Ashwagandha Root Extract Mitigates Fibromyalgia-like Symptoms via Neurochemical and Histological Modulation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Methanolic Extraction of Ashwagandha Roots
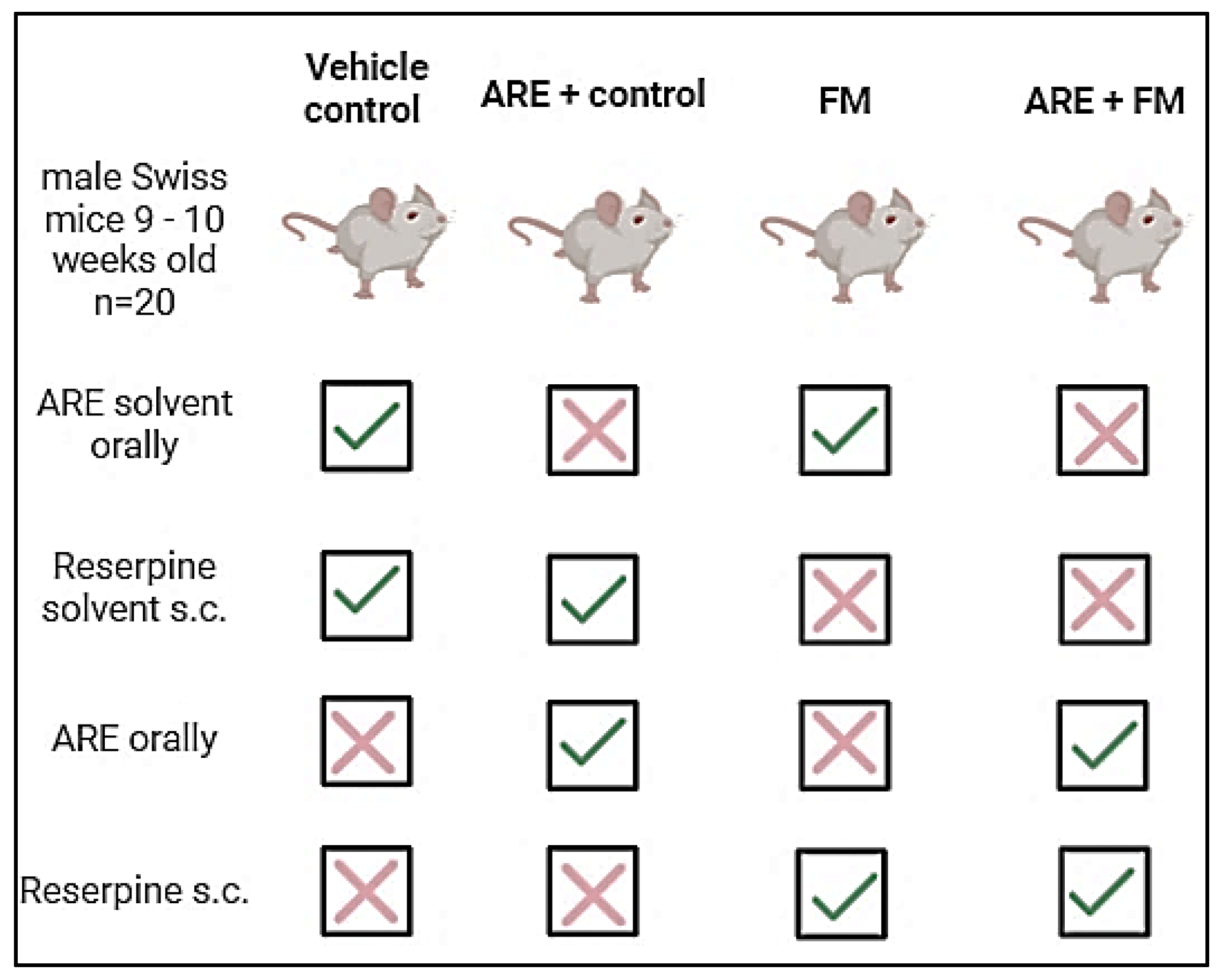
2.3. Experimental Design
2.4. Body Weight Assessment
2.5. Behavioral Assessments
2.5.1. Pain Behavior Evaluation
Von Frey Test
Hot Plate Test
2.5.2. Depression-like Behavior
Tail Suspension Test
Forced Swimming Test
Splash Test
2.5.3. Locomotor Activity
Open Field Test
Rotarod Test
Grip Strength Test
2.5.4. Spatial Working Memory
Spontaneous Alternation Test
2.6. Sampling
2.7. Histological Analysis
2.8. Biochemical Analysis
2.8.1. Serotonin and Norepinephrine Determination
2.8.2. Measurement of IL-1β and TNF-α Levels
2.8.3. Analysis of MDA and NO
2.9. Statistical Analysis
3. Results
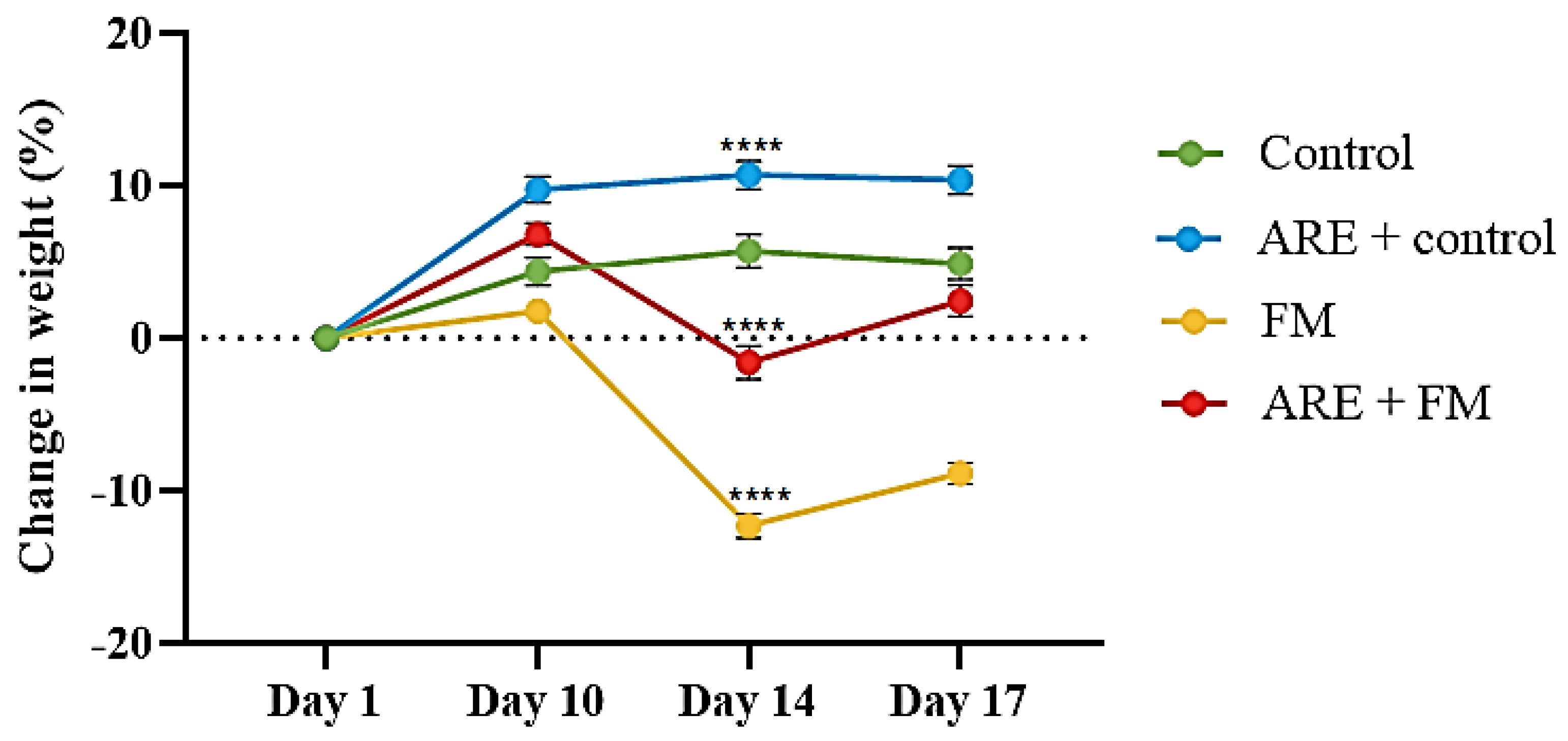
3.1. Effect of Ashwagandha Root Extract (ARE) on Body Weight
3.2. Behavioral Study
3.2.1. Mechanical Allodynia and Evoked Thermal Nociception in Mice
3.2.2. Antidepressant-like Effects of ARE
3.2.3. Effect of ARE on Motor Function
3.2.4. Effect of ARE on Spontaneous Alternation
3.3. Biochemical Results
3.3.1. Modulation of Serotonin and Norepinephrine Levels by ARE
3.3.2. Alterations in Brain IL-1β and TNF-α Levels
3.3.3. Alterations in MDA and NO Levels
3.3.4. Histological Alterations in the Hippocampus
3.3.5. Histological Alterations in the Thalamus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FMS | Fibromyalgia syndrome |
| FIQR | Fibromyalgia impact questionnaire revised |
| ARE | Ashwagandha |
| IL-1β | Interleukin-1 beta |
| TNFα | Tumor necrosis factor alpha |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| CRP | C-reactive protein |
| ROS | Reactive oxygen species |
| TST | Tail suspension test |
| FST | Forced swim test |
| ST | Splash test |
References
- Berwick, R.; Barker, C.; Goebel, A. The diagnosis of fibromyalgia syndrome. Clin. Med. J. R. Coll. Physicians Lond. 2022, 22, 570–574. [Google Scholar] [CrossRef]
- Brum, E.S.; Becker, G.; Fialho, M.F.P.; Oliveira, S.M. Animal models of fibromyalgia: What is the best choice? Pharmacol. Ther. 2022, 230, 107959. [Google Scholar] [CrossRef]
- Bawazir, Y. Prevalence of fibromyalgia syndrome in Saudi Arabia: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 1–9. [Google Scholar] [CrossRef]
- Siracusa, R.; Di Paola, R.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- García Rodríguez, D.F.; Abud Mendoza, C. Physiopathology of fibromyalgia. Reumatol. Clin. (Engl. Ed.) 2020, 16, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Priego, L.N.; Cueto-Ureña, C.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M. Fibromyalgia: A review of the patho-physiological mechanisms and multidisciplinary treatment strategies. Biomedicines 2024, 12, 1543. [Google Scholar] [CrossRef]
- Alfaro-Rodríguez, A.; Reyes-Long, S.; Roldan-Valadez, E.; González-Torres, M.; Bonilla-Jaime, H.; Bandala, C.; Avila-Luna, A.; Bueno-Nava, A.; Cabrera-Ruiz, E.; Sanchez-Aparicio, P.; et al. Association of the Serotonin and Kynurenine Pathways as Possible Therapeutic Targets to Modulate Pain in Patients with Fibromyalgia. Pharmaceuticals 2024, 17, 1205. [Google Scholar] [CrossRef] [PubMed]
- Loçasso, F.A.; Filho, H.A.; Alvarenga, R.M.P.; Schimidt, S.L.; Fiorelli, F.K.; Ramos, P.d.S.; Leidersnaider, S.C.L.; Blum, K.; Lewandrowski, K.-U.; Cunha-Junior, E.F.; et al. Assessing the Impact of IL-6 and Serotonin on Pain and Symptomatology in Fibromyalgia: An Exploratory Clinical Study. J. Pers. Med. 2024, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- O’mAhony, L.F.; Srivastava, A.; Mehta, P.; Ciurtin, C. Is fibromyalgia associated with a unique cytokine profile? A systematic review and meta-analysis. Rheumatology 2021, 60, 2602–2614. [Google Scholar] [CrossRef]
- Ernberg, M.; Christidis, N.; Ghafouri, B.; Bileviciute-Ljungar, I.; Löfgren, M.; Bjersing, J.; Palstam, A.; Larsson, A.; Mannerkorpi, K.; Gerdle, B.; et al. Plasma Cytokine Levels in Fibromyalgia and Their Response to 15 Weeks of Progressive Resistance Exercise or Relaxation Therapy. Mediat. Inflamm. 2018, 2018, 3985154. [Google Scholar] [CrossRef]
- González-Álvarez, M.E.; Riquelme-Aguado, V.; González-Pérez, Á.; Murillo-Llergo, R.; Manjón-Olmedillas, M.; Turroni, S.; Rossettini, G.; Villafañe, J.H. Association Between Systemic Neuroinflammation, Pain Perception and Clinical Status in Fibromyalgia Patients: Cross-Sectional Study. Cells 2024, 13, 1719. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Atamer, Y.; Sarac, S.; Asık, H.K.; Sahbaz, T. Serum paraoxonase activities, nitric oxide, and malondialdehyde levels are altered in patients with primary fibromyalgia syndrome. Ir. J. Med Sci. 2023, 192, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef]
- Zahiruddin, S.; Basist, P.; Parveen, A.; Parveen, R.; Khan, W.; Gaurav; Ahmad, S. Ashwagandha in brain disorders: A review of recent developments. J. Ethnopharmacol. 2020, 257, 112876. [Google Scholar] [CrossRef]
- Birla, H.; Keswani, C.; Rai, S.N.; Singh, S.S.; Zahra, W.; Dilnashin, H.; Rathore, A.S.; Singh, S.P. Neuroprotective effects of Withania somnifera in BPA induced-cognitive dysfunction and oxidative stress in mice. Behav. Brain Funct. 2019, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- AboTaleb, H.A.; Alturkistani, H.A.; El-Aziz, G.S.A.; Hindi, E.A.; Halawani, M.M.; Al-Thepyani, M.A.; Alghamdi, B.S. The Antinociceptive Effects and Sex-Specific Neurotransmitter Modulation of Metformin in a Mouse Model of Fibromyalgia. Cells 2024, 13, 1986. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cano, R.; Boivin, B.; Bullock, D.; Cornelissen, L.; Andrews, N.; Costigan, M. Up–Down Reader: An Open Source Program for Efficiently Processing 50% von Frey Thresholds. Front. Pharmacol. 2018, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Ashagrie, G.; Abebe, A.; Umer, S. Analgesic and Anti-Inflammatory Activities of 80% Methanol Extract and Solvent Fractions of Ehretia cymosa Thonn (Boraginaceae) Leaves in Rodents. J. Exp. Pharmacol. 2023, 15, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- AboTaleb, H.A.; Hindi, E.A.; El-Aziz, G.S.A.; Alturkistani, H.A.; Halawani, M.M.; Al-Thepyani, M.A.; Alghamdi, B.S. Evaluation of reserpine-induced fibromyalgia in mice: A comparative behavioral, neurochemical, and histological assessment of two doses. IBRO Neurosci. Rep. 2024. [Google Scholar] [CrossRef]
- Lefter, R.; Ciobica, A.; Timofte, D.; Ababei, D.; Dobrin, R.; Luca, A.; Trifan, A.; Stanciu, C.; Sfarti, C. A new biological approach in generating an irritable bowel syndrome rat model - focusing on depression in sucrose splash test and body weight change. Rom. Biotechnol. Lett. 2020, 25, 1554–1562. [Google Scholar] [CrossRef]
- Alzahrani, N.A.; Bahaidrah, K.A.; Mansouri, R.A.; Alsufiani, H.M.; Alghamdi, B.S. Investigation of the optimal dose for experimental lipopolysaccharide-induced recognition memory impairment: Behavioral and histological studies. J. Integr. Neurosci. 2022, 21, 49. [Google Scholar] [CrossRef]
- Lubrich, C.; Giesler, P.; Kipp, M. Motor Behavioral Deficits in the Cuprizone Model: Validity of the Rotarod Test Paradigm. Int. J. Mol. Sci. 2022, 23, 11342. [Google Scholar] [CrossRef]
- Owendoff, G.; Ray, A.; Bobbili, P.; Clark, L.; Baumann, C.W.; Clark, B.C.; Arnold, W.D. Optimization and construct validity of approaches to preclinical grip strength testing. J. Cachex Sarcopenia Muscle 2023, 14, 2439–2445. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Lee, Y.-B.; Lee, B.; Lee, D. A quantitative analysis of spontaneous alternation behaviors on a Y-maze reveals adverse effects of acute social isolation on spatial working memory. Sci. Rep. 2023, 13, 14722. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone of Elsevier: Philadelphia, PA, USA, 2013; pp. 172–186. ISBN 978-0-7020-5032-9. [Google Scholar]
- Treister-Goltzman, Y.; Peleg, R. Fibromyalgia and Mortality: A Systematic Review and Meta-Analysis. RMD Open 2023, 9, e003005. [Google Scholar] [CrossRef]
- Di Carlo, M.; Bianchi, B.; Salaffi, F.; Pellegrino, G.; Iannuccelli, C.; Giorgi, V.; Sarzi-Puttini, P. Fibromyalgia: One Year in Review 2024. Clin. Exp. Rheumatol. 2024, 42, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Bianchi, M.; Fear, E.J.; Giorgi, L.; Rossi, L. Management of Fibromyalgia: Novel Nutraceutical Therapies Beyond Traditional Pharmaceuticals. Nutrients 2025, 17, 530. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-K.; Kim, Y.R.; Kim, Y.H.; Yang, C.; Seo, C.-S.; Jung, I.C.; Jang, I.-S.; Kim, S.-H.; Lee, M.Y. Antidepressant-Like Effects of Gyejibokryeong-hwan in a Mouse Model of Reserpine-Induced Depression. BioMed Res. Int. 2018, 2018, 5845491. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Kaur, A.; Garg, S.; Singh, A.P.; Bhatti, R. Protective Effect of Esculetin, Natural Coumarin in Mice Model of Fibromyalgia: Targeting Pro-Inflammatory Cytokines and MAO-A. Neurochem. Res. 2020, 45, 2364–2374. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract. J. Evid.-Based Complement. Altern. Med. 2016, 22, 96–106. [Google Scholar] [CrossRef]
- Kaur, J.; Seshadri, S.; Golla, K.H.; Sampara, P. Efficacy and safety of standardized Ashwagandha (Withania somnifera) root extract on reducing stress and anxiety in domestic dogs: A randomized controlled trial. J. Veter. Behav. 2022, 51, 8–15. [Google Scholar] [CrossRef]
- Telega, L.M.; Berti, R.; Blazhenets, G.; Domogalla, L.-C.; Steinacker, N.; Omrane, M.A.; Meyer, P.T.; Coenen, V.A.; Eder, A.-C.; Döbrössy, M.D. Reserpine-induced rat model for depression: Behavioral, physiological and PET-based dopamine receptor availability validation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 133, 111013. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhong, Z.; Lu, S.; Zhang, Y. Repeated reserpine treatment induces depressive-like behaviors accompanied with hippocampal impairment and synapse deficit in mice. Brain Res. 2023, 1819, 148541. [Google Scholar] [CrossRef]
- KrishnaRaju, A.V.; Somepalli, V.; Thanawala, S.; Shah, R. Efficacy and Anti-Inflammatory Activity of Ashwagandha Sustained-Release Formulation on Depression and Anxiety Induced by Chronic Unpredictable Stress: In vivo and in vitro Studies. J. Exp. Pharmacol. 2023, 15, 291–305. [Google Scholar] [CrossRef]
- Arisha, S.M. Alpha-lipoic acid-role in improving both reserpine toxicity and paroxetine treatment in the cerebral cortex of albino rats; histological, ultrastructural, immunohistohemical and biochemical studies. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Coope, O.C.; Salguero, A.R.; Spurr, T.; Calvente, A.P.; Farre, A.D.; Fisas, E.J.; Lloyd, B.; Gooderick, J.; Sangrà, M.A.; Roman-Viñas, B. Effects of Root Extract of Ashwagandha (Withania somnifera) on Perception of Recovery and Muscle Strength in Female Athletes. Eur. J. Sport Sci. 2025, 25, e12265. [Google Scholar] [CrossRef]
- Noshahr, Z.S.; Shahraki, M.R.; Ahmadvand, H.; Nourabadi, D.; Nakhaei, A. Protective effects of Withania somnifera root on inflammatory markers and insulin resistance in fructose-fed rats. Rep. Biochem. Mol. Biol. 2015, 3, 62–67. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4757043/ (accessed on 1 September 2025).
- Trofin, D.-M.; Sardaru, D.-P.; Trofin, D.; Onu, I.; Tutu, A.; Onu, A.; Onită, C.; Galaction, A.I.; Matei, D.V. Oxidative Stress in Brain Function. Antioxidants 2025, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Al Sharie, S.; Varga, S.J.; Al-Husinat, L.; Sarzi-Puttini, P.; Araydah, M.; Bal’awi, B.R.; Varrassi, G. Unraveling the Complex Web of Fibromyalgia: A Narrative Review. Medicina 2024, 60, 272. [Google Scholar] [CrossRef]
- Mandela, P.; Chandley, M.; Xu, Y.-Y.; Zhu, M.-Y.; Ordway, G.A. Reserpine-induced reduction in norepinephrine transporter function requires catecholamine storage vesicles. Neurochem. Int. 2010, 56, 760–767. [Google Scholar] [CrossRef]
- Dawane, J.; Seok, S.; Dhande, P.; Langade, D.; Han, H.; Kim, S.-B.; Ju, J.-Y. Evaluation of the Anxiolytic and Antidepressant Effects of Standardized Ashwagandha (Withania somnifera) Root Extract in Wistar Rats. Prev. Nutr. Food Sci. 2024, 29, 414–421. [Google Scholar] [CrossRef]
- Szot, P.; White, S.S.; Greenup, J.L.; Leverenz, J.B.; Peskind, E.R.; Raskind, M.A. Compensatory Changes in the Noradrenergic Nervous System in the Locus Ceruleus and Hippocampus of Postmortem Subjects with Alzheimer’s Disease and Dementia with Lewy Bodies. J. Neurosci. 2006, 26, 467–478. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Fibromyalgia and Inflammation: Unrevealing the Connection. Cells 2025, 14, 271. [Google Scholar] [CrossRef]
- Davis, L.; Kuttan, G. Immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2000, 71, 193–200. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.M.; Lacerda, A.C.R.; Ribeiro, V.G.C.; Figueiredo, P.H.S.; Fonseca, S.F.; Lage, V.K.d.S.; Costa, H.S.; Lima, V.P.; Sañudo, B.; Bernardo-Filho, M.; et al. Oxidative Stress Biomarkers and Quality of Life Are Contributing Factors of Muscle Pain and Lean Body Mass in Patients with Fibromyalgia. Biology 2022, 11, 935. [Google Scholar] [CrossRef]
- Leon-Llamas, J.L.; Villafaina, S.; Murillo-Garcia, A.; Gusi, N. Impact of Fibromyalgia in the Hippocampal Subfields Volumes of Women—An MRI Study. Int. J. Environ. Res. Public Health 2021, 18, 1549. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.-S.; Han, K.; Sim, W.; Suh, H.J.; Ahn, Y. Ashwagandha (Withania somnifera (L.) dunal) root extract containing withanolide a alleviates depression-like behavior in mice by enhancing the brain-derived neurotrophic factor pathway under unexpected chronic mild stress. J. Ethnopharmacol. 2024, 340, 119224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, R.; Nazmi, A.; Lakhanpal, D.; Kataria, H.; Kaur, G. Glioprotective Effects of Ashwagandha Leaf Extract against Lead Induced Toxicity. BioMed Res. Int. 2014, 2014, 182029. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanyn, R.F.; Batawi, A.H.; AL-Thepyani, M.A.; Tash, R.; Almuhammadi, A.; Alsabban, A.H.; Alghamdi, B.S. Ashwagandha Root Extract Mitigates Fibromyalgia-like Symptoms via Neurochemical and Histological Modulation in Mice. Cells 2025, 14, 1478. https://doi.org/10.3390/cells14181478
Hasanyn RF, Batawi AH, AL-Thepyani MA, Tash R, Almuhammadi A, Alsabban AH, Alghamdi BS. Ashwagandha Root Extract Mitigates Fibromyalgia-like Symptoms via Neurochemical and Histological Modulation in Mice. Cells. 2025; 14(18):1478. https://doi.org/10.3390/cells14181478
Chicago/Turabian StyleHasanyn, Razan Fawaz, Ashwaq H. Batawi, Mona A. AL-Thepyani, Reham Tash, Asma Almuhammadi, Ashwaq Hassan Alsabban, and Badrah S. Alghamdi. 2025. "Ashwagandha Root Extract Mitigates Fibromyalgia-like Symptoms via Neurochemical and Histological Modulation in Mice" Cells 14, no. 18: 1478. https://doi.org/10.3390/cells14181478
APA StyleHasanyn, R. F., Batawi, A. H., AL-Thepyani, M. A., Tash, R., Almuhammadi, A., Alsabban, A. H., & Alghamdi, B. S. (2025). Ashwagandha Root Extract Mitigates Fibromyalgia-like Symptoms via Neurochemical and Histological Modulation in Mice. Cells, 14(18), 1478. https://doi.org/10.3390/cells14181478






