MyD88 Contributes to TLR3-Mediated NF-κB Activation and Cytokine Production in Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bone Marrow Derived Macrophages (BMDMs) Culture
2.3. ELISA Analysis
2.4. Cytotoxicity
2.5. Western Blotting
2.6. RNA Extraction and RT-qPCR
2.7. Plasmid Construction and Transient Expression of TLR3 in CHO Cells
2.8. Co-Immunoprecipitation
2.9. MyD88 Oligomerization by Crosslinking
2.10. Quantification and Statistical Analysis
3. Results
3.1. NF-κB Activation Is Significantly Reduced in MyD88-/- Cells Treated with Poly(I:C)
3.2. MyD88 Contributes to the Transcription of Cytokines
3.3. Cytokine Release Is Significantly Reduced in MyD88-/- Cells
3.4. Attenuated Cytokine Responses in MyD88-/- Mice
3.5. MyD88 Participation in TLR3 Signaling Extends Beyond Poly(I:C)
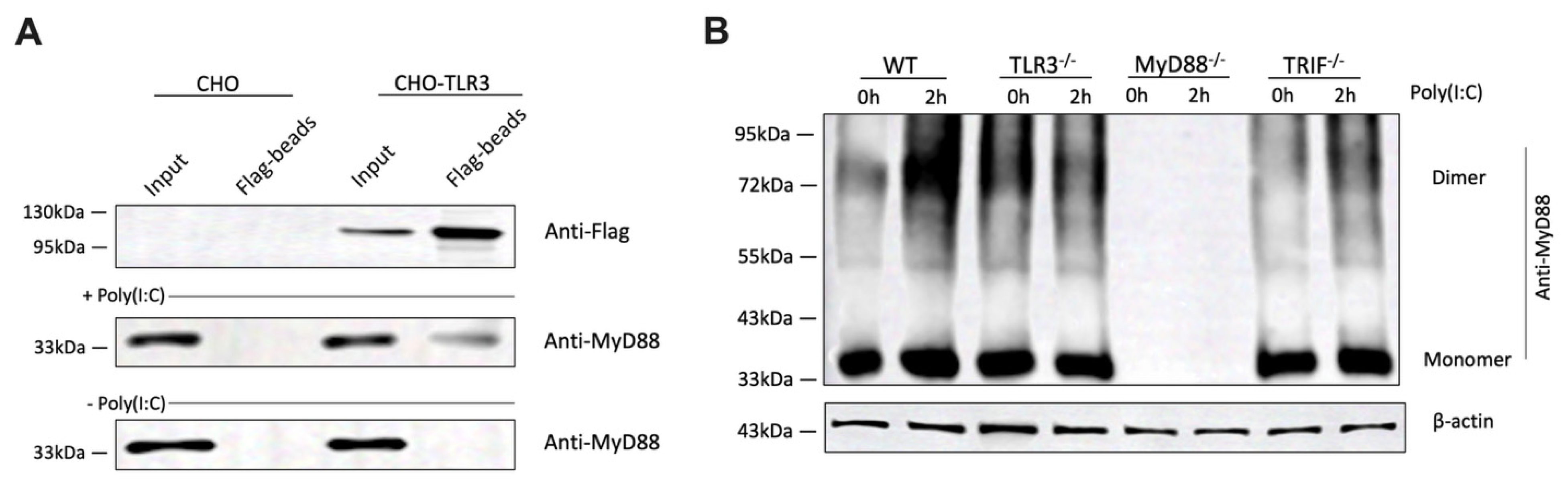
3.6. Poly(I:C) Stimulation Enhances the Direct Association Between TLR3 and MyD88
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLR3 | Toll-like receptor 3 |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| MyD88 | myeloid differentiation primary response 88 |
| poly(I:C) | polyinosinic-polycytidylic acid |
| poly(A:U) | polyadenylic–polyuridylic acid |
| BMDMs | bone marrow-derived macrophages |
| TLRs | Toll-like receptors |
| PRRs | pattern recognition receptors |
| PAMPs | pathogen-associated molecular patterns |
| LPSs | lipopolysaccharides |
| IFN-I | type I interferons |
| dsRNA | double-stranded RNA |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| IκB | Inhibitor of kappa B |
| MAPKs | mitogen-activated protein kinases |
| TNF-α | tumor necrosis factor-alpha |
| IRF3 | interferon regulatory factor 3 |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| qPCR | quantitative PCR |
| WT | Wild type |
| IL | interleukin |
| LDH | lactate dehydrogenase |
| DSS | disuccinimidyl suberate |
References
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M. Toll-Like Receptors: General Molecular and Structural Biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Wu, C.; Lu, W.; Zhang, Y.; Zhang, G.; Shi, X.; Hisada, Y.; Grover, S.P.; Zhang, X.; Li, L.; Xiang, B.; et al. Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis. Immunity 2019, 50, 1401–1411.E4. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, G.; Dong, B.; Pandeya, A.; Cui, J.; Valenca, S.d.S.; Yang, L.; Qi, J.; Chai, Z.; Wu, C.; et al. Pyroptosis of pulmonary fibroblasts and macrophages through NLRC4 inflammasome leads to acute respiratory failure. Cell Rep. 2025, 44, 115479. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chai, Z.; Pandeya, A.; Yang, L.; Zhang, Y.; Zhang, G.; Wu, C.; Li, Z.; Wei, Y. Caspase-11 and NLRP3 exacerbate systemic Klebsiella infection through reducing mitochondrial ROS production. Front. Immunol. 2025, 16, 1516120. [Google Scholar] [CrossRef]
- Lannoy, V.; Côté-Biron, A.; Asselin, C.; Rivard, N. TIRAP, TRAM, and Toll-Like Receptors: The Untold Story. Mediat. Inflamm. 2023, 2023, 2899271. [Google Scholar] [CrossRef]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leucoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, K.S.; Peroulis, M.; Schizas, D.; Kapelouzou, A. MYD88 and Proinflammatory Chemokines in Aortic Atheromatosis: Exploring Novel Statin Effects. Int. J. Mol. Sci. 2023, 24, 9248. [Google Scholar] [CrossRef]
- Shafeghat, M.; Kazemian, S.; Aminorroaya, A.; Aryan, Z.; Rezaei, N. Toll-like receptor 7 regulates cardiovascular diseases. Int. Immunopharmacol. 2022, 113, 109390. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Nilsen, N.J.; Vladimer, G.I.; Stenvik, J.; Orning, M.P.; Zeid-Kilani, M.V.; Bugge, M.; Bergstroem, B.; Conlon, J.; Husebye, H.; Hise, A.G.; et al. A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J. Biol. Chem. 2015, 290, 3209–3222. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef]
- Oshiumi, H.; Matsumoto, M.; Funami, K.; Akazawa, T.; Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3–mediated interferon-β induction. Nat. Immunol. 2003, 4, 161–167. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Vercammen, E.; Staal, J.; Beyaert, R. Sensing of viral infection and activation of innate immunity by toll-like receptor 3. Clin. Microbiol. Rev. 2008, 21, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Herman, M.; Ciancanelli, M.J.; Pérez de Diego, R.; Sancho-Shimizu, V.; Abel, L.; Casanova, J.L. TLR3 immunity to infection in mice and humans. Curr. Opin. Immunol. 2013, 25, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Winkelmann, E.R.; Gorder, S.R.; Mason, P.W.; Milligan, G.N. TLR3- and MyD88-dependent signaling differentially influences the development of West Nile virus-specific B cell responses in mice following immunization with RepliVAX WN, a single-cycle flavivirus vaccine candidate. J. Virol. 2013, 87, 12090–12101. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, K.; Du, X.; Georgel, P.; Janssen, E.; Tabeta, K.; Kim, S.O.; Goode, J.; Lin, P.; Mann, N.; Mudd, S.; et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 2003, 424, 743–748. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, H.Y.; Hsueh, Y.P. TLR3 downregulates expression of schizophrenia gene Disc1 via MYD88 to control neuronal morphology. EMBO Rep. 2017, 18, 169–183. [Google Scholar] [CrossRef]
- Johnson, A.C.; Li, X.; Pearlman, E. MyD88 Functions as a Negative Regulator of TLR3/TRIF-induced Corneal Inflammation by Inhibiting Activation of c-Jun N-terminal Kinase *. J. Biol. Chem. 2008, 283, 3988–3996. [Google Scholar] [CrossRef]
- Siednienko, J.; Gajanayake, T.; Fitzgerald, K.A.; Moynagh, P.; Miggin, S.M. Absence of MyD88 Results in Enhanced TLR3-Dependent Phosphorylation of IRF3 and Increased IFN-β and RANTES Production. J. Immunol. 2011, 186, 2514–2522. [Google Scholar] [CrossRef]
- Thierry, S.; Maadadi, S.; Berton, A.; Dimier, L.; Perret, C.; Vey, N.; Ourfali, S.; Saccas, M.; Caron, S.; Boucard-Jourdin, M.; et al. TL-532, a novel specific Toll-like receptor 3 agonist rationally designed for targeting cancers: Discovery process and biological characterization. Microb. Cell 2023, 10, 117–132. [Google Scholar] [CrossRef]
- Komal, A.; Noreen, M.; El-Kott, A.F. TLR3 agonists: RGC100, ARNAX, and poly-IC: A comparative review. Immunol. Res. 2021, 69, 312–322. [Google Scholar] [CrossRef]
- Mian, M.F.; Ahmed, A.N.; Rad, M.; Babaian, A.; Bowdish, D.; Ashkar, A.A. Length of dsRNA (poly I:C) drives distinct innate immune responses, depending on the cell type. J. Leukoc. Biol. 2013, 94, 1025–1036. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, X.; Liao, C.; Zhang, Y.; Shen, S.; Zhang, R.; Li, G.; Yin, H. MyD88 self-assembles into supramolecular filaments to amplify NF-κB signaling. Fundam. Res. 2025, in press. [CrossRef]
- Guven-Maiorov, E.; Keskin, O.; Gursoy, A.; VanWaes, C.; Chen, Z.; Tsai, C.-J.; Nussinov, R. The Architecture of the TIR Domain Signalosome in the Toll-like Receptor-4 Signaling Pathway. Sci. Rep. 2015, 5, 13128. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ye, J.; Wang, H.; Sun, M.; Zhang, Y.; Sang, X.; Zhuang, Z. Application of toll-like receptors (TLRs) and their agonists in cancer vaccines and immunotherapy. Front. Immunol. 2023, 14, 1227833. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Cha, S.B.; Lee, S.H.; Bae, H.S.; Ham, C.S.; Lee, M.G.; Kim, D.H.; Han, S.H. A novel defined TLR3 agonist as an effective vaccine adjuvant. Front. Immunol. 2023, 14, 1075291. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tatematsu, M.; Nishikawa, F.; Azuma, M.; Ishii, N.; Morii-Sakai, A.; Shime, H.; Seya, T. Defined TLR3-specific adjuvant that induces NK and CTL activation without significant cytokine production in vivo. Nat. Commun. 2015, 6, 6280. [Google Scholar] [CrossRef]
- Sartorius, R.; Trovato, M.; Manco, R.; D’Apice, L.; De Berardinis, P. Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. npj Vaccines 2021, 6, 127. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, P. Machine Learning Methods for Prediction of COVID-19 Patient Length of Stay: Using Texas PUDF Data. In Proceedings of the 2023 3rd International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Tenerife, Spain, 19–21 July 2023; pp. 1–7. [Google Scholar]
- Thomas, R.; Connolly, K.J.; Brekk, O.R.; Hinrich, A.J.; Hastings, M.L.; Isacson, O.; Hallett, P.J. Viral-like TLR3 induction of cytokine networks and α-synuclein are reduced by complement C3 blockade in mouse brain. Sci. Rep. 2023, 13, 15164. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Wang, J.; Chen, J.; Su, J.; Tang, Y.; Wu, X.; Ma, X.; Chen, F.; Ruan, C.; Zheng, X.L.; et al. Plasma levels of complement activation fragments C3b and sC5b-9 significantly increased in patients with thrombotic microangiopathy after allogeneic stem cell transplantation. Ann. Hematol. 2017, 96, 1849–1855. [Google Scholar] [CrossRef]
- Qi, J.; Pan, T.; You, T.; Tang, Y.; Chu, T.; Chen, J.; Fan, Y.; Hu, S.; Yang, F.; Ruan, C.; et al. Upregulation of HIF-1α contributes to complement activation in transplantation-associated thrombotic microangiopathy. Br. J. Haematol. 2022, 199, 603–615. [Google Scholar] [CrossRef]
- Zuo, W.; Wakimoto, M.; Kozaiwa, N.; Shirasaka, Y.; Oh, S.-W.; Fujiwara, S.; Miyachi, H.; Kogure, A.; Kato, H.; Fujita, T. PKR and TLR3 trigger distinct signals that coordinate the induction of antiviral apoptosis. Cell Death Dis. 2022, 13, 707. [Google Scholar] [CrossRef]
- Bianchi, F.; Pretto, S.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol. Ther. 2017, 18, 747–756. [Google Scholar] [CrossRef]
- Teixeira, H.S.; Zhao, J.; Kazmierski, E.; Kinane, D.F.; Benakanakere, M.R. TLR3-Dependent Activation of TLR2 Endogenous Ligands via the MyD88 Signaling Pathway Augments the Innate Immune Response. Cells 2020, 9, 1910. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.I.; Zacca, E.R.; Núñez, N.G.; Ranocchia, R.P.; Maccioni, M.; Maletto, B.A.; Pistoresi-Palencia, M.C.; Morón, G. TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8α+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface. J. Immunol. 2013, 190, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Hoshino, K.; Saito, M.; Yano, T.; Sasaki, I.; Yamazaki, C.; Akira, S.; Kaisho, T. Immunoadjuvant effects of polyadenylic:polyuridylic acids through TLR3 and TLR7. Int. Immunol. 2008, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
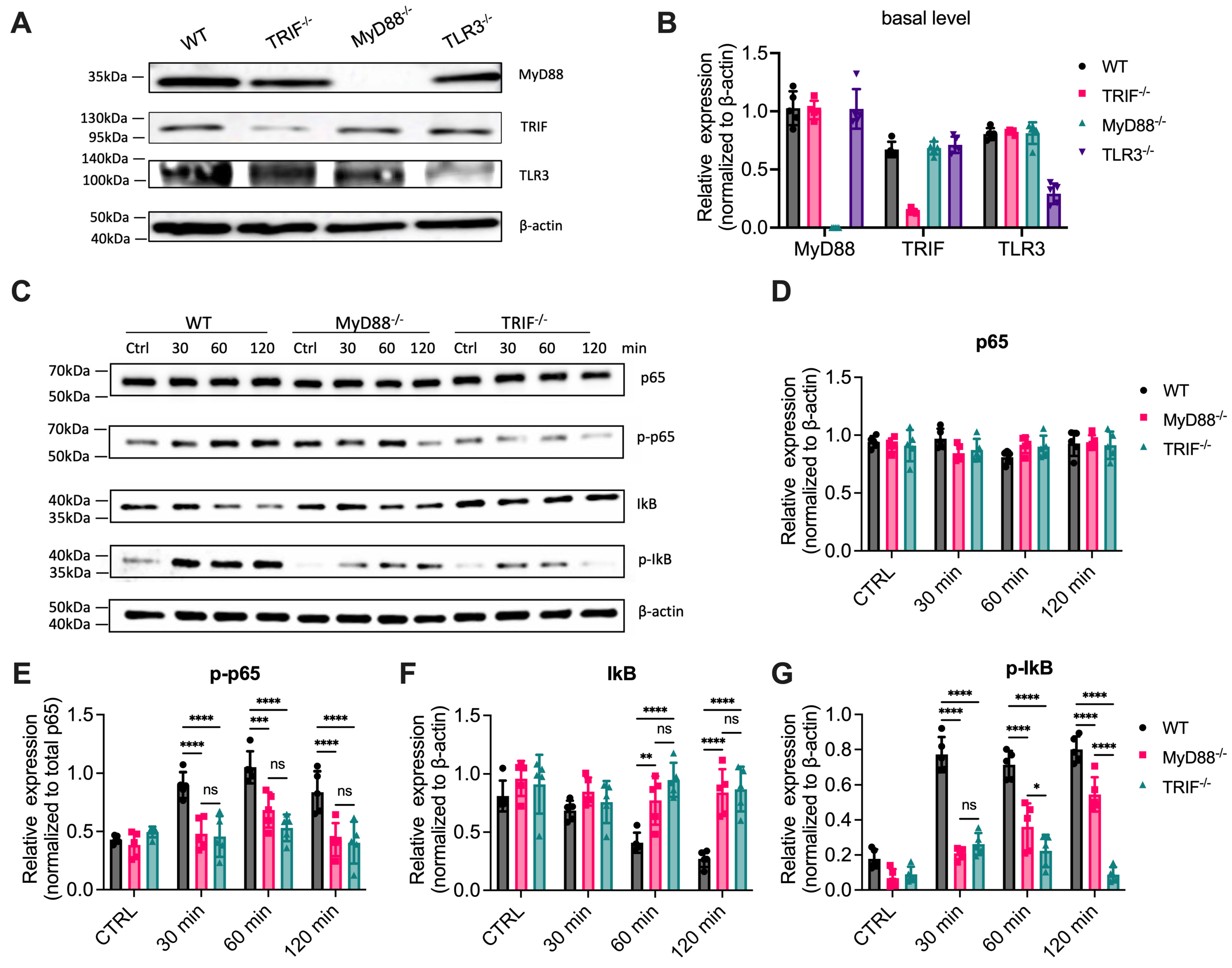
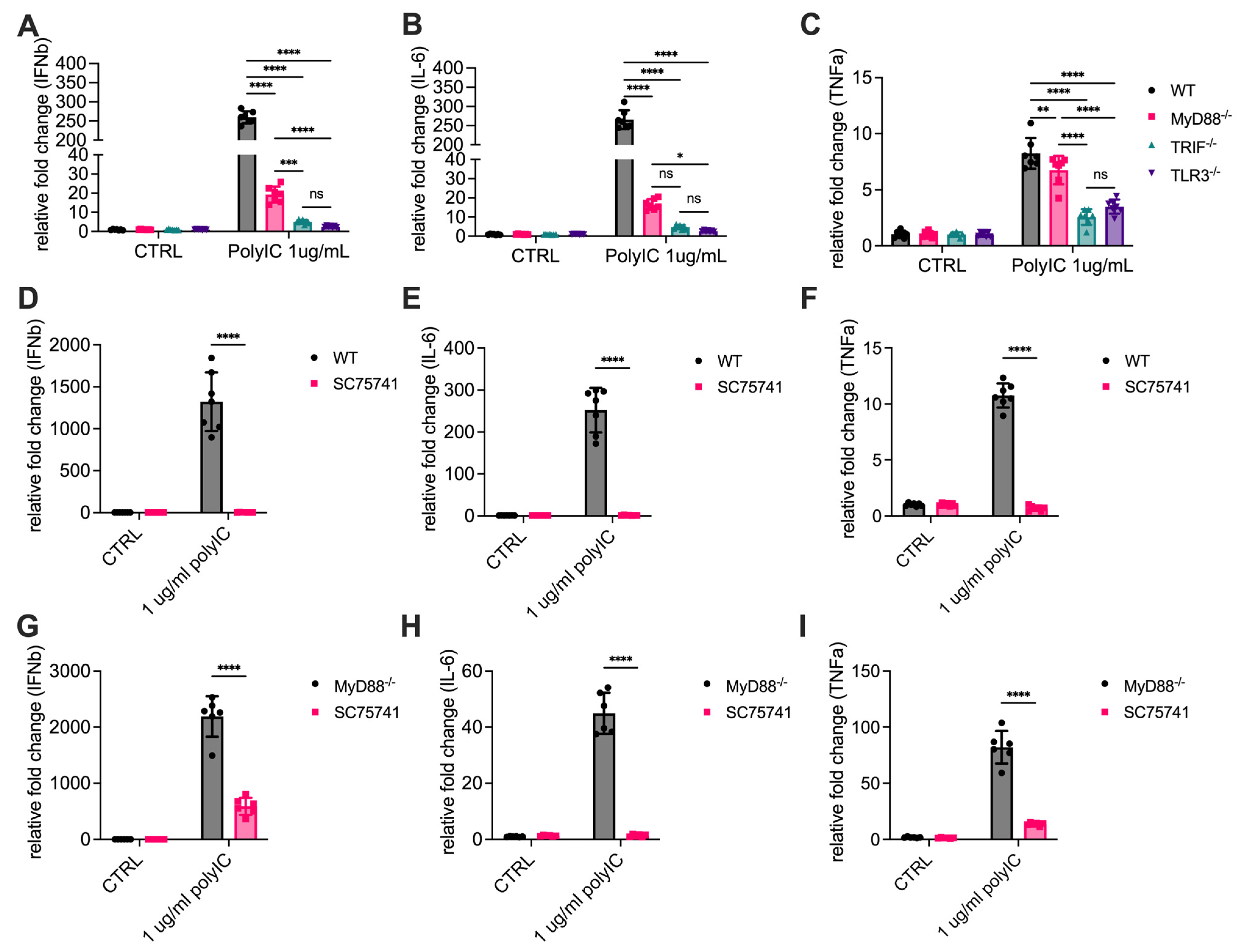

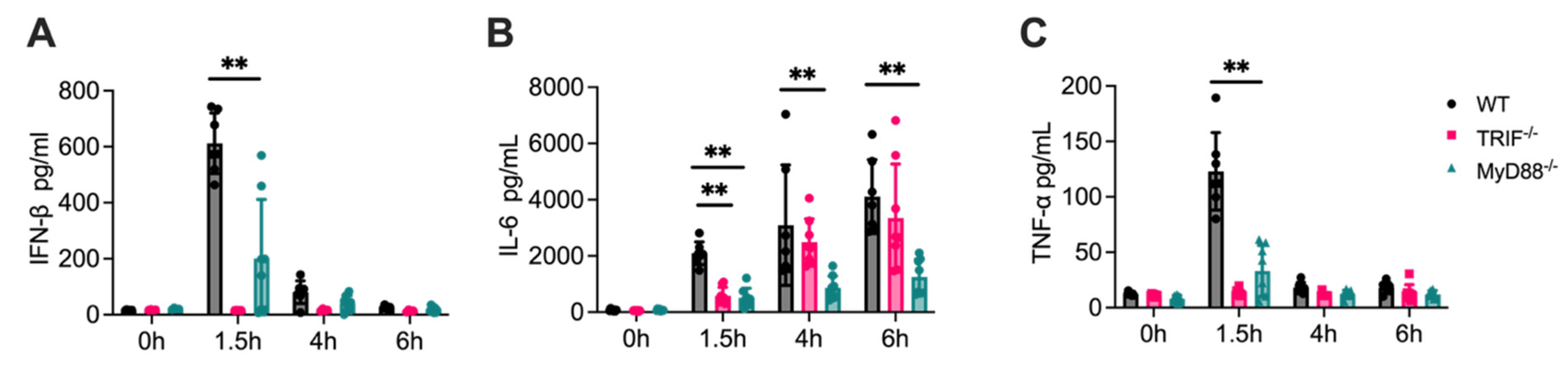
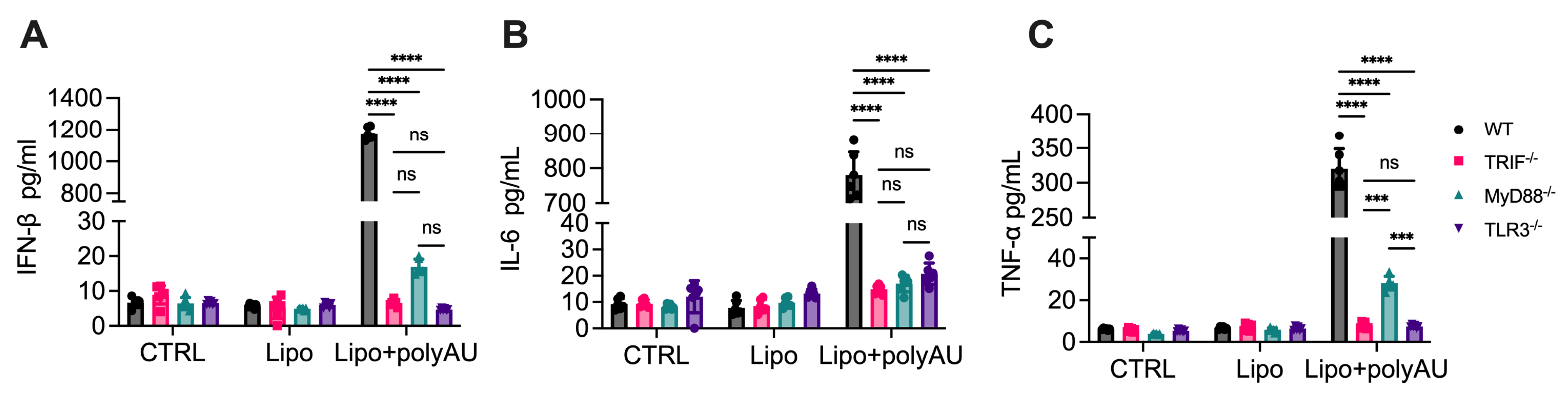
| Antibody | Clonality | Clone ID | Host Species | Target Epitope | Vendor | Catalog # | Lot # |
|---|---|---|---|---|---|---|---|
| TLR3 | Monoclonal | 40C1285.6 | mouse | Amino acids 55–85 | Novus Biologicals | NBP2-24875 | C-5 |
| MyD88 | Monoclonal | D80F5 | rabbit | Cys233 | CST | 4283 | / |
| TRIF | Polyclonal | / | rabbit | amino acids 100–180 | Novus Biologicals | NB120-13810SS | B-4 |
| p65 | Monoclonal | D14E12 | rabbit | Glu498 | CST | 8242 | / |
| p-p65 | Polyclonal | / | rabbit | Ser536 | CST | 3031 | / |
| IκB | Monoclonal | L35A5 | mouse | Amino-terminal | CST | 4814 | / |
| p-IκB | Monoclonal | 14D4 | rabbit | Ser32 | CST | 2859 | / |
| Flag | Monoclonal | M2 | Mouse | FLAG | Sigma-Aldrich | F1804 | / |
| β-actin | Monoclonal | 13E5 | rabbit | amino-terminal | CST | 4970 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Z.; Zhou, Y.; Yang, L.; Zhang, Y.; Hossain, S.; Hajimirzaei, S.; Qi, J.; Zhang, G.; Wei, Y.; Li, Z. MyD88 Contributes to TLR3-Mediated NF-κB Activation and Cytokine Production in Macrophages. Cells 2025, 14, 1507. https://doi.org/10.3390/cells14191507
Chai Z, Zhou Y, Yang L, Zhang Y, Hossain S, Hajimirzaei S, Qi J, Zhang G, Wei Y, Li Z. MyD88 Contributes to TLR3-Mediated NF-κB Activation and Cytokine Production in Macrophages. Cells. 2025; 14(19):1507. https://doi.org/10.3390/cells14191507
Chicago/Turabian StyleChai, Zhuodong, Yuqi Zhou, Ling Yang, Yan Zhang, Sukria Hossain, Sahelosadat Hajimirzaei, Jiaqian Qi, Guoying Zhang, Yinan Wei, and Zhenyu Li. 2025. "MyD88 Contributes to TLR3-Mediated NF-κB Activation and Cytokine Production in Macrophages" Cells 14, no. 19: 1507. https://doi.org/10.3390/cells14191507
APA StyleChai, Z., Zhou, Y., Yang, L., Zhang, Y., Hossain, S., Hajimirzaei, S., Qi, J., Zhang, G., Wei, Y., & Li, Z. (2025). MyD88 Contributes to TLR3-Mediated NF-κB Activation and Cytokine Production in Macrophages. Cells, 14(19), 1507. https://doi.org/10.3390/cells14191507







