Defined Composition of Culture Media Promotes Rodent Neonatal Cardiomyocyte Maturation and Enables Functional Neuro-Cardiac Co-Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Origin of Animals
2.3. Isolation and Culture of Newborn Rat Ventricular Cardiomyocytes
2.4. Establishment of SN/CM Co-Cultures
2.5. Immunofluorescence in Cultured Cells
2.6. Morphometric Analyses
2.7. Transmission Electron Microscopy
2.8. Real-Time Quantitative PCR
2.9. Western Blot in Neonatal CMs
2.10. Real-Time Ca2+ Imaging in Neonatal CM
2.11. Real-Time Ca2+ Imaging in SN/CM Co-Cultures
2.12. Combined Live Imaging Ca2+ Dynamics and Scanning Ion Conductance Microscopy (SICM) in CM-SN Co-Cultures
2.13. Statistical Analysis
3. Results
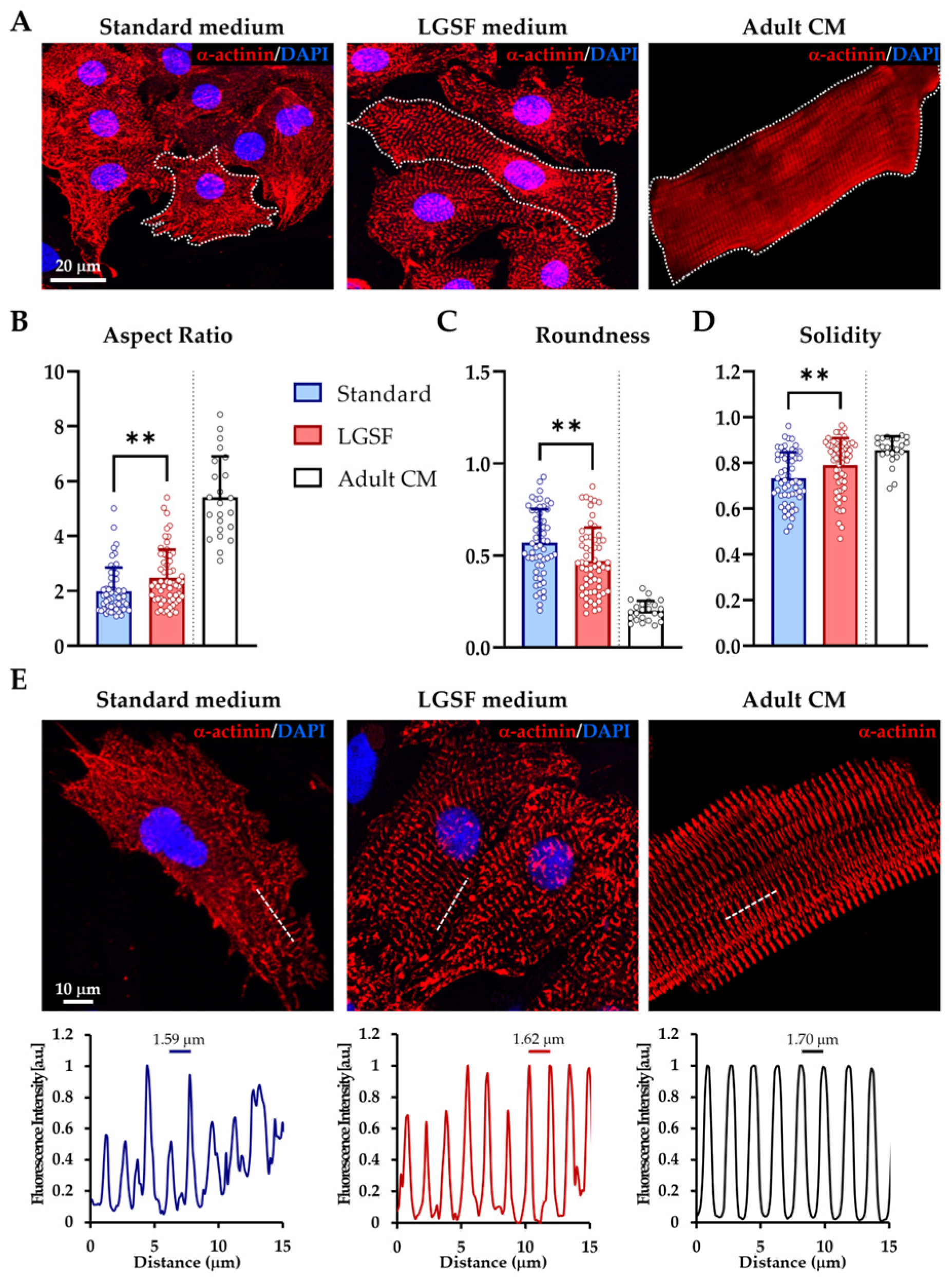
3.1. Low-Glucose, Serum-Deprived Culture Medium Drives Structural and Functional Maturation of Rat Neonatal Cardiomyocytes
3.1.1. LGSF Medium Improves Cell Geometry and Sarcomeric Organization
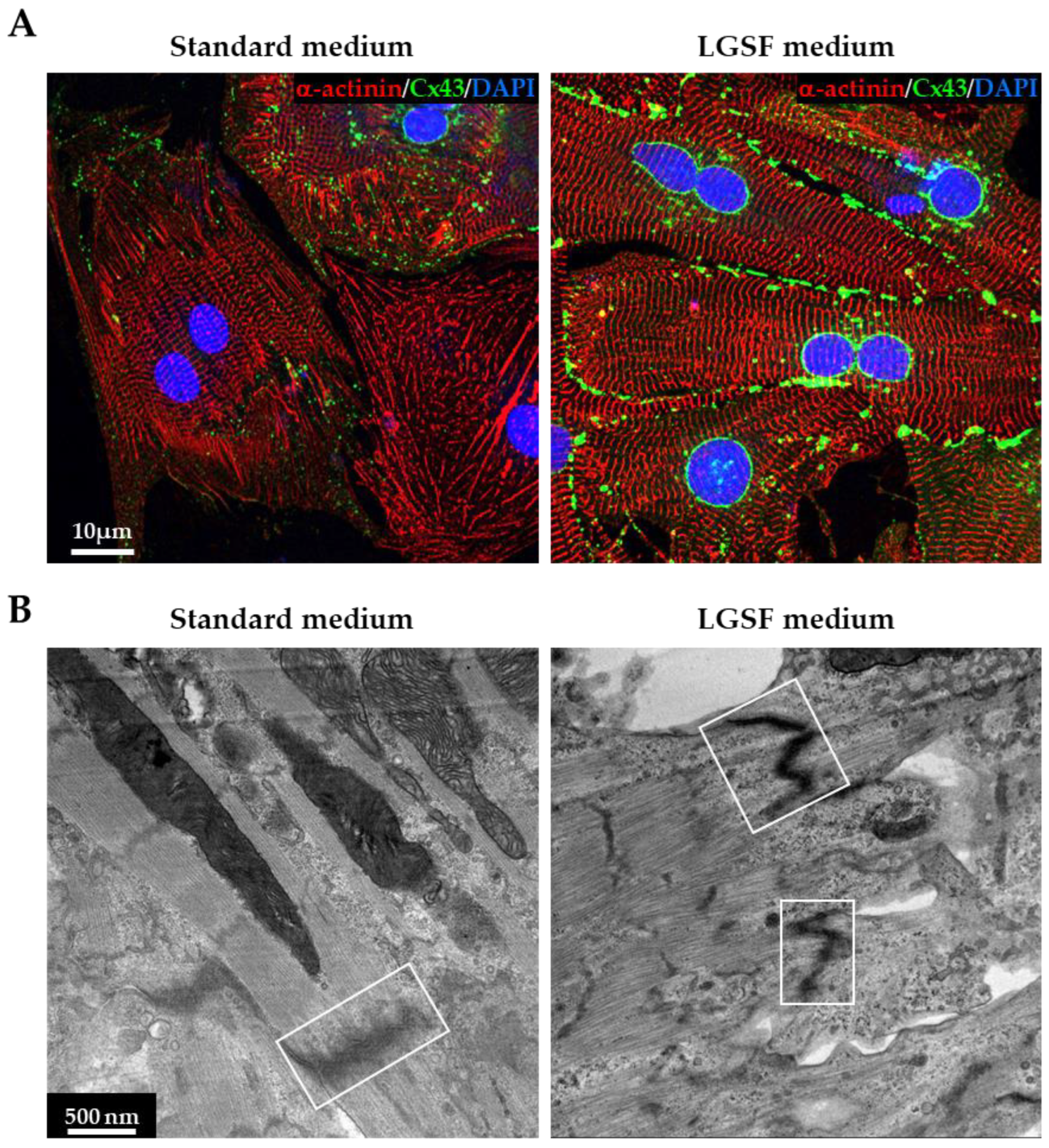
3.1.2. LGSF Medium Promotes Intercellular Structure Maturation
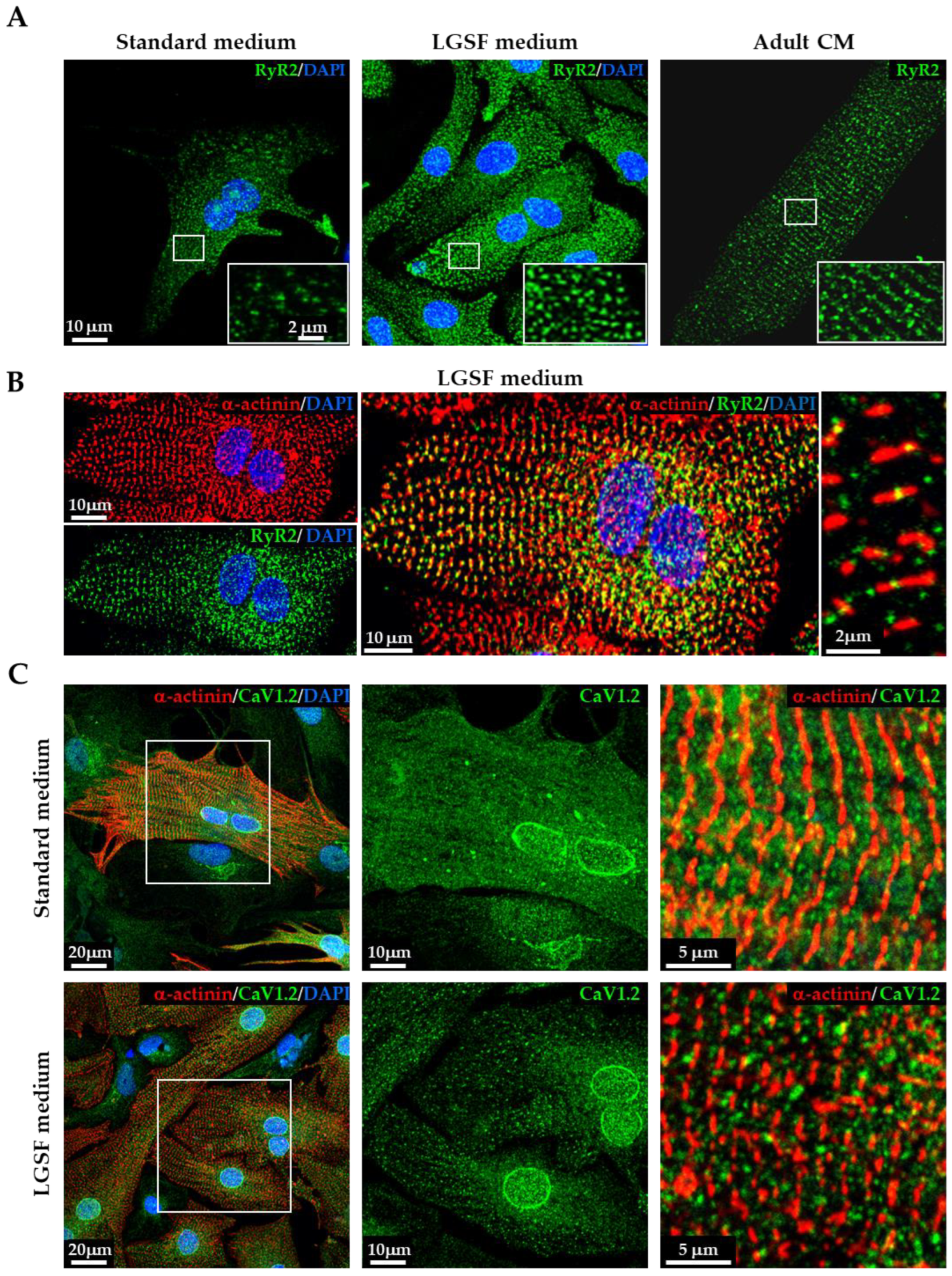
3.1.3. LGSF Medium Influences Intracellular Organelle Maturation and Distribution
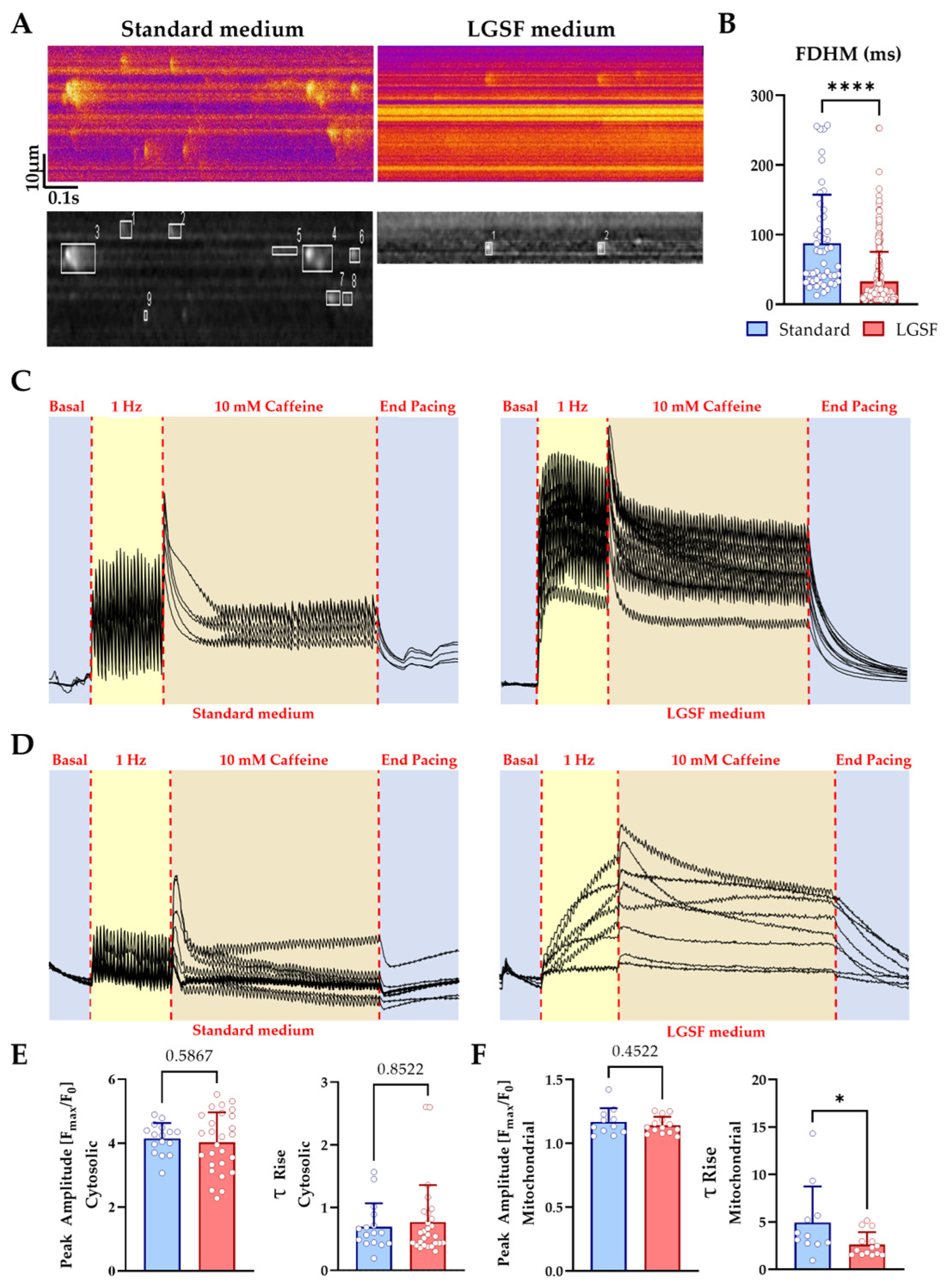
3.1.4. LGSF Medium Improves Cytosolic and Mitochondrial Ca2+ Handling in Neonatal Cardiomyocytes
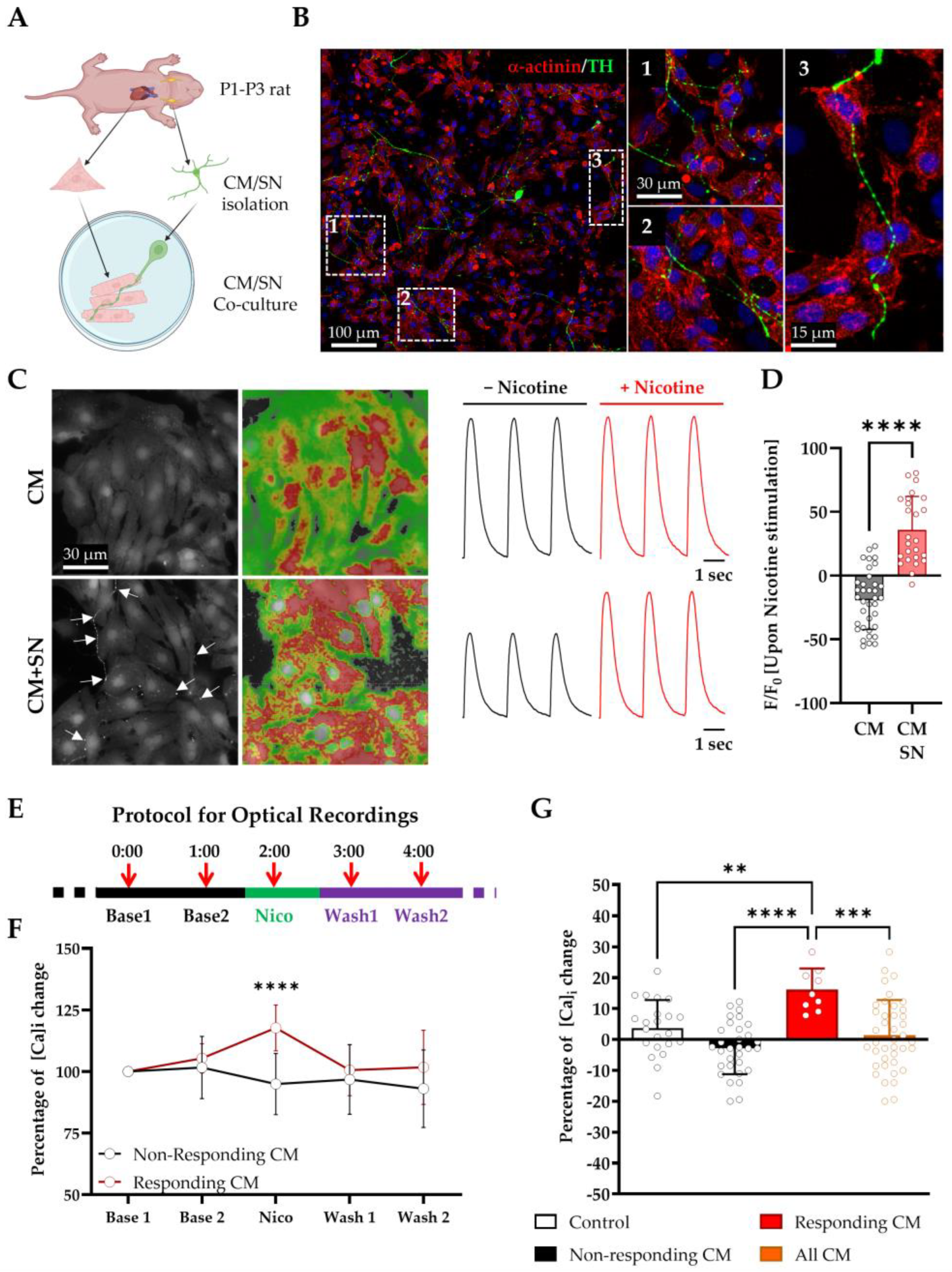
3.2. LGSF Medium Supports the Establishment of Functional Sympathetic Neuron–Cardiomyocyte Co-Cultures
- (i)
- LGSF medium supports SN survival, axonal growth, and the functional innervation of CMs;
- (ii)
- SNs form anatomically and functionally defined NCJs;
- (iii)
- SN activation modulates CM Ca2+ handling in a spatially restricted and reversible manner.
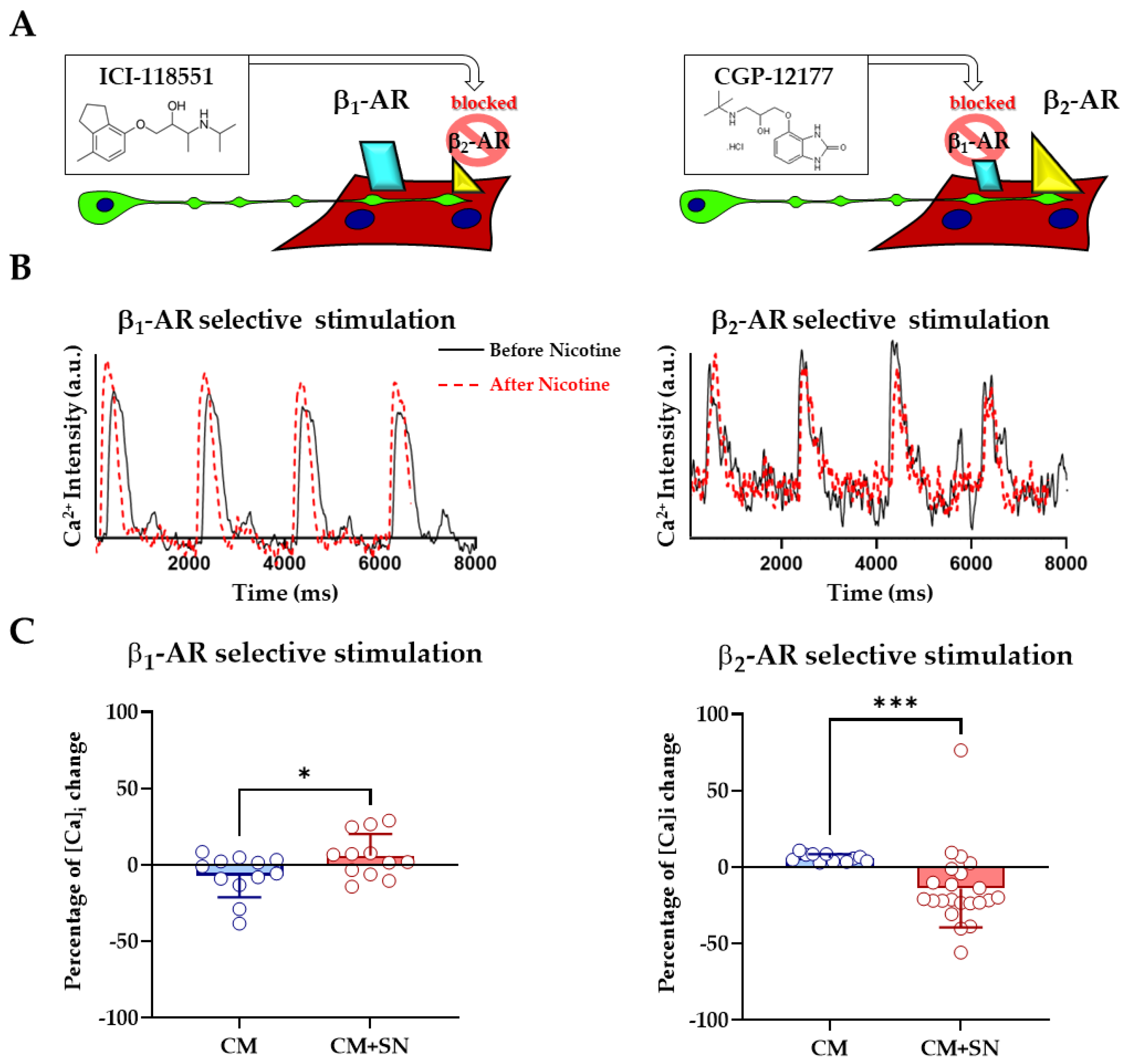
3.3. β1- and β2- Adrenergic Receptors Differentially Contribute to Sympathetic Neuron-Evoked Ca2+ Responses in Cardiomyocytes
4. Discussion
4.1. LGSF Medium Promotes Structural and Functional Maturation of Neonatal Cardiomyocytes
4.2. LGSF Conditions Improve Excitation–Contraction Coupling and Ca2+ Cycling
- (i)
- Higher spatial confinement of Ca2+ sparks;
- (ii)
- Reduced spontaneous wave activity;
- (iii)
- Improved mitochondrial Ca2+ uptake velocity.
4.3. A Functional In Vitro Model of Sympathetic Innervation
4.4. Receptor-Specific Dissection of β-Adrenergic Signaling
4.5. Limitations and Outlook
4.6. Comparison with Other Protocols
5. Conclusions
- (i)
- Enhances the structural and functional maturation of neonatal cardiomyocytes (CMs) beyond what is achievable with standard protocols;
- (ii)
- Supports the formation of a physiologically relevant neuro-cardiac junction (NCJ) with sympathetic neurons (SN);
- (iii)
- Enables the dissection of receptor-specific β-AR signaling in a controlled, synaptic environment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Louch, W.E.; Sheehan, K.A.; Wolska, B.M. Methods in Cardiomyocyte Isolation, Culture, and Gene Transfer. J. Mol. Cell. Cardiol. 2011, 51, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, Ó.; Wang, M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A Review of Protocols for Human IPSC Culture, Cardiac Differentiation, Subtype-Specification, Maturation, and Direct Reprogramming. STAR Protoc. 2022, 3, 101560. [Google Scholar] [CrossRef] [PubMed]
- Ewoldt, J.K.; DePalma, S.J.; Jewett, M.E.; Karakan, M.Ç.; Lin, Y.M.; Mir Hashemian, P.; Gao, X.; Lou, L.; McLellan, M.A.; Tabares, J.; et al. Induced Pluripotent Stem Cell-Derived Cardiomyocyte in Vitro Models: Benchmarking Progress and Ongoing Challenges. Nat. Methods 2024, 22, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Harary, I.; Farley, B. In Vitro Studies of Single Isolated Beating Heart Cells. Science 1960, 131, 1674–1675. [Google Scholar] [CrossRef]
- Franzoso, M.; Zaglia, T.; Mongillo, M. Putting Together the Clues of the Everlasting Neuro-Cardiac Liaison. Biochim. Biophys. Acta 2016, 1863, 1904–1915. [Google Scholar] [CrossRef]
- Habecker, B.A.; Anderson, M.E.; Birren, S.J.; Fukuda, K.; Herring, N.; Hoover, D.B.; Kanazawa, H.; Paterson, D.J.; Ripplinger, C.M. Molecular and Cellular Neurocardiology: Development, and Cellular and Molecular Adaptations to Heart Disease. J. Physiol. 2016, 594, 3853–3875. [Google Scholar] [CrossRef]
- Habecker, B.A.; Bers, D.M.; Birren, S.J.; Chang, R.; Herring, N.; Kay, M.W.; Li, D.; Mendelowitz, D.; Mongillo, M.; Montgomery, J.M.; et al. Molecular and Cellular Neurocardiology in Heart Disease. J. Physiol. 2025, 603, 1689–1728. [Google Scholar] [CrossRef]
- Zaglia, T.; Mongillo, M. Cardiac Sympathetic Innervation, from a Different Point of (Re)View. J. Physiol. 2017, 595, 3919–3930. [Google Scholar] [CrossRef]
- Prando, V.; Da Broi, F.; Franzoso, M.; Plazzo, A.P.; Pianca, N.; Francolini, M.; Basso, C.; Kay, M.W.; Zaglia, T.; Mongillo, M. Dynamics of Neuroeffector Coupling at Cardiac Sympathetic Synapses. J. Physiol. 2018, 596, 2055–2075. [Google Scholar] [CrossRef]
- Pianca, N.; Di Bona, A.; Lazzeri, E.; Costantini, I.; Franzoso, M.; Prando, V.; Armani, A.; Rizzo, S.; Fedrigo, M.; Angelini, A.; et al. Cardiac Sympathetic Innervation Network Shapes the Myocardium by Locally Controlling Cardiomyocyte Size through the Cellular Proteolytic Machinery. J. Physiol. 2019, 597, 3639–3656. [Google Scholar] [CrossRef]
- Dokshokova, L.; Franzoso, M.; Di Bona, A.; Moro, N.; Sanchez Alonso, J.L.; Prando, V.; Sandre, M.; Basso, C.; Faggian, G.; Abriel, H.; et al. Nerve Growth Factor Transfer from Cardiomyocytes to Innervating Sympathetic Neurons Activates TrkA Receptors at the Neuro-Cardiac Junction. J. Physiol. 2022, 600, 2853–2875. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Beanlands, R.S.; Dasilva, J.N. Altered Sympathetic Nervous System Signaling in the Diabetic Heart: Emerging Targets for Molecular Imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 314–334. [Google Scholar]
- Fukuda, K.; Kanazawa, H.; Aizawa, Y.; Ardell, J.L.; Shivkumar, K. Cardiac Innervation and Sudden Cardiac Death. Circ. Res. 2015, 116, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.T.; Ripplinger, C.M.; Myles, R.C.; Habecker, B.A. Molecular Mechanisms of Sympathetic Remodeling and Arrhythmias. Circ. Arrhythmia Electrophysiol. 2016, 9, e001359. [Google Scholar] [CrossRef] [PubMed]
- Clyburn, C.; Sepe, J.J.; Habecker, B.A. What Gets on the Nerves of Cardiac Patients? Pathophysiological Changes in Cardiac Innervation. J. Physiol. 2022, 600, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Winbo, A.; Ramanan, S.; Eugster, E.; Jovinge, S.; Skinner, J.R.; Montgomery, J.M. Functional Coculture of Sympathetic Neurons and Cardiomyocytes Derived from Human-Induced Pluripotent Stem Cells. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H927–H937. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Garcia-Pak, I.H.; Li, W.; Uosaki, H.; Tampakakis, E.; Zou, J.; Lin, Y.; Patterson, K.; Kwon, C.; Mukouyama, Y.S. Sympathetic Neurons Regulate Cardiomyocyte Maturation in Culture. Front. Cell Dev. Biol. 2022, 10, 850645. [Google Scholar] [CrossRef]
- Li, N.; Edel, M.; Liu, K.; Denning, C.; Betts, J.; Neely, O.C.; Li, D.; Paterson, D.J. Human Induced Pluripotent Stem Cell-Derived Cardiac Myocytes and Sympathetic Neurons in Disease Modelling. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220173. [Google Scholar] [CrossRef]
- Mohammadi, N.; Fedele, L.; Chakravarthy, P.; Leonov, V.; Tsansizi, L.; Gu, H.; Seyedmousavi, S.; Cosson, M.V.; Bernardo, A.S.; Gorelik, J.; et al. Sympathetic Neurons Can Modify the Intrinsic Structural and Functional Properties of Human Pluripotent Stem Cell-Derived Cardiomyocytes. J. Physiol. 2025, 603, 2089–2118. [Google Scholar] [CrossRef]
- Campo, A.; Mongillo, M. Imaging Intracellular Ca2+ in Cardiomyocytes with Genetically Encoded Fluorescent Probes. In Methods in Molecular Biology; Humana Press Inc.: Humana, New York, NY, 2019; Volume 1925, pp. 111–125. [Google Scholar]
- Zareen, N.; Greene, L.A. Protocol for Culturing Sympathetic Neurons from Rat Superior Cervical Ganglia (SCG). J. Vis. Exp. 2009, 23, e988. [Google Scholar] [CrossRef]
- Zaglia, T.; Milan, G.; Ruhs, A.; Franzoso, M.; Bertaggia, E.; Pianca, N.; Carpi, A.; Carullo, P.; Pesce, P.; Sacerdoti, D.; et al. Atrogin-1 Deficiency Promotes Cardiomyopathy and Premature Death via Impaired Autophagy. J. Clin. Investig. 2014, 124, 2410–2424. [Google Scholar] [CrossRef] [PubMed]
- Picht, E.; Zima, A.V.; Blatter, L.A.; Bers, D.M. SparkMaster: Automated Calcium Spark Analysis with ImageJ. Am. J. Physiol. Cell Physiol. 2007, 293, C1073–C1081. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Bünemann, M.; Schmitteckert, E.; Lohse, M.J.; Engelhardt, S. Cyclic AMP Imaging in Adult Cardiac Myocytes Reveals Far-Reaching Β1-Adrenergic but Locally Confined Β2-Adrenergic Receptor-Mediated Signaling. Circ. Res. 2006, 99, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, V.O.; Moshkov, A.; Lyon, A.R.; Miragoli, M.; Novak, P.; Paur, H.; Lohse, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Β2-Adrenergic Receptor Redistribution in Heart Failure Changes CAMP Compartmentation. Science 2010, 327, 1653–1657. [Google Scholar] [CrossRef]
- Porter, G.A.; Hom, J.R.; Hoffman, D.L.; Quintanilla, R.A.; Bentley, K.L.d.M.; Sheu, S.S. Bioenergetics, Mitochondria, and Cardiac Myocyte Differentiation. Prog. Pediatr. Cardiol. 2011, 31, 75–81. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, G.; Li, L.; Fernández-Velasco, M.; Rueda, A.; Lefebvre, F.; Wang, Y.; Mateo, P.; Cassan, C.; Gellen, B.; Benitah, J.P.; et al. Reconciling Depressed Ca2+ Sparks Occurrence with Enhanced RyR2 Activity in Failing Mice Cardiomyocytes. J. Gen. Physiol. 2015, 146, 295–306. [Google Scholar] [CrossRef]
- Araujo, P.A.; Muñoz, M.; Quan, J.; Contreras, J.E. Connexin-43 Remodelling and Arrhythmias: Hemichannels as Key Drivers of Cardiac Dysfunction. J. Physiol. 2025, 603, 4293–4306. [Google Scholar] [CrossRef]
- Devic, E.; Xiang, Y.; Gould, D.; Kobilka, B. β-Adrenergic Receptor Subtype-Specific Signaling in Cardiac Myocytes from Β1 and Β2 Adrenoceptor Knockout Mice. Mol. Pharmacol. 2001, 60, 577–583. [Google Scholar] [CrossRef]
- Paur, H.; Wright, P.T.; Sikkel, M.B.; Tranter, M.H.; Mansfield, C.; O’Gara, P.; Stuckey, D.J.; Nikolaev, V.O.; Diakonov, I.; Pannell, L.; et al. High Levels of Circulating Epinephrine Trigger Apical Cardiodepression in a β 2-Adrenergic Receptor/Gi-Dependent Manner: A New Model of Takotsubo Cardiomyopathy. Circulation 2012, 126, 697–706. [Google Scholar] [CrossRef]
- Setterberg, I.E.; Le, C.; Frisk, M.; Li, J.; Louch, W.E. The Physiology and Pathophysiology of T-Tubules in the Heart. Front. Physiol. 2021, 12, 718404. [Google Scholar] [CrossRef]
- Mitcheson, J. Cultured Adult Cardiac Myocytes Future Applications, Culture Methods, Morphological and Electrophysiological Properties. Cardiovasc. Res. 1998, 39, 280–300. [Google Scholar] [CrossRef]
- Iyer, V.R.; Eisen, M.B.; Ross, D.T.; Schuler, G.; Moore, T.; Lee, J.C.F.; Trent, J.M.; Staudt, L.M.; Hudson, J.; Boguski, M.S.; et al. The Transcriptional Program in the Response of Human Fibroblasts to Serum. Science 1999, 283, 83–87. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Spafford, M.A.; Marsh, D.R. Glycolysis Is Predominant Source of Myocardial ATP Production Immediately after Birth. Am. J. Physiol. Heart Circ. Physiol. 1991, 261, H1698–H1705. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Jaswal, J.S. Energy Metabolic Phenotype of the Cardiomyocyte during Development, Differentiation, and Postnatal Maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chen, S.Y.; Fu, R.H.; Huang, Y.C.; Chen, S.Y.; Shyu, W.C.; Lin, S.Z.; Liu, S.P. Differentiation of Embryonic Stem Cells into Cardiomyocytes Used to Investigate the Cardioprotective Effect of Salvianolic Acid B through BNIP3 Involved Pathway. Cell Transplant. 2015, 24, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hodgkinson, C.P.; Lu, K.; Payne, A.J.; Pratt, R.E.; Dzau, V.J. Selenium Augments MicroRNA Directed Reprogramming of Fibroblasts to Cardiomyocytes via Nanog. Sci. Rep. 2016, 6, 23017. [Google Scholar] [CrossRef]
- Ross, R.S.; Pham, C.; Shai, S.Y.; Goldhaber, J.I.; Fenczik, C.; Glembotski, C.C.; Ginsberg, M.H.; Loftus, J.C. β Integrins Participate in the Hypertrophic Response of Rat Ventricular Myocytes. Circ. Res. 1998, 82, 1160–1172. [Google Scholar] [CrossRef]
- Pham, C.G.; Harpf, A.E.; Keller, R.S.; Vu, H.T.; Shai, S.Y.; Loftus, J.C.; Ross, R.S. Striated Muscle-Specific β(1D)-Integrin and FAK Are Involved in Cardiac Myocyte Hypertrophic Response Pathway. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2916–H2926. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Witschas, K.; Ribeiro, I.; Zuzarte, M.; Catarino, S.; Ribeiro-Rodrigues, T.; Caramelo, F.; Aasen, T.; Carreira, I.M.; Goncalves, L.; et al. Cx43 Can Form Functional Channels at the Nuclear Envelope and Modulate Gene Expression in Cardiac Cells. Open Biol. 2023, 13, 230258. [Google Scholar] [CrossRef]
- Ong, S.-B.; Hausenloy, D.J. Mitochondrial Morphology and Cardiovascular Disease. Cardiovasc. Res. 2010, 88, 16–29. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Q.; Zhou, L.; Liu, K.; Jiao, K. Complex Regulation of Mitochondrial Function during Cardiac Development. J. Am. Heart Assoc. 2019, 8, e012731. [Google Scholar] [CrossRef]
- Lu, X.; Thai, P.N.; Lu, S.; Pu, J.; Bers, D.M. Intrafibrillar and Perinuclear Mitochondrial Heterogeneity in Adult Cardiac Myocytes. J. Mol. Cell. Cardiol. 2019, 136, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.M.; Lederer, W.J. Changes in the Calcium Current of Rat Heart Ventricular Myocytes during Development. J. Physiol. 1988, 406, 115–146. [Google Scholar] [CrossRef]
- Klitzner, T.S. Maturational Changes in Excitation-Contraction Coupling in Mammalian Myocardium. JACC 1991, 17, 218–225. [Google Scholar] [CrossRef]
- Poindexter, B.J.; Smith, J.R.; Buja, L.M.; Bick, R.J. Calcium Signaling Mechanisms in Dedifferentiated Cardiac Myocytes: Comparison with Neonatal and Adult Cardiomyocytes. Cell Calcium 2001, 30, 373–382. [Google Scholar] [CrossRef]
- Birkedal, R.; Laasmaa, M.; Branovets, J.; Vendelin, M. Ontogeny of Cardiomyocytes: Ultrastructure Optimization to Meet the Demand for Tight Communication in Excitation–Contraction Coupling and Energy Transfer. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210321. [Google Scholar] [CrossRef]
- Waddell, H.M.M.; Mereacre, V.; Alvarado, F.J.; Munro, M.L. Clustering Properties of the Cardiac Ryanodine Receptor in Health and Heart Failure. J. Mol. Cell. Cardiol. 2023, 185, 38–49. [Google Scholar] [CrossRef]
- Myagmar, B.E.; Flynn, J.M.; Cowley, P.M.; Swigart, P.M.; Montgomery, M.D.; Thai, K.; Nair, D.; Gupta, R.; Deng, D.X.; Hosoda, C.; et al. Adrenergic Receptors in Individual Ventricular Myocytes: The Beta-1 and Alpha-1B Are in All Cells, the Alpha-1A Is in a Subpopulation, and the Beta-2 and Beta-3 Are Mostly Absent. Circ. Res. 2017, 120, 1103–1115. [Google Scholar] [CrossRef]
- Shcherbakova, O.G.; Hurt, C.M.; Xiang, Y.; Dell’Acqua, M.L.; Zhang, Q.; Tsien, R.W.; Kobilka, B.K. Organization of β-Adrenoceptor Signaling Compartments by Sympathetic Innervation of Cardiac Myocytes. J. Cell Biol. 2007, 176, 521–533. [Google Scholar] [CrossRef]
- Xiang, Y.K. Compartmentalization of β-Adrenergic Signals in Cardiomyocytes. Circ. Res. 2011, 109, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Q.; Wang, L.P.; Gong, Y.Y.; Fan, X.X.; Zhu, S.Y.; Wang, X.T.; Wang, Y.P.; Li, L.L.; Xing, X.; Liu, X.X.; et al. Β2-Adrenergic Stimulation Compartmentalizes Β1 Signaling into Nanoscale Local Domains by Targeting the C-Terminus of Β1-Adrenoceptors. Circ. Res. 2019, 124, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pu, W.T. Cardiomyocyte Maturation: New Phase in Development. Circ. Res. 2020, 126, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Vunjak-Novakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced Maturation of Human Cardiac Tissue Grown from Pluripotent Stem Cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Mills, R.J.; Titmarsh, D.M.; Koenig, X.; Parker, B.L.; Ryall, J.G.; Quaife-Ryan, G.A.; Voges, H.K.; Hodson, M.P.; Ferguson, C.; Drowley, L.; et al. Functional Screening in Human Cardiac Organoids Reveals a Metabolic Mechanism for Cardiomyocyte Cell Cycle Arrest. Proc. Natl. Acad. Sci. USA 2017, 114, E8372–E8381. [Google Scholar] [CrossRef]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hörmann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human IPSC-Derived Cardiomyocytes. Cell Rep. 2020, 32, 107925. [Google Scholar] [CrossRef]

| Component | Standard Medium (% v/v) | LGSF Medium (% v/v) | ||
|---|---|---|---|---|
| 1st Day | 2nd Day | 1st Day | 2nd Day | |
| DMEM (25 mM HEPES) | 67 | 75 | – | – |
| M199 | 17.5 | 17 | – | – |
| Horse Serum (HS) | 10 | 5 | – | – |
| Newborn Calf Serum (NCS) | 5 | 0.5 | – | – |
| MEM | – | – | 88.8 | 98.8 |
| Fetal Bovine Serum (FBS) | – | – | 10 | 0 |
| Non-Essential Amino Acids (NEAA) | – | – | 0.1 | 0.1 |
| ITS-X Supplement | – | – | 0 | 0.1 |
| L-Glutamine | 1 | 1 | – | – |
| Penicillin/Streptomycin | 1 | 1 | 1 | 1 |
| Antibody | Host | Application | Dilution | Supplier | RRID |
|---|---|---|---|---|---|
| α-Actinin | M | IF | 1:200 | SA | AB_2221571 |
| WB | 1:1000 | ||||
| Actin | Rb | WB | 1:2500 | SA | AB_476738 |
| RyR2 | Rb | IF | 1:200 | SA | AB_1856527 |
| CaV1.2 (LTCC) | Rb | IF | 1:100 | Alomone | AB_2039771 |
| Connexin-43 | Rb | IF | 1:200 | SA | AB_2294609 |
| TOM20 | Rb | IF | 1:200 | SCB | AB_2207533 |
| Thyrosine Hydroxylase | Rb | IF | 1:200 | SA | AB_390204 |
| SERCA2A | M | WB | 1:1000 | Invitrogen | AB_325502 |
| Anti-Rb-488 | G | IF | 1:200 | JL | AB_2338052 |
| Anti-M-488 | G | IF | 1:200 | JL | AB_2338840 |
| Anti-M-Cy3 | G | IF | 1:200 | JL | AB_2338692 |
| Anti-Rb-Cy3 | G | IF | 1:200 | JL | AB_2338006 |
| Anti-M-HRP | G | WB | 1:5000 | BioRad | AB_2921252 |
| Anti-Rb-HRP | G | WB | 1:5000 | BioRad | AB_11125142 |
| Gene (Rat) | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Adra1a | GGTTGCTTCGTCCTCTGCT | GAAATCCGGGAAGAAAGACC |
| Adra1b | CCCTTCTTCATCGCTCTCC | GGATTGAGGCAGCTGTTGA |
| Adra1d | TTCTTCTTCGTCCTGCCTCT | AGCGGGTTCACACAGCTATT |
| Adrb1 | AGAGCAGAAGGCGCTCAAG | AGCCAGCAGAGCGTGAAC |
| Adrb2 | TGCTATCACATCGCCCTTC | ACCACTCGGGCCTTATTCTT |
| Gapdh | CACCATCTTCCAGGAGCGAG | CCTTCTCCATGGTGGTGAAGAC |
| Parameter | Neonatal CM in Standard Medium | Neonatal CM in LGSF Medium | Adult CM | p-Value (Standard vs. LGSF) |
|---|---|---|---|---|
| Aspect Ratio | 2.00 ± 0.85 | 2.48 ± 1.03 | 5.41 ± 1.49 | ≤0.01 |
| Roundness | 0.57 ± 0.18 | 0.47 ± 0.18 | 0.19 ± 0.05 | ≤0.01 |
| Solidity | 0.73 ± 0.11 | 0.79 ± 0.11 | 0.85 ± 0.06 | ≤0.01 |
| Cell Area (μm2) | 800.8 ± 325.1 | 1017.9 ± 311.5 | 2109.47 ± 664.3 | ≤0.001 |
| Sarcomere distance | 1.59 ± 0.17 | 1.62 ± 0.10 | 1.70 ± 0.14 | >0.9999 |
| Mitochondrial Content [%] | 14.1 ± 7.9 | 22.8 ± 3.9 | ~30% [26] | ≤0.05 |
| Spark FDHM [ms] | 87.7 ± 69.8 | 32.8 ± 42.6 | ~20–30 [27] | ≤0.0001 |
| Spark FWHM [μm] | 4.2 ± 3.1 | 2.3 ± 2.0 | ~2 [27] | ≤0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borile, G.; Dokshokova, L.; Moro, N.; Campo, A.; Prando, V.; Sanchez-Alonso, J.L.; Gorelik, J.; Faggian, G.; Mongillo, M.; Zaglia, T. Defined Composition of Culture Media Promotes Rodent Neonatal Cardiomyocyte Maturation and Enables Functional Neuro-Cardiac Co-Culture. Cells 2025, 14, 1434. https://doi.org/10.3390/cells14181434
Borile G, Dokshokova L, Moro N, Campo A, Prando V, Sanchez-Alonso JL, Gorelik J, Faggian G, Mongillo M, Zaglia T. Defined Composition of Culture Media Promotes Rodent Neonatal Cardiomyocyte Maturation and Enables Functional Neuro-Cardiac Co-Culture. Cells. 2025; 14(18):1434. https://doi.org/10.3390/cells14181434
Chicago/Turabian StyleBorile, Giulia, Lolita Dokshokova, Nicola Moro, Antonio Campo, Valentina Prando, Jose L. Sanchez-Alonso, Julia Gorelik, Giuseppe Faggian, Marco Mongillo, and Tania Zaglia. 2025. "Defined Composition of Culture Media Promotes Rodent Neonatal Cardiomyocyte Maturation and Enables Functional Neuro-Cardiac Co-Culture" Cells 14, no. 18: 1434. https://doi.org/10.3390/cells14181434
APA StyleBorile, G., Dokshokova, L., Moro, N., Campo, A., Prando, V., Sanchez-Alonso, J. L., Gorelik, J., Faggian, G., Mongillo, M., & Zaglia, T. (2025). Defined Composition of Culture Media Promotes Rodent Neonatal Cardiomyocyte Maturation and Enables Functional Neuro-Cardiac Co-Culture. Cells, 14(18), 1434. https://doi.org/10.3390/cells14181434









