Abstract
Hereditary spastic paraplegia (HSP) and hereditary ataxias (HA) are clinically and genetically heterogeneous neurodegenerative disorders that primarily affect motor coordination and neural integrity. Despite distinct pathological features, such as pyramidal tract degeneration in HSP and spinocerebellar pathway involvement in HA, these conditions share overlapping genetic pathways and mechanisms. The fruit fly Drosophila melanogaster has emerged as a powerful model organism for investigating the molecular basis of rare diseases, including HSP and HA. Its genetic tractability, rapid life cycle, and high degree of gene conservation with humans make it a cost-effective and ethically viable platform for disease modelling. In this review, we provide a comprehensive overview of Drosophila-based models for HSP and HA. We highlight the use of advanced genetic tools, including RNA interference, CRISPR/Cas9, and the GAL4/UAS system, as well as behavioral and neuroanatomical assays to model disease features. Furthermore, we discuss the application of genetic “avatars” and high-throughput drug screening platforms to test therapeutic candidates. Collectively, these models have deepened our understanding of the pathophysiology of HSP and HA, offering valuable insights for the development of targeted therapies and approaches to personalized medicine.
1. Introduction
The term hereditary spastic paraplegia (HSP) refers to a clinically and genetically heterogeneous group of neurodegenerative disorders characterized by progressive spasticity and weakness of the lower limbs [1]. Most patients exhibit the same clinical features, including bilateral spasticity of the legs, particularly evident while walking, muscle weakness, hypertone in the lower limbs, hyperreflexia, and a bilateral Babinski sign [2]. Post-mortem analyses conducted on patient tissues have revealed the presence of axonal degeneration that begins at the distal end and slowly progresses toward the cell body; this phenomenon is why these conditions are often referred to as “dying-back” axonopathies [3]. In complicated forms, alterations may also affect other brain structures, such as the cerebellum, cerebral cortex, basal ganglia, and white matter [4]. Based on symptomatology HSP can be classified as either pure or complicated. It is defined as pure if these symptoms represent the only observed clinical manifestations, whereas it is considered complicated when additional neurological or extra-neurological symptoms are present. These may include cognitive/mental impairment, cerebellar ataxia, peripheral neuropathy, epilepsy, optic atrophy, retinal alterations, cataracts, dystonia, and hypokinetic movement disorders [5]. The current genetic classification of the different forms of HSP is based on the type of inheritance, chromosomal locus, and the responsible mutation. HSP can be inherited in an autosomal dominant (AD), autosomal recessive (AR), X-linked, or maternal (mitochondrial) pattern [1]. The prevalence of AD HSP has been reported to range between 0.5 and 5.5 cases per 100,000 individuals. In contrast, AR HSP prevalence varies from 0.3 to 5.3 per 100,000 in hospital-based cohorts, and between 0.6 and 2.6 per 100,000 in studies incorporating multiple data sources. X-linked and mitochondrial forms of HSP are relatively rare and are predominantly associated with congenital presentations [6]. HSP and hereditary ataxia (HA) share overlapping clinical and genetic features, as both involve progressive neurodegeneration affecting motor pathways including the corticospinal tracts and cerebellum; furthermore, certain genes can cause phenotypes spanning from spastic paraplegia to cerebellar ataxia, reflecting a disease spectrum with shared molecular mechanisms (Figure 1). However, in HSP there is degeneration of the pyramidal tract, while in HA there is progressive degeneration of the spinocerebellar tract and Purkinje cells [7]. Furthermore, HSP and HA share pathological mechanisms and cellular pathways [8].
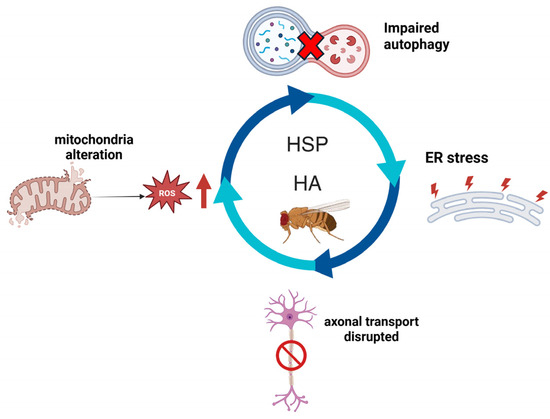
Figure 1.
The figure shows the main molecular pathways shared by HSP and HA and conserved in Drosophila model. Created with Biorender.com.
Regarding main features in HA, patients show a progressive loss of coordination and movement ability, as well as limited eye movement [9]. The classification of HA is based on the mode of transmission and is divided into autosomal dominant cerebellar ataxia, autosomal recessive, X-linked HA, and ataxias with mitochondrial gene mutations [10]. The AD forms are characterized by trinucleotide repeat expansions, the most common being the CAG triplet expansions within the coding region of the gene [9]. Among the AR forms, Friedreich’s ataxia (FRDA) is the most common, with a population frequency of 1–2:50,000 [9,10,11]. Patients with the X-linked form present with early onset, dysmetria, and dysdiadochokinesia. Progressive forms of ataxia can also be often associated with mitochondrial dysfunction [12]. The Drosophila melanogaster model is a powerful tool to study genetically and clinically heterogeneous disorders. It is widely used to investigate rare human diseases and to assess the pathogenicity of specific genetic variants [13]. Drosophila is a small fly belonging to the order Diptera and the family Drosophilidae, commonly known as the fruit fly [14]. This small invertebrate has led to many important discoveries in the field of biology over the years, and it is still used today as a model organism in over 1800 laboratories worldwide, thanks to numerous seminal work that make it ideal for research (Figure 2). It is small, easy to handle, and cost-effective to maintain in the laboratory [14]. It has a short lifespan, a high reproductive capacity, and an external development, allowing researchers to monitor each stage of its growth. Additionally, its genome has been fully sequenced, and homologs have been identified by nearly 75% of genes associated with human diseases. With the combination of genome-wide genetic screening, advanced sequencing techniques (such as RNA-seq and ChIP-seq), and metabolomics analyses, Drosophila has become a widely used model for studying human diseases, including those affecting the central and peripheral nervous systems [15]. Despite significant differences in the overall anatomy of Drosophila and human brains, they share many conserved genetic, cellular, electrophysiological, and chemical characteristics. Like vertebrates, fruit flies rely on a diverse range of neuronal types to process information [16].
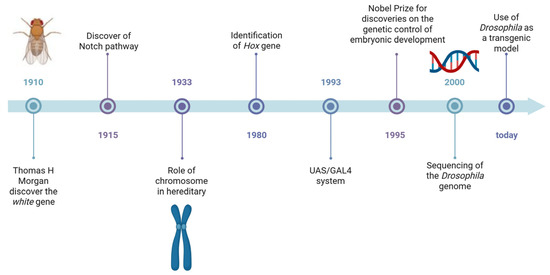
Figure 2.
Timeline of major discoveries made using Drosophila as animal model. Created with Biorender.com.
2. Modelling Diseases in Drosophila
Two alternative techniques can be used to study diseases like HA and HSP in Drosophila: forward genetics and reverse genetics. The term forward genetics refers to a technique that involves generating random mutations, with flies then identified by analyzing their phenotype [16]. These mutations can be induced using chemical or insertional mutagenesis techniques [17]. Reverse genetics begins with a gene of interest (such as a Drosophila homolog of a human disease gene) and tests its function by disrupting or modifying it. Unlike forward genetics, which proceed from phenotype to gene, reverse genetics follow the opposite logic, moving from gene to phenotype. A variety of genetic tools are available to manipulate gene function in Drosophila [15]. One widely used approach is the CRISPR/Cas9 system, which enables targeted gene knockout or the introduction of loss-of-function mutations, thereby allowing replication of causative variants identified in human disorders. Alternatively, researchers can employ transposon-mediated mutagenesis or excision of existing transposable elements. Another key methodology is the GAL4-UAS system, a binary expression tool that permits spatial and temporal control of transgene expression. This system can be employed to ectopically express either wild-type or mutant genes, or to drive RNA interference (RNAi) constructs placed under the UAS promoter. In the latter case, GAL4-driven expression of UAS-RNAi transgenes results in gene silencing and facilitates the generation of loss-of-function phenotypes. Such RNAi-mediated downregulation has been extensively used to model HSPs in Drosophila, as it enables the reduction in target gene expression in specific tissues or developmental stages [18]. Similarly, models of HAs often exploit the GAL4-UAS system to achieve either RNAi-mediated knockdown or the overexpression of mutant proteins, thereby recapitulating both loss-of-function and gain-of-function mechanisms [19]. Together, these complementary genetic strategies provide powerful means to model disease phenotypes in vivo and to investigate the molecular and cellular consequences of gene inactivation or toxic protein expression. These models exhibit various phenotypes, including motor and locomotion deficits. They also show alterations at the neuromuscular junction (NMJ), such as changes in synaptic bouton number, size, and branching, as well as functional impairments in synaptic transmissions. These motor alterations can be easily analyzed in Drosophila using techniques that assess movement ability, such as the climbing assay, crawling assay, and NMJ assay. In the case of the climbing assay, adult flies are used; they are placed in a tube, gently tapped to the bottom, and their ability to climb up the walls above a mark (usually at 6 cm from the bottom) within a defined time interval (usually 8–10 s) is measured. A reduction in performance is indicative of motor deficits, neurodegeneration, neuromuscular dysfunctions, or aging (Figure 3) [20]. The crawling assay, on the other hand, is used to analyze the locomotion of larvae, typically at the third instar stage. In this test, larvae are placed on a flat surface (such as an agar-coated Petri dish), and their movement is observed or recorded over a defined period. This assay is particularly useful for identifying early motor alterations caused by genetic mutations (Figure 3) [21]. Another test that can be employed in these models is the NMJ assay, a combination of morphological and functional analyses aimed at assessing NMJ integrity. Morphological evaluation involves dissection, fixation, and immunostaining of larval or adult tissues using synaptic markers, followed by fluorescence microscopy to quantify synaptic bouton number, size, and branching, while functional assessment can include electrophysiological recordings of synaptic transmission (Figure 3) [22]. Together, these approaches provide a comprehensive evaluation of synaptic structure and function, essential for understanding neuromuscular deficits in HA and HSP models [23].
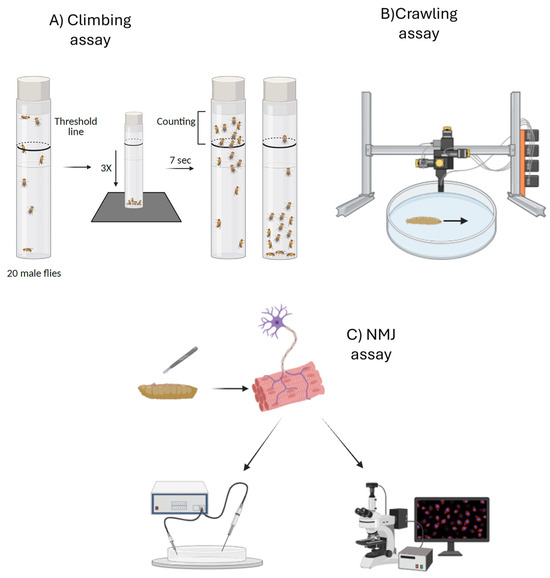
Figure 3.
The figure shows three alternative techniques that can be used in Drosophila models for HA and HSP. From left to right, we see the climbing assay (A), a behavioral test used to assess motor function by evaluating the flies’ ability to climb up a test tube over time [20]. The crawling assay is another behavioral test, performed on larvae (B), where the animal’s movement across a Petri dish is monitored [21]. Finally, the NMJ assay (C) is a morphological and functional test in which the larva is dissected, the neuromuscular junction is isolated, and immunofluorescence or electrophysiological analyses can be performed on it [22]. All these tests allow the assessment of motor function and the detection of early alterations in ataxia and paraparesis models. Created with Biorender.com.
These models are crucial for understanding the mechanisms underlying neurodegeneration in HSP and HA and provide insights for therapeutic development. In this review, we examine and illustrate the different Drosophila models generated for various forms of HSP and HA, highlighting the genetic strategies used to recreate disease-relevant phenotypes, the observed phenotypic manifestations, and the insights gained into underlying pathogenic mechanisms [24]. Additionally, we discuss how these models have been employed to identify genetic modifiers and potential therapeutic targets, emphasizing the versatility and power of Drosophila as a tool for studying neurodegenerative disorders.
3. Investigating Visual Dysfunction in Drosophila Models of HA and HSP
Patients affected by HA and HSP may present significant visual impairments, although the frequency and severity of these manifestations vary widely depending on the genetic subtype. Among ataxias, some of the forms most frequently associated with visual deficits include spinocerebellar ataxia 7 (SCA7), FRDA, and to a lesser extent, SCA1, SCA3, and SCA6. SCA7 is notably associated with pigmentary retinopathy and progressive macular degeneration, which can lead to complete blindness in patients, often preceding the onset of cerebellar motor symptoms [25]. Visual disturbances have also been described in SCA1 and SCA3, where defects in visual synaptic transmission, photoreceptor degeneration, and reduced retinal integrity have been reported. In FRDA, an autosomal recessive disorder caused by mutations in the FXN gene, optic atrophy, color vision deficits, and abnormalities in visual evoked potentials have been documented, suggesting impaired conduction along the optic nerve and visual cortex [26]. In fact, certain forms of HSP in patients, particularly those linked to mutations in SPG7, SPG11, and SPG15, also present with optic atrophy and retinal abnormalities, including reduced visual acuity, optic disc pallor, and in some cases, cortical blindness. The Drosophila eye has a modular organization; the retina consists of approximately 750 ommatidia, each separated from its neighbor and containing eight distinct types of photoreceptors [27]. This architecture allows for easy detection of morphological changes induced by genetic mutations, such as cellular disorganization, apoptosis, or neuro-axonal degeneration. Furthermore, although it is not part of the central nervous system, the eye is innervated, enabling its use as an efficient platform for both genetic and pharmacological screening [28]. Visual alterations are observed in many Drosophila models replicating forms of HA and HSP. In the Drosophila model of sca7, expression of expanded human ATXN7 induces retinal degeneration, neuronal apoptosis, ommatidial disorganization, and reduced electrophysiological response to light [19]. Similarly, degenerative ocular phenotypes, including the so-called “rough eye” phenotypes, have been observed in models of sca3, sca13, sca1, and sca31, reflecting neuronal dysfunction in the retina and altered visual signal transduction [29,30,31]. As for HSP, visual impairments are especially evident in spg7 and spg4 models. In spg7, photoreceptor synaptic terminals were found to be disorganized and displayed accumulations of swollen and morphologically abnormal mitochondria [32]. The spg4 model also shows significant visual alterations, such as defective ommatidial morphology and reduced eye size [33].
These findings underscore the importance of the visual system as a vulnerable and clinically relevant target in various forms of HA and HSP. The use of Drosophila melanogaster as a model organism, particularly through eye-specific assays, proves to be a powerful tool to dissect the molecular mechanisms underlying visual neurodegeneration
4. Drosophila in the Study of HSP Disorders
A total of 18 orthologous SPG genes have been identified in Drosophila, including 7 dominant and 11 recessive forms (https://flybase.org/lists/FBhh/; accesed on 17 June 2025) (Table 1). Drosophila melanogaster models of HSP have revolutionized our understanding of the cellular and molecular mechanisms of these rare neurodegenerative disorders, which affect the central nervous system, primarily motor neurons and spinocerebellar nerve fibres. Among the most studied and common forms are the genes SPAST (SPG4), ATLASTIN (SPG3A) and SPG7 [34].
Many of these genes encode proteins involved in intracellular trafficking of proteins and organelles. The human SPAST gene encodes spastin, an ATP-dependent microtubule severing protein and shares sequence similarity with the N-terminal microtubule interacting and trafficking domain of the protein associated with SPG20 [35]. The protein spastin plays a critical role in ER-to-Golgi membrane trafficking and in the completion of cytokinetic abscission, and evidence further suggests its involvement in axonal outgrowth and branching [35]. There is a single fly orthologue of SPAST, spas, which has been studied to better understand the role of spastin in neurodegeneration. Loss of spas function in the fly nervous system is not lethal, but affected flies progressively develop movement defects until they become completely immobile. Neurodegeneration is visible in the fly brain, with a clear presence of neurons undergoing apoptosis, these features being reminiscent of those observed in HSP patients [36]. In addition to knockdown analyses, overexpression of human wildtype and mutant SPAST was also performed in flies (Table 1). There is a functional equivalence between human and fly genes, suggesting conserved functionality between the two species [37]. An additional frequent form is SPG3A caused by mutations in the ATL1 gene, which encodes atlastin, a dynamin-related GTPase that plays a role in formation of the ER network and in axon elongation in neurons [38]. In Drosophila, atlastin knockdown mediated by RNAi leads to loss of atlastin in neurons and ensuing ER morphological defects (Table 1) [39], synaptic dysfunction, and microtubule disorganization. It has been shown that both downregulation and overexpression of atlastin in Drosophila motor neurons lead to impaired locomotion. Larvae exhibit reduced crawling speed and contraction frequency, while adult flies show a progressive decline in climbing ability highlighting the critical role of this protein in neuronal health [40]. Among the most common HSP recessive forms, we list SPG7, encoding paraplegin, a component of the m-AAA protease, an ATP-dependent proteolytic complex of the mitochondrial inner membrane and the protein has roles in diverse cellular processes including membrane trafficking, intracellular motility, organelle biogenesis, protein folding, and proteolysis [41]. In Drosophila there is a single orthologue, spg7 [32]. To generate a knock-out spg7 fruit fly model, the CRISPR/Cas9 gene editing technique has been adopted. The mutant flies showed a shorter lifespan, motor impairment, neuronal and muscular degeneration, and finally the presence of dysmorphic mitochondria, indicating that the phenotypes of the spg7 mutants are associated with mitochondrial dysfunction (Table 1) [32]. SPG11 CRISPR/Cas9 and SPG15 RNAi Drosophila knockdown models present autophagosome accumulation, enlarged lysosomes, a reduced number of free lysosomes, autophagic lysosomal reformation defects in larval muscles, and locomotor deficits [42]. SPG11 and SPG15 encode proteins that are essential for the proper functioning of the autophagy-lysosome pathway. SPG11 is involved in the formation and trafficking of autolysosomes and in maintaining lysosomal homeostasis, while SPG15 plays a key role in autophagic lysosomal reformation and the maturation of lysosomes. The loss of either protein disrupts lysosomal clearance and autophagic flux, leading to the accumulation of autophagosomes and impaired muscle function, which in turn contributes to locomotor deficits observed in the fly models. Moreover, the specific function of reticulon-2 (RTN2) has been most clearly defined in Drosophila, making this model a paradigmatic exemplar for the field. Espadas et al. demonstrated that Drosophila reticulon drives dynamic constriction and fission of ER membranes, establishing a mechanistic basis for reticulon-dependent ER remodeling [43]. Building on this, Pérez-Moreno et al. showed that the Drosophila SPG12/RTN2 orthologue, reticulon-like 1 (Rtnl1), governs presynaptic ER organization and Ca2+ dynamics, directly linking reticulon activity to neuronal physiology [44]. Together, these studies position Drosophila as the key reference system for understanding RTN2/reticulon function in ER dynamics, and for other HSPs proteins involved in ER network.

Table 1.
Summary table of HSP/SPG genes relevant to Drosophila research, including conserved orthologues and loci without orthologue, the genetic models developed, methodologies employed, and main phenotypes observed.
Table 1.
Summary table of HSP/SPG genes relevant to Drosophila research, including conserved orthologues and loci without orthologue, the genetic models developed, methodologies employed, and main phenotypes observed.
| Human Genes | Protein Function | Identity and Similarity | Drosophila Genes | Type of Model | Behavior | Morphology | References |
|---|---|---|---|---|---|---|---|
| SPG3A/ATL1 | Membrane-anchored dynamin-like GTPase mediating GTP-dependent fusion of ER membranes; essential for maintaining a continuous tubular ER network and axonal homeostasis | 52–57% and 71–77% | atlastin | Loss of function | reduce climbing performance | muscular ER fragmentation at the NMJs, and progressive degeneration of dopaminergic neurons | [45,46,47,48] |
| SPG4/SPAST | ATP-dependent microtubule severing enzyme that preferentially cuts polyglutamylated microtubules; regulates axonal microtubule dynamics and transport | 44% and 55% | spas | Loss of function and gain of function | adults have severe movement defects, cannot fly, and have weak legs | larvae have altered NMJs in which presynaptic boutons are more numerous and smaller than in wild type | [36,49,50,51,52] |
| SPG7 | Catalytic component of the m-AAA protease, a protease that plays a key role in proteostasis of inner mitochondrial membrane proteins, and which is essential for axonal and neuron development | 58% and 75% | spg7 | Loss of function | shortened lifespan, climbing or flight defects, sensitivity to stressors | photoreceptor synaptic terminal disorganized, degeneration of indirect flight muscle and mitochondrial trafficking defects | [32] |
| SPG10/KIF5A | Microtubule-dependent motor required for slow axonal transport of neurofilament proteins | 60% and 76% | khc | Gain of function | complete paralysis, reduced lifespan, no stable flight | axons innervating posterior segments are considerably longer | [53,54,55] |
| SPG11 | play a role in neurite plasticity by maintaining cytoskeleton stability and regulating synaptic vesicle transport | spg11 | Loss of function | locomotor deficit | autophagosome accumulation, enlarged lysosomes, reduced free lysosomes, autophagic reformation defects | [43] | |
| SPG12/RTN2 | ER-shaping protein of the reticulon family; regulates curvature of ER tubules and ER network homeostasis | 30% and 46% | rtnl1/rtnl2 | Loss of function | locomotion impairment | ER stress response, abnormalities of ER marker, MT cytoskeleton and mitochondria | [43,44,56] |
| SPG15/ZFYVE26 | Phosphatidylinositol 3-phosphate-binding protein required for the abscission step in cytokinesis: recruited to the midbody during cytokinesis and acts as a regulator of abscission. May also be required for efficient homologous recombination DNA double-strand break repair | 30% and 50% | sptz | Loss of function | locomotor deficit | autophagosome accumulation, enlarged lysosomes, reduced free lysosomes, autophagic reformation defects | [57] |
| SPG17/BSCL2 | Plays a crucial role in the formation of lipid droplets (LDs) which are storage organelles at the center of lipid and energy homeostasis | 31% and 51% | seipin | Loss of function | reduced locomotor activity | mild triacylglycerol storage phenotype | [58] |
| SPG20/SPART | Lipophagy receptor that plays an important role in lipid droplet (LD) turnover in motor neurons | 25% and 40% | spartin | Loss of function | locomotion impairment | neurodegeneration, progressive vacuolization in adult brain, reduced neurotransmitter release | [59] |
| SPG30/KIF1A | Kinesin motor with a plus-end-directed microtubule motor activity | - | no orthologue | Loss of function | embryos are paralyzed and fail to hatch | nerve outgrowth fails, synaptic bouton defects, loss of SVs and AZs at NMJ | [60,61] |
| SPG31/REEP1 | ER membrane protein linking ER tubules to microtubules; required for ER shaping, remodeling, and axonal maintenance | 50% and 70% | reepA | Loss of function | locomotor dysfunction, shortened lifespan | expansion of ER sheet-like structures | [62,63] |
| SPG35/FA2H | Catalyzes the hydroxylation of free fatty acids at the C-2 position to produce 2-hydroxy fatty acids, which are building blocks of sphingolipids and glycosphingolipids common in neural tissue and epidermis | 60% and 75% | fa2h | Loss of function | flying disability, behavioral abnormalities | mitochondrial dynamics and autophagy alterations | [64] |
| SPG39/PNPLA6 | Catalyzes the hydrolysis of several naturally occurring membrane-associated lipids | 45% and 65% | sws | Loss of function | progressive behavioral defects | neurodegeneration with vacuole formation | [65,66,67] |
| SPG61/ARL6IP1 | Positively regulates SLC1A1/EAAC1-mediated glutamate transport by increasing its affinity for glutamate in a PKC activity-dependent manner | 29% and 61% | arl6IP1 | Loss of function | significant locomotor deficit | ER and mitochondrial disorganization, disrupted lipid droplets | [68,69] |
| SPG76/CAPN1 | Calcium-regulated non-lysosomal thiol-protease which catalyzes limited proteolysis of substrates involved in cytoskeletal remodeling and signal transduction | 45% and 64% | calpA/caplB | Loss of function | locomotor defects | axonal abnormalities | [70] |
| SPG77/FARS2 | Is responsible for the charging of tRNA (Phe) with phenylalanine in mitochondrial translation | 65% AND 80% | pheRS-m | knockout | developmental delay, seizures | mitochondrial tRNAphe and oxidative phosphorylation defects | [71,72] |
| SPG78/ATP13A2 | ATPase which acts as a lysosomal polyamine exporter with high affinity for spermine | - | no orthologue | knockdown | [73] | ||
| SPG92/FICD | Protein that can both mediate the addition of adenosine 5’-monophosphate (AMP) to specific residues of target proteins (AMPylation), and the removal of the same modification from target proteins (de-AMPylation), depending on the context | 35% and 60% | fic | knockout | [74] |
5. Genetic Modelling of HA in Drosophila
A significant number of SCAs are caused by abnormal expansions of CAG triplet repeats in their respective genes, resulting in the production of proteins containing expanded polyglutamine (polyQ) tracts. These expansions promote the formation of toxic intracellular aggregates, alterations in nucleocytoplasmic trafficking, transcriptional dysregulation, and progressive loss of neuronal function [75]. To understand the molecular mechanisms underlying these diseases and to test potential therapeutic strategies, the use of genetically tractable experimental models is essential. In this context, Drosophila melanogaster has emerged as a highly valuable model system due to its well-defined genetics, considerable homology with human genes implicated in SCAs, and the availability of powerful genetic tools that enable detailed analysis of the molecular pathways involved in neurodegeneration [75]. Despite lacking a cerebellar structure homologous to that of vertebrates, Drosophila has a complex central nervous system with well-characterized neural circuits and quantifiable motor behaviors that are affected by neurodegeneration. Thanks to the evolutionary conservation of many human genes, the availability of high-throughput genetic tools, and the feasibility of large-scale experiments, Drosophila has been widely used to develop transgenic models of various forms of HA [76]. Approximately 22 orthologous genes have been identified in Drosophila, including 11 dominant and 11 recessive forms (https://flybase.org/lists/FBhh/) (Table 2). The most common dominant ataxias include SCA1, SCA2, SCA3, SCA6, and SCA7, while the most studied recessive forms include FRDA and ataxia-telangiectasia (AT) [9]. One of the first Drosophila models developed to study SCA1 was based on the expression of human ATXN1 [82Q] and [30Q] using the UAS-GAL4 system; the protein ataxin1 binds RNA, associates with large protein complexes, and interacts with a vast network of proteins and is thought to be involved in transcriptional repression and to regulate Notch and Capicua [77]. This model is known for reproducing pathological features such as nuclear inclusions, retinal degeneration, and impaired locomotion [78]. Notably, neurotoxicity is polyQ-length dependent, with a marked decline in locomotor ability observed as the flies age. The reduced lifespan of these flies further highlights the progressive nature of the disease. In the case of SCA2, the model developed by Lessing and Bonini, induced protein aggregation and neurodegeneration by expressing mutant ATXN2 in Drosophila neurons, where ATXN2 functions as an RNA-binding protein involved in stress granule assembly and the regulation of RNA metabolism [79,80]. Motor dysfunction was a consistent finding, accompanied by reduced climbing ability as the primary behavioral phenotype. Additionally, reduced lifespan was observed, reflecting the neurodegenerative progression in both the nervous system and muscle tissue. For SCA3, Drosophila models expressing the C-terminal fragment of human ATXN3 with 78 [81] or 82 CAG repeats [82] led to retinal degeneration, abnormal eye morphology, and neuronal death. These flies showed reduced motility and exhibited the well-known “rough eye” phenotype, with locomotor dysfunction and loss of climbing ability as further key phenotypic features. ATXN3 is a deubiquitinase that normally participates in protein quality control and ubiquitin-mediated proteostasis, and its polyglutamine-expanded form disrupts these processes, thereby promoting neuronal toxicity. Regarding FRDA, Drosophila models with RNAi-mediated downregulation of the frataxin homolog (fh) have demonstrated mitochondrial dysfunction, oxidative stress, and impaired locomotion [83,84]. Consistent with these findings, frataxin is a nuclear-encoded mitochondrial iron chaperone that plays a key role in iron-sulfur cluster biogenesis and heme biosynthesis, processes whose disruption underlies the observed defects. These models typically show reduced climbing ability and a shortened lifespan, mirroring the clinical presentation of FRDA, which is closely tied to mitochondrial and neuronal damage. The ATM protein is a member of the phosphatidylinositol 3-kinase family and responds to DNA damage by phosphorylating key substrates involved in DNA repair and cell cycle control. Consistent with this function, Drosophila models of AT targeting the ATM homolog tefu exhibit neurodevelopmental defects and neuronal degeneration [85,86], along with reduced lifespan, increased sensitivity to DNA damage, and impaired repair pathways, thereby recapitulating key aspects of the human disease.

Table 2.
Summary table of HA genes relevant to Drosophila research, including conserved orthologues and loci without orthologue, the genetic models developed, methodologies employed, and main phenotypes observed.
In summary, across these various models, common behavioral traits such as impaired locomotion and shortened lifespan are consistently observed. In addition, many of these models share neurological and mitochondrial defects, such as reduced climbing, and oxidative stress sensitivity, all of which provide valuable insights into the molecular and cellular underpinnings of these diseases.
6. Commonalities and Differences
Across the spectrum of SCA and autosomal recessive cerebellar ataxia (ARCA) models, several shared phenotypic and molecular characteristics have been observed. Locomotor deficits and shortened lifespan are among the most consistently reported behavioral phenotypes. These impairments, evident in models of SCA1 (Atx-1), SCA2 (Atx-2), SCA17 (Tbp), and recessive forms like FRDA (fh) and SCAR25 (Atg5), indicate broad neurodegenerative processes affecting motor coordination and survival [78,83,89,106,123]. Neurodegeneration, particularly in the eye and brain, is a hallmark of many of these models, such as SCA3, SCA7, and dentatorubral-pallidoluysian atrophy (DRPLA), where retinal and neural tissue show progressive collapse, vacuolization, or loss of structure [82,100,114]. In models like SCA5 and SCA12, synaptic and axonal transport defects have also been documented, often accompanying reduced synaptic terminal growth and mitochondrial abnormalities [94,95,96,97,98,99,100,101,102,103]. These synaptic changes often mirror findings in patients and emphasize the utility of Drosophila in identifying disease-related cellular phenotypes. At the cellular and molecular levels, mitochondrial dysfunction and oxidative stress sensitivity are commonly observed, where mutations disrupt mitochondrial dynamics, energy metabolism, and autophagy pathways [115,119,123]. These alterations frequently coincide with ER stress, altered cytoskeletal organization, and impaired organelle transport. Such convergence on mitochondrial and ER dysfunction points to their central role in the pathogenesis of ataxias. Some ataxia models reveal developmental abnormalities and failure to hatch, especially in early-onset or severe phenotypes like those of SCAR21 (yata) and SCA12 (tws), reflecting disruptions in neuronal development or apoptosis pathways [103,120]. Finally, defects in NMJ morphology and function, such as reduced synaptic branching and altered plasticity, are reported in SCAR24, SCAN3, and SETX models, offering insight into motor system vulnerability in ataxias [122,128,129]. Drosophila melanogaster models of HSPs reveal numerous shared phenotypic and cellular features that mirror the neurological impairments observed in human patients. A common behavioral hallmark is locomotor impairment, reported across most models [43,44,45,46], frequently accompanied by reduced climbing or flight ability, paralysis, or decreased lifespan. Morphologically, a consistent finding is NMJ disruption, as seen in models like spas, spg7, spartin (SPG20) [32,49,59]. Another prevalent feature is ER and mitochondrial dysfunction, often accompanied by oxidative stress, this is particularly evident in models of atl (SPG3A), rtnl1/rtnl2 (SPG12), reepA (SPG31), fa2h (SPG35), arl6IP1 (SPG61), and fic (SPG92) [45,46,56,62,64,68,74]. Mitochondrial dynamics, autophagy defects, and ER stress responses are recurring pathological signatures, such as autophagosome accumulation, lysosomal enlargement, and impaired organelle reformation. Some models, such as pheRS-m (SPG77), even display early developmental abnormalities and seizure-like activity, reflecting mitochondrial translation and oxidative phosphorylation defects [71].
In summary, despite the genetic and clinical heterogeneity of HA and HSP, Drosophila melanogaster models have uncovered a set of strikingly convergent phenotypic and molecular features. Locomotor impairment is a unifying behavioral hallmark across both disease groups, reflecting widespread dysfunction of motor circuits. Neurodegeneration, particularly affecting the eye, brain, and NMJ, emerges as a shared pathological outcome, often accompanied by disrupted synaptic integrity and axonal transport. At the subcellular level, mitochondrial dysfunction, oxidative stress, and ER stress are consistently observed across many models, indicating that defects in energy metabolism and organelle homeostasis are central drivers of neurodegeneration in both HA and HSP. Moreover, early developmental defects and failure to hatch in more severe or early-onset forms highlight the impact of these mutations on neurodevelopmental pathways. These commonalities underscore the value of Drosophila as a cross-disease platform, not only for dissecting shared pathogenic mechanisms but also for identifying potential therapeutic targets that may benefit a broad spectrum of hereditary neurodegenerative disorders.
7. Conclusions and Future Perspective
For many years, researchers have used fruit flies to explore how developmental signaling pathways work and to better understand the roles of genes linked to human diseases [16], including rare neurodegenerative disorders such as HSP and HA. Reaching a diagnosis and proposing treatments for patients affected by rare and ultra-rare diseases is particularly challenging [130]. In this context, Drosophila models offer important advantages, including genetic tractability, short life cycle and the possibility to investigate disease mechanisms in a whole-organism context. These features enable the development of innovative research strategies for studying conditions that are often difficult to address using cell cultures or vertebrate models, due to limitations in complexity, ethical concerns, and longer experimental times [131]. Many genetic techniques were originally developed in the fruit fly and later adapted for use in mammalian model systems, for example, techniques to introduce transgenes into the genome using pronuclear injection or viral-mediated transgenesis [132,133], as well as the Gal4/UAS system, RNAi, and CRISPR/Cas9. These techniques are key tools for “humanizing” Drosophila, allowing researchers to replicate human pathological mutations and study rare variants of human proteins [134,135]. By taking advantage of these available genetic tools, researchers have been able to model and characterize many forms of both HSP and HA, enabling the identification of disease-causing genes, the investigation of molecular mechanisms, and the establishment of genotype–phenotype correlations.
The use of Drosophila has certain limitations. Although flies do not possess human-like cognitive abilities, they can perform some complex behaviors, including learning and memory. Additionally, despite a high degree of gene conservation, approximately 40% of human genes are either absent in Drosophila or perform different functions, which can restrict the modeling of certain disease mechanisms.
Anatomical differences between humans and flies, such as the absence of a cerebral cortex, myelinated neurons, and a cerebellum, further limit the study of processes that rely on these structures. Similarly, variations in cellular composition, organ complexity, and physiology may influence the manifestation of phenotypes and responses to pharmacological treatments, potentially reducing translational relevance [130]. Furthermore, some pathways or disease processes that involve higher-order cognitive functions or specific tissue interactions cannot be fully replicated in flies. Despite these constraints, Drosophila remains a powerful model for investigating conserved molecular pathways, genetic interactions, and basic cellular mechanisms underlying human diseases [130]. In Drosophila, wrapping glia plays a crucial role in ensuring proper neuronal signaling. They regulate axon diameter and conduction speed, and their differentiation depends on gap junctions and FGF signaling. Importantly, beyond influencing conduction speed, wrapping glia are essential for the precision of neuronal signaling and coordinated locomotor patterns. These findings highlight how glial cells actively shape neuronal communication, an aspect relevant to understanding glial contributions to neurodegenerative and neuromuscular disorders [136]. These limitations can be overcome by employing murine models, which possess a fully myelinated central nervous system and brain structures homologous to those in humans. In mice, behavioral and neuropathological phenotypes such as spasticity, tremors, and motor coordination deficits can be accurately reproduced, allowing for detailed functional validation of disease genes and therapeutic interventions [131]. Despite these challenges, numerous studies have demonstrated that drug responses in Drosophila models often parallel those observed in humans, particularly in neurodegenerative and oncological diseases, highlighting their utility as a cost-effective and rapid screening platform [134]. To further enhance translational relevance, human cellular models, especially patient-derived induced pluripotent stem cells (iPSCs), are employed to study patient-specific phenotypes such as mitochondrial dysfunction, axonal degeneration, or protein misfolding within a human genetic background [137]. These cellular systems also support high-throughput drug screening under conditions closer to human physiology, thus complementing whole-animal studies and improving predictive power for clinical application. Therefore, the integration of Drosophila, murine, and cellular models offers a powerful complementary approach: flies for high-speed genetic analysis, mice for behavioral and systemic validation, and human cell cultures for mechanistic studies and personalized therapeutic testing. This combined strategy enhances our ability to elucidate disease mechanisms and bridge the gap between basic research and clinical translation in complex neurogenetic diseases. Drosophila melanogaster is an experimental model widely used for the validation of genetic mutations, thanks to its well-characterized genetics, rapid life cycle, and ease of manipulation. The genes involved in human genetic diseases are conserved in Drosophila allowing researchers to use this model to validate the role of these variants in a living biological context [138]. In the context of HSPs, a notable example is represented by mutations in the KIF5A gene (SPG10) [54]. The patient-derived p.N256S variant has been modeled in Drosophila through ectopic expression of the mutated human kinesin heavy chain (KHC) orthologue. This UAS- GAL4 approach allowed the reproduction of key pathological features, including impaired axonal transport, axonal swellings, and motor deficits, thus faithfully mimicking the human HSP phenotype [54]. Similarly, recently identified mutations in the FICD gene, responsible for a recessive form of HSP, have been investigated in Drosophila by generating knockout models lacking the endogenous fic gene [74]. These flies displayed reduced levels of Binding immunoglobulin protein (BiP), an essential for proper protein folding and stress response, increased oxidative stress, and locomotor defects, thereby highlighting the pathogenic contribution of impaired protein folding and ER stress in HSP [74]. For HA, Drosophila models have also provided relevant insights. Loss of the mitochondrial protein VPS13D, mutated in patients with spastic-ataxic syndromes, was studied through knockdown in flies, which exhibited abnormal mitochondrial morphology and defective axonal transport. These findings supported a mitochondrial-based mechanism underlying disease progression [119]. Taken together, these examples illustrate how Drosophila models, whether based on overexpression of human mutated genes or on targeted loss of endogenous orthologs, have contributed to the identification of novel disease-causing mutations, clarified the molecular pathways involved, and strengthened genotype–phenotype correlations in both HSP and HA [135]. An additional strength of the Drosophila model is its ability to be used in high-throughput drug screenings, enabling the identification of compounds capable of modulating the neurological defects observed in rare diseases [75]. By combining high-throughput compatibility with robust phenotypic assays, it accelerates target identification and preclinical validation, optimizing resources before vertebrate testing, Drosophila has been widely used for both primary screens and secondary validation of biologically active compounds [24]. In the field of HSP, 6 autophagy modulating compounds were tested in SPG15 RNAi Drosophila model and one of these rescued lysosomal parameters, autophagic lysosomal reformation defects and locomotor deficits [42]. In the field of ataxias, models of FRDA generated via RNAi have been employed to perform secondary drug screening. This model enabled the testing of 12 drugs that showed specificity (they did not rescue another slow-growing strain) and were not toxic at high doses. Two compounds rescued the developmental delay, and one of these also ameliorated cardiac dilation defects in adult Drosophila with fxn RNAi [138]. Another example of Drosophila uses in drug screening involved fmr1(-/-) mutants, a model for studying Fragile X Syndrome. A high-throughput approach was employed, screening approximately 2000 FDA-approved drugs. From these, 15 compounds were selected for further analysis [24]. In addition to being an excellent platform for primary screening, the fruit fly can also be effectively used in a secondary validation phase. This approach enables the rapid selection of a smaller, higher-quality subset of active compounds from a larger pool, which can then undergo medicinal chemistry optimization and subsequent testing in murine models. This strategy helps optimize resources and accelerates the overall drug discovery process. The development of Drosophila “avatar” models, genetically engineered to carry patient-specific mutations, represents an emerging and powerful tool in the era of precision medicine. These models allow functional validation of individual genetic variants identified in patients, offering a rapid and cost-effective strategy to assess variant pathogenicity and to test personalized therapeutic options [139]. In avatar flies, human mutations are introduced into the homologous fly gene (when conserved), or human transgenes carrying the mutant sequence are expressed in relevant tissues. These models preserve whole-organism context and are particularly useful for evaluating complex phenotypes such as locomotion, neurodegeneration, seizure susceptibility, and drug response [139]. In cancer research, the pioneering work of Ross Cagan and collaborators has demonstrated the feasibility of using Drosophila avatars to screen drug combinations tailored to individual patients’ genetic profiles [140]. Notably, such models have led to the identification of personalized multi-drug regimens with clinical benefit in advanced, treatment-resistant cancers. For example, in a patient with metastatic colorectal cancer harboring a KRAS mutation, a fly avatar enabled the discovery of a combination therapy (trametinib and zoledronate) that achieved a 45% reduction in tumor burden [141]. Beyond oncology the group led by Richard Burke used fly avatars carrying ATP7A and ATP7B mutations to uncover pathogenic mechanisms and identify candidate compounds [142]. The application of this approach to HA and HSP holds strong potential. Several Drosophila models have been developed that recapitulate patient-relevant aspects of these diseases. For instance, in HSP, mutations in the gene atlastin have been extensively studied. The in vivo effects of four pathogenic missense mutations were analyzed using two complementary approaches: CRISPR/Cas9 editing to introduce the variants into the endogenous gene, and transgenesis to generate Drosophila lines overexpressing atlastin carrying the patient-derived pathogenic variants [48]. Similarly, in FRDA, CRISPR/Cas9-mediated introduction of GAA repeat expansions into the fly frataxin orthologue leads to phenotypes including reduced lifespan, motor impairment, and oxidative stress [115]. Models of SCA7 have also been generated through expression of mutant ataxin-7, producing neurodegenerative phenotypes useful for genetic modifier screens [99]. These examples highlight the suitability of Drosophila avatars for dissecting the functional impact of patient-specific variants and for high-throughput in vivo drug screening.
The future of Drosophila “avatars” in precision medicine is particularly promising, one major avenue being the functional characterization of variants of uncertain significance (VUS) identified through patient genome sequencing [143]. Fly avatars allow rapid in vivo assessment of variant pathogenicity, offering a cost-effective approach to prioritize variants for further study in mammalian systems or patient-derived cellular models. Advances in genome editing technologies, such as CRISPR/Cas9, base editors, and prime editors, will enable increasingly precise introduction of patient-specific mutations, including point mutations, indels, and regulatory region variants. This precision will allow modeling of compound heterozygous mutations, complex alleles, or combinations of multiple patient-specific variants, expanding the ability to replicate real patient genotypes in vivo. Integration with multi-omics approaches, such as transcriptomics, proteomics, and metabolomics, can deepen the understanding of disease mechanisms in fly avatars [139]. By correlating molecular changes with phenotypic outcomes, researchers can identify biomarkers, disease pathways, and potential therapeutic targets more efficiently. Coupled with automated high-throughput behavioral and physiological assays, these approaches can reveal subtle neurobehavioral or organ-specific defects that are otherwise difficult to detect. Drosophila avatars also offer a powerful platform for personalized drug discovery and repurpose [144]. Large-scale chemical libraries, including FDA-approved drugs, can be screened in patient-specific fly models to identify compounds that rescue disease phenotypes. Future directions may include the combination of avatars with organ-specific modeling, such as tissue-specific expression of human mutations, optogenetic manipulation of neuronal circuits, or modeling interactions between multiple organs or cell types.
In conclusion, Drosophila melanogaster represents an extremely versatile and powerful model for studying rare genetic diseases, thanks to its unique combination of advanced genetic tools, low experimental costs, rapid life cycle, and high conservation of fundamental biological mechanisms with humans, making it a valuable resource for translational research and the development of targeted therapies.
Author Contributions
Conceptualization, F.M.S., R.V. and M.M.; methodology, R.V.; investigation, R.V.; resources, F.M.S. and M.M.; writing—original draft preparation, R.V.; writing—review and editing, F.M.S., M.M., M.T.B. and C.V.; visualization, M.T.B. and C.V.; supervision, F.M.S. and M.M.; project administration, F.M.S. and M.M.; funding acquisition, F.M.S. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ricerca Corrente 2024-2025, RC 5 × 1000 to MTB, F.M.S and M.M. FMS is also supported by Telethon grant GJC21131.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lo Giudice, T.; Lombardi, F.; Santorelli, F.M.; Kawarai, T.; Orlacchio, A. Hereditary spastic paraplegia: Clinical-genetic characteristics and evolving molecular mechanisms. Exp. Neurol. 2014, 261, 518–539. [Google Scholar] [CrossRef]
- Boutry, M.; Morais, S.; Stevanin, G. Update on the Genetics of Spastic Paraplegias. Curr. Neurol. Neurosci. Rep. 2019, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Deluca, G.C.; Ebers, G.C.; Esiri, M.M. The extent of axonal loss in the long tracts in hereditary spastic paraplegia. Neuropathol. Appl. Neurobiol. 2004, 30, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Hensiek, A.; Kirker, S.; Reid, E. Diagnosis, investigation and management of hereditary spastic paraplegias in the era of next-generation sequencing. J. Neurol. 2015, 262, 1601–1612. [Google Scholar] [CrossRef]
- Panza, E.; Meyyazhagan, A.; Orlacchio, A. Hereditary spastic paraplegia: Genetic heterogeneity and common pathways. Exp. Neurol. 2022, 357, 114203. [Google Scholar] [CrossRef]
- Elsayed, L.E.O.; Eltazi, I.Z.; Ahmed, A.E.; Stevanin, G. Insights into Clinical, Genetic, and Pathological Aspects of Hereditary Spastic Paraplegias: A Comprehensive Overview. Front. Mol. Biosci. 2021, 8, 690899. [Google Scholar] [CrossRef]
- Naef, V.; Mero, S.; Fichi, G.; D’Amore, A.; Ogi, A.; Gemignani, F.; Santorelli, F.M.; Marchese, M. Swimming in Deep Water: Zebrafish Modeling of Complicated Forms of Hereditary Spastic Paraplegia and Spastic Ataxia. Front. Neurosci. 2019, 13, 1311. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Schüle, R. Overcoming the divide between ataxias and spastic paraplegias: Shared phenotypes, genes, and pathways. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 332–345. [Google Scholar] [CrossRef]
- Pilotto, F.; Del Bondio, A.; Puccio, H. Hereditary Ataxias: From Bench to Clinic, Where Do We Stand? Cells 2024, 13, 319. [Google Scholar] [CrossRef]
- Finsterer, J. Ataxias with autosomal, X-chromosomal or maternal inheritance. The Canadian journal of neurological sciences. Le J. Can. Des Sci. Neurol. 2009, 36, 409–428. [Google Scholar] [CrossRef]
- Embiruçu, E.K.; Martyn, M.L.; Schlesinger, D.; Kok, F. Autosomal recessive ataxias: 20 types and counting. Arq. De Neuro-Psiquiatr. 2009, 67, 1143–1156. [Google Scholar] [CrossRef]
- Bustamante-Barrientos, F.A.; Luque-Campos, N.; Araya, M.J.; Lara-Barba, E.; de Solminihac, J.; Pradenas, C.; Molina, L.; Herrera-Luna, Y.; Utreras-Mendoza, Y.; Elizondo-Vega, R.; et al. Mitochondrial dysfunction in neurodegenerative disorders: Potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J. Transl. Med. 2023, 21, 613. [Google Scholar] [CrossRef]
- Oriel, C.; Lasko, P. Recent Developments in Using Drosophila as a Model for Human Genetic Disease. Int. J. Mol. Sci. 2018, 19, 2041. [Google Scholar] [CrossRef]
- Hales, K.G.; Korey, C.A.; Larracuente, A.M.; Roberts, D.M. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 2015, 201, 815–842. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, H. Drosophila as a Model Organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar] [CrossRef]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Lin, S.C.; Chang, Y.Y.; Chan, C.C. Strategies for gene disruption in Drosophila. Cell Biosci. 2014, 4, 63. [Google Scholar] [CrossRef]
- Trotta, N.; Orso, G.; Rossetto, M.G.; Daga, A.; Broadie, K. The hereditary spastic paraplegia gene, spastin, regulates microtubule stability to modulate synaptic structure and function. Curr. Biol. CB 2004, 14, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Sujkowski, A.; Ranxhi, B.; Bangash, Z.R.; Chbihi, Z.M.; Prifti, M.V.; Qadri, Z.; Alam, N.; Todi, S.V.; Tsou, W.L. Progressive degeneration in a new Drosophila model of spinocerebellar ataxia type 7. Sci. Rep. 2024, 14, 14332. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Post, Y.; Paululat, A. Muscle Function Assessment Using a Drosophila Larvae Crawling Assay. Bio-Protoc. 2018, 8, e2933. [Google Scholar] [CrossRef]
- Imlach, W.; McCabe, B.D. Electrophysiological methods for recording synaptic potentials from the NMJ of Drosophila larvae. J. Vis. Exp. JoVE 2009, 24, 1109. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Johnson, T.K.; Xie, W. Immunohistochemical Analysis of the Drosophila Larval Neuromuscular Junction. Methods Mol. Biol. 2024, 2746, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Jackson, S.M.; Whitworth, A.J.; Greene, J.C.; Libby, R.T.; Baccam, S.L.; Pallanck, L.J.; La Spada, A.R. A SCA7 CAG/CTG repeat expansion is stable in Drosophila melanogaster despite modulation of genomic context and gene dosage. Gene 2005, 347, 35–41. [Google Scholar] [CrossRef]
- Rodden, L.N.; McIntyre, K.; Keita, M.; Wells, M.; Park, C.; Profeta, V.; Waldman, A.; Rummey, C.; Balcer, L.J.; Lynch, D.R. Retinal hypoplasia and degeneration result in vision loss in Friedreich ataxia. Ann. Clin. Transl. Neurol. 2023, 10, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Paulk, A.; Millard, S.S.; van Swinderen, B. Vision in Drosophila: Seeing the world through a model’s eyes. Annu. Rev. Entomol. 2013, 58, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Prifti, M.V.; Nuga, O.; Dulay, R.O.; Patel, N.C.; Kula, T.; Libohova, K.; Jackson-Butler, A.; Tsou, W.L.; Richardson, K.; Todi, S.V. Insights into dentatorubral-pallidoluysian atrophy from a new Drosophila model of disease. Neurobiol. Dis. 2025, 207, 106834. [Google Scholar] [CrossRef]
- Sutton, J.R.; Blount, J.R.; Libohova, K.; Tsou, W.L.; Joshi, G.S.; Paulson, H.L.; Costa, M.D.C.; Scaglione, K.M.; Todi, S.V. Interaction of the polyglutamine protein ataxin-3 with Rad23 regulates toxicity in Drosophila models of Spinocerebellar Ataxia Type 3. Hum. Mol. Genet. 2017, 26, 1419–1431. [Google Scholar] [CrossRef]
- Ishiguro, T.; Sato, N.; Ueyama, M.; Fujikake, N.; Sellier, C.; Kanegami, A.; Tokuda, E.; Zamiri, B.; Gall-Duncan, T.; Mirceta, M.; et al. Regulatory Role of RNA Chaperone TDP-43 for RNA Misfolding and Repeat-Associated Translation in SCA31. Neuron 2017, 94, 108–124.e7. [Google Scholar] [CrossRef]
- Khare, S.; Nick, J.A.; Zhang, Y.; Galeano, K.; Butler, B.; Khoshbouei, H.; Rayaprolu, S.; Hathorn, T.; Ranum, L.P.W.; Smithson, L.; et al. A KCNC3 mutation causes a neurodevelopmental, non-progressive SCA13 subtype associated with dominant negative effects and aberrant EGFR trafficking. PLoS ONE 2017, 12, e0173565. [Google Scholar] [CrossRef]
- Pareek, G.; Thomas, R.E.; Pallanck, L.J. Loss of the Drosophila m-AAA mitochondrial protease paraplegin results in mitochondrial dysfunction, shortened lifespan, and neuronal and muscular degeneration. Cell Death Dis. 2018, 9, 304. [Google Scholar] [CrossRef]
- Ozdowski, E.F.; Gayle, S.; Bao, H.; Zhang, B.; Sherwood, N.T. Loss of Drosophila melanogaster p21-activated kinase 3 suppresses defects in synapse structure and function caused by spastin mutations. Genetics 2011, 189, 123–135. [Google Scholar] [CrossRef]
- Awuah, W.A.; Tan, J.K.; Shkodina, A.D.; Ferreira, T.; Adebusoye, F.T.; Mazzoleni, A.; Wellington, J.; David, L.; Chilcott, E.; Huang, H.; et al. Hereditary spastic paraplegia: Novel insights into the pathogenesis and management. SAGE Open Med. 2023, 12, 20503121231221941. [Google Scholar] [CrossRef]
- Solowska, J.M.; Baas, P.W. Hereditary spastic paraplegia SPG4: What is known and not known about the disease. Brain A J. Neurol. 2015, 138 Pt 9, 2471–2484. [Google Scholar] [CrossRef]
- Orso, G.; Martinuzzi, A.; Rossetto, M.G.; Sartori, E.; Feany, M.; Daga, A. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J. Clin. Investig. 2005, 115, 3026–3034. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Ozdowski, E.F.; Kotowski, I.K.; Marchuk, D.A.; Sherwood, N.T. Functional conservation of human Spastin in a Drosophila model of autosomal dominant-hereditary spastic paraplegia. Hum. Mol. Genet. 2010, 19, 1883–1896. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Zhu, P.P.; Parker, R.L.; Blackstone, C. Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network. J. Clin. Investig. 2010, 120, 1097–1110. [Google Scholar] [CrossRef]
- Orso, G.; Pendin, D.; Liu, S.; Tosetto, J.; Moss, T.J.; Faust, J.E.; Micaroni, M.; Egorova, A.; Martinuzzi, A.; McNew, J.A.; et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature 2009, 460, 978–983. [Google Scholar] [CrossRef]
- De Gregorio, C.; Delgado, R.; Ibacache, A.; Sierralta, J.; Couve, A. Drosophila Atlastin in motor neurons is required for locomotion and presynaptic function. J. Cell Sci. 2017, 130, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Casari, G.; Marconi, R.; Adam, M.P.; Feldman, J.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E. Amemiya A Spastic Paraplegia 7. In GeneReviews®; University of Washington: Seattle, WA, USA, 2006. [Google Scholar]
- Vantaggiato, C.; Guarato, G.; Brivio, F.; Panzeri, E.; Speltoni, B.; Gumeni, S.; Orso, G.; Santorelli, F.M.; Bassi, M.T. Naringenin and SMER28 target lysosomal reformation and rescue SPG11 and SPG15 hereditary spastic paraplegia phenotypes. Pharmacol. Res. 2025, 218, 107836. [Google Scholar] [CrossRef]
- Espadas, J.; Pendin, D.; Bocanegra, R.; Escalada, A.; Misticoni, G.; Trevisan, T.; Velasco Del Olmo, A.; Montagna, A.; Bova, S.; Ibarra, B.; et al. Dynamic constriction and fission of endoplasmic reticulum membranes by reticulon. Nat. Commun. 2019, 10, 5327. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.J.; Smith, R.C.; Oliva, M.K.; Gallo, F.; Ojha, S.; Müller, K.H.; O’Kane, C.J. Drosophila SPG12 ortholog, reticulon-like 1, governs presynaptic ER organization and Ca2+ dynamics. J. Cell Biol. 2023, 222, e202112101. [Google Scholar] [CrossRef] [PubMed]
- Candia, N.; Ibacache, A.; Medina-Yáñez, I.; Olivares, G.H.; Ramírez, M.; Vega-Macaya, F.; Couve, A.; Sierralta, J.; Olguín, P. Identification of atlastin genetic modifiers in a model of hereditary spastic paraplegia in Drosophila. Hum. Genet. 2023, 142, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Paik, D.; Bang, S.; Kang, J.; Chun, B.; Lee, S.; Bae, E.; Chung, J.; Kim, J. Loss of spastic paraplegia gene atlastin induces age-dependent death of dopaminergic neurons in Drosophila. Neurobiol. Aging 2008, 29, 84–94. [Google Scholar] [CrossRef]
- Xu, S.; Stern, M.; McNew, J.A. Beneficial effects of rapamycin in a Drosophila model for hereditary spastic paraplegia. J. Cell Sci. 2017, 130, 453–465. [Google Scholar] [CrossRef]
- Montagna, A.; Vajente, N.; Pendin, D.; Daga, A. In vivo Analysis of CRISPR/Cas9 Induced Atlastin Pathological Mutations in Drosophila. Front. Neurosci. 2020, 14, 547746. [Google Scholar] [CrossRef]
- Sardina, F.; Carsetti, C.; Giorgini, L.; Fattorini, G.; Cestra, G.; Rinaldo, C. Cul-4 inhibition rescues spastin levels and reduces defects in hereditary spastic paraplegia models. Brain: A J. Neurol. 2024, 147, 3534–3546. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, N.T.; Sun, Q.; Xue, M.; Zhang, B.; Zinn, K. Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2004, 2, e429. [Google Scholar] [CrossRef]
- Rao, K.; Stone, M.C.; Weiner, A.T.; Gheres, K.W.; Zhou, C.; Deitcher, D.L.; Levitan, E.S.; Rolls, M.M. Spastin, atlastin, and ER relocalization are involved in axon but not dendrite regeneration. Mol. Biol. Cell 2016, 27, 3245–3256. [Google Scholar] [CrossRef]
- Baxter, S.L.; Allard, D.E.; Crowl, C.; Sherwood, N.T. Cold temperature improves mobility and survival in Drosophila models of autosomal-dominant hereditary spastic paraplegia (AD-HSP). Dis. Models Mech. 2014, 7, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Soustelle, L.; Aimond, F.; López-Andrés, C.; Brugioti, V.; Raoul, C.; Layalle, S. ALS-Associated KIF5A Mutation Causes Locomotor Deficits Associated with Cytoplasmic Inclusions, Alterations of Neuromuscular Junctions, and Motor Neuron Loss. J. Neurosci. Off. J. Soc. Neurosci. 2023, 43, 8058–8072. [Google Scholar] [CrossRef]
- Füger, P.; Sreekumar, V.; Schüle, R.; Kern, J.V.; Stanchev, D.T.; Schneider, C.D.; Karle, K.N.; Daub, K.J.; Siegert, V.K.; Flötenmeyer, M.; et al. Spastic paraplegia mutation N256S in the neuronal microtubule motor KIF5A disrupts axonal transport in a Drosophila HSP model. PLoS Genet. 2012, 8, e1003066. [Google Scholar] [CrossRef]
- Djagaeva, I.; Rose, D.J.; Lim, A.; Venter, C.E.; Brendza, K.M.; Moua, P.; Saxton, W.M. Three routes to suppression of the neurodegenerative phenotypes caused by kinesin heavy chain mutations. Genetics 2012, 192, 173–183. [Google Scholar] [CrossRef]
- O’Sullivan, N.C.; Jahn, T.R.; Reid, E.; O’Kane, C.J. Reticulon-like-1, the Drosophila orthologue of the hereditary spastic paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum. Mol. Genet. 2012, 21, 3356–3365. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Orso, G.; Guarato, G.; Brivio, F.; Napoli, B.; Panzeri, E.; Masotti, S.; Santorelli, F.M.; Lamprou, M.; Gumeni, S.; et al. Rescue of lysosomal function as therapeutic strategy for SPG15 hereditary spastic paraplegia. Brain A J. Neurol. 2023, 146, 1103–1120. [Google Scholar] [CrossRef]
- Tian, Y.; Bi, J.; Shui, G.; Liu, Z.; Xiang, Y.; Liu, Y.; Wenk, M.R.; Yang, H.; Huang, X. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011, 7, e1001364. [Google Scholar] [CrossRef] [PubMed]
- Nahm, M.; Lee, M.J.; Parkinson, W.; Lee, M.; Kim, H.; Kim, Y.J.; Kim, S.; Cho, Y.S.; Min, B.M.; Bae, Y.C.; et al. Spartin regulates synaptic growth and neuronal survival by inhibiting BMP-mediated microtubule stabilization. Neuron 2013, 77, 680–695. [Google Scholar] [CrossRef]
- Zhang, Y.V.; Hannan, S.B.; Stapper, Z.A.; Kern, J.V.; Jahn, T.R.; Rasse, T.M. The Drosophila KIF1A Homolog unc-104 Is Important for Site-Specific Synapse Maturation. Front. Cell. Neurosci. 2016, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hannan, S.; Kern, J.; Stanchev, D.T.; Koç, B.; Jahn, T.R.; Rasse, T.M. The KIF1A homolog Unc-104 is important for spontaneous release, postsynaptic density maturation and perisynaptic scaffold organization. Sci. Rep. 2017, 7, 38172. [Google Scholar] [CrossRef]
- Napoli, B.; Gumeni, S.; Forgiarini, A.; Fantin, M.; De Filippis, C.; Panzeri, E.; Vantaggiato, C.; Orso, G. Naringenin Ameliorates Drosophila ReepA Hereditary Spastic Paraplegia-Linked Phenotypes. Front. Neurosci. 2019, 13, 1202. [Google Scholar] [CrossRef]
- Oliva, M.K.; Pérez-Moreno, J.J.; O’Shaughnessy, J.; Wardill, T.J.; O’Kane, C.J. Endoplasmic Reticulum Lumenal Indicators in Drosophila Reveal Effects of HSP-Related Mutations on Endoplasmic Reticulum Calcium Dynamics. Front. Neurosci. 2020, 14, 816. [Google Scholar] [CrossRef]
- Mandik, F.; Kanana, Y.; Rody, J.; Misera, S.; Wilken, B.; Laabs von Holt, B.H.; Klein, C.; Vos, M. A new model for fatty acid hydroxylase-associated neurodegeneration reveals mitochondrial and autophagy abnormalities. Front. Cell Dev. Biol. 2022, 10, 1000553. [Google Scholar] [CrossRef]
- Sujkowski, A.; Rainier, S.; Fink, J.K.; Wessells, R.J. Delayed Induction of Human NTE (PNPLA6) Rescues Neurodegeneration and Mobility Defects of Drosophila swiss cheese (sws) Mutants. PLoS ONE 2015, 10, e0145356. [Google Scholar] [CrossRef]
- Sunderhaus, E.R.; Law, A.D.; Kretzschmar, D. Disease-Associated PNPLA6 Mutations Maintain Partial Functions When Analyzed in Drosophila. Front. Neurosci. 2019, 13, 1207. [Google Scholar] [CrossRef]
- Melentev, P.A.; Ryabova, E.V.; Surina, N.V.; Zhmujdina, D.R.; Komissarov, A.E.; Ivanova, E.A.; Boltneva, N.P.; Makhaeva, G.F.; Sliusarenko, M.I.; Yatsenko, A.S.; et al. Loss of swiss cheese in Neurons Contributes to Neurodegeneration with Mitochondria Abnormalities, Reactive Oxygen Species Acceleration and Accumulation of Lipid Droplets in Drosophila Brain. Int. J. Mol. Sci. 2021, 22, 8275. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.J.; Garcia-Pardo, M.E.; Cole, N.B.; Batnasan, B.; Heneghan, S.; Sohail, A.; Blackstone, C.; O’Sullivan, N.C. Liver X receptor-agonist treatment rescues degeneration in a Drosophila model of hereditary spastic paraplegia. Acta Neuropathol. Commun. 2022, 10, 40. [Google Scholar] [CrossRef]
- Fowler, P.C.; Byrne, D.J.; Blackstone, C.; O’Sullivan, N.C. Loss of the Mitochondrial Fission GTPase Drp1 Contributes to Neurodegeneration in a Drosophila Model of Hereditary Spastic Paraplegia. Brain Sci. 2020, 10, 646. [Google Scholar] [CrossRef]
- Gan-Or, Z.; Bouslam, N.; Birouk, N.; Lissouba, A.; Chambers, D.B.; Vérièpe, J.; Androschuk, A.; Laurent, S.B.; Rochefort, D.; Spiegelman, D.; et al. Mutations in CAPN1 Cause Autosomal-Recessive Hereditary Spastic Paraplegia. Am. J. Hum. Genet. 2016, 98, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jin, X.; Xu, M.; Xi, Y.; Lu, W.; Yang, X.; Guan, M.X.; Ge, W. FARS2 deficiency in Drosophila reveals the developmental delay and seizure manifested by aberrant mitochondrial tRNA metabolism. Nucleic Acids Res. 2021, 49, 13108–13121. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Li, R.; He, C.; Chen, Q.; Xu, C.; Shen, L.; Chen, K.; Wu, Y. Hedgehog pathway is negatively regulated during the development of Drosophila melanogaster PheRS-m (Drosophila homologs gene of human FARS2) mutants. Hum. Cell 2023, 36, 121–131. [Google Scholar] [CrossRef]
- Schuurs-Hoeijmakers, J.H.; Geraghty, M.T.; Kamsteeg, E.J.; Ben-Salem, S.; de Bot, S.T.; Nijhof, B.; van de Vondervoort, I.I.; van der Graaf, M.; Nobau, A.C.; Otte-Höller, I.; et al. Mutations in DDHD2, encoding an intracellular phospholipase A (1), cause a recessive form of complex hereditary spastic paraplegia. Am. J. Hum. Genet. 2012, 91, 1073–1081. [Google Scholar] [CrossRef]
- Lobato, A.G.; Ortiz-Vega, N.N.; Canic, T.; Tao, X.; Bucan, N.; Ruan, K.; Rebelo, A.P.; Schule, R.; Zuchner, S.; Syed, S.; et al. Loss of Fic causes progressive neurodegeneration in a Drosophila model of hereditary spastic paraplegia. Biochimica et biophysica acta. Mol. Basis Dis. 2024, 1870, 167348. [Google Scholar] [CrossRef]
- Mundwiler, A.; Shakkottai, V.G. Autosomal-dominant cerebellar ataxias. Handb. Clin. Neurol. 2018, 147, 173–185. [Google Scholar] [CrossRef]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nature reviews. Neuroscience 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Lu, B.; Vogel, H. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. 2009, 4, 315–342. [Google Scholar] [CrossRef]
- Bergeron, D.; Lapointe, C.; Bissonnette, C.; Tremblay, G.; Motard, J.; Roucou, X. An out-of-frame overlapping reading frame in the ataxin-1 coding sequence encodes a novel ataxin-1 interacting protein. J. Biol. Chem. 2013, 288, 21824–21835. [Google Scholar] [CrossRef]
- Fernandez-Funez, P.; Nino-Rosales, M.L.; de Gouyon, B.; She, W.C.; Luchak, J.M.; Martinez, P.; Turiegano, E.; Benito, J.; Capovilla, M.; Skinner, P.J.; et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 2000, 408, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lessing, D.; Bonini, N.M. Polyglutamine genes interact to modulate the severity and progression of neurodegeneration in Drosophila. PLoS Biol. 2008, 6, e29. [Google Scholar] [CrossRef] [PubMed]
- Warrick, J.M.; Paulson, H.L.; Gray-Board, G.L.; Bui, Q.T.; Fischbeck, K.H.; Pittman, R.N.; Bonini, N.M. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 1998, 93, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Warrick, J.M.; Morabito, L.M.; Bilen, J.; Gordesky-Gold, B.; Faust, L.Z.; Paulson, H.L.; Bonini, N.M. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol. Cell 2005, 18, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.R.; Kirby, K.; Hilliker, A.J.; Phillips, J.P. RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum. Mol. Genet. 2005, 14, 3397–3405. [Google Scholar] [CrossRef]
- Navarro, J.A.; Ohmann, E.; Sanchez, D.; Botella, J.A.; Liebisch, G.; Moltó, M.D.; Ganfornina, M.D.; Schmitz, G.; Schneuwly, S. Altered lipid metabolism in a Drosophila model of Friedreich’s ataxia. Hum. Mol. Genet. 2010, 19, 2828–2840. [Google Scholar] [CrossRef]
- Petersen, A.J.; Rimkus, S.A.; Wassarman, D.A. ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, E656–E664. [Google Scholar] [CrossRef]
- Bolus, H.; Crocker, K.; Boekhoff-Falk, G.; Chtarbanova, S. Modeling Neurodegenerative Disorders in Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 3055. [Google Scholar] [CrossRef]
- Al-Ramahi, I.; Lam, Y.C.; Chen, H.K.; de Gouyon, B.; Zhang, M.; Pérez, A.M.; Branco, J.; de Haro, M.; Patterson, C.; Zoghbi, H.Y.; et al. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J. Biol. Chem. 2006, 281, 26714–26724. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.M.; Snoddy, C.A.; York, P.M.; Davis, S.M.; Hunter, M.F.; Krishnan, N. Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase. Biology 2024, 13, 854. [Google Scholar] [CrossRef]
- Petrauskas, A.; Fortunati, D.L.; Kandi, A.R.; Pothapragada, S.S.; Agrawal, K.; Singh, A.; Huelsmeier, J.; Hillebrand, J.; Brown, G.; Chaturvedi, D.; et al. Structured and disordered regions of Ataxin-2 contribute differently to the specificity and efficiency of mRNP granule formation. PLoS Genet. 2024, 20, e1011251. [Google Scholar] [CrossRef]
- Del Castillo, U.; Norkett, R.; Lu, W.; Serpinskaya, A.; Gelfand, V.I. Ataxin-2 is essential for cytoskeletal dynamics and neurodevelopment in Drosophila. iScience 2021, 25, 103536. [Google Scholar] [CrossRef]
- Sujkowski, A.; Richardson, K.; Prifti, M.V.; Wessells, R.J.; Todi, S.V. Endurance exercise ameliorates phenotypes in Drosophila models of spinocerebellar ataxias. eLife 2022, 11, e75389. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Prifti, M.V.; Sujkowski, A.; Libohova, K.; Blount, J.R.; Hong, L.; Tsou, W.L.; Todi, S.V. Drosophila as a Model of Unconventional Translation in Spinocerebellar Ataxia Type 3. Cells 2022, 11, 1223. [Google Scholar] [CrossRef]
- Denha, S.A.; Atang, A.E.; Hays, T.S.; Avery, A.W. β-III-spectrin N-terminus is required for high-affinity actin binding and SCA5 neurotoxicity. Sci. Rep. 2022, 12, 1726. [Google Scholar] [CrossRef]
- Avery, A.W.; Thomas, D.D.; Hays, T.S. β-III-spectrin spinocerebellar ataxia type 5 mutation reveals a dominant cytoskeletal mechanism that underlies dendritic arborization. Proc. Natl. Acad. Sci. USA 2017, 114, E9376–E9385. [Google Scholar] [CrossRef]
- Lorenzo, D.N.; Li, M.G.; Mische, S.E.; Armbrust, K.R.; Ranum, L.P.; Hays, T.S. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J. Cell Biol. 2010, 189, 143–158. [Google Scholar] [CrossRef]
- Tsou, W.L.; Qiblawi, S.H.; Hosking, R.R.; Gomez, C.M.; Todi, S.V. Polyglutamine length-dependent toxicity from α1ACT in Drosophila models of spinocerebellar ataxia type 6. Biol. Open 2016, 5, 1770–1775. [Google Scholar] [CrossRef]
- Tsou, W.L.; Hosking, R.R.; Burr, A.A.; Sutton, J.R.; Ouyang, M.; Du, X.; Gomez, C.M.; Todi, S.V. DnaJ-1 and karyopherin α3 suppress degeneration in a new Drosophila model of Spinocerebellar Ataxia Type 6. Hum. Mol. Genet. 2015, 24, 4385–4396. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Rosenfeld, J.A.; Yamamoto, S.; Harel, T.; Zuo, Z.; Hall, M.; Wierenga, K.J.; Pastore, M.T.; Bartholomew, D.; Delgado, M.R.; et al. Members of the UDN Clinically severe CACNA1A alleles affect synaptic function and neurodegeneration differentially. PLoS Genet. 2017, 13, e1006905. [Google Scholar] [CrossRef]
- Nath, S.; Caron, N.S.; May, L.; Gluscencova, O.B.; Kolesar, J.; Brady, L.; Kaufman, B.A.; Boulianne, G.L.; Rodriguez, A.R.; Tarnopolsky, M.A.; et al. Functional characterization of variants of unknown significance in a spinocerebellar ataxia patient using an unsupervised machine learning pipeline. Hum. Genome Var. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Latouche, M.; Lasbleiz, C.; Martin, E.; Monnier, V.; Debeir, T.; Mouatt-Prigent, A.; Muriel, M.P.; Morel, L.; Ruberg, M.; Brice, A.; et al. A conditional pan-neuronal Drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Mutsuddi, M.; Marshall, C.M.; Benzow, K.A.; Koob, M.D.; Rebay, I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr. Biol. CB 2004, 14, 302–308. [Google Scholar] [CrossRef]
- Tripathi, B.K.; Das, R.; Mukherjee, A.; Mutsuddi, M. Interaction of Spoonbill with Prospero in Drosophila: Implications in neuroblast development. Genesis 2017, 55, e23049. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lee, C.M.; Lee, L.C.; Tung, L.C.; Hsieh-Li, H.M.; Lee-Chen, G.J.; Su, M.T. Mitochondrial dysfunction and oxidative stress contribute to the pathogenesis of spinocerebellar ataxia type 12 (SCA12). J. Biol. Chem. 2011, 286, 21742–21754. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Iradi, C.; Bickford, J.S.; Khare, S.; Hall, A.; Nick, J.A.; Salmasinia, D.; Wawrowsky, K.; Bannykh, S.; Huynh, D.P.; Rincon-Limas, D.E.; et al. KCNC3(R420H), a K(+) channel mutation causative in spinocerebellar ataxia 13 displays aberrant intracellular trafficking. Neurobiol. Dis. 2014, 71, 270–279. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hasan, G. Functional complementation of Drosophila itpr mutants by rat Itpr1. J. Neurogenet. 2012, 26, 328–337. [Google Scholar] [CrossRef]
- Montalvo-Méndez, R.J.; Cárdenas-Tueme, M.; Reséndez-Pérez, D. Drosophila in the study of hTBP protein interactions in the development and modeling of SCA17. Drosophila en el estudio de las interacciones proteicas de hTBP en el desarrollo y modelaje de SCA17. Gac. Medica De Mex. 2024, 160, 1–8. [Google Scholar] [CrossRef]
- Patel, N.; Alam, N.; Libohova, K.; Dulay, R.; Todi, S.V.; Sujkowski, A. Phenotypic defects from the expression of wild-type and pathogenic TATA-binding proteins in new Drosophila models of Spinocerebellar Ataxia Type 17. G3: Genes Genomes Genet. 2023, 13, jkad180. [Google Scholar] [CrossRef]
- Hsu, T.C.; Wang, C.K.; Yang, C.Y.; Lee, L.C.; Hsieh-Li, H.M.; Ro, L.S.; Chen, C.M.; Lee-Chen, G.J.; Su, M.T. Deactivation of TBP contributes to SCA17 pathogenesis. Hum. Mol. Genet. 2014, 23, 6878–6893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, J.; Jegga, A.G.; Zhang, M.; Deng, J.; Liu, J.; Gordon, C.B.; Aronow, B.J.; Lu, L.J.; Zhang, B.; Ma, J. A Drosophila model of the neurodegenerative disease SCA17 reveals a role of RBP-J/Su(H) in modulating the pathological outcome. Hum. Mol. Genet. 2011, 20, 3424–3436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pareek, G.; Pallanck, L.J. Inactivation of the mitochondrial protease Afg3l2 results in severely diminished respiratory chain activity and widespread defects in mitochondrial gene expression. PLoS Genet. 2020, 16, e1009118. [Google Scholar] [CrossRef]
- Shibata, T.; Nagano, K.; Ueyama, M.; Ninomiya, K.; Hirose, T.; Nagai, Y.; Ishikawa, K.; Kawai, G.; Nakatani, K. Small molecule targeting r(UGGAA)n disrupts RNA foci and alleviates disease phenotype in Drosophila model. Nat. Commun. 2021, 12, 236. [Google Scholar] [CrossRef]
- Tripathy, D.; Vignoli, B.; Ramesh, N.; Polanco, M.J.; Coutelier, M.; Stephen, C.D.; Canossa, M.; Monin, M.L.; Aeschlimann, P.; Turberville, S.; et al. Mutations in TGM6 induce the unfolded protein response in SCA35. Hum. Mol. Genet. 2017, 26, 3749–3762. [Google Scholar] [CrossRef]
- Nisoli, I.; Chauvin, J.P.; Napoletano, F.; Calamita, P.; Zanin, V.; Fanto, M.; Charroux, B. Neurodegeneration by polyglutamine Atrophin is not rescued by induction of autophagy. Cell Death Differ. 2010, 17, 1577–1587. [Google Scholar] [CrossRef]
- Charroux, B.; Fanto, M. The fine line between waste disposal and recycling: DRPLA fly models illustrate the importance of completing the autophagy cycle for rescuing neurodegeneration. Autophagy 2010, 6, 667–669. [Google Scholar] [CrossRef]
- Navarro, J.A.; Llorens, J.V.; Soriano, S.; Botella, J.A.; Schneuwly, S.; Martínez-Sebastián, M.J.; Moltó, M.D. Overexpression of human and fly frataxins in Drosophila provokes deleterious effects at biochemical, physiological and developmental levels. PLoS ONE 2011, 6, e21017. [Google Scholar] [CrossRef]
- Russi, M.; Martin, E.; D’Autréaux, B.; Tixier, L.; Tricoire, H.; Monnier, V. A Drosophila model of Friedreich ataxia with CRISPR/Cas9 insertion of GAA repeats in the frataxin gene reveals in vivo protection by N-acetyl cysteine. Hum. Mol. Genet. 2020, 29, 2831–2844. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, S.A.; Katzenberger, R.J.; Trinh, A.T.; Dodson, G.E.; Tibbetts, R.S.; Wassarman, D.A. Mutations in String/CDC25 inhibit cell cycle re-entry and neurodegeneration in a Drosophila model of Ataxia telangiectasia. Genes Dev. 2008, 22, 1205–1220. [Google Scholar] [CrossRef]
- Petersen, A.J.; Katzenberger, R.J.; Wassarman, D.A. The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics 2013, 194, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Seong, E.; Insolera, R.; Dulovic, M.; Kamsteeg, E.J.; Trinh, J.; Brüggemann, N.; Sandford, E.; Li, S.; Ozel, A.B.; Li, J.Z.; et al. Mutations in VPS13D lead to a new recessive ataxia with spasticity and mitochondrial defects. Ann. Neurol. 2018, 83, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Uchida, A.; Komatsu, A.; Suzuki, E.; Ibuki, I.; Asada, M.; Shiwaku, H.; Tamura, T.; Hoshino, M.; Okazawa, H.; et al. Loss of yata, a novel gene regulating the subcellular localization of APPL, induces deterioration of neural tissues and lifespan shortening. PLoS ONE 2009, 4, e4466. [Google Scholar] [CrossRef]
- Duan, R.; Shi, Y.; Yu, L.; Zhang, G.; Li, J.; Lin, Y.; Guo, J.; Wang, J.; Shen, L.; Jiang, H.; et al. UBA5 Mutations Cause a New Form of Autosomal Recessive Cerebellar Ataxia. PLoS ONE 2016, 11, e0149039. [Google Scholar] [CrossRef]
- Pan, X.; Alvarez, A.N.; Ma, M.; Lu, S.; Crawford, M.W.; Briere, L.C.; Kanca, O.; Yamamoto, S.; Sweetser, D.A.; Wilson, J.L.; et al. Allelic strengths of encephalopathy-associated UBA5 variants correlate between in vivo and in vitro assays. eLife 2023, 12, RP89891. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sandford, E.; Gatica, D.; Qiu, Y.; Liu, X.; Zheng, Y.; Schulman, B.A.; Xu, J.; Semple, I.; Ro, S.H.; et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife 2016, 5, e12245. [Google Scholar] [CrossRef]
- Eidhof, I.; Baets, J.; Kamsteeg, E.J.; Deconinck, T.; van Ninhuijs, L.; Martin, J.J.; Schüle, R.; Züchner, S.; De Jonghe, P.; Schenck, A.; et al. GDAP2 mutations implicate susceptibility to cellular stress in a new form of cerebellar ataxia. Brain A J. Neurol. 2018, 141, 2592–2604. [Google Scholar] [CrossRef]
- Rebelo, A.P.; Eidhof, I.; Cintra, V.P.; Guillot-Noel, L.; Pereira, C.V.; Timmann, D.; Traschütz, A.; Schöls, L.; Coarelli, G.; Durr, A.; et al. Biallelic loss-of-function variations in PRDX3 cause cerebellar ataxia. Brain A J. Neurol. 2021, 144, 1467–1481. [Google Scholar] [CrossRef]
- Yu, L.; Li, G.; Deng, J.; Jiang, X.; Xue, J.; Zhu, Y.; Huang, W.; Tang, B.; Duan, R. The UFM1 cascade times mitosis entry associated with microcephaly. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 1319–1330. [Google Scholar] [CrossRef]
- Choudhury, S.D.; Vs, A.; Mushtaq, Z.; Kumar, V. Altered translational repression of an RNA-binding protein, Elav by AOA2-causative Senataxin mutation. Synapse 2017, 71, e21969. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Choudhury, S.D.; Gangwar, S.K.; Orso, G.; Kumar, V. Human Senataxin Modulates Structural Plasticity of the Neuromuscular Junction in Drosophila through a Neuronally Conserved TGFβ Signalling Pathway. Neuro-Degener. Dis. 2016, 16, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Okunushi, R.; Hara, T.; Hashiguchi, A.; Yuan, J.; Yoshimura, A.; Murayama, K.; Ohtake, A.; Ando, M.; Hiramatsu, Y.; et al. Mutations in COA7 cause spinocerebellar ataxia with axonal neuropathy. Brain A J. Neurol. 2018, 141, 1622–1636. [Google Scholar] [CrossRef]
- Casas-Tintó, S. Drosophila as a Model for Human Disease: Insights into Rare and Ultra-Rare Diseases. Insects 2024, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; Sanchez-Pernaute, R. Mice in translational neuroscience: What R we doing? Prog. Neurobiol. 2022, 217, 102330. [Google Scholar] [CrossRef]
- Chen-Tsai, R.Y. Integrase-Mediated Targeted Transgenics Through Pronuclear Microinjection. Methods Mol. Biol. 2020, 2066, 35–46. [Google Scholar] [CrossRef]
- Schilit, S.L.P.; Ohtsuka, M.; Quadros, R.M.; Gurumurthy, C.B. Pronuclear Injection-Based Targeted Transgenesis. Curr. Protoc. Hum. Genet. 2016, 91, 15.10.1–15.10.28. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Wangler, M.F.; Yamamoto, S. The fruit fly at the interface of diagnosis and pathogenic mechanisms of rare and common human diseases. Hum. Mol. Genet. 2019, 28, R207–R214. [Google Scholar] [CrossRef]
- Ma, M.; Moulton, M.J.; Lu, S.; Bellen, H.J. ‘Fly-ing’ from rare to common neurodegenerative disease mechanisms. Trends Genet. TIG 2022, 38, 972–984. [Google Scholar] [CrossRef]
- Kottmeier, R.; Bittern, J.; Schoofs, A.; Scheiwe, F.; Matzat, T.; Pankratz, M.; Klämbt, C. Wrapping glia regulates neuronal signaling speed and precision in the peripheral nervous system of Drosophila. Nat. Commun. 2020, 11, 4491. [Google Scholar] [CrossRef]
- Heiduschka, S.; Prigione, A. iPSC models of mitochondrial diseases. Neurobiol. Dis. 2025, 207, 106822. [Google Scholar] [CrossRef]
- Seguin, A.; Monnier, V.; Palandri, A.; Bihel, F.; Rera, M.; Schmitt, M.; Camadro, J.M.; Tricoire, H.; Lesuisse, E. A Yeast/Drosophila Screen to Identify New Compounds Overcoming Frataxin Deficiency. Oxidative Med. Cell. Longev. 2015, 2015, 565140. [Google Scholar] [CrossRef]
- Verheyen, E.M. The power of Drosophila in modeling human disease mechanisms. Dis. Models Mech. 2022, 15, dmm049549. [Google Scholar] [CrossRef] [PubMed]
- Rudrapatna, V.A.; Cagan, R.L.; Das, T.K. Drosophila cancer models. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2012, 241, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Bangi, E.; Murgia, C.; Teague, A.G.; Sansom, O.J.; Cagan, R.L. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 2016, 7, 13615. [Google Scholar] [CrossRef]
- Zhang, B.; Burke, R. Copper homeostasis and the ubiquitin proteasome system. Met. Integr. Biometal Sci. 2023, 15, mfad010. [Google Scholar] [CrossRef] [PubMed]
- Rohde, P.D.; Østergaard, S.; Kristensen, T.N.; Sørensen, P.; Loeschcke, V.; Mackay, T.F.C.; Sarup, P. Functional Validation of Candidate Genes Detected by Genomic Feature Models. G3 Genes Genomes Genet. 2018, 8, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Giacomotto, J.; Ségalat, L. High-throughput screening and small animal models where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).