Morphological and Transcriptomic Analyses of the Adrenal Gland in Acomys cahirinus: A Novel Model for Murine Adrenal Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Issues
2.2. Histological Procedure
2.3. Immunofluorescence Analysis
2.4. RNA Sequencing and Analysis
2.5. Validation of RNA-Seq Data
2.6. Statistical Analysis
3. Results
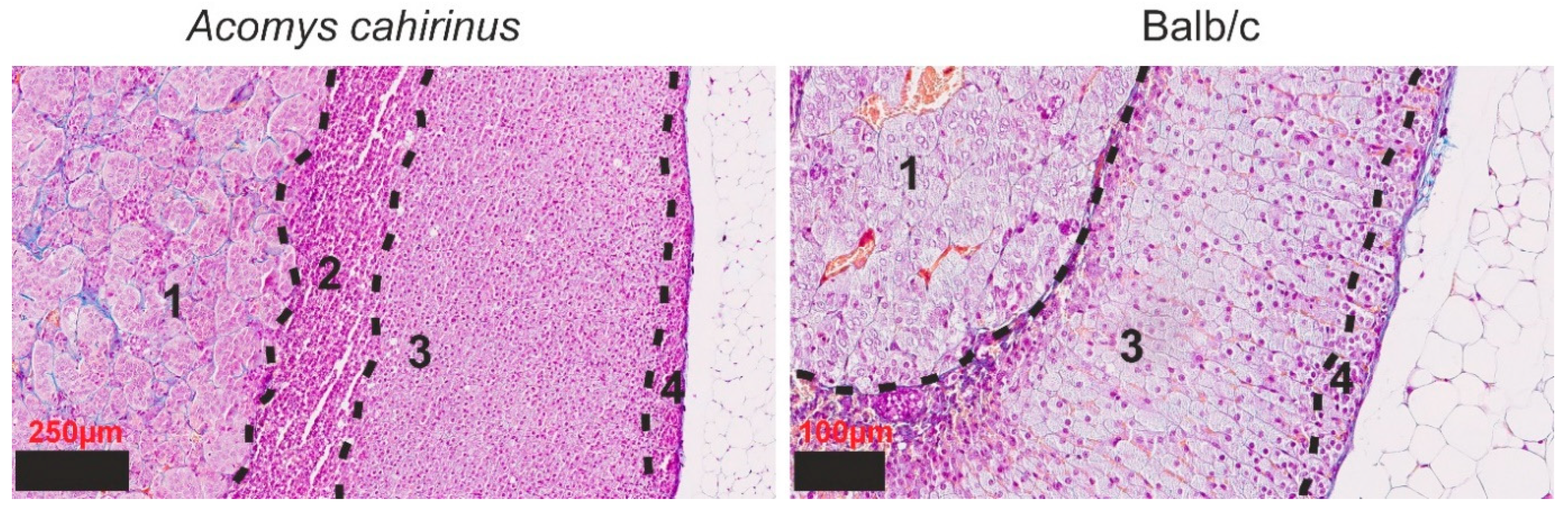
3.1. Histological Examination
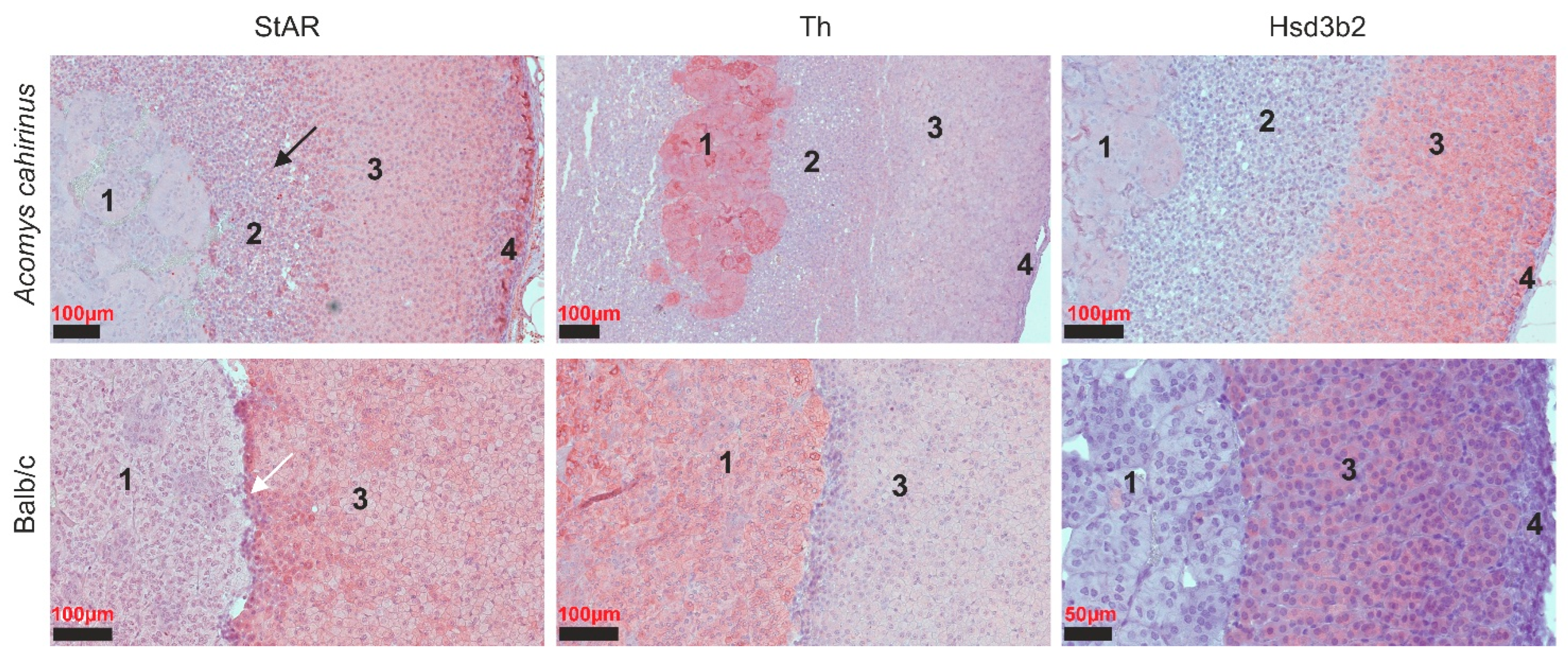
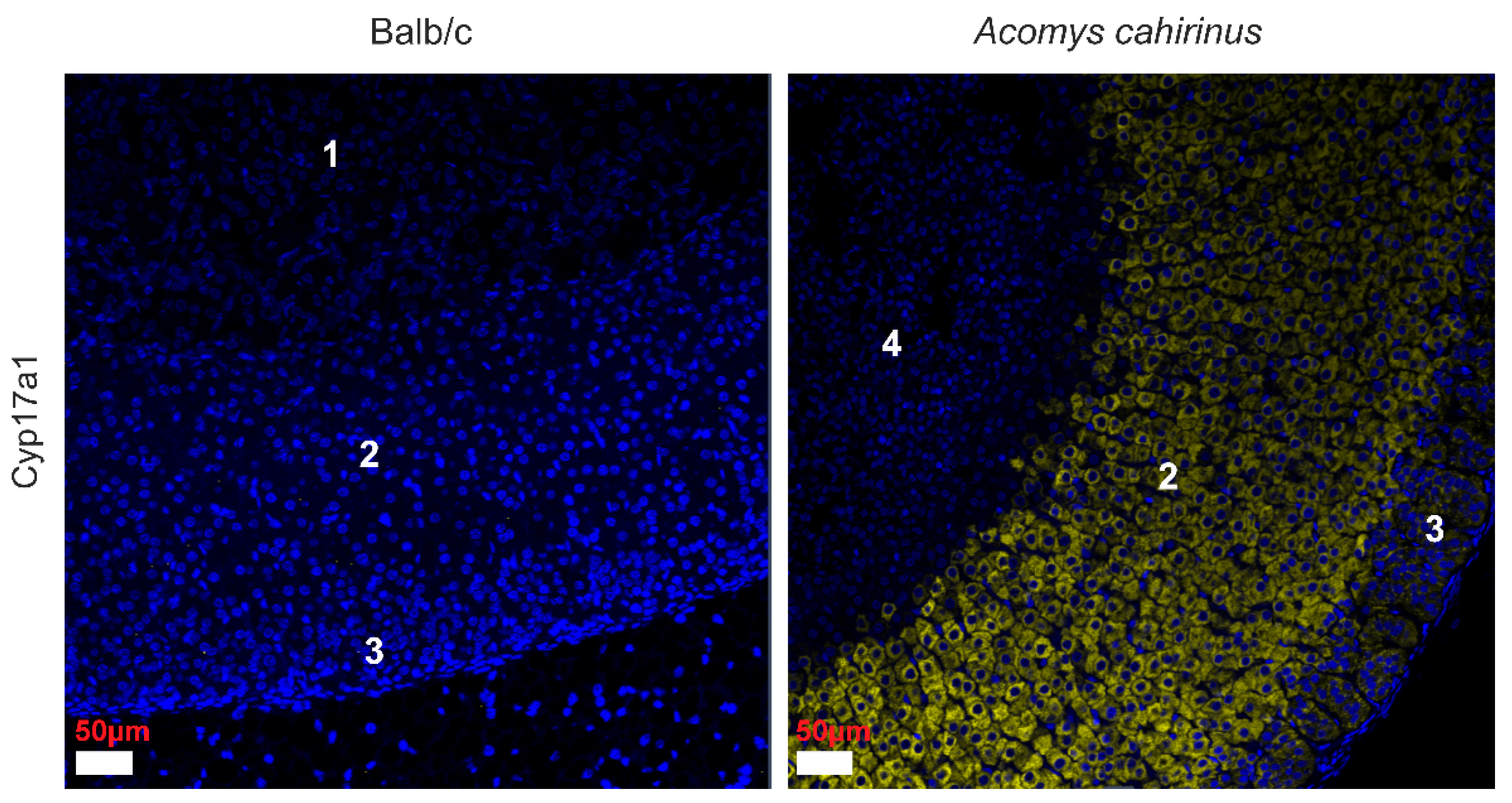
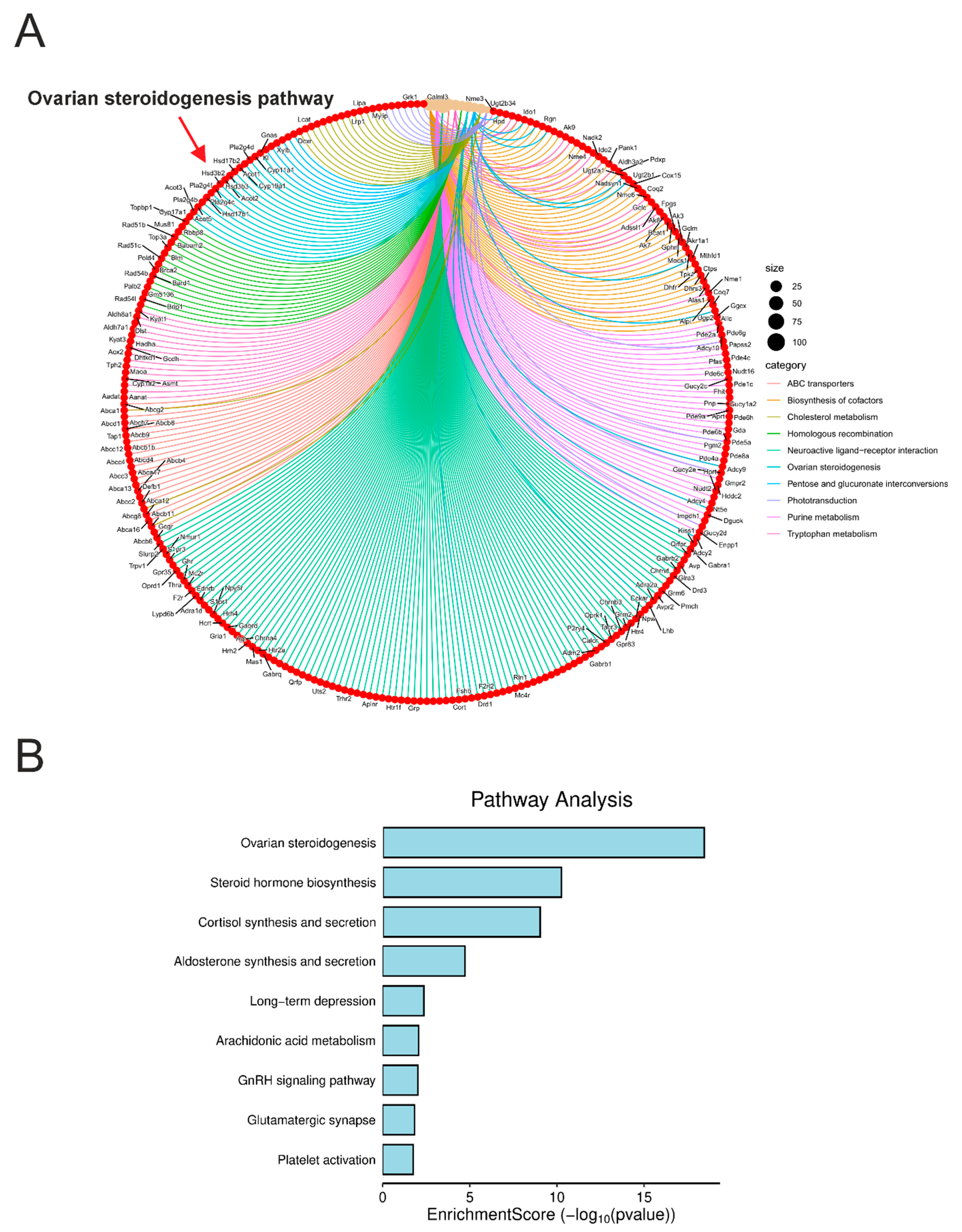
3.2. Differential Gene Expression in the Adrenal Glands of Acomys Cahirinus and Balb/C Mice
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yates, R.; Katugampola, H.; Cavlan, D.; Cogger, K.; Meimaridou, E.; Hughes, C.; Metherell, L.; Guasti, L.; King, P. Adrenocortical development, maintenance, and disease. Curr. Top. Dev. Biol. 2013, 106, 239–312. [Google Scholar] [CrossRef] [PubMed]
- El Ghorayeb, N.; Bourdeau, I.; Lacroix, A. Role of ACTH and Other Hormones in the Regulation of Aldosterone Production in Primary Aldosteronism. Front. Endocrinol. 2016, 7, 72. [Google Scholar] [CrossRef]
- Hyatt, P.J.; Bhatt, K.; Tait, J.F. Steroid biosynthesis by zona fasciculata and zona reticularis cells purified from the mammalian adrenal cortex. J. Steroid Biochem. 1983, 19, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Zhang, A.; Hernandez, S.; Marck, B.T.; Zhang, X.; Tamae, D.; Biehl, H.E.; Tretiakova, M.; Bartlett, J.; Burns, J.; et al. Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 426–439. [Google Scholar] [CrossRef]
- Quinn, T.A.; Ratnayake, U.; Castillo-Melendez, M.; Moritz, K.M.; Dickinson, H.; Walker, D.W. Adrenal steroidogenesis following prenatal dexamethasone exposure in the spiny mouse. J. Endocrinol. 2014, 221, 347–362. [Google Scholar] [CrossRef][Green Version]
- Mitani, F. Functional zonation of the rat adrenal cortex: The development and maintenance. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 163–183. [Google Scholar] [CrossRef]
- Missaghian, E.; Kempná, P.; Dick, B.; Hirsch, A.; Alikhani-Koupaei, R.; Jégou, B.; Mullis, P.E.; Frey, B.M.; Flück, C.E. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009, 202, 99–109. [Google Scholar] [CrossRef]
- Keeney, D.S.; Jenkins, C.M.; Waterman, M.R. Developmentally regulated expression of adrenal 17 alpha-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology 1995, 136, 4872–4879. [Google Scholar] [CrossRef]
- Maden, M.; Brant, J.O.; Rubiano, A.; Sandoval, A.G.W.; Simmons, C.; Mitchell, R.; Collin-Hooper, H.; Jacobson, J.; Omairi, S.; Patel, K. Perfect chronic skeletal muscle regeneration in adult spiny mice, Acomys cahirinus. Sci. Rep. 2018, 8, 8920. [Google Scholar] [CrossRef]
- Seifert, A.W.; Kiama, S.G.; Seifert, M.G.; Goheen, J.R.; Palmer, T.M.; Maden, M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012, 489, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.X.; Harn, H.I.; Ou, K.L.; Lei, M.; Chuong, C.M. Comparative regenerative biology of spiny (Acomys cahirinus) and laboratory (Mus musculus) mouse skin. Exp. Dermatol. 2019, 28, 442–449. [Google Scholar] [CrossRef]
- Okamura, D.M.; Brewer, C.M.; Wakenight, P.; Bahrami, N.; Bernardi, K.; Tran, A.; Olson, J.; Shi, X.; Yeh, S.Y.; Piliponsky, A.; et al. Spiny mice activate unique transcriptional programs after severe kidney injury regenerating organ function without fibrosis. iScience 2021, 24, 103269. [Google Scholar] [CrossRef]
- Qi, Y.; Dasa, O.; Maden, M.; Vohra, R.; Batra, A.; Walter, G.; Yarrow, J.F.; Aranda, J.M.; Raizada, M.K.; Pepine, C.J. Functional heart recovery in an adult mammal, the spiny mouse. Int. J. Cardiol. 2021, 338, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Ratnayake, U.; Dickinson, H.; Nguyen, T.H.; McIntosh, M.; Castillo-Melendez, M.; Conley, A.J.; Walker, D.W. Ontogeny of the adrenal gland in the spiny mouse, with particular reference to production of the steroids cortisol and dehydroepiandrosterone. Endocrinology 2013, 154, 1190–1201. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Takahashi, H.; Kato, S.; Murata, M.; Carninci, P. CAGE (cap analysis of gene expression): A protocol for the detection of promoter and transcriptional networks. Methods Mol. Biol. 2012, 786, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Kouno, T.; Moody, J.; Kwon, A.T.; Shibayama, Y.; Kato, S.; Huang, Y.; Böttcher, M.; Motakis, E.; Mendez, M.; Severin, J.; et al. C1 CAGE detects transcription start sites and enhancer activity at single-cell resolution. Nat. Commun. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; de Hoon, M.J.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V.; Lizio, M.; Itoh, M.; et al. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [CrossRef]
- Alam, T.; Agrawal, S.; Severin, J.; Young, R.S.; Andersson, R.; Arner, E.; Hasegawa, A.; Lizio, M.; Ramilowski, J.A.; Abugessaisa, I.; et al. Comparative transcriptomics of primary cells in vertebrates. Genome Res. 2020, 30, 951–961. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Ekstrand, B.; Zamaratskaia, G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase: A review. Int. J. Mol. Sci. 2013, 14, 17926–17942. [Google Scholar] [CrossRef]
- Chetyrkin, S.V.; Belyaeva, O.V.; Gough, W.H.; Kedishvili, N.Y. Characterization of a novel type of human microsomal 3alpha-hydroxysteroid dehydrogenase: Unique tissue distribution and catalytic properties. J. Biol. Chem. 2001, 276, 22278–22286. [Google Scholar] [CrossRef]
- Burris-Hiday, S.D.; Scott, E.E. Steroidogenic cytochrome P450 17A1 structure and function. Mol. Cell. Endocrinol. 2021, 528, 111261. [Google Scholar] [CrossRef]
- Yi, M.; Negishi, M.; Lee, S.J. Estrogen Sulfotransferase (SULT1E1): Its Molecular Regulation, Polymorphisms, and Clinical Perspectives. J. Pers. Med. 2021, 11, 194. [Google Scholar] [CrossRef]
- Udhane, S.; Kempna, P.; Hofer, G.; Mullis, P.E.; Flück, C.E. Differential regulation of human 3β-hydroxysteroid dehydrogenase type 2 for steroid hormone biosynthesis by starvation and cyclic AMP stimulation: Studies in the human adrenal NCI-H295R cell model. PLoS ONE 2013, 8, e68691. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kim, J.; Yu, J. Androgen receptor genomic regulation. Transl. Androl. Urol. 2013, 2, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.G.; Zou, M.; Yao, G.X.; Ma, W.B.; Zhu, Q.L.; Li, X.Q.; Chen, Z.J.; Sun, Y. Androgenic regulation of beta-defensins in the mouse epididymis. Reprod. Biol. Endocrinol. 2014, 12, 76. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Berthon, A.; Settas, N.; Delaney, A.; Giannakou, A.; Demidowich, A.; Faucz, F.R.; Seminara, S.B.; Chen, M.E.; Stratakis, C.A. Kisspeptin deficiency leads to abnormal adrenal glands and excess steroid hormone secretion. Hum. Mol. Genet. 2020, 29, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Rotinen, M.; Celay, J.; Alonso, M.M.; Arrazola, A.; Encio, I.; Villar, J. Estradiol induces type 8 17beta-hydroxysteroid dehydrogenase expression: Crosstalk between estrogen receptor alpha and C/EBPbeta. J. Endocrinol. 2009, 200, 85–92. [Google Scholar] [CrossRef]
- Fomitcheva, J.; Baker, M.E.; Anderson, E.; Lee, G.Y.; Aziz, N. Characterization of Ke 6, a new 17beta-hydroxysteroid dehydrogenase, and its expression in gonadal tissues. J. Biol. Chem. 1998, 273, 22664–22671. [Google Scholar] [CrossRef] [PubMed]
- Bellofiore, N.; McKenna, J.; Ellery, S.; Temple-Smith, P. The Spiny Mouse-A Menstruating Rodent to Build a Bridge From Bench to Bedside. Front. Reprod. Health 2021, 3, 784578. [Google Scholar] [CrossRef] [PubMed]
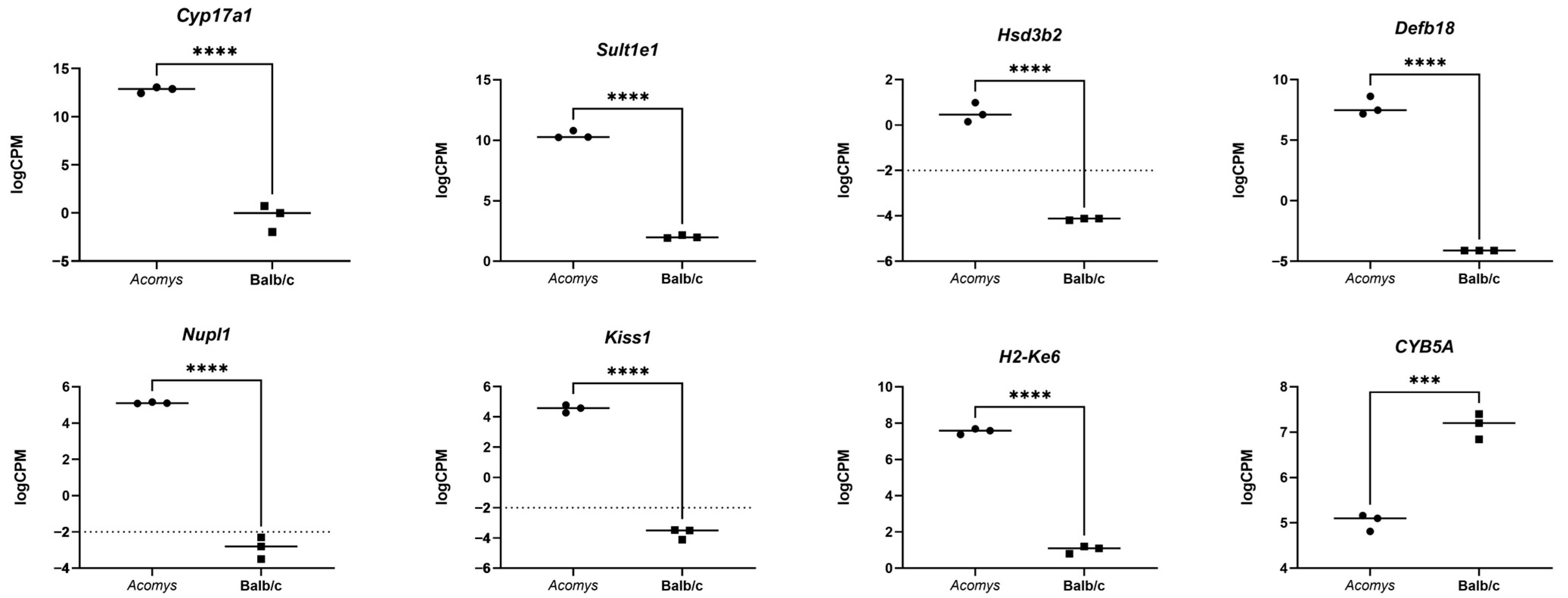
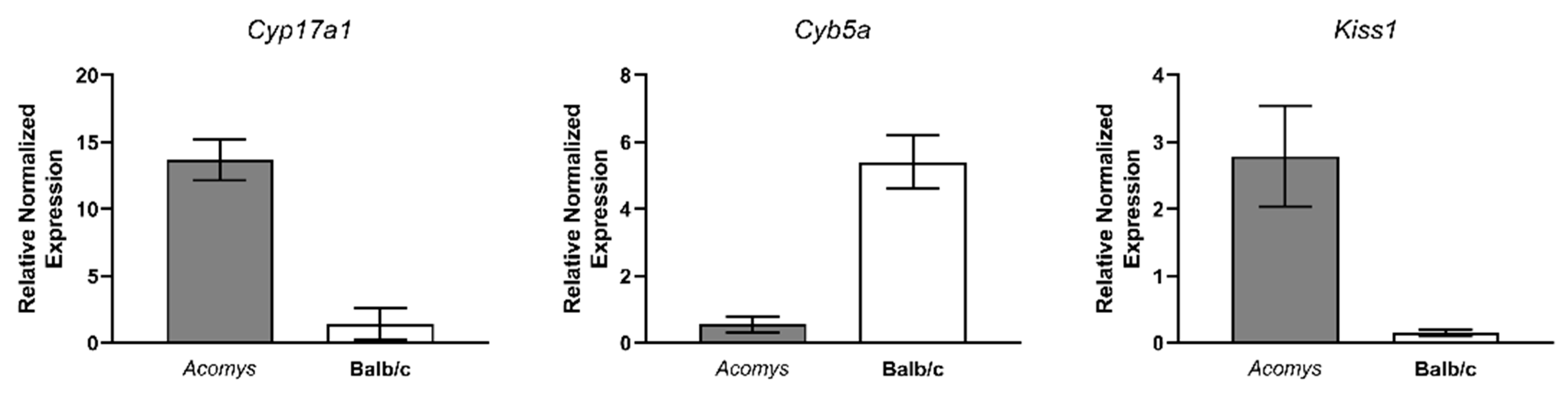
| Gene | Balb/c | Acomys cahirinus | DE |
|---|---|---|---|
| Cyp17a1 | Not expressed | Active | - |
| Sult1e1 | Not expressed | Active | - |
| Hsd3b2 | Not expressed | Active | - |
| Defb18 | Not expressed | Active | - |
| Nupl1 | Active | Active | n/s (p-value > 0,08) |
| Kiss1 | Not expressed | Active | - |
| H2-Ke6 | Active | Active | Acomys > Balb/c 1.8 times; p = 2.6 × 10−10 |
| Cyb5a | Active | Active | Acomys < Balb/c 2.7 times; \ p = 7.7 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilyalova, A.; Bilyalov, A.; Kozlova, O.; Filatov, N.; Filimoshina, D.; Gazizova, G.; Deviatiiarov, R.; Titova, A.; Bydanov, A.; Mukhamedshina, Y.; et al. Morphological and Transcriptomic Analyses of the Adrenal Gland in Acomys cahirinus: A Novel Model for Murine Adrenal Physiology. Cells 2025, 14, 1431. https://doi.org/10.3390/cells14181431
Bilyalova A, Bilyalov A, Kozlova O, Filatov N, Filimoshina D, Gazizova G, Deviatiiarov R, Titova A, Bydanov A, Mukhamedshina Y, et al. Morphological and Transcriptomic Analyses of the Adrenal Gland in Acomys cahirinus: A Novel Model for Murine Adrenal Physiology. Cells. 2025; 14(18):1431. https://doi.org/10.3390/cells14181431
Chicago/Turabian StyleBilyalova, Alina, Airat Bilyalov, Olga Kozlova, Nikita Filatov, Daria Filimoshina, Guzel Gazizova, Ruslan Deviatiiarov, Angelina Titova, Andrey Bydanov, Yana Mukhamedshina, and et al. 2025. "Morphological and Transcriptomic Analyses of the Adrenal Gland in Acomys cahirinus: A Novel Model for Murine Adrenal Physiology" Cells 14, no. 18: 1431. https://doi.org/10.3390/cells14181431
APA StyleBilyalova, A., Bilyalov, A., Kozlova, O., Filatov, N., Filimoshina, D., Gazizova, G., Deviatiiarov, R., Titova, A., Bydanov, A., Mukhamedshina, Y., Shagimardanova, E., Kiyasov, A., Tychinin, D., Woroncow, M., & Gusev, O. (2025). Morphological and Transcriptomic Analyses of the Adrenal Gland in Acomys cahirinus: A Novel Model for Murine Adrenal Physiology. Cells, 14(18), 1431. https://doi.org/10.3390/cells14181431