Endodontic Regeneration Therapy: Current Strategies and Tissue Engineering Solutions
Abstract
1. Introduction
2. Current Endodontic Therapy for Promoting Pulp Wound Healing
2.1. Vital Pulp Therapy
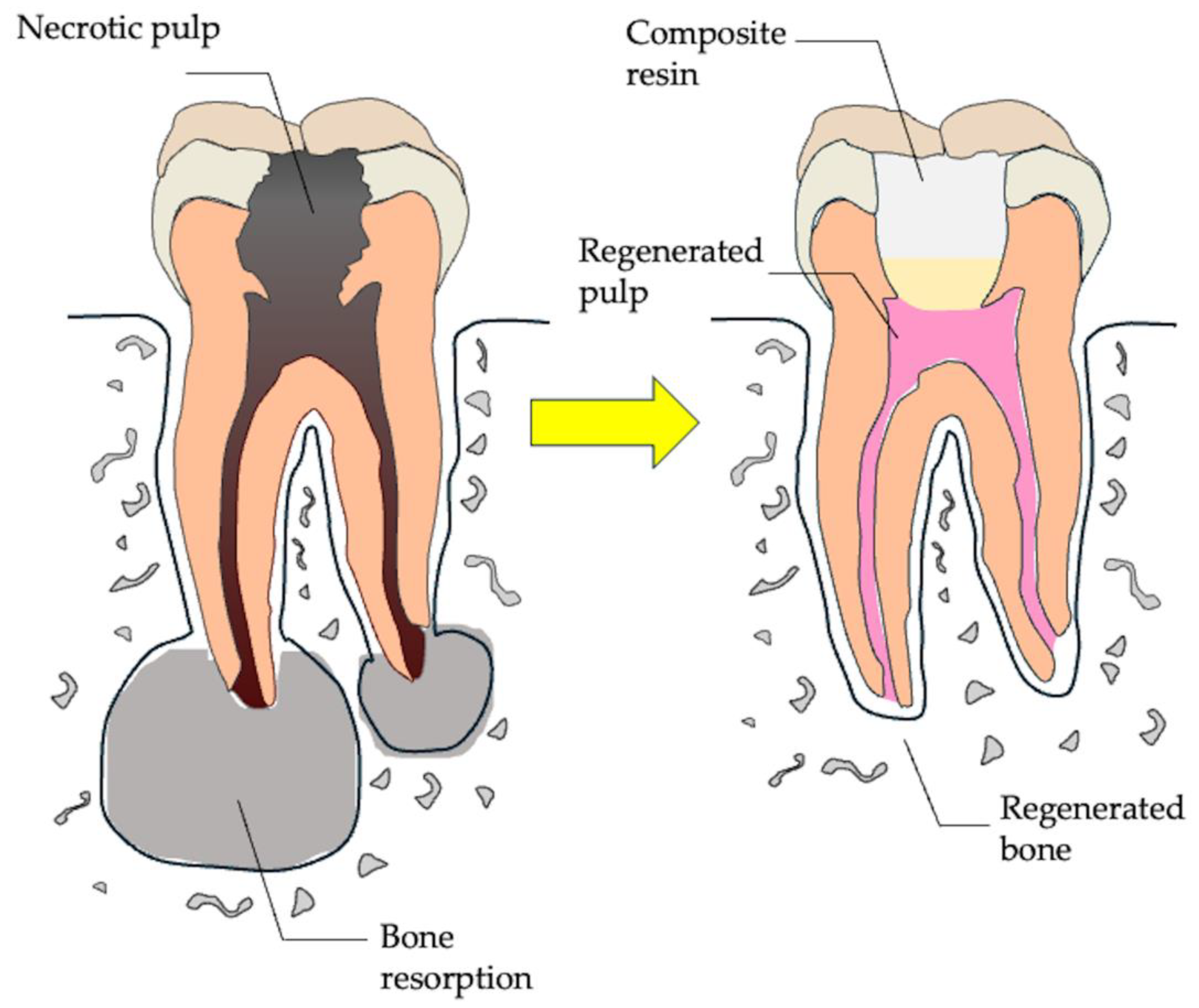
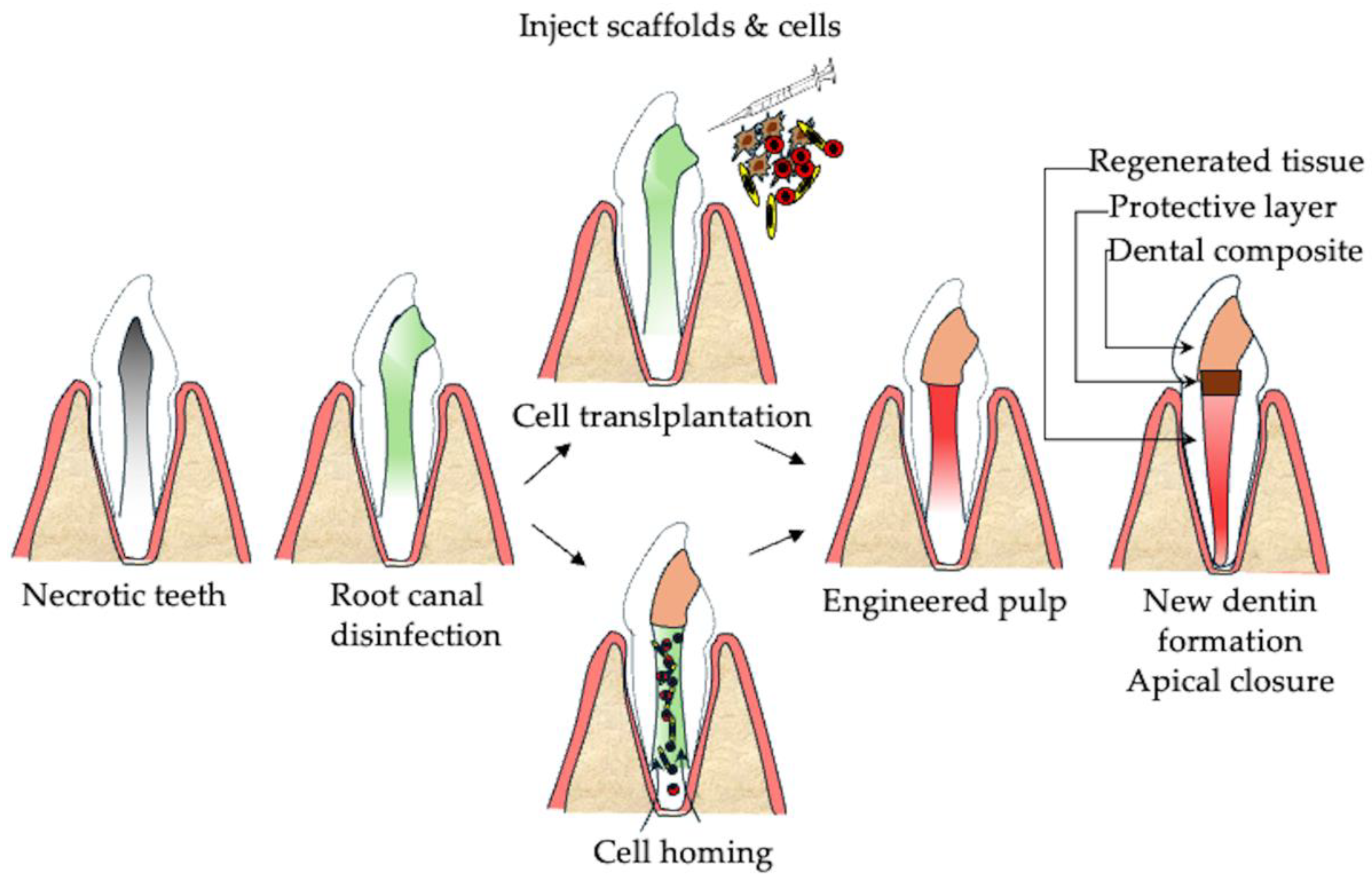
2.2. Regenerative Endodontics
3. Dental Pulp Regeneration Therapy
3.1. Tooth Development
3.2. Tissue Engineering Technology of Tooth Regeneration
3.3. Cell-Based Approaches for Pulp Regeneration
| Author | Transplant Group | Origin of the Cells | Type of Transplantation | Outcome |
|---|---|---|---|---|
| Nakashima et al., 2017 [56] | Irreversible pulpitis of mature single root canal (n = 5) | Autologous mobilized dental pulp stem cells (mobilized DPSCs) from permanent dental pulp | Mobilized DPSCs + granulocyte colony-stimulating factor (G-CSF) + atelocollagen sponge | Pulp sensibility was restored in four out of five patients via EPT. MRI signal intensity was comparable between test and control teeth, while CBCT revealed lateral dentin formation in three out of five cases. |
| Xuan et al., 2018 [54] | Traumatic immature incisor with pulp necrosis (n = 26) | Autologous stem cells from human exfoliated deciduous dental pulp (SHEDs) | SHED (1 × 108 cells) were implanted into injured teeth | Dental pulp tissue regeneration after 12 months follow-up. Increase vascular formation by laser Doppler flowmetry. Increased root length and reduced the width of the apical foramen by CBCT analysis. |
| Brizuela et al., 2020 [57] | Mature teeth with pulp necrosis or apical periodontitis (n = 18) | Allogenic human umbilical cord mesenchymal stem cells (UC-MSCs) | Encapsulated UC-MSCs + platelet-poor plasma | Test groups showed increased positive pulp responses and perfusion units via laser Doppler flowmetry at 12 months follow-up. |
| Gomez-Sosa et al., 2024 [55] | Immature teeth with pulp necrosis or apical periodontitis (n = 15) | Allogenic bone marrow mesenchymal stem cells (BM-MSCs) | BM-MSCs + autologous platelet-rich plasma (PRP) | Clinical and radiographic evaluation of the treated teeth showed periapical lesion healing, sensitivity to cold and electricity, decreased width of the apical foramen, and mineralization within the canal space. |
| Kim et al., 2025 [58] | Mature teeth with pulp necrosis or apical periodontitis (n = 6) | Autologous dental pulp stem cells from permanent dental pulp | Autologous minced pulp grafting in teeth | After 19 to 42 months, periapical lesions were resolved in all teeth, both clinically and radiographically, with one tooth regaining sensibility, while three out of six teeth showed calcification. |
4. Bone Regeneration Therapy for Apical Periodontitis
4.1. Pathology of Apical Periodontitis
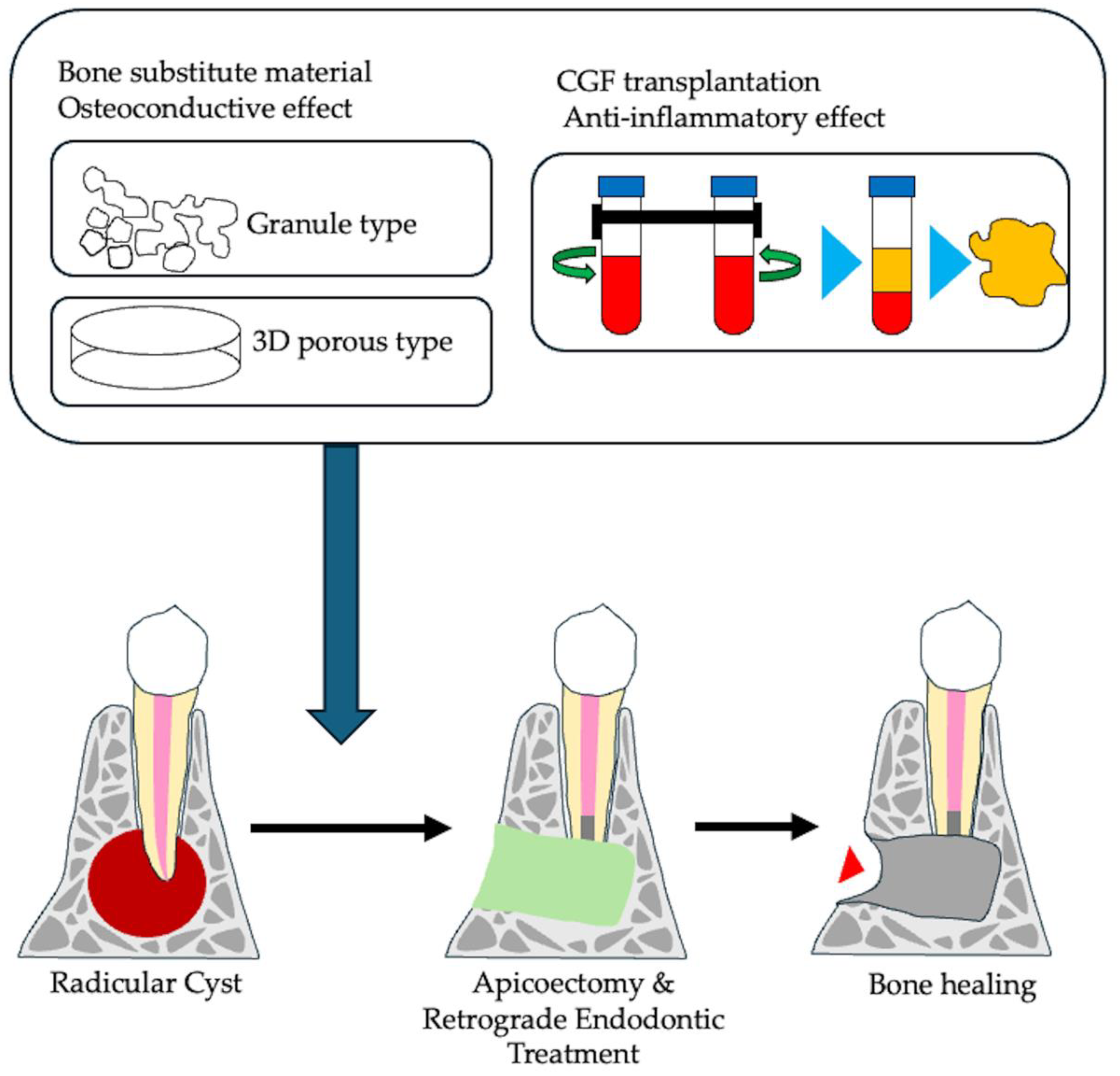
4.2. Current Approach for Promoting Bone Healing of Apical Periodontitis
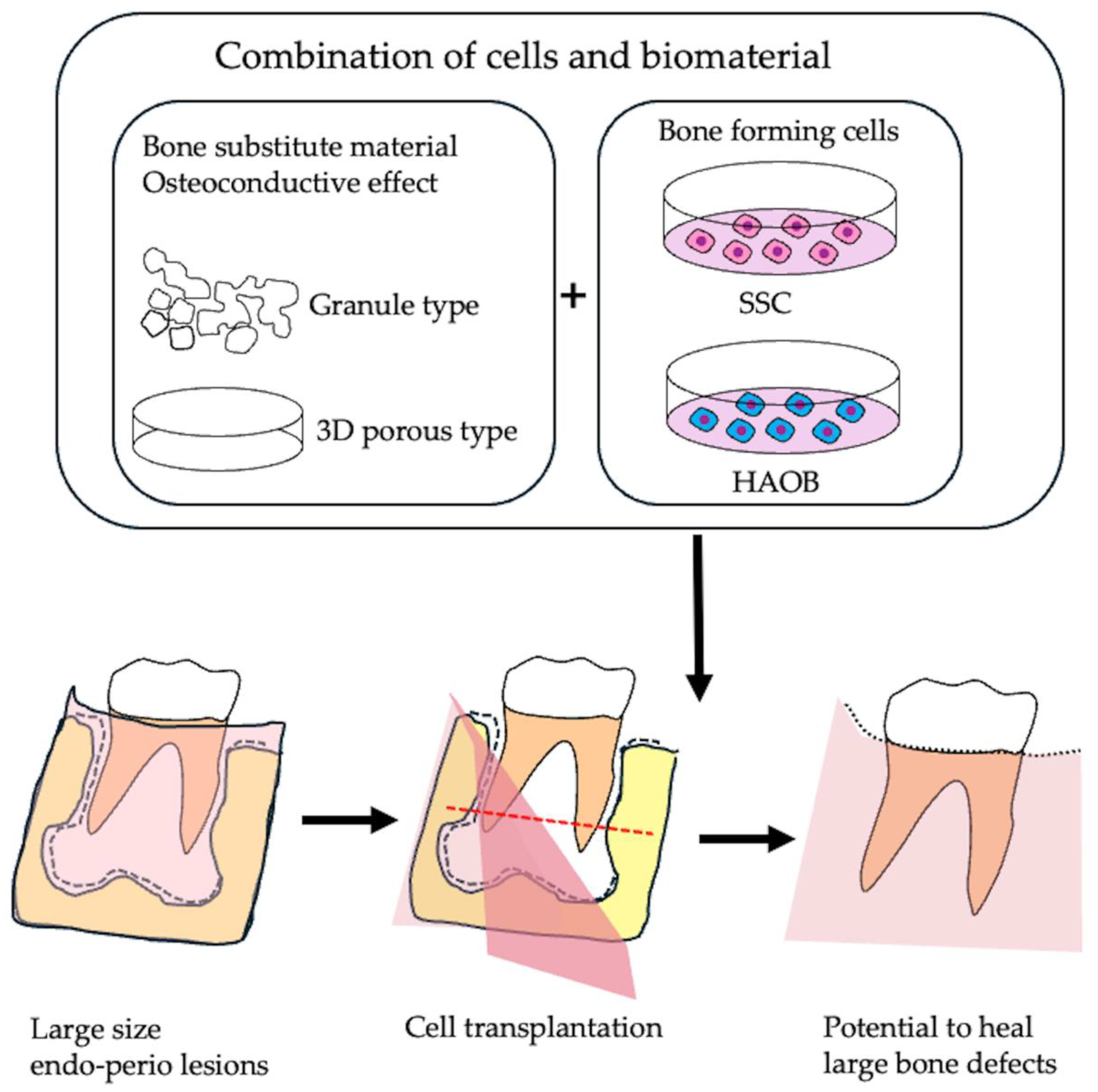
5. Bone Tissue Engineering of Apical Periodontitis
5.1. Bone Substitute Material
5.2. Application of Skeletal Stem Cells as Bone Forming Cells
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tjäderhane, L.; Carrilho, M.R.; Breschi, L.; Tay, F.R.; Pashley, D.H. Dentin basic structure and composition—An overview. Endod. Topics 2009, 20, 3–29. [Google Scholar] [CrossRef]
- Yu, C.; Abbott, P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef]
- Park, S.H.; Ye, L.; Love, R.M.; Farges, J.C.; Yumoto, H. Inflammation of the Dental Pulp. Mediat. Inflamm. 2015, 2015, 980196. [Google Scholar] [CrossRef]
- Bjørndal, L. Dentin and pulp reactions to caries and operative treatment: Biological variables affecting treatment outcome. Endo. Topics 2002, 2, 10–23. [Google Scholar] [CrossRef]
- Abbott, P.; Yu, C. A clinical classification of the status of the pulp and the root canal system. Aust. Dent. J. 2007, 52, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, M.; Tayeed, H.; Kurtzman, G.; Abbas, F.M.; Ardakani, M.T. Histopathological investigation of dental pulp reactions related to periodontitis. Eur. Endod. J. 2021, 6, 164. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, A.; Latronico, F.; Pirani, C.; Greco, M.F.; Corrente, M.; Prati, C. Symptomatic and asymptomatic apical periodontitis associated with red complex bacteria: Clinical and microbiological evaluation. Odontology 2013, 101, 84–88. [Google Scholar] [CrossRef]
- Nair, P. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef]
- Ardila, C.M.; Vivares-Builes, A.M. Clinical Efficacy of Treatment of Endodontic-Periodontal Lesions: A Systematic Scoping Review of Experimental Studies. Int. J. Environ. Res. Public Health 2022, 19, 13649. [Google Scholar] [CrossRef]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Clin. Periodontol. 2018, 45, S78–S94. [Google Scholar] [CrossRef]
- Friedman, S.; Mor, C. The success of endodontic therapy—Healing and functionality. J. Calif. Dent. Assoc. 2004, 32, 493–503. [Google Scholar] [CrossRef]
- Imura, N.; Pinheiro, E.T.; Gomes, B.P.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. The outcome of endodontic treatment: A retrospective study of 2000 cases performed by a specialist. J. Endod. 2007, 33, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Kamboozia, A.; Punnia-Moorthy, A. The fate of teeth in mandibular fracture lines: A clinical and radiographic follow-up study. Int. J. Oral Maxillofac. Surg. 1993, 22, 97–101. [Google Scholar] [CrossRef]
- Ye, L.; Cao, L.; Song, W.; Yang, C.; Tang, Q.; Yuan, Z. Interaction between apical periodontitis and systemic disease. Int. J. Mol. Med. 2023, 52, 60. [Google Scholar] [CrossRef]
- Sabeti, M.; Ghobrial, D.; Zanjir, M.; da Costa, B.R.; Young, Y.; Azarpazhooh, A. Treatment outcomes of regenerative endodontic therapy in immature permanent teeth with pulpal necrosis: A systematic review and network meta-analysis. Int. Endod. J. 2024, 57, 238–255. [Google Scholar] [CrossRef]
- Yan, H.; De Deus, G.; Kristoffersen, I.M.; Wiig, E.; Reseland, J.E.; Johnsen, G.F.; Silva, E.J.; Haugen, H.J. Regenerative endodontics by cell homing: A review of recent clinical trials. J. Endod. 2023, 49, 4–17. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional dental pulp regeneration: Basic research and clinical translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- AlJasser, R.; Bukhary, S.; AlSarhan, M.; Alotaibi, D.; AlOraini, S.; Habib, S.R. Regenerative therapy modality for treatment of true combined endodontic-periodontal lesions: A randomized controlled clinical trial. Int. J. Environ. Res. Public Health 2021, 18, 6220. [Google Scholar] [CrossRef]
- Aguilar, P.; Linsuwanont, P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: A systematic review. J. Endod. 2011, 37, 581–587. [Google Scholar] [CrossRef]
- Witherspoon, D.E. Vital pulp therapy with new materials: New directions and treatment perspectives—Permanent teeth. Pediatr. Dent. 2008, 30, 220–224. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- European Society of Endodontology (ESE); Duncan, H.; Galler, K.; Tomson, P.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar]
- Hirschberg, C.S.; Galicia, J.C.; Ruparel, N.B. AAE Position Statement on Vital Pulp Therapy. J. Endod. 2021, 47, 1340–1344. [Google Scholar]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M.; Kawana, K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2021, 25, 2101–2112. [Google Scholar] [CrossRef]
- Santos, J.M.; Pereira, J.F.; Marques, A.; Sequeira, D.B.; Friedman, S. Vital pulp therapy in permanent mature posterior teeth with symptomatic irreversible pulpitis: A systematic review of treatment outcomes. Medicina 2021, 57, 573. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Abuzaid, A.M.; Khader, Y.S. A randomized controlled clinical trial of pulpotomy versus root canal therapy in mature teeth with irreversible pulpitis: Outcome, quality of life, and patients’ satisfaction. J. Endod. 2023, 49, 624–631. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part I: Chemical, physical, and antibacterial properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef]
- Kim, S.; Malek, M.; Sigurdsson, A.; Lin, L.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388. [Google Scholar] [CrossRef]
- Iwaya, S.i.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dental Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Nada, O.A.; El Backly, R.M. Stem Cells From the Apical Papilla (SCAP) as a Tool for Endogenous Tissue Regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef]
- Chrepa, V.; Pitcher, B.; Henry, M.A.; Diogenes, A. Survival of the Apical Papilla and Its Resident Stem Cells in a Case of Advanced Pulpal Necrosis and Apical Periodontitis. J. Endod. 2017, 43, 561–567. [Google Scholar] [CrossRef]
- Lin, L.; Ricucci, D.; Huang, G.J. Regeneration of the dentine–pulp complex with revitalization/revascularization therapy: Challenges and hopes. Int. Endod. J. 2014, 47, 713–724. [Google Scholar] [CrossRef]
- Diogenes, A.; Ruparel, N.B.; Shiloah, Y.; Hargreaves, K.M. Regenerative endodontics: A way forward. J. Am. Dent. Assoc. 2016, 147, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G. Infection and pulp regeneration. Dent. J. 2016, 4, 4. [Google Scholar] [CrossRef]
- Fouad, A. The microbial challenge to pulp regeneration. Adv. Dent. Res. 2011, 23, 285–289. [Google Scholar] [CrossRef]
- American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure; American Association of Endodontists: Chicago, IL, USA, 2016. [Google Scholar]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Abo-Heikal, M.M.; El-Shafei, J.M.; Shouman, S.A.; Roshdy, N.N. Evaluation of the efficacy of injectable platelet-rich fibrin versus platelet-rich plasma in the regeneration of traumatized necrotic immature maxillary anterior teeth: A randomized clinical trial. Dent. Traumatol. 2024, 40, 61–75. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, H.; Peng, C. Continued root development of immature permanent teeth after regenerative endodontics with or without a collagen membrane: A randomized, controlled clinical trial. Int. J. Paediatr. Dent. 2022, 32, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sosa, J.F.; Diaz-Solano, D.; Wittig, O.; Cardier, J.E. Dental pulp regeneration induced by allogenic mesenchymal stromal cell transplantation in a mature tooth: A case report. J. Endod. 2022, 48, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parada, C.; Chai, Y. Cellular and molecular mechanisms of tooth root development. Development 2017, 144, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Kumar, G. Epithelial-mesenchymal interactions in odontogenesis: Part-2. J. Oral Maxillofac. Pathol. 2005, 9, 55–58. [Google Scholar]
- Thesleff, I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 2003, 116, 1647–1648. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Jones, T.J. The epithelial-mesenchymal interactions: Insights into physiological and pathological aspects of oral tissues. Oncol. Rev. 2014, 8, 239. [Google Scholar]
- Oshima, M.; Mizuno, M.; Imamura, A.; Ogawa, M.; Yasukawa, M.; Yamazaki, H.; Morita, R.; Ikeda, E.; Nakao, K.; Takano-Yamamoto, T. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS ONE 2011, 6, e21531. [Google Scholar] [CrossRef]
- Shi, X.; Hu, X.; Jiang, N.; Mao, J. Regenerative endodontic therapy: From laboratory bench to clinical practice. J. Adv. Res. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Acevedo-Jake, A.M.; Griffith, A.; Kadincesme, N.; Dabek, K.; Hindi, D.; Kim, K.K.; Kobayashi, Y.; Shimizu, E.; Kumar, V. Cells and material-based strategies for regenerative endodontics. Bioact. Mater. 2022, 14, 234–249. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sosa, J.F.; Cardier, J.E.; Wittig, O.; Diaz-Solano, D.; Lara, E.; Duque, K.; Ramos-Gonzalez, G. Allogeneic Bone Marrow Mesenchymal Stromal Cell Transplantation Induces Dentin Pulp Complex-like Formation in Immature Teeth with Pulp Necrosis and Apical Periodontitis. J. Endod. 2024, 50, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramirez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef]
- Kim, U.; Kim, S.; Choi, S.M.; Kang, M.K.; Chang, I.; Kim, E. Regenerative endodontic procedures with minced pulp tissue graft in mature permanent teeth: A clinical study. J. Endod. 2025, 51, 43–53.e2. [Google Scholar] [CrossRef]
- Hong, J.W.; Lim, J.H.; Chung, C.J.; Kang, T.J.; Kim, T.Y.; Kim, Y.S.; Roh, T.S.; Lew, D.H. Immune tolerance of human dental pulp-derived mesenchymal stem cells mediated by CD4+ CD25+ FoxP3+ regulatory T-cells and induced by TGF-β1 and IL-10. Yonsei Med. J. 2017, 58, 1031. [Google Scholar] [CrossRef]
- Kwack, K.H.; Lee, H.-W. Clinical potential of dental pulp stem cells in pulp regeneration: Current endodontic progress and future perspectives. Front. Cell Dev. Biol. 2022, 10, 857066. [Google Scholar] [CrossRef]
- Galler, K.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Duncan, H.F. Present status and future directions—Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in Regenerative Dentistry Approaches: An Update. Int. Dent. J. 2024, 74, 25–34. [Google Scholar]
- KOYANAGI, K.; MAEDA, M.; SEKIYA, M.; NISHIDA, T.; IGARASHI, M. Development of Multilayered Dental Pulp Cell Sheets and Consideration for Calcification Ability. Oper. Dent. Endodontol. Periodontol. 2021, 1, 22–29. [Google Scholar]
- Liu, F.; Xiao, J.; Chen, L.-H.; Pan, Y.-Y.; Tian, J.-Z.; Zhang, Z.-R.; Bai, X.-C. Self-assembly of differentiated dental pulp stem cells facilitates spheroid human dental organoid formation and prevascularization. World J. Stem Cells 2024, 16, 287. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Liu, Q.; Chen, B.; Liu, X.; Pathak, J.L.; Watanabe, N.; Li, J. Dental pulp stem cells-derived cannabidiol-treated organoid-like microspheroids show robust osteogenic potential via upregulation of WNT6. Commun. Biol. 2024, 7, 972. [Google Scholar]
- Zhao, F.; Zhang, Z.; Guo, W. The 3-dimensional printing for dental tissue regeneration: The state of the art and future challenges. Front. Bioeng. Biotechnol. 2024, 12, 1356580. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar]
- Wince, C.; Kassa, C.T.; Insper, J.; Amzallag, D.; Consonlandich, W.; Tortamano, A.C.A.; Magalhães, F.D.; Lestido, V.; Pavani, C.; Prates, R.A. Antimicrobial photodynamic therapy effects mediated by methylene blue in surfactant medium as an adjuvant treatment of teeth with apical periodontitis and presence of fistula–Protocol for randomized, controlled, double-blind clinical trial. PLoS ONE 2024, 19, e0315169. [Google Scholar]
- Halboub, E.; Al-Maswary, A.; Mashyakhy, M.; Al-Qadhi, G.; Al-Maweri, S.A.; Ba-Hattab, R.; Abdulrab, S. The Potential Association Between Inflammatory Bowel Diseases and Apical Periodontitis: A Systematic Review and Meta-Analysis. Eur. Endod. J. 2023, 9, 8–17. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, D.; Zhou, L.; Wang, Q.; Wang, T.; Liang, S. Genetic association between immune-mediated inflammatory diseases and peripheral artery disease: A Mendelian Randomization Study. Sci. Rep. 2025, 15, 3891. [Google Scholar]
- Petty, L.E.; Silva, R.; de Souza, L.C.; Vieira, A.R.; Shaw, D.M.; Below, J.E.; Letra, A. Genome-wide association study identifies novel risk loci for apical periodontitis. J. Endod. 2023, 49, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Niu, T.; Terwedow, H.A.; Xu, X.; Feng, Y.; Li, Z.; Brain, J.D.; Rosen, C.J.; Laird, N.; Xu, X. Variation in genes involved in the RANKL/RANK/OPG bone remodeling pathway are associated with bone mineral density at different skeletal sites in men. Hum. Genet. 2006, 118, 568–577. [Google Scholar] [CrossRef]
- Küchler, E.C.; Hannegraf, N.D.; Lara, R.M.; Reis, C.L.B.; de Oliveira, D.S.B.; Mazzi-Chaves, J.F.; Andrades, K.M.R.; de Lima, L.F.; Salles, A.G.; Antunes, L.A.A. Investigation of genetic polymorphisms in BMP2, BMP4, SMAD6, and RUNX2 and persistent apical periodontitis. J. Endod. 2021, 47, 278–285. [Google Scholar] [CrossRef]
- Setzer, F.C.; Shah, S.B.; Kohli, M.R.; Karabucak, B.; Kim, S. Outcome of endodontic surgery: A meta-analysis of the literature—Part 1: Comparison of traditional root-end surgery and endodontic microsurgery. J. Endod. 2010, 36, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Tsesis, I.; Rosen, E.; Taschieri, S.; Strauss, Y.T.; Ceresoli, V.; Del Fabbro, M. Outcomes of surgical endodontic treatment performed by a modern technique: An updated meta-analysis of the literature. J. Endod. 2013, 39, 332–339. [Google Scholar] [CrossRef]
- Von Arx, T.; Peñarrocha, M.; Jensen, S. Endodontic surgery prognostic factors. J. Endod. 2010, 36, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Shinbori, N.; Grama, A.M.; Patel, Y.; Woodmansey, K.; He, J. Clinical outcome of endodontic microsurgery that uses EndoSequence BC root repair material as the root-end filling material. J. Endod. 2015, 41, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Skoglund, A.; Persson, G. A follow-up study of apicoectomized teeth with total loss of the buccal bone plate. Oral Maxillofac. Pathol. 1985, 59, 78–81. [Google Scholar] [CrossRef]
- Taschieri, S.; Corbella, S.; Tsesis, I.; Bortolin, M.; Del Fabbro, M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment of through-and-through lesions: A retrospective study at 4-year follow-up. Oral Maxillofac. Surg. 2011, 15, 153–159. [Google Scholar] [CrossRef]
- Dhamija, R.; Tewari, S.; Sangwan, P.; Duhan, J.; Mittal, S. Impact of platelet-rich plasma in the healing of through-and-through periapical lesions using 2-dimensional and 3-dimensional evaluation: A randomized controlled trial. J. Endod. 2020, 46, 1167–1184, Erratum in J. Endod. 2021, 47, 336–338. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, N.M.; Ranganath, A.; Jacob, B. Concentrated growth factor–surgical management of large periapical lesion using a novel platelet concentrate in combination with bone graft. Ann. Maxillofac. Surg. 2020, 10, 246–250. [Google Scholar]
- Malli Sureshbabu, N.; Selvarasu, K.; V, J.K.; Nandakumar, M.; Selvam, D. Concentrated growth factors as an ingenious biomaterial in regeneration of bony defects after periapical surgery: A report of two cases. Case Rep. Dent. 2019, 2019, 7046203. [Google Scholar] [CrossRef]
- Yahata, Y.; Handa, K.; Ohkura, N.; Okamoto, M.; Ohshima, J.; Itoh, S.; Kawashima, N.; Tanaka, T.; Sato, N.; Noiri, Y. Autologous concentrated growth factor mediated accelerated bone healing in root-end microsurgery: A multicenter randomized clinical trial. Regen. Ther. 2023, 24, 377–384. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Wang, H.-L. Periodontal and endodontic regeneration. J. Endod. 2009, 35, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.; Khan, Y.; El-Amin, S.F. Bone graft substitutes. Expert Rev. Med. Devices 2006, 3, 49–57. [Google Scholar] [CrossRef]
- Vaishnavi, C.; Mohan, B.; Narayanan, L.L. Treatment of endodontically induced periapical lesions using hydroxyapatite, platelet-rich plasma, and a combination of both: An: In vivo: Study. J. Conserv. Dent. 2011, 14, 140–146. [Google Scholar] [CrossRef]
- Kurmanalina, M.A.; Uraz, R.M.; Skagers, A.; Locs, J.; Taganiyazova, A.A.; Omargali, A.E. Radiological evaluation of endodontic treatment of chronic apical periodontitis using biphasic calcium phosphate biomaterial. Eurasian J. Anal. Chem. 2018, 13, 95170. [Google Scholar] [CrossRef]
- Hulbert, S.; Young, F.; Mathews, R.; Klawitter, J.; Talbert, C.; Stelling, F. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Jarcho, M. Biomaterial aspects of calcium phosphates: Properties and applications. Dent. Clin. N. Am. 1986, 30, 25–47. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Mukherji, A.; Rath, S.K. Calcium sulfate in periodontics: A time tested versatile alloplast. J. Sci. Soc. 2016, 43, 18–23. [Google Scholar] [CrossRef]
- Sukumar, S.; Drízhal, I.; Paulusová, V.; Bukac, J. Surgical treatment of periodontal intrabony defects with calcium sulphate in combination with beta-tricalcium phosphate: Clinical observations two years post-surgery. Acta Medica (Hradec Kral.) 2011, 54, 13–20. [Google Scholar] [CrossRef]
- Sasaki, T.; Niizuma, K.; Kanoke, A.; Matsui, K.; Ogita, S.; Rashad, S.; Kawai, T.; Watanabe, M.; Endo, H.; Takahashi, T. Octacalcium phosphate collagen composite (OCP/Col) enhance bone regeneration in a rat model of skull defect with dural defect. Heliyon 2020, 6, e03347. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.i.; Sumita, Y.; Kajii, F.; Tanaka, H.; Kamakura, S.; Asahina, I. First clinical application of octacalcium phosphate collagen composite on bone regeneration in maxillary sinus floor augmentation: A prospective, single-arm, open-label clinical trial. J. Biomed. Mater. Res. 2020, 108, 243–252. [Google Scholar] [CrossRef]
- Yamaki, D.; Fukuba, S.; Okada, M.; Takeuchi, S.; Hoshi, S.; Matsuura, T.; Iwata, T. Octacalcium phosphate collagen composite for periodontal regeneration in a canine one-wall intrabony defect. J. Periodontal Res. 2024, 59, 521–529. [Google Scholar] [CrossRef]
- Kaida, N.; Matsunaga, S.; Tachiki, C.; Otsu, Y.; Sugahara, K.; Kasahara, N.; Abe, S.; Katakura, A.; Yamamoto, H.; Nishii, Y. Ridge preservation using octacalcium phosphate collagen to induce new bone containing a vascular network of mainly Type H vessels. Sci. Rep. 2024, 14, 25335. [Google Scholar] [CrossRef] [PubMed]
- Kibe, T.; Maeda-Iino, A.; Takahashi, T.; Kamakura, S.; Suzuki, O.; Nakamura, N. A follow-up study on the clinical outcomes of alveolar reconstruction using octacalcium phosphate granules and atelocollagen complex. Oral Maxillofac. Surg. 2021, 79, 2462–2471. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Sotome, S.; Asou, Y.; Kikuchi, M.; Koyama, Y.; Ogawa, T.; Tanaka, J.; Shinomiya, K. Effects of pore size and implant volume of porous hydroxyapatite/collagen (HAp/Col) on bone formation in a rabbit bone defect model. J. Med. Dent. Sci. 2008, 55, 91–99. [Google Scholar]
- Maehara, H.; Sotome, S.; Yoshii, T.; Torigoe, I.; Kawasaki, Y.; Sugata, Y.; Yuasa, M.; Hirano, M.; Mochizuki, N.; Kikuchi, M. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J. Orthop. Res. 2010, 28, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shirosaki, Y.; Oshima, S.; Tsuru, K.; Koyama, Y.; Aizawa, M.; Kikuchi, M. Initial bone tissue reactions of hydroxyapatite/collagen–(3-glycidoxypropyl) trimethoxysilane injectable bone paste. J. Biomed. Mater. Res. 2024, 112, e35451. [Google Scholar] [CrossRef]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Nepola, J.C.; Petersen, E.B.; DeVries-Watson, N.; Grosland, N.; Fredericks, D.C. Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine posterolateral fusion model. Iowa Orthop. J. 2019, 39, 9. [Google Scholar]
- Noguchi, T.; Kitaura, H.; Marahleh, A.; Agista, A.Z.; Ohsaki, Y.; Shirakawa, H.; Mizoguchi, I. Fermented Rice Bran Supplementation Inhibits LPS-Induced Osteoclast Formation and Bone Resorption in Mice. Nutrients 2023, 15, 3044. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Handa, K.; Venkataiah, V.S.; Hasegawa, T.; Njuguna, M.M.; Yahata, Y.; Saito, M. Comparison of the vertical bone defect healing abilities of carbonate apatite, β-tricalcium phosphate, hydroxyapatite and bovine-derived heterogeneous bone. Dent. Mater. J. 2020, 39, 309–318. [Google Scholar] [CrossRef]
- YildirimM, S. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. Clin. Oral Impl. Res. 2000, 11, 217–229. [Google Scholar] [CrossRef]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J. Periodontol. 2001, 72, 152–159. [Google Scholar]
- Merkx, M.; Maltha, J.; Stoelinga, P. Assessment of the value of anorganic bone additives in sinus floor augmentation: A review of clinical reports. Int. J. Oral Maxillofac. Surg. 2003, 32, 1–6. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Fichtner, G.; Schultze-Mosgau, S.; Wiltfang, J. Histologic findings in sinus augmentation with autogenous bone chips versus a bovine bone substitute. Int. J. Oral Maxillofac. Implants. 2003, 18, 53–58. [Google Scholar] [PubMed]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the Human Skeletal Stem Cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef]
- Marecic, O.; Tevlin, R.; McArdle, A.; Seo, E.Y.; Wearda, T.; Duldulao, C.; Walmsley, G.G.; Nguyen, A.; Weissman, I.L.; Chan, C.K. Identification and characterization of an injury-induced skeletal progenitor. Proc. Natl. Acad. Sci. USA 2015, 112, 9920–9925. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Worthley, D.L.; Churchill, M.; Compton, J.T.; Tailor, Y.; Rao, M.; Si, Y.; Levin, D.; Schwartz, M.G.; Uygur, A.; Hayakawa, Y. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 2015, 160, 269–284. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T. Identification and specification of the mouse skeletal stem cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Yu, Y.; Fu, J.; Zhang, S.; Li, M.; Yang, J.; Zhang, X.; Liu, X.; Lv, F. Identification of human cranio-maxillofacial skeletal stem cells for mandibular development. Sci. Adv. 2025, 11, eado7852. [Google Scholar] [CrossRef]
- Suzuki, S.; Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Njuguna, M.M.; Nozawa, R.; Kakiuchi, Y.; Nakano, M.; Handa, K. Correction of large jawbone defect in the mouse using immature osteoblast–like cells and a 3D polylactic acid scaffold. Proc. Natl. Acad. Sci. USA Nexus 2022, 1, pgac151. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyaw, M.S.; Kamano, Y.; Yahata, Y.; Tanaka, T.; Sato, N.; Toyama, F.; Noguchi, T.; Saito, M.; Nakano, M.; Harada, F.; et al. Endodontic Regeneration Therapy: Current Strategies and Tissue Engineering Solutions. Cells 2025, 14, 422. https://doi.org/10.3390/cells14060422
Kyaw MS, Kamano Y, Yahata Y, Tanaka T, Sato N, Toyama F, Noguchi T, Saito M, Nakano M, Harada F, et al. Endodontic Regeneration Therapy: Current Strategies and Tissue Engineering Solutions. Cells. 2025; 14(6):422. https://doi.org/10.3390/cells14060422
Chicago/Turabian StyleKyaw, Moe Sandar, Yuya Kamano, Yoshio Yahata, Toshinori Tanaka, Nobuya Sato, Fusami Toyama, Tomose Noguchi, Marina Saito, Masato Nakano, Futaba Harada, and et al. 2025. "Endodontic Regeneration Therapy: Current Strategies and Tissue Engineering Solutions" Cells 14, no. 6: 422. https://doi.org/10.3390/cells14060422
APA StyleKyaw, M. S., Kamano, Y., Yahata, Y., Tanaka, T., Sato, N., Toyama, F., Noguchi, T., Saito, M., Nakano, M., Harada, F., & Saito, M. (2025). Endodontic Regeneration Therapy: Current Strategies and Tissue Engineering Solutions. Cells, 14(6), 422. https://doi.org/10.3390/cells14060422





