; Hao, Y.; Lin, S.; Gu, J.; Chang, S.; Lan, L.; Zhao, G.; Zhang, D. Paeonol Relieves Chronic Neuropathic Pain by Reducing Communication Between Schwann Cells and Macrophages in the Dorsal Root Ganglia After Injury. Int. J. Mol. Sci. 2025, 26, 3964. [Google Scholar] [CrossRef]
- Qu, Y.; Cai, R.; Li, Q.; Wang, H.; Lu, L. Neuroinflammation signatures in dorsal root ganglia following chronic constriction injury. Heliyon 2024, 10, e31481. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Hamel, K.A.; Morvan, M.G.; Yu, S.; Leff, J.; Guan, Z.; Braz, J.M.; Basbaum, A.I. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat. Commun. 2020, 11, 264. [Google Scholar] [CrossRef]
- Feng, R.; Muraleedharan Saraswathy, V.; Mokalled, M.H.; Cavalli, V. Self-renewing macrophages in dorsal root ganglia contribute to promote nerve regeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2215906120. [Google Scholar] [CrossRef]
- Brown, A.G. Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Fowler, C.J.; Griffiths, D.; De Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef]
- Kuru, M. Nervous control of micturition. Physiol. Rev. 1965, 45, 425–494. [Google Scholar] [CrossRef] [PubMed]
- Uher, E.-M.; Swash, M. Sacral reflexes: Physiology and clinical application. Dis. Colon Rectum 1998, 41, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.; Morales, H. Spinal Cord Anatomy and Clinical Syndromes. Semin. Ultrasound CT MRI 2016, 37, 360–371. [Google Scholar] [CrossRef]
- Cramer, G.D.; Darby, S.A. Clinical Anatomy of the Spine, Spinal Cord, and ANS; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Green, T.R.; Rowe, R.K. Quantifying microglial morphology: An insight into function. Clin. Exp. Immunol. 2024, 216, 221–229. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Edler, M.K.; Mhatre-Winters, I.; Richardson, J.R. Microglia in Aging and Alzheimer’s Disease: A Comparative Species Review. Cells 2021, 10, 1138. [Google Scholar] [CrossRef]
- Pottorf, T.S.; Lane, E.L.; Haley-Johnson, Z.; Amores-Sanchez, V.; Ukmar, D.N.; Correa-Torres, P.M.; Alvarez, F.J. Dual Role of Microglial TREM2 in Neuronal Degeneration and Regeneration After Axotomy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Hakim, R.; Zachariadis, V.; Sankavaram, S.R.; Han, J.; Harris, R.A.; Brundin, L.; Enge, M.; Svensson, M. Spinal Cord Injury Induces Permanent Reprogramming of Microglia into a Disease-Associated State Which Contributes to Functional Recovery. J. Neurosci. 2021, 41, 8441–8459. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef]
- Samant, R.R.; Standaert, D.G.; Harms, A.S. The emerging role of disease-associated microglia in Parkinson’s disease. Front. Cell. Neurosci. 2024, 18, 1476461. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhao, S.; Bosco, D.B.; Nguyen, A.; Wu, L.J. Microglial TREM2 in amyotrophic lateral sclerosis. Dev. Neurobiol. 2022, 82, 125–137. [Google Scholar] [CrossRef]
- Wang, H. Microglia heterogeneity in Alzheimer’s disease: Insights from single-cell technologies. Front. Synaptic Neurosci. 2021, 13, 773590. [Google Scholar] [CrossRef]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-associated microglia: A universal immune sensor of neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, W.M.; Andhey, P.S.; Swain, A.; Levy, T.; Miller, K.R.; Poliani, P.L.; Cominelli, M.; Grover, S.; Gilfillan, S.; et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 2020, 26, 131–142. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; Jun, G.; DeStefano, A.L.; Bis, J.C.; Beecham, G.W. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef]
- Song, W.M.; Colonna, M. The identity and function of microglia in neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef]
- McQuade, A.; Kang, Y.J.; Hasselmann, J.; Jairaman, A.; Sotelo, A.; Coburn, M.; Shabestari, S.K.; Chadarevian, J.P.; Fote, G.; Tu, C.H.; et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Commun. 2020, 11, 5370. [Google Scholar] [CrossRef]
- Ennerfelt, H.; Frost, E.L.; Shapiro, D.A.; Holliday, C.; Zengeler, K.E.; Voithofer, G.; Bolte, A.C.; Lammert, C.R.; Kulas, J.A.; Ulland, T.K. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 2022, 185, 4135–4152.e4122. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, M. The role of microglial TREM2 in development: A path toward neurodegeneration? Glia 2024, 72, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Jay, T.R.; von Saucken, V.E.; Muñoz, B.; Codocedo, J.F.; Atwood, B.K.; Lamb, B.T.; Landreth, G.E. TREM2 is required for microglial instruction of astrocytic synaptic engulfment in neurodevelopment. Glia 2019, 67, 1873–1892. [Google Scholar] [CrossRef]
- Vecchiarelli, H.A.; Bisht, K.; Sharma, K.; Weiser Novak, S.; Traetta, M.E.; Garcia-Segura, M.E.; St-Pierre, M.K.; Savage, J.C.; Willis, C.; Picard, K. Dark Microglia Are Abundant in Normal Postnatal Development, where they Remodel Synapses via Phagocytosis and Trogocytosis, and Are Dependent on TREM2. bioRxiv 2024. [Google Scholar] [CrossRef]
- St-Pierre, M.K.; Šimončičová, E.; Bögi, E.; Tremblay, M.-È. Shedding light on the dark side of the microglia. ASN Neuro 2020, 12, 1759091420925335. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.H.; Barres, B.A.; Stevens, B. The complement system: An unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012, 35, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.S.; Eyo, U.B. Microglia and Astrocytes in Postnatal Neural Circuit Formation. Glia 2025, 73, 232–250. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Perrucci, F.; Morini, R.; Erreni, M.; Mahoney, M.; Witkowska, A.; Carey, A.; Faggiani, E.; Schuetz, L.T.; Mason, S.; et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020, 39, e105380. [Google Scholar] [CrossRef]
- Shen, Y.; Lue, L.F.; Yang, L.B.; Roher, A.; Kuo, Y.M.; Strohmeyer, R.; Goux, W.J.; Lee, V.; Johnson, G.V.; Webster, S.D. Complement activation by neurofibrillary tangles in Alzheimer’s disease. Neurosci. Lett. 2001, 305, 165–168. [Google Scholar] [CrossRef]
- Jiang, H.; Burdick, D.; Glabe, C.G.; Cotman, C.W.; Tenner, A.J. beta-Amyloid activates complement by binding to a specific region of the collagen-like domain of the C1q A chain. J. Immunol. 1994, 152, 5050–5059. [Google Scholar] [CrossRef]
- Rogers, J.; Cooper, N.R.; Webster, S.; Schultz, J.; McGeer, P.L.; Styren, S.D.; Civin, W.H.; Brachova, L.; Bradt, B.; Ward, P. Complement activation by beta-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10016–10020. [Google Scholar] [CrossRef]
- Zhong, L.; Sheng, X.; Wang, W.; Li, Y.; Zhuo, R.; Wang, K.; Zhang, L.; Hu, D.-D.; Hong, Y.; Chen, L. TREM2 receptor protects against complement-mediated synaptic loss by binding to complement C1q during neurodegeneration. Immunity 2023, 56, 1794–1808. [Google Scholar] [CrossRef]
- Rueda-Carrasco, J.; Sokolova, D.; Lee, S.E.; Childs, T.; Jurčáková, N.; Crowley, G.; De Schepper, S.; Ge, J.Z.; Lachica, J.I.; Toomey, C.E.; et al. Microglia-synapse engulfment via PtdSer-TREM2 ameliorates neuronal hyperactivity in Alzheimer’s disease models. EMBO J. 2023, 42, e113246. [Google Scholar] [CrossRef]
- Fracassi, A.; Marcatti, M.; Tumurbaatar, B.; Woltjer, R.; Moreno, S.; Taglialatela, G. TREM2-induced activation of microglia contributes to synaptic integrity in cognitively intact aged individuals with Alzheimer’s neuropathology. Brain Pathol. 2023, 33, e13108. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Wu, T.; Tsai, M.C.; Graykowski, D.; Gandham, V.D.; Rose, C.M.; Bakalarski, C.E.; Ngu, H.; Wang, Y.; Pandey, S.; et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2022, 2, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Mao, W.; Voskobiynyk, Y.; Necula, D.; Lew, I.; Petersen, C.; Zahn, A.; Yu, G.Q.; Yu, X.; Smith, N.; et al. Alzheimer risk-increasing TREM2 variant causes aberrant cortical synapse density and promotes network hyperexcitability in mouse models. Neurobiol. Dis. 2023, 186, 106263. [Google Scholar] [CrossRef] [PubMed]
- Freria, C.M.; Brennan, F.H.; Sweet, D.R.; Guan, Z.; Hall, J.C.; Kigerl, K.A.; Nemeth, D.P.; Liu, X.; Lacroix, S.; Quan, N. Serial Systemic Injections of Endotoxin (LPS) Elicit Neuroprotective Spinal Cord Microglia through IL-1-Dependent Cross Talk with Endothelial Cells. J. Neurosci. 2020, 40, 9103–9120. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Lénárt, N.; Fekete, R.; László, Z.I.; Lele, Z.; Orsolits, B.; Molnár, G.; Heindl, S.; Schwarcz, A.D.; et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 2020, 367, 528–537. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Tozaki-Saitoh, H.; Takeda, H.; Inoue, K. The Role of Microglial Purinergic Receptors in Pain Signaling. Molecules 2022, 27, 1919. [Google Scholar] [CrossRef]
- Tozaki-Saitoh, H.; Tsuda, M.; Miyata, H.; Ueda, K.; Kohsaka, S.; Inoue, K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J. Neurosci. 2008, 28, 4949–4956. [Google Scholar] [CrossRef]
- Tsuda, M.; Masuda, T.; Kitano, J.; Shimoyama, H.; Tozaki-Saitoh, H.; Inoue, K. IFN-γ receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 8032–8037. [Google Scholar] [CrossRef]
- Masuda, T.; Ozono, Y.; Mikuriya, S.; Kohro, Y.; Tozaki-Saitoh, H.; Iwatsuki, K.; Uneyama, H.; Ichikawa, R.; Salter, M.W.; Tsuda, M. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. 2016, 7, 12529. [Google Scholar] [CrossRef]
- Boakye, P.A.; Tang, S.J.; Smith, P.A. Mediators of Neuropathic Pain; Focus on Spinal Microglia, CSF-1, BDNF, CCL21, TNF-α, Wnt Ligands, and Interleukin 1β. Front. Pain Res. 2021, 2, 698157. [Google Scholar] [CrossRef] [PubMed]
- Sideris-Lampretsas, G.; Malcangio, M. Microglial heterogeneity in chronic pain. Brain Behav. Immun. 2021, 96, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Basbaum, A.; Guan, Z. Contribution of colony-stimulating factor 1 to neuropathic pain. Pain Rep. 2021, 6, e883. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Tsuda, M. Role of microglia and P2X4 receptors in chronic pain. Pain Rep. 2021, 6, e864. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central nervous system targets: Glial cell mechanisms in chronic pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef]
- Ferrini, F.; De Koninck, Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013, 2013, 429815. [Google Scholar] [CrossRef]
- Malcangio, M.; Sideris-Lampretsas, G. How microglia contribute to the induction and maintenance of neuropathic pain. Nat. Rev. Neurosci. 2025, 26, 263–275. [Google Scholar] [CrossRef]
- Long, J.; Tian, G.; He, K.; Su, Y.; Wang, Z.; Huang, L.; Yao, Y.; Li, X.; Lin, Y. The role of microglia in neuropathic pain: A systematic review of animal experiments. Brain Res. Bull. 2025, 28, 111410. [Google Scholar] [CrossRef]
- Kobayashi, M.; Konishi, H.; Sayo, A.; Takai, T.; Kiyama, H. TREM2/DAP12 signal elicits proinflammatory response in microglia and exacerbates neuropathic pain. J. Neurosci. 2016, 36, 11138–11150. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Huang, Y.; Liu, W.; Cai, G.; Huang, S.; Zeng, Y.; Ren, S.; Zhan, H.; Wu, W. Resveratrol mediates mechanical allodynia through modulating inflammatory response via the TREM2-autophagy axis in SNI rat model. J. Neuroinflamm. 2020, 17, 311. [Google Scholar] [CrossRef]
- Yousefpour, N.; Locke, S.; Deamond, H.; Wang, C.; Marques, L.; St-Louis, M.; Ouellette, J.; Khoutorsky, A.; De Koninck, Y.; Ribeiro-da-Silva, A. Time-dependent and selective microglia-mediated removal of spinal synapses in neuropathic pain. Cell Rep. 2023, 42, 112010. [Google Scholar] [CrossRef]
- Kohno, K.; Shirasaka, R.; Yoshihara, K.; Mikuriya, S.; Tanaka, K.; Takanami, K.; Inoue, K.; Sakamoto, H.; Ohkawa, Y.; Masuda, T.; et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science 2022, 376, 86–90. [Google Scholar] [CrossRef]
- Tsuda, M.; Masuda, T.; Kohno, K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023, 46, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, W.; Kooijman, L.; Schetters, S.; Orre, M.; Hol, E.M. Transcriptional profiling of CD11c-positive microglia accumulating around amyloid plaques in a mouse model for Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.T.; Yin, Z.; Guneykaya, D.; Gauthier, C.D.; Hayes, J.P.; D’Hary, A.; Butovsky, O.; Moalem-Taylor, G. Sex-specific transcriptome of spinal microglia in neuropathic pain due to peripheral nerve injury. Glia 2021, 70, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Tang, S.Q.; He, W.Y.; He, J.; Wang, Y.H.; Wang, H. Contribution of Trem2 signaling to the development of painful diabetic neuropathy by mediating microglial polarization in mice. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Guruprasad, B.; Chaudhary, P.; Choedon, T.; Kumar, V.L. Artesunate ameliorates functional limitations in Freund’s complete adjuvant-induced monoarthritis in rat by maintaining oxidative homeostasis and inhibiting COX-2 expression. Inflammation 2015, 38, 1028–1035. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Gao, T.; Zhang, H.; Li, J.; Wang, G.; Wang, C.; Li, Y. Artesunate reduces remifentanil-induced hyperalgesia and peroxiredoxin-3 hyperacetylation via modulating spinal metabotropic glutamate receptor 5 in rats. Neuroscience 2022, 487, 88–98. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Zhang, H.; Wang, Y.; Gao, T.; Zhao, Y.; Wang, G.; Yu, Y.; Wang, C.; Li, Y. Artesunate therapy alleviates fracture-associated chronic pain after orthopedic surgery by suppressing CCL21-dependent TREM2/DAP12 inflammatory signaling in mice. Front. Pharmacol. 2022, 13, 894963. [Google Scholar] [CrossRef]
- Uzun, T.; Toptas, O.; Saylan, A.; Carver, H.; Turkoglu, S.A. Evaluation and Comparison of the Effects of Artesunate, Dexamethasone, and Tacrolimus on Sciatic Nerve Regeneration. J. Oral Maxillofac. Surg. 2019, 77, 1092.e1091–1092.e1012. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.T.; Qian, N.S.; Zhang, T.; Li, J.M.; Li, X.K.; Wang, P.; Zhao, D.S.; Huang, G.; Zhang, L.; Fei, Z. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS ONE 2013, 8, e60733. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Zhou, Y.; Cui, W.Q.; Hu, X.M.; Du, L.X.; Mi, W.L.; Chu, Y.X.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav. Immun. 2018, 68, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, L.D.C.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef]
- Pevida, M.; Lastra, A.; Hidalgo, A.; Baamonde, A.; Menéndez, L. Spinal CCL2 and microglial activation are involved in paclitaxel-evoked cold hyperalgesia. Brain Res. Bull. 2013, 95, 21–27. [Google Scholar] [CrossRef]
- Zheng, F.; Xiao, W.H.; Bennett, G. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience 2011, 176, 447–454. [Google Scholar] [CrossRef]
- Rotterman, T.M.; MacPherson, K.P.; Tansey, M.G.; Alvarez, F.J. Motor circuit synaptic plasticity after peripheral nerve injury depends on a central neuroinflammatory response and a CCR2 mechanism. J. Neurosci. 2019, 39, 3412–3433. [Google Scholar]
- Rotterman, T.M.; Haley-Johnson, Z.; Pottorf, T.S.; Chopra, T.; Chang, E.; Zhang, S.; McCallum, W.M.; Fisher, S.; Franklin, H.; Alvarez, M. Modulation of central synapse remodeling after remote peripheral injuries by the CCL2-CCR2 axis and microglia. Cell Rep. 2024, 43, 113776. [Google Scholar] [CrossRef]
- Rotterman, T.M.; García, V.V.; Housley, S.N.; Nardelli, P.; Sierra, R.; Fix, C.E.; Cope, T.C. Structural preservation does not ensure function at sensory Ia–motoneuron synapses following peripheral nerve injury and repair. J. Neurosci. 2023, 43, 4390–4404. [Google Scholar] [CrossRef]
- Rotterman, T.M.; Alvarez, F.J. Microglia dynamics and interactions with motoneurons axotomized after nerve injuries revealed by two-photon imaging. Sci. Rep. 2020, 10, 8648. [Google Scholar] [CrossRef] [PubMed]
- Rotterman, T.M.; Akhter, E.T.; Lane, A.R.; MacPherson, K.P.; García, V.V.; Tansey, M.G.; Alvarez, F.J. Spinal Motor Circuit Synaptic Plasticity after Peripheral Nerve Injury Depends on Microglia Activation and a CCR2 Mechanism. J. Neurosci. 2019, 39, 3412–3433. [Google Scholar] [CrossRef] [PubMed]
- Manich, G.; Gómez-López, A.R.; Almolda, B.; Villacampa, N.; Recasens, M.; Shrivastava, K.; González, B.; Castellano, B. Differential Roles of TREM2+ Microglia in Anterograde and Retrograde Axonal Injury Models. Front. Cell. Neurosci. 2020, 14, 567404. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Konishi, H.; Takai, T.; Kiyama, H. A DAP12-dependent signal promotes pro-inflammatory polarization in microglia following nerve injury and exacerbates degeneration of injured neurons. Glia 2015, 63, 1073–1082. [Google Scholar] [CrossRef]
- Lieberman, A. The axon reaction: A review of the principal features of perikaryal responses to axon injury. Int. Rev. Neurobiol. 1971, 14, 49–124. [Google Scholar]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef]
- Castro, R.W.; Lopes, M.C.; De Biase, L.M.; Valdez, G. Aging spinal cord microglia become phenotypically heterogeneous and preferentially target motor neurons and their synapses. Glia 2024, 72, 206–221. [Google Scholar] [CrossRef]
- García-Domínguez, M. Pathological and Inflammatory Consequences of Aging. Biomolecules 2025, 15, 404. [Google Scholar] [CrossRef]
- Castro, R.W.; Lopes, M.C.; Settlage, R.E.; Valdez, G. Aging alters mechanisms underlying voluntary movements in spinal motor neurons of mice, primates, and humans. JCI Insight 2023, 8, e168448. [Google Scholar] [CrossRef]
- You, J.; Youssef, M.M.; Santos, J.R.; Lee, J.; Park, J. Microglia and astrocytes in amyotrophic lateral sclerosis: Disease-associated states, pathological roles, and therapeutic potential. Biology 2023, 12, 1307. [Google Scholar] [CrossRef]
- Clarke, B.E.; Patani, R. The microglial component of amyotrophic lateral sclerosis. Brain J. Neurol. 2020, 143, 3526–3539. [Google Scholar] [CrossRef]
- Brites, D.; Vaz, A.R. Microglia centered pathogenesis in ALS: Insights in cell interconnectivity. Front. Cell. Neurosci. 2014, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.S.; Beers, D.R.; Zhao, W.; Appel, S.H. Microglia in ALS: The good, the bad, and the resting. J. Neuroimmune Pharmacol. 2009, 4, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Dols-Icardo, O.; Montal, V.; Sirisi, S.; López-Pernas, G.; Cervera-Carles, L.; Querol-Vilaseca, M.; Muñoz, L.; Belbin, O.; Alcolea, D.; Molina-Porcel, L. Motor cortex transcriptome reveals microglial key events in amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e829. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Zhang, M.D.; Xu, H.F.; Ye, H.Q.; Chen, D.F.; Wang, P.S.; Bao, Z.W.; Zou, S.M.; Lv, Y.T.; Wu, Z.Y. Single-Nucleus RNA Sequencing Reveals the Spatiotemporal Dynamics of Disease-Associated Microglia in Amyotrophic Lateral Sclerosis. Research 2024, 7, 0548. [Google Scholar] [CrossRef]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; Phatnani, H.; Kwan, J.; Sareen, D.; et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e1165. [Google Scholar] [CrossRef]
- Jauregui, C.; Blanco-Luquin, I.; Macías, M.; Roldan, M.; Caballero, C.; Pagola, I.; Mendioroz, M.; Jericó, I. Exploring the Disease-Associated microglia state in amyotrophic lateral sclerosis. Biomedicines 2023, 11, 2994. [Google Scholar] [CrossRef]
- Maniatis, S.; Äijö, T.; Vickovic, S.; Braine, C.; Kang, K.; Mollbrink, A.; Fagegaltier, D.; Andrusivová, Ž.; Saarenpää, S.; Saiz-Castro, G.; et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 2019, 364, 89–93. [Google Scholar] [CrossRef]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 Mutations in Familial and Sporadic Amyotrophic Lateral Sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef]
- Prasad, A.; Bharathi, V.; Sivalingam, V.; Girdhar, A.; Patel, B.K. Molecular mechanisms of TDP-43 misfolding and pathology in amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Scotter, E.L.; Chen, H.J.; Shaw, C.E. TDP-43 proteinopathy and ALS: Insights into disease mechanisms and therapeutic targets. Neurotherapeutics 2015, 12, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Adkins, R.; Yakura, J. Definition of complete spinal cord injury. Spinal Cord. 1991, 29, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, E.S. Diagnosis and prognosis of acute cervical spinal cord injury. Clin. Orthop. Relat. Res. 1975, 112, 9–15. [Google Scholar] [CrossRef]
- Klose, K.J.; Green, B.A.; Smith, R.S.; Adkins, R.H.; MacDonald, A.M. University of Miami Neuro-Spinal Index (UMNI): A quantitative method for determining spinal cord function. Paraplegia 1980, 18, 331–336. [Google Scholar] [CrossRef]
- Maynard, F.M., Jr.; Bracken, M.B.; Creasey, G.; Ditunno, J.F., Jr.; Donovan, W.H.; Ducker, T.B.; Garber, S.L.; Marino, R.J.; Stover, S.L.; Tator, C.H. International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997, 35, 266–274. [Google Scholar] [CrossRef]
- Cheriyan, T.; Ryan, D.; Weinreb, J.; Cheriyan, J.; Paul, J.; Lafage, V.; Kirsch, T.; Errico, T. Spinal cord injury models: A review. Spinal Cord 2014, 52, 588–595. [Google Scholar] [CrossRef]
- Hall, M. Second Memoir on some principles of the pathology of the nervous system. Med. Chir. Trans. 1840, 23, 121. [Google Scholar] [CrossRef]
- Ko, H.Y. Revisit Spinal Shock: Pattern of Reflex Evolution during Spinal Shock. Korean J. Neurotrauma 2018, 14, 47–54. [Google Scholar] [CrossRef]
- Bastian, H.C. On the symptomatology of total transverse lesions of the spinal cord; with special reference to the condition of the various reflexes. Med. Chir. Trans. 1890, 73, 151. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Lindan, R.; Joiner, E.; Freehafer, A.; Hazel, C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Spinal Cord 1980, 18, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Widerström-Noga, E.G.; Felipe-Cuervo, E.; Yezierski, R.P. Relationships among clinical characteristics of chronic pain after spinal cord injury. Arch. Phys. Med. Rehabil. 2001, 82, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Tator, C.H.; Fehlings, M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef]
- Brennan, F.H.; Li, Y.; Wang, C.; Ma, A.; Guo, Q.; Li, Y.; Pukos, N.; Campbell, W.A.; Witcher, K.G.; Guan, Z.; et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 2022, 13, 4096. [Google Scholar] [CrossRef]
- Swarts, E.A.; Brennan, F.H. Evolving insights on the role of microglia in neuroinflammation, plasticity, and regeneration of the injured spinal cord. Front. Immunol. 2025, 16, 1621789. [Google Scholar] [CrossRef]
- Kroner, A.; Almanza, J.R. Role of microglia in spinal cord injury. Neurosci. Lett. 2019, 709, 134370. [Google Scholar] [CrossRef]
- Zhou, X.; He, X.; Ren, Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen. Res. 2014, 9, 1787–1795. [Google Scholar] [CrossRef]
- Brockie, S.; Hong, J.; Fehlings, M.G. The role of microglia in modulating neuroinflammation after spinal cord injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J.; Ding, Y.; Wang, L.; Zhu, Y.J. Current knowledge of microglia in traumatic spinal cord injury. Front. Neurol. 2022, 12, 796704. [Google Scholar] [CrossRef] [PubMed]
- Brockie, S.; Zhou, C.; Fehlings, M.G. Resident immune responses to spinal cord injury: Role of astrocytes and microglia. Neural Regen. Res. 2024, 19, 1678–1685. [Google Scholar] [CrossRef]
- Zha, X.; Zheng, G.; Skutella, T.; Kiening, K.; Unterberg, A.; Younsi, A. Microglia: A promising therapeutic target in spinal cord injury. Neural Regen. Res. 2025, 20, 454–463. [Google Scholar] [CrossRef]
- Zhao, T.; Di, J.; Kang, Y.; Zhang, H.; Yao, S.; Liu, B.; Rong, L. TREM2 Impedes Recovery After Spinal Cord Injury by Regulating Microglial Lysosomal Membrane Permeabilization-Mediated Autophagy; Wiley: Oxford, UK, 2025; p. e70047. [Google Scholar]
- Burgess, M.; Wicks, K.; Gardasevic, M.; Mace, K.A. Cx3CR1 expression identifies distinct macrophage populations that contribute differentially to inflammation and repair. Immunohorizons 2019, 3, 262–273. [Google Scholar] [CrossRef]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.Y. Tissue-specific role of CX3CR1 expressing immune cells and their relationships with human disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef]
- Flores, A.J.; Lavemia, C.; Owens, P.W. Anatomy and physiology of peripheral nerve injury and repair. Am. J. Orthop. Belle Mead 2000, 29, 167–178. [Google Scholar]
- Geuna, S.; Raimondo, S.; Ronchi, G.; Di Scipio, F.; Tos, P.; Czaja, K.; Fornaro, M. Histology of the peripheral nerve and changes occurring during nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 27–46. [Google Scholar]
- Boissaud-Cooke, M.; Pidgeon, T.E.; Tunstall, R. Chapter 37—The Microcirculation of Peripheral Nerves: The Vasa Nervorum. In Nerves and Nerve Injuries; Tubbs, R.S., Rizk, E., Shoja, M.M., Loukas, M., Barbaro, N., Spinner, R.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 507–523. [Google Scholar]
- Appenzeller, O.; Dhital, K.K.; Cowen, T.; Burnstock, G. The nerves to blood vessels supplying blood to nerves: The innervation of vasa nervorum. Brain Res. 1984, 304, 383–386. [Google Scholar] [CrossRef]
- Sladjana, U.Z.; Ivan, J.D.; Bratislav, S.D. Microanatomical structure of the human sciatic nerve. Surg. Radiol. Anat. 2008, 30, 619–626. [Google Scholar] [CrossRef]
- Brosius Lutz, A.; Lucas, T.A.; Carson, G.A.; Caneda, C.; Zhou, L.; Barres, B.A.; Buckwalter, M.S.; Sloan, S.A. An RNA-sequencing transcriptome of the rodent Schwann cell response to peripheral nerve injury. J. Neuroinflamm. 2022, 19, 105. [Google Scholar] [CrossRef]
- Gerber, D.; Pereira, J.A.; Gerber, J.; Tan, G.; Dimitrieva, S.; Yángüez, E.; Suter, U. Transcriptional profiling of mouse peripheral nerves to the single-cell level to build a sciatic nerve ATlas (SNAT). eLife 2021, 10, e58591. [Google Scholar] [CrossRef]
- Wang, P.L.; Yim, A.K.; Kim, K.W.; Avey, D.; Czepielewski, R.S.; Colonna, M.; Milbrandt, J.; Randolph, G.J. Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 2020, 11, 2552. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L. Peripheral Nerve Macrophages and Their Implications in Neuroimmunity; Washington University in St. Louis: St. Louis, MO, USA, 2021. [Google Scholar]
- Tsuchiya, T.; Miyawaki, S.; Teranishi, Y.; Ohara, K.; Hirano, Y.; Ogawa, S.; Torazawa, S.; Sakai, Y.; Hongo, H.; Ono, H.; et al. Current molecular understanding of central nervous system schwannomas. Acta Neuropathol. Commun. 2025, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Baruah, P.; Mahony, C.; Marshall, J.L.; Smith, C.G.; Monksfield, P.; Irving, R.I.; Dumitriu, I.E.; Buckley, C.D.; Croft, A.P. Single-cell RNA sequencing analysis of vestibular schwannoma reveals functionally distinct macrophage subsets. Br. J. Cancer 2024, 130, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Katzenelenbogen, Y.; Sheban, F.; Yalin, A.; Yofe, I.; Svetlichnyy, D.; Jaitin, D.A.; Bornstein, C.; Moshe, A.; Keren-Shaul, H.; Cohen, M. Coupled scRNA-Seq and intracellular protein activity reveal an immunosuppressive role of TREM2 in cancer. Cell 2020, 182, 872–885.e819. [Google Scholar] [CrossRef]
- Nakamura, K.; Smyth, M.J. TREM2 marks tumor-associated macrophages. Signal Transduct. Target. Ther. 2020, 5, 233. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, Y.; Guo, L.; Liu, Y. Identification of key genes and immune infiltration based on weighted gene co-expression network analysis in vestibular schwannoma. Medicine 2023, 102, e33470. [Google Scholar] [CrossRef]
- Olsson, Y.; Sjöstrand, J. Origin of macrophages in Wallerian degeneration of peripheral nerves demonstrated autoradiographically. Exp. Neurol. 1969, 23, 102–112. [Google Scholar] [CrossRef]
- Monaco, S.; Gehrmann, J.; Raivich, G.; Kreutzberg, G.W. MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J. Neurocytol. 1992, 21, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Calavia, N.G.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 21, 8652. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, Q.; Parkinson, D.B.; Dun, X.P. Analysis of Schwann cell migration and axon regeneration following nerve injury in the sciatic nerve bridge. Front. Mol. Neurosci. 2019, 12, 308. [Google Scholar] [CrossRef]
- Witzel, C.; Rohde, C.; Brushart, T.M. Pathway sampling by regenerating peripheral axons. J. Comp. Neurol. 2005, 485, 183–190. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Žygelytė, E.; Grenier, J.K.; Edwards, M.G.; Cheetham, J. Temporal changes in macrophage phenotype after peripheral nerve injury. J. Neuroinflamm. 2018, 15, 185. [Google Scholar] [CrossRef]
- Rios, R.; Jablonka-Shariff, A.; Broberg, C.; Snyder-Warwick, A.K. Macrophage roles in peripheral nervous system injury and pathology: Allies in neuromuscular junction recovery. Mol. Cell. Neurosci. 2021, 111, 103590. [Google Scholar] [CrossRef]
- Ydens, E.; Cauwels, A.; Asselbergh, B.; Goethals, S.; Peeraer, L.; Lornet, G.; Almeida-Souza, L.; Van Ginderachter, J.A.; Timmerman, V.; Janssens, S. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J. Neuroinflamm. 2012, 9, 176. [Google Scholar] [CrossRef]
- Shiraishi, W.; Yamasaki, R.; Hashimoto, Y.; Ko, S.; Kobayakawa, Y.; Isobe, N.; Matsushita, T.; Kira, J.I. Clearance of peripheral nerve misfolded mutant protein by infiltrated macrophages correlates with motor neuron disease progression. Sci. Rep. 2021, 11, 16438. [Google Scholar] [CrossRef]
- Graber, D.J.; Hickey, W.F.; Harris, B.T. Progressive changes in microglia and macrophages in spinal cord and peripheral nerve in the transgenic rat model of amyotrophic lateral sclerosis. J. Neuroinflamm. 2010, 7, 8. [Google Scholar] [CrossRef]
- Kano, O.; Beers, D.R.; Henkel, J.S.; Appel, S.H. Peripheral nerve inflammation in ALS mice: Cause or consequence. Neurology 2012, 78, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Muriana, A.; Mancuso, R.; Francos-Quijorna, I.; Olmos-Alonso, A.; Osta, R.; Perry, V.H.; Navarro, X.; Gomez-Nicola, D.; López-Vales, R. CSF1R blockade slows the progression of amyotrophic lateral sclerosis by reducing microgliosis and invasion of macrophages into peripheral nerves. Sci. Rep. 2016, 6, 25663. [Google Scholar] [CrossRef] [PubMed]
- Chiot, A.; Zaïdi, S.; Iltis, C.; Ribon, M.; Berriat, F.; Schiaffino, L.; Jolly, A.; de la Grange, P.; Mallat, M.; Bohl, D. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nat. Neurosci. 2020, 23, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Msheik, Z.; El Massry, M.; Rovini, A.; Billet, F.; Desmoulière, A. The macrophage: A key player in the pathophysiology of peripheral neuropathies. J. Neuroinflamm. 2022, 19, 97. [Google Scholar] [CrossRef]
- MacIntosh, B.R.; Gardiner, P.F.; McComas, A.J. Skeletal Muscle: Form and Function; Human Kinetics: Champaign, IL, USA, 2006. [Google Scholar]
- Lieber, R.L. Skeletal Muscle Structure, Function, and Plasticity; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002. [Google Scholar]
- Liddel, E.; Sherrington, C. Reflexes in response to stretch (myotatic reflexes). Proc. R. Soc. Lond. Ser. B 1924, 96, 212–242. [Google Scholar]
- Eccles, J.; Eccles, R.M.; Lundberg, A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J. Physiol. 1957, 138, 227. [Google Scholar] [CrossRef]
- Mendell, L.M.; Henneman, E. Terminals of single Ia fibers: Location, density, and distribution within a pool of 300 homonymous motoneurons. J. Neurophysiol. 1971, 34, 171–187. [Google Scholar] [CrossRef]
- Bączyk, M.; Manuel, M.; Roselli, F.; Zytnicki, D. Diversity of Mammalian Motoneurons and Motor Units. In Vertebrate Motoneurons; Springer: Berlin/Heidelberg, Germany, 2022; pp. 131–150. [Google Scholar]
- Kernell, D. The Motoneurone and its Muscle Fibres; Oxford University Press UK: Oxford, UK, 2006. [Google Scholar]
- Manuel, M.; Zytnicki, D. Alpha, beta and gamma motoneurons: Functional diversity in the motor system’s final pathway. J. Integr. Neurosci. 2011, 10, 243–276. [Google Scholar] [CrossRef]
- Alhindi, A.; Boehm, I.; Forsythe, R.O.; Miller, J.; Skipworth, R.J.E.; Simpson, H.; Jones, R.A.; Gillingwater, T.H. Terminal Schwann cells at the human neuromuscular junction. Brain Commun. 2021, 3, fcab081. [Google Scholar] [CrossRef]
- Balice-Gordon, R.J. Schwann cells: Dynamic roles at the neuromuscular junction. Curr. Biol. 1996, 6, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.L.; Valdez, G. Origin, identity, and function of terminal Schwann cells. Trends Neurosci. 2024, 47, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Hastings, R.L.; Mikesh, M.; Lee, Y.i.; Thompson, W.J. Morphological remodeling during recovery of the neuromuscular junction from terminal Schwann cell ablation in adult mice. Sci. Rep. 2020, 10, 11132. [Google Scholar] [CrossRef] [PubMed]
- Muir, A.; Kanji, A.; Allbrook, D. The structure of the satellite cells in skeletal muscle. J. Anat. 1965, 99, 435. [Google Scholar]
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493. [Google Scholar] [CrossRef]
- Rubenstein, A.B.; Smith, G.R.; Raue, U.; Begue, G.; Minchev, K.; Ruf-Zamojski, F.; Nair, V.D.; Wang, X.; Zhou, L.; Zaslavsky, E.; et al. Single-cell transcriptional profiles in human skeletal muscle. Sci. Rep. 2020, 10, 229. [Google Scholar] [CrossRef]
- De Micheli, A.J.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef]
- Kang, H.; Tian, L.; Mikesh, M.; Lichtman, J.W.; Thompson, W.J. Terminal Schwann Cells Participate in Neuromuscular Synapse Remodeling during Reinnervation following Nerve Injury. J. Neurosci. 2014, 34, 6323–6333. [Google Scholar] [CrossRef]
- Reynolds, M.; Woolf, C. Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J. Neurocytol. 1992, 21, 50–66. [Google Scholar] [CrossRef]
- Bermedo-García, F.; Zelada, D.; Martínez, E.; Tabares, L.; Henríquez, J.P. Functional regeneration of the murine neuromuscular synapse relies on long-lasting morphological adaptations. BMC Biol. 2022, 20, 158. [Google Scholar] [CrossRef]
- Son, Y.J.; Thompson, W.J. Schwann cell processes guide regeneration of peripheral axons. Neuron 1995, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Chen, M.; Rao, M.; Lin, Y.; Zhang, M.; Xu, R.; Hu, X.; Chen, R.; Chai, W.; Huang, X. Deciphering immune landscape remodeling unravels the underlying mechanism for synchronized muscle and bone aging. Adv. Sci. 2024, 11, 2304084. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.A.; Wallace, E.C.; Spence, B.D.; Buras, E.; Aguilar, C.A. Spatiotemporal mapping of immune and stem cell dysregulation after volumetric muscle loss. JCI Insight 2023, 8, e162835. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Alabdullatif, S.; Homma, S.T.; Alekseyev, Y.O.; Zhou, L. Expansion and pathogenic activation of skeletal muscle–resident macrophages in mdx5cv/Ccr2−/− mice. Proc. Natl. Acad. Sci. USA 2025, 122, e2410095122. [Google Scholar] [CrossRef]
- Essex, A.L.; Huot, J.R.; Deosthale, P.; Wagner, A.; Figueras, J.; Davis, A.; Damrath, J.; Pin, F.; Wallace, J.; Bonetto, A.; et al. Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) R47H Variant Causes Distinct Age- and Sex-Dependent Musculoskeletal Alterations in Mice. J. Bone Min. Res. 2022, 37, 1366–1381. [Google Scholar] [CrossRef]
- Tacconi, S.; Giudetti, A.M.; Blangero, F.; Meugnier, E.; El-jaafari, A.; Longo, S.; Angilé, F.; Fanizzi, F.P.; Canaple, L.; Jalabert, A.; et al. LAM/TREM2+ macrophages release extracellular vesicles and extracellular lipid droplets which modulate the phenotype of recipient macrophages and homeostasis of skeletal muscle cells. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Santosa, K.B.; Jablonka-Shariff, A.; Vannucci, B.; Fuchs, A.; Turnbull, I.; Pan, D.; Wood, M.D.; Snyder-Warwick, A.K. Macrophage-derived vascular endothelial growth factor-A is integral to neuromuscular junction reinnervation after nerve injury. J. Neurosci. 2020, 40, 9602–9616. [Google Scholar] [CrossRef]
- Van Dyke, J.M.; Smit-Oistad, I.M.; Macrander, C.; Krakora, D.; Meyer, M.G.; Suzuki, M. Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS). Exp. Neurol. 2016, 277, 275–282. [Google Scholar] [CrossRef]
- Chiu, I.M.; Phatnani, H.; Kuligowski, M.; Tapia, J.C.; Carrasco, M.A.; Zhang, M.; Maniatis, T.; Carroll, M.C. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20960–20965. [Google Scholar] [CrossRef]
- Dachs, E.; Hereu, M.; Piedrafita, L.; Casanovas, A.; Calderó, J.; Esquerda, J.E. Defective neuromuscular junction organization and postnatal myogenesis in mice with severe spinal muscular atrophy. J. Neuropathol. Exp. Neurol. 2011, 70, 444–461. [Google Scholar] [CrossRef]
- Deczkowska, A.; Weiner, A.; Amit, I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef]
- Chtarto, A.; Humbert-Claude, M.; Bockstael, O.; Das, A.T.; Boutry, S.; Breger, L.S.; Klaver, B.; Melas, C.; Barroso-Chinea, P.; Gonzalez-Hernandez, T. A regulatable AAV vector mediating GDNF biological effects at clinically-approved sub-antimicrobial doxycycline doses. Mol. Ther. Methods Clin. Dev. 2016, 3, 16027. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Xu, Y.; Zhang, Y.; Zhu, C. A comprehensive review of AAV-mediated strategies targeting microglia for therapeutic intervention of neurodegenerative diseases. J. Neuroinflamm. 2024, 21, 232. [Google Scholar] [CrossRef]
- Masoudi, N.; Willen, J.; Daniels, C.; Jenkins, B.A.; Furber, E.C.; Kothiya, M.; Banjoko, M.B.; Gowda, R.; Hendricks, J.; Fang, Y.-Y.; et al. Microglial-targeted Gene Therapy: Developing a Disease Modifying Treatment for ALSP Associated with CSF1R Mutations (ALSP-CSF1R) (P11-4.012). Neurology 2024, 102, 3061. [Google Scholar] [CrossRef]
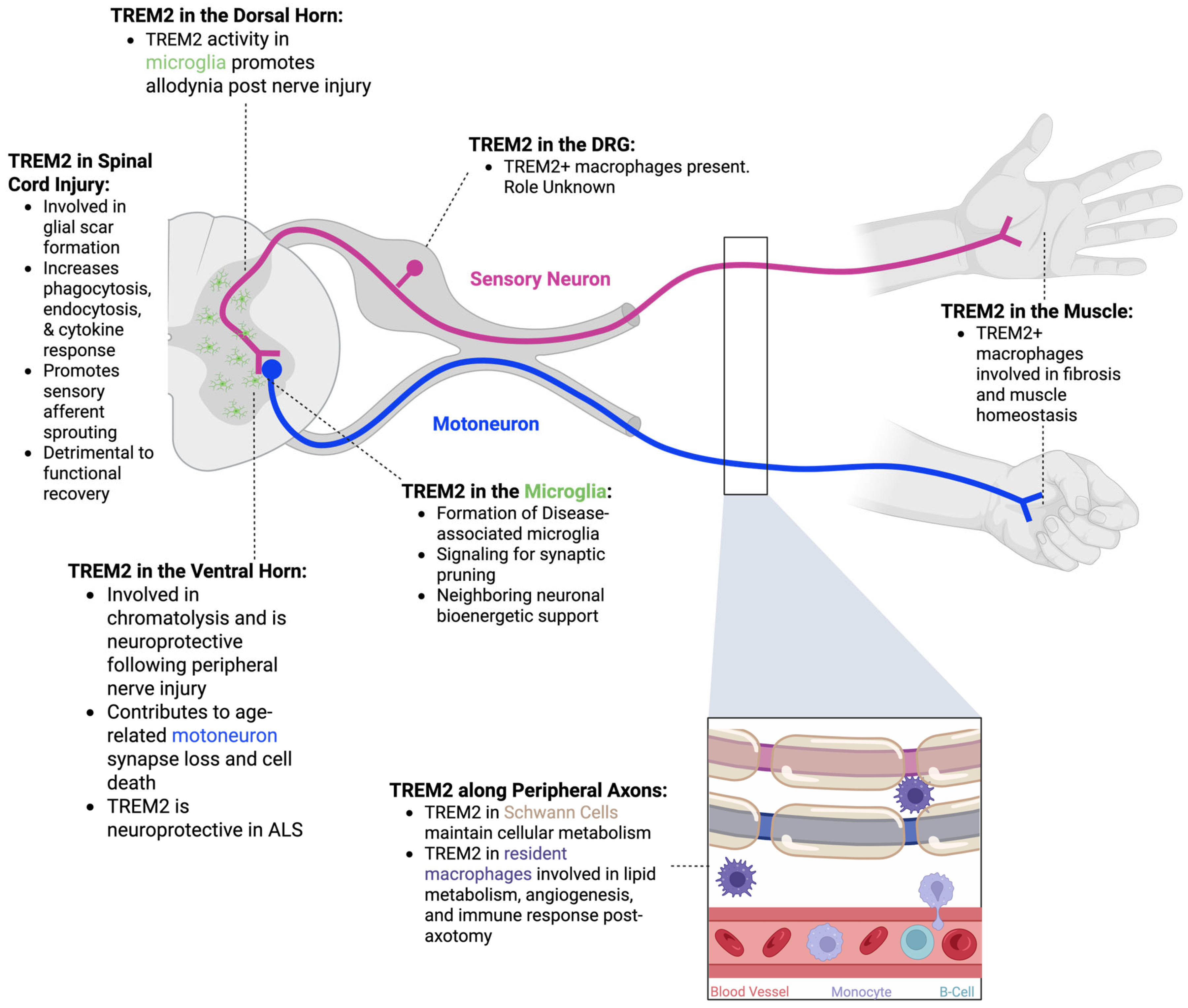

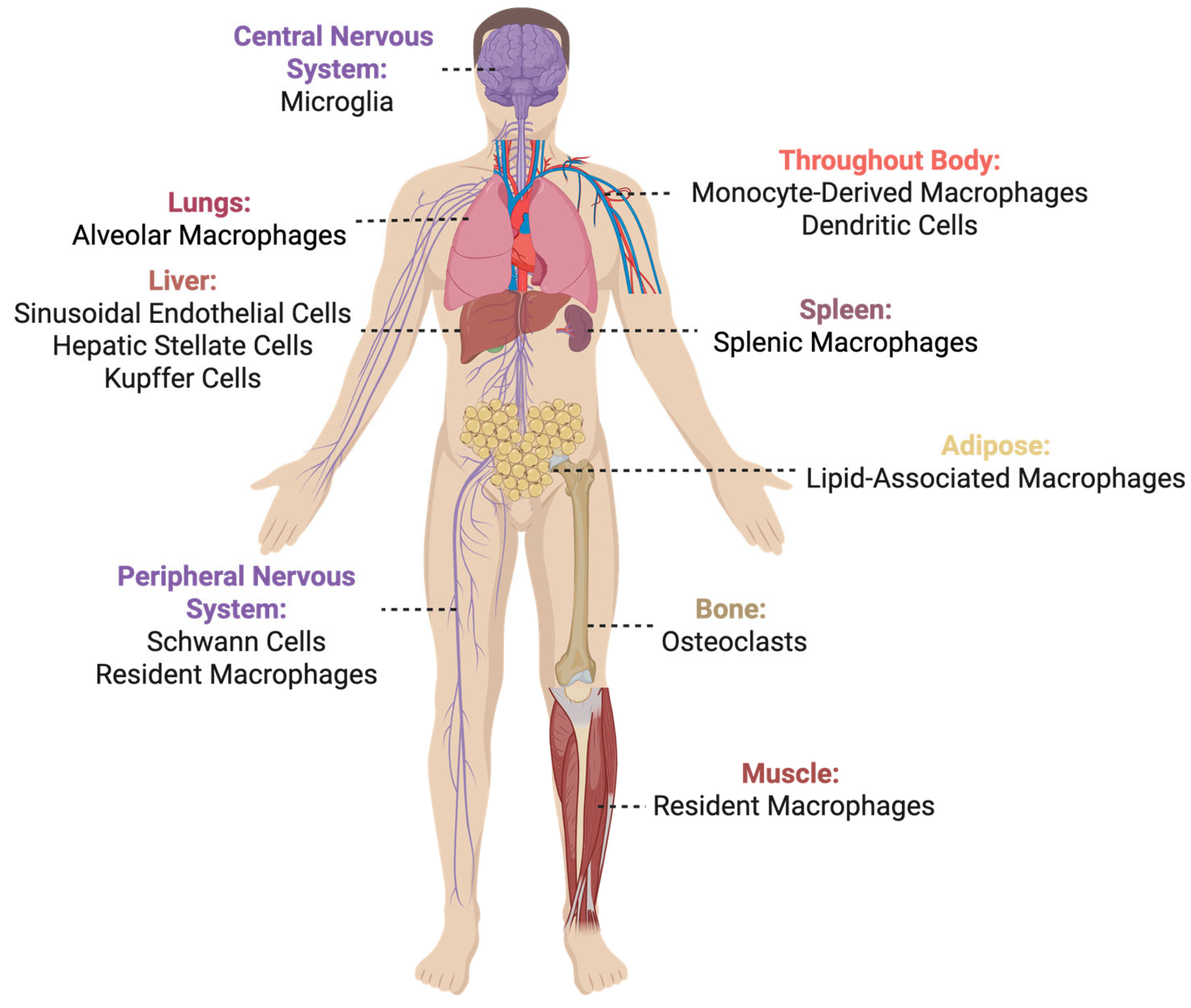
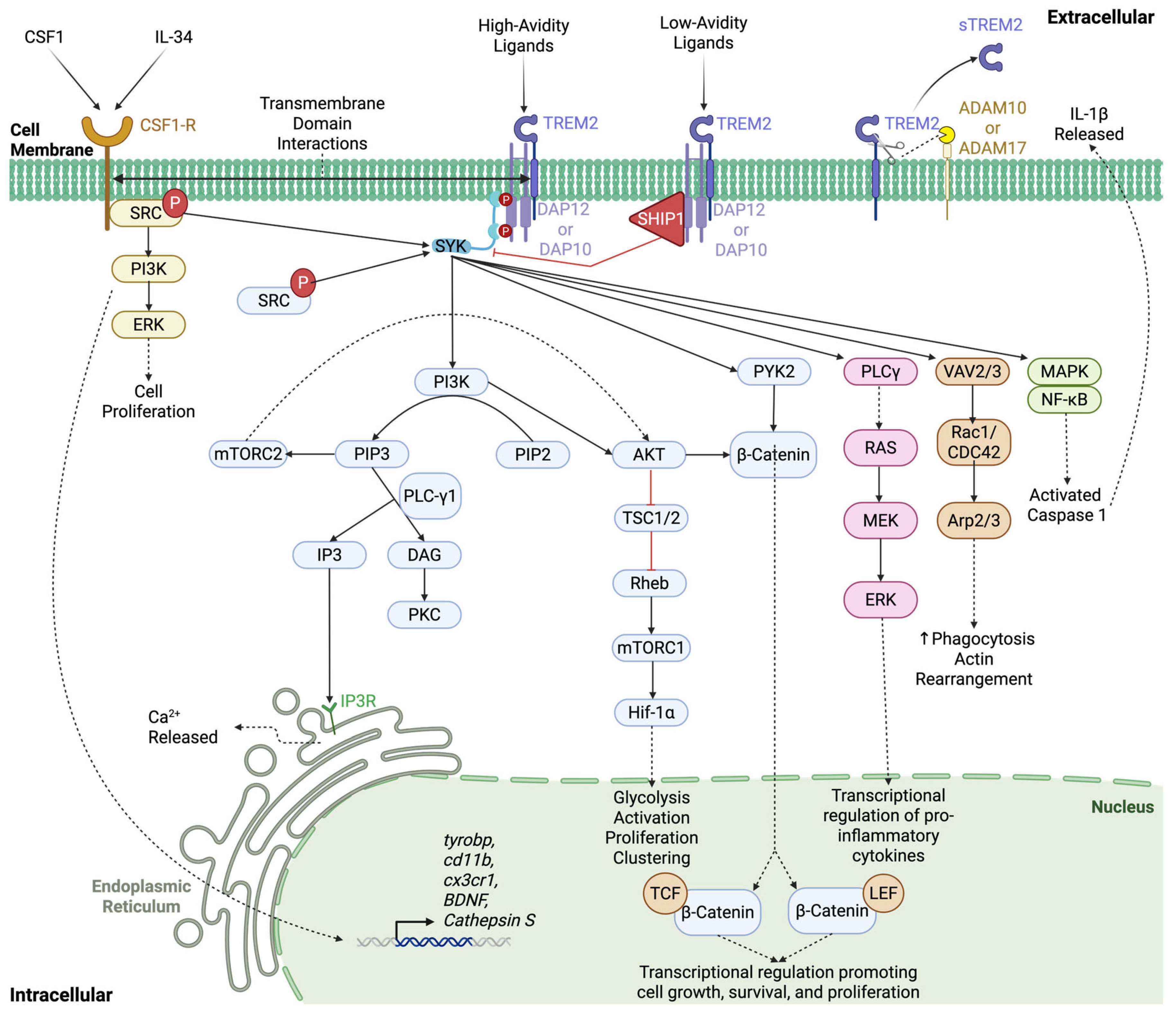

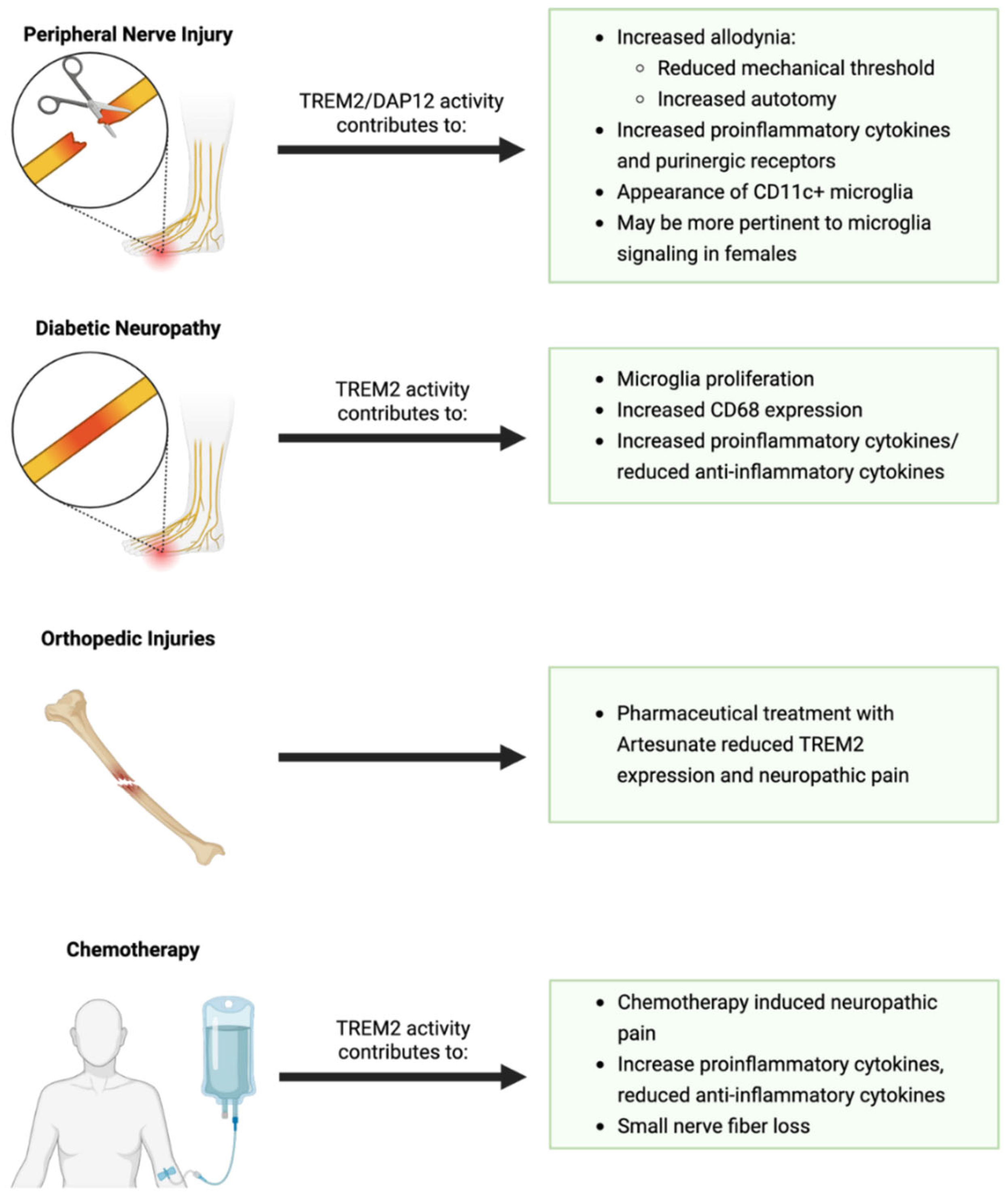
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pottorf, T.S.; Lane, E.L.; Alvarez, F.J. Is TREM2 a Stretch: Implications of TREM2 Along Spinal Cord Circuits in Health, Aging, Injury, and Disease. Cells 2025, 14, 1520. https://doi.org/10.3390/cells14191520
Pottorf TS, Lane EL, Alvarez FJ. Is TREM2 a Stretch: Implications of TREM2 Along Spinal Cord Circuits in Health, Aging, Injury, and Disease. Cells. 2025; 14(19):1520. https://doi.org/10.3390/cells14191520
Chicago/Turabian StylePottorf, Tana S., Elizabeth L. Lane, and Francisco J. Alvarez. 2025. "Is TREM2 a Stretch: Implications of TREM2 Along Spinal Cord Circuits in Health, Aging, Injury, and Disease" Cells 14, no. 19: 1520. https://doi.org/10.3390/cells14191520
APA StylePottorf, T. S., Lane, E. L., & Alvarez, F. J. (2025). Is TREM2 a Stretch: Implications of TREM2 Along Spinal Cord Circuits in Health, Aging, Injury, and Disease. Cells, 14(19), 1520. https://doi.org/10.3390/cells14191520






