The Form and Function of Retinal Ganglion Cells in Diabetes
Abstract
1. Introduction
2. Apoptosis of RGCs and Other Neural Cells in Diabetes
3. Mechanisms of RGC Loss in Diabetes
4. Thinning of the Nerve Fiber Layer Reflects the Loss of RGCs
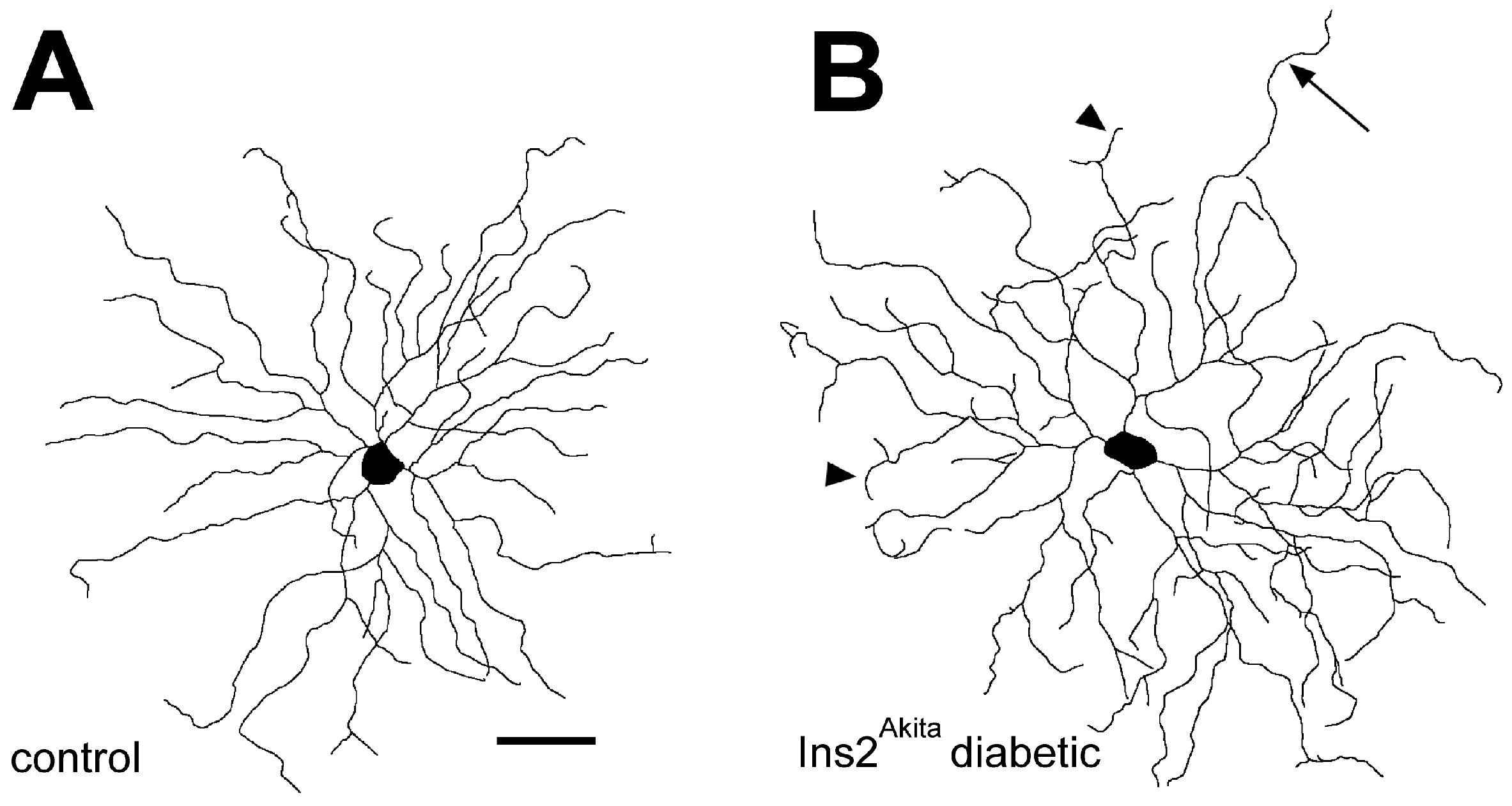
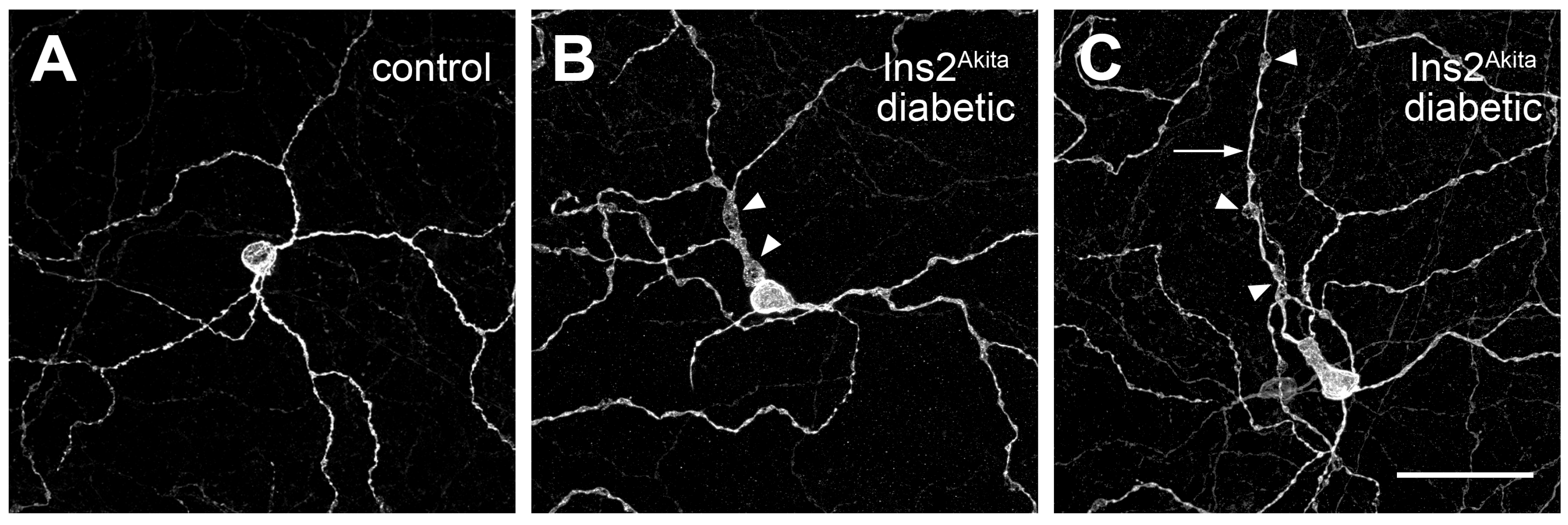
5. Diabetes Alters the Dendritic Field Morphology of RGCs
6. RGC Pathology Reduces the Scotopic Threshold Response
7. Diabetes Alters the Pattern-ERG Response
8. Diabetes Alters the Single-Cell Electrophysiology of RGCs
9. Diabetes Causes Pathological Changes Within the Optic Nerve
10. Diabetes Compromises the Function of Intrinsically Photosensitive RGCs
11. Neuroprotection of RGCs
12. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedenwald, J.S. Diabetic retinopathy. Am. J. Ophthalmol. 1950, 33, 1187–1199. [Google Scholar] [CrossRef]
- Friedenwald, J.; Day, R. The vascular lesions of diabetic retinopathy. Bull. Johns Hopkins Hosp. 1950, 86, 253–254. [Google Scholar]
- Engerman, R.L.; Bloodworth, J.M., Jr. Experimental diabetic retinopathy in dogs. Arch. Ophthalmol. 1965, 73, 205–210. [Google Scholar] [CrossRef]
- Wolter, J.R. Diabetic retinopathy. Am. J. Ophthalmol. 1961, 51, 1123–1139. [Google Scholar] [CrossRef]
- Bloodworth, J.M., Jr. Diabetic retinopathy. Diabetes 1962, 11, 1–22. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.K.; Abramoff, M.D. Diabetic retinopathy is a neurodegenerative disorder. Vision Res. 2017, 139, 101–107. [Google Scholar] [CrossRef]
- Barber, A.J. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 283–290. [Google Scholar] [CrossRef]
- Barber, A.J. Diabetic retinopathy: Recent advances towards understanding neurodegeneration and vision loss. Sci. China Life Sci. 2015, 58, 541–549. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita Mouse as a Model of Early Retinal Complications in Diabetes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2210–2218. [Google Scholar] [CrossRef]
- Gastinger, M.J.; Singh, R.S.; Barber, A.J. Loss of Cholinergic and Dopaminergic Amacrine Cells in Streptozotocin-Diabetic Rat and Ins2Akita-Diabetic Mouse Retinas. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3143–3150. [Google Scholar] [CrossRef]
- Qin, Y.; Xu, G.; Wang, W. Dendritic abnormalities in retinal ganglion cells of three-month diabetic rats. Curr. Eye Res. 2006, 31, 967–974. [Google Scholar] [CrossRef]
- Gastinger, M.J.; Kunselman, A.R.; Conboy, E.E.; Bronson, S.K.; Barber, A.J. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2635–2642. [Google Scholar] [CrossRef]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- Yang, J.H.; Kwak, H.W.; Kim, T.G.; Han, J.; Moon, S.W.; Yu, S.Y. Retinal Neurodegeneration in Type II Diabetic Otsuka Long-Evans Tokushima Fatty Rats. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3844–3851. [Google Scholar] [CrossRef]
- Lee, V.K.; Hosking, B.M.; Holeniewska, J.; Kubala, E.C.; Lundh von Leithner, P.; Gardner, P.J.; Foxton, R.H.; Shima, D.T. BTBR ob/ob mouse model of type 2 diabetes exhibits early loss of retinal function and retinal inflammation followed by late vascular changes. Diabetologia 2018, 61, 2422–2432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Y.; Xie, P.; Cheng, H.; Song, Q.; Su, T.; Yuan, S.; Liu, Q. Retinal Neurodegeneration in db/db Mice at the Early Period of Diabetes. J. Ophthalmol. 2015, 2015, 757412. [Google Scholar] [CrossRef]
- Abu-El-Asrar, A.M.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, D.; Chen, X.; Zhao, L.; Tian, Q.; Liu, C.; Zhou, B.L. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Mol. Vis. 2012, 18, 1411–1420. [Google Scholar]
- Fernandez, D.C.; Sande, P.H.; de Zavalia, N.; Belforte, N.; Dorfman, D.; Casiraghi, L.P.; Golombek, D.; Rosenstein, R.E. Effect of experimental diabetic retinopathy on the non-image-forming visual system. Chronobiol. Int. 2013, 30, 583–597. [Google Scholar] [CrossRef]
- Hombrebueno, J.R.; Chen, M.; Penalva, R.G.; Xu, H. Loss of synaptic connectivity, particularly in second order neurons is a key feature of diabetic retinal neuropathy in the Ins2Akita mouse. PLoS ONE 2014, 9, e97970. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Du, J.; Song, J.; Li, Y.; Wu, M.; Zhou, J.; Wu, S. Loss-of-function mutation of serine racemase attenuates retinal ganglion cell loss in diabetic mice. Exp. Eye Res. 2018, 175, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Xu, Y.; Xiong, S.; Zhang, J.; Gu, Q.; Ke, B.; Xu, X. Involvement of ciliary neurotrophic factor in early diabetic retinal neuropathy in streptozotocin-induced diabetic rats. Eye 2018, 32, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Church, K.A.; Rodriguez, D.; Vanegas, D.; Gutierrez, I.L.; Cardona, S.M.; Madrigal, J.L.M.; Kaur, T.; Cardona, A.E. Models of microglia depletion and replenishment elicit protective effects to alleviate vascular and neuronal damage in the diabetic murine retina. J. Neuroinflamm. 2022, 19, 300. [Google Scholar] [CrossRef]
- Piri, N.; Kwong, J.M.; Song, M.; Caprioli, J. Expression of hermes gene is restricted to the ganglion cells in the retina. Neurosci. Lett. 2006, 405, 40–45. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Abbas, S.N. Diabetes-induced mitochondrial dysfunction in the retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5327–5334. [Google Scholar] [CrossRef]
- Adamiec-Mroczek, J.; Zajac-Pytrus, H.; Misiuk-Hojlo, M. Caspase-Dependent Apoptosis of Retinal Ganglion Cells During the Development of Diabetic Retinopathy. Adv. Clin. Exp. Med. 2015, 24, 531–535. [Google Scholar] [CrossRef]
- Park, H.L.; Kim, J.H.; Park, C.K. Different contributions of autophagy to retinal ganglion cell death in the diabetic and glaucomatous retinas. Sci. Rep. 2018, 8, 13321. [Google Scholar] [CrossRef]
- Giardino, I.; Fard, A.K.; Hatchell, D.L.; Brownlee, M. Aminoguanidine inhibits reactive oxygen species formation, lipid peroxidation, and oxidant-induced apoptosis. Diabetes 1998, 47, 1114–1120. [Google Scholar] [CrossRef]
- Baynes, J.W.; Thorpe, S.R. Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 1999, 48, 1–9. [Google Scholar] [CrossRef]
- Alka, K.; Kumar, J.; Kowluru, R.A. Impaired mitochondrial dynamics and removal of the damaged mitochondria in diabetic retinopathy. Front. Endocrinol. 2023, 14, 1160155. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, P.; Kowluru, R.A. Homocysteine and mitochondrial quality control in diabetic retinopathy. Eye Vision 2024, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Malaviya, P.; Kumar, J.; Kowluru, R.A. Role of ferroptosis in mitochondrial damage in diabetic retinopathy. Free Radic. Biol. Med. 2024, 225, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.H.; Hu, J.; Li, S.; Wu, Q.; Lu, P. P66Shc expression in diabetic rat retina. BMC Ophthalmol. 2018, 18, 58. [Google Scholar] [CrossRef]
- Mishra, M.; Duraisamy, A.J.; Bhattacharjee, S.; Kowluru, R.A. Adaptor Protein p66Shc: A Link Between Cytosolic and Mitochondrial Dysfunction in the Development of Diabetic Retinopathy. Antioxid. Redox Signal. 2019, 30, 1621–1634. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, W.; Zhao, Y.; Shu, X.; Wang, W.; Wang, D.; Yang, Y.; He, Z.; Wang, X.; Ying, Y. GSK3beta-mediated tau hyperphosphorylation triggers diabetic retinal neurodegeneration by disrupting synaptic and mitochondrial functions. Mol. Neurodegener. 2018, 13, 62. [Google Scholar] [CrossRef]
- Haider, S.Z.; Sadanandan, N.P.; Joshi, P.G.; Mehta, B. Early Diabetes Induces Changes in Mitochondrial Physiology of Inner Retinal Neurons. Neuroscience 2019, 406, 140–149. [Google Scholar] [CrossRef]
- Santiago, A.R.; Rosa, S.C.; Santos, P.F.; Cristovao, A.J.; Barber, A.J.; Ambrosio, A.F. Elevated glucose changes the expression of ionotropic glutamate receptor subunits and impairs calcium homeostasis in retinal neural cells. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4130–4137. [Google Scholar] [CrossRef]
- Alam, N.M.; Mills, W.C.t.; Wong, A.A.; Douglas, R.M.; Szeto, H.H.; Prusky, G.T. A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Dis. Models Mech. 2015, 8, 701–710. [Google Scholar]
- Daniel, A.; Premilovac, D.; Foa, L.; Feng, Z.; Shah, K.; Zhang, Q.; Woolley, K.L.; Bye, N.; Smith, J.A.; Gueven, N. Novel Short-Chain Quinones to Treat Vision Loss in a Rat Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 20. [Google Scholar] [CrossRef]
- Lam, C.H.; Zuo, B.; Chan, H.H.; Leung, T.W.; Abokyi, S.; Catral, K.P.C.; Tse, D.Y. Coenzyme Q10 eyedrops conjugated with vitamin E TPGS alleviate neurodegeneration and mitochondrial dysfunction in the diabetic mouse retina. Front. Cell. Neurosci. 2024, 18, 1404987. [Google Scholar] [CrossRef]
- Xu, Z.; Wei, Y.; Gong, J.; Cho, H.; Park, J.K.; Sung, E.R.; Huang, H.; Wu, L.; Eberhart, C.; Handa, J.T.; et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia 2014, 57, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mishra, M. Epigenetic regulation of redox signaling in diabetic retinopathy: Role of Nrf2. Free Radic. Biol. Med. 2017, 103, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Zhong, Q.; Kowluru, R.A. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: Implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic. Biol. Med. 2014, 75, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Zhong, Q.; Kowluru, R.A. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7256–7265. [Google Scholar] [CrossRef]
- Fang, J.; Bai, W.; Yang, L. Astaxanthin inhibits oxidative stress and apoptosis in diabetic retinopathy. Acta Histochem. 2023, 125, 152069. [Google Scholar] [CrossRef]
- Yeh, P.T.; Huang, H.W.; Yang, C.M.; Yang, W.S.; Yang, C.H. Astaxanthin Inhibits Expression of Retinal Oxidative Stress and Inflammatory Mediators in Streptozotocin-Induced Diabetic Rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef]
- Dong, L.Y.; Jin, J.; Lu, G.; Kang, X.L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef]
- Chous, A.P.; Richer, S.P.; Gerson, J.D.; Kowluru, R.A. The Diabetes Visual Function Supplement Study (DiVFuSS). Br. J. Ophthalmol. 2016, 100, 227–234. [Google Scholar] [CrossRef]
- Baccouche, B.; Benlarbi, M.; Barber, A.J.; Ben Chaouacha-Chekir, R. Short-Term Administration of Astaxanthin Attenuates Retinal Changes in Diet-Induced Diabetic Psammomys obesus. Curr. Eye Res. 2018, 43, 1177–1189. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoo, W.S.; Choi, M.; Chung, I.; Yoo, J.M.; Choi, W.S. Increased O-GlcNAcylation of NF-kappaB Enhances Retinal Ganglion Cell Death in Streptozotocin-induced Diabetic Retinopathy. Curr. Eye Res. 2016, 41, 249–257. [Google Scholar] [CrossRef]
- Miller, W.P.; Mihailescu, M.L.; Yang, C.; Barber, A.J.; Kimball, S.R.; Jefferson, L.S.; Dennis, M.D. The Translational Repressor 4E-BP1 Contributes to Diabetes-Induced Visual Dysfunction. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1327–1337. [Google Scholar] [CrossRef]
- Miller, W.P.; Yang, C.; Mihailescu, M.L.; Moore, J.A.; Dai, W.; Barber, A.J.; Dennis, M.D. Deletion of the Akt/mTORC1 Repressor REDD1 Prevents Visual Dysfunction in a Rodent Model of Type 1 Diabetes. Diabetes 2018, 67, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Dierschke, S.K.; Miller, W.P.; Favate, J.S.; Shah, P.; Imamura Kawasawa, Y.; Salzberg, A.C.; Kimball, S.R.; Jefferson, L.S.; Dennis, M.D. O-GlcNAcylation alters the selection of mRNAs for translation and promotes 4E-BP1-dependent mitochondrial dysfunction in the retina. J. Biol. Chem. 2019, 294, 5508–5520. [Google Scholar] [CrossRef]
- Miller, W.P.; Toro, A.L.; Barber, A.J.; Dennis, M.D. REDD1 Activates a ROS-Generating Feedback Loop in the Retina of Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2369–2379. [Google Scholar] [CrossRef]
- Xu, Y.; Ola, M.S.; Berkich, D.A.; Gardner, T.W.; Barber, A.J.; Palmieri, F.; Hutson, S.M.; Lanoue, K.F. Energy sources for glutamate neurotransmission in the retina: Absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J. Neurochem. 2007, 101, 120–131. [Google Scholar] [CrossRef]
- Barber, A.J.; Robinson, W.F.; Jackson, G.R. Neurodegeneration in Diabetic Retinopathy. In Visual Dysfunction in Diabetes: The Science of Patient Impairment and Health Care; Tombran-Tink, J., Barnstable, C.J., Gardner, T.W., Eds.; Springer: New York, NY, USA, 2012; pp. 189–209. [Google Scholar]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Lieth, E.; LaNoue, K.F.; Antonetti, D.A.; Ratz, M. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Exp. Eye Res. 2000, 70, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Engerman, R.L.; Case, G.L.; Kern, T.S. Retinal glutamate in diabetes and effect of antioxidants. Neurochem. Int. 2001, 38, 385–390. [Google Scholar] [CrossRef]
- Lieth, E.; LaNoue, K.F.; Berkich, D.A.; Xu, B.; Ratz, M.; Taylor, C.; Hutson, S.M. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J. Neurochem. 2001, 76, 1712–1723. [Google Scholar] [CrossRef]
- Hutson, S.M.; Lieth, E.; LaNoue, K.F. Function of leucine in excitatory neurotransmitter metabolism in the central nervous system. J. Nutr. 2001, 131, 846S–850S. [Google Scholar] [CrossRef]
- Gowda, K.; Zinnanti, W.J.; LaNoue, K.F. The influence of diabetes on glutamate metabolism in retinas. J. Neurochem. 2011, 117, 309–320. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Wu, Y.; Zhang, F. Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism. Nutrients 2023, 15, 30. [Google Scholar] [CrossRef]
- Lau, J.C.; Kroes, R.A.; Moskal, J.R.; Linsenmeier, R.A. Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol. Vis. 2013, 19, 1538–1553. [Google Scholar] [PubMed]
- Chihara, E.; Matsuoka, T.; Ogura, Y.; Matsumura, M. Retinal nerve fiber layer defect as an early manifestation of diabetic retinopathy. Ophthalmology 1993, 100, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Ozdek, S.; Lonneville, Y.H.; Onol, M.; Yetkin, I.; Hasanreisoglu, B.B. Assessment of nerve fiber layer in diabetic patients with scanning laser polarimetry. Eye 2002, 16, 761–765. [Google Scholar] [CrossRef]
- Biallosterski, C.; van Velthoven, M.E.; Michels, R.P.; Schlingemann, R.O.; DeVries, J.H.; Verbraak, F.D. Decreased optical coherence tomography-measured pericentral retinal thickness in patients with diabetes mellitus type 1 with minimal diabetic retinopathy. Br. J. Ophthalmol. 2007, 91, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Cabrera DeBuc, D.; Somfai, G.M. Early detection of retinal thickness changes in diabetes using Optical Coherence Tomography. Med. Sci. Monit. 2010, 16, MT15–MT21. [Google Scholar]
- van Dijk, H.W.; Kok, P.H.B.; Garvin, M.; Sonka, M.; Devries, J.H.; Michels, R.P.J.; van Velthoven, M.E.J.; Schlingemann, R.O.; Verbraak, F.D.; Abramoff, M.D. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3404–3409. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Garvin, M.K.; Sonka, M.; Lee, K.; Devries, J.H.; Michels, R.P.; van Velthoven, M.E.; Schlingemann, R.O.; et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3660–3665. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Kok, P.H.; Stehouwer, M.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Early neurodegeneration in the retina of type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
- Hammoum, I.; Benlarbi, M.; Dellaa, A.; Szabo, K.; Dekany, B.; Csaba, D.; Almasi, Z.; Hajdu, R.I.; Azaiz, R.; Charfeddine, R.; et al. Study of retinal neurodegeneration and maculopathy in diabetic Meriones shawi: A particular animal model with human-like macula. J. Comp. Neurol. 2017, 525, 2890–2914. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.; Zou, B.; Chan, H.H.; Tse, D.Y. Functional and structural changes in the neuroretina are accompanied by mitochondrial dysfunction in a type 2 diabetic mouse model. Eye Vision 2023, 10, 37. [Google Scholar] [CrossRef]
- van Dijk, H.W.; Verbraak, F.D.; Stehouwer, M.; Kok, P.H.; Garvin, M.K.; Sonka, M.; DeVries, J.H.; Schlingemann, R.O.; Abramoff, M.D. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011, 51, 224–228. [Google Scholar] [CrossRef]
- Montesano, G.; Ometto, G.; Higgins, B.E.; Das, R.; Graham, K.W.; Chakravarthy, U.; McGuiness, B.; Young, I.S.; Kee, F.; Wright, D.M.; et al. Evidence for Structural and Functional Damage of the Inner Retina in Diabetes With No Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 35. [Google Scholar] [CrossRef]
- Zhu, T.; Ma, J.; Li, Y.; Zhang, Z. Association between retinal neuronal degeneration and visual function impairment in type 2 diabetic patients without diabetic retinopathy. Sci. China Life Sci. 2015, 58, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, M.W.; Byeon, S.H.; Kim, S.S.; Koh, H.J.; Lee, S.C.; Kim, M. Associations between Individual Retinal Layer Thicknesses and Diabetic Peripheral Neuropathy Using Retinal Layer Segmentation Analysis. Retina 2018, 38, 2190–2196. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Yan, Y.; Shen, X. Diabetic macular morphology changes may occur in the early stage of diabetes. BMC Ophthalmol. 2016, 16, 12. [Google Scholar] [CrossRef]
- De Clerck, E.E.; Schouten, J.S.; Berendschot, T.T.; Kessels, A.G.; Nuijts, R.M.; Beckers, H.J.; Schram, M.T.; Stehouwer, C.D.; Webers, C.A. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: A systematic review. Lancet Diabetes Endocrinol. 2015, 3, 653–663. [Google Scholar] [CrossRef]
- De Clerck, E.E.; Schouten, J.S.; Berendschot, T.T.; Beckers, H.J.; Schaper, N.C.; Schram, M.T.; Stehouwer, C.D.; Webers, C.A. Loss of Temporal Peripapillary Retinal Nerve Fibers in Prediabetes or Type 2 Diabetes Without Diabetic Retinopathy: The Maastricht StudyTemporal RNFL Thinning in Prediabetes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1017–1027. [Google Scholar] [CrossRef]
- Sohn, E.H.; van Dijk, H.W.; Jiao, C.; Kok, P.H.; Jeong, W.; Demirkaya, N.; Garmager, A.; Wit, F.; Kucukevcilioglu, M.; van Velthoven, M.E.; et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc. Natl. Acad. Sci. USA 2016, 113, E2655–E2664. [Google Scholar] [CrossRef]
- Lee, M.W.; Lim, H.B.; Kim, M.S.; Park, G.S.; Nam, K.Y.; Lee, Y.H.; Kim, J.Y. Effects of prolonged type 2 diabetes on changes in peripapillary retinal nerve fiber layer thickness in diabetic eyes without clinical diabetic retinopathy. Sci. Rep. 2021, 11, 6813. [Google Scholar] [CrossRef]
- Moghimi, S.; Zangwill, L.M.; Penteado, R.C.; Hasenstab, K.; Ghahari, E.; Hou, H.; Christopher, M.; Yarmohammadi, A.; Manalastas, P.I.C.; Shoji, T.; et al. Macular and Optic Nerve Head Vessel Density and Progressive Retinal Nerve Fiber Layer Loss in Glaucoma. Ophthalmology 2018, 125, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lin, C.; Weinreb, R.N.; Lai, G.; Chiu, V.; Leung, C.K. Risk of Visual Field Progression in Glaucoma Patients with Progressive Retinal Nerve Fiber Layer Thinning: A 5-Year Prospective Study. Ophthalmology 2016, 123, 1201–1210. [Google Scholar] [CrossRef]
- Miki, A.; Medeiros, F.A.; Weinreb, R.N.; Jain, S.; He, F.; Sharpsten, L.; Khachatryan, N.; Hammel, N.; Liebmann, J.M.; Girkin, C.A.; et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology 2014, 121, 1350–1358. [Google Scholar] [CrossRef]
- Sung, K.R.; Sun, J.H.; Na, J.H.; Lee, J.Y.; Lee, Y. Progression detection capability of macular thickness in advanced glaucomatous eyes. Ophthalmology 2012, 119, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wall, K.; Arend, L.P.; von der Emde, L.; Sasmannshausen, M.; Holz, F.G.; Ach, T. Characterization of the Disorganization of the Inner Retinal Layers in Diabetics Using Increased Axial Resolution Optical Coherence Tomography. TVST 2025, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Koprich, J.B.; Siddiqi, H.; Isacson, O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J. Neurosci. 2009, 29, 3365–3373. [Google Scholar] [CrossRef]
- Anderson, E.E.; Greferath, U.; Fletcher, E.L. Changes in morphology of retinal ganglion cells with eccentricity in retinal degeneration. Cell Tissue Res. 2016, 364, 263–271. [Google Scholar] [CrossRef]
- Kern, T.S.; Barber, A.J. Retinal ganglion cells in diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef]
- Meyer-Rusenberg, B.; Pavlidis, M.; Stupp, T.; Thanos, S.; Meyer-Rusenberg, B.; Pavlidis, M.; Stupp, T.; Thanos, S. Pathological changes in human retinal ganglion cells associated with diabetic and hypertensive retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Son, J.R.; Lee, M.J.; Jeon, C.J. Changes in Starburst Amacrine Cells in Mice with Diabetic Retinopathy. Front. Biosci. 2023, 28, 92. [Google Scholar] [CrossRef]
- Ivanova, E.; Bianchimano, P.; Corona, C.; Eleftheriou, C.G.; Sagdullaev, B.T. Optogenetic Stimulation of Cholinergic Amacrine Cells Improves Capillary Blood Flow in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 44. [Google Scholar] [CrossRef] [PubMed]
- Enzsoly, A.; Szabo, A.; Szabo, K.; Szel, A.; Nemeth, J.; Lukats, A. Novel features of neurodegeneration in the inner retina of early diabetic rats. Histol. Histopathol. 2015, 30, 971–985. [Google Scholar]
- VanGuilder, H.D.; Brucklacher, R.M.; Patel, K.; Ellis, R.W.; Freeman, W.M.; Barber, A.J. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur. J. Neurosci. 2008, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, T.S.; Weibley, B.N.; Kimball, S.R.; Barber, A.J. Post-translational processing of synaptophysin in the rat retina is disrupted by diabetes. PLoS ONE 2012, 7, e44711. [Google Scholar] [CrossRef]
- Gaspar, J.M.; Baptista, F.I.; Galvao, J.; Castilho, A.F.; Cunha, R.A.; Ambrosio, A.F. Diabetes differentially affects the content of exocytotic proteins in hippocampal and retinal nerve terminals. Neuroscience 2010, 169, 1589–1600. [Google Scholar] [CrossRef]
- Sundstrom, J.M.; Hernandez, C.; Weber, S.R.; Zhao, Y.; Dunklebarger, M.; Tiberti, N.; Laremore, T.; Simo-Servat, O.; Garcia-Ramirez, M.; Barber, A.J.; et al. Proteomic Analysis of Early Diabetic Retinopathy Reveals Mediators of Neurodegenerative Brain Diseases. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2264–2274. [Google Scholar] [CrossRef]
- Shankar, U.; Gunasundari, R. A Review on Electrophysiology Based Detection of Diabetic Retinopathy. Procedia Comput. Sci. 2015, 48, 630–637. [Google Scholar] [CrossRef][Green Version]
- Bui, B.V.; Fortune, B. Ganglion cell contributions to the rat full-field electroretinogram. J. Physiol. 2004, 555, 153–173. [Google Scholar] [CrossRef]
- Kohzaki, K.; Vingrys, A.J.; Bui, B.V.; Kohzaki, K.; Vingrys, A.J.; Bui, B.V. Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3595–3604. [Google Scholar] [CrossRef]
- Bui, B.V.; Loeliger, M.; Thomas, M.; Vingrys, A.J.; Rees, S.M.; Nguyen, C.T.O.; He, Z.; Tolcos, M. Investigating structural and biochemical correlates of ganglion cell dysfunction in streptozotocin-induced diabetic rats. Exp. Eye Res. 2009, 88, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Saul, A.; Tawfik, A.; Zorrilla, E.P.; Ganapathy, V.; Smith, S.B. Diabetes accelerates retinal ganglion cell dysfunction in mice lacking sigma receptor 1. Mol. Vis. 2012, 18, 2860–2870. [Google Scholar]
- Nasralah, Z.; Robinson, W.; Jackson, G.; Barber, A. Measuring Visual Function in Diabetic Retinopathy: Progress in Basic and Clinical Research. Clin. Exp. Ophthalmol. 2013, 4, 1000306. [Google Scholar]
- Castoldi, V.; Zerbini, G.; Maestroni, S.; Vigano, I.; Rama, P.; Leocani, L. Topical Nerve Growth Factor (NGF) restores electrophysiological alterations in the Ins2Akita mouse model of diabetic retinopathy. Exp. Eye Res. 2023, 237, 109693. [Google Scholar] [CrossRef]
- Abraham, F.A.; Haimovitz, J.; Berezin, M. The photopic and scotopic visual thresholds in diabetics without diabetic retinopathy. Metab. Pediatr. Syst. Ophthalmol. 1988, 11, 76–77. [Google Scholar]
- Aylward, G.W.; Billson, F.A. The scotopic threshold response in diabetic retinopathy—A preliminary report. Aust. N. Z. J. Ophthalmol. 1989, 17, 369–372. [Google Scholar] [CrossRef]
- Parisi, V.; Uccioli, L. Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab. Res. Rev. 2001, 17, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Berardi, N.; Domenici, L.; Gravina, A.; Maffei, L. Pattern ERG in rats following section of the optic nerve. Exp. Brain Res. 1990, 79, 539–546. [Google Scholar] [CrossRef]
- Ding, Y.; Yuan, S.; Liu, X.; Mao, P.; Zhao, C.; Huang, Q.; Zhang, R.; Fang, Y.; Song, Q.; Yuan, D.; et al. Protective effects of astragaloside IV on db/db mice with diabetic retinopathy. PLoS ONE 2014, 9, e112207. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Xu, Y.; Hang, H.; Liu, X.; Chen, X.; Xie, P.; Yuan, S.; Zhang, W.; Lin, X.; Liu, Q. Edaravone protect against retinal damage in streptozotocin-induced diabetic mice. PLoS ONE 2014, 9, e99219. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulou, S.; Wikstrom, P.; Bucolo, C.; Romano, G.L.; Micale, V.; Svensson, R.; Spyridakos, D.; Mastrodimou, N.; Georgakis, S.; Verginis, P.; et al. Topically Administered NOX4 Inhibitor, GLX7013114, Is Efficacious in Treating the Early Pathological Events of Diabetic Retinopathy. Diabetes 2023, 72, 638–652. [Google Scholar] [CrossRef]
- Boschi, M.C.; Frosini, R.; Mencucci, R.; Sodi, A. The influence of early diabetes on the pattern electroretinogram. Doc. Ophthalmol. 1989, 71, 369–374. [Google Scholar] [CrossRef]
- Falsini, B.; Porciatti, V.; Scalia, G.; Caputo, S.; Minnella, A.; Di Leo, M.A.; Ghirlanda, G. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc. Ophthalmol. 1989, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ghirlanda, G.; Di Leo, M.A.; Caputo, S.; Falsini, B.; Porciatti, V.; Marietti, G.; Greco, A.V. Detection of inner retina dysfunction by steady-state focal electroretinogram pattern and flicker in early IDDM. Diabetes 1991, 40, 1122–1127. [Google Scholar] [CrossRef]
- Parisi, V.; Uccioli, L.; Monticone, G.; Parisi, L.; Manni, G.; Ippoliti, D.; Menzinger, G.; Bucci, M.G. Electrophysiological assessment of visual function in IDDM patients. Electroencephalogr. Clin. Neurophysiol. 1997, 104, 171–179. [Google Scholar] [CrossRef]
- Prager, T.C.; Garcia, C.A.; Mincher, C.A.; Mishra, J.; Chu, H.H. The pattern electroretinogram in diabetes. Am. J. Ophthalmol. 1990, 109, 279–284. [Google Scholar] [CrossRef]
- Di Leo, M.A.; Falsini, B.; Caputo, S.; Ghirlanda, G.; Porciatti, V.; Greco, A.V. Spatial frequency-selective losses with pattern electroretinogram in type 1 (insulin-dependent) diabetic patients without retinopathy. Diabetologia 1990, 33, 726–730. [Google Scholar] [CrossRef][Green Version]
- Caputo, S.; Di Leo, M.A.; Falsini, B.; Ghirlanda, G.; Porciatti, V.; Minella, A.; Greco, A.V. Evidence for early impairment of macular function with pattern ERG in type I diabetic patients. Diabetes Care 1990, 13, 412–418. [Google Scholar] [CrossRef]
- Deak, K.; Fejes, I.; Janaky, M.; Varkonyi, T.; Benedek, G.; Braunitzer, G. Further Evidence for the Utility of Electrophysiological Methods for the Detection of Subclinical Stage Retinal and Optic Nerve Involvement in Diabetes. Med. Princ. Pract. 2016, 25, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Mermeklieva, E.A. Pattern electroretinography and retinal changes in patients with diabetes mellitus type 2. Neurophysiol. Clin. 2019, 49, 209–215. [Google Scholar] [CrossRef]
- Lasta, M.; Pemp, B.; Schmidl, D.; Boltz, A.; Kaya, S.; Palkovits, S.; Werkmeister, R.; Howorka, K.; Popa-Cherecheanu, A.; Garhofer, G.; et al. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 842–847. [Google Scholar] [CrossRef]
- Lecleire-Collet, A.; Audo, I.; Aout, M.; Girmens, J.F.; Sofroni, R.; Erginay, A.; Le Gargasson, J.F.; Mohand-Said, S.; Meas, T.; Guillausseau, P.J.; et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2861–2867. [Google Scholar] [CrossRef]
- Kocer, A.M.; Sekeroglu, M.A. Evaluation of the neuronal and microvascular components of the macula in patients with diabetic retinopathy. Doc. Ophthalmol. 2021, 143, 193–205. [Google Scholar] [CrossRef]
- Polat Gultekin, B.; Hamurcu, M. Evaluation of optical coherence tomography angiography and pattern and flash electroretinography in diabetes mellitus without retinopathy. Ann. Med. 2024, 56, 2397573. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, K.I.; Veselovskaya, N.N.; Maslov, V.Y.; Fedulova, S.A.; Veselovsky, M.S. Electrical Properties of Retinal Ganglion Cells of the Rats with Streptozotocin-Induced Diabetes Mellitus. Int. J. Phys. Pathophysiol. 2014, 5, 57–63. [Google Scholar] [CrossRef]
- Martyniuk, N.Y.; Maslov, V.Y.; Purnyn, H.E.; Fedulova, S.A.; Veselovsky, N.S. Changes in Ongoing Activity and Electrophysiological Characteristics of the Rat Retinal Ganglion Cells in Diabetes Mellitus. Int. J. Phys. Pathophysiol. 2018, 9, 315–324. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Weng, S.J.; Yang, X.L.; Zhang, D.Q.; Zhong, Y.M. Hyperactivity of ON-type retinal ganglion cells in streptozotocin-induced diabetic mice. PLoS ONE 2013, 8, e76049. [Google Scholar] [CrossRef] [PubMed]
- Moore-Dotson, J.M.; Eggers, E.D. Reductions in Calcium Signaling Limit Inhibition to Diabetic Retinal Rod Bipolar Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4063–4073. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Xu, L.; Zhang, H. Visual response properties of retinal ganglion cells in the royal college of surgeons dystrophic rat. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3579–3585. [Google Scholar] [CrossRef]
- Xiao, C.; He, M.; Nan, Y.; Zhang, D.; Chen, B.; Guan, Y.; Pu, M. Physiological effects of superoxide dismutase on altered visual function of retinal ganglion cells in db/db mice. PLoS ONE 2012, 7, e30343. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, C.; Pu, M. High glucose levels impact visual response properties of retinal ganglion cells in C57 mice-An in vitro physiological study. Sci. China Life Sci. 2017, 60, 1428–1435. [Google Scholar] [CrossRef]
- Cui, R.Z.; Wang, L.; Qiao, S.N.; Wang, Y.C.; Wang, X.; Yuan, F.; Weng, S.J.; Yang, X.L.; Zhong, Y.M. ON-Type Retinal Ganglion Cells are Preferentially Affected in STZ-Induced Diabetic Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; So, C.; Qiu, C.; Zhang, T.; Yang, K.; Pan, F. Diminished light sensitivities of ON alpha retinal ganglion cells observed in a mouse model of hyperglycemia. Exp. Eye Res. 2024, 248, 110113. [Google Scholar] [CrossRef]
- Trenholm, S.; Awatramani, G.B. Origins of spontaneous activity in the degenerating retina. Front. Cell. Neurosci. 2015, 9, 277. [Google Scholar] [CrossRef]
- Flood, M.D.; Wellington, A.J.; Cruz, L.A.; Eggers, E.D. Early diabetes impairs ON sustained ganglion cell light responses and adaptation without cell death or dopamine insensitivity. Exp. Eye Res. 2020, 200, 108223. [Google Scholar] [CrossRef]
- Flood, M.D.; Wellington, A.J.; Eggers, E.D. Impaired Light Adaptation of ON-Sustained Ganglion Cells in Early Diabetes Is Attributable to Diminished Response to Dopamine D4 Receptor Activation. Investig. Ophthalmol. Vis. Sci. 2022, 63, 33. [Google Scholar] [CrossRef] [PubMed]
- MacIsaac, A.R.; Wellington, A.J.; Filicetti, K.; Eggers, E.D. Impaired dopamine signaling in early diabetic retina: Insights from D1R and D4R agonist effects on whole retina responses. Exp. Eye Res. 2024, 247, 110049. [Google Scholar] [CrossRef] [PubMed]
- Motz, C.T.; Chesler, K.C.; Allen, R.S.; Bales, K.L.; Mees, L.M.; Feola, A.J.; Maa, A.Y.; Olson, D.E.; Thule, P.M.; Iuvone, P.M.; et al. Novel Detection and Restorative Levodopa Treatment for Preclinical Diabetic Retinopathy. Diabetes 2020, 69, 1518–1527. [Google Scholar] [CrossRef]
- Aung, M.H.; Park, H.N.; Han, M.K.; Obertone, T.S.; Abey, J.; Aseem, F.; Thule, P.M.; Iuvone, P.M.; Pardue, M.T. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J. Neurosci. 2014, 34, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Sandell, J.H.; Maunsell, J.H. Functions of the ON and OFF channels of the visual system. Nature 1986, 322, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H. The ON and OFF channels of the visual system. Trends Neurosci. 1992, 15, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Moore-Dotson, J.M.; Beckman, J.J.; Mazade, R.E.; Hoon, M.; Bernstein, A.S.; Romero-Aleshire, M.J.; Brooks, H.L.; Eggers, E.D. Early Retinal Neuronal Dysfunction in Diabetic Mice: Reduced Light-Evoked Inhibition Increases Rod Pathway Signaling. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1418–1430. [Google Scholar] [CrossRef]
- Castilho, A.; Ambrosio, A.F.; Hartveit, E.; Veruki, M.L. Disruption of a neural microcircuit in the rod pathway of the mammalian retina by diabetes mellitus. J. Neurosci. 2015, 35, 5422–5433. [Google Scholar] [CrossRef]
- Castilho, A.; Madsen, E.; Ambrosio, A.F.; Veruki, M.L.; Hartveit, E. Diabetic hyperglycemia reduces Ca2+ permeability of extrasynaptic AMPA receptors in AII amacrine cells. J. Neurophysiol. 2015, 114, 1545–1553. [Google Scholar] [CrossRef]
- Patterson, S.S.; Cai, Y.; Yang, Q.; Merigan, W.H.; Williams, D.R. Asymmetric Activation of Retinal ON and OFF Pathways by AOSLO Raster-Scanned Visual Stimuli. bioRxiv 2024. [Google Scholar] [CrossRef]
- Freed, M.A. Asymmetry between ON and OFF α ganglion cells of mouse retina: Integration of signal and noise from synaptic inputs. J. Physiol. 2017, 595, 6979–6991. [Google Scholar] [CrossRef]
- Eggers, E.D.; Carreon, T.A. The effects of early diabetes on inner retinal neurons. Vis. Neurosci. 2020, 37, E006. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, Y.; Yoshida, M.; Chen, H.; Yang, J.F.; Kim, T.S.; Cang, J.; Troy, J.B.; Liu, X. Sustained ocular hypertension induces dendritic degeneration of mouse retinal ganglion cells that depends on cell type and location. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, R.S.; Chen, B.Y.; Tay, D.K.; Chan, H.H.; Pu, M.L.; So, K.F. Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2951–2958. [Google Scholar] [CrossRef]
- LaVail, J.H.; Tauscher, A.N.; Aghaian, E.; Harrabi, O.; Sidhu, S.S. Axonal transport and sorting of herpes simplex virus components in a mature mouse visual system. J. Virol. 2003, 77, 6117–6126. [Google Scholar] [CrossRef]
- Fahy, E.T.; Chrysostomou, V.; Crowston, J.G. Mini-Review: Impaired Axonal Transport and Glaucoma. Curr. Eye Res. 2016, 41, 273–283. [Google Scholar] [CrossRef]
- Scott, T.M.; Foote, J.; Peat, B.; Galway, G. Vascular and neural changes in the rat optic nerve following induction of diabetes with streptozotocin. J. Anat. 1986, 144, 145–152. [Google Scholar]
- Howell, S.J.; Mekhail, M.N.; Azem, R.; Ward, N.L.; Kern, T.S. Degeneration of retinal ganglion cells in diabetic dogs and mice: Relationship to glycemic control and retinal capillary degeneration. Mol. Vis. 2013, 19, 1413–1421. [Google Scholar]
- Li, S.; Wang, X.; Yang, J.; Lei, H.; Wang, X.; Xiang, Y. Metabolic profile of visual cortex in diabetic rats measured with in vivo proton MRS. NMR Biomed. 2017, 30, e3783. [Google Scholar] [CrossRef] [PubMed]
- Medori, R.; Autilio-Gambetti, L.; Monaco, S.; Gambetti, P. Experimental diabetic neuropathy: Impairment of slow transport with changes in axon cross-sectional area. Proc. Natl. Acad. Sci. USA 1985, 82, 7716–7720. [Google Scholar] [CrossRef]
- Tsukada, T.; Chihara, E. Changes in components of fast axonally transported proteins in the optic nerves of diabetic rabbits. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1115–1122. [Google Scholar]
- Zhang, L.; Ino-ue, M.; Dong, K.; Yamamoto, M. Retrograde axonal transport impairment of large- and medium-sized retinal ganglion cells in diabetic rat. Curr. Eye Res. 2000, 20, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ino-Ue, M.; Zhang, L.; Naka, H.; Kuriyama, H.; Yamamoto, M. Polyol metabolism of retrograde axonal transport in diabetic rat large optic nerve fiber. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4055–4058. [Google Scholar]
- Fernandez, D.C.; Pasquini, L.A.; Dorfman, D.; Aldana Marcos, H.J.; Rosenstein, R.E. Ischemic conditioning protects from axoglial alterations of the optic pathway induced by experimental diabetes in rats. PLoS ONE 2012, 7, e51966. [Google Scholar] [CrossRef]
- Dorfman, D.; Aranda, M.L.; Rosenstein, R.E. Enriched Environment Protects the Optic Nerve from Early Diabetes-Induced Damage in Adult Rats. PLoS ONE 2015, 10, e0136637. [Google Scholar] [CrossRef]
- Foxton, R.; Osborne, A.; Martin, K.R.; Ng, Y.S.; Shima, D.T. Distal retinal ganglion cell axon transport loss and activation of p38 MAPK stress pathway following VEGF-A antagonism. Cell Death Dis. 2016, 7, e2212. [Google Scholar] [CrossRef]
- Delcroix, J.D.; Tomlinson, D.R.; Fernyhough, P. Diabetes and axotomy-induced deficits in retrograde axonal transport of nerve growth factor correlate with decreased levels of p75LNTR protein in lumbar dorsal root ganglia. Brain Res. Mol. Brain Res. 1997, 51, 82–90. [Google Scholar] [CrossRef]
- Hellweg, R.; Raivich, G.; Hartung, H.D.; Hock, C.; Kreutzberg, G.W. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp. Neurol. 1994, 130, 24–30. [Google Scholar] [CrossRef]
- Fernyhough, P.; Diemel, L.T.; Hardy, J.; Brewster, W.J.; Mohiuddin, L.; Tomlinson, D.R. Human recombinant nerve growth factor replaces deficient neurotrophic support in the diabetic rat. Eur. J. Neurosci. 1995, 7, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.K.; Geddis, M.S.; Rosario, R.; Schmidt, A.M. Impaired slow axonal transport in diabetic peripheral nerve is independent of RAGE. Eur. J. Neurosci. 2013, 38, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Ma, Z.Z.; Song, C.; Li, X.H.; Li, Y.Z.; Liu, Y.Y. Optic nerve lesions in diabetic rats: Blood flow to the optic nerve, permeability of micro blood vessels and histopathology. Int. J. Ophthalmol. 2010, 3, 291–294. [Google Scholar] [PubMed]
- Sredy, J.; Sawicki, D.R.; Notvest, R.R. Polyol pathway activity in nervous tissues of diabetic and galactose-fed rats: Effect of dietary galactose withdrawal or tolrestat intervention therapy. J. Diabet. Complicat. 1991, 5, 42–47. [Google Scholar] [CrossRef]
- Mendonca, H.R.; Carvalho, J.N.A.; Abreu, C.A.; Mariano de Souza Aguiar Dos Santos, D.; Carvalho, J.R.; Marques, S.A.; da Costa Calaza, K.; Martinez, A.M.B. Lack of Galectin-3 attenuates neuroinflammation and protects the retina and optic nerve of diabetic mice. Brain Res. 2018, 1700, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, O.L.; Capponi, J.A.; Di Martino, I.; Labal, E.S.; Sirois, P. Oxidative stress in the optic nerve and cortical visual area of steptozotocin-induced diabetic Wistar rats: Blockade with a selective bradykinin B1 receptor antagonist. Neuropeptides 2017, 66, 97–102. [Google Scholar]
- Casselini, C.M.; Parson, H.K.; Frizzi, K.E.; Marquez, A.; Smith, D.R.; Guernsey, L.; Nemmani, R.; Tayarani, A.; Jolivalt, C.G.; Weaver, J.; et al. A muscarinic receptor antagonist reverses multiple indices of diabetic peripheral neuropathy: Preclinical and clinical studies using oxybutynin. Acta Neuropathol. 2024, 147, 60. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-containing retinal ganglion cells: Architecture, projections, and intrinsic photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef]
- Perez-Leon, J.A.; Warren, E.J.; Allen, C.N.; Robinson, D.W.; Brown, R.L. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur. J. Neurosci. 2006, 24, 1117–1123. [Google Scholar] [CrossRef]
- Sondereker, K.B.; Stabio, M.E.; Renna, J.M. Crosstalk: The diversity of melanopsin ganglion cell types has begun to challenge the canonical divide between image-forming and non-image-forming vision. J. Comp. Neurol. 2020, 528, 2044–2067. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Chen, W.Y.; Han, X.; Cui, L.J.; Yu, C.X.; Sheng, W.L.; Yu, J.; Yuan, F.; Zhong, Y.M.; Yang, X.L.; Weng, S.J. Cell-Subtype-Specific Remodeling of Intrinsically Photosensitive Retinal Ganglion Cells in Streptozotocin-Induced Diabetic Mice. Diabetes 2021, 70, 1157–1169. [Google Scholar] [CrossRef]
- Lahouaoui, H.; Coutanson, C.; Cooper, H.M.; Bennis, M.; Dkhissi-Benyahya, O. Clock genes and behavioral responses to light are altered in a mouse model of diabetic retinopathy. PLoS ONE 2014, 9, e101584. [Google Scholar] [CrossRef]
- Lahouaoui, H.; Coutanson, C.; Cooper, H.M.; Bennis, M.; Dkhissi-Benyahya, O. Diabetic retinopathy alters light-induced clock gene expression and dopamine levels in the mouse retina. Mol. Vis. 2016, 22, 959–969. [Google Scholar] [PubMed]
- Kumar, S.; Zhuo, L. Quantitative analysis of pupillary light reflex by real-time autofluorescent imaging in a diabetic mouse model. Exp. Eye Res. 2011, 92, 164–172. [Google Scholar] [CrossRef]
- Straub, R.H.; Zietz, B.; Palitzsch, K.D.; Scholmerich, J. Impact of disease duration on cardiovascular and pupillary autonomic nervous function in IDDM and NIDDM patients. Diabetes Care 1996, 19, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, F.; Karavanaki, K.; Greenwood, R.; Baum, J.D. Consistency of pupillary abnormality in children and adolescents with diabetes. Diabet. Med. 1997, 14, 849–853. [Google Scholar] [CrossRef]
- Feigl, B.; Zele, A.J.; Fader, S.M.; Howes, A.N.; Hughes, C.E.; Jones, K.A.; Jones, R. The post-illumination pupil response of melanopsin-expressing intrinsically photosensitive retinal ganglion cells in diabetes. Acta Ophthalmol. 2012, 90, e230–e234. [Google Scholar] [CrossRef]
- Ortube, M.C.; Kiderman, A.; Eydelman, Y.; Yu, F.; Aguilar, N.; Nusinowitz, S.; Gorin, M.B. Comparative regional pupillography as a noninvasive biosensor screening method for diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 9–18. [Google Scholar] [CrossRef]
- Ishibashi, F.; Kojima, R.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. The Preferential Impairment of Pupil Constriction Stimulated by Blue Light in Patients with Type 2 Diabetes without Autonomic Neuropathy. J. Diabetes Res. 2017, 2017, 6069730. [Google Scholar] [CrossRef] [PubMed]
- Bista Karki, S.; Coppell, K.J.; Mitchell, L.V.; Ogbuehi, K.C. Dynamic Pupillometry in Type 2 Diabetes: Pupillary Autonomic Dysfunction and the Severity of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 3923–3930. [Google Scholar] [CrossRef]
- Tan, T.E.; Finkelstein, M.T.; Tan, G.S.W.; Tan, A.C.S.; Chan, C.M.; Mathur, R.; Wong, E.Y.M.; Cheung, C.M.G.; Wong, T.Y.; Milea, D.; et al. Retinal neural dysfunction in diabetes revealed with handheld chromatic pupillometry. Clin. Exp. Ophthalmol. 2022, 50, 745–756. [Google Scholar] [CrossRef]
- Hall, C.A.; Chilcott, R.P. Eyeing up the Future of the Pupillary Light Reflex in Neurodiagnostics. Diagnostics 2018, 8, 13. [Google Scholar] [CrossRef]
- Dumpala, S.; Zele, A.J.; Feigl, B. Outer Retinal Structure and Function Deficits Contribute to Circadian Disruption in Patients With Type 2 Diabetes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Litsea japonica extract inhibits neuronal apoptosis and the accumulation of advanced glycation end products in the diabetic mouse retina. Mol. Med. Rep. 2015, 12, 1075–1081. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Tan, H.Y.; Li, S.; Wang, N.; Zhang, Y.; Feng, Y. Neuroprotective effect of He-Ying-Qing-Re formula on retinal ganglion cell in diabetic retinopathy. J. Ethnopharmacol. 2018, 214, 179–189. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Barber, A.J.; Spagnuolo, C.; Russo, G.L.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E. Nrf2 as molecular target for polyphenols: A novel therapeutic strategy in diabetic retinopathy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 293–312. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Shafighi, N.; Barber, A.J. Nabavi SM. Anthocyanins as a potential therapy for diabetic retinopathy. Curr. Med. Chem. 2015, 22, 51–58. [Google Scholar] [CrossRef]
- Robledinos-Anton, N.; Fernandez-Gines, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qi, E.; Liu, X.; Cui, L.; Fan, X.; Wei, T.; Hu, Y. The lack of homology domain and leucine rich repeat protein phosphatase 2 ameliorates visual impairment in rats with diabetic retinopathy through regulation of the AKT-GSK-3β-Nrf2 signal cascade. Toxicol. Appl. Pharmacol. 2024, 482, 116766. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Baccouche, B. Neurodegeneration in diabetic retinopathy: Potential for novel therapies. Vision Res. 2017, 139, 82–92. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial effect of zeaxanthin on retinal metabolic abnormalities in diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef]
- Yang, F.; Yu, J.; Ke, F.; Lan, M.; Li, D.; Tan, K.; Ling, J.; Wang, Y.; Wu, K.; Li, D. Curcumin Alleviates Diabetic Retinopathy in Experimental Diabetic Rats. Ophthalmic Res. 2018, 60, 43–54. [Google Scholar] [CrossRef]
- Yang, X.; Huo, F.; Liu, B.; Liu, J.; Chen, T.; Li, J.; Zhu, Z.; Lv, B. Crocin Inhibits Oxidative Stress and Pro-inflammatory Response of Microglial Cells Associated with Diabetic Retinopathy Through the Activation of PI3K/Akt Signaling Pathway. J. Mol. Neurosci. 2017, 61, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xu, G.; Jiang, T.; Qin, Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6541–6556. [Google Scholar] [CrossRef]
- Zhang, Q.; Guy, K.; Pagadala, J.; Jiang, Y.; Walker, R.J.; Liu, L.; Soderland, C.; Kern, T.S.; Ferry, R., Jr.; He, H.; et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3004–3013. [Google Scholar] [CrossRef]
- Foureaux, G.; Nogueira, B.S.; Coutinho, D.C.; Raizada, M.K.; Nogueira, J.C.; Ferreira, A.J. Activation of endogenous angiotensin converting enzyme 2 prevents early injuries induced by hyperglycemia in rat retina. Braz. J. Med. Biol. Res. 2015, 48, 1109–1114. [Google Scholar] [CrossRef]
- Hernandez, C.; Bogdanov, P.; Corraliza, L.; Garcia-Ramirez, M.; Sola-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simo, R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef]
- Liu, Y.; Leo, L.F.; McGregor, C.; Grivitishvili, A.; Barnstable, C.J.; Tombran-Tink, J. Pigment epithelium-derived factor (PEDF) peptide eye drops reduce inflammation, cell death and vascular leakage in diabetic retinopathy in Ins2(Akita) mice. Mol. Med. 2012, 18, 1387–1401. [Google Scholar] [CrossRef]
- Afarid, M.; Namvar, E.; Sanie-Jahromi, F. Diabetic Retinopathy and BDNF: A Review on Its Molecular Basis and Clinical Applications. J. Ophthalmol. 2020, 2020, 1602739. [Google Scholar] [CrossRef]
- Le, Y.Z.; Xu, B.; Chucair-Elliott, A.J.; Zhang, H.; Zhu, M. VEGF Mediates Retinal Muller Cell Viability and Neuroprotection through BDNF in Diabetes. Biomolecules 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.E.; Sandirasegarane, L.; Wolpert, E.B.; Klinger, M.; Simpson, I.A.; Barber, A.J.; Antonetti, D.A.; Kester, M.; Gardner, T.W. Characterization of insulin signaling in rat retina in vivo and ex vivo. Am. J. Physiol.—Endocrinol. Metab. 2003, 285, E763–E774. [Google Scholar] [CrossRef]
- Reiter, C.E.; Wu, X.; Sandirasegarane, L.; Nakamura, M.; Gilbert, K.A.; Singh, R.S.; Fort, P.E.; Antonetti, D.A.; Gardner, T.W. Diabetes reduces basal retinal insulin receptor signaling: Reversal with systemic and local insulin. Diabetes 2006, 55, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.J.; Nakamura, M.; Wolpert, E.B.; Reiter, C.E.N.; Seigel, G.M.; Antonetti, D.A.; Gardner, T.W. Insulin Rescues Retinal Neurons from Apoptosis by a Phosphatidylinositol 3-Kinase/Akt-mediated Mechanism That Reduces the Activation of Caspase-3. J. Biol. Chem. 2001, 276, 32814–32821. [Google Scholar] [CrossRef]
- Faiq, M.A.; Sengupta, T.; Nath, M.; Velpandian, T.; Saluja, D.; Dada, R.; Dada, T.; Chan, K.C. Ocular manifestations of central insulin resistance. Nerual Regen. 2023, 18, 1139–1146. [Google Scholar] [CrossRef]
- Zerbini, G.; Maestroni, S.; Vigano, I.; Mosca, A.; Paleari, R.; Gabellini, D.; Galbiati, S.; Rama, P. Progressive Thinning of Retinal Nerve Fiber Layer/Ganglion Cell Layer (RNFL/GCL) as Biomarker and Pharmacological Target of Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 12672. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Abdel-Mottaleb, Y.; Elkazaz, A.Y.; Atef, H.; Lashine, R.M.; Youssef, A.M.; Ezzat, W.; El-Ghaiesh, S.H.; Elshaer, R.E.; El-Shafey, M.; et al. Carbamazepine Alleviates Retinal and Optic Nerve Neural Degeneration in Diabetic Mice via Nerve Growth Factor-Induced PI3K/Akt/mTOR Activation. Front. Neurosci. 2019, 13, 1089. [Google Scholar] [CrossRef]
- Ibán-Arias, R.; Lisa, S.; Mastrodimou, N.; Kokona, D.; Koulakis, E.; Iordanidou, P.; Kouvarakis, A.; Fothiadaki, M.; Papadogkonaki, S.; Sotiriou, A.; et al. The Synthetic Microneurotrophin BNN27 Affects Retinal Function in Rats With Streptozotocin-Induced Diabetes. Diabetes 2017, 67, 321–333. [Google Scholar] [CrossRef]
- Iban-Arias, R.; Lisa, S.; Poulaki, S.; Mastrodimou, N.; Charalampopoulos, I.; Gravanis, A.; Thermos, K. Effect of topical administration of the microneurotrophin BNN27 in the diabetic rat retina. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2429–2436. [Google Scholar] [CrossRef]
- Kokkali, M.; Karali, K.; Thanou, E.; Papadopoulou, M.A.; Zota, I.; Tsimpolis, A.; Efstathopoulos, P.; Calogeropoulou, T.; Li, K.W.; Sidiropoulou, K.; et al. Multimodal beneficial effects of BNN27, a nerve growth factor synthetic mimetic, in the 5xFAD mouse model of Alzheimer’s disease. Mol. Psychiatry 2025, 30, 2265–2283. [Google Scholar] [CrossRef] [PubMed]
- LaNoue, K.F.; Berkich, D.A.; Conway, M.; Barber, A.J.; Hu, L.Y.; Taylor, C.; Hutson, S. Role of specific aminotransferases in de novo glutamate synthesis and redox shuttling in the retina. J. Neurosci. Res. 2001, 66, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Alhomida, A.S.; LaNoue, K.F. Gabapentin Attenuates Oxidative Stress and Apoptosis in the Diabetic Rat Retina. Neurotox. Res. 2019, 36, 81–90. [Google Scholar] [CrossRef]
- Kusari, J.; Zhou, S.; Padillo, E.; Clarke, K.G.; Gil, D.W. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5152–5159. [Google Scholar] [CrossRef]
- Wang, L.; Deng, Q.Q.; Wu, X.H.; Yu, J.; Yang, X.L.; Zhong, Y.M. Upregulation of glutamate-aspartate transporter by glial cell line-derived neurotrophic factor ameliorates cell apoptosis in neural retina in streptozotocin-induced diabetic rats. CNS Neurosci. Ther. 2013, 19, 945–953. [Google Scholar] [PubMed]
- Gu, L.; Xu, H.; Wang, F.; Xu, G.; Sinha, D.; Wang, J.; Xu, J.Y.; Tian, H.; Gao, F.; Li, W.; et al. Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8208–8222. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Wikstrom, P.; Walum, E.; Georgakis, S.; Thermos, K. Investigation of the Effects of a Novel NOX2 Inhibitor, GLX7013170, against Glutamate Excitotoxicity and Diabetes Insults in the Retina. Pharmaceuticals 2024, 17, 393. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Zhang, Y.; Li, M.; Huang, X.; Yang, Y.; Zeng, J.; Zhao, Y.; Wang, X.; Zhang, W.; Ying, Y. Topical ocular administration of the GLP-1 receptor agonist liraglutide arrests hyperphosphorylated tau-triggered diabetic retinal neurodegeneration via activation of GLP-1R/Akt/GSK3beta signaling. Neuropharmacology 2019, 153, 1–12. [Google Scholar] [CrossRef]
- Boccaccini, A.; Cavaterra, D.; Carnevale, C.; Tanga, L.; Marini, S.; Bocedi, A.; Lacal, P.M.; Manni, G.; Graziani, G.; Sbardella, D.; et al. Novel frontiers in neuroprotective therapies in glaucoma: Molecular and clinical aspects. Mol. Asp. Med. 2023, 94, 101225. [Google Scholar] [CrossRef]
- Rossetti, L.; Goni, F.; Montesano, G.; Stalmans, I.; Topouzis, F.; Romano, D.; Galantin, E.; Delgado-Gonzales, N.; Giammaria, S.; Coco, G.; et al. The effect of citicoline oral solution on quality of life in patients with glaucoma: The results of an international, multicenter, randomized, placebo-controlled cross-over trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Prinz, J.; Prokosch, V.; Liu, H.; Walter, P.; Fuest, M.; Migliorini, F. Efficacy of citicoline as a supplement in glaucoma patients: A systematic review. PLoS ONE 2023, 18, e0291836. [Google Scholar] [CrossRef] [PubMed]
- Hui, F.; Tang, J.; Williams, P.A.; McGuinness, M.B.; Hadoux, X.; Casson, R.J.; Coote, M.; Trounce, I.A.; Martin, K.R.; van Wijngaarden, P.; et al. Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin. Exp. Ophthalmol. 2020, 48, 903–914. [Google Scholar] [CrossRef]
- De Moraes, C.G.; John, S.W.M.; Williams, P.A.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M. Nicotinamide and Pyruvate for Neuroenhancement in Open-Angle Glaucoma: A Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 11–18. [Google Scholar] [CrossRef]
- Scuteri, D.; Bagetta, G.; Nucci, C.; Aiello, F.; Cesareo, M.; Tonin, P.; Corasaniti, M.T. Evidence on the neuroprotective properties of brimonidine in glaucoma. Prog. Brain Res. 2020, 257, 155–166. [Google Scholar]
- Otsubo, M.; Sase, K.; Tsukahara, C.; Fujita, N.; Arizono, I.; Tokuda, N.; Kitaoka, Y. Axonal protection by combination of ripasudil and brimonidine with upregulation of p-AMPK in TNF-induced optic nerve degeneration. Int. Ophthalmol. 2024, 44, 173. [Google Scholar] [CrossRef] [PubMed]
- Trento, M.; Durando, O.; Lavecchia, S.; Charrier, L.; Cavallo, F.; Costa, M.A.; Hernandez, C.; Simo, R.; Porta, M., for the EUROCONDOR Trial Investigators. Vision related quality of life in patients with type 2 diabetes in the EUROCONDOR trial. Endocrine 2017, 57, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Hernandez, C.; Porta, M.; Bandello, F.; Grauslund, J.; Harding, S.P.; Aldington, S.J.; Egan, C.; Frydkjaer-Olsen, U.; Garcia-Arumi, J.; et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes 2019, 68, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Kim, J.H.; Han, J.-S.; Park, C.K. Exploring Neuroprotective Effects of Topical Brimonidine in Experimental Diabetic Retinopathy. In Vivo 2024, 38, 1609–1620. [Google Scholar] [CrossRef]
- Zuclich, J.A.; Stolarski, D.J. Retinal damage induced by red diode laser. Health Phys. 2001, 81, 8–14. [Google Scholar] [CrossRef]
- Gold, G.; Giannakopoulos, P.; Herrmann, F.R.; Bouras, C.; Kövari, E. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain 2007, 130, 2830–2836. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barber, A.J. The Form and Function of Retinal Ganglion Cells in Diabetes. Cells 2025, 14, 1455. https://doi.org/10.3390/cells14181455
Barber AJ. The Form and Function of Retinal Ganglion Cells in Diabetes. Cells. 2025; 14(18):1455. https://doi.org/10.3390/cells14181455
Chicago/Turabian StyleBarber, Alistair J. 2025. "The Form and Function of Retinal Ganglion Cells in Diabetes" Cells 14, no. 18: 1455. https://doi.org/10.3390/cells14181455
APA StyleBarber, A. J. (2025). The Form and Function of Retinal Ganglion Cells in Diabetes. Cells, 14(18), 1455. https://doi.org/10.3390/cells14181455




