Mechanotransduction-Mediated Expansion of Rabbit Vocal Fold Epithelial Cells via ROCK Inhibition and Stromal Cell-Derived Paracrine Signals
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation and VF Tissue Harvesting
2.2. Histological Staining of Vocal Fold Tissue
2.3. Media Composition for Culturing VF Epithelial Cells
2.4. Mitomycin C (MMC) Treatment to STO Cells
2.5. Preparation of MMC Treated STO-Conditioned Media
2.6. VF Cell Isolation and Culture
2.7. Differential Adhesion-Based Removal of Fibroblasts from the Co-Culture
2.8. Magnetic-Activated Cell Sorting of VF-Derived Cells
2.9. Calculation of Population Doubling (PD) and PD Time
2.10. Immunofluorescence Staining
2.11. Cell Proliferation Assay
2.12. EdU Assay
2.13. Cell Cycle and Flow Cytometry Analyses
2.14. Quantitative Real-Time PCR (qPCR)
2.15. Preparation of Polyacrylamide (PAA) Gel
2.16. IF Staining Images Analyses
- Set 1: anti-YAP (clone 63.7, Cat. # sc-101199, Santa Cruz Biotechnology, Dallas, TX, USA) and anti-FAK (phospho Y397, clone EP2160Y, Cat. # ab81298, Abcam, Cambridge, UK)
- Set 2: Paxillin monoclonal antibody (clone 5H11, Cat. #MA5-13356, Thermo Fisher Scientific, dilution 1:200) and anti-p63 (clone EPR5701, Cat. # ab124762, Abcam, dilution 1:200)
- Set 3: anti-BCL-2 (clone 124, Cat. # sc-7382, Santa Cruz Biotechnology, Dallas, TX, USA) and anti-cytokeratin 14 (clone SP53, Cat. # ab119695, Abcam, dilution 1:200)
2.17. Cellular Forces Analysis
2.18. Statistical Analysis
3. Results
3.1. Rabbit VF Epithelial Cell Isolation, Purification, Characterization, and Maintenance
3.2. Role of ROCKi, EGF, and STO-CM in Driving Proliferation of rbVFEs
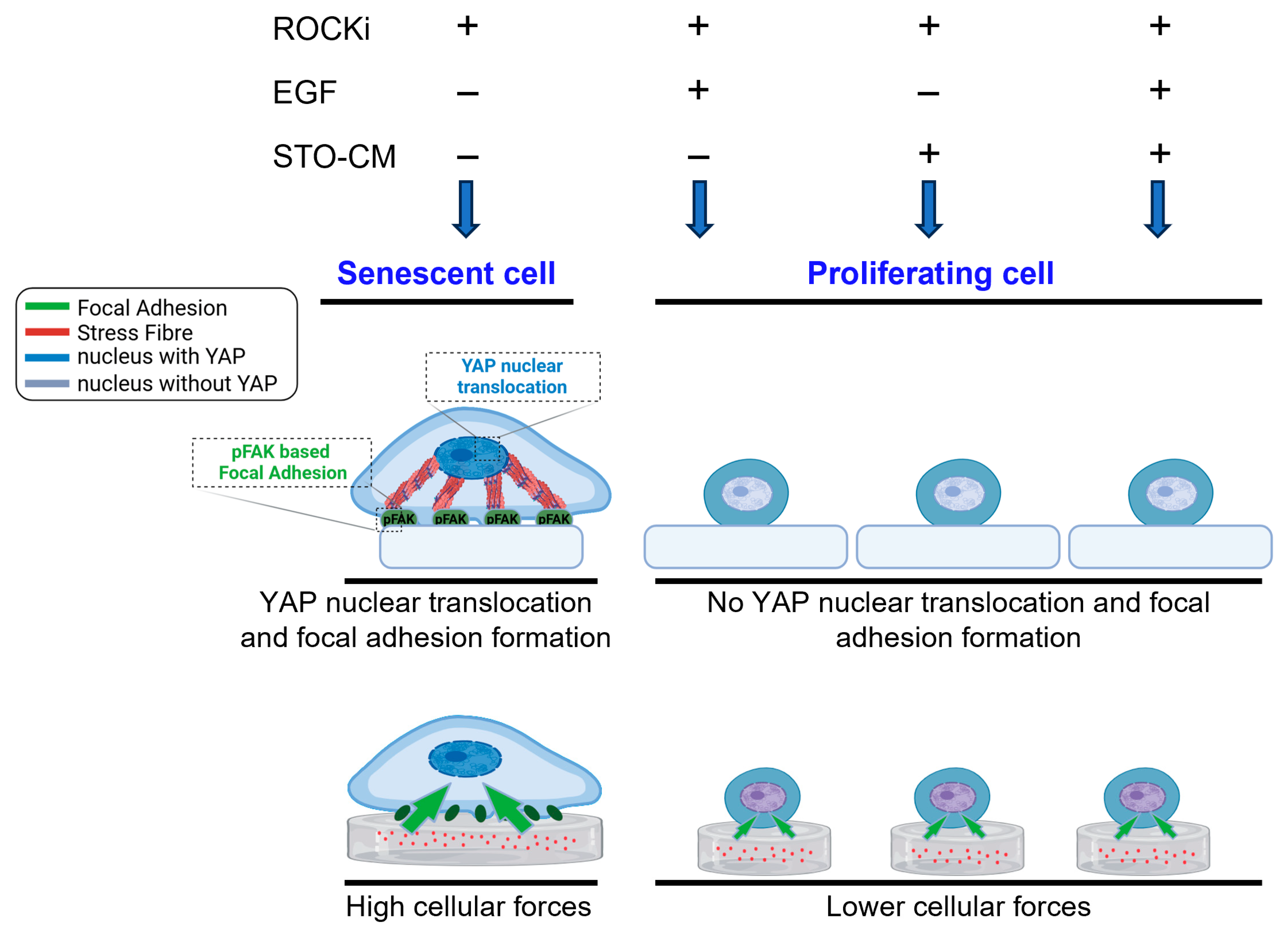
3.3. Role of ROCKi, EGF, and STO-CM in Modulating YAP Activation and Adhesion Dynamics and Cellular Senescence
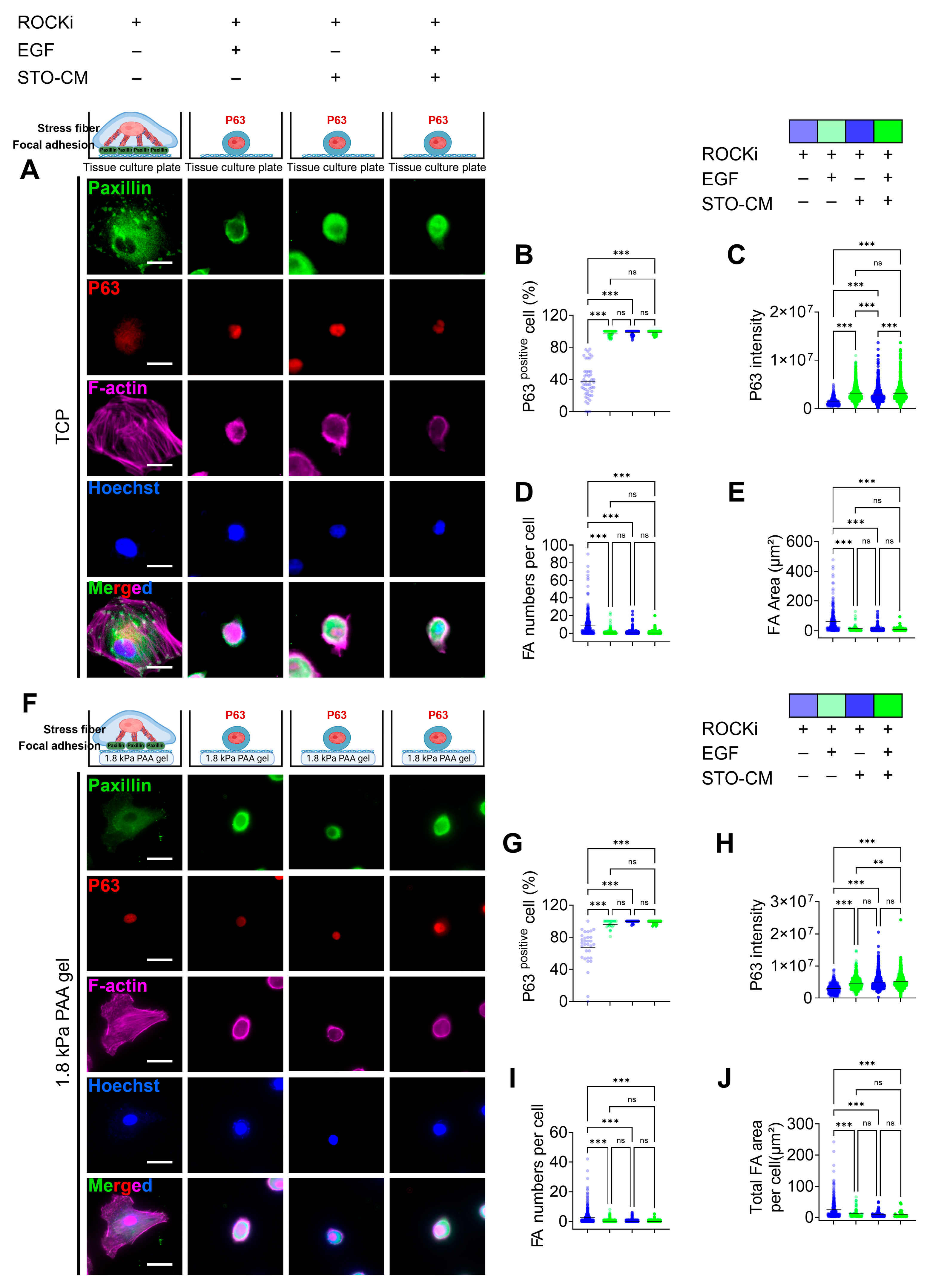
3.4. Correlation Between Paxillin-Based Focal Adhesion Dynamics and Self-Renewal Marker (p63) Expression
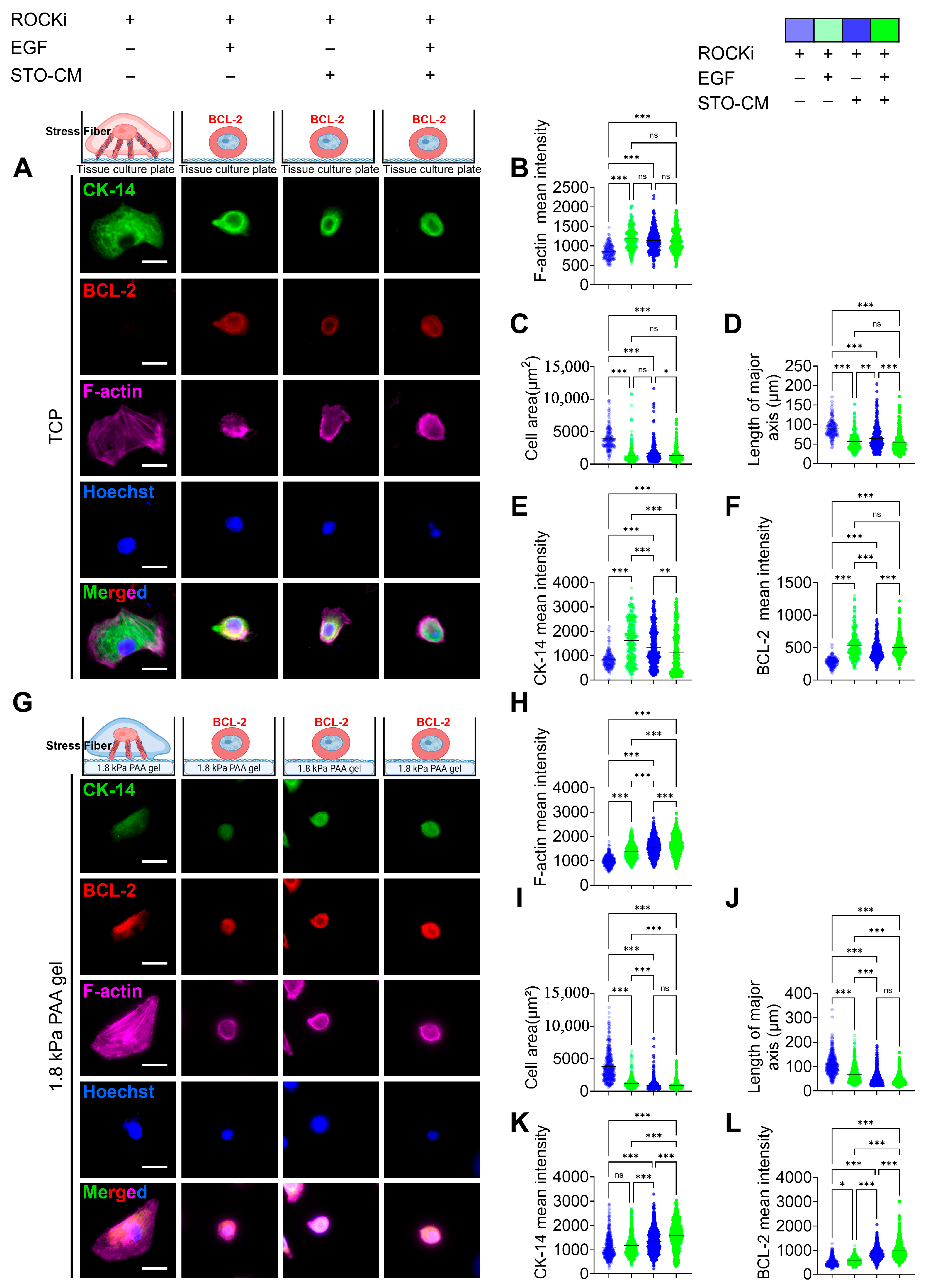
3.5. Effect of ROCKi, EGF, and STO-CM in Cytoskeletal Protein Remodeling and Apoptosis Marker Expression
3.6. Cellular Traction Forces and Intracellular Tension During Senescence or Self-Renewal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VF | Vocal fold |
| VFEs | VF-derived epithelial cells |
| rb | Rabbit |
| ROCKi | Rho kinase inhibitor |
| EGF | Epidermal growth factor |
| STO | SIM mouse embryo-derived thioguanine and ouabain-resistant (STO) fibroblasts |
| CM | Conditioned media |
| YAP | Yes-associated protein |
| FA | Focal adhesion |
| FAK | Focal adhesion kinase |
| Krt | Keratin |
| iPSCs | Induced pluripotent stem cells |
| ECM | Extracellular matrix |
| PD | Population doubling |
| PAA | Polyacrylamide |
| TFM | Traction force microscopy |
| IFM | Intracellular force microscopy |
| TGF-β | Transforming growth factor β |
| bFGF | Basic fibroblast growth factor |
| IGF-1 | Insulin-like growth factor 1 |
| HGF | Hepatocyte growth factor |
| VEGF | Vascular endothelial growth factor |
| IGFBP-6 | Insulin-like growth factor binding protein |
| CSF-1 | Macrophage colony-stimulating factor |
| PEDF | Pigment epithelium-derived factor |
| SCF | Stem cell factor |
| IFN-γ | Interferon gamma |
| NMII | Non-muscle myosin II |
| ROCK | Rho kinase |
References
- Lungova, V.; Chen, X.; Wang, Z.; Kendziorski, C.; Thibeault, S.L. Human induced pluripotent stem cell-derived vocal fold mucosa mimics development and responses to smoke exposure. Nat. Commun. 2019, 10, 4161. [Google Scholar] [CrossRef]
- Erickson-DiRenzo, E.; Leydon, C.; Thibeault, S.L. Methodology for the establishment of primary porcine vocal fold epithelial cell cultures. Laryngoscope 2019, 129, E355–E364. [Google Scholar] [CrossRef]
- Ravikrishnan, A.; Fowler, E.W.; Stuffer, A.J.; Jia, X. Hydrogel-supported, engineered model of vocal fold epithelium. ACS Biomater. Sci. Eng. 2021, 7, 4305–4317. [Google Scholar] [CrossRef]
- Mizuta, M.; Kurita, T.; Kimball, E.E.; Rousseau, B. Structurally and functionally characterized in vitro model of rabbit vocal fold epithelium. Tissue Cell 2017, 49, 427–434. [Google Scholar] [CrossRef]
- Ling, C.; Li, Q.; Brown, M.E.; Kishimoto, Y.; Toya, Y.; Devine, E.E.; Choi, K.-O.; Nishimoto, K.; Norman, I.G.; Tsegyal, T. Bioengineered vocal fold mucosa for voice restoration. Sci. Transl. Med. 2015, 7, 314ra187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lungova, V.; Zhang, H.; Mohanty, C.; Kendziorski, C.; Thibeault, S.L. Novel immortalized human vocal fold epithelial cell line: In vitro tool for mucosal biology. FASEB J. 2021, 35, e21243. [Google Scholar] [CrossRef]
- Rousseau, B.; Kojima, T.; Novaleski, C.K.; Kimball, E.E.; Valenzuela, C.V.; Mizuta, M.; Daniero, J.J.; Garrett, C.G.; Sivasankar, M.P. Recovery of vocal fold epithelium after acute phonotrauma. Cells Tissues Organs 2017, 204, 93–104. [Google Scholar] [CrossRef]
- Gaston, J.; Quinchia Rios, B.; Bartlett, R.; Berchtold, C.; Thibeault, S.L. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS ONE 2012, 7, e30965. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Thibeault, S.L. Biophysical aspects of mechanotransduction in cells and their physiological/biological implications in vocal fold vibration: A narrative review. Front. Cell Dev. Biol. 2025, 13, 1501341. [Google Scholar] [CrossRef] [PubMed]
- Van Aelst, L.; D’Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 1997, 11, 2295–2322. [Google Scholar] [CrossRef] [PubMed]
- Dakic, A.; DiVito, K.; Fang, S.; Suprynowicz, F.; Gaur, A.; Li, X.; Palechor-Ceron, N.; Simic, V.; Choudhury, S.; Yu, S.; et al. ROCK inhibitor reduces Myc-induced apoptosis and mediates immortalization of human keratinocytes. Oncotarget 2016, 7, 66740–66753. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Q.; Xu, F.; Xue, Y.; Zhen, Z.; Deng, Y.; Liu, M.; Chen, J.; Liu, S.; Qiu, M.; et al. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol. Cancer Res. 2009, 7, 570–580. [Google Scholar] [CrossRef]
- Reynolds, S.D.; Rios, C.; Wesolowska-Andersen, A.; Zhuang, Y.; Pinter, M.; Happoldt, C.; Hill, C.L.; Lallier, S.W.; Cosgrove, G.P.; Solomon, G.M.; et al. Airway progenitor clone formation is enhanced by Y-27632–dependent changes in the transcriptome. Am. J. Respir. Cell Mol. Biol. 2016, 55, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Kümper, S.; Mardakheh, F.K.; McCarthy, A.; Yeo, M.; Stamp, G.W.; Paul, A.; Worboys, J.; Sadok, A.; Jørgensen, C.; Guichard, S.; et al. Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. eLife 2016, 5, e12203. [Google Scholar] [CrossRef] [PubMed]
- Moujaber, O.; Fishbein, F.; Omran, N.; Liang, Y.; Colmegna, I.; Presley, J.F.; Stochaj, U. Cellular senescence is associated with reorganization of the microtubule cytoskeleton. Cell. Mol. Life Sci. 2019, 76, 1169–1183. [Google Scholar] [CrossRef]
- Pospelova, T.V.; Chitikova, Z.V.; Pospelov, V.A. An integrated approach for monitoring cell senescence. In Cell Senescence: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; pp. 383–408. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive cell growth causes cytoplasm dilution and contributes to senescence. Cell 2019, 176, 1083–1097.e18. [Google Scholar] [CrossRef]
- Cheung, T.M.; Yan, J.B.; Fu, J.J.; Huang, J.; Yuan, F.; Truskey, G.A. Endothelial cell senescence increases traction forces due to age-associated changes in the glycocalyx and SIRT1. Cell. Mol. Bioeng. 2015, 8, 63–75. [Google Scholar] [CrossRef]
- Grandy, C.; Port, F.; Radzinski, M.; Singh, K.; Erz, D.; Pfeil, J.; Reichmann, D.; Gottschalk, K.-E. Remodeling of the focal adhesion complex by hydrogen-peroxide-induced senescence. Sci. Rep. 2023, 13, 9735. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Liu, X.; Krawczyk, E.; Suprynowicz, F.A.; Palechor-Ceron, N.; Yuan, H.; Dakic, A.; Simic, V.; Zheng, Y.-L.; Sripadhan, P.; Chen, C.; et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 2017, 12, 439–451. [Google Scholar] [CrossRef]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.A.; Nealon, C.; Dakic, A.; Simic, V.; et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012, 180, 599–607. [Google Scholar] [CrossRef]
- Li, P.; Wang, S.; Zhan, L.; He, X.; Chi, G.; Lv, S.; Xu, Z.; Xia, Y.; Teng, S.; Li, L.; et al. Efficient feeder cells preparation system for large-scale preparation and application of induced pluripotent stem cells. Sci. Rep. 2017, 7, 12266. [Google Scholar] [CrossRef]
- Talbot, N.C.; Sparks, W.O.; Powell, A.M.; Kahl, S.; Caperna, T.J. Quantitative and semiquantitative immunoassay of growth factors and cytokines in the conditioned medium of STO and CF-1 mouse feeder cells. Vitr. Cell. Dev. Biol. Anim. 2012, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Scadden, D.T.; Gilliland, D.G. The leukemic stem cell niche: Current concepts and therapeutic opportunities. Blood J. Am. Soc. Hematol. 2009, 114, 1150–1157. [Google Scholar] [CrossRef]
- Murakami, T.; Saitoh, I.; Inada, E.; Kurosawa, M.; Iwase, Y.; Noguchi, H.; Terao, Y.; Yamasaki, Y.; Hayasaki, H.; Sato, M. STO feeder cells are useful for propagation of primarily cultured human deciduous dental pulp cells by eliminating contaminating bacteria and promoting cellular outgrowth. Cell Med. 2013, 6, 75–81. [Google Scholar] [CrossRef]
- Aframian, D.; David, R.; Ben-Bassat, H.; Shai, E.; Deutsch, D.; Baum, B.; Palmon, A. Characterization of murine autologous salivary gland graft cells: A model for use with an artificial salivary gland. Tissue Eng. 2004, 10, 914–920. [Google Scholar] [CrossRef]
- Rheinwatd, J.G.; Green, H. Seria cultivation of strains of human epidemal keratinocytes: The formation keratinizin colonies from single cell is. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Ai, G.; Shao, X.; Meng, M.; Song, L.; Qiu, J.; Wu, Y.; Zhou, J.; Cheng, J.; Tong, X. Epidermal growth factor promotes proliferation and maintains multipotency of continuous cultured adipose stem cells via activating STAT signal pathway in vitro. Medicine 2017, 96, e7607. [Google Scholar] [CrossRef] [PubMed]
- Tropepe, V.; Sibilia, M.; Ciruna, B.G.; Rossant, J.; Wagner, E.F.; van der Kooy, D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev. Biol. 1999, 208, 166–188. [Google Scholar] [CrossRef] [PubMed]
- Leydon, C.; Selekman, J.A.; Palecek, S.; Thibeault, S.L. Human embryonic stem cell-derived epithelial cells in a novel in vitro model of vocal mucosa. Tissue Eng. Part A 2013, 19, 2233–2241. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Li, R.; Park, C.Y.; Fredberg, J.J.; Janmey, P.A. A novel method to make viscoelastic polyacrylamide gels for cell culture and traction force microscopy. APL Bioeng. 2020, 4, 036104. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Karadaghy, A.; Zustiak, S. Simple polyacrylamide-based multiwell stiffness assay for the study of stiffness-dependent cell responses. J. Vis. Exp. 2015, 97, 52643. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Naleway, S.E.; Maker, Y.N.; Kang, K.Y.; Lee, J.; Ha, J.; Hur, S.S.; Chien, S.; McKittrick, J. 3D printed templating of extrinsic freeze-casting for macro–microporous biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 2122–2133. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.S.; Jeong, J.H.; Ban, M.J.; Park, J.H.; Yoon, J.K.; Hwang, Y. Traction force microscopy for understanding cellular mechanotransduction. BMB Rep. 2020, 53, 74. [Google Scholar] [CrossRef]
- Hur, S.S.; Zhao, Y.; Li, Y.-S.; Botvinick, E.; Chien, S. Live cells exert 3-dimensional traction forces on their substrata. Cell. Mol. Bioeng. 2009, 2, 425–436. [Google Scholar] [CrossRef]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 3594. [Google Scholar] [CrossRef]
- Kasai, Y.; Morino, T.; Mori, E.; Yamamoto, K.; Kojima, H. ROCK inhibitor combined with Ca2+ controls the myosin II activation and optimizes human nasal epithelial cell sheets. Sci. Rep. 2020, 10, 16853. [Google Scholar] [CrossRef] [PubMed]
- Dollner, R.; Granzow, C.; Helmke, B.M.; Ruess, A.; Schad, A.; Dietz, A. The impact of stromal cell contamination on chemosensitivity testing of head and neck carcinoma. Anticancer Res. 2004, 24, 325–332. [Google Scholar]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef]
- Nelson, W.G.; Sun, T.-T. The 50-and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: Cell culture studies. J. Cell Biol. 1983, 97, 244–251. [Google Scholar] [CrossRef]
- Gao, L.; Nath, S.C.; Jiao, X.; Zhou, R.; Nishikawa, S.; Krawetz, R.; Li, X.; Rancourt, D.E. Post-passage rock inhibition induces cytoskeletal aberrations and apoptosis in human embryonic stem cells. Stem Cell Res. 2019, 41, 101641. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Tissue culture as a hostile environment: Identifying conditions for breast cancer progression studies. Cancer Cell 2007, 12, 100–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krawczyk, E.; Hong, S.-H.; Galli, S.; Trinh, E.; Wietlisbach, L.; Misiukiewicz, S.F.; Tilan, J.U.; Chen, Y.-S.; Schlegel, R.; Kitlinska, J. Murine neuroblastoma cell lines developed by conditional reprogramming preserve heterogeneous phenotypes observed in vivo. Lab. Investig. 2020, 100, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Blahnova, V.H.; Dankova, J.; Rampichova, M.; Filova, E. Combinations of growth factors for human mesenchymal stem cell proliferation and osteogenic differentiation. Bone Jt. Res. 2020, 9, 412–420. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, J.; Wang, Z.-Y. A switch role of Src in the biphasic EGF signaling of ER-negative breast cancer cells. PLoS ONE 2012, 7, e41613. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.-L. The Hippo–YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef]
- Mo, J.-S.; Yu, F.-X.; Gong, R.; Brown, J.H.; Guan, K.-L. Regulation of the Hippo–YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012, 26, 2138–2143. [Google Scholar] [CrossRef]
- Gregorieff, A.; Liu, Y.; Inanlou, M.R.; Khomchuk, Y.; Wrana, J.L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 2015, 526, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Dumbauld, D.W.; Lee, T.T.; Singh, A.; Scrimgeour, J.; Gersbach, C.A.; Zamir, E.A.; Fu, J.; Chen, C.S.; Curtis, J.E.; Craig, S.W.; et al. How vinculin regulates force transmission. Proc. Natl. Acad. Sci. USA 2013, 110, 9788–9793. [Google Scholar] [CrossRef]
- Cho, K.A.; Ryu, S.J.; Oh, Y.S.; Park, J.H.; Lee, J.W.; Kim, H.-P.; Kim, K.T.; Jang, I.S.; Park, S.C. Morphological adjustment of senescent cells by modulating caveolin-1 status. J. Biol. Chem. 2004, 279, 42270–42278. [Google Scholar] [CrossRef] [PubMed]
- Chantachotikul, P.; Liu, S.; Furukawa, K.; Deguchi, S. AP2A1 is upregulated upon replicative senescence of human fibroblasts to strengthen focal adhesions via integrin β1 translocation along stress fibers. bioRxiv 2023, preprint. [Google Scholar] [CrossRef]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef]
- Kassianidou, E.; Kumar, S. A biomechanical perspective on stress fiber structure and function. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.M.; Tu, V.C.; Catania, J.; Burton, M.; Toussaint, O.; Dilley, T. Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide. J. Cell Sci. 2000, 113, 4087–4097. [Google Scholar] [CrossRef]
- Georges, P.C.; Janmey, P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005, 98, 1547–1553. [Google Scholar] [CrossRef]
- Pelham, R.J., Jr.; Wang, Y.-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 1997, 94, 13661–13665. [Google Scholar] [CrossRef]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate compliance versus ligand density in cell on gel responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Chowdhury, F.; Li, Y.; Poh, Y.-C.; Yokohama-Tamaki, T.; Wang, N.; Tanaka, T.S. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PLoS ONE 2010, 5, e15655. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Shoham, N.; Mor-Yossef Moldovan, L.; Benayahu, D.; Gefen, A. Multiscale modeling of tissue-engineered fat: Is there a deformation-driven positive feedback loop in adipogenesis? Tissue Eng. Part A 2015, 21, 1354–1363. [Google Scholar] [CrossRef]
- Chowdhury, F.; Na, S.; Li, D.; Poh, Y.-C.; Tanaka, T.S.; Wang, F.; Wang, N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010, 9, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Downing, T.L.; Soto, J.; Morez, C.; Houssin, T.; Fritz, A.; Yuan, F.; Chu, J.; Patel, S.; Schaffer, D.V.; Li, S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013, 12, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, J.H.; Ahn, K.S.; Shim, H.W.; Yoon, J.Y.; Hyun, J.; Lee, J.H.; Jang, S.; Yoo, K.H.; Jang, Y.K. Cyclic Stretch Promotes Cellular Reprogramming Process through Cytoskeletal-Nuclear Mechano-Coupling and Epigenetic Modification. Adv. Sci. 2023, 10, 2303395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, H.; Li, H.; Wu, Y. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J. Cell. Mol. Med. 2017, 21, 1073–1084. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK–ERK linkage. Oncogene 2009, 28, 4326–4343. [Google Scholar] [CrossRef]
- Li, X.; McLain, C.; Samuel, M.S.; Olson, M.F.; Radice, G.L. Actomyosin-mediated cellular tension promotes Yap nuclear translocation and myocardial proliferation through α5 integrin signaling. Development 2023, 150, dev201013. [Google Scholar] [CrossRef]
- Katsuda, T.; Kawamata, M.; Hagiwara, K.; Takahashi, R.-U.; Yamamoto, Y.; Camargo, F.D.; Ochiya, T. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell 2017, 20, 41–55. [Google Scholar] [CrossRef]
- Shutova, M.; Yang, C.; Vasiliev, J.M.; Svitkina, T. Functions of nonmuscle myosin II in assembly of the cellular contractile system. PLoS ONE 2012, 7, e40814. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M.; Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996, 133, 1403–1415. [Google Scholar] [CrossRef]
- Kuo, J.-C.; Han, X.; Hsiao, C.-T.; Yates III, J.R.; Waterman, C.M. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 2011, 13, 383–393. [Google Scholar] [CrossRef]
- Schiller, H.B.; Friedel, C.C.; Boulegue, C.; Fässler, R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011, 12, 259–266. [Google Scholar] [CrossRef]
- Watanabe, T.; Hosoya, H.; Yonemura, S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol. Biol. Cell 2007, 18, 605–616. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Zareno, J.; Whitmore, L.; Choi, C.K.; Horwitz, A.F. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007, 176, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Biais, N.; Giannone, G.; Tanase, M.; Jiang, G.; Hofman, J.M.; Wiggins, C.H.; Silberzan, P.; Buguin, A.; Ladoux, B. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 2006, 91, 3907–3920. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Koach, M.A.; Whitmore, L.; Lamers, M.L.; Horwitz, A.F. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 2008, 183, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Goeckeler, Z.M.; Bridgman, P.C.; Wysolmerski, R.B. Nonmuscle myosin II is responsible for maintaining endothelial cell basal tone and stress fiber integrity. Am. J. Physiol. Cell Physiol. 2008, 295, C994–C1006. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, H.; Henis, Y.I.; Geiger, B.; Bershadsky, A.D. The heel and toe of the cell’s foot: A multifaceted approach for understanding the structure and dynamics of focal adhesions. Cell Motil. Cytoskelet. 2009, 66, 1017–1029. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, S.; Kim, J.H.; Jeong, J.Y.; Hur, S.S.; Lee, S.W.; Hwang, Y. Mechanotransduction-Mediated Expansion of Rabbit Vocal Fold Epithelial Cells via ROCK Inhibition and Stromal Cell-Derived Paracrine Signals. Cells 2025, 14, 1412. https://doi.org/10.3390/cells14181412
Thapa S, Kim JH, Jeong JY, Hur SS, Lee SW, Hwang Y. Mechanotransduction-Mediated Expansion of Rabbit Vocal Fold Epithelial Cells via ROCK Inhibition and Stromal Cell-Derived Paracrine Signals. Cells. 2025; 14(18):1412. https://doi.org/10.3390/cells14181412
Chicago/Turabian StyleThapa, Samjhana, Joo Hyun Kim, Jun Yeong Jeong, Sung Sik Hur, Seung Won Lee, and Yongsung Hwang. 2025. "Mechanotransduction-Mediated Expansion of Rabbit Vocal Fold Epithelial Cells via ROCK Inhibition and Stromal Cell-Derived Paracrine Signals" Cells 14, no. 18: 1412. https://doi.org/10.3390/cells14181412
APA StyleThapa, S., Kim, J. H., Jeong, J. Y., Hur, S. S., Lee, S. W., & Hwang, Y. (2025). Mechanotransduction-Mediated Expansion of Rabbit Vocal Fold Epithelial Cells via ROCK Inhibition and Stromal Cell-Derived Paracrine Signals. Cells, 14(18), 1412. https://doi.org/10.3390/cells14181412





