1. Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignant tumors worldwide and an important cause of cancer-related mortality, accounting for 8.3% and ranking as the third leading cause of all cancer-related deaths [
1]. It is particularly prevalent in regions with high rates of chronic hepatitis B and C infections, such as Asia and Africa [
2,
3]. The primary risk factors for HCC include chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, cirrhosis, long-term alcohol consumption, aflatoxin exposure, and non-alcoholic fatty liver disease (NAFLD) [
4]. Despite recent advances in diagnostic techniques and treatment options, the overall survival rate for HCC patients remains low, largely due to the high frequency of metastasis and recurrence. The most common sites of HCC metastasis include the lungs, lymph nodes, and bones, with pulmonary metastasis being particularly frequent [
5]. Therefore, identifying effective therapeutic strategies to inhibit HCC metastasis and improve patient outcomes is of utmost importance.
The mulberry tree, scientifically named
Morus alba L., is commonly known as “mulberry” or “sang shu”. Originally native to China, this versatile plant has been widely cultivated and naturalized across many countries. It is highly valued as an important herbal resource [
5]. The mulberry tree has a long-standing history in traditional Chinese medicine and is renowned for its extensive health benefits. These benefits include antioxidant, cholesterol-lowering, anti-atherosclerotic, weight management, blood sugar regulation, immune-modulating, lipid-lowering, neuroprotective, and liver-protective effects [
6]. These therapeutic properties are primarily due to the diverse bioactive compounds found within the plant, such as flavonoids, anthocyanins, phenolic acids, flavonols, and volatile aromatic compounds [
7].
Morus alba L., including Kuwanon derivatives such as Kuwanon C and G, has shown anticancer activity [
8]. Nevertheless, Kuwanon A (KA) has not been extensively and in-depth investigated for its anticancer activity yet. Previous studies have demonstrated that KA induces cytotoxic endoplasmic reticulum stress and apoptosis in gastric cancer cells and enhances the sensitivity of tumor cells to the clinical drug 5-fluorouracil [
9]. Additionally, KA has been proven to inhibit the growth of melanoma cells [
10]. These findings suggest that KA has potential as a therapeutic agent for various cancers. However, the specific mechanisms by which KA exerts its anticancer effects, particularly in hepatocellular carcinoma, remain to be fully elucidated.
The
14-3-3 gene encodes a member of the 14-3-3 protein family, which is involved in a wide range of cellular processes, including cell cycle regulation, apoptosis, and signal transduction [
11]. YWHAB, whose full name is tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein beta, is a member of the 14-3-3 protein family. It is known to interact with various proteins and modulate their functions through protein-protein interactions [
12]. In cancer, YWHAB has been implicated in promoting cell proliferation, survival, and metastasis [
13,
14]. High expression levels of YWHAB have been associated with poor prognosis in several types of cancer, including hepatocellular carcinoma [
15]. By regulating key signaling pathways such as the EGFR/PI3K/AKT pathway, YWHAB can influence tumor progression and resistance to therapy [
16,
17,
18]. Therefore, targeting YWHAB represents a promising strategy for cancer treatment.
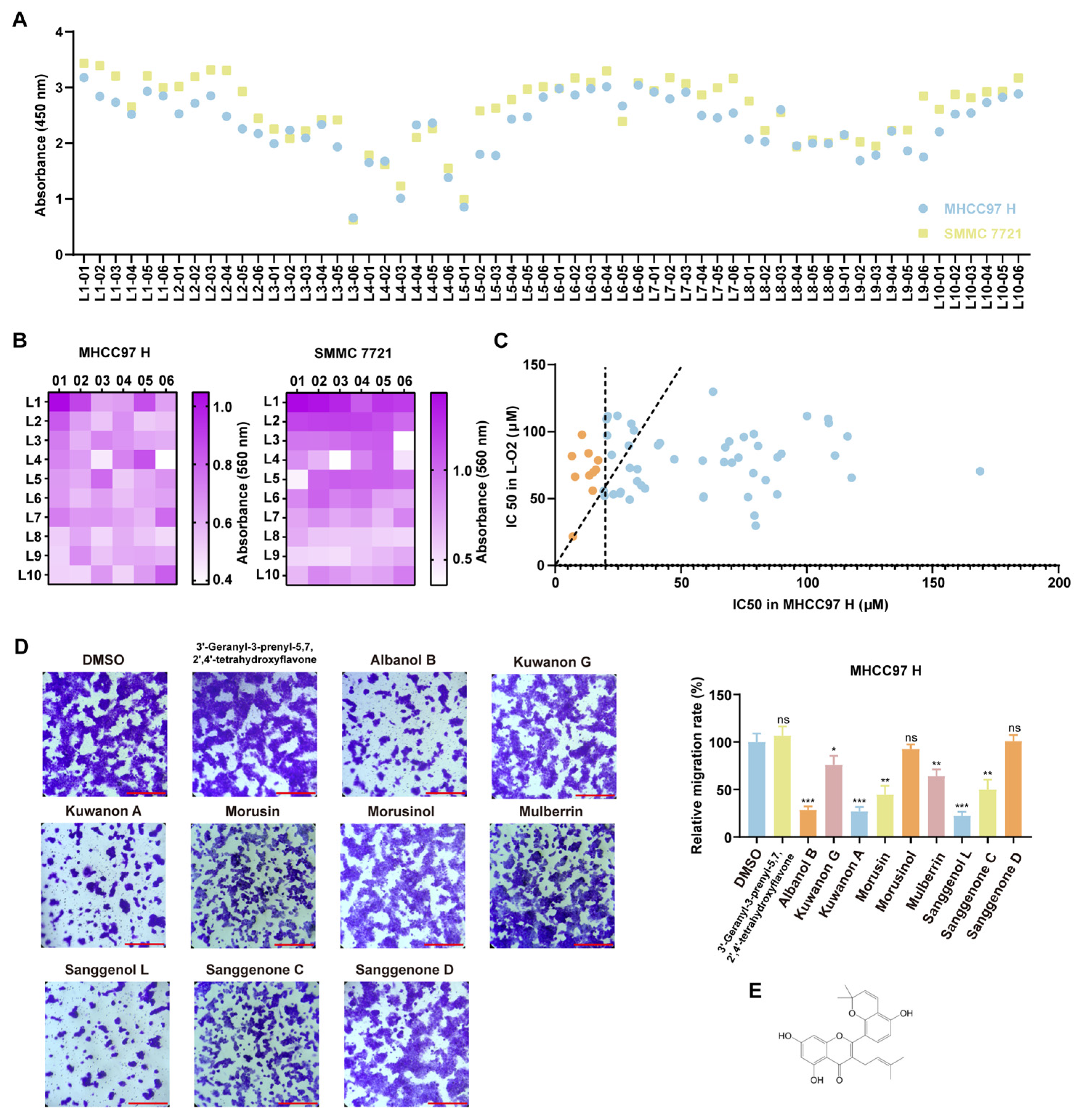
In this study, we screened 623 mulberry metabolites and identified Kuwanon A (KA) as an active compound that effectively inhibits the progression of hepatocellular carcinoma (HCC) and suppresses the metastasis of highly metastatic HCC cells both in vitro and in vivo. Our results demonstrate that KA specifically targeted YWHAB and mediated the dissociation of its dimer. Thereby affecting downstream biological functions. The phosphorylation signaling cascade of the classical MAPK pathway, including Raf/MEK/ERK, is significantly inhibited [
12,
19]. Then it led to cell cycle arrest and the inhibition of migration and invasion. When combined with the clinical drug sorafenib, KA exhibits a synergistic effect in inhibiting HCC cell proliferation, migration, and invasion. These findings collectively suggest that Kuwanon A can act as a MAPK signaling pathway inhibitor and exert antitumor effects in HCC. KA held significant promise as a potential anticancer agent for cancer therapy.
2. Materials and Methods
2.1. Reagents and Antibodies
Kuwanon A (CAS No. 62949-77-3) was procured from LEMEITIAN MEDICINE (Chengdu, China) and dissolved in DMSO. Sorafenib (CAS No. 284461-73-0) was obtained from MedChemExpress (Monmouth Junction, NJ, USA). The antibodies anti-cyclin D1 (Cat No. 26939-1-AP), anti-cyclin E2 (Cat No. 11935-1-AP), anti-CDK 4 (Cat No. 11026-1-AP), anti-cyclin B1 (Cat No. 55004-1-AP), anti-CDK1 (Cat No. 19532-1-AP), anti-ERK 1/2 (Cat No. 11257-1-AP), anti-Phospho-ERK1/2 (Thr202/Tyr204) (Cat No. 28733-1-AP), anti-c-Myc (Cat No. 10828-1-AP), anti-Alpha Tubulin (Cat No. 80762-1-RR), anti-Lamin A/C (Cat No. 10298-1-AP), anti-MYC tag (Cat No. 60003-2-Ig), and anti-DYKDDDDK tag (Cat No. 20543-1-AP) were sourced from Proteintech (Wuhan, China). Additional antibodies, including anti-MEK 1/2 (Cat#9122S), anti-Phospho-MEK 1/2 (Ser217/221) (Cat#9121S), anti-N-Cadherin (Cat#13116T), and anti-E-Cadherin (Cat#3195T) were acquired from Cell Signaling Technology (Boston, MA, USA). The antibody anti-YWHAB (Cat No. ab32560) was acquired from Abcam (Cambridge, UK). MTT (Cat#M5655) and DMSO (Cat#D5879) reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA). Reagents such as DAPI (Cat#C1002), the Cell Counting Kit-8 (Cat#C0038), the Matrix-Gel™ Basement Membrane Matrix (Cat#C0371-1ml), the BeyoClick™ EdU Cell Proliferation Kit with Alexa Fluor 488 (Cat#C0071S), the BCA Protein Assay Kit (Cat#P0012), the Hematoxylin and Eosin Staining Kit (Cat# C0105M), the Nuclear and Cytoplasmic Protein Extraction Kit (Cat#P0027), the Cell Cycle and Apoptosis Analysis Kit (Cat#C1052), the Cell Lysis Buffer for Western and IP (Cat#P0013), the RIPA Lysis Buffer (Cat#P0013B), the Crystal Violet Staining Solution (Cat#C0121), HRP goat anti-mouse antibody (Cat#A0126), HRP goat anti-rabbit antibody (Cat#A0208), and Alexa Fluor 488-labeled Goat Anti-Rabbit IgG (Cat#A0423) were sourced from Beyotime (Shanghai, China). The Blue/Clear Native PAGE Electrophoresis Kit (Cat#RTD6140) was acquired from Real Times (Beijing, China). The Epoxy-Activated Sepharose 6B (Cat#17048001) was obtained from Cytia (Washington, DC, USA). Finally, the transfection reagent Lipofectamine™ 2000 was purchased from Thermo Fisher Scientific (New York, NY, USA).
2.2. Cell Culture
HCC cell lines (MHCC97 H and SMMC 7721), normal human liver cells (L-O2), and human embryonic kidney cells (HEK293T, HEK293FT) were purchased from the American Type Culture Collection (University Blvd Manassas, VA, USA). All cell lines were confirmed to be mycoplasma-free and cultured at 37 °C and 5% carbon dioxide.
2.3. Cell Proliferation Analysis
Cell viability was assessed using MTT experiments. Cells, together with KA, were seeded into 96-well plates at a density of 2000 cells per well with three replicates per condition. DMSO was used as a control. At designated time points, 20 μL of MTT (Sigma, St. Louis, MO, USA) solution was added to each well and incubated for 3 h. After removing the culture medium, 200 μL of DMSO was added to dissolve the formazan crystals. The absorbance was then measured using a microplate reader at a wavelength of 560 nm.
2.4. Colony Formation Assay
Using a colony formation assay, the effect of KA on the colony-forming ability of HCC cells was evaluated. A total of 1000–2000 cells, along with KA, were seeded into each well of a six-well plate. During this period, fresh culture media and KA were replaced multiple times. After 2 to 3 weeks of incubation, the colonies were stained with crystal violet (Beyotime, Shanghai, China) and subsequently quantified in each well using ImageJ 1.52a software.
2.5. CCK8 Assay
The CCK8 assay is used to investigate the effect of compounds on cell viability by measuring the amount of formazan dye. Firstly, 8000 cells were inoculated into each well of the 96-well plate with three replicates per condition. At the same time, add the corresponding mulberry active substances. After 48 h of incubation, 20 μL of the Cell Counting Kit-8 (CCK8) reagent (Beyotime, Shanghai, China) was added to each well. After being cultured under 37 °C and 5% carbon dioxide for 2–3 h, the absorbance was measured at 450 nm.
2.6. Transwell Assay
The Transwell assay measured the migration and invasion of cells by calculating the number of cells that passed through the material. Prior to the Transwell migration assay, cells were serum-starved in FBS-free culture medium for 24 h. The cells were then detached using trypsin, washed 1–2 times with PBS, and resuspended in DMEM. Approximately 50,000–100,000 cells were seeded into the upper chamber of the Transwell insert. The lower chamber was filled with 600 μL of complete medium containing the test compound. The Transwell system was incubated for 12 h under standard culture conditions (37 °C, 5% CO2). After incubation, the migrated cells on the lower membrane surface were fixed with 4% paraformaldehyde (PFA) and stained with 0.1% crystal violet. Non-migrated cells remaining in the upper chamber were carefully removed with a cotton swab. Finally, the stained cells were visualized under a microscope. If matrix gel was needed, it should be added according to the instructions (Beyotime, Shanghai, China) before the cells were seeded.
2.7. EdU Staining
EdU staining was employed to assess cell proliferation following the manufacturer’s guidelines (Beyotime, Shanghai, China). A total of 2 × 104 cells, together with KA or DMSO, were seeded into 24-well plates and cultured for 48 h. Subsequently, the cells were incubated with 10 mM EDU for 2 h, followed by fixation with 4% PFA for 15 min, permeabilization with 0.3% Triton X-100 for 10 min, and reaction with Click reaction cocktails for 30 min. Finally, the nuclei were stained with DAPI for 30 min at room temperature before microscopic examination.
2.8. Flow Cytometry
Cells were digested, centrifuged, and resuspended in PBS buffer following 48 h of treatment with either KA or DMSO. Prior to cell cycle analysis, the cells were fixed in 75% ethanol for a minimum of 24 h, followed by labeling with PI and RNase (Beyotime, Shanghai, China). Subsequently, the cells were analyzed using flow cytometry. Each group consisted of three replicates.
2.9. Transfection and Infection
Short hairpin (sh) RNA targeting YWHAB was cloned into the pLKO.1-puro plasmid, with the sequences listed in
Supplementary Table S1. Flag-tagged YWHAB, MYC-tagged YWHAB, and Flag-tagged Raf1 plasmids were purchased from Miaoling (Wuhan, China). Transfection was performed by introducing the specified plasmids into HEK293FT cells using Lipofectamine 2000 (Thermo, New York, NY, USA), following the manufacturer’s instructions. Viral supernatant or cells were collected two days post-transfection. For infection, HCC cells were treated with viral supernatant and polybrene (Sigma, Virginia Beach, VA, USA). After two rounds of infection, the cells were stably selected by using puromycin (Sigma, Virginia Beach, VA, USA).
2.10. Western Blot
Cells were harvested using a cell scrape and washed three times with PBS buffer, then lysed on ice in RIPA lysis buffer (Beyotime, Shanghai, China). Protein concentration was determined using a BCA protein assay kit (Beyotime, Shanghai, China). Subsequently, 60 µg of protein was mixed with 5× loading buffer and heated in a 100 °C water bath for 15 min. The samples were subjected to SDS-PAGE electrophoresis, followed by transfer onto a PVDF membrane (Millipore, Darmstadt, Germany). The membrane was blocked with 5% BSA for 2 h at room temperature and incubated sequentially with primary and HRP-conjugated secondary antibodies. Finally, signal detection was performed using an ECL detection system (Clinx, Shanghai, China).
2.11. Label Free Analysis
After two days of treatment with KA or DMSO, MHCC97 H cells were collected and analyzed using label-free quantitative proteomic analysis conducted by Shanghai Applied Protein Technology Biotechnology Corporation. The process involved protein extraction, peptide enzymatic hydrolysis, liquid chromatography-tandem mass spectrometry (LC-MS/MS) data collection, and database retrieval. To identify proteins with significant differences between groups, the criteria for screening included a fold change (FC) greater than 2.0 (upregulated > 2.0 or down-regulated < 0.5) and a p-value of less than 0.05.
2.12. Molecular Docking and PyMOL Visualization
The 3D structure of the protein was downloaded from the RCSB Protein Data Bank (
https://www.rcsb.org/) (accessed on 18 May 2024), and the molecular structure of KA was obtained from TCMSP (
https://www.tcmsp-e.com/) (accessed on 18 May 2024). In PyMOL, we removed the ligands from the protein and then used them for drug pocket prediction on the website (
https://playmolecule.org/deepsite/) (accessed on 18 May 2024). According to the potential drug pocket, the molecular docking was performed in Autodock. We selected the docking results with lower binding energy and more hydrogen bonds. After exporting the structure of the docking result, we conducted the visual analysis in PyMOL.
2.13. Animal Studies and Animal Ethics
Thirty-six female BALB/c-nu mice, four weeks old (supplied by Slike Jingda Laboratory Animal Co., Ltd., Changsha, China; Animal qualification number: SCXK-2019-0004), were housed in an SPF room. Based on previous experimental observations, female mice exhibited less individual variability and lower aggression, particularly after tumor inoculation. Moreover, they displayed stable immune responses and lower rejection reactions. Therefore, female mice were selected for this study; 12 mice were used to establish the hepatocellular carcinoma xenografts. MHCC97 H cells (1 × 105 cells) were slowly injected into the left lobe of the mouse liver after being anesthetized with isoflurane. Three days later, the mice received intraperitoneal injections of KA (30 mg/kg) every two days for 45 days. Control mice were administered DMSO injections. Recorded the survival status of the animals. Prior to liver collection, the mice were anesthetized with isoflurane to minimize pain and euthanized by cervical dislocation. Ethics approval serial number: IACUC-20240613-03. Animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006).
2.14. Hematoxylin-Eosin Staining
The liver tissue was fixed in 4% PFA and then prepared into paraffin sections through tissue dehydration, transparency, and wax dip. After baking the slices in the oven, they were dewaxed in xylene and then rehydrated with a series of ethanol gradients. After staining the nuclei with hematoxylin, the slices were differentiated with HCL for a few seconds. After being blued in ammonia solution, the eosin stain was used to stain the cytoplasm of the tissue slices. Lastly, the slices were dehydrated and covered.
2.15. Immunohistochemistry
We prepared the tissue slices until rehydration as described in 2.14. Subsequently, we restored the antigen conformation and blocked endogenous peroxidase. The tissue slices were incubated with the corresponding primary antibody overnight after being blocked with 5% goat serum. The diaminobenzidine (DAB) reaction was conducted after incubation of the secondary antibody at room temperature. Subsequently, the nuclei of tissue slices were stained with hematoxylin. After the dehydration, the tissue slices were covered and observed.
2.16. Cell Wound Healing Assay
We seeded the cells in 6-well plates and allowed them to adhere and grow to full confluence. Then, we gently scratched the cell monolayer in a straight line using a sterile pipette tip. We rinsed the wells with PBS to remove detached cells and debris. We captured bright-field images of the scratched area at baseline (0 h). We replaced the medium with DMEM containing KA or DMSO and continued incubation. We re-imaged the same wound areas at 24 h and 48 h post-scratch. We quantified cell migration by measuring wound closure (%) over time using image analysis software.
2.17. Cell Thermal Shift Assay (CETSA)
The cells were treated with a high concentration (3–4 times the IC50) of the drug for 2–4 h before being digested with trypsin. Then the cells were washed 1–2 times with PBS. Resuspend the cells in 450 µL of PBS. Subsequently, divided them into eight portions, each containing 50 µL. Set the appropriate temperature range and gradient. In our research, the range of 44–65 °C was used. Each portion of cells was treated at the specified temperature for 3–5 min. Then, the cells were repeatedly frozen and thawed using liquid nitrogen. After high-speed centrifugation (20,000× g, 20 min), we took the top layer of supernatant. After adding an appropriate amount of loading buffer, we conducted the Western Blot assay. Quantitative analysis was used to determine whether the compound has enhanced the thermal stability of the target protein.
2.18. Native-PAGE
Blue/Clear Native PAGE (Real Times, Beijing, China) is an electrophoretic technique used to separate protein complexes from biological samples. Instead of SDS, Coomassie Brilliant Blue G-250 binds to the protein, imparting a negative charge, allowing separation in the PAGE gel based on the molecular weight of the proteins. HCC cells treated with KA or DMSO for 48 h were collected using a cell scraper and lysed on ice in IP cell lysis buffer for one hour. A suitable amount of 4× BN/CN-PAGE protein loading buffer and 5% G-250 stain (for loading) was added to the supernatant of the protein sample, followed by BN-PAGE electrophoresis in the Native PAGE gel. After electrophoresis and membrane transfer, the PVDF membrane (Millipore, Darmstadt, Germany) was blocked with 5% BSA for two hours at room temperature. HPR-linked primary and secondary antibodies were incubated for detection, and protein bands were visualized using the ECL detection system (Clinx, Shanghai, China)
2.19. Immunofluorescence
Immunofluorescence was used to explore the effect of KA or Sorafenib on the nuclear translocation of ERK. After laying the glass coverslip in 24-well plates, the cells were seeded. Cells were treated with a certain concentration of KA or Sorafenib for 48 h. Firstly, fix it with 4%PFA, and then permeabilize with 0.2% Triton X-100. After blocking with 5% goat serum, the primary antibody was incubated overnight. The next day, incubate with the fluorescent secondary antibody (Alexa Fluor 488-labeled Goat Anti-Rabbit IgG, Beyotime, Shanghai, China) of the same genus. Confocal observation of fluorescence signals was conducted after staining the nucleus with DAPI.
2.20. Nuclear and Cytoplasmic Protein Extraction
After treating HCC cells with KA or DMSO, the cells were collected by cell scraping. Adding an appropriate amount of PMSF and reagent A (Nuclear and Cytoplasmic Protein Extraction Kit, Beyotime, Shanghai, China). Vortexed and then ice bathed for 15 min. Adding the reagent B provided in the kit mentioned before. Then vortexed and ice bathed for 1 min. Vortexed again. Centrifuged at 14,000× g for 5 min. The supernatant was cytoplasmic protein. Adding a certain amount of nuclear protein extraction reagent to the precipitate. Multiple vortices within 30 min. Centrifuged at 14,000× g for 10 min. The obtained supernatant was the nuclear protein.
2.21. In Vivo Imaging
MHCC97 H cells labeled with luciferase (1 × 105 cells) were injected into the 4- to 5-week-old female BALB/c-nu mice (SPF grade, average weight 16–18 g) through intravenous injection. Following the injection, luciferase substrate was delivered to the mice via intraperitoneal injection. After a 5-min period of anesthesia with isoflurane, the mice were imaged using an in vivo small animal imaging system. The drug treatment was the same as previously mentioned (2.13), and DMSO was used as a control. To monitor the progression and location of the HCC cells, in vivo imaging was conducted on days 0, 14, 21, and 35 after cell injection.
2.22. Epoxy-Activated Sepharose 6B Affinity Media
Epoxy-activated Sepharose 6B (Cytia, Washington, DC, USA) affinity media was used to bind KA molecules following the media’s absorbency swelling. The Sepharose 6B media was co-incubated with KA or DMSO and gently agitated overnight at room temperature. The product was then thoroughly washed through at least three cycles. Any remaining active groups were blocked using 1 M ethanolamine, incubated for 4 h at 37 °C. The 6B media, now bound with KA or DMSO, were further incubated with the cell lysate sample overnight, with gentle agitation at 4 °C. The media was washed again through a minimum of three cycles. A suitable amount of loading buffer was added, and the samples were heated in a 100 °C water bath for 15 min. The resulting supernatant was then ready for use in SDS-PAGE electrophoresis.
2.23. Statistics Analysis
All data were presented independently three times and analyzed using GraphPad Prism 8. Results were expressed as Mean ± SD. Statistical significance was assessed for independent samples using the Student’s unpaired t-test, with significance defined as p < 0.05 (p < 0.05: *, p < 0.01: **, p < 0.001: ***).
4. Discussion
In our research, we provided mechanistic insights into the anticancer effects of Kuwanon A in hepatocellular carcinoma cells. Through compound bank screening, we selected KA and further characterized YWHAB as its target protein using LC-MS/MS. We demonstrated that KA repressed the MAPK pathway, which had been enriched in proteomics, by directly targeting YWHAB and disrupting its dimerization. It suggested that KA effectively blocked the Raf/MEK/ERK signaling cascade, which was crucial for cell cycle progression and migration/invasion of HCC cells. Additionally, the combination of KA with sorafenib, a clinically approved drug for HCC treatment, demonstrated significant synergistic effects on the inhibition of cell proliferation, migration, and invasion. The enhanced inhibition of MEK1/2 and ERK1/2 phosphorylation observed in the combination treatment suggests that KA may potentiate the effects of sorafenib by targeting overlapping pathways [
25,
26]. In a word, our research has supported the anti-proliferative and anti-metastatic capabilities of KA in HCC.
Hepatocellular carcinoma (HCC) accounts for up to 90% of all liver cancer cases, with the vast majority developing from pre-existing chronic liver diseases. In developing countries, hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are major contributors to HCC, whereas in developed countries, non-alcoholic steatohepatitis (NASH) plays a more prominent role [
4,
5]. Despite recent advances in diagnostic techniques and therapeutic strategies, the prognosis for HCC patients remains poor, largely due to the high frequency of metastasis and recurrence [
27]. The most common sites of HCC metastasis include the lungs, lymph nodes, and bones, with pulmonary metastasis being particularly frequent [
27,
28]. This metastatic potential significantly contributes to the high mortality rate associated with HCC.
The current treatment options for HCC are limited and often insufficient to achieve long-term remission. Surgical resection and liver transplantation are the primary curative treatments, but they are only suitable for a minority of patients with early-stage disease. For advanced HCC, targeted therapies such as sorafenib and lenvatinib have shown some efficacy, but their benefits are often modest, and resistance frequently develops [
29,
30]. Immunotherapies, including immune checkpoint inhibitors, have also shown promise, but their response rates are variable, and they are associated with significant side effects [
31]. Therefore, there is an urgent need for novel therapeutic agents that can effectively inhibit HCC progression and metastasis, particularly in high-risk populations. Our research also focused on this aspect and investigated the role of the mulberry active substances in enhancing the sensitivity to chemotherapy. We have revealed that the combination of KA with sorafenib can significantly inhibit the proliferation and epithelial-mesenchymal transition (EMT) of HCC cells, as evidenced by a series of experiments. In our research, we found that the combination of KA with sorafenib can significantly exhibit anti-migration and anti-invasion capabilities that sorafenib alone did not possess. Previous studies on the functions of sorafenib have mainly focused on its role in angiogenesis and proliferation inhibition. An important study on the mechanism of sorafenib’s action had suggested that sorafenib is a multi-kinase inhibitor, including Raf kinase and various receptor tyrosine kinases [
24]. Numerous early studies have demonstrated that sorafenib inhibited the proliferation of HCC cells by blocking the Raf/MEK/ERK signaling pathway and decreased microvascular density by suppressing VEGFR and PDGFR signaling [
32,
33,
34,
35]. However, there has been little discussion about the role of sorafenib in inhibiting migration and invasion or in preventing pulmonary metastasis. At the same time, recent studies on sorafenib resistance have proposed various mechanisms for the migration and invasion of resistant HCC cells. There are some studies focused on the inhibition of the mTOR-related signaling pathway [
36]. At the same time, some studies had also suggested that inhibiting the accumulation of c-Myc is associated with overcoming sorafenib resistance in advanced HCC patients [
37], which was in line with our research findings. KA can enhance the inhibitory effect of sorafenib on c-Myc expression, as well as the migration and invasion of HCC cells, which further supports its potential as an anti-metastatic agent. However, this method does not explain why sorafenib, when administered as a monotherapy, can effectively suppress the expression of c-Myc but has no significant impact on the levels of N-cadherin and E-cadherin. This suggested that sorafenib, as a multi-kinase inhibitor, may modulate EMT-related proteins and migration through alternative mechanisms, such as the activation of the STAT3 or EGFR/beta-catenin pathway, as mentioned in some studies [
38,
39]. Further investigation into the broader mechanisms of sorafenib may contribute to overcoming chemotherapeutic resistance in HCC.
The research on Kuwanon A in tumors is not extensive, though there are articles suggesting its anti-tumor function in melanoma and gastric cancer [
9,
10]. KA might suppress melanoma cell proliferation and migration by promoting β-catenin protein degradation via the E3 ubiquitin ligase synoviolin 1 (SYVN1) [
9]. However, there was no direct evidence to prove that SYVN1 was the specific target of KA. The other research revealed that KA could induce unfolded protein response (UPR) and endoplasmic reticulum (ER) stress, potentially resulting in DNA damage, autophagy, and cell death [
10]. But no detailed discussion was conducted regarding the mechanism. Our research not only supplemented the functional study of KA in HCC but also analyzed the protein targets and mechanisms. We have demonstrated the interaction between YWHAB and KA in detail. It also fully elaborated that KA inhibited the proliferation, migration, and invasion of HCC cells by suppressing the MAPK signaling pathway.
The target protein YWHAB mentioned in our research has been involved in many studies, including investigations related to neurodegenerative diseases and some kinds of cancer. A study on Alzheimer’s disease found significant associations between cerebrospinal fluid (CSF) 14-3-3β levels and CSF biomarkers of p-tau, t-tau, pTau/Aβ42 ratios, and GAP-43, as well as other Alzheimer’s disease biomarkers such as Aβ-PET. Authors proposed the association between CSF 14-3-3β and progressive cognitive decline in Alzheimer’s disease [
40]. In breast cancer cells, IRX5 could inhibit the migration and invasion of cells by inducing YWHAB, which contradicts our research findings [
13]. However, more research has shown that high levels of YWHAB promote tumor progression. A study related to colon cancer had proved that YWHAB knockdown inhibited cell proliferation whilst promoting cell cycle arrest and apoptosis through PIK3R2 [
14]. Additionally, the reduction of YWHAB contributed to etoposide-induced lung cancer apoptosis [
17]. What is more, the identification and validation of novel biomarkers of HCC had demonstrated that YWHAB was upregulated in HCC samples, and higher expression levels were associated with advanced tumor stages and T grades [
15]. Regarding this discrepancy, we have conducted some analyses and suppositions. As YWHAB has a strong affinity for proteins containing phosphorylated serine or phosphorylated threonine, it is involved in regulating numerous signaling pathways and protein function [
12]. As a result, depending on the specific biological context, YWHAB can exhibit dual functional roles, either promoting or suppressing tumorigenesis. For instance, YWHAB regulated the Hippo pathway by interacting and promoting the cytoplasmic retention of YAP [
41]. There is also evidence suggesting that YWHAB can activate the PI3K/AKT pathway and promote tumor progression. These findings suggested that YWHAB may have pleiotropic functions, potentially exerting different effects under varying conditions or cellular contexts.
Our research still has some limitations. To make the conclusions more rigorous, animal experiments used to prove the synergistic effect of KA and sorafenib in inhibiting the proliferation and migration/invasion of HCC in vivo are necessary. Additionally, more research is needed to explore how KA can address sorafenib’s limitation in migration inhibition during combined medication. Additionally, this study was conducted solely in cell cultures and mice models. Our findings indicated the potential and promise of Kuwanon A, a bioactive compound from mulberry, as an anticancer agent. However, its clinical relevance remains to be substantiated by more extensive data and further research.
In conclusion, our study demonstrated that KA, a natural compound derived from mulberry root bark, had significant anticancer effects in HCC cells by targeting YWHAB and disrupting the MAPK signaling pathway. The synergistic interaction with sorafenib further highlighted its potential as a therapeutic agent. These findings provided a possibility for further investigation of KA as a novel therapeutic option for HCC.