The Role of Nesprin-4 in Breast Cancer Migration and Invasion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture
2.2. Mycoplasma Testing
2.3. Plasmids and Transfections
2.4. Quantitative Real-Time PCR
2.5. Protein Detection by Immunoblotting
2.6. Immunofluorescence Microscopy
2.7. Migration Assays
2.8. Transwell Invasion Assay
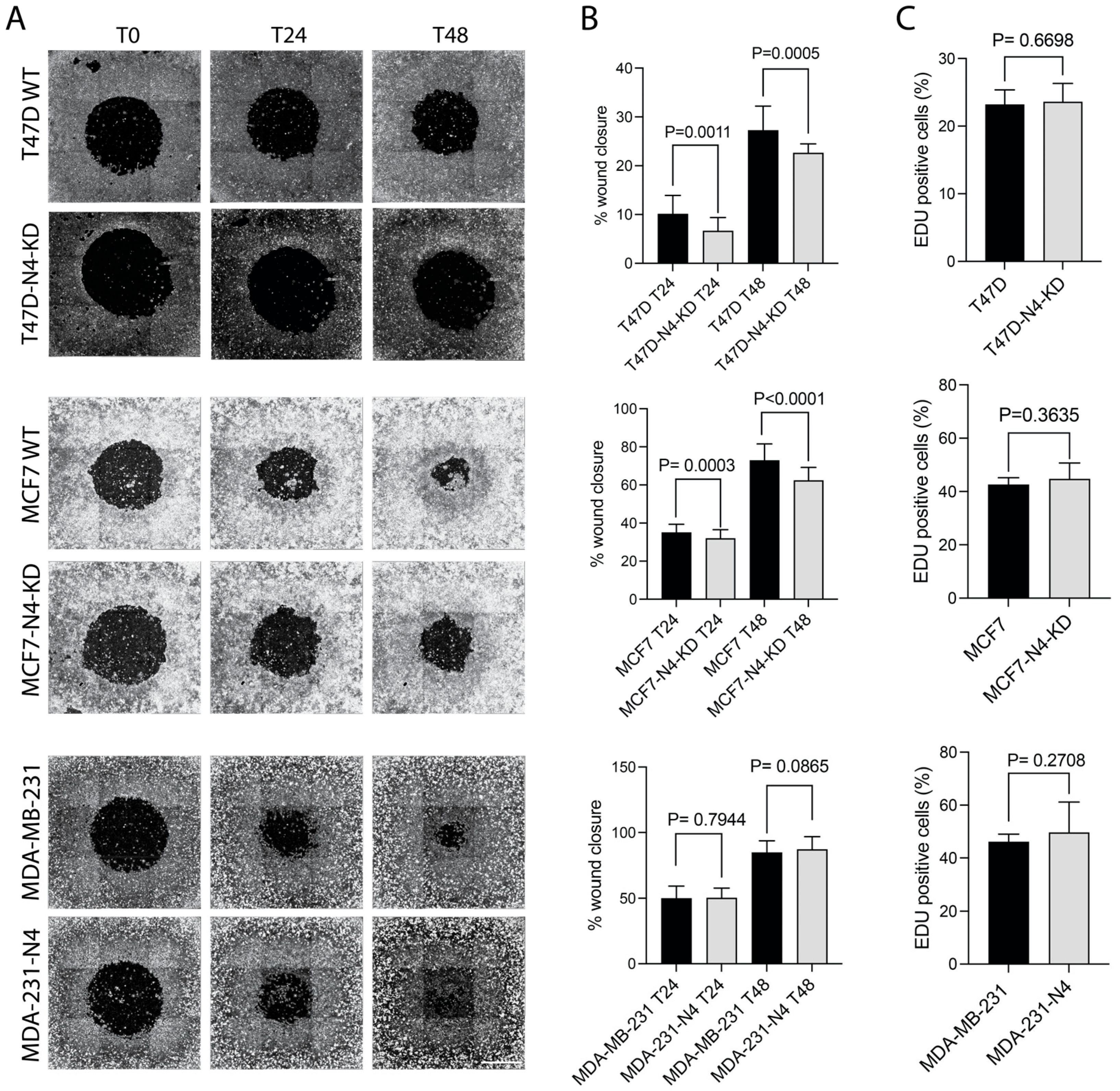
2.9. EdU Cell Proliferation Assay
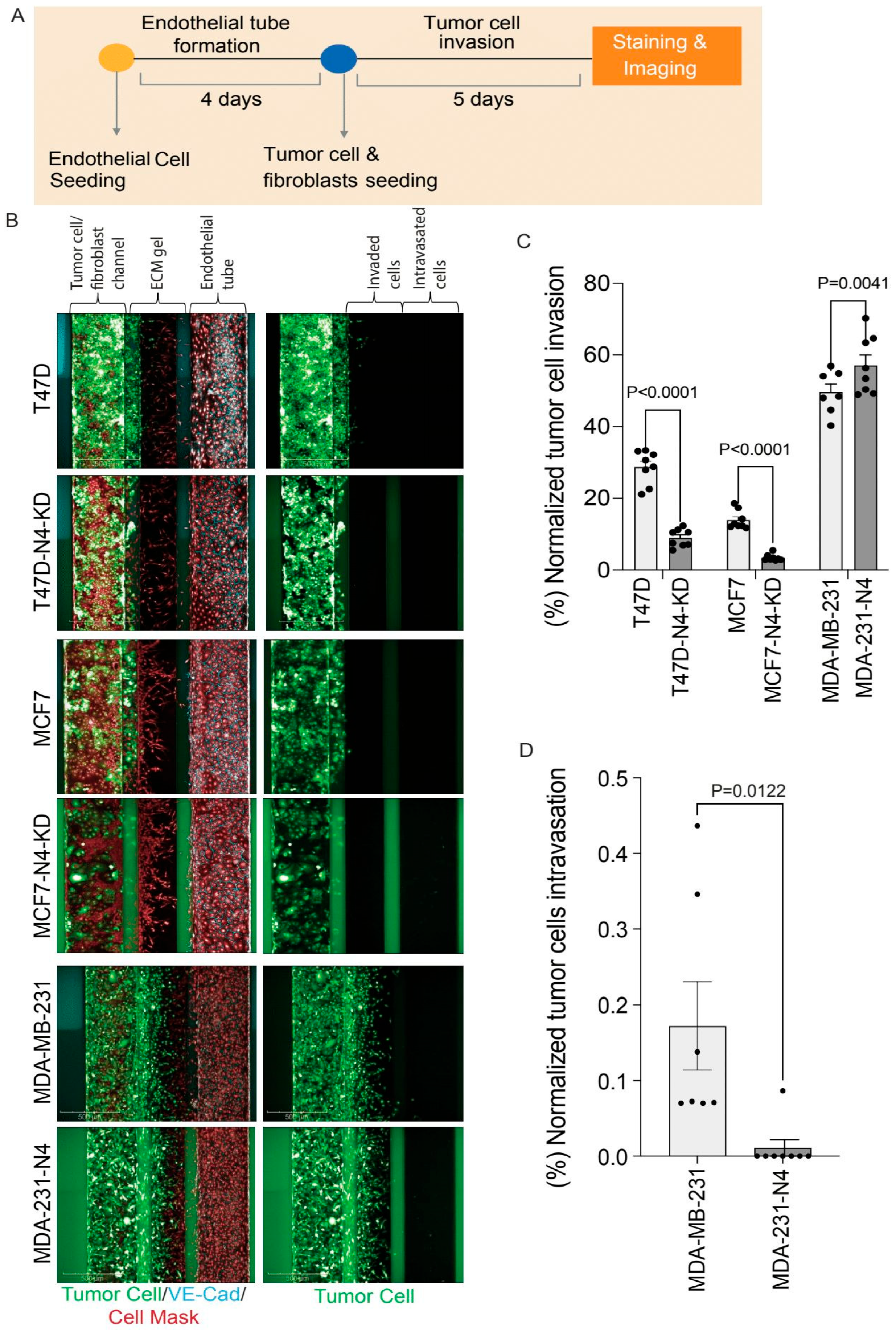
2.10. Organ-on-Chip Assay
2.11. Data Acquisition and Statistical Analysis
3. Results
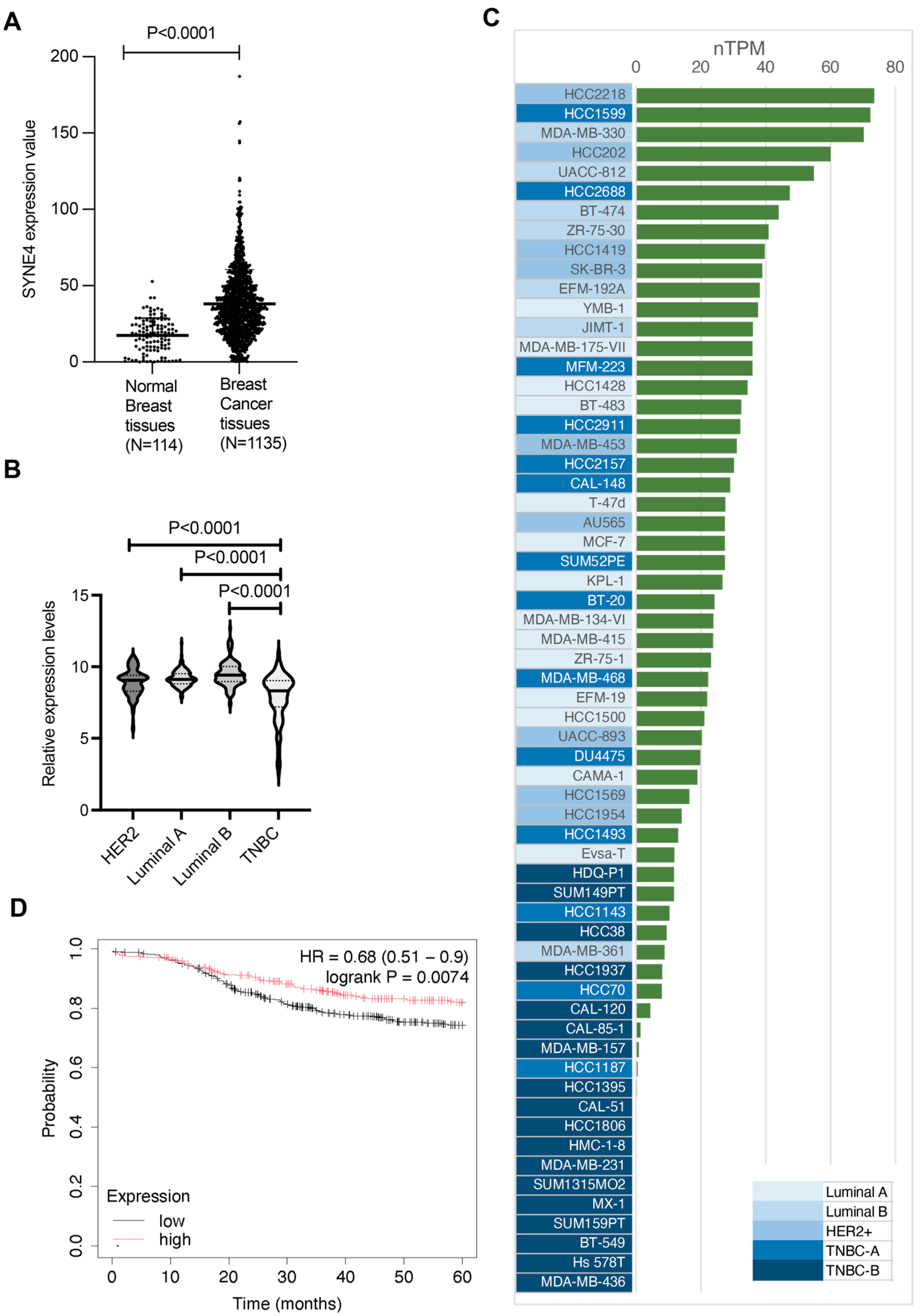
3.1. Nesprin-4 Expression Is Elevated in Breast Cancer
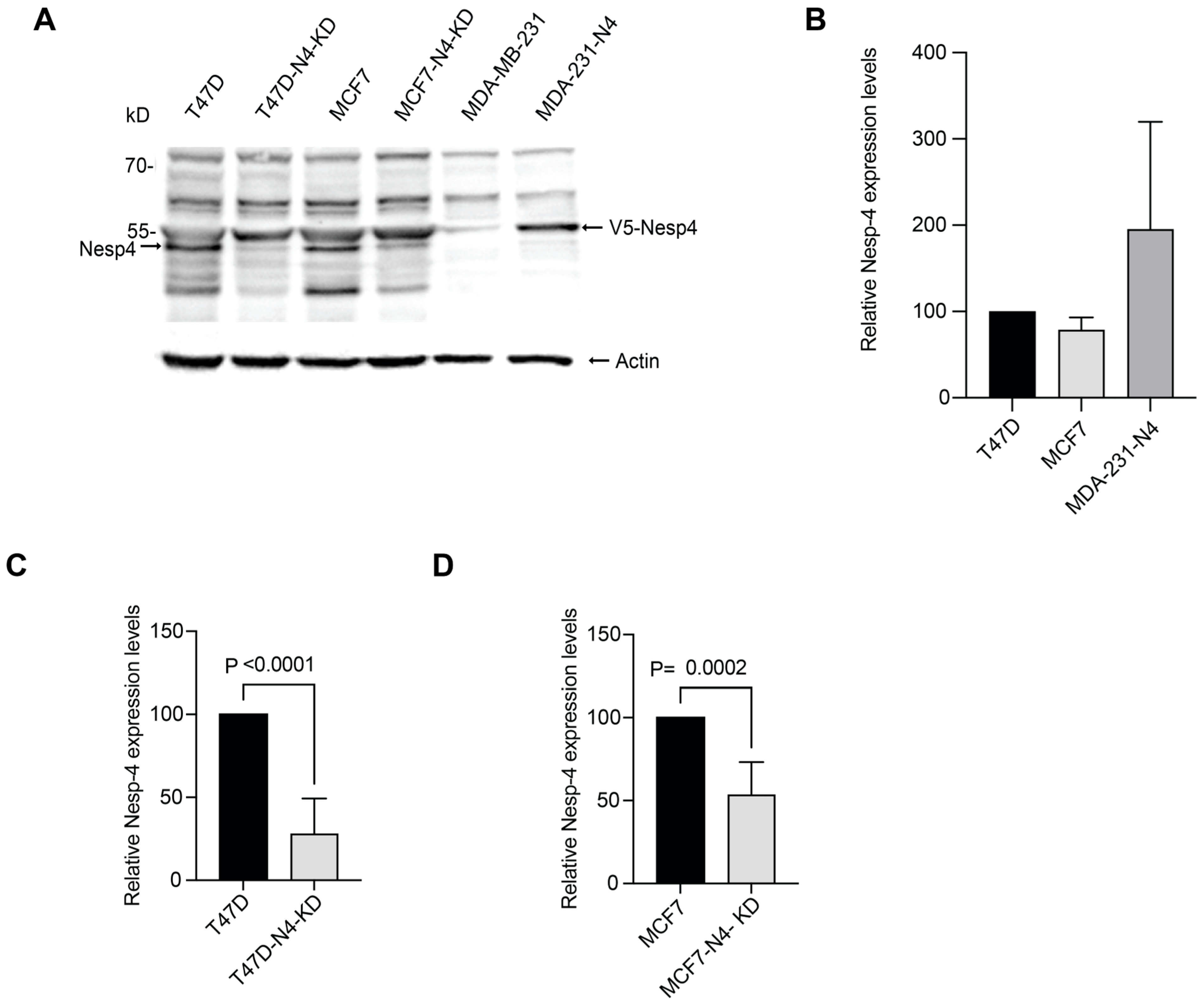
3.2. Nesprin-4 Expression Levels in Breast Cancer Cell Lines
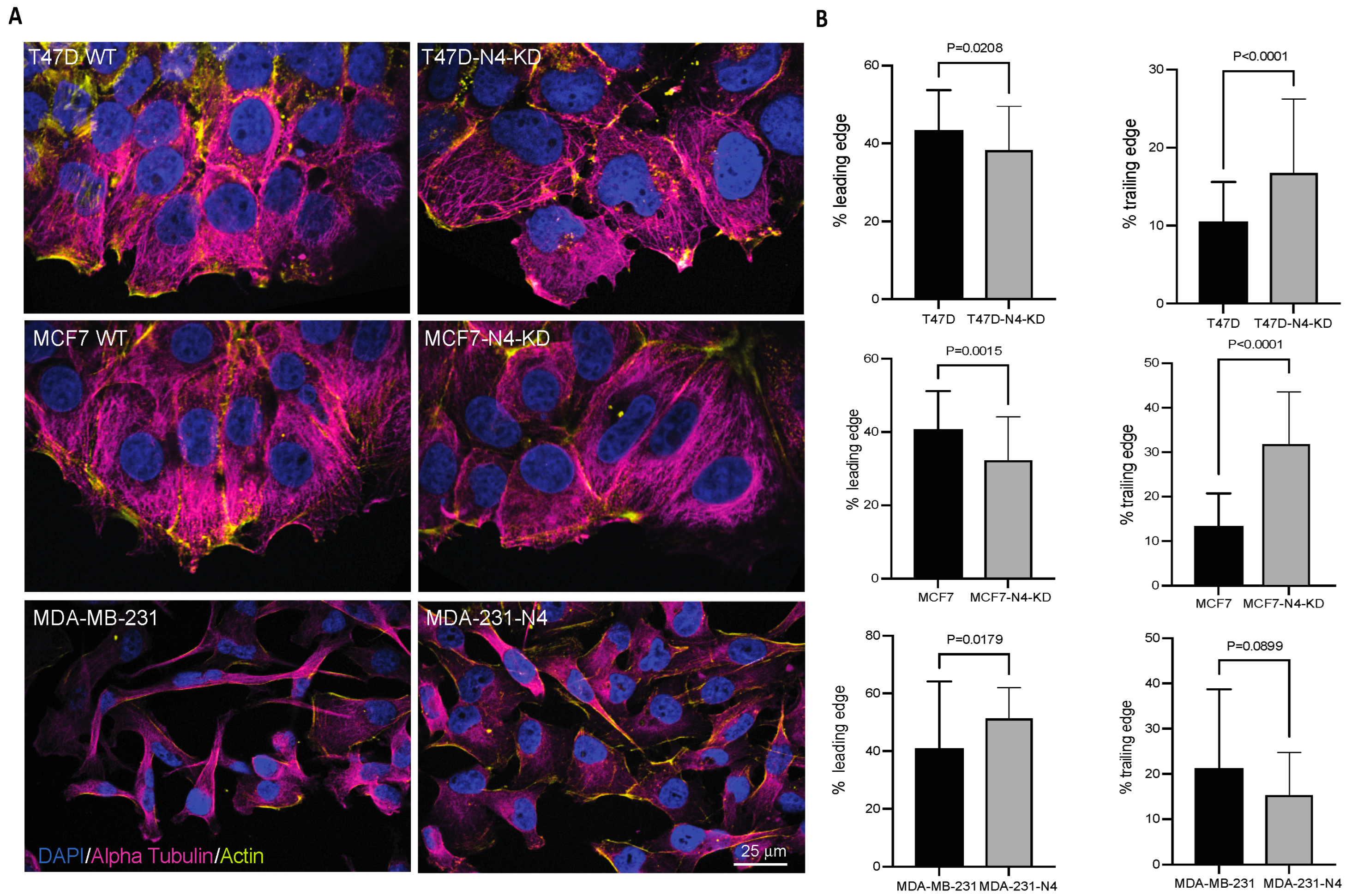
3.3. Nesprin-4 Promotes Two-Dimensional (2D) Cell Migration
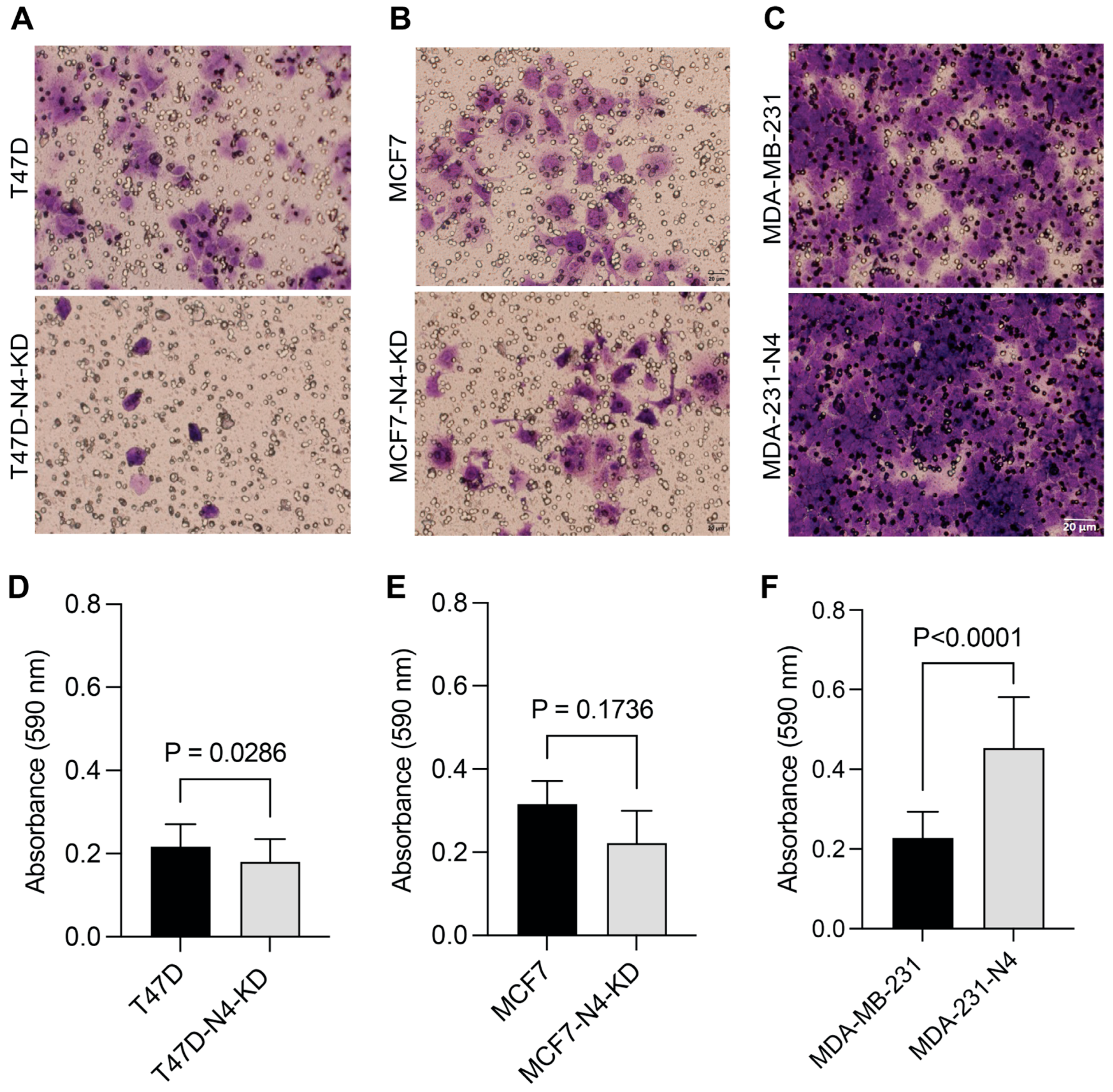
3.4. Nesprin-4 Promotes Three-Dimensional (3D) Invasion
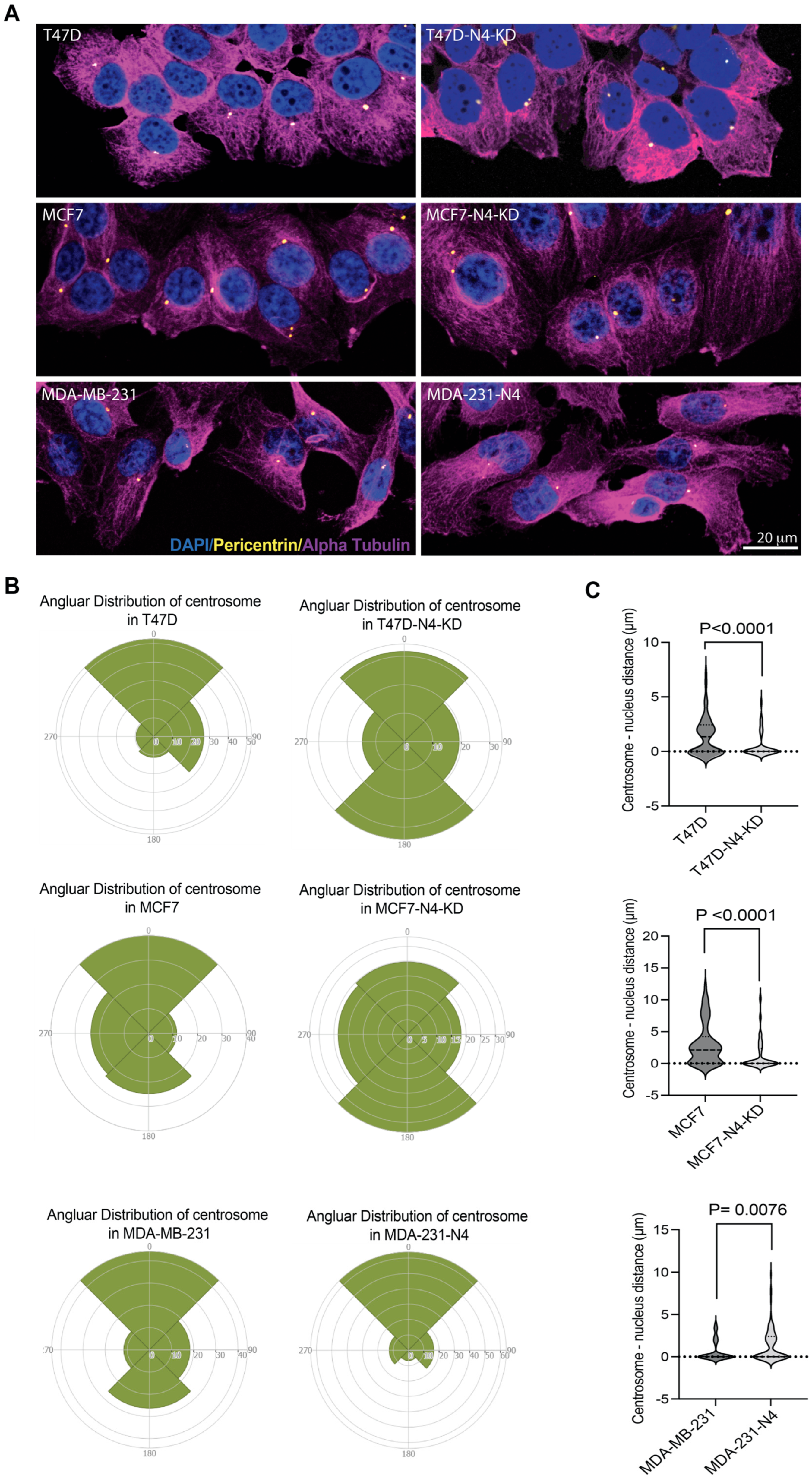
3.5. Nesprin-4 Affects Nuclear Positioning During Breast Cancer Cell Migration
3.6. Nesprin-4 Expression Affects Centrosome Localization in Breast Cancer Cells
3.7. Nesprin-4 Promotes Invasion but Blocks Intravasation in a Metastasis-on-Chip Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMT | Epithelial-to-Mesenchymal Transition |
| MET | Mesenchymal-to-Epithelial Transition |
| EMP | Epithelial–Mesenchymal Plasticity |
| ECM | Extracellular Matrix |
| LINC | Linker of Nucleoskeleton and Cytoskeleton |
| TNBC | Triple-Negative Breast Cancer |
| N4 | nesprin-4 |
| KD | Knockdown |
References
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of Deaths from Cancer Caused by Metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Sporn, M.B. The War on Cancer. Lancet 1996, 347, 1377–1381. [Google Scholar] [CrossRef]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global Breast Cancer Incidence and Mortality Trends by Region, Age-Groups, and Fertility Patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of Cancer Metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Javanmardi, Y.; Watson, S.A.; Serwinski, B.; Djordjevic, B.; Li, W.; Aref, A.R.; Jenkins, R.W.; Moeendarbary, E. Mechanical Signatures in Cancer Metastasis. NPJ Biol. Phys. Mech. 2025, 2, 3. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Kang, Y. How Important Is EMT for Cancer Metastasis? PLoS Biol. 2024, 22, e3002487. [Google Scholar] [CrossRef]
- Wolf, K.; te Lindert, M.; Krause, M.; Alexander, S.; te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical Limits of Cell Migration: Control by ECM Space and Nuclear Deformation and Tuning by Proteolysis and Traction Force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef]
- McGregor, A.L.; Hsia, C.-R.; Lammerding, J. Squish and Squeeze—The Nucleus as a Physical Barrier during Migration in Confined Environments. Curr. Opin. Cell Biol. 2016, 40, 32–40. [Google Scholar] [CrossRef]
- Fischer, T.; Hayn, A.; Mierke, C.T. Effect of Nuclear Stiffness on Cell Mechanics and Migration of Human Breast Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 393. [Google Scholar] [CrossRef]
- Harada, T.; Swift, J.; Irianto, J.; Shin, J.W.; Spinler, K.R.; Athirasala, A.; Diegmiller, R.; Dingal, P.C.D.P.; Ivanovska, I.L.; Discher, D.E. Nuclear Lamin Stiffness Is a Barrier to 3D Migration, but Softness Can Limit Survival. J. Cell Biol. 2014, 204, 669–682. [Google Scholar] [CrossRef]
- Fu, Y.; Chin, L.K.; Bourouina, T.; Liu, A.Q.; VanDongen, A.M.J. Nuclear Deformation during Breast Cancer Cell Transmigration. Lab. Chip 2012, 12, 3774. [Google Scholar] [CrossRef]
- Davidson, P.M.; Denais, C.; Bakshi, M.C.; Lammerding, J. Nuclear Deformability Constitutes a Rate-Limiting Step During Cell Migration in 3-D Environments. Cell Mol. Bioeng. 2014, 7, 293–306. [Google Scholar] [CrossRef]
- Alisafaei, F.; Jokhun, D.S.; Shivashankar, G.V.; Shenoy, V.B. Regulation of Nuclear Architecture, Mechanics, and Nucleocytoplasmic Shuttling of Epigenetic Factors by Cell Geometric Constraints. Proc. Natl. Acad. Sci. USA 2019, 116, 13200–13209. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, Á.; Toseland, C.P. Regulation of Nuclear Mechanics and the Impact on DNA Damage. Int. J. Mol. Sci. 2021, 22, 3178. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, S.M.; Koo, P.K.; Zhao, Y.; Mochrie, S.G.J.; King, M.C. The Tethering of Chromatin to the Nuclear Envelope Supports Nuclear Mechanics. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.D.; Liu, P.Z.; Banigan, E.J.; Almassalha, L.M.; Backman, V.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin Histone Modifications and Rigidity Affect Nuclear Morphology Independent of Lamins. Mol. Biol. Cell 2018, 29, 220–233. [Google Scholar] [CrossRef]
- Guilluy, C.; Osborne, L.D.; Van Landeghem, L.; Sharek, L.; Superfine, R.; Garcia-Mata, R.; Burridge, K. Isolated Nuclei Adapt to Force and Reveal a Mechanotransduction Pathway in the Nucleus. Nat. Publ. Group 2014, 16, 376–381. [Google Scholar] [CrossRef]
- Swift, J.; Discher, D.E. The Nuclear Lamina Is Mechano-Responsive to ECM Elasticity in Mature Tissue. J. Cell Sci. 2014, 127, 3005–3015. [Google Scholar] [CrossRef]
- Davidson, P.M.; Lammerding, J. Broken Nuclei–Lamins, Nuclear Mechanics, and Disease. Trends Cell Biol. 2014, 24, 247–256. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. The Nuclear Lamins: Flexibility in Function. Nat. Rev. Mol. Cell Biol. 2012, 14, 13–24. [Google Scholar] [CrossRef]
- Denais, C.M.; Gilbert, R.M.; Isermann, P.; McGregor, A.L.; te Lindert, M.; Weigelin, B.; Davidson, P.M.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear Envelope Rupture and Repair during Cancer Cell Migration. Science 2016, 352, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell 2019, 49, 920–935.e5. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [PubMed]
- Lammerding, J.; Lee, R.T. Mechanical Properties of Interphase Nuclei Probed by Cellular Strain Application. Methods Mol. Biol. 2009, 464, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Q.; Sun, J.; Wang, A.; Shi, Y.; Ju, Y.; Morita, Y.; Song, G. Decreased Nuclear Stiffness via FAK-ERK1/2 Signaling Is Necessary for Osteopontin-Promoted Migration of Bone Marrow-Derived Mesenchymal Stem Cells. Exp. Cell Res. 2017, 355, 172–181. [Google Scholar] [CrossRef]
- Vahabikashi, A.; Sivagurunathan, S.; Nicdao, F.A.S.; Han, Y.L.; Park, C.Y.; Kittisopikul, M.; Wong, X.; Tran, J.R.; Gundersen, G.G.; Reddy, K.L.; et al. Nuclear Lamin Isoforms Differentially Contribute to LINC Complex-Dependent Nucleocytoskeletal Coupling and Whole-Cell Mechanics. Proc. Natl. Acad. Sci. USA 2022, 119, e2121816119. [Google Scholar] [CrossRef]
- Ghosh, S.; Cuevas, V.C.; Seelbinder, B.; Neu, C.P. Image-Based Elastography of Heterochromatin and Euchromatin Domains in the Deforming Cell Nucleus. Small 2021, 17, e2006109. [Google Scholar] [CrossRef]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; Arcos, J.M.G.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817.e22. [Google Scholar] [CrossRef]
- Crisp, M.; Liu, Q.; Roux, K.; Rattner, J.B.; Shanahan, C.; Burke, B.; Stahl, P.D.; Hodzic, D. Coupling of the Nucleus and Cytoplasm: Role of the LINC Complex. J. Cell Biol. 2006, 172, 41–53. [Google Scholar] [CrossRef]
- Horn, H.F. LINC Complex Proteins in Development and Disease. In Mouse Models of the Nuclear Envelopathies and Related Diseases, 1st ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 109. [Google Scholar] [CrossRef]
- Alam, S.G.; Zhang, Q.; Prasad, N.; Li, Y.; Chamala, S.; Kuchibhotla, R.; KC, B.; Aggarwal, V.; Shrestha, S.; Jones, A.L.; et al. The Mammalian LINC Complex Regulates Genome Transcriptional Responses to Substrate Rigidity. Sci. Rep. 2016, 6, 38063. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.L.; Jaalouk, D.E.; Shanahan, C.M.; Burke, B.; Roux, K.J.; Lammerding, J. The Interaction between Nesprins and Sun Proteins at the Nuclear Envelope Is Critical for Force Transmission between the Nucleus and Cytoskeleton. J. Biol. Chem. 2011, 286, 26743–26753. [Google Scholar] [CrossRef]
- Bouzid, T.; Kim, E.; Riehl, B.D.; Esfahani, A.M.; Rosenbohm, J.; Yang, R.; Duan, B.; Lim, J.Y. The LINC Complex, Mechanotransduction, and Mesenchymal Stem Cell Function and Fate. J. Biol. Eng. 2019, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Luxton, G.W.G.; Gomes, E.R.; Folker, E.S.; Worman, H.; Gundersen, G.G. TAN Lines: A Novel Nuclear Envelope Structure Involved in Nuclear Positioning. Nucleus 2011, 2, 173–181. [Google Scholar] [CrossRef]
- Borrego-Pinto, J.; Jegou, T.; Osorio, D.S.; Aurade, F.; Gorjanacz, M.; Koch, B.; Mattaj, I.W.; Gomes, E.R. Samp1 Is a Component of TAN Lines and Is Required for Nuclear Movement. J. Cell Sci. 2012, 125, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Calero-Cuenca, F.J.; Janota, C.S.; Gomes, E.R. Dealing with the Nucleus during Cell Migration. Curr. Opin. Cell Biol. 2018, 50, 35–41. [Google Scholar] [CrossRef]
- Luxton, G.W.G.; Gomes, E.R.; Folker, E.S.; Vintinner, E.; Gundersen, G.G. Linear Arrays of Nuclear Envelope Proteins Harness Retrograde Actin Flow for Nuclear Movement. Science 2010, 329, 956–959. [Google Scholar] [CrossRef]
- Cain, N.E.; Jahed, Z.; Schoenhofen, A.; Valdez, V.A.; Elkin, B.; Hao, H.; Harris, N.J.; Herrera, L.A.; Woolums, B.M.; Mofrad, M.R.K.; et al. Conserved SUN-KASH Interfaces Mediate LINC Complex-Dependent Nuclear Movement and Positioning. Curr. Biol. 2018, 28, 3086–3097. [Google Scholar] [CrossRef]
- Schliwa, M.; Euteneuer, U.; Gräf, R.; Ueda, M. Centrosomes, Microtubules and Cell Migration. Biochem. Soc. Symp. 1999, 65, 223–231. [Google Scholar]
- Luxton, G.G.; Gundersen, G.G. Orientation and Function of the Nuclear–Centrosomal Axis during Cell Migration. Curr. Opin. Cell Biol. 2011, 23, 579–588. [Google Scholar] [CrossRef]
- Olins, A.L.; Hoang, T.V.; Zwerger, M.; Herrmann, H.; Zentgraf, H.; Noegel, A.A.; Karakesisoglou, I.; Hodzic, D.; Olins, D.E. The LINC-Less Granulocyte Nucleus. Eur. J. Cell Biol. 2009, 88, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.F.; Brownstein, Z.; Lenz, D.R.; Shivatzki, S.; Dror, A.A.; Dagan-Rosenfeld, O.; Friedman, L.M.; Roux, K.J.; Kozlov, S.; Jeang, K.-T.; et al. The LINC Complex Is Essential for Hearing. J. Clin. Investig. 2013, 123, 740–750. [Google Scholar] [CrossRef]
- Roux, K.J.; Crisp, M.L.; Liu, Q.; Kim, D.; Kozlov, S.; Stewart, C.L.; Burke, B. Nesprin 4 Is an Outer Nuclear Membrane Protein That Can Induce Kinesin-Mediated Cell Polarization. Proc. Natl. Acad. Sci. USA 2009, 106, 2194–2199. [Google Scholar] [CrossRef]
- Chiba, K.; Ori-McKenney, K.M.; Niwa, S.; McKenney, R.J. Synergistic Autoinhibition and Activation Mechanisms Control Kinesin-1 Motor Activity. Cell Rep. 2022, 39, 110900. [Google Scholar] [CrossRef]
- Ozer, L.Y.; Fayed, H.S.; Ericsson, J.; Al Haj Zen, A. Development of a Cancer Metastasis-on-Chip Assay for High Throughput Drug Screening. Front. Oncol. 2023, 13, 1269376. [Google Scholar] [CrossRef]
- Mohammed, A.A. The Clinical Behavior of Different Molecular Subtypes of Breast Cancer. Cancer Treat. Res. Commun. 2021, 29, 100469. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Exon Publications: Brisbane, Australia, 2022; pp. 31–42. [Google Scholar] [CrossRef]
- Cai, S.; Zuo, W.; Lu, X.; Gou, Z.; Zhou, Y.; Liu, P.; Pan, Y.; Chen, S. The Prognostic Impact of Age at Diagnosis Upon Breast Cancer of Different Immunohistochemical Subtypes: A Surveillance, Epidemiology, and End Results (SEER) Population-Based Analysis. Front. Oncol. 2020, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- SenGupta, S.; Parent, C.A.; Bear, J.E. The Principles of Directed Cell Migration. Nat. Rev. Mol. Cell Biol. 2021, 22, 529–547. [Google Scholar] [CrossRef]
- Petrie, R.J.; Yamada, K.M. At the Leading Edge of Three-Dimensional Cell Migration. J. Cell Sci. 2012, 125, 5917–5926. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Ren, Y.; Liu, S.; Ba, Y.; Zuo, A.; Luo, P.; Cheng, Q.; Xu, H.; Han, X. Multi-Stage Mechanisms of Tumor Metastasis and Therapeutic Strategies. Signal Transduct. Target. Ther. 2024, 9, 270. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Sznurkowska, M.K.; Aceto, N. The Gate to Metastasis: Key Players in Cancer Cell Intravasation. FEBS J. 2022, 289, 4336–4354. [Google Scholar] [CrossRef]
- Ni, B.-S.; Tzao, C.; Huang, J.-H. Plug-and-Play In Vitro Metastasis System toward Recapitulating the Metastatic Cascade. Sci. Rep. 2019, 9, 18110. [Google Scholar] [CrossRef]
- Gimpel, P.; Lee, Y.L.; Sobota, R.M.; Calvi, A.; Koullourou, V.; Patel, R.; Mamchaoui, K.; Nédélec, F.; Shackleton, S.; Schmoranzer, J.; et al. Nesprin-1α-Dependent Microtubule Nucleation from the Nuclear Envelope via Akap450 Is Necessary for Nuclear Positioning in Muscle Cells. Curr. Biol. 2017, 27, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Umeshima, H.; Hirano, T.; Kengaku, M. Microtubule-Based Nuclear Movement Occurs Independently of Centrosome Positioning in Migrating Neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 16182–16187. [Google Scholar] [CrossRef]
- Zheng, Y.; Buchwalter, R.A.; Zheng, C.; Wight, E.M.; Chen, J.V.; Megraw, T.L. A Perinuclear Microtubule-Organizing Centre Controls Nuclear Positioning and Basement Membrane Secretion. Nat. Cell Biol. 2020, 22, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, P.; Kotila, T.; Jégou, A.; Romet-Lemonne, G. Biochemical and Mechanical Regulation of Actin Dynamics. Nat. Rev. Mol. Cell Biol. 2022, 23, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Sahabandu, N.; Okada, K.; Khan, A.; Elnatan, D.; Starr, D.A.; Ori-McKenney, K.M.; Luxton, G.W.; McKenney, R.J. Active Microtubule-Actin Cross-Talk Mediated by a Nesprin-2G–Kinesin Complex. Sci. Adv. 2025, 11. [Google Scholar] [CrossRef]
- Pimm, M.L.; Henty-Ridilla, J.L. New Twists in Actin–Microtubule Interactions. Mol. Biol. Cell 2021, 32, 211–217. [Google Scholar] [CrossRef]
- Wittmann, T.; Waterman-Storer, C.M. Cell Motility: Can Rho GTPases and Microtubules Point the Way? J. Cell Sci. 2001, 114, 3795–3803. [Google Scholar] [CrossRef]
- Krendel, M.; Zenke, F.T.; Bokoch, G.M. Nucleotide Exchange Factor GEF-H1 Mediates Cross-Talk between Microtubules and the Actin Cytoskeleton. Nat. Cell Biol. 2002, 4, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Omelchenko, T.; Vasiliev, J.M.; Gelfand, I.M.; Feder, H.H.; Bonder, E.M. Mechanisms of Polarization of the Shape of Fibroblasts and Epitheliocytes: Separation of the Roles of Microtubules and Rho-Dependent Actin–Myosin Contractility. Proc. Natl. Acad. Sci. USA 2002, 99, 10452–10457. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the Tumour Transition States Occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Kröger, C.; Afeyan, A.; Mraz, J.; Eaton, E.N.; Reinhardt, F.; Khodor, Y.L.; Thiru, P.; Bierie, B.; Ye, X.; Burge, C.B.; et al. Acquisition of a Hybrid E/M State Is Essential for Tumorigenicity of Basal Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 7353–7362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sammak, B.F.; Yildiz Ozer, L.; Fayed, H.S.; Kafour, N.M.; Ericsson, J.; Al Haj Zen, A.; Horn, H.F. The Role of Nesprin-4 in Breast Cancer Migration and Invasion. Cells 2025, 14, 1484. https://doi.org/10.3390/cells14191484
Al-Sammak BF, Yildiz Ozer L, Fayed HS, Kafour NM, Ericsson J, Al Haj Zen A, Horn HF. The Role of Nesprin-4 in Breast Cancer Migration and Invasion. Cells. 2025; 14(19):1484. https://doi.org/10.3390/cells14191484
Chicago/Turabian StyleAl-Sammak, Badria Fouad, Lutfiye Yildiz Ozer, Hend Salah Fayed, Nada Mohamed Kafour, Johan Ericsson, Ayman Al Haj Zen, and Henning F. Horn. 2025. "The Role of Nesprin-4 in Breast Cancer Migration and Invasion" Cells 14, no. 19: 1484. https://doi.org/10.3390/cells14191484
APA StyleAl-Sammak, B. F., Yildiz Ozer, L., Fayed, H. S., Kafour, N. M., Ericsson, J., Al Haj Zen, A., & Horn, H. F. (2025). The Role of Nesprin-4 in Breast Cancer Migration and Invasion. Cells, 14(19), 1484. https://doi.org/10.3390/cells14191484








