The Pituitary Immune Environment and Immunotherapy: From Hypophysitis to Pituitary Neuroendocrine Tumors
Abstract
1. Introduction
ICIs and Their Mechanisms of Action
2. Animal Models of Autoimmune Hypophysitis
3. Immune Checkpoint Inhibitor-Induced Hypophysitis
3.1. Epidemiology of IIH
3.2. Pathophysiology of IIH
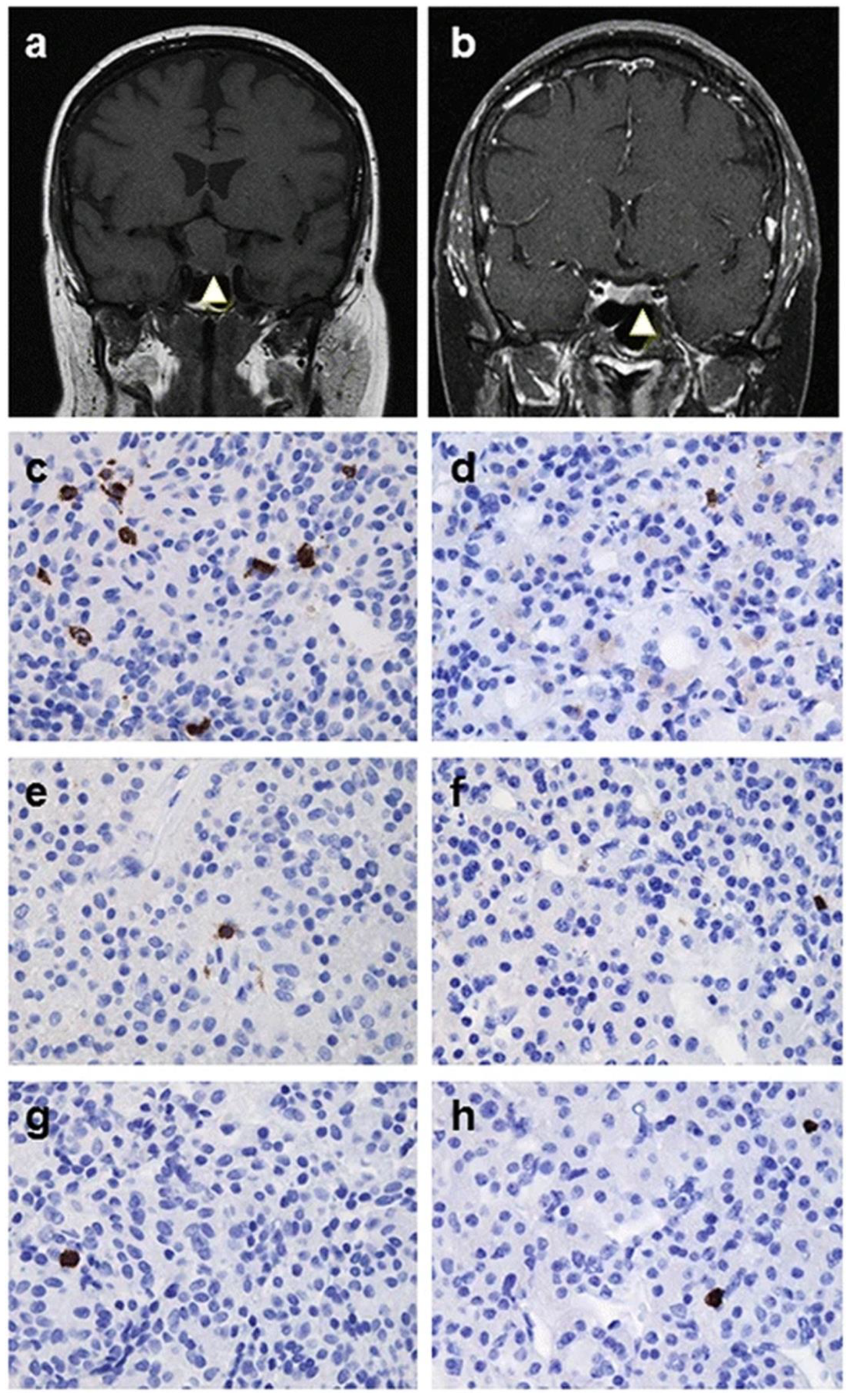
3.3. Clinical Case of IIH
3.4. Clinical Presentation of IIH
3.5. Diagnosis of IIH
3.6. Predictors of IIH
3.7. Management of IIH
4. Immune Microenvironment and Pituitary Neuroendocrine Tumor (PitNET) with PDL-1 as a Biomarker
4.1. Immune Microenvironment of PitNETs
4.2. PD-L1 as a Biomarker
4.3. A Review of Immunotherapy in the Management of Aggressive and Metastatic PitNETs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICIs | immune checkpoint inhibitors |
| IIH | ICI-induced hypophysitis |
| PitNETs | pituitary neuroendocrine tumors |
| PDL-1 | programmed cell death ligand 1 |
| FDA | Food and Drug Administration |
| CTLA-4 | cytotoxic T-lymphocyte-associated antigen 4 |
| LAG-3 | lymphocyte-activating gene-3 |
| PD-1 | programmed cell death protein 1 |
| APCs | antigen presenting cells |
| MHC | major histocompatibility complex |
| TCR | T-cell receptor |
| IRAK1 | interleukin-1 receptor-associated kinase 1 |
| LINH | lymphocytic infundibulo-neurohypophysitis |
| AVP | arginine vasopressin |
| irAEs | immune-related adverse events |
| ADCC | antibody-dependent cellular cytotoxicity |
| GNAL | guanine nucleotide-binding protein G (olf) subunit alpha |
| ITM2B | integral membrane protein 2B |
| ACTH | adrenocorticotropic hormone |
| POMC | Proopiomelanocortin |
| NK | natural killer |
References
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., III; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, V.; La Sala, D.; Cozzi, R.; Scavuzzo, F.; De Geronimo, V.; Poggi, M.; Vitale, M.; Tortora, A. Immunotherapy-Related Hypophysitis: A Narrative Review. Cancers 2025, 17, 436. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Pareja, N.; Faje, A.T.; Miller, K.K. Pituitary Complications of Checkpoint Inhibitor Use. Endocrinology 2024, 165, bqae084. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-cosibelimab-ipdl-metastatic-or-locally-advanced-cutaneous-squamous-cell-carcinoma (accessed on 30 April 2025).
- Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots-tevimbra (accessed on 30 April 2025).
- Fukuda, I. Immune Checkpoint Inhibitors and Associated Pituitary Dysfunctions: A Mini-Review. J. Nippon Med. Sch. 2023, 90, 149–156. [Google Scholar] [CrossRef]
- Lopes, S.; Pabst, L.; Bahougne, T.; Barthélémy, P.; Guitton, R.; Didier, K.; Geoffrois, L.; Granel-Brocard, F.; Mennecier, B.; Mascaux, C.; et al. Central nervous system complications of immune checkpoint inhibitors: A comprehensive review. Crit. Rev. Oncol. Hematol. 2025, 206, 104595. [Google Scholar] [CrossRef]
- Kotwal, A. Hypophysitis from immune checkpoint inhibitors: Challenges in diagnosis and management. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 427–434. [Google Scholar] [CrossRef]
- Caturegli, P.; Newschaffer, C.; Olivi, A.; Pomper, M.G.; Burger, P.C.; Rose, N.R. Autoimmune hypophysitis. Endocr. Rev. 2005, 26, 599–614. [Google Scholar] [CrossRef]
- Tzou, S.C.; Lupi, I.; Landek, M.; Gutenberg, A.; Tzou, Y.M.; Kimura, H.; Pinna, G.; Rose, N.R.; Caturegli, P. Autoimmune hypophysitis of SJL mice: Clinical insights from a new animal model. Endocrinology 2008, 149, 3461–3469. [Google Scholar] [CrossRef]
- Lupi, I.; Zhang, J.; Gutenberg, A.; Landek-Salgado, M.; Tzou, S.C.; Mori, S.; Caturegli, P. From pituitary expansion to empty sella: Disease progression in a mouse model of autoimmune hypophysitis. Endocrinology 2011, 152, 4190–4198. [Google Scholar] [CrossRef]
- Landek-Salgado, M.A.; Rose, N.R.; Caturegli, P. Placenta suppresses experimental autoimmune hypophysitis through soluble TNF receptor 1. J. Autoimmun. 2012, 38, J88–J96. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Gutenberg, A.; Chen, T.Y.; Tsai, N.M.; Lee, C.J.; Cheng, Y.C.; Cheng, W.H.; Tzou, Y.M.; Caturegli, P.; Tzou, S.C. In situ activation of pituitary-infiltrating T lymphocytes in autoimmune hypophysitis. Sci. Rep. 2017, 7, 43492. [Google Scholar] [CrossRef]
- Chalan, P.; Thomas, N.; Caturegli, P. Th17 cells contribute to the pathology of autoimmune hypophysitis. J. Immunol. 2021, 206, 2536–2543. [Google Scholar] [CrossRef]
- Huang, H.C.; Chen, Y.T.; Lin, H.H.; Li, Z.Q.; Yang, J.M.; Tzou, S.C. Inhibition of IRAK1 is an effective therapy for autoimmune hypophysitis in mice. Int. J. Mol. Sci. 2022, 23, 14958. [Google Scholar] [CrossRef] [PubMed]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- Mihic-Probst, D.; Reinehr, M.; Dettwiler, S.; Kolm, I.; Britschgi, C.; Kudura, K.; Maggio, E.M.; Lenggenhager, D.; Rushing, E.J. The role of macrophages type 2 and T-regs in immune checkpoint inhibitor related adverse events. Imunobiology 2020, 225, 152009. [Google Scholar] [CrossRef] [PubMed]
- Iwama, S.; Sugimura, Y.; Kiyota, A.; Kato, T.; Enomoto, A.; Suzuki, H.; Iwata, N.; Takeuchi, S.; Nakashima, K.; Takagi, H.; et al. Rabphilin-3A as a Targeted Autoantigen in Lymphocytic Infundibulo-neurohypophysitis. J. Clin. Endocrinol. Metab. 2015, 100, E946–E954. [Google Scholar] [CrossRef]
- Arihara, Z.; Sakurai, K.; Niitsuma, S.; Sato, R.; Yamada, S.; Inoshita, N.; Iwata, N.; Fujisawa, H.; Watanabe, T.; Suzuki, A.; et al. Studies on anti-rabphilin-3A antibodies in 15 consecutive patients presenting with central diabetes insipidus at a single referral center. Sci. Rep. 2022, 12, 4440. [Google Scholar] [CrossRef]
- Yasuda, Y.; Iwama, S.; Kiyota, A.; Izumida, H.; Nakashima, K.; Iwata, N.; Ito, Y.; Morishita, Y.; Goto, M.; Suga, H.; et al. Critical role of rabphilin-3A in the pathophysiology of experimental lymphocytic neurohypophysitis. J. Pathol. 2018, 244, 469–478. [Google Scholar] [CrossRef]
- Esteves-Ferreira, S.; Rosinha, P. Immune checkpoint inhibitor-induced hypophysitis: Clinical and biochemical features. J. Cancer Res. Clin. Oncol. 2023, 149, 7925–7932. [Google Scholar] [CrossRef]
- Cardona, Z.; Sosman, J.A.; Chandra, S.; Huang, W. Endocrine side effects of immune checkpoint inhibitors. Front. Endocrinol. 2023, 14, 1157805. [Google Scholar] [CrossRef] [PubMed]
- Amereller, F.; Deutschbein, T.; Joshi, M.; Schopohl, J.; Schilbach, K.; Detomas, M.; Duffy, L.; Carroll, P.; Papa, S.; Störmann, S. Differences between immunotherapy-induced and primary hypophysitis-a multicenter retrospective study. Pituitary 2022, 25, 152–158. [Google Scholar] [CrossRef]
- Tahir, S.A.; Gao, J.; Miura, Y.; Blando, J.; Tidwell, R.S.S.; Zhao, H.; Subudhi, S.K.; Tawbi, H.; Keung, E.; Wargo, J.; et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc. Natl. Acad. Sci. USA 2019, 116, 22246–22251. [Google Scholar] [CrossRef]
- Takahashi, Y. The novel concept of “Onco-Immuno-Endocrinology” led to the discovery of new clinical entity “paraneoplastic autoimmune hypophysitis”. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101663. [Google Scholar] [CrossRef]
- Langlois, F.; Varlamov, E.V.; Fleseriu, M. Hypophysitis, the Growing Spectrum of a Rare Pituitary Disease. J. Clin. Endocrinol. Metab. 2022, 107, 10–28. [Google Scholar] [CrossRef]
- Urai, S.; Iguchi, G.; Kanie, K.; Bando, H.; Yamamoto, M.; Oi, Y.; Kashitani, Y.; Iida, K.; Kanzawa, M.; Fukuoka, H.; et al. Clinical features of anti-pituitary-specific transcription factor-1 (PIT-1) hypophysitis: A new aspect of paraneoplastic autoimmune condition. Eur. J. Endocrinol. 2024, 190, K1–K7. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Chen, X.; Liu, K.; Zhou, Y.; Wang, S. Pituitary-related immune adverse events induced by programmed death Protein-1 inhibitors differ clinically from hypophysitis. Clin. Endocrinol. 2024, 101, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.; Reynolds, K.; Zubiri, L.; Lawrence, D.; Cohen, J.V.; Sullivan, R.J.; Nachtigall, L.; Tritos, N. Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab associated hypophysitis. Eur. J. Endocrinol. 2019, 181, 211–219. [Google Scholar] [CrossRef]
- Lu, D.; Yao, J.; Yuan, G.; Gao, Y.; Zhang, J.; Guo, X. Immune checkpoint inhibitor-associated new-onset hypophysitis: A retrospective analysis using the FAERS. Endocrine 2024, 86, 342–348. [Google Scholar] [CrossRef]
- Chamorro-Pareja, N.; Faje, A.T.; Miller, K.K. The Risk of Adrenal Insufficiency after Treatment with Relatlimab in Combination with Nivolumab is Higher than Expected. J. Clin. Endocrinol. Metab. 2025, 26, dgaf122. [Google Scholar] [CrossRef]
- Maccio, U.; Wicki, A.; Ruschitzka, F.; Beuschlein, F.; Wolleb, S.; Varga, Z.; Moch, H. Frequency and Consequences of Immune Checkpoint Inhibitor-Associated Inflammatory Changes in Different Organs: An Autopsy Study Over 13-Years. Mod. Pathol. 2025, 38, 100683. [Google Scholar] [CrossRef]
- Mizukoshi, T.; Fukuoka, H.; Takahashi, Y. Immune checkpoint inhibitor-related hypophysitis. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101668. [Google Scholar] [CrossRef]
- Iglesias, P.; Sánchez, J.C.; Díez, J.J. Isolated ACTH deficiency induced by cancer immunotherapy: A systematic review. Pituitary 2021, 24, 630–643. [Google Scholar] [CrossRef]
- Di Dalmazi, G.; Ippolito, S.; Lupi, I.; Caturegli, P. Hypophysitis induced by immune checkpoint inhibitors: A 10-year assessment. Expert Rev. Endocrinol. Metab. 2019, 14, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Okuji, T.; Ito, M.; Onoue, T.; Goto, M.; Sugiyama, M.; Tsunekawa, T.; et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study. J. Immunother. Cancer 2020, 8, e000779. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iwama, S.; Sugiyama, D.; Yasuda, Y.; Okuji, T.; Ito, M.; Ito, S.; Sugiyama, M.; Onoue, T.; Takagi, H.; et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e002493. [Google Scholar] [CrossRef]
- Mitri, F.; Machiraju, D.; Naoum, C.; Hassel, J.C. Early Serum Markers for Immune Checkpoint Inhibitor Induced Hypophysitis in Melanoma Patients. Cancers 2024, 16, 1340. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, S.M.C.; Sheriff, N.; Tran, C.H.; Menzies, A.M.; Tsang, V.H.M.; Long, G.V.; Tonks, K.T.T. Fall in thyroid stimulating hormone (TSH) may be an early marker of ipilimumab-induced hypophysitis. Pituitary 2018, 21, 274–282. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Lai, Z.M.; Spain, L.; Greener, V.; Turajlic, S.; Larkin, J.; Morganstein, D.L. Predicting development of ipilimumab-induced hypophysitis: Utility of T4 and TSH index but not TSH. J. Endocrinol. Investig. 2021, 44, 195–203. [Google Scholar] [CrossRef]
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Freedman, C.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018, 124, 3706–3714. [Google Scholar] [CrossRef]
- Lopes-Pinto, M.; Lacerda-Nobre, E.; Silva, A.L.; Tortosa, F.; Marques, P. The Role of Programmed Cell Death Ligand 1 Expression in Pituitary Tumours: Lessons from the Current Literature. Neuroendocrinology 2024, 114, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Q.; Adam, B.; Jack, A.S.; Lam, A.; Broad, R.W.; Chik, C.L. Immune Cell Infiltrates in Pituitary Adenomas: More Macrophages in Larger Adenomas and More T Cells in Growth Hormone Adenomas. Endocr. Pathol. 2015, 26, 263–272. [Google Scholar] [CrossRef]
- Lopes-Pinto, M.; Lacerda-Nobre, E.; Silva, A.L.; Marques, P. Therapeutical Usefulness of PD-1/PD-L1 Inhibitors in Aggressive or Metastatic Pituitary Tumours. Cancers 2024, 16, 3033. [Google Scholar] [CrossRef]
- Kennedy, R.; Awada, H.; Vura, N.; Ciltea, D.; Morocco, M. Endocrinopathies from checkpoint inhibitors: Incidence, outcomes, and management. Cleve. Clin. J. Med. 2023, 90, 307–317. [Google Scholar] [CrossRef]
- Guo, X.; Chang, M.; Li, W.; Qian, Z.; Guo, H.; Xie, C.; Bi, W.L.; Xing, B.; Zhang, F.; Huang, Y. Immune atlas of pituitary neuroendocrine tumors highlights endocrine-driven immune signature and therapeutic implication. Cell Rep. 2025, 44, 115584. [Google Scholar] [CrossRef]
- Luo, M.; Tang, R.; Wang, H. Tumor immune microenvironment in pituitary neuroendocrine tumors (PitNETs): Increased M2 macrophage infiltration and PD-L1 expression in PIT1-lineage subset. J. Neuro-Oncol. 2023, 163, 663–674. [Google Scholar] [CrossRef]
- Vela-Patiño, S.; Salazar, M.I.; Taniguchi-Ponciano, K.; Vadillo, E.; Gomez-Apo, E.; Escobar-España, A.; Perez-Koldenkova, V.; Bonifaz, L.; Aguilar-Flores, C.; Marrero-Rodríguez, D.; et al. The Immune Microenvironment Landscape of Pituitary NeuroEndocrine Tumors, a Transcriptomic Approach. Genes 2024, 15, 531. [Google Scholar] [CrossRef]
- Lin, S.; Dai, Y.; Han, C.; Han, T.; Zhao, L.; Wu, R.; Liu, J.; Zhang, B.; Huang, N.; Liu, Y.; et al. Single-cell transcriptomics reveal distinct immune-infiltrating phenotypes and macrophage-tumor interaction axes among different lineages of pituitary neuroendocrine tumors. Genome. Med. 2024, 16, 60. [Google Scholar] [CrossRef]
- Nie, D.; Xue, Y.; Fang, Q.; Cheng, J.; Li, B.; Wang, D.; Li, C.; Gui, S.; Zhang, Y.; Zhao, P.l. Immune Checkpoints: Therapeutic Targets for Pituitary Tumors. Dis. Markers. 2021, 2021, 5300381. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.C.M.; Derks, J.L.; Thunnissen, E.; van Suylen, R.J.; den Bakker, M.A.; Groen, H.J.M.; Smit, E.F.; Damhuis, R.A.; van den Broek, E.C.; Stallinga, C.M.; et al. Prevalence and prognostic value of PD-L1 expression in molecular subtypes of metastatic large cell neuroendocrine carcinoma (LCNEC). Lung Cancer 2019, 130, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Arpin, D.; Charpentier, M.C.; Bernardi, M.; Monnet, I.; Boni, A.; Watkin, E.; Goubin-Versini, I.; Lamy, R.; Gérinière, L.; Geier, M.; et al. PD-L1-expression patterns in large-cell neuroendocrine carcinoma of the lung: Potential implications for use of immunotherapy in these patients: The GFPC 03-2017 “EPNEC” study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920937972. [Google Scholar] [CrossRef]
- Eichhorn, F.; Harms, A.; Warth, A.; Muley, T.; Winter, H.; Eichhorn, M.E. PD-L1 expression in large cell neuroendocrine carcinoma of the lung. Lung Cancer 2018, 118, 76–82. [Google Scholar] [CrossRef]
- Roberts, J.A.; Gonzalez, R.S.; Das, S.; Berlin, J.; Shi, C. Expression of PD-1 and PD-L1 in poorly differentiated neuroendocrine carcinomas of the digestive system: A potential target for anti-PD-1/PD-L1 therapy. Hum. Pathol. 2017, 70, 49–54. [Google Scholar] [CrossRef]
- Cossu, G.; La Rosa, S.; Brouland, J.P.; Pitteloud, N.; Harel, E.; Santoni, F.; Brunner, M.; Daniel, R.T.; Messerer, M. PD-L1 Expression in Pituitary Neuroendocrine Tumors/Pituitary Adenomas. Cancers 2023, 15, 4471. [Google Scholar] [CrossRef]
- Lin, A.L.; Jonsson, P.; Tabar, V.; Yang, T.J.; Cuaron, J.; Beal, K.; Cohen, M.; Postow, M.; Rosenblum, M.; Shia, J.; et al. Marked Response of a Hypermutated ACTH-Secreting Pituitary Carcinoma to Ipilimumab and Nivolumab. J. Clin. Endocrinol. Metab. 2018, 103, 3925–3930. [Google Scholar] [CrossRef]
- Caccese, M.; Barbot, M.; Ceccato, F.; Padovan, M.; Gardiman, M.P.; Fassan, M.; Denaro, L.; Emanuelli, E.; D’aVella, D.; Scaroni, C.; et al. Rapid disease progression in patient with mismatch-repair deficiency pituitary ACTH-secreting adenoma treated with checkpoint inhibitor pembrolizumab. Anti-Cancer Drugs 2020, 31, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, C.; Ilie, M.D.; Salle, H.; Nassouri, A.S.; Gaillard, S.; Deluche, E.; Assaker, R.; Mortier, L.; Cortet, C.; Raverot, G. Immunotherapy in Corticotroph and Lactotroph Aggressive Tumors and Carcinomas: Two Case Reports and a Review of the Literature. J. Pers. Med. 2020, 10, 88. [Google Scholar] [CrossRef]
- Lamb, L.S.; Sim, H.W.; McCormack, A.I. Case Report: A Case of Pituitary Carcinoma Treated With Sequential Dual Immunotherapy and Vascular Endothelial Growth Factor Inhibition Therapy. Front. Endocrinol. 2020, 11, 576027. [Google Scholar] [CrossRef]
- Majd, N.; Waguespack, S.G.; Janku, F.; Fu, S.; Penas-Prado, M.; Xu, M.; Alshawa, A.; Kamiya-Matsuoka, C.; Raza, S.M.; McCutcheon, I.E.; et al. Efficacy of pembrolizumab in patients with pituitary carcinoma: Report of four cases from a phase II study. J. Immunother. Cancer. 2020, 8, e001532. [Google Scholar] [CrossRef] [PubMed]
- Sol, B.; de Filette, J.M.K.; Awada, G.; Raeymaeckers, S.; Aspeslagh, S.; Andreescu, C.E.; Neyns, B.; Velkeniers, B. Immune checkpoint inhibitor therapy for ACTH-secreting pituitary carcinoma: A new emerging treatment? Eur. J. Endocrinol. 2021, 184, K1–K5. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Manzoor, S.; Rothman, Y.; Hagen, M.; Pater, L.; Golnik, K.; Mahammedi, A.; Lin, A.L.; Bhabhra, R.; Forbes, J.A.; et al. Complete Response of a Patient With a Mismatch Repair Deficient Aggressive Pituitary Adenoma to Immune Checkpoint Inhibitor Therapy: A Case Report. Neurosurgery 2022, 91, e51–e56. [Google Scholar] [CrossRef]
- Burman, P.; Trouillas, J.; Losa, M.; McCormack, A.; Petersenn, S.; Popovic, V.; Theodoropoulou, M.; Raverot, G.; Dekkers, O.M.; ESE survey collaborators. Aggressive pituitary tumours and carcinomas, characteristics and management of 171 patients. Eur. J. Endocrinol. 2022, 187, 593–605. [Google Scholar] [CrossRef]
- Ilie, M.D.; Villa, C.; Cuny, T.; Cortet, C.; Assie, G.; Baussart, B.; Cancel, M.; Chanson, P.; Decoudier, B.; Deluche, E.; et al. Real-life efficacy and predictors of response to immunotherapy in pituitary tumors: A cohort study. Eur. J. Endocrinol. 2022, 187, 685–696. [Google Scholar] [CrossRef]
- Feola, T.; Carbonara, F.; Verrico, M.; Di Crescenzo, R.M.; Gianno, F.; Colonnese, C.; Arcella, A.; de Alcubierre, D.; Tomao, S.; Esposito, V.; et al. Immunotherapy for Aggressive and Metastatic Pituitary Neuroendocrine Tumors (PitNETs): State-of-the Art. Cancers 2022, 14, 4093. [Google Scholar] [CrossRef] [PubMed]
- Goichot, B.; Taquet, M.C.; Baltzinger, P.; Baloglu, S.; Gravaud, M.; Malouf, G.G.; Noël, G.; Imperiale, A. Should pituitary carcinoma be treated using a NET-like approach? A case of complete remission of a metastatic malignant prolactinoma with multimodal therapy including immunotherapy. Clin. Endocrinol. 2023, 98, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.J.; Zohdy, Y.M.; Porto, E.; Revuelta Barbero, J.M.; Bray, D.; Maldonado, J.; Rodas, A.; Mayol, M.; Morales, B.; Neill, s.; et al. Therapeutic response to pazopanib: Case report and literature review on molecular abnormalities of aggressive prolactinomas. Front. Endocrinol. 2023, 14, 1195792. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, W.; Hong, A.R.; Yoon, J.H.; Kim, H.K.; Kang, H.C. Remarkable response of ACTH-secreting pituitary carcinoma to immune checkpoint inhibitors: A case report. J. Endocr. Soc. 2023, 7 (Suppl. S1), bvad114.1099. [Google Scholar] [CrossRef]
- de Alcubierre, D.; Carretti, A.L.; Ducray, F.; Jouanneau, E.; Raverot, G.; Ilie, M.D. Aggressive pituitary tumors and carcinomas: Medical treatment beyond temozolomide. Minerva Endocrinol. 2024, 49, 321–334. [Google Scholar] [CrossRef]
- Lin, A.L.; Rudneva, V.; Newton, A.; Majd, N.K.; Magge, R.; Sengupta, S.; Goichot, B.; Piotrowski, A.; Gavrilovic, I.; Cooper, O.; et al. Immune checkpoint inhibitor therapy for aggressive pituitary neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2025, 20, dgaf178. [Google Scholar] [CrossRef]
| Ref | Age a | Sex | PitNET | Function | Carcinoma | PD-L1 | Treatment | Imaging Response | Hormone Response |
|---|---|---|---|---|---|---|---|---|---|
| [58] | 35 | F | ACTH | ACTH | Yes | <1% | Dual to Niv | PR → DPR | PR → PD → SD |
| [59] | 47 | M | ACTH | Silent to ACTH | No | Neg | Pembro | PD | PD |
| [60,66] | 60 | F | ACTH | ACTH | Yes | Neg | Dual to Nivo | DPR → PD | PD |
| [60,66] | 68 | M | PRL | PRL | No | Neg | Dual | PD | PR → PD |
| [61] | 72 | F | PRL | Silent | Yes | <1% | Dual to Nivo to Dual | PR → PD | Silent tumor |
| [62] | 30s | M | ACTH | ACTH | Yes | Neg | Pembro | PR | CR |
| [62] | 20s | F | ACTH | ACTH | Yes | Neg | Pembro | PR | PD → PR |
| [62] | Teens | M | ACTH | Silent | Yes | Neg | Pembro | PD | Silent tumor |
| [62] | 50s | F | PRL | PRL | Yes | Neg | Pembro | SD | PD |
| [63] | 48 | M | ACTH | ACTH | Yes | NA | Dual to Niv | CR | PR |
| [64] | 57 | M | ACTH | Silent | Yes | NA | Dual | PD | Silent tumor |
| [65] | NR | NA | ACTH | ACTH | No | 15% | ICI not specified | PD | NA |
| [65] | NR | NA | ACTH | ACTH | Yes | NA | Dual | PD | NA |
| [65] | NR | NA | ACTH | ACTH | Yes | NA | Dual | PD | NA |
| [66] | 66 | M | ACTH | ACTH | No | NA | Dual | SD → PD | PD |
| [66] | 73 | M | PRL | Silent | No | 10% | Dual to Nivo | SD → PD | Silent tumor |
| [66] | 73 | F | PRL | PRL | No | 0% | Dual | SD → PD | PD |
| [66] | 72 | F | ACTH | ACTH | No | 0% | Dual | SD → PD | PD |
| [66] | 44 | M | ACTH | ACTH to Silent | No | 0% | Nivo to Ipi | SD | Silent tumor |
| [66] | 31 | F | ACTH | ACTH | No | 0% | Dual to Nivo | SD → PD | NA |
| [66] | 75 | M | PRL | PRL | No | 40% | Dual to Nivo | PD | PR → PD |
| [66] | 43 | M | ACTH | ACTH | Yes | 0% | Dual | PD | NA |
| [66] | 54 | F | ACTH | ACTH | No | 0% | Dual | SD → PD | SD → PD |
| [66] | 39 | M | PRL | PRL | Yes | NA | Dual to Nivo to Ipi | SD → PD | PR → PD |
| [66] | 38 | M | ACTH | ACTH | Yes | 0% | Dual to Nivo | PR | CR |
| [66] | 52 | M | ACTH | ACTH | Yes | 0% | Dual to Nivo | DPR | NA |
| [67] | 57 | F | Pit-1 | Silent | Yes | 95% | Pembro | PR | Silent tumor |
| [66,68] | 54 | M | PRL | PRL | Yes | 95% | Dual to Nivo | PR | CR |
| [69] | 61 | M | PRL | PRL | No | Pos | Pembro | PD | NA |
| [70] | 70 | F | ACTH | ACTH | Yes | NA | Dual to Nivo | PR | CR |
| [71] | 51 | M | ACTH | Silent | Yes | 0% | Dual | PD | Silent tumor |
| [72] b | 31–79 | M—4 c F—5 | ACTH 4 c PRL 4 GH 1 | Silent/partial 4, ACTH 1 c | ACTH 2 PRL 2 | NA | Dual to Nivo | SD—6 PD—3 | >50% ↓, none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tateno, T.; Shahidi, M.; Lu, J.-Q.; Chik, C. The Pituitary Immune Environment and Immunotherapy: From Hypophysitis to Pituitary Neuroendocrine Tumors. Cells 2025, 14, 1450. https://doi.org/10.3390/cells14181450
Tateno T, Shahidi M, Lu J-Q, Chik C. The Pituitary Immune Environment and Immunotherapy: From Hypophysitis to Pituitary Neuroendocrine Tumors. Cells. 2025; 14(18):1450. https://doi.org/10.3390/cells14181450
Chicago/Turabian StyleTateno, Toru, Mariam Shahidi, Jian-Qiang Lu, and Constance Chik. 2025. "The Pituitary Immune Environment and Immunotherapy: From Hypophysitis to Pituitary Neuroendocrine Tumors" Cells 14, no. 18: 1450. https://doi.org/10.3390/cells14181450
APA StyleTateno, T., Shahidi, M., Lu, J.-Q., & Chik, C. (2025). The Pituitary Immune Environment and Immunotherapy: From Hypophysitis to Pituitary Neuroendocrine Tumors. Cells, 14(18), 1450. https://doi.org/10.3390/cells14181450






