Inflammation in Cerebral Cavernous Malformations: Differences Between Malformation Related Epilepsy vs. Symptomatic Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Clinical Data Collection
2.3. Histopathological Data Collection
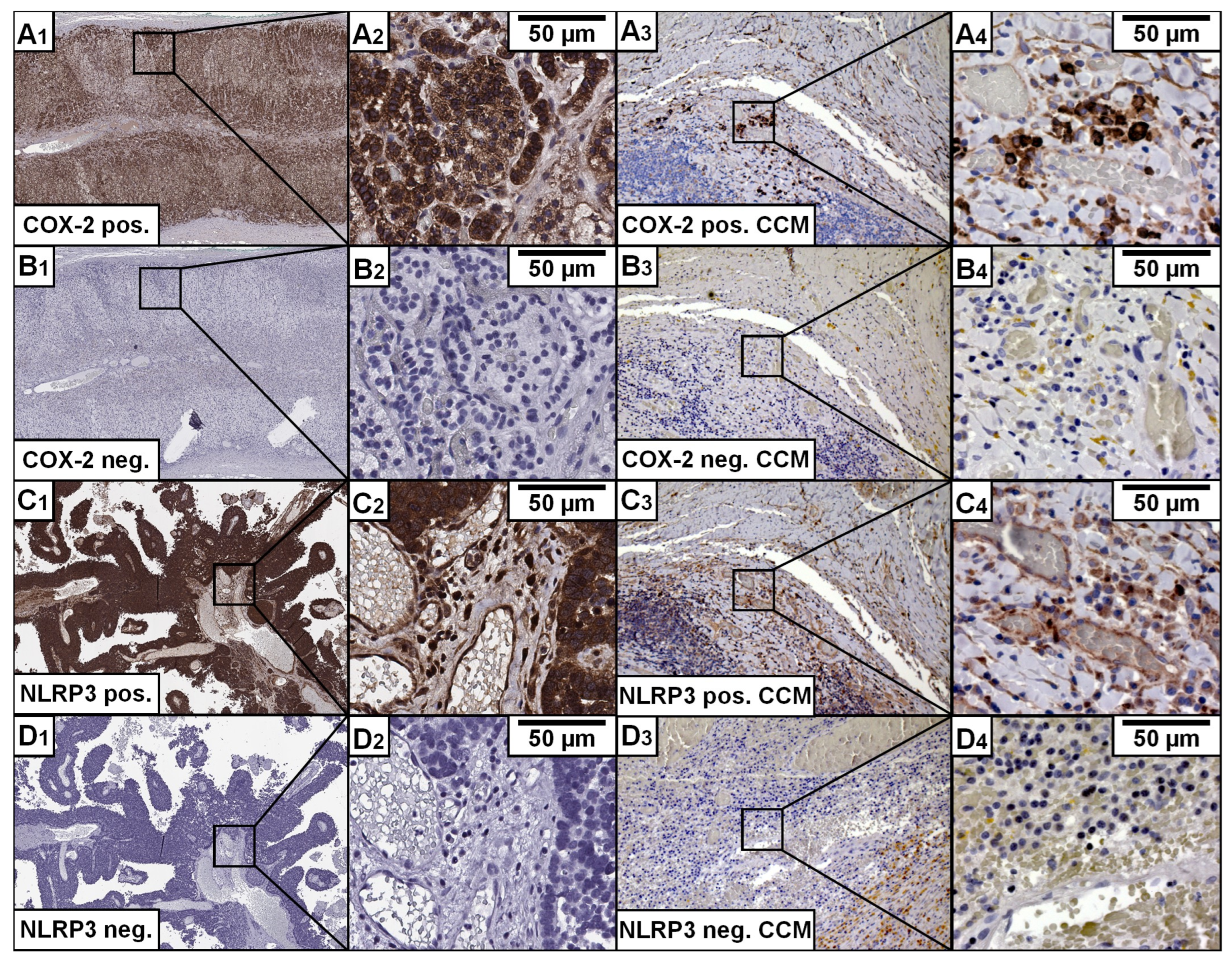
2.4. Immunohistochemical Staining
2.5. Histological Image Analysis
2.6. Radiological Data Collection and Measurements
2.7. Statistical Analysis
3. Results
3.1. Study Cohort
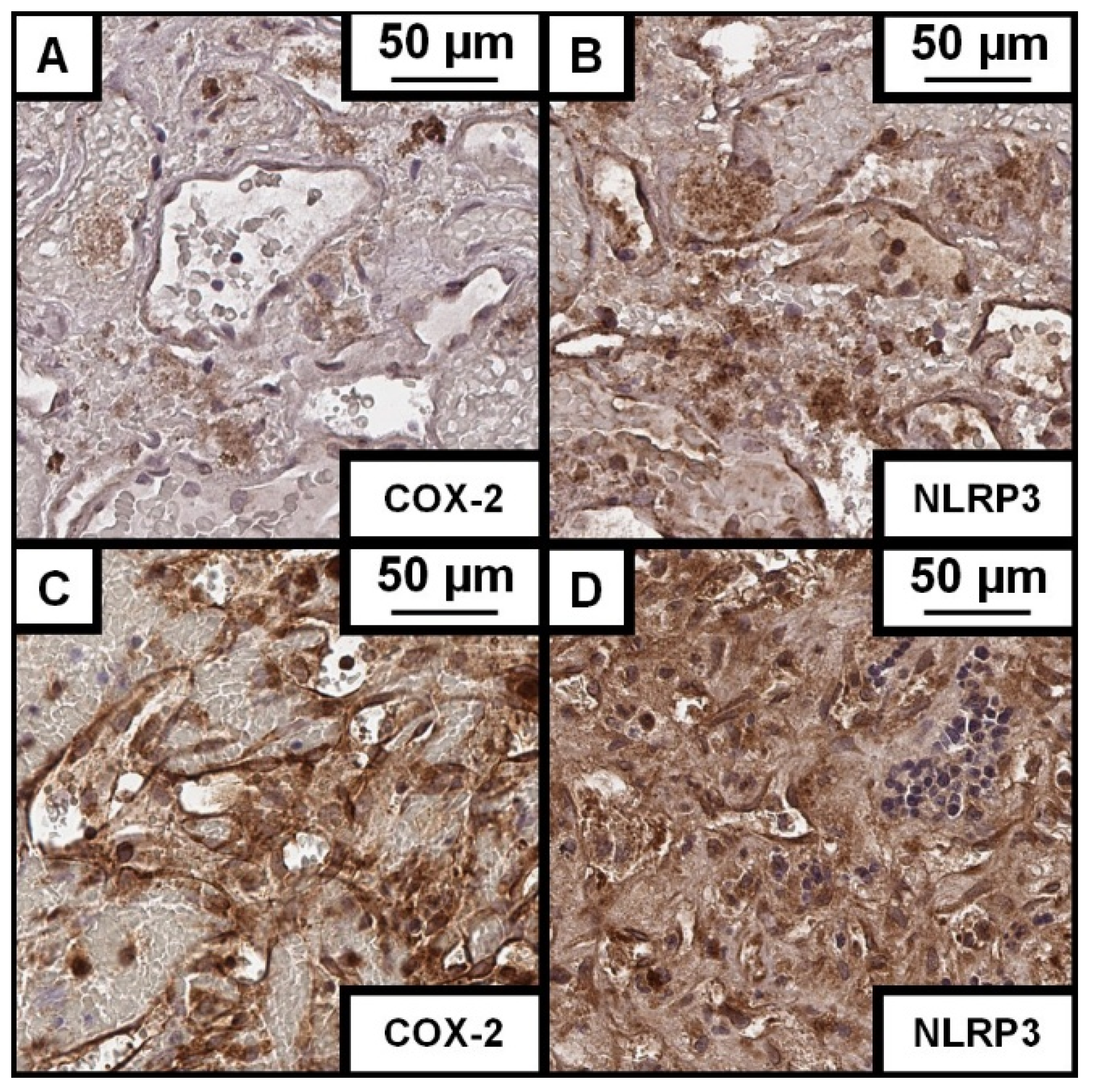
3.2. Histological Characteristics
3.3. CCM and Its Association with NLRP3
3.4. CCM and Its Association with COX-2
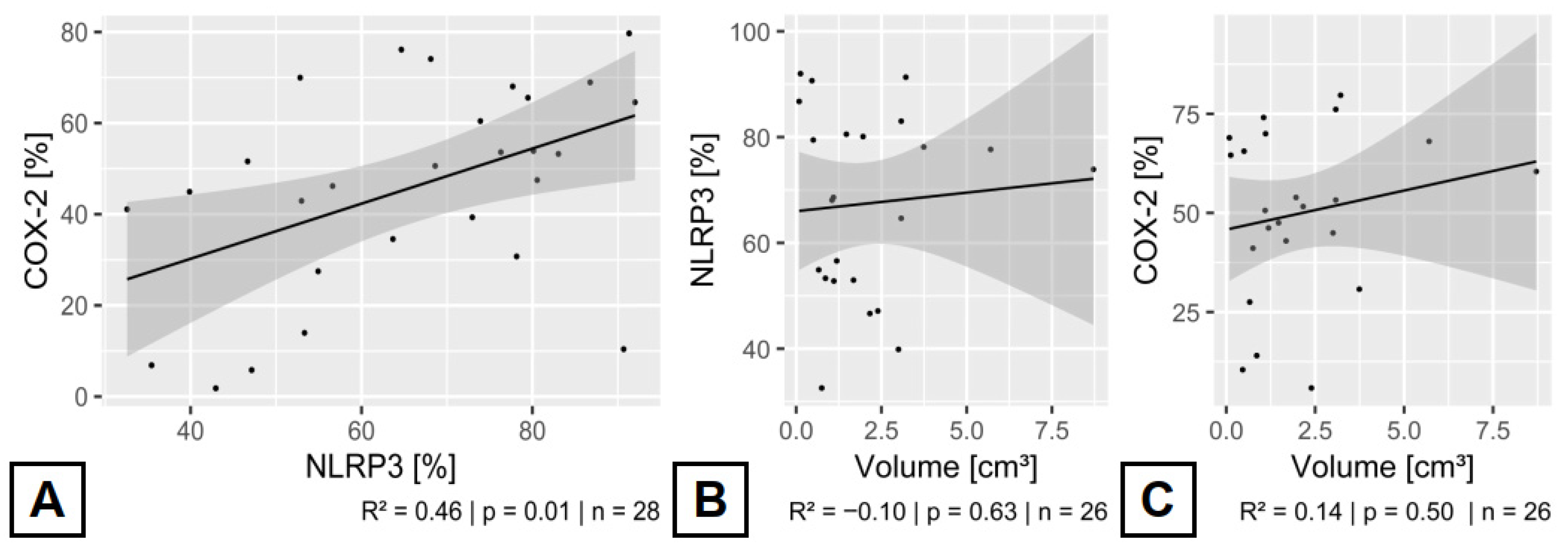
3.5. Relationship Between the Expressed Inflammatory Enzymes in CCMs
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caton, M.T.; Karsonovich, T.; Shenoy, V.S. Cerebral Cavernous Malformations. In StatPearls; Caton, M.T., Karsonovich, T., Shenoy, V.S., Eds.; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Spiegler, S.; Rath, M.; Paperlein, C.; Felbor, U. Cerebral Cavernous Malformations: An Update on Prevalence, Molecular Genetic Analyses, and Genetic Counselling. Mol. Syndromol. 2018, 9, 60–69. [Google Scholar] [CrossRef]
- Fischer, A.; Zalvide, J.; Faurobert, E.; Albiges-Rizo, C.; Tournier-Lasserve, E. Cerebral cavernous malformations: From CCM genes to endothelial cell homeostasis. Trends Mol. Med. 2013, 19, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Dammann, P.; Santos, A.N.; Wan, X.-Y.; Zhu, Y.; Sure, U. Cavernous Malformations: Updates in Surgical Management and Biology. Neurosurg. Clin. N. Am. 2022, 33, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Lant, B.; Pal, S.; Chapman, E.M.; Yu, B.; Witvliet, D.; Choi, S.; Zhao, L.; Albiges-Rizo, C.; Faurobert, E.; Derry, W.B. Interrogating the ccm-3 Gene Network. Cell Rep. 2018, 24, 2857–2868.e4. [Google Scholar] [CrossRef]
- Mikati, A.G.; Khanna, O.; Zhang, L.; Girard, R.; Shenkar, R.; Guo, X.; Shah, A.; Larsson, H.B.W.; Tan, H.; Li, L.; et al. Vascular permeability in cerebral cavernous malformations. J. Cereb. Blood Flow Metab. 2015, 35, 1632–1639. [Google Scholar] [CrossRef]
- Denier, C.; Labauge, P.; Bergametti, F.; Marchelli, F.; Riant, F.; Arnoult, M.; Maciazek, J.; Vicaut, E.; Brunereau, L.; Tournier-Lasserve, E. Genotype-phenotype correlations in cerebral cavernous malformations patients. Ann. Neurol. 2006, 60, 550–556. [Google Scholar] [CrossRef]
- Choquet, H.; Pawlikowska, L.; Lawton, M.T.; Kim, H. Genetics of cerebral cavernous malformations: Current status and future prospects. J. Neurosurg. Sci. 2015, 59, 211–220. [Google Scholar]
- Gore, A.V.; Lampugnani, M.G.; Dye, L.; Dejana, E.; Weinstein, B.M. Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. Dis. Model. Mech. 2008, 1, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, G.G.; Golanov, E.; Awad, I.A.; Young, W.L. Biology of vascular malformations of the brain. Stroke 2009, 40, e694–e702. [Google Scholar] [CrossRef]
- Goitre, L.; De Luca, E.; Braggion, S.; Trapani, E.; Guglielmotto, M.; Biasi, F.; Forni, M.; Moglia, A.; Trabalzini, L.; Retta, S.F. KRIT1 loss of function causes a ROS-dependent upregulation of c-Jun. Free Radic. Biol. Med. 2014, 68, 134–147. [Google Scholar] [CrossRef]
- Lai, C.C.; Nelsen, B.; Frias-Anaya, E.; Gallego-Gutierrez, H.; Orecchioni, M.; Herrera, V.; Ortiz, E.; Sun, H.; Mesarwi, O.A.; Ley, K.; et al. Neuroinflammation Plays a Critical Role in Cerebral Cavernous Malformation Disease. Circ. Res. 2022, 131, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ramirez, M.A.; Lai, C.C.; Soliman, S.I.; Hale, P.; Pham, A.; Estrada, E.J.; McCurdy, S.; Girard, R.; Verma, R.; Moore, T.; et al. Astrocytes propel neurovascular dysfunction during cerebral cavernous malformation lesion formation. J. Clin. Investig. 2021, 131, e139570. [Google Scholar] [CrossRef]
- Ramsay, R.G.; Ciznadija, D.; Vanevski, M.; Mantamadiotis, T. Transcriptional regulation of cyclo-oxygenase expression: Three pillars of control. Int. J. Immunopathol. Pharmacol. 2003, 16, 59–67. [Google Scholar] [PubMed]
- Rodemerk, J.; Junker, A.; Chen, B.; Pierscianek, D.; Dammann, P.; Darkwah Oppong, M.; Radbruch, A.; Forsting, M.; Maderwald, S.; Quick, H.H.; et al. Pathophysiology of Intracranial Aneurysms: COX-2 Expression, Iron Deposition in Aneurysm Wall, and Correlation With Magnetic Resonance Imaging. Stroke 2020, 51, 2505–2513. [Google Scholar] [CrossRef]
- Jabbarli, R.; Rauschenbach, L.; Dinger, T.F.; Darkwah Oppong, M.; Rodemerk, J.; Pierscianek, D.; Dammann, P.; Junker, A.; Sure, U.; Wrede, K.H. In the wall lies the truth: A systematic review of diagnostic markers in intracranial aneurysms. Brain Pathol. 2020, 30, 437–445. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Barrington, J.; Lemarchand, E.; Allan, S.M. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 2017, 27, 205–212. [Google Scholar] [CrossRef]
- Russell-Puleri, S.; Dela Paz, N.G.; Adams, D.; Chattopadhyay, M.; Cancel, L.; Ebong, E.; Orr, A.W.; Frangos, J.A.; Tarbell, J.M. Fluid shear stress induces upregulation of COX-2 and PGI2 release in endothelial cells via a pathway involving PECAM-1, PI3K, FAK, and p38. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H485–H500. [Google Scholar] [CrossRef] [PubMed]
- Retta, S.F.; Glading, A.J. Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: Two sides of the same coin. Int. J. Biochem. Cell Biol. 2016, 81, 254–270. [Google Scholar] [CrossRef]
- Sano, F.; Shigetomi, E.; Shinozaki, Y.; Tsuzukiyama, H.; Saito, K.; Mikoshiba, K.; Horiuchi, H.; Cheung, D.L.; Nabekura, J.; Sugita, K.; et al. Reactive astrocyte-driven epileptogenesis is induced by microglia initially activated following status epilepticus. JCI Insight 2021, 6, e135391. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, A.; Salvati, L.F.; Perrelli, A.; Ferraris, C.; Massara, A.; Minardi, M.; Aruta, G.; Rosso, M.; Massa Micon, B.; Garbossa, D.; et al. Distant Recurrence of a Cerebral Cavernous Malformation in the Vicinity of a Developmental Venous Anomaly: Case Report of Local Oxy-Inflammatory Events. Int. J. Mol. Sci. 2022, 23, 14643. [Google Scholar] [CrossRef]
- Petersen, T.A.; Morrison, L.A.; Schrader, R.M.; Hart, B.L. Familial versus sporadic cavernous malformations: Differences in developmental venous anomaly association and lesion phenotype. AJNR Am. J. Neuroradiol. 2010, 31, 377–382. [Google Scholar] [CrossRef]
- Rodemerk, J.; Oppong, M.D.; Junker, A.; Deuschl, C.; Forsting, M.; Zhu, Y.; Dammann, P.; Uerschels, A.; Jabbarli, R.; Sure, U.; et al. Ischemia-induced inflammation in arteriovenous malformations. Neurosurg Focus 2022, 53, E3. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; van de Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Hua, K.-F.; Chou, J.-C.; Ka, S.-M.; Tasi, Y.-L.; Chen, A.; Wu, S.-H.; Chiu, H.-W.; Wong, W.-T.; Wang, Y.-F.; Tsai, C.-L.; et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1β production. J. Cell. Physiol. 2015, 230, 863–874. [Google Scholar] [CrossRef]
- Sokolowska, M.; Chen, L.-Y.; Liu, Y.; Martinez-Anton, A.; Qi, H.-Y.; Logun, C.; Alsaaty, S.; Park, Y.H.; Kastner, D.L.; Chae, J.J.; et al. Prostaglandin E2 Inhibits NLRP3 Inflammasome Activation through EP4 Receptor and Intracellular Cyclic AMP in Human Macrophages. J. Immunol. 2015, 194, 5472–5487. [Google Scholar] [CrossRef]
- Li, Y.; Srinath, A.; Alcazar-Felix, R.J.; Hage, S.; Bindal, A.; Lightle, R.; Shenkar, R.; Shi, C.; Girard, R.; Awad, I.A. Inflammatory Mechanisms in a Neurovascular Disease: Cerebral Cavernous Malformation. Brain Sci. 2023, 13, 1336. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, F.; Liang, J.; Deng, X.; Zhang, Y.; Ding, G.; Zhang, A.; Jia, Z.; Huang, S. Activation of COX-2/mPGES-1/PGE2 Cascade via NLRP3 Inflammasome Contributes to Albumin-Induced Proximal Tubule Cell Injury. Cell. Physiol. Biochem. 2017, 42, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Peng, Z.; Ren, J.; Zhang, H. Cerebral Cavernous Malformation: Immune and Inflammatory Perspectives. Front. Immunol. 2022, 13, 922281. [Google Scholar] [CrossRef] [PubMed]

| Manifestation | NLRP3 | COX-2 | NLRP3 vs. COX-2 With Manifestation | NLRP3 vs. COX-2 Without Manifestation |
|---|---|---|---|---|
| Pathology | ||||
| Gliosis | p = 0.81, OR: 1.001, 95% CI: 0.991–1.012 | p = 0.59, OR: 0.997, 95% CI: 0.989–1.006 | p = 0.001 66.3 ± 18.2% vs. 44.4 ± 23.0% | p = 0.16 64.5 ± 17.5% vs. 49.5 ± 22.4% |
| Edema in adjacent parenchyma | p = 0.26, OR: 1.001, 95% CI: 0.995–1.018 | p = 0.78, OR: 1.001, 95% CI: 0.992–1.010 | p = 0.003 69.9 ± 17.8% vs. 47.1 ± 17.4% | p = 0.04 62.2 ± 17.3% vs. 44.7 ± 26.8% |
| Clinical data | ||||
| Age | p = 0.87, OR: 0.981, 95% CI: 0.716–1.345 | p = 0.81, OR: 1.039, 95% CI: 0.812–1.331 | Patients over 29 years: p = 0.10 61.7 ± 17.1% vs. 48.8 ± 21.8% | Patients under 30 Years: p = 0.002 69.3 ± 18.0% vs. 43.2 ± 23.5% |
| BMI | p = 0.64, OR: 0.69, 95% CI: 0.36–1.34 | p = 0.054, OR: 0.64, 95% CI: 0.406–1.010 | ---///--- | ---///--- |
| Body Height | p = 0.14, OR: 1.46, 95% CI: 0.89–2.42 | p = 0.06, OR: 1.39, 95% CI: 0.97–2.00 | ---///--- | ---///--- |
| Sex | p = 0.93, OR: 0.999, 95% CI: 0.988–1.011 | p = 0.50, OR: 1.002, 95% CI: 0.994–1.011 | Female: p = 0.006 65.5 ± 17.6% vs. 48.2 ± 17.3% | Male: p = 0.03 66.1 ± 18.6% vs. 42.2 ± 29.5% |
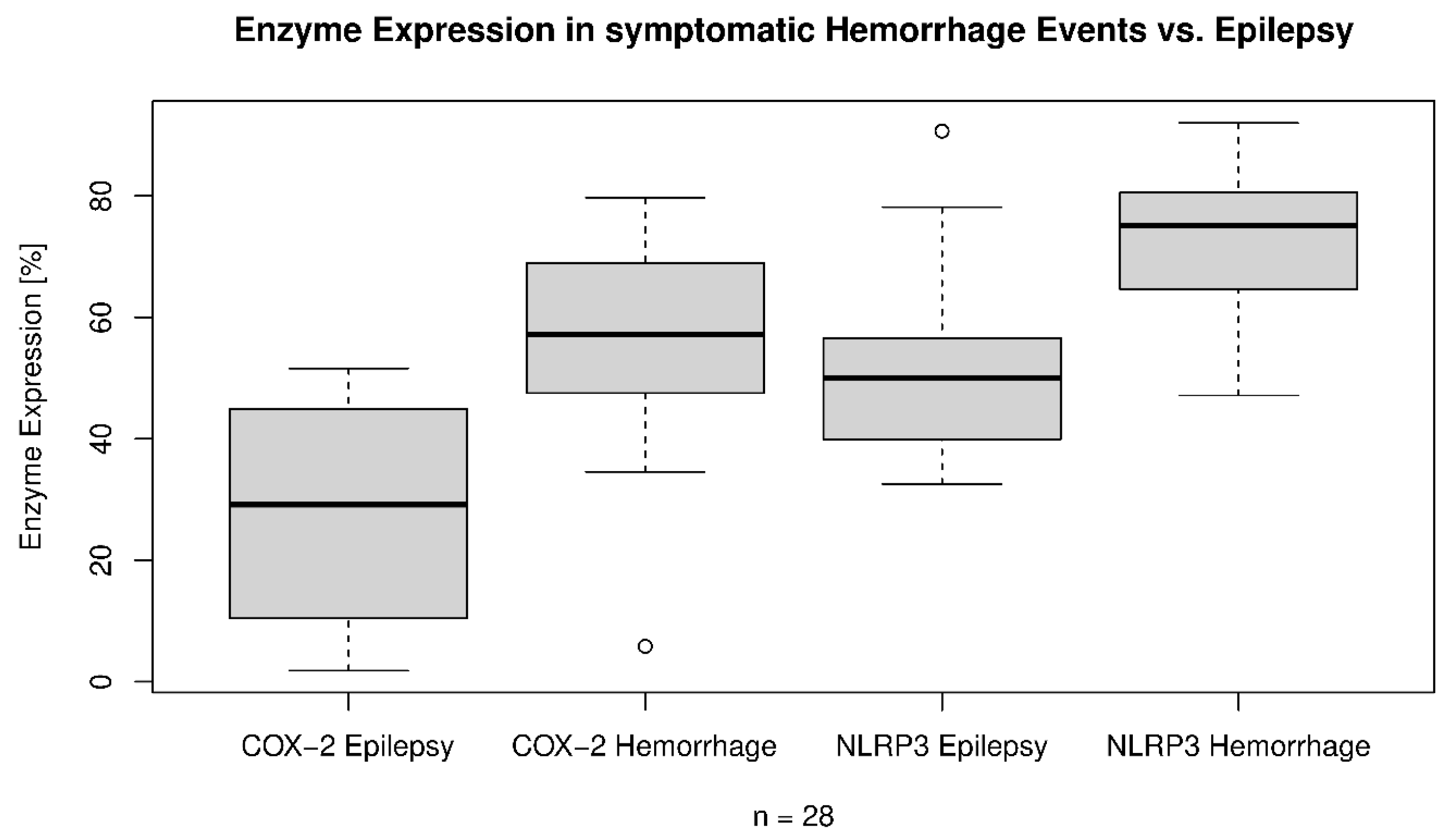
| Symptomatic hemorrhage | p = 0.002, OR: 1.015, 95% CI: 1.005–1.024 | p = < 0.001, OR: 1.013, 95% CI: 1.006–1.020 | p = 0.003 72.8 ± 12.9% vs. 56.0 ± 18.0% | p = 0.006 53.1 ± 18.5% vs. 27.5 ± 18.2% |
| CCM related epilepsy | p = 0.01, OR: 0.986, 95% CI: 0.976–0.996 | p = 0.03, OR: 0.991, 95% CI: 0.982–0.999 | p = 0.01 58.6 ± 18.9% vs. 38.1 ± 25.4% | p < 0.001 75.3 ± 10.3% vs. 57.1 ± 12.8% |
| Multiple CCMs | p = 0.60, OR: 0.997, 95% CI: 0.989–1.005 | p = 0.41, OR: 1.002, 95% CI: 0.996–1.008 | p = 0.66 60.7 ± 19.8% vs. 54.6 ± 17.6% | p < 0.001 66.6 ± 17.6% vs. 44.4 ± 23.2% |
| Radiology | ||||
| Volume | p = 0.89, OR: 1.069, 95% CI: 0.82–1.39 | p = 0.82, OR: 0.97, 95% CI: 0.78–1.21 | ---///--- | ---///--- |
| Radiographic hemorrhage | p = 0.051, OR: 1.010, 95% CI: 0.999–1.021 | p = 0.001, OR: 1.012, 95% CI: 1.005–1.019 | p = 0.01 70.6 ± 14.9% vs. 55.5 ± 18.2% | p = 0.004 57.1 ± 19.7% vs. 28.4 ± 19.3% |
| DVA | p = 0.90, OR: 0.99, 95% CI: 0.988–1.010 | p = 0.87, OR: 1.00, 95% CI: 0.992–1.009 | p = 0.044 65.2 ± 14.9% vs. 46.8 ± 20.1% | p = 0.006 66.0 ± 19.2% vs. 45.3 ± 24.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodemerk, J.; Engel, A.; Horstmann, J.L.H.; Rauschenbach, L.; Darkwah Oppong, M.; Santos, A.N.; Junker, A.; Deuschl, C.; Forsting, M.; Zhu, Y.; et al. Inflammation in Cerebral Cavernous Malformations: Differences Between Malformation Related Epilepsy vs. Symptomatic Hemorrhage. Cells 2025, 14, 1510. https://doi.org/10.3390/cells14191510
Rodemerk J, Engel A, Horstmann JLH, Rauschenbach L, Darkwah Oppong M, Santos AN, Junker A, Deuschl C, Forsting M, Zhu Y, et al. Inflammation in Cerebral Cavernous Malformations: Differences Between Malformation Related Epilepsy vs. Symptomatic Hemorrhage. Cells. 2025; 14(19):1510. https://doi.org/10.3390/cells14191510
Chicago/Turabian StyleRodemerk, Jan, Adrian Engel, Julius L. H. Horstmann, Laurèl Rauschenbach, Marvin Darkwah Oppong, Alejandro N. Santos, Andreas Junker, Cornelius Deuschl, Michael Forsting, Yuan Zhu, and et al. 2025. "Inflammation in Cerebral Cavernous Malformations: Differences Between Malformation Related Epilepsy vs. Symptomatic Hemorrhage" Cells 14, no. 19: 1510. https://doi.org/10.3390/cells14191510
APA StyleRodemerk, J., Engel, A., Horstmann, J. L. H., Rauschenbach, L., Darkwah Oppong, M., Santos, A. N., Junker, A., Deuschl, C., Forsting, M., Zhu, Y., Jabbarli, R., Wrede, K. H., Schmidt, B., Sure, U., & Dammann, P. (2025). Inflammation in Cerebral Cavernous Malformations: Differences Between Malformation Related Epilepsy vs. Symptomatic Hemorrhage. Cells, 14(19), 1510. https://doi.org/10.3390/cells14191510








