Dicer1 Depletion Leads to DNA Damage Accumulation and Cell Death in a RET/PTC3 Papillary Thyroid Cancer Mouse Model, Thereby Inhibiting Tumor Progression
Abstract
1. Introduction
2. Materials and Methods
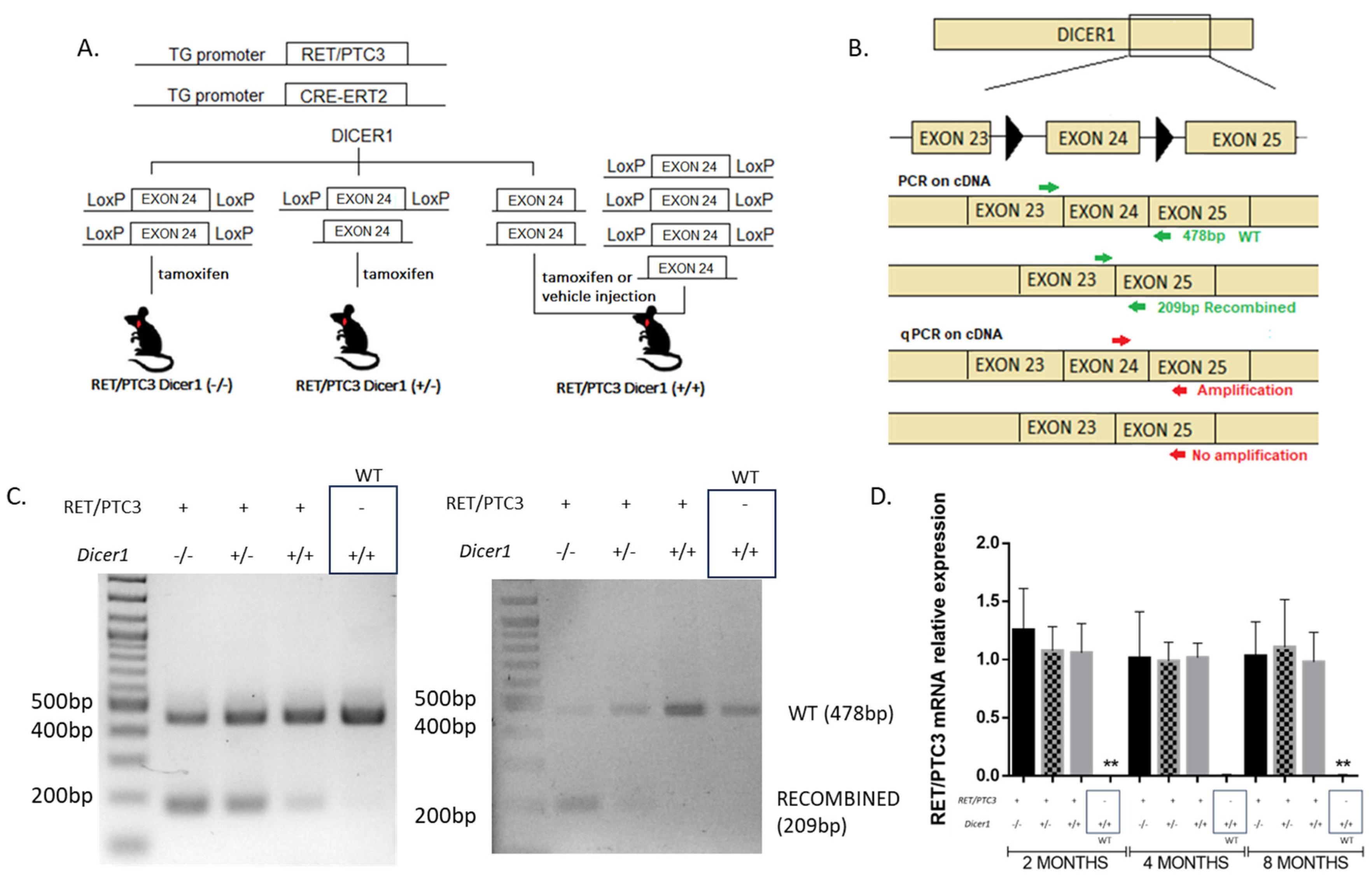
2.1. Generation of the RET/PTC3 Dicer1(−/−) or RET/PTC3 Dicer1(+/−) Mice and Tissue and Blood Collection
2.2. RNA Extraction and Quantitative PCR Amplification
2.3. Flow Cytometry Assays
2.4. Histology and Immunofluorescence
2.5. TUNEL Staining for DNA Fragmentation Detection
2.6. Statistical Analyses
3. Results
3.1. Cre-Mediated Recombination of Dicer1 in the Thyroid Is Confirmed
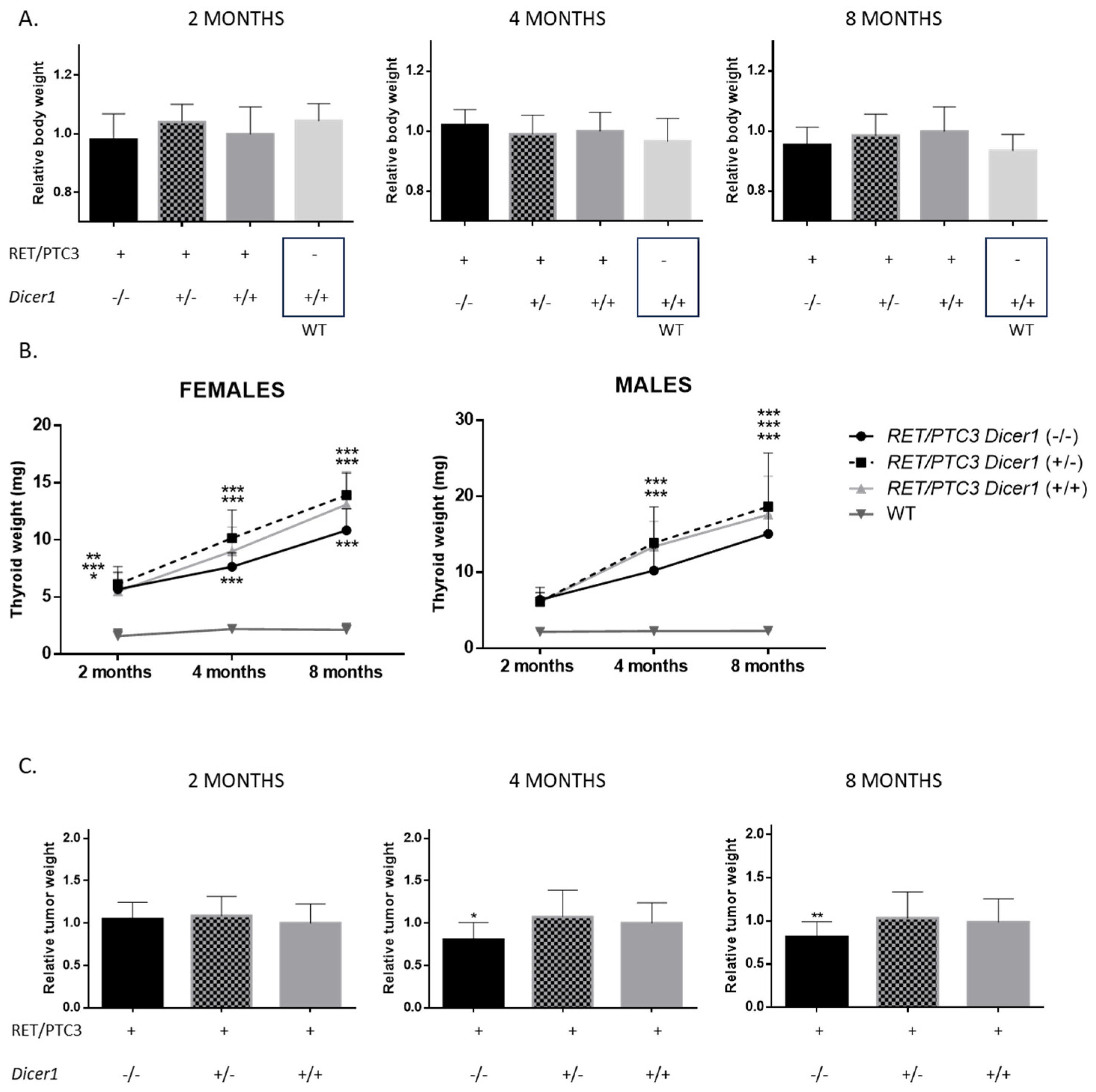
3.2. Total Dicer1 Inactivation Leads to Reduced Thyroid Tumor Size
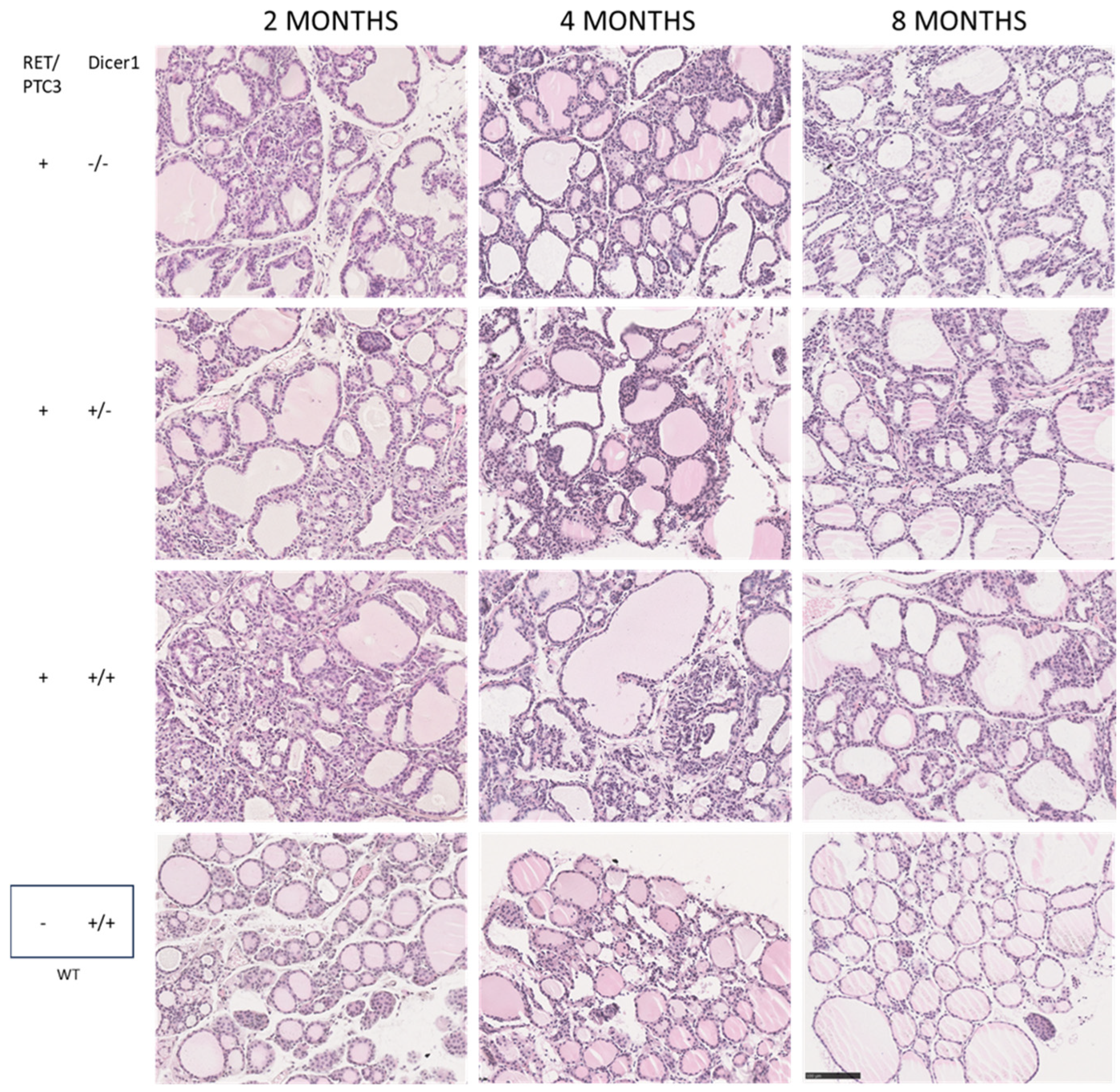
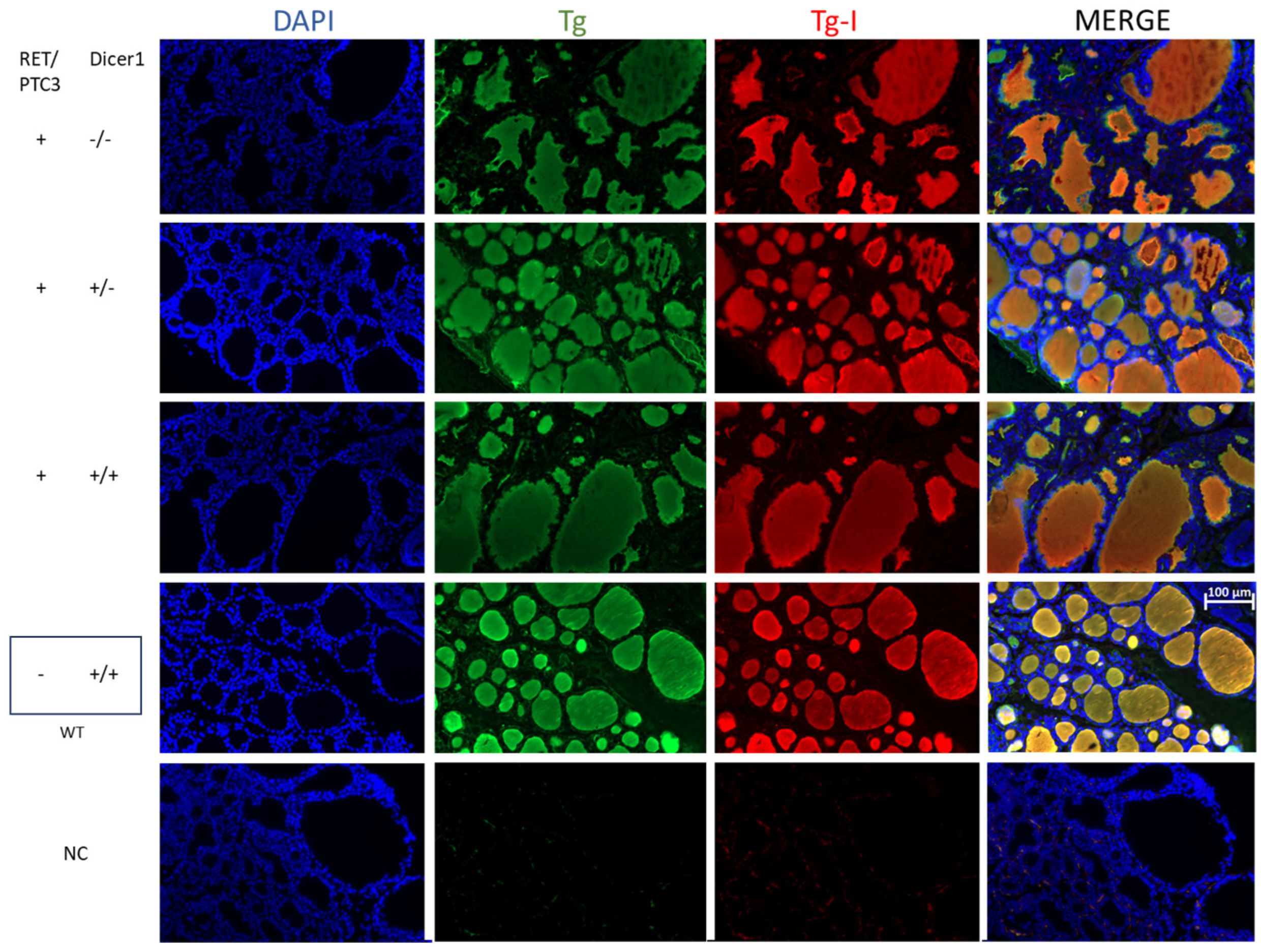
3.3. Although the Thyroid Follicular Structure Is Altered in Thyroid Tumors, Hormone Production Is Sustained
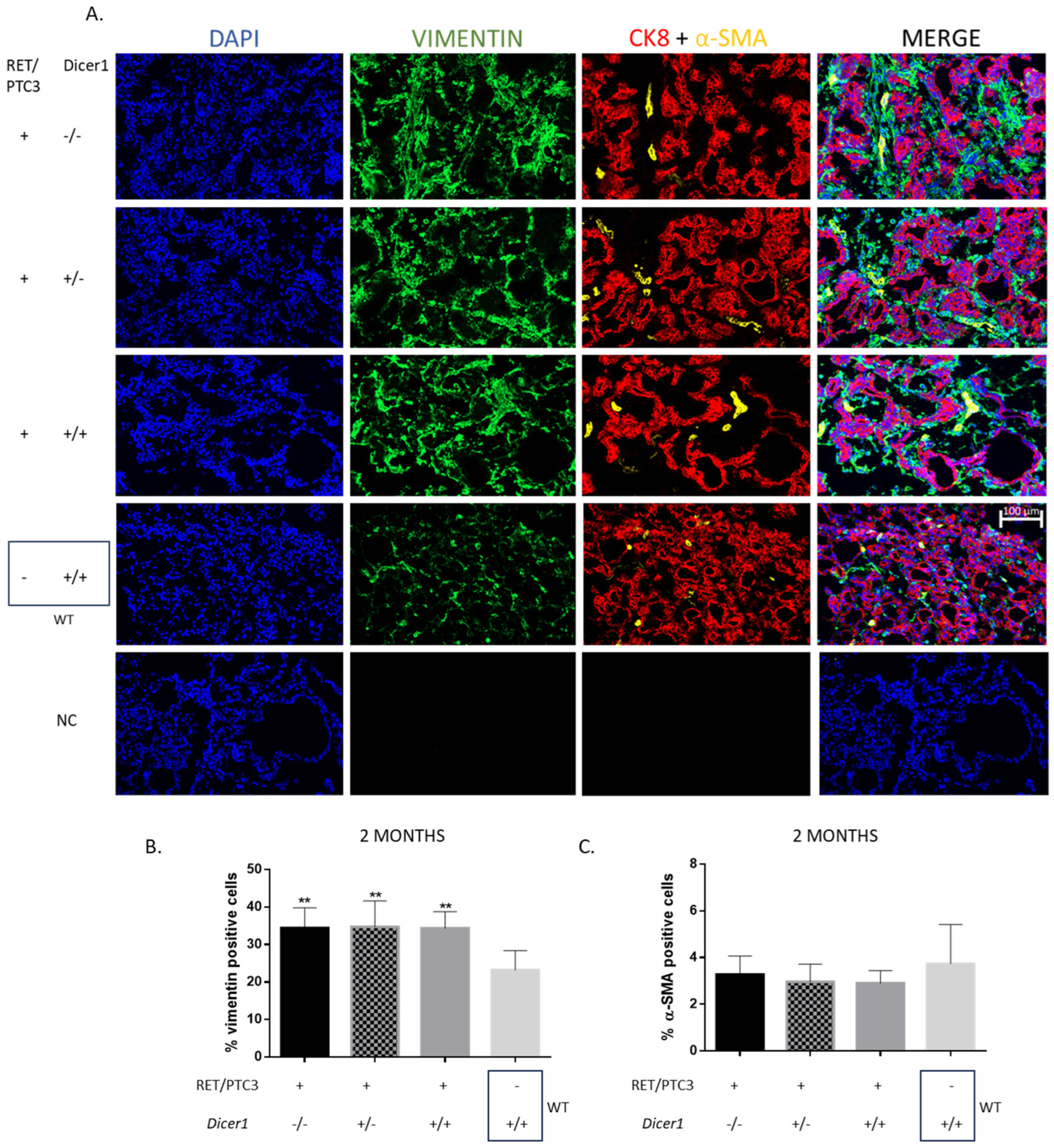
3.4. RET/PTC3 Tumors Show Increased Proportion of Vimentin-Positive Cells, Regardless of Dicer1 Inactivation
3.5. Dicer1 Inactivation Does Not Affect the Altered Thyroid-Specific or Redox Homeostasis Gene Expression Patterns Observed in RET/PTC3 Thyroid Tumors
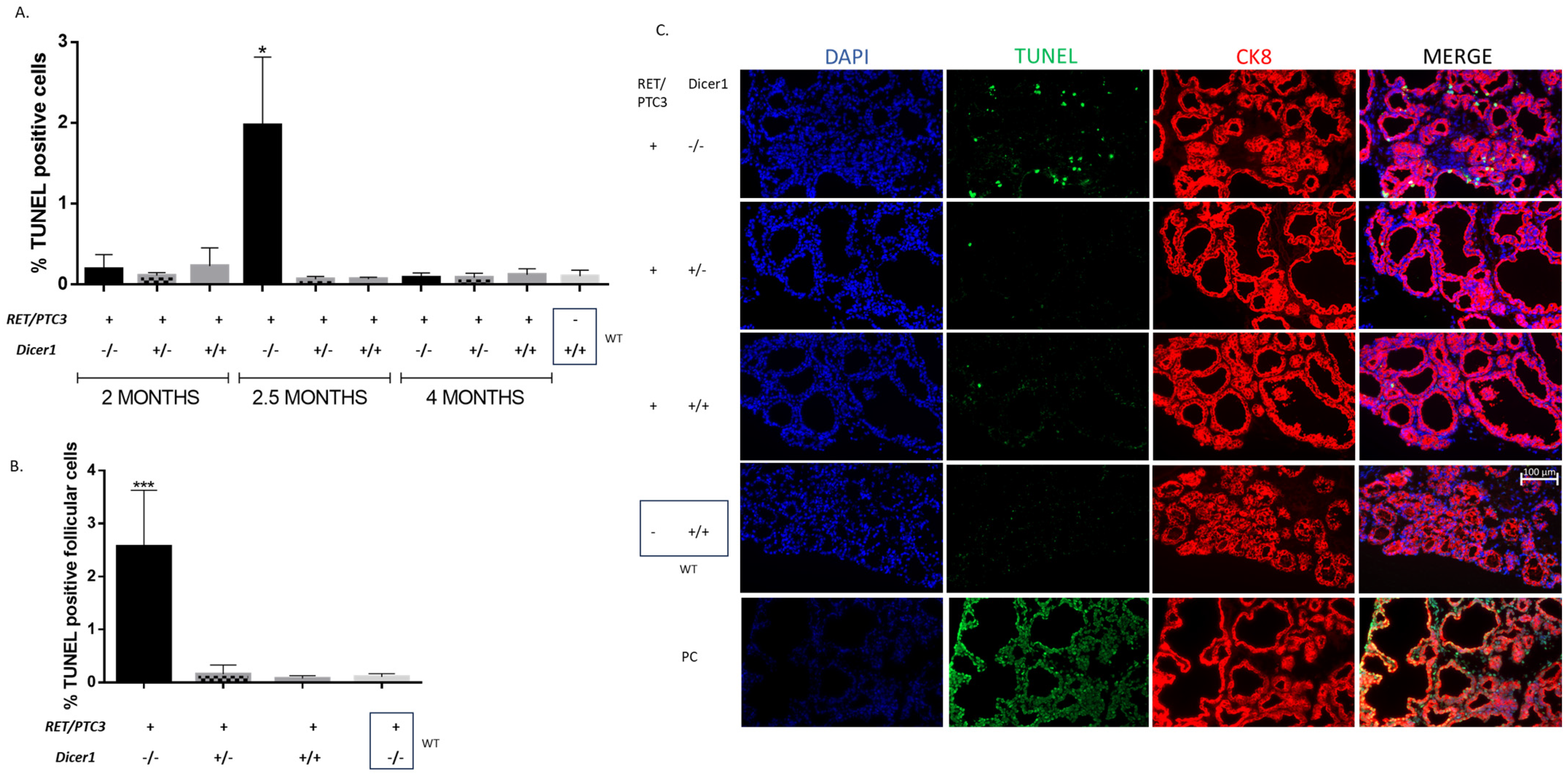
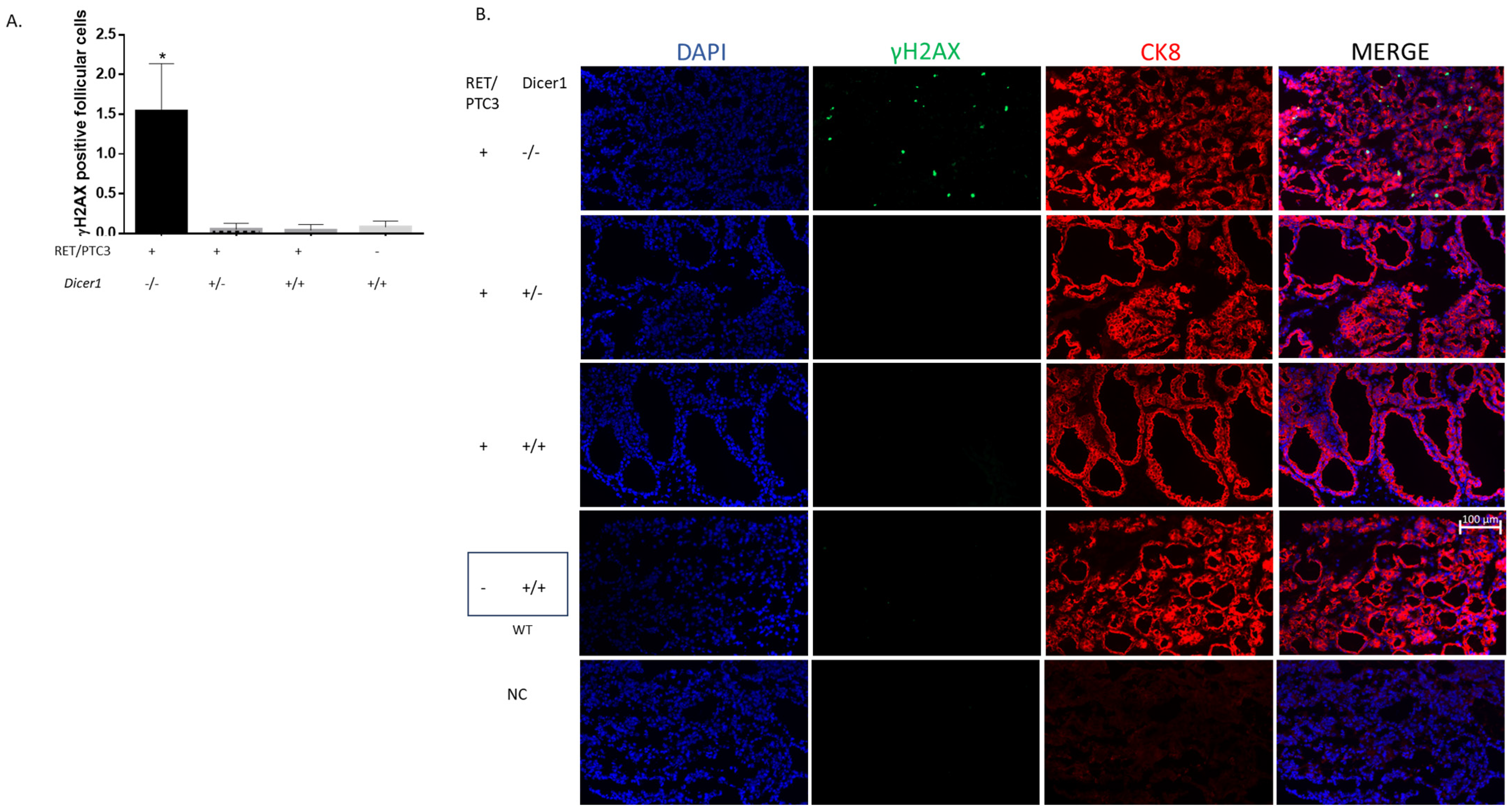
3.6. Dicer1(−/−) Tumor Cells Accumulate DNA Damage, Triggering Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiology Biomarkers Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Fiorentino, V.; Aquila, M.D.; Musarra, T.; Martini, M.; Capodimonti, S.; Fadda, G.; Curatolo, M.; Traini, E.; Raffaelli, M.; Lombardi, C.P.; et al. The Role of Cytology in the Diagnosis of Subcentimeter Thyroid Lesions. Diagnostics 2021, 11, 1043. [Google Scholar] [CrossRef]
- Forma, A.; Kłodnicka, K.; Pająk, W.; Flieger, J.; Teresińska, B.; Januszewski, J.; Baj, J. Thyroid Cancer: Epidemiology, Classification, Risk Factors, Diagnostic and Prognostic Markers, and Current Treatment Strategies. Int. J. Mol. Sci. 2025, 26, 5173. [Google Scholar] [CrossRef]
- Tarabichi, M.; Demetter, P.; Craciun, L.; Maenhaut, C.; Detours, V. Thyroid cancer under the scope of emerging technologies. Mol. Cell. Endocrinol. 2022, 541, 111491. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Dell’aQuila, M.; Granitto, A.; Martini, M.; Capodimonti, S.; Cocomazzi, A.; Musarra, T.; Fiorentino, V.; Pontecorvi, A.; Lombardi, C.P.; Fadda, G.; et al. PD-L1 and thyroid cytology: A possible diagnostic and prognostic marker. Cancer Cytopathol. 2019, 128, 177–189. [Google Scholar] [CrossRef] [PubMed]
- A Lebbink, C.; Links, T.P.; Czarniecka, A.; Dias, R.P.; Elisei, R.; Izatt, L.; Krude, H.; Lorenz, K.; Luster, M.; Newbold, K.; et al. 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur. Thyroid. J. 2022, 11. [Google Scholar] [CrossRef]
- Landa, I.; Cabanillas, M.E. Genomic alterations in thyroid cancer: Biological and clinical insights. Nat. Rev. Endocrinol. 2023, 20, 93–110. [Google Scholar] [CrossRef]
- Xing, M. BRAF mutation in thyroid cancer. Endocr.-Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef]
- Howell, G.M.; Hodak, S.P.; Yip, L. RAS Mutations in Thyroid Cancer. Oncol. 2013, 18, 926–932. [Google Scholar] [CrossRef]
- E Nikiforov, Y. RET/PTC Rearrangement in Thyroid Tumors. Endocr. Pathol. 2002, 13, 03–16. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M.; Carrington, J.C. Principles of MicroRNA–Target Recognition. PLOS Biol. 2005, 3, e85. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.M.; Mott, J.L. Overview of MicroRNA Biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef]
- Saiselet, M.; Gacquer, D.; Spinette, A.; Craciun, L.; Decaussin-Petrucci, M.; Andry, G.; Detours, V.; Maenhaut, C. New global analysis of the microRNA transcriptome of primary tumors and lymph node metastases of papillary thyroid cancer. BMC Genom. 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Taheri, M. The role of microRNAs in the pathogenesis of thyroid cancer. Non-coding RNA Res. 2020, 5, 88–98. [Google Scholar] [CrossRef]
- Papaioannou, M.; Chorti, A.G.; Chatzikyriakidou, A.; Giannoulis, K.; Bakkar, S.; Papavramidis, T.S. MicroRNAs in Papillary Thyroid Cancer: What Is New in Diagnosis and Treatment. Front. Oncol. 2022, 11, 755097. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Priest, J.R.; Duchaine, T.F. DICER1: Mutations, microRNAs and mechanisms. Nat. Rev. Cancer 2014, 14, 662–672. [Google Scholar] [CrossRef]
- Francia, S.; Michelini, F.; Saxena, A.; Tang, D.; de Hoon, M.; Anelli, V.; Mione, M.; Carninci, P.; di Fagagna, F.D. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 2012, 488, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ba, Z.; Gao, M.; Wu, Y.; Ma, Y.; Amiard, S.; White, C.I.; Danielsen, J.M.R.; Yang, Y.-G.; Qi, Y. A Role for Small RNAs in DNA Double-Strand Break Repair. Cell 2012, 149, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Francia, S.; Cabrini, M.; Matti, V.; Oldani, A.; di Fagagna, F.D. DICER, DROSHA and DNA damage response RNAs are necessary for the secondary recruitment of DNA damage response factors. J. Cell Sci. 2016, 129, 1468–1476. [Google Scholar] [CrossRef]
- Undeutsch, H.; Löf, C.; Pakarinen, P.; Poutanen, M.; Kero, J. Thyrocyte-Specific Dicer1 Deficiency Alters Thyroid Follicular Organization and Prevents Goiter Development. Endocrinology 2015, 156, 1590–1601. [Google Scholar] [CrossRef] [PubMed]
- Frezzetti, D.; Reale, C.; Calì, G.; Nitsch, L.; Fagman, H.; Nilsson, O.; Scarfò, M.; De Vita, G.; Di Lauro, R.; Pfeffer, S. The microRNA-Processing Enzyme Dicer Is Essential for Thyroid Function. PLOS ONE 2011, 6, e27648. [Google Scholar] [CrossRef]
- Rodriguez, W.; Jin, L.; Janssens, V.; Pierreux, C.; Hick, A.-C.; Urizar, E.; Costagliola, S.; Pfeffer, S. Deletion of the RNaseIII Enzyme Dicer in Thyroid Follicular Cells Causes Hypothyroidism with Signs of Neoplastic Alterations. PLOS ONE 2012, 7, e29929. [Google Scholar] [CrossRef]
- Swahari, V.; Nakamura, A.; Deshmukh, M. The paradox of dicer in cancer. Mol. Cell. Oncol. 2016, 3, e1155006. [Google Scholar] [CrossRef]
- Caroleo, A.M.; De Ioris, M.A.; Boccuto, L.; Alessi, I.; Del Baldo, G.; Cacchione, A.; Agolini, E.; Rinelli, M.; Serra, A.; Carai, A.; et al. DICER1 Syndrome and Cancer Predisposition: From a Rare Pediatric Tumor to Lifetime Risk. Front. Oncol. 2021, 10, e614541. [Google Scholar] [CrossRef]
- Kock, L.; Wu, M.K.; Foulkes, W.D. Ten years of DICER1 mutations: Provenance, distribution, and associated phenotypes. Hum. Mutat. 2019, 40, 1939–1953. [Google Scholar] [CrossRef]
- Karube, Y.; Tanaka, H.; Osada, H.; Tomida, S.; Tatematsu, Y.; Yanagisawa, K.; Yatabe, Y.; Takamizawa, J.; Miyoshi, S.; Mitsudomi, T.; et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 2005, 96, 111–115. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Sun, X.-J.; Fu, W.-N.; Guan, Y.; Gao, F.; Wang, Y.; Sun, K.-L. Decreased expression of DICER1 in gastric cancer. Chin. Med J. 2007, 120, 2099–2104. [Google Scholar] [CrossRef]
- Pampalakis, G.; Diamandis, E.P.; Katsaros, D.; Sotiropoulou, G. Down-regulation of dicer expression in ovarian cancer tissues. Clin. Biochem. 2010, 43, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Erler, P.; Keutgen, X.M.; Crowley, M.J.; Zetoune, T.; Kundel, A.; Kleiman, D.; Beninato, T.; Scognamiglio, T.; Elemento, O.; Zarnegar, R.; et al. Dicer expression and microRNA dysregulation associate with aggressive features in thyroid cancer. Surgery 2014, 156, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, G.R.V.; Ribeiro, T.C.; Faria, A.M.; Mariani, B.M.P.; Lerario, A.M.; Zerbini, M.C.N.; Soares, I.C.; Wakamatsu, A.; Alves, V.A.F.; Mendonca, B.B.; et al. Low DICER1 expression is associated with poor clinical outcome in adrenocortical carcinoma. Oncotarget 2015, 6, 22724–22733. [Google Scholar] [CrossRef] [PubMed]
- Ravi, A.; Gurtan, A.M.; Kumar, M.S.; Bhutkar, A.; Chin, C.; Lu, V.; Lees, J.A.; Jacks, T.; Sharp, P.A. Proliferation and Tumorigenesis of a Murine Sarcoma Cell Line in the Absence of DICER1. Cancer Cell 2012, 21, 848–855. [Google Scholar] [CrossRef]
- Kumar, M.S.; Pester, R.E.; Chen, C.Y.; Lane, K.; Chin, C.; Lu, J.; Kirsch, D.G.; Golub, T.R.; Jacks, T. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009, 23, 2700–2704. [Google Scholar] [CrossRef]
- Lambertz, I.; Nittner, D.; Mestdagh, P.; Denecker, G.; Vandesompele, J.; A Dyer, M.; Marine, J.-C. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2009, 17, 633–641. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Otsuka, M.; Kishikawa, T.; Takata, A.; Ohno, M.; Shibata, C.; Kang, Y.J.; Yoshida, H.; Koike, K.; Wang, Y. Unique Haploinsufficient Role of the MicroRNA-Processing Molecule Dicer1 in a Murine Colitis-Associated Tumorigenesis Model. PLOS ONE 2013, 8, e71969. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, H.; Zhang, L.; Dakhova, O.; Zhang, Y.; Lewis, M.T.; Creighton, C.J.; Ittmann, M.M.; Xin, L. A dosage-dependent pleiotropic role of Dicer in prostate cancer growth and metastasis. Oncogene 2013, 33, 3099–3108. [Google Scholar] [CrossRef]
- Arrate, M.P.; Vincent, T.; Odvody, J.; Kar, R.; Jones, S.N.; Eischen, C.M. MicroRNA Biogenesis Is Required for Myc-Induced B-Cell Lymphoma Development and Survival. Cancer Res. 2010, 70, 6083–6092. [Google Scholar] [CrossRef]
- Hanna, J.A.; Drummond, C.J.; Garcia, M.R.; Go, J.C.; Finkelstein, D.; Rehg, J.E.; Hatley, M.E. Biallelic Dicer1 Loss Mediated by aP2-Cre Drives Angiosarcoma. Cancer Res. 2017, 77, 6109–6118. [Google Scholar] [CrossRef]
- Powell, D.J., Jr.; Russell, J.; Nibu, K.; Li, G.; Rhee, E.; Liao, M.; Goldstein, M.; Keane, W.M.; Santoro, M.; Fusco, A.; et al. The RET/PTC3 oncogene: Metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res. 1998, 58, 5523–5528. [Google Scholar] [PubMed]
- Undeutsch, H.; Löf, C.; Offermanns, S.; Kero, J. A mouse model with tamoxifen-inducible thyrocyte-specific cre recombinase activity. Genesis 2014, 52, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Harfe, B.D.; McManus, M.T.; Mansfield, J.H.; Hornstein, E.; Tabin, C.J. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl. Acad. Sci. USA 2005, 102, 10898–10903. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, H.; De Deken, X.; Mayo, O.B.; Raad, H.; Weiss, M.; Liao, X.-H.; Refetoff, S. Mice Deficient in Dual Oxidase Maturation Factors Are Severely Hypothyroid. Mol. Endocrinol. 2012, 26, 481–492. [Google Scholar] [CrossRef]
- Delys, L.; Detours, V.; Franc, B.; Thomas, G.; Bogdanova, T.; Tronko, M.; Libert, F.; E Dumont, J.; Maenhaut, C. Gene expression and the biological phenotype of papillary thyroid carcinomas. Oncogene 2007, 26, 7894–7903. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Burniat, A.; Jin, L.; Detours, V.; Driessens, N.; Goffard, J.-C.; Santoro, M.; Rothstein, J.; Dumont, J.E.; Miot, F.; Corvilain, B. Gene Expression in RET/PTC3 and E7 Transgenic Mouse Thyroids: RET/PTC3 But Not E7 Tumors Are Partial and Transient Models of Human Papillary Thyroid Cancers. Endocrinology 2008, 149, 5107–5117. [Google Scholar] [CrossRef]
- Jin, L.; Burniat, A.; Dumont, J.-E.; Miot, F.; Corvilain, B.; Franc, B. Human thyroid tumours, the puzzling lessons from E7 and RET/PTC3 transgenic mice. Br. J. Cancer 2008, 99, 1874–1883. [Google Scholar] [CrossRef][Green Version]
- Maenhaut, C.; Christophe, D.; Vassart, G. Ontogeny, anatomy, metabolism and physiology of the thyroid. In Thyroid Disease Manager; De Groot, L.J., Ed.; Endocrine Education Inc.: Washington, DC, USA, 2015; Chapter 2. [Google Scholar][Green Version]
- Rojo-Pardillo, M.; Godefroid, L.; Dom, G.; Lefort, A.; Libert, F.; Robaye, B.; Maenhaut, C. Understanding the Dosage-Dependent Role of Dicer1 in Thyroid Tumorigenesis. Int. J. Mol. Sci. 2024, 25, 10701. [Google Scholar] [CrossRef]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front. Oncol. 2022, 12, 862743. [Google Scholar] [CrossRef]
- Boiteux, S.; Radicella, J. The Human OGG1 Gene: Structure, Functions, and Its Implication in the Process of Carcinogenesis. Arch. Biochem. Biophys. 2000, 377, 1–8. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Doroshow, J.H.; Chu, F.F. The beginning of GPX2 and 30 years later. Free Radic Biol Med. 2022, 188, 419–433. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Merritt, W.M.; Lin, Y.G.; Han, L.Y.; Kamat, A.A.; Spannuth, W.A.; Schmandt, R.; Urbauer, D.; Pennacchio, L.A.; Cheng, J.-F.; Nick, A.M.; et al. Dicer, Drosha, and Outcomes in Patients with Ovarian Cancer. New Engl. J. Med. 2008, 359, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Khoshnaw, S.M.; Rakha, E.A.; Abdel-Fatah, T.M.; Nolan, C.C.; Hodi, Z.; Macmillan, D.R.; Ellis, I.O.; Green, A.R. Loss of Dicer expression is associated with breast cancer progression and recurrence. Breast Cancer Res. Treat. 2012, 135, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Theotoki, E.I.; Pantazopoulou, V.I.; Georgiou, S.; Kakoulidis, P.; Filippa, V.; Stravopodis, D.J.; Anastasiadou, E. Dicing the Disease with Dicer: The Implications of Dicer Ribonuclease in Human Pathologies. Int. J. Mol. Sci. 2020, 21, 7223. [Google Scholar] [CrossRef]
- Song, A.J.; Palmiter, R.D. Detecting and Avoiding Problems When Using the Cre–lox System. Trends Genet. 2018, 34, 333–340. [Google Scholar] [CrossRef]
- Durante, C.; Puxeddu, E.; Ferretti, E.; Morisi, R.; Moretti, S.; Bruno, R.; Barbi, F.; Avenia, N.; Scipioni, A.; Verrienti, A.; et al. BRAF Mutations in Papillary Thyroid Carcinomas Inhibit Genes Involved in Iodine Metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2840–2843. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Magdeldin, T.; Stamati, K.; Nyga, A.; Loizidou, M.; Emberton, M.; Cheema, U. Cancer-associated fibroblasts mediate cancer progression and remodel the tumouroid stroma. Br. J. Cancer 2020, 123, 1178–1190. [Google Scholar] [CrossRef]
- Moyer, A.; Tanaka, K.; Cheng, E.H. Apoptosis in Cancer Biology and Therapy. Annu. Rev. Pathol. Mech. Dis. 2025, 20, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2022, 28, 20–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, J.A.; Schandl, C.A.; Young, K.K.; Vesely, J.; Willingham, M.C. Major DNA Fragmentation Is a Late Event in Apoptosis. J. Histochem. Cytochem. 1997, 45, 923–934. [Google Scholar] [CrossRef]
- Driessens, N.; Versteyhe, S.; Ghaddhab, C.; Burniat, A.; De Deken, X.; Van Sande, J.; Dumont, J.-E.; Miot, F.; Corvilain, B. Hydrogen peroxide induces DNA single- and double-strand breaks in thyroid cells and is therefore a potential mutagen for this organ. Endocrine-Related Cancer 2009, 16, 845–856. [Google Scholar] [CrossRef]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.-C.; et al. Roles of Hydrogen Peroxide in Thyroid Physiology and Disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef]
- Lyle, S.; Hoover, K.; Colpan, C.; Zhu, Z.; Matijasevic, Z.; Jones, S.N.; Dadras, S.S. Dicer Cooperates with p53 to Suppress DNA Damage and Skin Carcinogenesis in Mice. PLOS ONE 2014, 9, e100920. [Google Scholar] [CrossRef][Green Version]
- Swahari, V.; Nakamura, A.; Baran-Gale, J.; Garcia, I.; Crowther, A.J.; Sons, R.; Gershon, T.R.; Hammond, S.; Sethupathy, P.; Deshmukh, M. Essential Function of Dicer in Resolving DNA Damage in the Rapidly Dividing Cells of the Developing and Malignant Cerebellum. Cell Rep. 2016, 14, 216–224. [Google Scholar] [CrossRef]
- Jing, X.; Arya, V.; Reynolds, K.S.; Rogers, H. Clinical Pharmacology of RNA Interference-Based Therapeutics: A Summary Based on Food and Drug Administration-Approved Small Interfering RNAs. Drug Metab. Dispos. 2023, 51, 193–198. [Google Scholar] [CrossRef]
- Knauf, J.A.; Ma, X.; Smith, E.P.; Zhang, L.; Mitsutake, N.; Liao, X.-H.; Refetoff, S.; Nikiforov, Y.E.; Fagin, J.A. Targeted Expression of BRAFV600E in Thyroid Cells of Transgenic Mice Results in Papillary Thyroid Cancers that Undergo Dedifferentiation. Cancer Res. 2005, 65, 4238–4245. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Pardillo, M.; Augenlicht, A.; Dom, G.; Kero, J.; Robaye, B.; Maenhaut, C. Dicer1 Depletion Leads to DNA Damage Accumulation and Cell Death in a RET/PTC3 Papillary Thyroid Cancer Mouse Model, Thereby Inhibiting Tumor Progression. Cells 2025, 14, 1465. https://doi.org/10.3390/cells14181465
Rojo-Pardillo M, Augenlicht A, Dom G, Kero J, Robaye B, Maenhaut C. Dicer1 Depletion Leads to DNA Damage Accumulation and Cell Death in a RET/PTC3 Papillary Thyroid Cancer Mouse Model, Thereby Inhibiting Tumor Progression. Cells. 2025; 14(18):1465. https://doi.org/10.3390/cells14181465
Chicago/Turabian StyleRojo-Pardillo, Maria, Alice Augenlicht, Geneviève Dom, Jukka Kero, Bernard Robaye, and Carine Maenhaut. 2025. "Dicer1 Depletion Leads to DNA Damage Accumulation and Cell Death in a RET/PTC3 Papillary Thyroid Cancer Mouse Model, Thereby Inhibiting Tumor Progression" Cells 14, no. 18: 1465. https://doi.org/10.3390/cells14181465
APA StyleRojo-Pardillo, M., Augenlicht, A., Dom, G., Kero, J., Robaye, B., & Maenhaut, C. (2025). Dicer1 Depletion Leads to DNA Damage Accumulation and Cell Death in a RET/PTC3 Papillary Thyroid Cancer Mouse Model, Thereby Inhibiting Tumor Progression. Cells, 14(18), 1465. https://doi.org/10.3390/cells14181465






