Divergent Morphologies and Common Signaling Features of Active and Inactive Oncogenic RHOA Mutants in Yeast
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
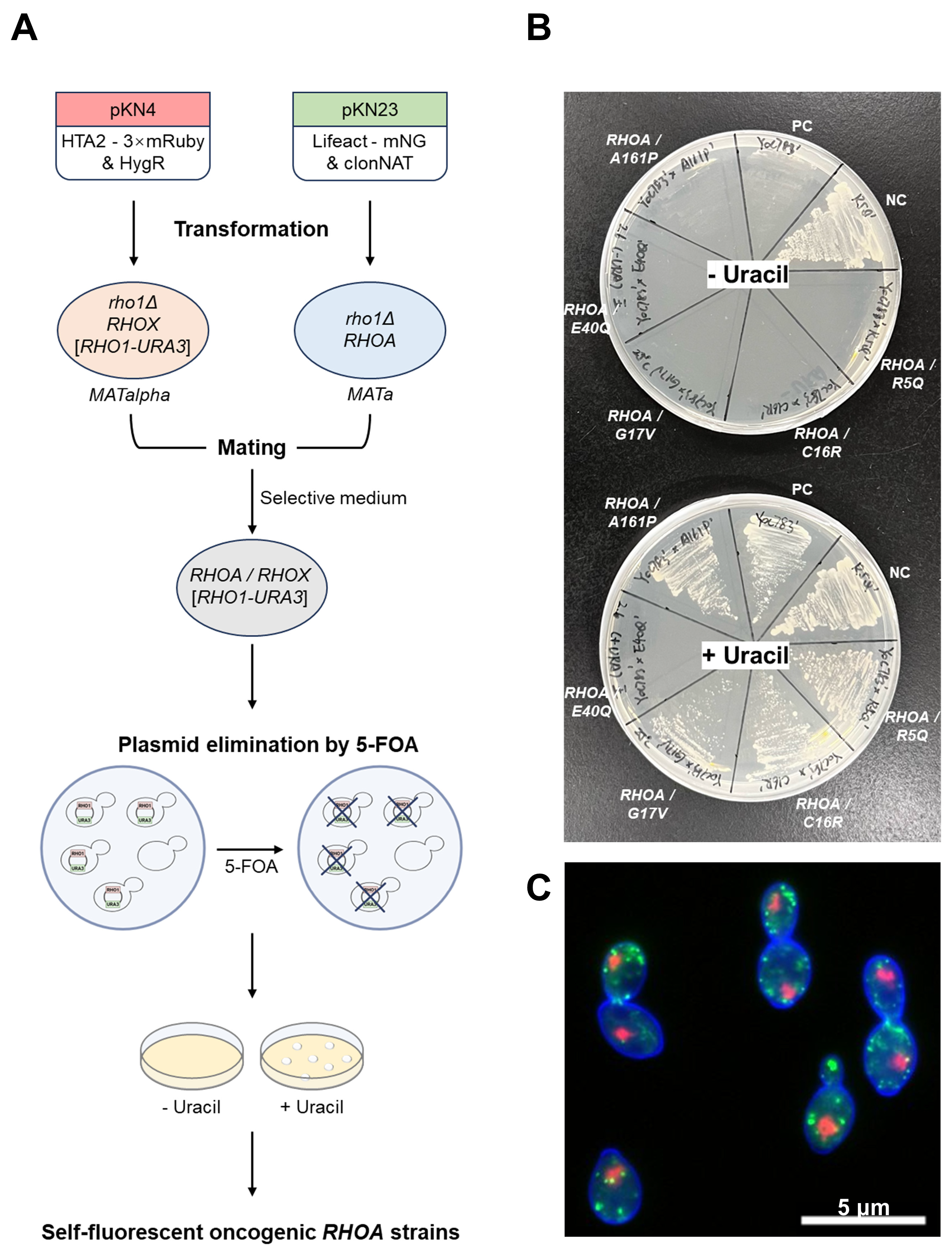
2.2. Transformation, Mating, and Screening
2.3. Colony PCR
2.4. Cell Wall Fluorescence Staining, Microscopy, and Image Processing
2.5. Data Normalization
2.6. Principal Component Analysis
2.7. Hierarchical Clustering
2.8. Generalized Linear Model Analysis
2.9. Linear Discriminant Analysis
2.10. Feature Selection and Interpretation
2.11. Cell Lysate Preparation and Immunoblotting Analysis
3. Results
3.1. Construction of Phenotypic Assay Strains Expressing Oncogenic RHOA Variants in Yeast
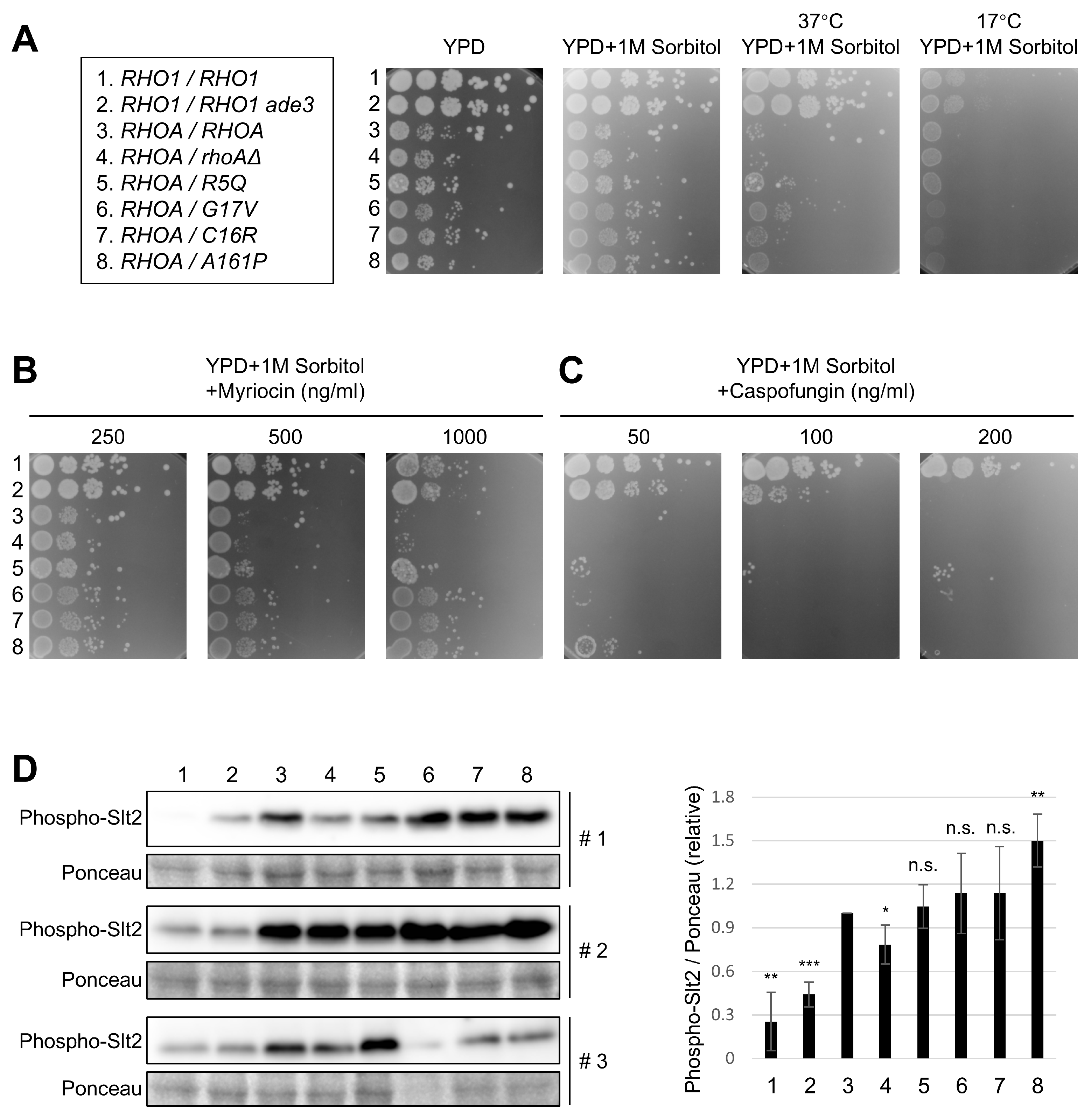
3.2. Growth Phenotypes of RHOA Mutants
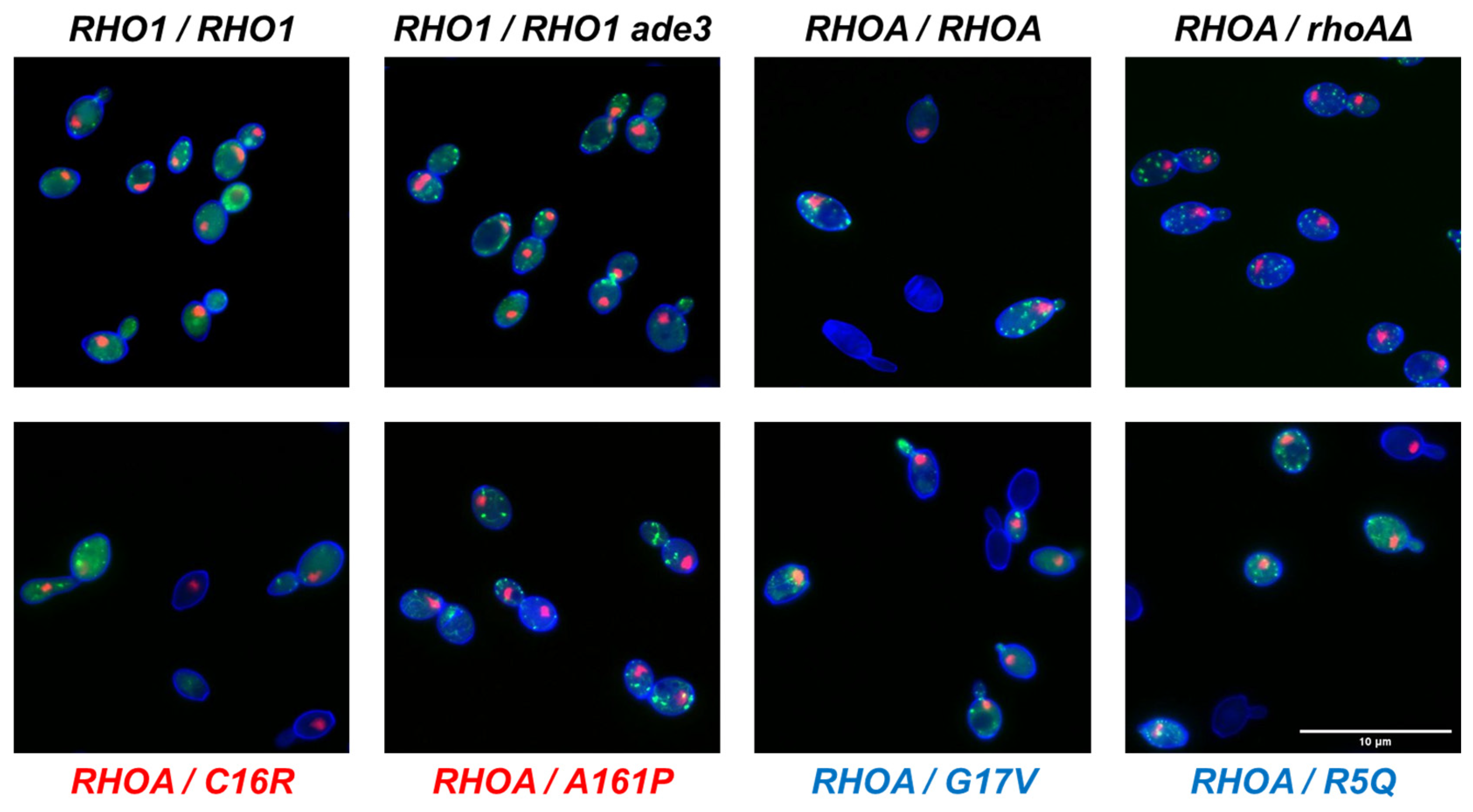
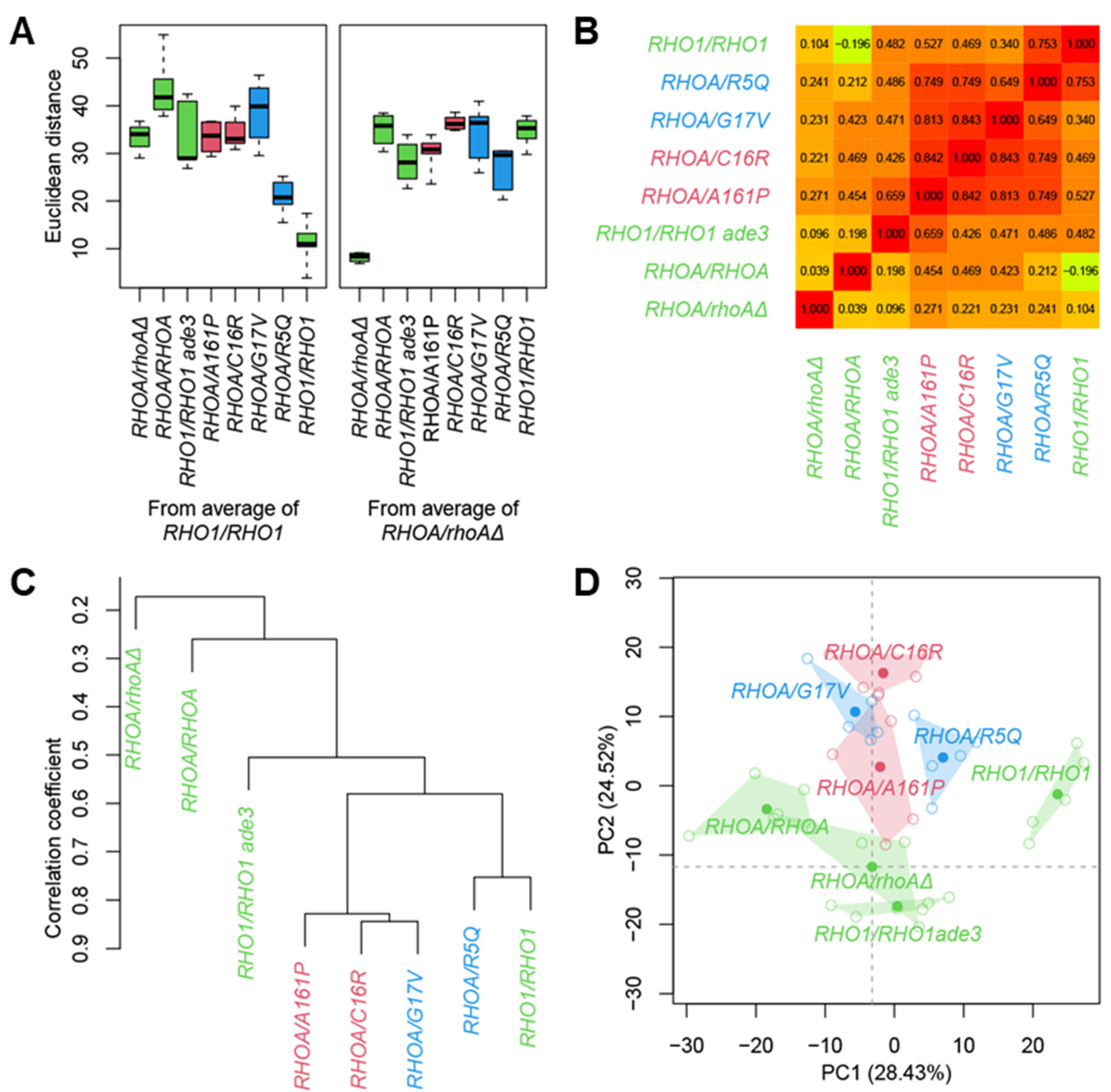
3.3. Morphological Profiling of RHOA Mutants
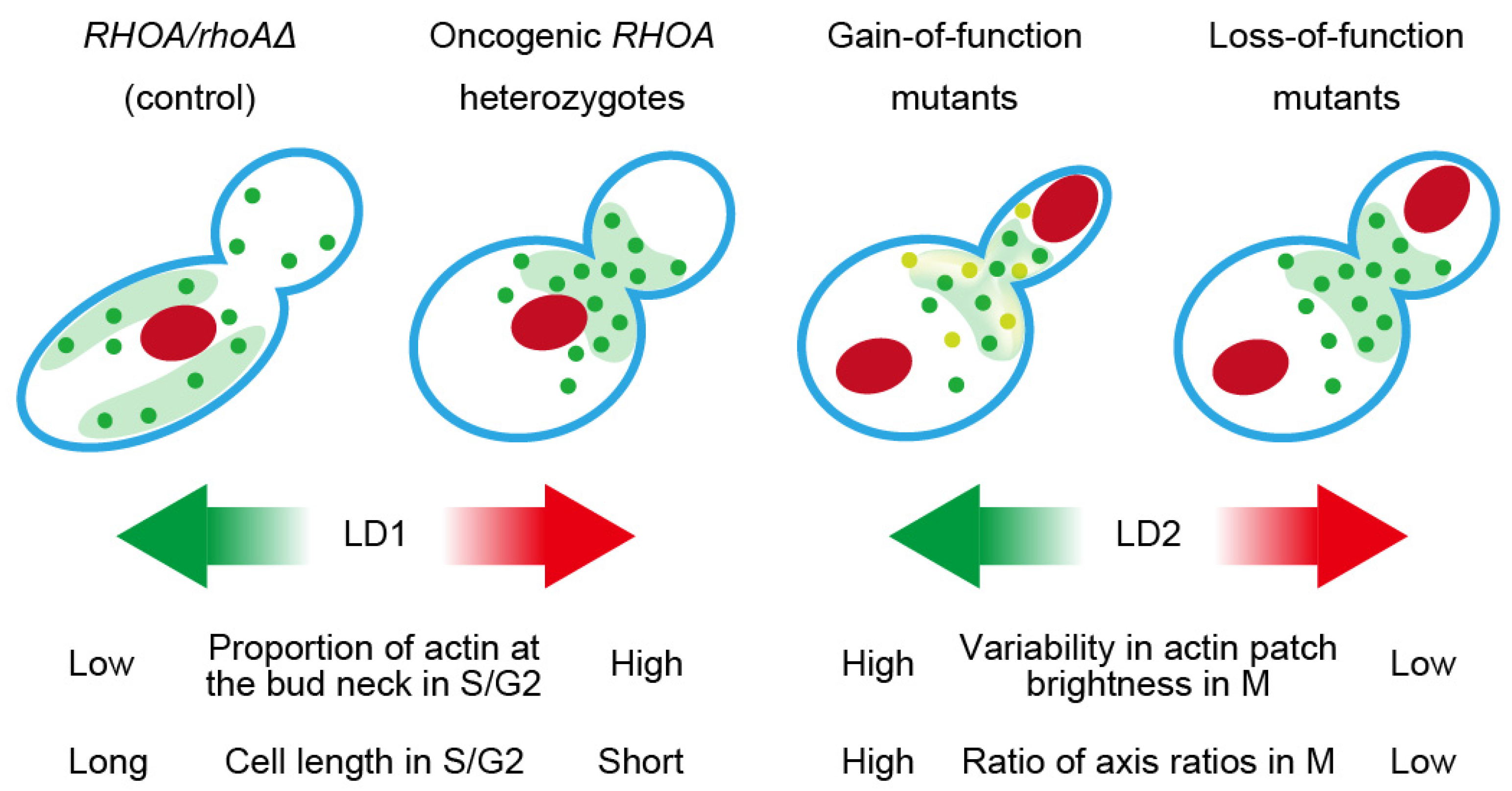
3.4. Differential Morphological Effects of Gain- and Loss-of-Function Mutants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| AITL | Angioimmunoblastic T-cell lymphoma |
| ATL | Adult T-cell leukemia/lymphoma |
| Con A | Concanavalin A |
| CWI | Cell wall integrity |
| FDR | False discovery rate |
| GLM | Generalized linear model |
| GTP | Guanosine triphosphate |
| GDP | Guanosine diphosphate |
| HBSS | Hanks’ Balanced Salt Solution |
| HTA2 | Histone H2A2 gene |
| HygR | Hygromycin B resistance |
| IC50 | Half-maximal inhibitory concentration |
| LD | Linear discriminant |
| LDA | Linear discriminant analysis |
| MAPK | Mitogen-activated protein kinase |
| PC | Principal component |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SD | Synthetic dextrose medium |
| SDCA | Synthetic dextrose casamino acids medium |
| TORC2 | Target of rapamycin complex 2 |
| YPD | Yeast extract peptone dextrose medium |
| 5-FOA | 5-Fluorotic acid |
References
- World Health Organization. Cancer. Available online: https://www.who.int/health-topics/cancer (accessed on 18 July 2025).
- Nagarajan, A.; Malvi, P.; Wajapeyee, N. Oncogene-Directed Alterations in Cancer Cell Metabolism. Trends Cancer 2016, 2, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Crosas-Molist, E.; Samain, R.; Kohlhammer, L.; Orgaz, J.L.; George, S.L.; Maiques, O.; Barcelo, J.; Sanz-Moreno, V. Rho GTPase Signaling in Cancer Progression and Dissemination. Physiol. Rev. 2022, 102, 455–510. [Google Scholar] [CrossRef]
- Sahai, E.; Marshall, C.J. RHO-GTPases and Cancer. Nat. Rev. Cancer 2002, 2, 133–142. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in Cell Biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Jung, H.; Yoon, S.R.; Lim, J.; Cho, H.J.; Lee, H.G. Dysregulation of Rho GTPases in Human Cancers. Cancers 2020, 12, 1179. [Google Scholar] [CrossRef]
- Matos, P. Small GTPases in Cancer: Still Signaling the Way. Cancers 2021, 13, 1500. [Google Scholar] [CrossRef]
- Aspenström, P. The Role of Fast-Cycling Atypical RHO GTPases in Cancer. Cancers 2022, 14, 1961. [Google Scholar] [CrossRef]
- Haga, R.B.; Ridley, A.J. Rho GTPases: Regulation and Roles in Cancer Cell Biology. Small GTPases 2016, 7, 207–221. [Google Scholar] [CrossRef]
- Warner, H.; Wilson, B.J.; Caswell, P.T. Control of Adhesion and Protrusion in Cell Migration by Rho GTPases. Curr. Opin. Cell Biol. 2019, 56, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Spiering, D.; Hodgson, L. Dynamics of the Rho-Family Small GTPases in Actin Regulation and Motility. Cell Adhes. Migr. 2011, 5, 170–180. [Google Scholar] [CrossRef]
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.N.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-Genome Sequencing and Comprehensive Molecular Profiling Identify New Driver Mutations in Gastric Cancer. Nat. Genet. 2014, 46, 573–582. [Google Scholar] [CrossRef]
- Nam, S.; Kim, J.H.; Lee, D.H. RHOA in Gastric Cancer: Functional Roles and Therapeutic Potential. Front. Genet. 2019, 10, 438. [Google Scholar] [CrossRef]
- Kataoka, K.; Ogawa, S. Variegated RHOA Mutations in Human Cancers. Exp. Hematol. 2016, 44, 1123–1129. [Google Scholar] [CrossRef]
- Kakiuchi, M.; Nishizawa, T.; Ueda, H.; Gotoh, K.; Tanaka, A.; Hayashi, A.; Yamamoto, S.; Tatsuno, K.; Katoh, H.; Watanabe, Y.; et al. Recurrent Gain-of-Function Mutations of RHOA in Diffuse-Type Gastric Carcinoma. Nat. Genet. 2014, 46, 583–587. [Google Scholar] [CrossRef]
- Rohde, M.; Richter, J.; Schlesner, M.; Betts, M.J.; Claviez, A.; Bonn, B.R.; Zimmermann, M.; Damm-Welk, C.; Russell, R.B.; Borkhardt, A.; et al. Recurrent RHOA Mutations in Pediatric Burkitt Lymphoma Treated According to the NHL-BFM Protocols. Genes Chromosomes Cancer 2014, 53, 911–916. [Google Scholar] [CrossRef]
- Palomero, T.; Couronné, L.; Khiabanian, H.; Kim, M.-Y.; Ambesi-Impiombato, A.; Perez-Garcia, A.; Carpenter, Z.; Abate, F.; Allegretta, M.; Haydu, J.E.; et al. Recurrent Mutations in Epigenetic Regulators, RHOA and FYN Kinase in Peripheral T Cell Lymphomas. Nat. Genet. 2014, 46, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Kontani, K.; Enami, T.; Kataoka, K.; Ishii, R.; Totoki, Y.; Kataoka, T.R.; Hirata, M.; Aoki, K.; Nakano, K.; et al. Variegated RHOA Mutations in Adult T-Cell Leukemia/Lymphoma. Blood 2016, 127, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Svensmark, J.H.; Brakebusch, C. Rho GTPases in Cancer: Friend or Foe? Oncogene 2019, 38, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S. Opposite RHOA Functions within the ATLL Category. Blood 2016, 127, 524–525. [Google Scholar] [CrossRef][Green Version]
- Sakata-Yanagimoto, M.; Enami, T.; Yoshida, K.; Shiraishi, Y.; Ishii, R.; Miyake, Y.; Muto, H.; Tsuyama, N.; Sato-Otsubo, A.; Okuno, Y.; et al. Somatic RHOA Mutation in Angioimmunoblastic T Cell Lymphoma. Nat. Genet. 2014, 46, 171–175. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Sung, M.K.; Lee, S.H.; Kim, S.; Lee, H.; Park, S.; Kim, S.C.; Lee, B.; Rho, K.; Lee, J.-E.; et al. A Recurrent Inactivating Mutation in RHOA GTPase in Angioimmunoblastic T Cell Lymphoma. Nat. Genet. 2014, 46, 371–375. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, M.; Inoue, A.; Kufareva, I.; Wang, Z.; Mikelis, C.M.; Drummond, R.A.; Avino, S.; Finkel, K.; Kalim, K.W.; DiPasquale, G.; et al. Inactivating Mutations in GNA13 and RHOA in Burkitt’s Lymphoma and Diffuse Large B-Cell Lymphoma: A Tumor Suppressor Function for the Gα13/RhoA Axis in B Cells. Oncogene 2016, 35, 3771–3780. [Google Scholar] [CrossRef]
- Laurent, J.M.; Young, J.H.; Kachroo, A.H.; Marcotte, E.M. Efforts to Make and Apply Humanized Yeast. Briefings in Functional Genomics 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, T.; Galli, A. Yeast as a Tool to Understand the Significance of Human Disease-Associated Gene Variants. Genes 2021, 12, 1303. [Google Scholar] [CrossRef]
- Kachroo, A.H.; Vandeloo, M.; Greco, B.M.; Abdullah, M. Humanized Yeast to Model Human Biology, Disease and Evolution. Dis. Model. Mech. 2022, 15, dmm049309. [Google Scholar] [CrossRef]
- Qadota, H.; Anraku, Y.; Botstein, D.; Ohya, Y. Conditional Lethality of a Yeast Strain Expressing Human RHOA in Place of RHO1. Proc. Natl. Acad. Sci. USA 1994, 91, 9317–9321. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Sese, J.; Yukawa, M.; Sano, F.; Nakatani, Y.; Saito, T.L.; Saka, A.; Fukuda, T.; Ishihara, S.; Oka, S.; et al. High-Dimensional and Large-Scale Phenotyping of Yeast Mutants. Proc. Natl. Acad. Sci. USA 2005, 102, 19015–19020. [Google Scholar] [CrossRef]
- Ohnuki, S.; Nogami, S.; Ohya, Y. A Microfluidic Device to Acquire High-Magnification Microphotographs of Yeast Cells. Cell Div. 2009, 4, 5. [Google Scholar] [CrossRef]
- Wadsworth, J.M.; Clarke, D.J.; McMahon, S.A.; Lowther, J.P.; Beattie, A.E.; Langridge-Smith, P.R.R.; Broughton, H.B.; Dunn, T.M.; Naismith, J.H.; Campopiano, D.J. The Chemical Basis of Serine Palmitoyltransferase Inhibition by Myriocin. J. Am. Chem. Soc. 2013, 135, 14276–14285. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Dei Cas, M.; Cianciolo, S.; Fidilio, A.; Lazzara, F.; Paroni, R.; Pignatello, R.; Strettoi, E.; Ghidoni, R.; Drago, F.; et al. Novel Ophthalmic Formulation of Myriocin: Implications in Retinitis Pigmentosa. Drug Deliv. 2019, 26, 237–243. [Google Scholar] [CrossRef]
- Hatakeyama, R.; Kono, K.; Yoshida, S. Ypk1 and Ypk2 Kinases Maintain Rho1 at the Plasma Membrane by Flippase-Dependent Lipid Remodeling after Membrane Stresses. J. Cell Sci. 2017, 130, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Onishi, J.; Meinz, M.; Thompson, J.; Curotto, J.; Dreikorn, S.; Rosenbach, M.; Douglas, C.; Abruzzo, G.; Flattery, A.; Kong, L.; et al. Discovery of Novel Antifungal (1,3)-β-d-Glucan Synthase Inhibitors. Antimicrob. Agents Chemother. 2000, 44, 368–377. [Google Scholar] [CrossRef] [PubMed]
- González-Rubio, G.; Sastre-Vergara, L.; Molina, M.; Martín, H.; Fernández-Acero, T. Substrates of the MAPK Slt2: Shaping Yeast Cell Integrity. J. Fungi 2022, 8, 368. [Google Scholar] [CrossRef]
- Martín, H.; Rodríguez-Pachón, J.M.; Ruiz, C.; Nombela, C.; Molina, M. Regulatory Mechanisms for Modulation of Signaling through the Cell Integrity Slt2-Mediated Pathway in Saccharomyces Cerevisiae*. J. Biol. Chem. 2000, 275, 1511–1519. [Google Scholar] [CrossRef]
- Ohnuki, S.; Ogawa, I.; Itto-Nakama, K.; Lu, F.; Ranjan, A.; Kabbage, M.; Gebre, A.A.; Yamashita, M.; Li, S.C.; Yashiroda, Y.; et al. High-Throughput Platform for Yeast Morphological Profiling Predicts the Targets of Bioactive Compounds. NPJ Syst. Biol. Appl. 2022, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Anowar, F.; Sadaoui, S.; Selim, B. Conceptual and Empirical Comparison of Dimensionality Reduction Algorithms (PCA, KPCA, LDA, MDS, SVD, LLE, ISOMAP, LE, ICA, t-SNE). Comput. Sci. Rev. 2021, 40, 100378. [Google Scholar] [CrossRef]
- Senoo, H.; Murata, D.; Wai, M.; Arai, K.; Iwata, W.; Sesaki, H.; Iijima, M. KARATE: PKA-Induced KRAS4B-RHOA-mTORC2 Supercomplex Phosphorylates AKT in Insulin Signaling and Glucose Homeostasis. Mol. Cell 2021, 81, 4622–4634.e8. [Google Scholar] [CrossRef]
- Qadota, H.; Python, C.P.; Inoue, S.B.; Arisawa, M.; Anraku, Y.; Zheng, Y.; Watanabe, T.; Levin, D.E.; Ohya, Y. Identification of Yeast Rho1p GTPase as a Regulatory Subunit of 1,3-β-Glucan Synthase. Science 1996, 272, 279–281. [Google Scholar] [CrossRef]
- Moreno-Velásquez, S.D.; Seidel, C.; Juvvadi, P.R.; Steinbach, W.J.; Read, N.D. Caspofungin-Mediated Growth Inhibition and Paradoxical Growth in Aspergillus Fumigatus Involve Fungicidal Hyphal Tip Lysis Coupled with Regenerative Intrahyphal Growth and Dynamic Changes in β-1,3-Glucan Synthase Localization. Antimicrob. Agents Chemother. 2017, 61, e00710–e00717. [Google Scholar] [CrossRef]
- Hu, X.; Yang, P.; Chai, C.; Liu, J.; Sun, H.; Wu, Y.; Zhang, M.; Zhang, M.; Liu, X.; Yu, H. Structural and Mechanistic Insights into Fungal β-1,3-Glucan Synthase FKS1. Nature 2023, 616, 190–198. [Google Scholar] [CrossRef]
- Schaefer, A.; Reinhard, N.R.; Hordijk, P.L. Toward Understanding RhoGTPase Specificity: Structure, Function and Local Activation. Small GTPases 2014, 5, e968004. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ohnuki, S.; Savchenko, A.; Aburatani, H.; Yoshida, S.; Hatakeyama, R.; Ohya, Y. Divergent Morphologies and Common Signaling Features of Active and Inactive Oncogenic RHOA Mutants in Yeast. Cells 2025, 14, 1439. https://doi.org/10.3390/cells14181439
Wang C, Ohnuki S, Savchenko A, Aburatani H, Yoshida S, Hatakeyama R, Ohya Y. Divergent Morphologies and Common Signaling Features of Active and Inactive Oncogenic RHOA Mutants in Yeast. Cells. 2025; 14(18):1439. https://doi.org/10.3390/cells14181439
Chicago/Turabian StyleWang, Chenwei, Shinsuke Ohnuki, Anna Savchenko, Hiroyuki Aburatani, Satoshi Yoshida, Riko Hatakeyama, and Yoshikazu Ohya. 2025. "Divergent Morphologies and Common Signaling Features of Active and Inactive Oncogenic RHOA Mutants in Yeast" Cells 14, no. 18: 1439. https://doi.org/10.3390/cells14181439
APA StyleWang, C., Ohnuki, S., Savchenko, A., Aburatani, H., Yoshida, S., Hatakeyama, R., & Ohya, Y. (2025). Divergent Morphologies and Common Signaling Features of Active and Inactive Oncogenic RHOA Mutants in Yeast. Cells, 14(18), 1439. https://doi.org/10.3390/cells14181439







