Myocardial Ischemia/Reperfusion Injury: Molecular Insights, Forensic Perspectives, and Therapeutic Horizons
Abstract
1. Introduction
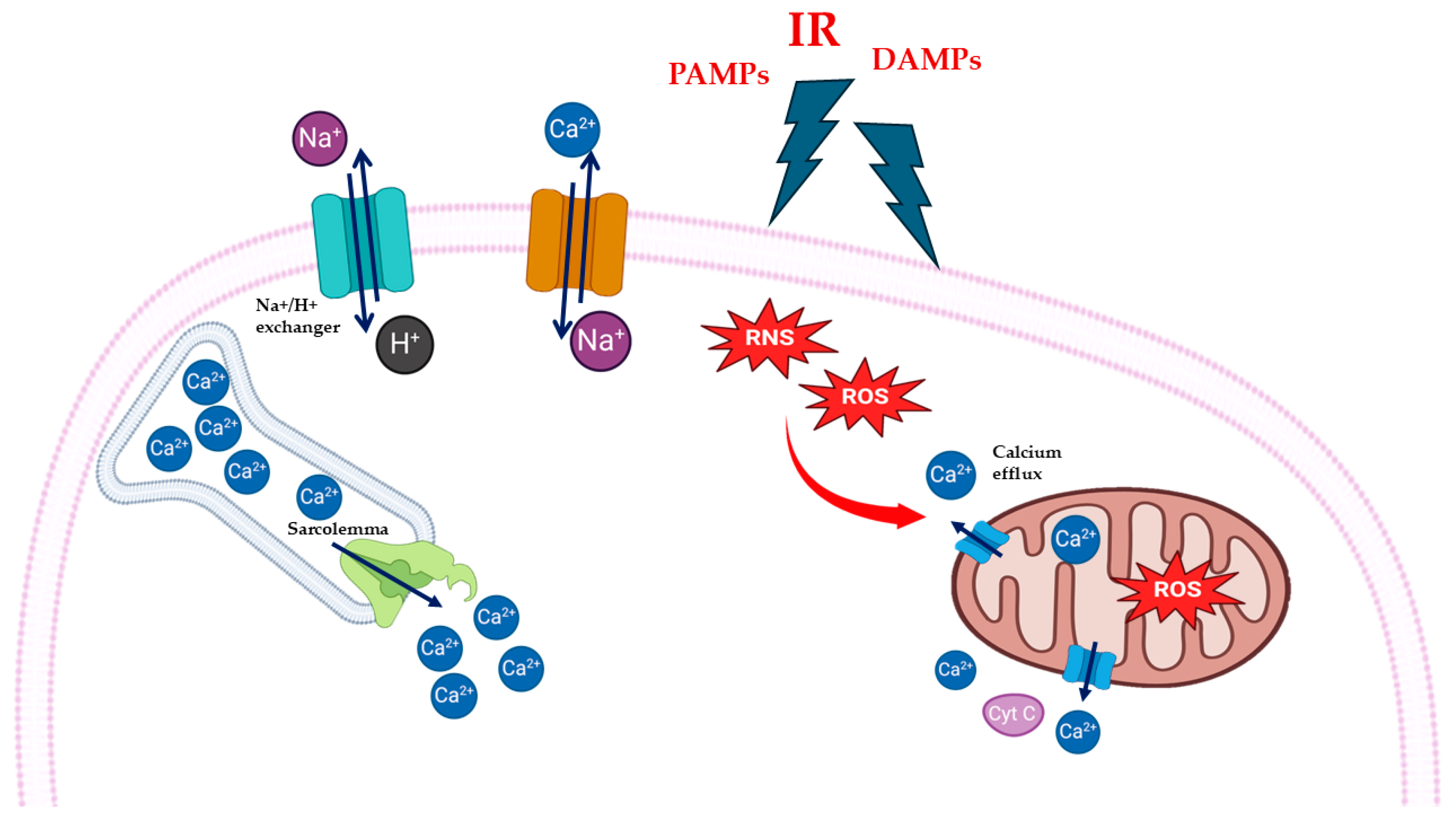
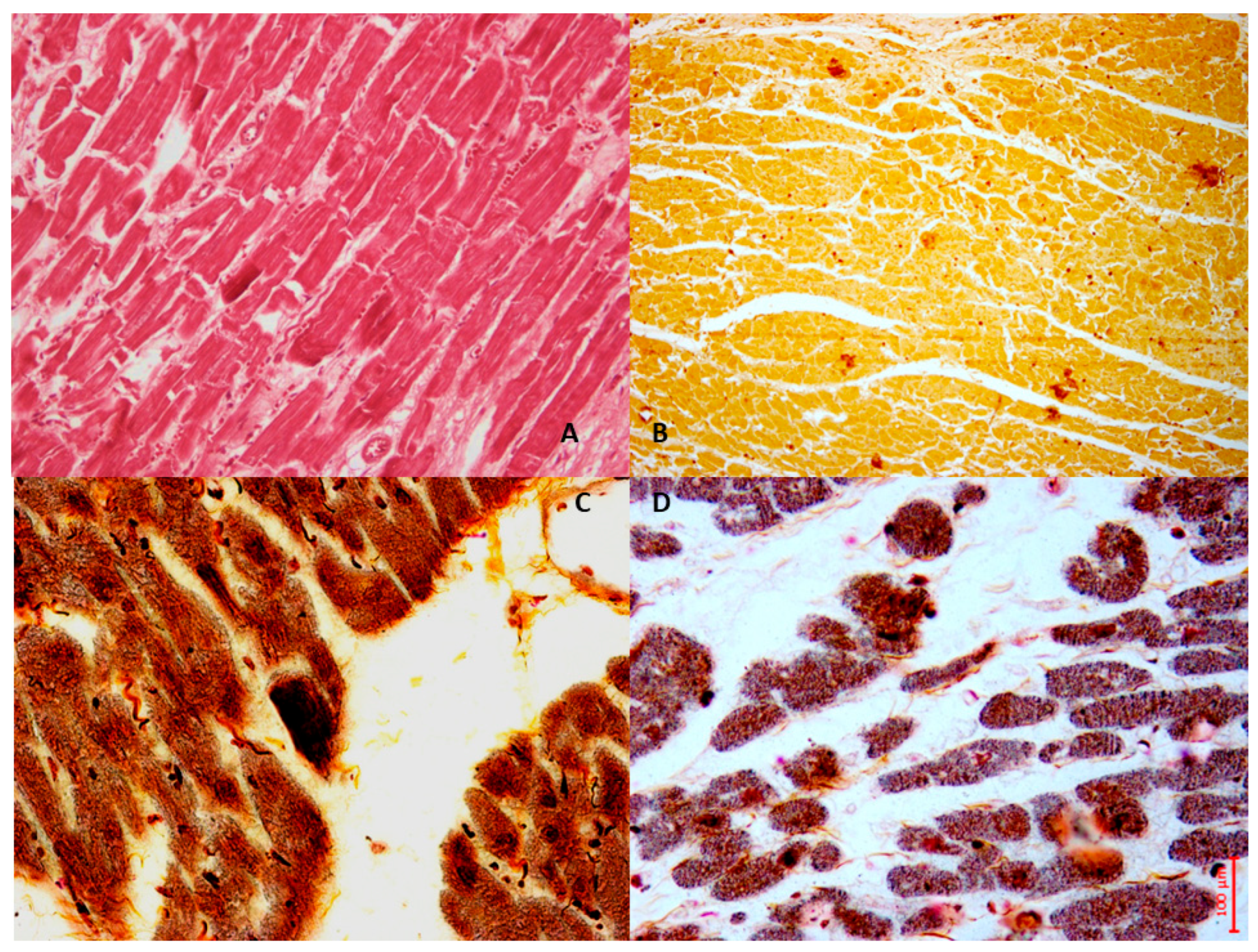
2. MIRI and Oxidative Stress: Mitochondria and Calcium Overload
3. MIRI, NLRP3 Inflammasome, and Cytokines: From Homeostasis to Dysregulation
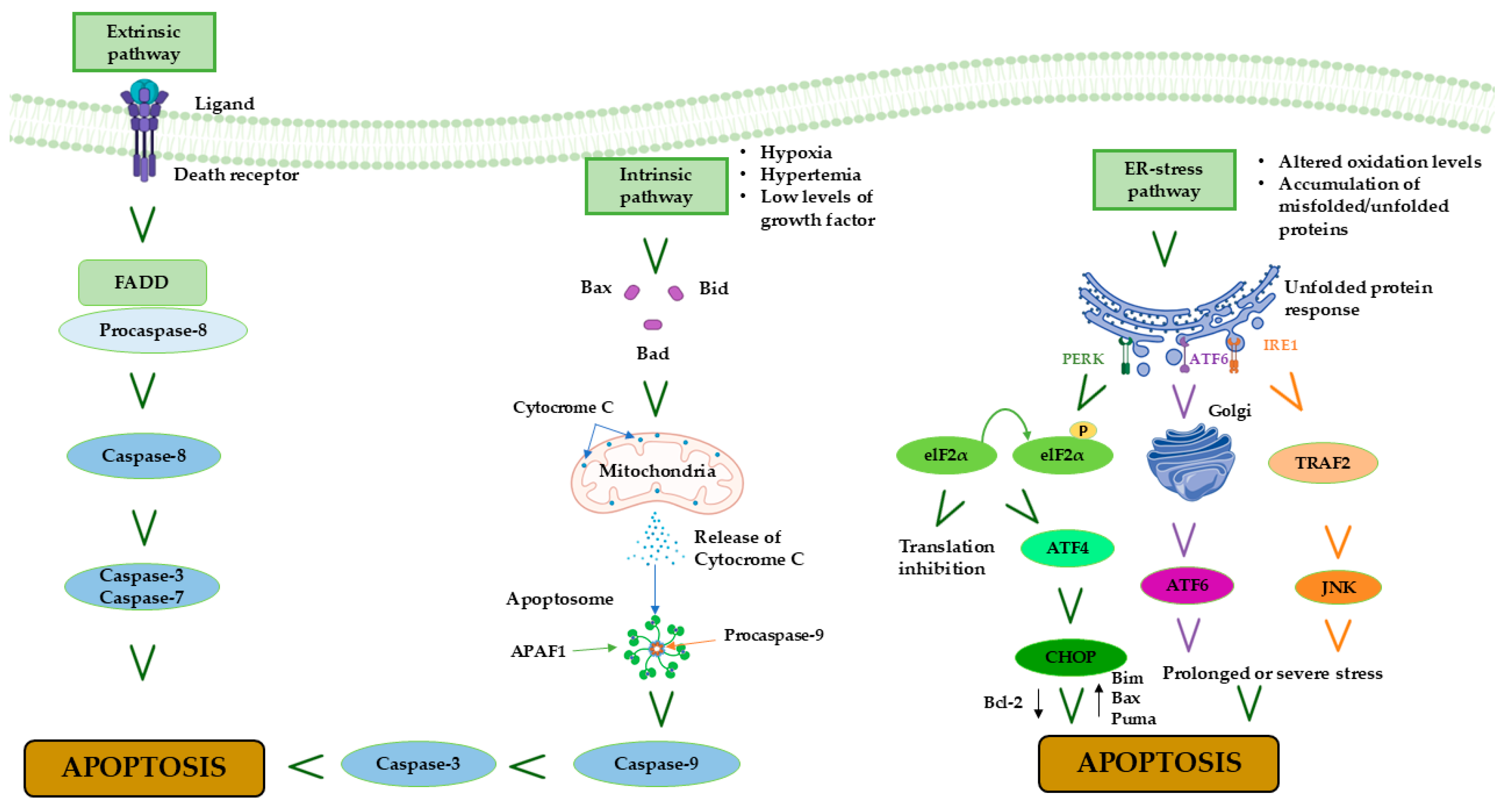
4. MIRI and Apoptosis
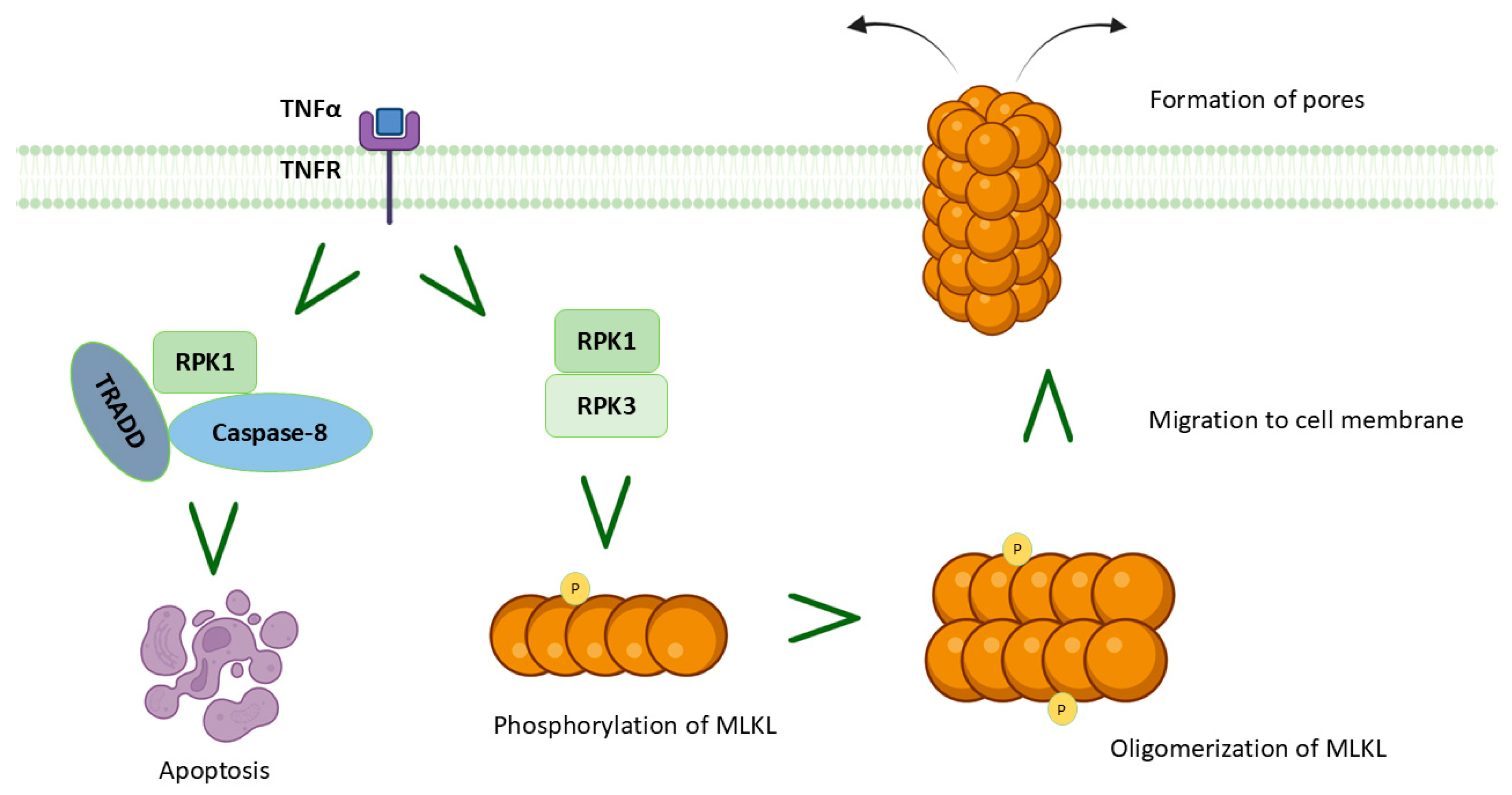
5. MIRI and Necroptosis
6. MIRI and Autophagy
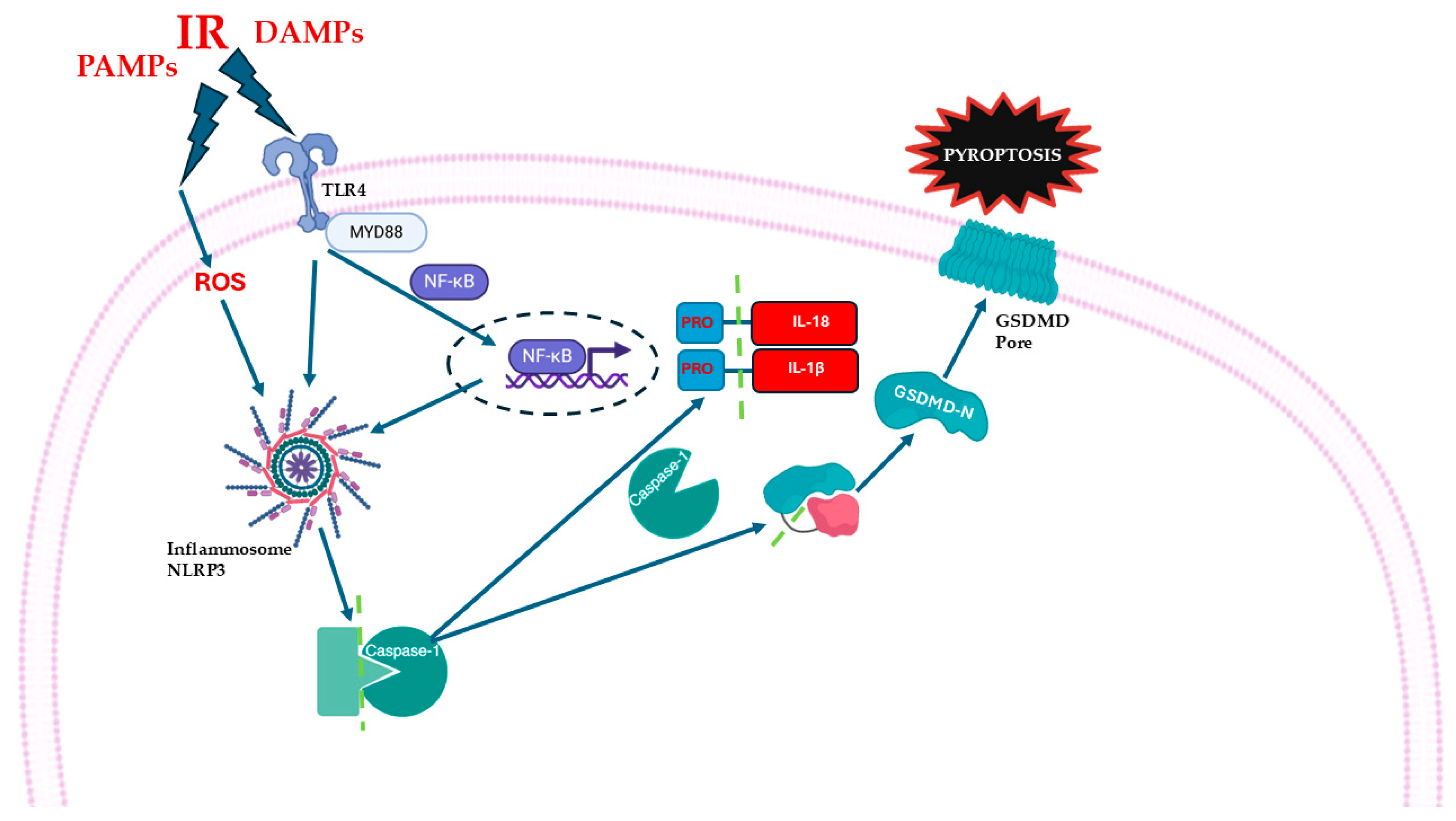
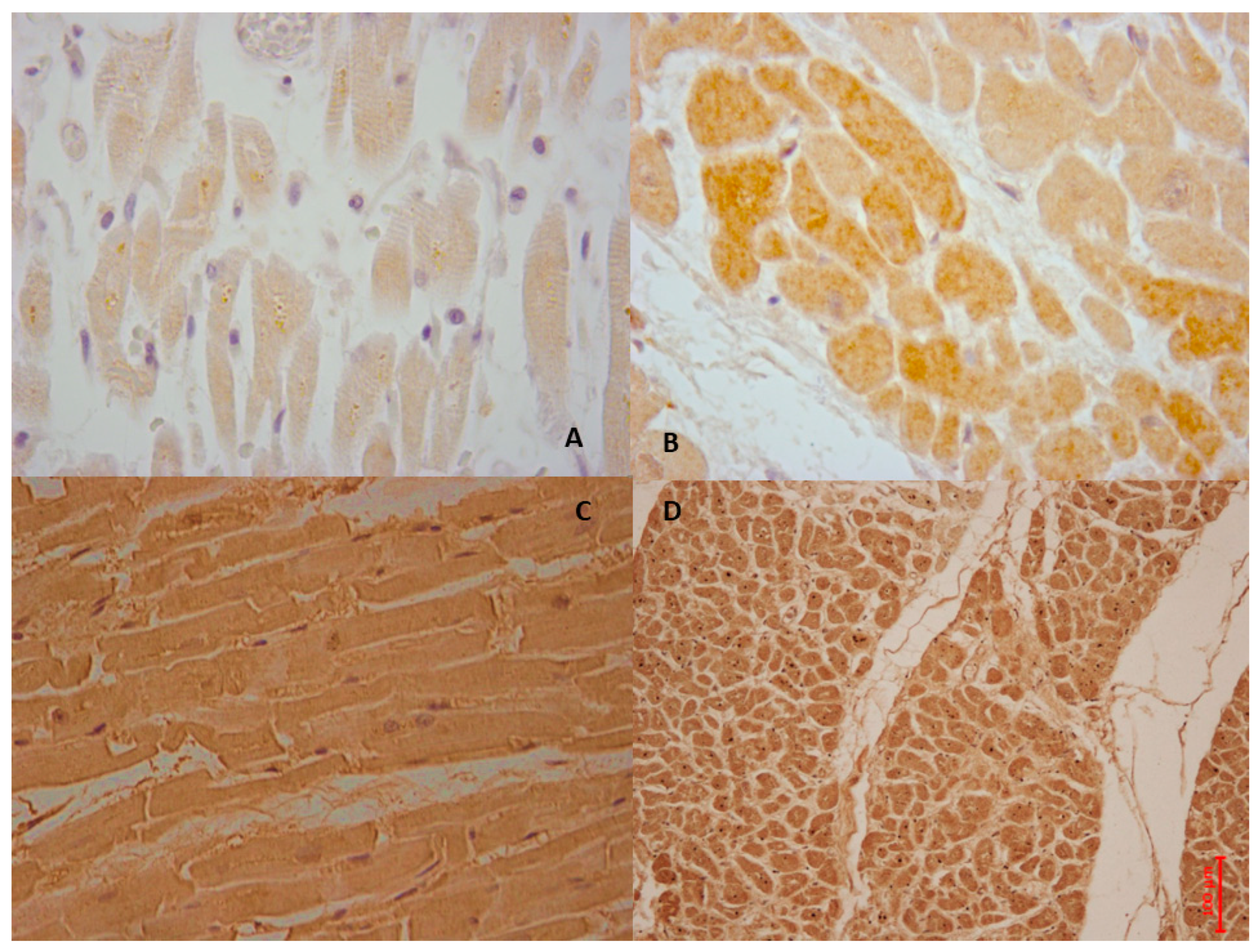
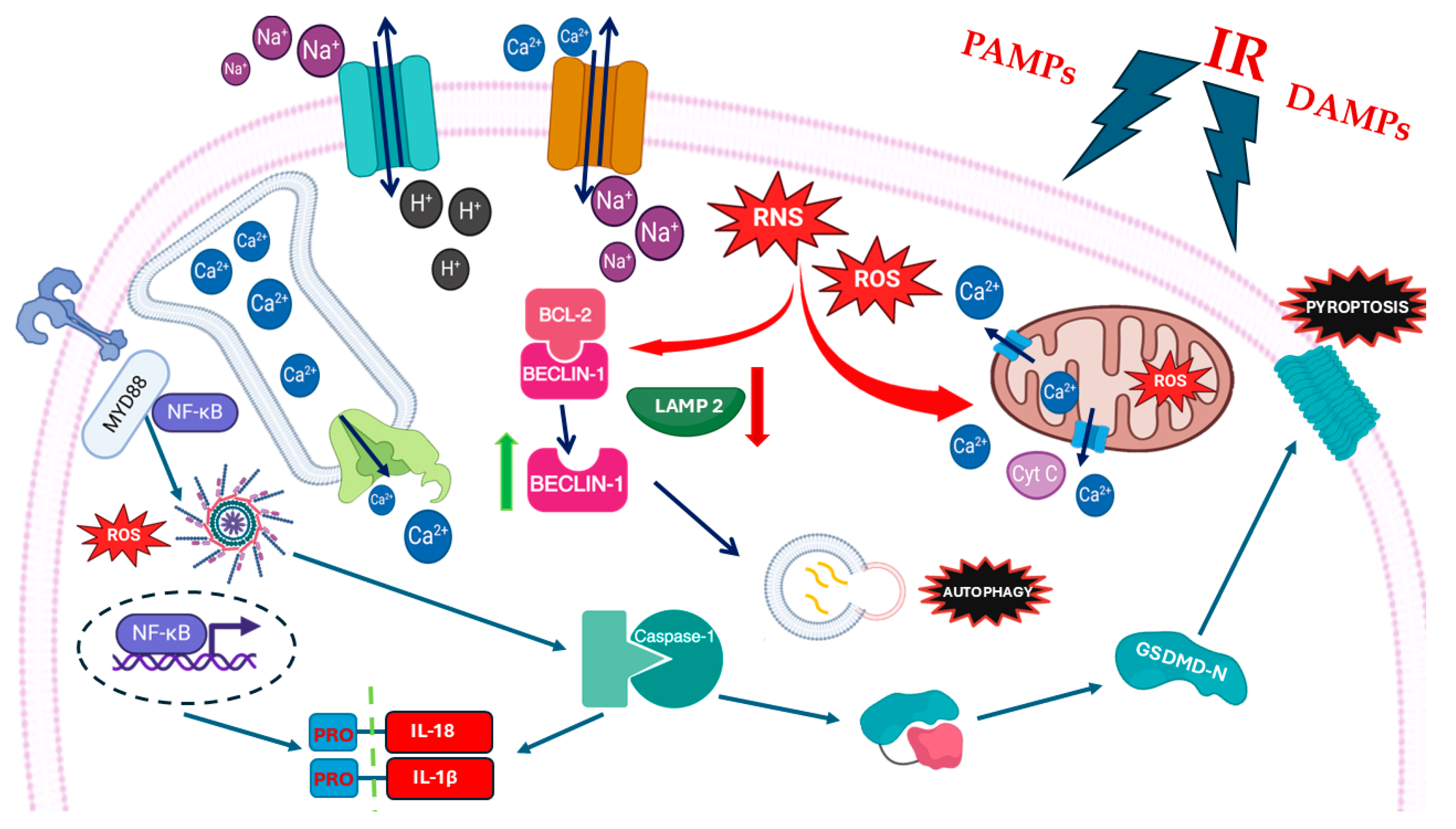
7. MIRI and Pyroptosis
8. Focus on Novel Therapeutic Strategies: Certainties and Hopes
8.1. Inflammation as an Actionable Target for the Secondary Prevention of Cardiovascular Events
8.2. MIRI Modulation in the Clinical Arena
8.3. What Hopes?
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Michaud, K.; Basso, C.; d’Amati, G.; Giordano, C.; Kholová, I.; Preston, S.D.; Rizzo, S.; Sabatasso, S.; Sheppard, M.N.; Vink, A.; et al. Association for European Cardiovascular Pathology (AECVP). Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification. Virchows Arch. 2020, 476, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fede, M.S.; Compagnucci, P.; Montana, A.; Dello Russo, A.; Giorgetti, R.; Busardò, F.P. Forensic perspectives on postmortem CIED interrogation: A systematic review and meta-analysis. Forensic Sci. Int. 2024, 359, 112001. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar]
- Tossetta, G.; Fantone, S.; Compagnucci, P.; Marzioni, D.; Montanari, E.; Neri, M.; Busardò, F.P.; Montana, A. γ-H2AX: A useful tool to detect DNA damage in sudden cardiac death heart tissues, an experimental study. Tissue Cell 2025, 96, 103042. [Google Scholar] [CrossRef]
- Keeley, E.C.; Boura, J.A.; Grines, C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet 2003, 361, 13–20. [Google Scholar] [CrossRef]
- Jeroudi, M.O.; Hartley, C.J.; Bolli, R. Myocardial reperfusion injury: Role of oxygen radicals and potential therapy with antioxidants. Am. J. Cardiol. 1994, 73, 2B–7B. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Kumar, A.; Connelly, K.; Vora, K.; Bainey, K.R.; Howarth, A.; Leipsic, J.; Betteridge-LeBlanc, S.; Prato, F.S.; Leong-Poi, H.; Main, A.; et al. The Canadian Cardiovascular Society Classification of Acute Atherothrombotic Myocardial Infarction Based on Stages of Tissue Injury Severity: An Expert Consensus Statement. Can. J. Cardiol. 2024, 40, 1–14. [Google Scholar] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar]
- Ibáñez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar]
- Wu, J.; Luo, J.; Cai, H.; Li, C.; Lei, Z.; Lu, Y.; Ni, L.; Cao, J.; Cheng, B.; Hu, X. Expression Pattern and Molecular Mechanism of Oxidative Stress-Related Genes in Myocardial Ischemia–Reperfusion Injury. J. Cardiovasc. Dev. Dis. 2023, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Fineschi, V.; Di Paolo, M.; Pomara, C.; Riezzo, I.; Turillazzi, E.; Cerretani, D. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar] [CrossRef]
- Pagliaro, P.; Penna, C. Inhibitors of NLRP3 Inflammasome in Ischemic Heart Disease: Focus on Functional and Redox Aspects. Antioxidants 2023, 12, 1396. [Google Scholar] [CrossRef]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [PubMed]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell. Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; He, F.; Cheng, C.; Xu, B.; Sheng, J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed. Pharmacother. 2021, 133, 110990. [Google Scholar] [CrossRef]
- Cleveland, J.C., Jr.; Meldrum, D.R.; Rowland, R.T.; Banerjee, A.; Harken, A.H. Adenosine preconditioning of human myocardium is dependent upon the ATP-sensitive K+ channel. J. Mol. Cell. Cardiol. 1997, 29, 175–182. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Schriewer, J.M.; Peek, C.B.; Bass, J.; Schumacker, P.T. ROS-mediated PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia-reperfusion. J. Am. Heart Assoc. 2013, 2, e000159. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, S.; Chen, X.; Fu, X.; Guo, S.; Jiang, Z.; Chen, K. MiR-219a-2 relieves myocardial ischemia-reperfusion injury by reducing calcium overload and cell apoptosis through HIF1α/NMDAR pathway. Exp. Cell Res. 2020, 395, 112172. [Google Scholar] [CrossRef] [PubMed]
- Cross, H.R.; Clarke, K.; Opie, L.H.; Radda, G.K. Is lactate-induced myocardial ischaemic injury mediated by decreased pH or increased intracellular lactate? J. Mol. Cell. Cardiol. 1995, 27, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C. Myocardial energy metabolism during ischemia and the mechanisms of metabolic therapies. J. Cardiovasc. Pharmacol. Ther. 2004, 9 (Suppl. S1), S31–S45. [Google Scholar] [CrossRef]
- Avkiran, M.; Ibuki, C.; Shimada, Y.; Haddock, P.S. Effects of acidic reperfusion on arrhythmias and Na(+)-K(+)-ATPase activity in regionally ischemic rat hearts. Am. J. Physiol. 1996, 270, H957–H964. [Google Scholar] [CrossRef]
- Garcia-Dorado, D.; Ruiz-Meana, M.; Inserte, J.; Rodriguez-Sinovas, A.; Piper, H.M. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc. Res. 2012, 94, 168–180. [Google Scholar] [CrossRef]
- Karmazyn, M. Mechanisms of protection of the ischemic and reperfused myocardium by sodium-hydrogen exchange inhibition. J. Thromb. Thrombolysis 1999, 8, 33–38. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T.; Humphreys, R.A.; Yoshida, H.; Kusumoto, K. The myocardial Na(+)-H(+) exchange: Structure, regulation, and its role in heart disease. Circ. Res. 1999, 85, 777–786. [Google Scholar] [CrossRef]
- Levitsky, J.; Gurell, D.; Frishman, W.H. Sodium ion/hydrogen ion exchange inhibition: A new pharmacologic approach to myocardial ischemia and reperfusion injury. J. Clin. Pharmacol. 1998, 38, 887–897. [Google Scholar] [CrossRef]
- Ye, J.; Wang, R.; Wang, M.; Fu, J.; Zhang, Q.; Sun, G.; Sun, X. Hydroxysafflor Yellow A Ameliorates Myocardial Ischemia/Reperfusion Injury by Suppressing Calcium Overload and Apoptosis. Oxid. Med. Cell. Longev. 2021, 2021, 6643615. [Google Scholar]
- Toldo, S.; Marchetti, C.; Mauro, A.G.; Chojnacki, J.; Mezzaroma, E.; Carbone, S.; Zhang, S.; Van Tassell, B.; Salloum, F.N.; Abbate, A. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int. J. Cardiol. 2016, 209, 215–220. [Google Scholar]
- Silvis, M.J.M.; Demkes, E.J.; Fiolet, A.T.L.; Dekker, M.; Bosch, L.; van Hout, G.P.J.; Timmers, L.; de Kleijn, D.P.V. Immunomodulation of the NLRP3 Inflammasome in Atherosclerosis, Coronary Artery Disease, and Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2021, 14, 23–34. [Google Scholar] [PubMed]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zeng, X.; Li, X.; Mehta, J.L.; Wang, X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res. Cardiol. 2017 113, 5. [CrossRef]
- Zhang, J.; Lu, Y.; Yu, P.; Li, Z.; Liu, Y.; Zhang, J.; Tang, X.; Yu, S. Therapeutic hypothermia alleviates myocardial ischaemia-reperfusion injury by inhibiting inflammation and fibrosis via the mediation of the SIRT3/NLRP3 signalling pathway. J. Cell. Mol. Med. 2022, 26, 4995–5007. [Google Scholar] [PubMed]
- Huang, Y.; Sun, X.; Juan, Z.; Zhang, R.; Wang, R.; Meng, S.; Zhou, J.; Li, Y.; Xu, K.; Xie, K. Dexmedetomidine attenuates myocardial ischemia-reperfusion injury in vitro by inhibiting NLRP3 Inflammasome activation. BMC Anesthesiol. 2021, 21, 104, Erratum in BMC Anesthesiol. 2021, 21, 141. [Google Scholar]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef]
- Sandanger, Ø.; Ranheim, T.; Vinge, L.E.; Bliksøen, M.; Alfsnes, K.; Finsen, A.V.; Dahl, C.P.; Askevold, E.T.; Florholmen, G.; Christensen, G.; et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2013, 99, 164–174. [Google Scholar]
- Sandanger, Ø.; Gao, E.; Ranheim, T.; Bliksøen, M.; Kaasbøll, O.J.; Alfsnes, K.; Nymo, S.H.; Rashidi, A.; Ohm, I.K.; Attramadal, H.; et al. NLRP3 inflammasome activation during myocardial ischemia reperfusion is cardioprotective. Biochem. Biophys. Res. Commun. 2016, 469, 1012–1020. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. New directions for protecting the heart against ischaemia-reperfusion injury: Targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc. Res. 2004, 61, 448–460. [Google Scholar] [CrossRef]
- Chong, A.J.; Shimamoto, A.; Hampton, C.R.; Takayama, H.; Spring, D.J.; Rothnie, C.L.; Yada, M.; Pohlman, T.H.; Verrier, E.D. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J. Thorac. Cardiovasc. Surg. 2004, 128, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jin, L.Y.; Ding, J.W.; Zhou, Y.Q.; Yang, J. Expression of Toll-like receptor 4 on peripheral blood mononuclear cells and its effects on patients with acute myocardial infarction treated with thrombolysis. Arch. Med. Res. 2010, 41, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Yu, P.; Chen, M.; Peng, Q.; Wang, Z.; Dong, N. Remote Ischaemic Preconditioning and Sevoflurane Postconditioning Synergistically Protect Rats from Myocardial Injury Induced by Ischemia and Reperfusion Partly via Inhibition TLR4/MyD88/NF-κB Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Ge, H.; Lin, Z.; Wang, H.; Lin, W.; Liu, Y.; Wu, G.; Xia, J.; Zhao, Q. The role of dendritic cells regulated by HMGB1/TLR4 signalling pathway in myocardial ischaemia reperfusion injury. J. Cell. Mol. Med. 2019, 23, 2849–2862. [Google Scholar] [CrossRef]
- Miao, Y.; Ding, Z.; Zou, Z.; Yang, Y.; Yang, M.; Zhang, X.; Li, Z.; Zhou, L.; Zhang, L.; Zhang, X.; et al. Inhibition of MyD88 by a novel inhibitor reverses two-thirds of the infarct area in myocardial ischemia and reperfusion injury. Am. J. Transl. Res. 2020, 12, 5151–5169. [Google Scholar]
- Yang, H.; Zhou, P.; Li, Q.; Zhou, X.; Li, J.; Wang, J.; Wang, J.; Zhao, Y.; Yang, B.; Zhang, B.; et al. TJ-M2010-5 Attenuates Severe Myocardial Ischemia/Reperfusion Injury in Heart Transplantation by Inhibiting MyD88 Homodimerization In Vivo. J. Cardiovasc. Transl. Res. 2022, 15, 1366–1376. [Google Scholar] [CrossRef]
- Baysa, A.; Maghazachi, A.A.; Sand, K.L.; Campesan, M.; Zaglia, T.; Mongillo, M.; Giorgio, M.; Di Lisa, F.; Gullestad, L.; Mariero, L.H.; et al. Toll-like receptor 9 signaling after myocardial infarction: Role of p66ShcA adaptor protein. Biochem. Biophys. Res. Commun. 2023, 644, 70–78. [Google Scholar] [CrossRef]
- Bliksøen, M.; Mariero, L.H.; Torp, M.K.; Baysa, A.; Ytrehus, K.; Haugen, F.; Seljeflot, I.; Vaage, J.; Valen, G.; Stensløkken, K.O. Extracellular mtDNA activates NF-κB via toll-like receptor 9 and induces cell death in cardiomyocytes. Basic. Res. Cardiol. 2016, 111, 42. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner Caspase-3, -6, and -7 Perform Distinct, Non-redundant Roles during the Demolition Phase of Apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Schlegel, R.A.; Williamson, P. Phosphatidylserine, a death knell. Cell Death Differ. 2001, 8, 551–563. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.M. Apoptosis in human acute myocardial infarction. Circulation 1997, 95, 320–323. [Google Scholar] [CrossRef]
- Freude, B.; Masters, T.N.; Robicsek, F.; Fokin, A.; Kostin, S.; Zimmermann, R.; Ullmann, C.; Lorenz-Meyer, S.; Schaper, J. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J. Mol. Cell. Cardiol. 2000, 32, 197–208. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Engler, R.L. Apoptosis in myocardial ischemia-reperfusion. Ann. N. Y. Acad. Sci. 1999, 874, 412–426. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Burleson, K.O.; Kloner, R.A.; Babior, B.M.; Engler, R.L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Investig. 1994, 94, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636. [Google Scholar] [CrossRef]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.; Roncarati, R.; Ross, J., Jr.; Pisani, A.; Stassi, G.; Todaro, M.; Trocha, S.; Drusco, A.; Gu, Y.; Russo, M.A.; et al. Heart-targeted overexpression of caspase3 in mice increases infarct size and depresses cardiac function. Proc. Natl. Acad. Sci. USA 2001, 98, 9977–9982. [Google Scholar] [CrossRef]
- Lee, P.; Sata, M.; Lefer, D.J.; Factor, S.M.; Walsh, K.; Kitsis, R.N. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H456–H463. [Google Scholar] [CrossRef]
- Jeremias, I.; Kupatt, C.; Martin-Villalba, A.; Habazettl, H.; Schenkel, J.; Boekstegers, P.; Debatin, K.M. Involvement of CD95/Apo1/Fas in Cell Death After Myocardial Ischemia. Circulation 2000, 102, 915–920. [Google Scholar] [CrossRef]
- Saelens, X.; Festjens, N.; Vande Walle, L.; Van Gurp, M.; Van Loo, G.; Vandenabeele, P. Toxic proteins released from mitochondria in cell death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef]
- Esposti, M.D. The roles of Bid. Apoptosis 2002, 7, 433–440. [Google Scholar] [CrossRef]
- Hochhauser, E.; Kivity, S.; Offen, D.; Maulik, N.; Otani, H.; Barhum, Y.; Pannet, H.; Shneyvays, V.; Shainberg, A.; Goldshtaub, V.; et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2351–H2359. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Weston, C.R.; Balmanno, K.; Chalmers, C.; Hadfield, K.; Molton, S.A.; Ley, R.; Wagner, E.F.; Cook, S.J. Activation of ERK1/2 by deltaRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 2003, 22, 1281–1293. [Google Scholar] [CrossRef]
- Mayo, L.D.; Donner, D.B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 98, 11598–11603. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Hausenloy, D.; Duchen, M.R.; Yellon, D.M. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int. J. Biochem. Cell Biol. 2006, 38, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Xie, X.; Chen, H.; Zhu, Q.; Ge, Z.; Wei, H.; Deng, J.; Xia, Z.; Lian, Q. Heme Oxygenase-1 Reduces Sepsis-Induced Endoplasmic Reticulum Stress and Acute Lung Injury. Mediat. Inflamm. 2018, 2018, 9413876. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, S.; Tang, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: Role of SIRT1 activation. Apoptosis 2017, 22, 942–954. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Gallaway, M.; Kumar, R. Unfolding the unfolded protein response: Unique insights into brain ischemia. Int. J. Mol. Sci. 2015, 16, 7133–7142. [Google Scholar] [CrossRef]
- Su, R.Y.; Geng, X.Y.; Yang, Y.; Yin, H.S. Nesfatin-1 inhibits myocardial ischaemia/reperfusion injury through activating Akt/ERK pathway-dependent attenuation of endoplasmic reticulum stress. J. Cell. Mol. Med. 2021, 25, 5050–5059. [Google Scholar] [CrossRef]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Vekich, J.A.; Belmont, P.J.; Thuerauf, D.J.; Glembotski, C.C. Protein disulfide isomerase-associated 6 is an ATF6-inducible ER stress response protein that protects cardiac myocytes from ischemia/reperfusion-mediated cell death. J. Mol. Cell. Cardiol. 2012, 53, 259–267. [Google Scholar] [CrossRef]
- Glembotski, C.C. Roles for ATF6 and the sarco/endoplasmic reticulum protein quality control system in the heart. J. Mol. Cell. Cardiol. 2014, 71, 11–15. [Google Scholar] [CrossRef]
- Deng, J. Advanced research on the regulated necrosis mechanism in myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2021, 334, 97–101. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, S.; Vanden Berghe, T.; Vandenabeele, P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017, 24, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef]
- Oerlemans, M.I.; Liu, J.; Arslan, F.; den Ouden, K.; van Middelaar, B.J.; Doevendans, P.A.; Sluijter, J.P. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res. Cardiol. 2012, 107, 270. [Google Scholar]
- Zhu, P.; Hu, S.; Jin, Q.; Li, D.; Tian, F.; Toan, S.; Li, Y.; Zhou, H.; Chen, Y. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: A mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018, 16, 157–168. [Google Scholar] [CrossRef]
- Wang, Y.S.; Yu, P.; Wang, Y.; Zhang, J.; Hang, W.; Yin, Z.X.; Liu, G.; Chen, J.; Werle, K.D.; Quan, C.S.; et al. AMP-activated protein kinase protects against necroptosis via regulation of Keap1-PGAM5 complex. Int. J. Cardiol. 2018, 259, 153–162. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, X. Potential relationship between autophagy and ferroptosis in myocardial ischemia/reperfusion injury. Genes Dis. 2022, 10, 2285–2295. [Google Scholar] [CrossRef]
- Chen, C.; Chen, W.; Li, Y.; Dong, Y.; Teng, X.; Nong, Z.; Pan, X.; Lv, L.; Gao, Y.; Wu, G. Hyperbaric oxygen protects against myocardial reperfusion injury via the inhibition of inflammation and the modulation of autophagy. Oncotarget 2017, 8, 111522–111534. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.B.; Gottlieb, R.A. Autophagy in ischemic heart disease. Circ. Res. 2009, 104, 150–158. [Google Scholar] [CrossRef]
- Yi, C.; Si, L.; Xu, J.; Yang, J.; Wang, Q.; Wang, X. Effect and mechanism of asiatic acid on autophagy in myocardial ischemia-reperfusion injury in vivo and in vitro. Exp. Ther. Med. 2020, 20, 54. [Google Scholar]
- Hariharan, N.; Zhai, P.; Sadoshima, J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid. Redox Signal. 2011, 14, 2179–2190. [Google Scholar] [CrossRef]
- Deng, R.M.; Zhou, J. The role of PI3K/AKT signaling pathway in myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2023, 123, 110714. [Google Scholar] [PubMed]
- Yan, L.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy 2009, 5, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Xuan, F.; Qin, F.; Huang, R. Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. Drug Des. Dev. Ther. 2015, 9, 5933–5945. [Google Scholar]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Chen, Y.; Cao, F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim. Biophys. Acta 2015, 1852, 271–276. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, H.; Yang, J.; Chen, J.; Yang, J.; Ding, J.W.; Li, S.; Wu, H.; Ding, H.S. Radioprotective 105 kDa protein attenuates ischemia/reperfusion-induced myocardial apoptosis and autophagy by inhibiting the activation of the TLR4/NF-κB signaling pathway in rats. Int. J. Mol. Med. 2016, 38, 885–893. [Google Scholar]
- Qiu, L.; Xu, C.; Xia, H.; Chen, J.; Liu, H.; Jiang, H. Downregulation of P300/CBP-Associated Factor Attenuates Myocardial Ischemia-Reperfusion Injury Via Inhibiting Autophagy. Int. J. Med. Sci. 2020, 17, 1196–1206. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Hill, J.A.; Diwan, A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 2012, 125, 3170–3181. [Google Scholar] [CrossRef]
- Yao, T.; Ying, X.; Zhao, Y.; Yuan, A.; He, Q.; Tong, H.; Ding, S.; Liu, J.; Peng, X.; Gao, E.; et al. Vitamin D receptor activation protects against myocardial reperfusion injury through inhibition of apoptosis and modulation of autophagy. Antioxid. Redox Signal. 2015, 22, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Diwan, A. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy 2012, 8, 1394–1396. [Google Scholar] [CrossRef]
- Brennan, M.A.; Cookson, B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000, 38, 31–40. [Google Scholar]
- Liu, Y.; Zhang, J.; Zhang, D.; Yu, P.; Zhang, J.; Yu, S. Research Progress on the Role of Pyroptosis in Myocardial Ischemia-Reperfusion Injury. Cells 2022, 11, 3271. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, X.; Chi, F.; Cong, N. Pyroptosis: A Newly Discovered Therapeutic Target for Ischemia-Reperfusion Injury. Biomolecules 2022, 12, 1625. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, H.; Liu, D.; Ye, M.; Li, Y.; Zhou, G.; Yang, Q.; Liu, Y.; Li, Y. cFLIPL alleviates myocardial ischemia-reperfusion injury by regulating pyroptosis. Cell Biol. Int. 2024, 48, 60–75. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, P.; Zhang, Y.; Luan, J.; Xu, C.; Wu, Z.; Ju, D.; Hu, W. GSDMD contributes to myocardial reperfusion injury by regulating pyroptosis. Front. Immunol. 2022, 13, 893914. [Google Scholar] [CrossRef] [PubMed]
- Neri, M.; Riezzo, I.; Pascale, N.; Pomara, C.; Turillazzi, E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediat. Inflamm. 2017, 2017, 7018393. [Google Scholar] [CrossRef] [PubMed]
- Inserte, J.; Hernando, V.; Garcia-Dorado, D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2012, 96, 23–31. [Google Scholar] [CrossRef]
- Hernando, V.; Inserte, J.; Sartório, C.L.; Parra, V.M.; Poncelas-Nozal, M.; Garcia-Dorado, D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J. Mol. Cell. Cardiol. 2010, 49, 271–279. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Jánossy, J.; Ubezio, P.; Apáti, A.; Magócsi, M.; Tompa, P.; Friedrich, P. Calpain as a multi-site regulator of cell cycle. Biochem. Pharmacol. 2004, 67, 1513–1521. [Google Scholar] [CrossRef]
- Ono, Y.; Sorimachi, H. Calpains: An elaborate proteolytic system. Biochim. Et Biophys. Acta 2012, 1824, 224–236. [Google Scholar] [CrossRef]
- Seki, S.; Horikoshi, K.; Takeda, H.; Izumi, T.; Nagata, A.; Okumura, H.; Taniguchi, M.; Mochizuki, S. Effects of sustained low-flow ischemia and reperfusion on Ca2+ transients and contractility in perfused rat hearts. Mol. Cell Biochem. 2001, 216, 111–119. [Google Scholar] [CrossRef]
- Neuhof, C.; Neuhof, H. Calpain system and its involvement in myocardial ischemia and reperfusion injury. World J. Cardiol. 2014, 6, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Casin, K.M.; Calvert, J.W. Dynamic Regulation of Cysteine Oxidation and Phosphorylation in Myocardial Ischemia-Reperfusion Injury. Cells 2021, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P.; CANTOS Investigators. Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. LoDoCo2 Trial Investigators. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Dalbeth, N.; Lauterio, T.J.; Wolfe, H.R. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 2014, 36, 1465–1479. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients with ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Wohlford, G.F.; Del Buono, M.G.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Kadariya, D.; Trankle, C.R.; Biondi-Zoccai, G.; Lipinski, M.J.; et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: Results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 503–510. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Damonte, J.I.; Moroni, F.; Chiabrando, J.G.; Markley, R.; Turlington, J.; Trankle, C.R.; Kang, L.; Biondi-Zoccai, G.; Kontos, M.C.; et al. Clinical and Pharmacological Implications of Time to Treatment with Interleukin-1 Blockade in ST-Segment Elevation Myocardial Infarction. J. Pharmacol. Exp. Ther. 2023, 386, 156–163. [Google Scholar] [CrossRef]
- Ridker, P.M.; Devalaraja, M.; Baeres, F.M.M.; Engelmann, M.D.M.; Hovingh, G.K.; Ivkovic, M.; Lo, L.; Kling, D.; Pergola, P.; Raj, D.; et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021, 397, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. From RESCUE to ZEUS: Will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc. Res. 2021, 117, e138–e140. [Google Scholar] [CrossRef]
- Kleveland, O.; Kunszt, G.; Bratlie, M.; Ueland, T.; Broch, K.; Holte, E.; Michelsen, A.E.; Bendz, B.; Amundsen, B.H.; Espevik, T.; et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: A double-blind, randomized, placebo-controlled phase 2 trial. Eur. Heart J. 2016, 37, 2406–2413. [Google Scholar] [CrossRef]
- Broch, K.; Anstensrud, A.K.; Woxholt, S.; Sharma, K.; Tøllefsen, I.M.; Bendz, B.; Aakhus, S.; Ueland, T.; Amundsen, B.H.; Damås, J.K.; et al. Randomized Trial of Interleukin-6 Receptor Inhibition in Patients with Acute ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1845–1855. [Google Scholar] [CrossRef]
- Ulander, L.; Tolppanen, H.; Hartman, O.; Rissanen, T.T.; Paakkanen, R.; Kuusisto, J.; Anttonen, O.; Nieminen, T.; Yrjölä, J.; Ryysy, R.; et al. Hydroxychloroquine reduces interleukin-6 levels after myocardial infarction: The randomized, double-blind, placebo-controlled OXI pilot trial. Int. J. Cardiol. 2021, 337, 21–27. [Google Scholar] [CrossRef]
- Sriranjan, R.; Zhao, T.X.; Tarkin, J.; Hubsch, A.; Helmy, J.; Vamvaka, E.; Jalaludeen, N.; Bond, S.; Hoole, S.P.; Knott, P.; et al. Low-dose interleukin 2 for the reduction of vascular inflammation in acute coronary syndromes (IVORY): Protocol and study rationale for a randomised, double-blind, placebo-controlled, phase II clinical trial. BMJ Open 2022, 12, e062602. [Google Scholar] [CrossRef]
- Zhao, T.X.; Kostapanos, M.; Griffiths, C.; Arbon, E.L.; Hubsch, A.; Kaloyirou, F.; Helmy, J.; Hoole, S.P.; Rudd, J.H.F.; Wood, G.; et al. Low-dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndromes (LILACS): Protocol and study rationale for a randomised, double-blind, placebo-controlled, phase I/II clinical trial. BMJ Open 2018, 8, e022452. [Google Scholar] [CrossRef]
- Mewton, N.; Roubille, F.; Bresson, D.; Prieur, C.; Bouleti, C.; Bochaton, T.; Ivanes, F.; Dubreuil, O.; Biere, L.; Hayek, A.; et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Engstrøm, T.; Kelbæk, H.; Helqvist, S.; Høfsten, D.E.; Kløvgaard, L.; Clemmensen, P.; Holmvang, L.; Jørgensen, E.; Pedersen, F.; Saunamaki, K.; et al. Effect of Ischemic Postconditioning during Primary Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 490–497. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R.; Bulluck, H.; Clayton, T.; Dana, A.; Dodd, M.; et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomized controlled trial. Lancet 2019, 394, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Lourenço, A.P.; Pereira, M.Á.; Azevedo, P.; Roncon-Albuquerque, R., Jr.; Marques, J.; Leite-Moreira, A.F. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res. Cardiol. 2018, 113, 14. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.M.; Lamas, D.; Gaspar, A.; Lourenço, A.P.; Antunes, N.; Marques, J.; Leite-Moreira, A.F. The impact of time-of-day reperfusion on remote ischemic conditioning in ST-elevation myocardial infarction: A RIC-STEMI substudy. Heart Vessels 2023, 38, 909–918. [Google Scholar] [CrossRef]
- Schäfer, A.; Akin, M.; Diekmann, J.; König, T. Intracoronary Application of Super-Saturated Oxygen to Reduce Infarct Size Following Myocardial Infarction. J. Clin. Med. 2022, 11, 1509. [Google Scholar] [CrossRef]
- David, S.W.; Khan, Z.A.; Patel, N.C.; Metzger, D.C.; Wood, F.O.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; et al. Evaluation of intracoronary hyperoxemic oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter. Cardiovasc. Interv. 2019, 93, 882–890. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef]
- Stone, G.W.; Martin, J.L.; de Boer, M.J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef]
- El Farissi, M.; Mast, T.P.; van de Kar, M.R.D.; Dillen, D.M.M.; Demandt, J.P.A.; Vervaat, F.E.; Eerdekens, R.; Dello, S.A.G.; Keulards, D.C.; Zelis, J.M.; et al. Hypothermia for Cardioprotection in Patients with St-Elevation Myocardial Infarction: Do Not Give It the Cold Shoulder Yet! J. Clin. Med. 2022, 11, 1082. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Liu, C.; Ma, Y.; Shi, Y.; Ye, Y.; Ma, X.; Liu, Y.; Luo, X.; Lin, F.; et al. Principle and design of clinical efficacy observation of extracorporeal cardiac shock wave therapy for patients with myocardial ischemia-reperfusion injury: A prospective randomized controlled trial protocol. PLoS ONE 2023, 18, e0294060. [Google Scholar] [CrossRef] [PubMed]
- Cung, T.T.; Morel, O.; Cayla, G.; Rioufol, G.; Garcia-Dorado, D.; Angoulvant, D.; Bonnefoy-Cudraz, E.; Guérin, P.; Elbaz, M.; Delarche, N.; et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015, 373, 1021–1031. [Google Scholar] [CrossRef]
- de Koning, M.L.Y.; van Dorp, P.; Assa, S.; Pundziute-Do Prado, G.; Voskuil, M.; Anthonio, R.L.; Veen, D.; Leiner, T.; Sibeijn-Kuiper, A.J.; van Goor, H.; et al. Sodium Thiosulfate in Acute Myocardial Infarction: A Randomized Clinical Trial. J. Am. Coll. Cardiol. Basic Trans. Sci. 2023, 8, 1285–1294. [Google Scholar]
- Ibanez, B.; Macaya, C.; Sánchez-Brunete, V.; Pizarro, G.; Fernández-Friera, L.; Mateos, A.; Fernández-Ortiz, A.; García-Ruiz, J.M.; García-Álvarez, A.; Iñiguez, A.; et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The Effect of Metoprolol in Cardioprotection during an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013, 128, 1495–1503. [Google Scholar] [PubMed]
- Díaz-Munoz, R.; Valle-Caballero, M.J.; Sanchez-Gonzalez, J.; Pizarro, G.; García-Rubira, J.C.; Escalera, N.; Fuster, V.; Fernández-Jiménez, R.; Ibanez, B. Intravenous metoprolol during ongoing STEMI ameliorates markers of ischemic injury: A METOCARD-CNIC trial electrocardiographic study. Basic Res. Cardiol. 2021, 116, 45. [Google Scholar] [CrossRef]
- Skrzypiec-Spring, M.; Urbaniak, J.; Sapa-Wojciechowska, A.; Pietkiewicz, J.; Orda, A.; Karolko, B.; Danielewicz, R.; Bil-Lula, I.; Woźniak, M.; Schulz, R.; et al. Matrix Metalloproteinase-2 Inhibition in Acute Ischemia-Reperfusion Heart Injury-Cardioprotective Properties of Carvedilol. Pharmaceuticals 2021, 14, 1276. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Osawa, K.; Miyoshi, T.; Mori, A.; Yoshikawa, M.; Oka, T.; Ichikawa, K.; Nakamura, K.; Ito, H. Efficacy and Safety of Early Intravenous Landiolol on Myocardial Salvage in Patients with ST-segment Elevation Myocardial Infarction before Primary Percutaneous Coronary Intervention: A Randomized Study. Acta Med. Okayama 2021, 75, 289–297. [Google Scholar] [PubMed]
- Qian, G.; Zhang, Y.; Dong, W.; Jiang, Z.C.; Li, T.; Cheng, L.Q.; Zou, Y.T.; Jiang, X.S.; Zhou, H.; A, X.; et al. Effects of Nicorandil Administration on Infarct Size in Patients with ST-Segment-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: The CHANGE Trial. J. Am. Heart Assoc. 2022, 11, e026232. [Google Scholar] [CrossRef]
- Wang, K.; Tang, R.; Wang, S.; Xiong, Y.; Wang, W.; Chen, G.; Zhang, K.; Li, P.; Tang, Y.D. Isoform-Selective HDAC Inhibitor Mocetinostat (MGCD0103) Alleviates Myocardial Ischemia/Reperfusion Injury Via Mitochondrial Protection Through the HDACs/CREB/PGC-1α Signaling Pathway. J. Cardiovasc. Pharmacol. 2022, 79, 217–228. [Google Scholar] [CrossRef]
- Xie, M.; Cho, G.W.; Kong, Y.; Li, D.L.; Altamirano, F.; Luo, X.; Morales, C.R.; Jiang, N.; Schiattarella, G.G.; May, H.I.; et al. Activation of Autophagic Flux Blunts Cardiac Ischemia/Reperfusion Injury. Circ. Res. 2021, 129, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Chen, G.; Xian, Y.; Zhang, H.; Wu, Y.; Yang, Y.; Wu, J.; Wang, C.; He, S.; et al. Traditional Chinese Medicine Compound (Tongxinluo) and Clinical Outcomes of Patients with Acute Myocardial Infarction: The CTS-AMI Randomized Clinical Trial. JAMA 2023, 330, 1534–1545. [Google Scholar] [CrossRef]
- Hartman, M.H.T.; Prins, J.K.B.; Schurer, R.A.J.; Lipsic, E.; Lexis, C.P.H.; van der Horst-Schrivers, A.N.A.; van Veldhuisen, D.J.; van der Horst, I.C.C.; van der Harst, P. Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: Data from the GIPS-III RCT. Clin. Res. Cardiol. 2017, 106, 939–946. [Google Scholar] [CrossRef]
- Osorio-Llanes, E.; Villamizar-Villamizar, W.; Ospino Guerra, M.C.; Díaz-Ariza, L.A.; Castiblanco-Arroyave, S.C.; Medrano, L.; Mengual, D.; Belón, R.; Castellar-López, J.; Sepúlveda, Y.; et al. Effects of Metformin on Ischemia/Reperfusion Injury: New Evidence and Mechanisms. Pharmaceuticals 2023, 16, 1121. [Google Scholar] [CrossRef]
- Rozemeijer, S.; Hemilä, H.; van Baaren, M.; de Man, A.M.E. Vitamin C may reduce troponin and CKMB levels after PCI and CABG: A meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 475. [Google Scholar] [CrossRef]
- Rozemeijer, S.; de Grooth, H.J.; Elbers, P.W.G.; Girbes, A.R.J.; den Uil, C.A.; Dubois, E.A.; Wils, E.J.; Rettig, T.C.D.; van Zanten, A.R.H.; Vink, R.; et al. Early high-dose vitamin C in post-cardiac arrest syndrome (VITaCCA): Study protocol for a randomized, double-blind, multi-center, placebo-controlled trial. Trials 2021, 22, 546. [Google Scholar] [CrossRef]
- Tanzilli, G.; Truscelli, G.; Arrivi, A.; Carnevale, R.; Placanica, A.; Viceconte, N.; Raparelli, V.; Mele, R.; Cammisotto, V.; Nocella, C.; et al. Glutathione infusion before primary percutaneous coronary intervention: A randomised controlled pilot study. BMJ Open 2019, 9, e025884. [Google Scholar] [CrossRef] [PubMed]
- Makkos, A.; Szántai, Á.; Pálóczi, J.; Pipis, J.; Kiss, B.; Poggi, P.; Ferdinandy, P.; Chatgilialoglu, A.; Görbe, A. A Comorbidity Model of Myocardial Ischemia/Reperfusion Injury and Hypercholesterolemia in Rat Cardiac Myocyte Cultures. Front. Physiol. 2020, 10, 1564. [Google Scholar] [CrossRef]
- Madsen, J.M.; Engstrøm, T.; Obling, L.E.R.; Zhou, Y.; Nepper-Christensen, L.; Beske, R.P.; Vejlstrup, N.G.; Bang, L.E.; Hassager, C.; Folke, F.; et al. Prehospital Pulse-Dose Glucocorticoid in ST-Segment Elevation Myocardial Infarction: The PULSE-MI Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Woxholt, S.; Ueland, T.; Aukrust, P.; Anstensrud, A.K.; Broch, K.; Tøllefsen, I.M.; Seljeflot, I.; Halvorsen, B.; Dahl, T.B.; Huse, C.; et al. Effect of tocilizumab on endothelial and platelet-derived CXC-chemokines and their association with inflammation and myocardial injury in STEMI patients undergoing primary PCI. Int. J. Cardiol. 2025, 418, 132613. [Google Scholar] [CrossRef]
- Kakavand, H.; Saadatagah, S.; Naderian, M.; Aghakouchakzadeh, M.; Jalali, A.; Sadri, F.; Amoli, A.I.; Hosseini, S.H.; Jenab, Y.; Pourhosseini, H.; et al. Evaluating the role of intravenous pentoxifylline administration on primary percutaneous coronary intervention success rate in patients with ST-elevation myocardial infarction (PENTOS-PCI). Naunyn. Schmiedebergs. Arch. Pharmacol. 2023, 396, 557–565. [Google Scholar] [CrossRef]
- Wang, X.; Guo, R.; Guo, Y.; Guo, Q.; Yan, Y.; Gong, W.; Zheng, W.; Wang, H.; Xu, L.; Ai, H.; et al. Rationale and design of the RESTORE trial: A multicenter, randomized, double-blinded, parallel-group, placebo-controlled trial to evaluate the effect of Shenfu injection on myocardial injury in STEMI patients after primary PCI. Am. Heart J. 2023, 260, 9–17. [Google Scholar] [CrossRef]
- Noaman, S.; Neil, C.; O’Brien, J.; Frenneaux, M.; Hare, J.; Wang, B.; Yee Tai, T.; Theuerle, J.; Shaw, J.; Stub, D.; et al. UpStreAm doxycycline in ST-eLeVation myocArdial infarction: TargetinG infarct hEaling and ModulatIon (SALVAGE-MI trial). Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 143–152. [Google Scholar] [CrossRef]
- Sezer, M.; Escaned, J.; Broyd, C.J.; Umman, B.; Bugra, Z.; Ozcan, I.; Sonsoz, M.R.; Ozcan, A.; Atici, A.; Aslanger, E.; et al. Gradual Versus Abrupt Reperfusion During Primary Percutaneous Coronary Interventions in ST-Segment-Elevation Myocardial Infarction (GUARD). J. Am. Heart Assoc. 2022, 11, e024172. [Google Scholar] [CrossRef]
- Adlam, D.; Zarebinski, M.; Uren, N.G.; Ptaszynski, P.; Oldroyd, K.G.; Munir, S.; Zaman, A.; Contractor, H.; Kiss, R.G.; Édes, I.; et al. A Randomized, double-blind, dose ranging clinical trial of intravenous FDY-5301 in acute STEMI patients undergoing primary PCI. Int. J. Cardiol. 2022, 347, 1–7. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Femminò, S.; Ravera, F.; Angelini, F.; Caccioppo, A.; Franchin, L.; Grosso, A.; Comità, S.; Cavallari, C.; Penna, C.; et al. Extracellular vesicles from patients with Acute Coronary Syndrome impact on ischemia-reperfusion injury. Pharmacol. Res. 2021, 170, 105715. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Vlastos, D.; Andreadou, I.; Gazouli, M.; Efentakis, P.; Varoudi, M.; Makavos, G.; Kapelouzou, A.; Lekakis, J.; Parissis, J.; et al. Vascular conditioning prevents adverse left ventricular remodelling after acute myocardial infarction: A randomised remote conditioning study. Basic Res. Cardiol. 2021, 116, 9. [Google Scholar] [CrossRef] [PubMed]
- Dallan, L.A.P.; Giannetti, N.S.; Rochitte, C.E.; Polastri, T.F.; San Martin, C.Y.B.; Hajjar, L.A.; Lima, F.G.; Nicolau, J.C.; de Oliveira, M.T., Jr.; Dae, M.; et al. Cooling as an Adjunctive Therapy to Percutaneous Intervention in Acute Myocardial Infarction: COOL-MI InCor Trial. Ther. Hypothermia Temp. Manag. 2021, 11, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Giblett, J.P.; Clarke, S.; Zhao, T.; McCormick, L.M.; Braganza, D.M.; Densem, C.G.; O’Sullivan, M.; Adlam, D.; Clarke, S.C.; Steele, J.; et al. The role of Glucagon-Like Peptide 1 Loading on periprocedural myocardial infarction During elective PCI (GOLD-PCI study): A randomized, placebo-controlled trial. Am. Heart J. 2019, 215, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Fröhlich, G.M.; Nicholas, J.M.; Mohdnazri, S.; Gamma, R.; Davies, J.; Sirker, A.; Mathur, A.; Blackman, D.; Garg, P.; et al. Mineralocorticoid receptor antagonist pre-treatment and early post-treatment to minimize reperfusion injury after ST-elevation myocardial infarction: The MINIMIZE STEMI trial. Am. Heart J. 2019, 211, 60–67. [Google Scholar] [CrossRef]
- Bulluck, H.; Fröhlich, G.M.; Mohdnazri, S.; Gamma, R.A.; Davies, J.R.; Clesham, G.J.; Sayer, J.W.; Aggarwal, R.K.; Tang, K.H.; Kelly, P.A.; et al. Mineralocorticoid receptor antagonist pretreatment to MINIMISE reperfusion injury after ST-elevation myocardial infarction (the MINIMISE STEMI Trial): Rationale and study design. Clin. Cardiol. 2015, 38, 259–266. [Google Scholar] [CrossRef]
- Jones, D.A.; Rathod, K.S.; Williamson, A.; Mand, K.; Harrington, D.; Andiapen, M.; van Eijl, S.; Westwood, M.; Antoniou, S.; Schilling, R.J.; et al. The effect of intracoronary sodium nitrite on the burden of ventricular arrhythmias following primary percutaneous coronary intervention for acute myocardial infarction. Int. J. Cardiol. 2019, 292, 284, Erratum for Int. J. Cardiol. 2018, 266, 1–6. [Google Scholar] [CrossRef]
- Jones, D.A.; Khambata, R.S.; Andiapen, M.; Rathod, K.S.; Mathur, A.; Ahluwalia, A. Intracoronary nitrite suppresses the inflammatory response following primary percutaneous coronary intervention. Heart 2017, 103, 508–516. [Google Scholar] [CrossRef]
- Nozari, Y.; Eshraghi, A.; Talasaz, A.H.; Bahremand, M.; Salamzadeh, J.; Salarifar, M.; Pourhosseini, H.; Jalali, A.; Mortazavi, S.H. Protection from Reperfusion Injury with Intracoronary N-Acetylcysteine in Patients with STEMI Undergoing Primary Percutaneous Coronary Intervention in a Cardiac Tertiary Center. Am. J. Cardiovasc. Drugs 2018, 18, 213–221. [Google Scholar] [CrossRef]
- Butt, N.; Bache-Mathiesen, L.K.; Nordrehaug, J.E.; Tuseth, V.; Munk, P.S.; Bonarjee, V.; Hall, T.S.; Jensen, S.E.; Halvorsen, S.; Firat, H.; et al. Administration of the MitochondrialPermeability Transition Pore Inhibitor, TRO40303, prior to Primary Percutaneous Coronary Intervention, Does Not Affect the Levels of Pro-Inflammatory Cytokines or Acute-Phase Proteins. Cardiology 2017, 138, 122–132. [Google Scholar] [CrossRef]
- Atar, D.; Arheden, H.; Berdeaux, A.; Bonnet, J.L.; Carlsson, M.; Clemmensen, P.; Cuvier, V.; Danchin, N.; Dubois-Randé, J.L.; Engblom, H.; et al. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur. Heart J. 2015, 36, 112–119. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P.; de la Torre-Hernandez, J.M.; Consuegra-Sanchez, L.; Piccolo, R.; Gonzalez-Gonzalez, J.; Garcia-Camarero, T.; Del Mar Garcia-Saiz, M.; Aldea-Perona, A.; Reiter, R.J.; et al. Usefulness of Early Treatment with Melatonin to Reduce Infarct Size in Patients with ST-Segment Elevation Myocardial Infarction Receiving Percutaneous Coronary Intervention (From the Melatonin Adjunct in the Acute Myocardial Infarction Treated with Angioplasty Trial). Am. J. Cardiol. 2017, 120, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early Use of N-acetylcysteine with Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction Reduces Myocardial Infarct Size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [PubMed]
- Hortmann, M.; Robinson, S.; Mohr, M.; Mauler, M.; Stallmann, D.; Reinöhl, J.; Duerschmied, D.; Peter, K.; Carr, J.; Gibson, C.M.; et al. The mitochondria-targeting peptide elamipretide diminishes circulating HtrA2 in ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 695–702. [Google Scholar] [CrossRef]
- Gibson, C.M.; Giugliano, R.P.; Kloner, R.A.; Bode, C.; Tendera, M.; Jánosi, A.; Merkely, B.; Godlewski, J.; Halaby, R.; Korjian, S.; et al. EMBRACE STEMI study: A Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur. Heart J. 2016, 37, 1296–1303. [Google Scholar] [CrossRef]
- Chen, W.R.; Chen, Y.D.; Tian, F.; Yang, N.; Cheng, L.Q.; Hu, S.Y.; Wang, J.; Yang, J.J.; Wang, S.F.; Gu, X.F. Effects of Liraglutide on Reperfusion Injury in Patients with ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2016, 9, e005146. [Google Scholar] [CrossRef]
- Kimura, K.; Nakao, K.; Shibata, Y.; Sone, T.; Takayama, T.; Fukuzawa, S.; Nakama, Y.; Hirayama, H.; Matsumoto, N.; Kosuge, M.; et al. AMITY Study Group. Randomized controlled trial of TY-51924, a novel hydrophilic NHE inhibitor, in acute myocardial infarction. J. Cardiol. 2016, 67, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dorado, D.; García-del-Blanco, B.; Otaegui, I.; Rodríguez-Palomares, J.; Pineda, V.; Gimeno, F.; Ruiz-Salmerón, R.; Elizaga, J.; Evangelista, A.; Fernandez-Avilés, F.; et al. Intracoronary injection of adenosine before reperfusion in patients with ST-segment elevation myocardial infarction: A randomized controlled clinical trial. Int. J. Cardiol. 2014, 177, 935–941. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Roe, M.; Aylward, P.; Galla, J.; Rynkiewicz, A.; Guetta, V.; Zelizko, M.; Kleiman, N.; White, H.; McErlean, E.; et al. PROTECTION AMI Investigators. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: Results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014, 35, 2516–2523. [Google Scholar]
- Lexis, C.P.; van der Horst, I.C.; Lipsic, E.; Wieringa, W.G.; de Boer, R.A.; van den Heuvel, A.F.; van der Werf, H.W.; Schurer, R.A.J.; Pundziute, G.; Tan, E.S.; et al. GIPS-III Investigators. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: The GIPS-III randomized clinical trial. JAMA 2014, 311, 1526–1535. [Google Scholar] [CrossRef]



| Study Name/ClinicalTrials.gov Identifier | Intervention/ Treatment | Intervention Target | Study Design | Timing of Administration of the Treatment | No. (Drug vs. Control) Patient Cohort | Primary Study End Point | Main Outcomes | Main Author, Date, Journal [Reference] |
|---|---|---|---|---|---|---|---|---|
| PULSE-MI (Pulse Glucocorticoid Therapy in Patients With ST-Segment Elevation Myocardial Infarction)/NCT05462730 | Methylprednisolone vs. placebo | Inflammation (genomic and non-genomic effect of glucocorticoids, decreasing vascular inflammation through membrane stabilization, and increasing contractility of the vascular smooth muscle cells) | Randomized, double-blind, placebo-controlled | In the pre-hospital setting, bolus infusion of 250 mg (1 × 4 mL) over a period of 5 min | 530 patients (378 patients completing the CMR at 3 m) with STEMI undergoing PPCI | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 3 months | No significant differences | Madsen JM, 2024, JAMA Cardiol. [164] |
| CTS/AMI (China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction)/NCT03792035 | Tongxinluo vs. placebo | Inflammation pyroptosis (reduction in pyroptosis of endothelial cells, in the activation of caspase-1, and in the release of inflammatory cytokines) | Randomized, double-blind, placebo-controlled | After randomization (loading dose of 2.08 g), followed by the maintenance dose of 1.04 g, t.i.d.) | 3777 patients (Tongxinluo: 1889 vs. 1888) with STEMI within 24 h of symptom onset | MACE at 30 d and at 1 y | Decrease in MACE both at 30 d and at 1 y in the Tongxinluo group compared with the placebo group | Yang Y, 2023, JAMA [157] |
| GIPS-IV (Groningen Intervention Study for the Preservation of Cardiac Function with STS after STEMI)/NCT02899364 | Sodium Thiosulfate (STS) vs. placebo | Oxidative stress, apoptosis, inflammation, mitochondrial and microvascular function (antioxidant and H2S donor) | Randomized, double-blind, placebo-controlled | Before and during PPCI, bolus infusion of 12.5 mg over 20–30 min, followed by 12.5 mg at 6 h post-PPCI | 373 patients (226, 116 vs. 110, completing the CMR at 4 m) with first-presentation STEMI within 12 h symptom onset, undergoing PPCI | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 4 m | No significant differences | de Koning MS, 2023, JACC Basic Transl Sci. [149] |
| Substudy of ASSAIL-MI (ASSessing the Effect of Anti-IL-6 Treatment in Myocardial Infarction: The ASSAIL-MI Trial)/NCT03004703 | Tocilizumab vs. placebo | Inflammation (antagonism of IL-6 receptor by a recombinant humanized monoclonal antibody) | Randomized, double-blind, placebo-controlled | Before PPCI, bolus infusion of 280 mg (14 mL) over a period of 1 h | 199 adult STEMI patients (101 vs. 98), within 6 h symptom onset before PPCI | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 3–7 d and at 6 m | Tocilizumab increased the concentrations of most cytokines (IL-6, IL-8 and IL-1ra) in the acute phase compared with placebo, but decreased neutrophils/CRP (markers of MI). In the ASSAIL-MI, Tocilizumab increased the MSI | Woxholt S, 2025, Int J Cardiol. [165]; Brock K, 2021, JACC. [133] |
| Substudy of RIC-STEMI (Remote Ischemic Conditioning in ST-elevation Myocardial Infarction)/NCT02313961 | RIC (3 cycles of manual inflation of a blood pressure cuff placed on the left lower limb to 200 mmHg for 5 min and then deflation to 0 mmHg for another 5 min) | Cardioprotection of brief cycles of non-lethal ischemia and reperfusion applied to a distant organ before, during or after a long period of myocardial ischemia | Randomized, open label | At the time of PPCI | 448 STEMI patients undergoing PPCI without (217) or with RIC (231); patients’ division also according to the time of PPCI (216 night-morning vs. 232 afternoon) | Composite: cardiac death or HF hospitalization on a minimum follow-up of 12 m | At 2.1 y of follow-up, improvement in outcomes in patients undergoing RIC as an adjunct to PPCI. The substudy showed a daytime variation in clinical results suggesting that the afternoon period enhances the cardioprotection induced by RIC | Pires CM, 2023, Heart Vessels [141]; Gaspar A, 2018, Basic Res Cardiol. [140] |
| PENTOS-PCI | Pentoxifylline vs. placebo | Inflammation, oxidative stress (methylxanthine derivative with known anti-inflammatory, antioxidant, vasodilator, and rheological properties) | Randomized, double-blind, placebo-controlled | During PPCI, 100 mg (bolus infusion of 50 mg followed by 50 mg in 30 min) | 161 (80 vs. 81) adult patients with STEMI eligible for PPCI | PCI’s success rate as measured by TIMI flow grade | No significant difference | Kakavand H, 2023, Naunyn Schmiedebergs Arch Pharmacol. [166] |
| RESTORE (Randomized Evaluation of Shenfu Injection to Reduce Myocardial Injury)/NCT04493840 | Shenfu vs. placebo | Inflammation, oxidative stress, apoptosis, calcium overload (scavenging free radicals, inhibiting inflammatory mediators, suppressing cell apoptosis, and inhibiting Ca 2+ overload) | Randomized, double-blind, placebo-controlled | Within 30 min before PPCI infusion of 80 mg (+70 mL 5% glucose injection) followed by once a day until 5 days after PPCI | 326 adult patients with first-time anterior STEMI undergoing PPCI within 12 h of symptom onset | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 5 ± 2 d | - | Wang X, 2023, Am Heart J. [167] |
| SALVAGE-MI (UpStreAm doxycycline in ST-eLeVation myocArdial infarction: targetinG infarct hEaling and ModulatIon) | Doxycycline | Inflammation, oxidative stress, apoptosis, and especially MMPs (inhibitory properties) | Randomized, double-blind, placebo-controlled | Before PPCI bolus infusion of 100 mg over 5 min followed by an oral dose of 100 mg (b.i.d.) for 7 d | 103 patients (50 vs. 53) with first-presentation STEMI within 12 h symptom onset, TIMI flow 0–1 undergoing PPCI | Reduction in final IS adjusted for area-at-risk (fIS/AAR) measured on 2 CMR (the first one, 1–2 w post-PPCI and the second one, between 3 and 6 m) | No significant difference | Noaman S, 2023, Eur Heart J Acute Cardiovasc Care [168] |
| CHANGE (China-Administration of Nicorandil Group)/NCT 03445728 | Nicorandil vs. placebo | Microvascular function (a hybrid ATP-sensitive potassium channel opening agent) | Randomized, double-blind, placebo-controlled | Before PPCI bolus infusion of 12 mg and then after PPCI, continuous infusion of 6 mg/h. up to 24 h | 238 STEMI patients (120 vs. 118) undergoing PPCI | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 7 d and at 6 m post-PPCI | Nicorandil lead to improved myocardial perfusion grade, increased left ventricular ejection fraction, and reduced myocardial IS | Qian G, 2022, J Am Heart Assoc. [154] |
| GUARD (Gradual Versus Abrupt Reperfusion During PPCI)/NCT 02732080 | Pressure-controlled reperfusion with delayed stenting (PCRDS) vs. standard PCI with immediate stenting | Microvascular integrity | Randomized, open label | After PCI | 30 adult STEMI patients undergoing PPCI with achieved TIMI flow 3 | Coronary zero flow pressure (Pzf) at the end of the 1 h intracoronary hemodynamic monitorization | PCRDS lead to better-preserved coronary microvascular integrity and smaller myocardial IS | Sezer M, 2022, J Am Heart Assoc. [169] |
| A Study of Acute Myocardial Infarction Using FDY-530/NCT03470441 | FDY-5301 vs. placebo | Oxidative stress (catalytic anti-peroxidant agent) | Randomized, double-blind, placebo-controlled | Before PPCI, infusion of 0.5, 1.0 or 2.0 mg/kg or placebo | 120 STEMI patients presenting within 12 h symptom onset undergoing PPCI | Incidence of cardiac arrhythmias of interest from 48 h to 14 d post-PPCI | No significant difference in the primary endpoint, but difference (without statistical significance) in reduction in IS and improvement of LVEF. Moreover, significant reduction in the levels of MPO, MMP2 and NTproBNP after PPCI | Adlam D, 2022, Int J Cardiol. [170] |
| GSH/EudraNCT2014-004486-25 | Glutathione vs. placebo | Oxidative stress, inflammation (modulation of innate immune cell recruitment and reduction in endothelial damage by reducing NOX2-mediated inflammatory process, and the deleterious effects of H2O2 generation on myocardium) | Randomized, double-blind, placebo-controlled | Before PPCI infusion of 2500 mg/25 mL over 10 min followed by infusion at the same dose at 24, 48 and 72 h | 100 adult STEMI patients undergoing PPCI | Reduction in plasma levels of oxidants and inflammatory markers (NOX2, TNFa, NO, H2O2) at 2 and 5 d | Early and prolonged glutathione infusion seems able to protect vital myocardial components and endothelial cell function against harmful pro-oxidant and inflammatory environments | Tanzilli G, 2021, JAHA [162] |
| VITaCCA (Vitamin C in Post-cardiac Arrest)/NCT03509662 | Vitamin C | Oxidative stress, endothelial function (scavenger of free radicals, reduction in the production of ROS) | Randomized, double-blind, placebo-controlled | Infusion of 1.5 gr or 5 gr b.i.d. (3 gr/d or 10 gr/d) for 4 d | 270 (90 vs. 90 vs. 90) comatose patients suffering an out-of-hospital cardiac arrest | ΔSOFA (the difference between SOFA admission and SOFA at 96 h) | - | Rozemeijer S, 2021, Trials [161] |
| METOCARD-CNIC (Effect of METOprolol in CARDioproteCtioN During an Acute Myocardial InfarCtion)/NCT01311700 | Metoprolol (beta-blocker) | MMPs (reduction in sarcomeric proteins cleavage with an amelioration of the cardiac contractile dysfunction) | Randomized, controlled parallel-group, observer-blinded | Before PPCI, intravenous infusion of up to three 5 mg i.v. dosages (2 min apart). 12–24 h post-reperfusion, oral treatment (25–100 mg/12 h), for all patients | 270 (131 vs. 139) anterior STEMI patients | Reduction in IS evaluated primarily by area of delayed enhancement on CMR at 5–7 d after PPCI and time-dependent progression of ischemic injury assessed by serial ECG | Early intravenous metoprolol before PPCI reduces IS and increases LVEF with no excess of adverse events during the first 24 h after STEMI. Moreover, ECG markers of myocardial ischemia ameliorate (ECG substudy) | Díaz -Munoz R, 2021, Basic Res Cardiol. [151]; Ibanez B, 2013, Circulation [150] |
| EUROCRIPS (Efficacy of RIPC to Reduce AKI for Patients Undergoing PCI)/NCT02195726 | RIC (5 min inflations of a blood pressure cuff to 200 mmHg around the upper non dominant arm) vs. sham-procedure | RISK pathway (EV-naive led to STAT-3 phosphorylation, while EV-RIPC to Erk-1/2 activation) | Randomized, double-blind, sham-controlled | Before PPCI, 4 cycles of RIC and then collection of serum EVs (extracellular vesicles) | 30 ACS patients undergoing PPCI | Incidence of acute kidney injury and of periprocedural MI at 24–48 h after PPCI | TnT is enriched in circulating EV from ACS patients; EV-naïve have a cardioprotective activity through SAFE pathways, lacking in EV-RIPC | D’Ascenzo F, 2021, Pharmacol Res. [171] |
| The Acute and Chronic Effects of Remote Ischemic Conditioning on Cardiovascular Function/NCT03984123 | RIC vs. sham procedure | Oxidative stress, endothelial function | Randomized, double-blind, sham-controlled | Within 48 h post-PPCI, one or two cycles of bilateral brachial cuff inflation | 270 STEMI patients undergoing PPCI | Changes of aortic stiffness, endothelial glycocalyx integrity, oxidative stress biomarkers, miRNA expression, Nitrate-nitrite-nitric oxide plasma concentrations at baseline, 10, 25, and 45 min | RIC evokes “vascular conditioning” likely by upregulation of cardioprotective microRNAs, NOx production, and oxidative stress reduction, facilitating reverse LV remodeling | Ikonomidis I, 2021, Basic Res Cardiol. [172] |
| COOL-MI (Hypothermia as an Adjunctive Therapy to Percutaneous Intervention in Patients With Acute Myocardial Infarction)/NCT02664194 | Endovascular hypothermia vs. sham procedure | Randomized, open label | Before reperfusion, 1 h or 3 h of intravascular hypothermia (1 L cold saline (1–4 °C) associated with the Proteus™ System, by cooling for at least 18 min with a target temperature of 32 °C ± 1 °C) | 70 Anterior or Inferior STEMI patients undergoing PPCI | Reduction in IS (in % of LV mass) as assessed by LGE on CMR, improvement of LVEF on CMR and incidence of MACE at 30 d after STEMI | No difference in IS or LVEF at 30 d nor in MACE, but there was a higher incidence of arrhythmia and in-hospital infection in the hypothermia group, with no increase in mortality | Dallan LAP, 2021, Ther Hypothermia Temp Manag. [173] | |
| COVERT-MI (COlchicine for Left VEntricular Remodeling Treatment in Acute Myocardial Infarction)/NCT03156816 | Colchicine (oral) vs. placebo | Inflammation (inhibition of NLRP3 inflammasome, and hence on the release of IL-1β and IL-18, as well as of other pro-inflammatory cytokines, such as IL-6 (surface expression and downstream pathway)) | Randomized, double-blind, placebo-controlled | At the time of revascularization (loading dose of 2 mg), followed by 0.5 mg b.i.d. for 5 d | 192 adult patients (101 vs. 91), with a first STEMI (initial TIMI flow ≤1), referred for PPCI | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 5 ± 2 d | No difference in IS (at CMR) neither at 5 d nor at 3 m between Colchicine and placebo | Mewton N, 2021, Circulation [137] |
| GOLD-PCI (GLP-1 Loading During Elective Percutaneous Coronary Intervention)/NCT02127996 | Glucagon-like peptide 1 (GLP-1) vs. placebo | Cardiac and vascular myocytes’ function (increased cGMP release, vasodilatation, and coronary flow through GLP-1R) | Randomized, double-blind, placebo-controlled | During PPCI infusion of 1.2 pmol/Kg/min | 192 adult patients (101 vs. 91), with a first STEMI (initial TIMI flow ≤1), referred for PPCI | Incidence of troponin I elevation at 6 h post-PPCI and MACE up to 6 m | - | Giblett JP, 2019, Am Heart J. [174] |
| MINIMIZE-STEMI (Early Mineralocorticoid Receptor Antagonist Treatment to Reduce Myocardial Infarct Size)/NCT01882179 | Mineralocorticoid receptor antagonist (MRA) vs. placebo | Inflammation, oxidative stress (Activity on adenosine receptor, protein kinase C, PI3-kinase, and ERK. Upregulation of phosphorylation of Akt and ERK1/2) | Randomized, double-blind, placebo-controlled | Before PPCI, infusion of potassium-canrenoate, followed by oral spironolactone 25 mg daily for 3 m (up titrated to 50 mg daily after 2 w, if possible) | 67 STEMI patients presenting within 12 h and with a proximal coronary artery occlusion with TIMI flow 0 | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 3 m after STEMI | No significant difference in the acute IS and final IS at 3 m, but there was an improvement in LVEF at 3 m | Bulluck H, 2019, Am Heart J [175]; Bulluck H, 2015, Clin Cardiol. [176] |
| NITRITE-AMI (Safety and Effectiveness of Intra-coronary Nitrite in Acute Myocardial Infarction)/NCT01584453 | Sodium nitrite vs. placebo | Inflammation | Randomized, double-blind, placebo-controlled | During PPCI, an intracoronary bolus infusion of 1.8 micromol in 10 mL over 1 m | 80 STEMI patients undergoing PPCI | IS measured by CK area under the curve at 48 h post PPCI and incidence of MACE at 3 y, markers of inflammation | Important reductions in neutrophil numbers and activation post-PPCI, associated with a reduction in both microvascular obstruction and IS | Jones DA, 2019, Int J Cardiol. [177]; Jones DA, 2017, Heart [178] |
| Effect of N-acetylcystein in Myocardial Infarction/NCT01741207 | N-acetylcysteine (NAC) vs. placebo | Oxidative stress | Randomized, double-blind, placebo-controlled | Before PPCI, bolus infusion of 100 mg/kg and then 480 mg intracoronary, followed by 10 mg/kg/h over 12 h after PPCI | 100 STEMI patients undergoing PPCI | Biomarkers of platelet activation (P selectin- CD40L-IL10- TGF-beta) after 24 h and Cardiac Necrosis Biomarkers (CKMB, troponin T) at 12 h, MACE at 30 d | NAC improved myocardial reperfusion markers and coronary blood flow, as revealed by differences in peak hs-TnT and TIMI flow grade 3 levels | Nozari Y, 2018, Am J Cardiovasc Drugs [179]; |
| MITOCARE | TRO40303 vs. placebo | Mitochondrial function (mPTP opening inhibitor) | Randomized, double-blind, placebo-controlled | Before PPCI, intravenous infusion and intracoronary bolus | 163 STEMI patients (83 vs. 80) with chest pain within 6 h before admission for PPCI | Serum levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-10, TNF), and of acute-phase proteins (hs-CRP) | No statistically significant differences | Butt N, 2017, Cardiology [180]; Atar D, 2015, Eur. Heart J [181] |
| MARIA (The Melatonin Adjunct in the Acute myocaRdial Infarction Treated With Angioplasty)/NCT00640094 | Melatonin vs. placebo | Oxidative stress (direct free radical scavenging activities and indirect actions in stimulating antioxidant enzymes) | Randomized, double-blind, placebo-controlled | During PPCI, intravenous infusion of 12 mg over 1 h, followed by intracoronary bolus of 2 mg | 146 STEMI patients presenting within 6 h of chest pain onset | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 5–7 d after reperfusion | Melatonin in patients with STEMI who presented early after symptom onset was associated with a significant reduction in the IS after PPCI | Dominguez-Rodriguez A, 2017, Am J Cardiol. [182] |
| NACIAM (N-acetylcysteine in Acute Myocardial Infarction) | N-acetylcysteine (NAC) vs. placebo | Oxidative stress | Randomized, double-blind, placebo-controlled | Intravenous high-dose (29 g over 2 d) with background low-dose nitroglycerin (7.2 mg over 2 d) | 112 STEMI patients undergoing PPCI, with 75 completing CMR follow-up (37 vs. 38) | Reduction in IS (in % of LV mass) as assessed by LGE on CMR at 3 m after STEMI | High-dose intravenous NAC administered with low-dose intravenous nitroglycerin is associated with reduced IS | Pasupathy S, 2017, Circulation [183] |
| EMBRACE (Evaluation of Myocardial Effects of MTP-131 for Reducing Reperfusion Injury in Patients With Acute Coronary Events)/NCT01572909 | Elamipretide (MTP-131) vs. placebo | Mitochondrial function, apoptosis (preserves the integrity of cardiolipin, enhances mitochondrial energetics, and improves myocyte survival during reperfusion) | Randomized, double-blind, placebo-controlled | Before PPCI, at least 15 min, but no more than 1 h, intravenous infusion at 0.05 mg/kg/hr for 1 h | 300 anterior STEMI patients | IS as measured by the AUC of serum CK-MB at 24 and 72 h post-PPCI | - | Hortmann M, 2019, Eur Heart J Acute Cardiovasc Care [184]; Gibson GM, 2016, Eur Heart J. [185] |
| Efficacy Study of Glucagonlike Peptide-1 to Treat Reperfusion Injury/NCT02001363 | Liraglutide (GLP-1) vs. placebo | Cardiac and vascular myocytes’ function (increased cGMP release, vasodilatation, and coronary flow through GLP-1R) | Randomized, double-blind, placebo-controlled | Before PPCI, within 30 min, subcutaneous bolus (1.8 mg) followed by maintained dose for 7 d after the PPCI (0.6 mg for 2 d, 1.2 mg for 2 d, followed by 1.8 mg for 3 d). | 96 STEMI patients undergoing PPCI | The salvage index measured by CMR after 3 m post-PPCI and final IS. MACE incidence at 6 m follow-up | The final IS and serum hs-CPR were lower in the liraglutide group. During a 6-m follow-up period, no difference in MACE incidence | Chen WR, 2016, Circ. Cardiovasc Imaging [186] |
| - | TY-51924 vs. placebo | Inhibition of activation of the Na(+)/H(+) exchanger (NHE) | Randomized, open label, placebo-controlled | During PPCI, intravenous injection of 10, 20, or 30 mg/kg | 105 patients with first anterior STEMI undergoing PPCI | MSI as determined by SPECT at 3–5 d after PPCI | No significant results | Kimura K, 2016, J. Cardiol. [187] |
| CIRCUS (Cyclosporine and Prognosis in Acute Myocardial Infarction Patients)/NCT01502774 | Cyclosporine A vs. placebo | Mitochondrial function, apoptosis (through an inhibitor of MPTP, actually not a blocker, but an increaser of the threshold of opening) | Randomized, double-blind, placebo-controlled | Before recanalization, an intravenous bolus injection of 2.5 mg/Kg | 970 anterior STEMI patients undergoing PPCI within 12 h after symptom onset | Combined incidence of total mortality; hospitalization for heart failure; LV remodeling at 1 y post-PPCI | No significant results at 1 y- follow-up | Cung T, 2015, NEJM [148] |
| DANAMI-3 (The Third DANish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction)/NCT01435408 | Ischemic postconditioning of the heart during PPCI | Randomized, double-blind, placebo-controlled | After PCI and before stent implantation 4 repeated 30 s balloon occlusions followed by 30 s of reperfusion | 1234 STEMI patients with onset of symptoms within 12 h and TIMI 0–1 | IS salvage index at 3 m and all-cause mortality, heart failure (postconditioning) at 2 y | No significant results | Engstrøm T, 2017, JAMA Cardiology [138] | |
| PROMISE (Myocardial Protection With Adenosine During Primary Percutaneous Coronary Intervention in Pts With STEMI)/NCT00781404 | Adenosine vs. placebo | Vascular function | Randomized, double-blind, placebo-controlled | Before PPCI, single intravenous infusion bolus of 0.45 mg/mL | 201 STEMI patients undergoing PPCI within 6 h of symptom onset | IS measured by CMR at 5–10 d after AMI | Intracoronary administration of Adenoisne prior to PPCI limits IS, and has a good effect on MSI and LVEF | Garcia-Dorado D, 2014, Int J Cardiol. [188] |
| PROTECTION AMI/NCT00785954 | Delcasertib | Selective inhibitor of delta-protein kinase C (delta-PKC) | Randomized, double-blind, placebo-controlled | Before PPCI, intravenous infusion of 50, 150, or 450 mg/h, continued for ∼2.5 h. | 1176 STEMI patients undergoing PPCI within 6 h of symptom onset | IS as assessed by CK-MB AUC | No reduction in biomarkers of MI | Lincoff AM, 2014, Eur Heart J. [189] |
| GIPS-III (Metabolic Modulation With Metformin to Reduce Heart Failure After Acute Myocardial Infarction: Glycometabolic Intervention as Adjunct to Primary Coronary Intervention in ST Elevation Myocardial Infarction)/NCT01217307 | Metformin vs. placebo | Oxidative stress, apoptosis, mitochondrial function (activation of the AMPK and RISK pathways, attenuation of mitochondrial dysfunction, decrease in myocardial oxidative stress, reduction in apoptosis) | Randomized, double-blind, placebo-controlled | After PPCI, oral treatment with 500 mg b.i.d. over 4 m | 380 (191 vs. 189) STEMI patients undergoing PPCI within 12 h of symptom onset | LVEF measured by CMR at 4 m after STEMI | No improvement in LVEF at 4 m, nor beneficial long-term effects at follow-up of 2 y | Lexis CP, 2014, JAMA [190]; Hartman MHT, 2017, Clin Res Cardiol. [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fede, M.S.; Daziani, G.; Tavoletta, F.; Montana, A.; Compagnucci, P.; Goteri, G.; Neri, M.; Busardò, F.P. Myocardial Ischemia/Reperfusion Injury: Molecular Insights, Forensic Perspectives, and Therapeutic Horizons. Cells 2025, 14, 1509. https://doi.org/10.3390/cells14191509
Fede MS, Daziani G, Tavoletta F, Montana A, Compagnucci P, Goteri G, Neri M, Busardò FP. Myocardial Ischemia/Reperfusion Injury: Molecular Insights, Forensic Perspectives, and Therapeutic Horizons. Cells. 2025; 14(19):1509. https://doi.org/10.3390/cells14191509
Chicago/Turabian StyleFede, Maria Sofia, Gloria Daziani, Francesco Tavoletta, Angelo Montana, Paolo Compagnucci, Gaia Goteri, Margherita Neri, and Francesco Paolo Busardò. 2025. "Myocardial Ischemia/Reperfusion Injury: Molecular Insights, Forensic Perspectives, and Therapeutic Horizons" Cells 14, no. 19: 1509. https://doi.org/10.3390/cells14191509
APA StyleFede, M. S., Daziani, G., Tavoletta, F., Montana, A., Compagnucci, P., Goteri, G., Neri, M., & Busardò, F. P. (2025). Myocardial Ischemia/Reperfusion Injury: Molecular Insights, Forensic Perspectives, and Therapeutic Horizons. Cells, 14(19), 1509. https://doi.org/10.3390/cells14191509











