ApoC3 Attenuates Platelet Activation Through GPIIb/IIIa Receptor Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Immunopurification of apoC3
2.3. Blood Collection and Platelet Preparation
2.4. Platelet Aggregation
2.5. ApoC3 Binding Assay
2.6. Thrombus Formation Under Flow Conditions
2.7. Necrotic-like and Apoptotic-like Processes
2.8. Expression of P-Selectin (CD62P)
2.9. GPIIb/IIIa (PAC-1) Activation
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
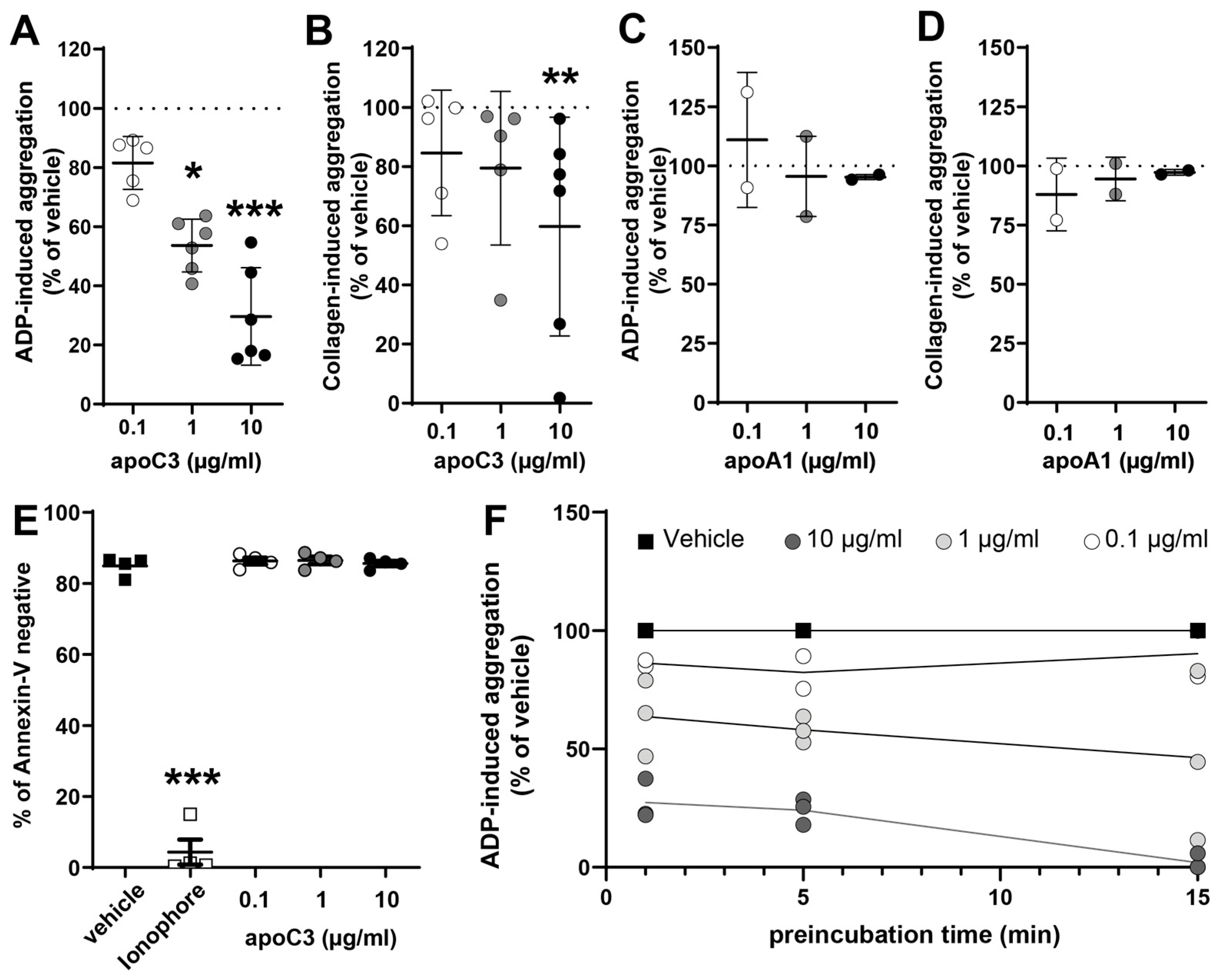
3.1. ApoC3 Exerts a Dose-Dependent Inhibitory Effect on Platelet Aggregation
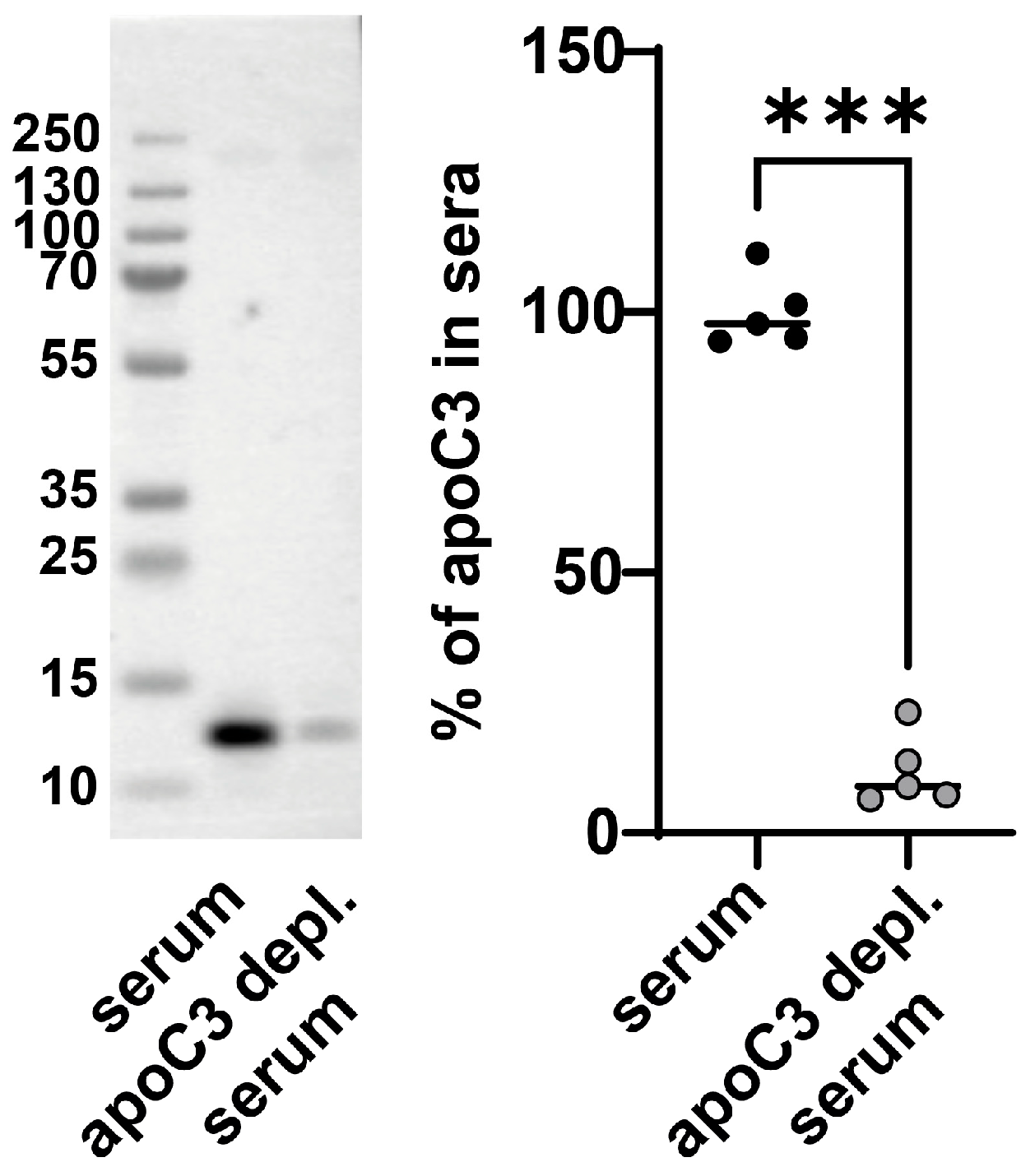
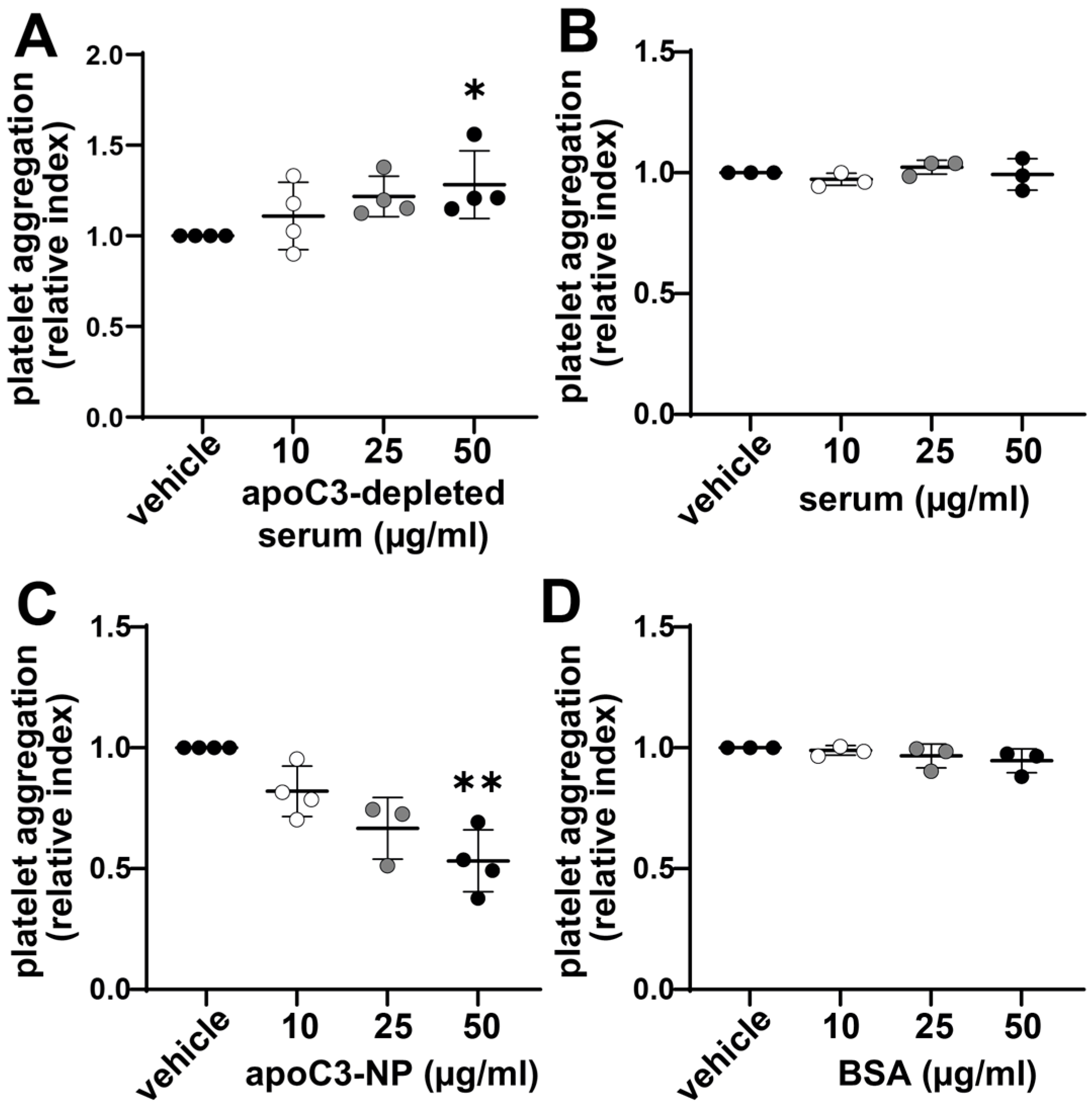
3.2. Enhanced Platelet Aggregation Is Linked to ApoC3 Depletion of Serum
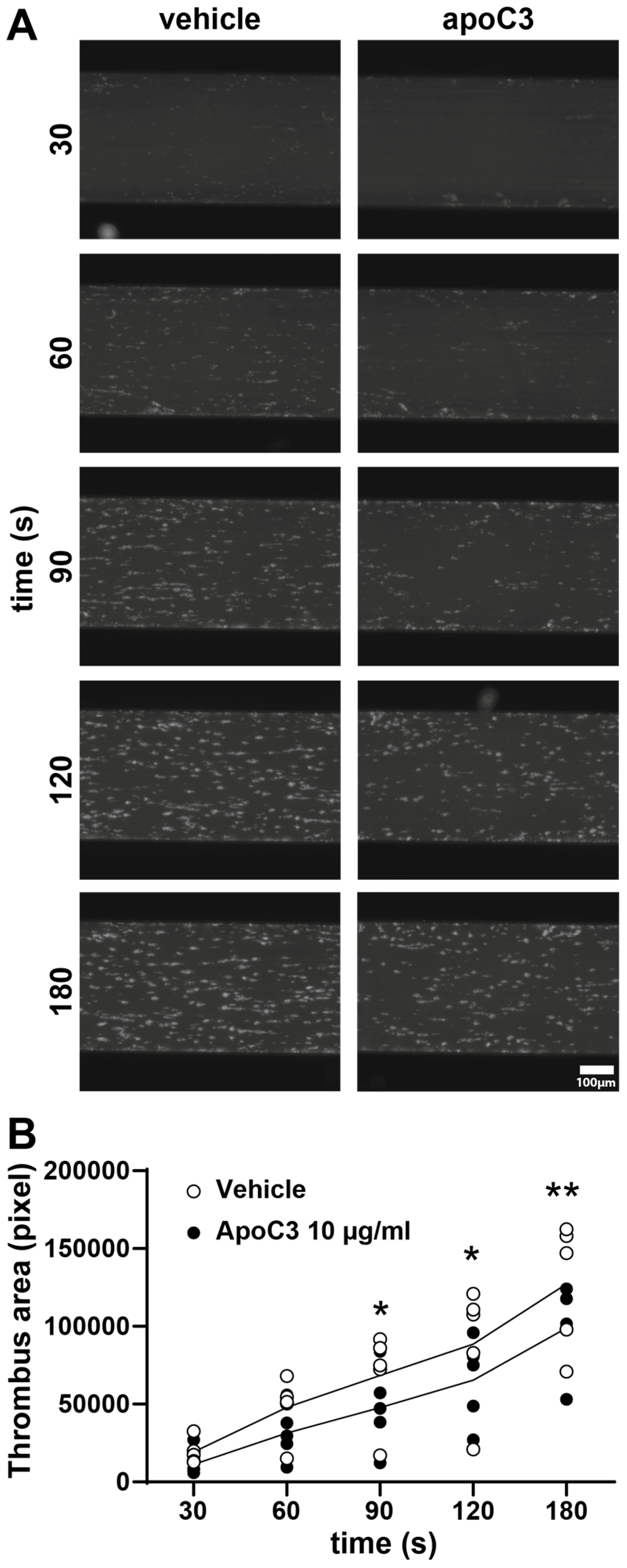
3.3. ApoC3 Inhibits Thrombus Formation Under Flow Conditions
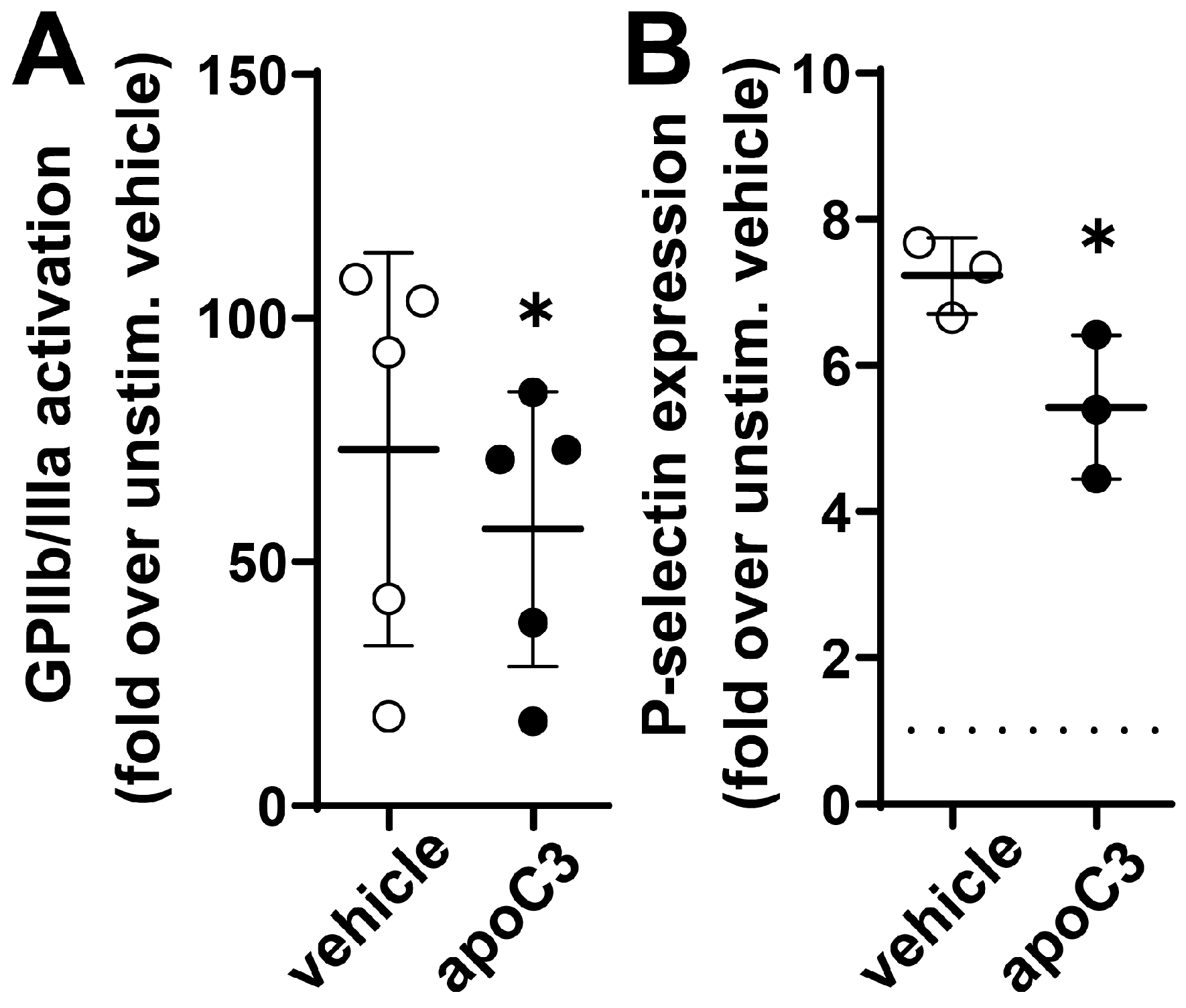
3.4. ApoC3 Reduces GPIIb/IIIa Activation and P-Selection Expression
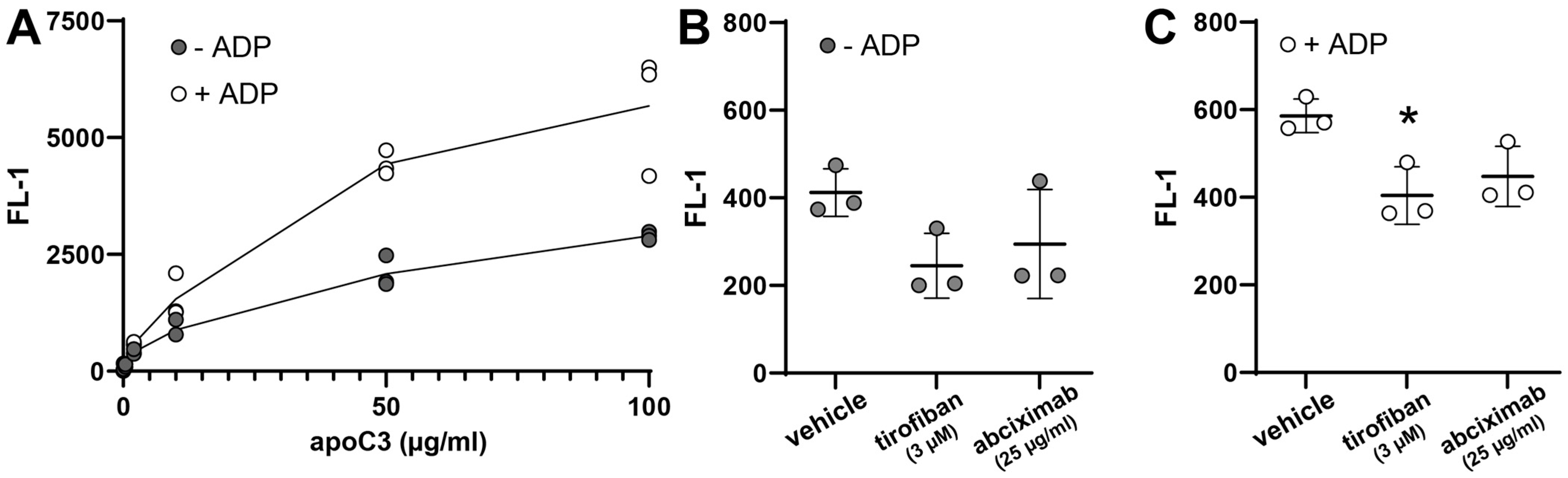
3.5. ApoC3 Directly Interacts with Glycoprotein IIb/IIIa (GPIIb/IIIa)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| apo | Apolipoprotein |
| ASO | Antisense oligonucleotides |
| CVD | Cardiovascular disease |
| GPIIb/IIIa | Glycoprotein type IIb/IIIa |
| LDLR | Low-density lipoprotein receptor |
| PRP | Platelet-rich plasma |
| siRNA | Small-interfering RNA |
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Bornfeldt, K.E. Apolipoprotein C3: Form begets function. J. Lipid Res. 2023, 65, 100475. [Google Scholar] [CrossRef]
- Scheffer, P.G.; Teerlink, T.; Dekker, J.M.; Bos, G.; Nijpels, G.; Diamant, M.; Kostense, P.J.; A Stehouwer, C.D.; Heine, R.J. Increased Plasma Apolipoprotein C-III Concentration Independently Predicts Cardiovascular Mortality: The Hoorn Study. Clin. Chem. 2008, 54, 1325–1330. [Google Scholar] [CrossRef]
- Van Capelleveen, J.C.; Lee, S.-R.; Verbeek, R.; Kastelein, J.J.; Wareham, N.; Stroes, E.S.; Hovingh, G.K.; Khaw, K.-T.; Boekholdt, S.M.; Witztum, J.L.; et al. Relationship of lipoprotein-associated apolipoprotein C-III with lipid variables and coronary artery disease risk: The EPIC-Norfolk prospective population study. J. Clin Lipidol. 2018, 12, 1493–1501.e11. [Google Scholar] [CrossRef]
- Von Ballmoos, M.C.W.; Haring, B.; Sacks, F.M. The risk of cardiovascular events with increased apolipoprotein CIII: A systematic review and meta-analysis. J. Clin. Lipidol. 2015, 9, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2019, 21, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Shao, B.; Kanter, J.E.; He, Y.; Vaisar, T.; Witztum, J.L.; Snell-Bergeon, J.; McInnes, G.; Bruse, S.; Gottesman, O.; et al. Apolipoprotein C3 induces inflammasome activation only in its delipidated form. Nat. Immunol. 2023, 24, 408–411. [Google Scholar] [CrossRef]
- Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Loss-of-Function Mutations in APOC3 and Risk of Ischemic Vascular Disease. N. Engl. J. Med. 2014, 371, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Arca, M.; Jones, A.; Soran, H.; Gouni-Berthold, I.; Stroes, E.S.; Alexander, V.J.; Jones, R.; Watts, L.; et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome: Long-term efficacy and safety data from patients in an open-label extension trial. J. Clin. Lipidol. 2023, 17, 342–355. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Marston, N.A.; Prohaska, T.A.; Alexander, V.J.; Zimerman, A.; Moura, F.A.; Murphy, S.A.; Goodrich, E.L.; Zhang, S.; Gaudet, D.; et al. Olezarsen for Hypertriglyceridemia in Patients at High Cardiovascular Risk. N. Engl. J. Med. 2024, 390, 1770–1780. [Google Scholar] [CrossRef]
- Stroes, E.S.; Alexander, V.J.; Karwatowska-Prokopczuk, E.; Hegele, R.A.; Arca, M.; Ballantyne, C.M.; Soran, H.; Prohaska, T.A.; Xia, S.; Ginsberg, H.N.; et al. Olezarsen, Acute Pancreatitis, and Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2024, 390, 1781–1792. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, J.; Zhang, L.; Miao, G.; Lai, P.; Zhang, W.; Liu, L.; Hou, X.; Wang, Y.; Huang, W.; et al. Targeting ApoC3 Paradoxically Aggravates Atherosclerosis in Hamsters With Severe Refractory Hypercholesterolemia. Front. Cardiovasc. Med. 2022, 9, 840358. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Kern, S.; Birner-Grünberger, R.; Curcic, S.; Heinemann, A.; Marsche, G. Refined purification strategy for reliable proteomic profiling of HDL2/3: Impact on proteomic complexity. Sci. Rep. 2016, 6, 38533. [Google Scholar] [CrossRef] [PubMed]
- Curcic, S.; Holzer, M.; Frei, R.; Pasterk, L.; Schicho, R.; Heinemann, A.; Marsche, G. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 184–193. [Google Scholar] [CrossRef]
- Curcic, S.; Holzer, M.; Pasterk, L.; Knuplez, E.; Eichmann, T.O.; Frank, S.; Zimmermann, R.; Schicho, R.; Heinemann, A.; Marsche, G. Secretory phospholipase A2 modified HDL rapidly and potently suppresses platelet activation. Sci. Rep. 2017, 7, 8030. [Google Scholar] [CrossRef]
- Torti, M.; Festetics, E.T.; Bertoni, A.; Sinigaglia, F.; Balduini, C. Agonist-induced Actin Polymerization Is Required for the Irreversibility of Platelet Aggregation. Thromb. Haemost. 1996, 76, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Knuplez, E.; Curcic, S.; Theiler, A.; Bärnthaler, T.; Trakaki, A.; Trieb, M.; Holzer, M.; Heinemann, A.; Zimmermann, R.; Sturm, E.M.; et al. Lysophosphatidylcholines inhibit human eosinophil activation and suppress eosinophil migration in vivo. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158686. [Google Scholar] [CrossRef]
- Philipose, S.; Konya, V.; Sreckovic, I.; Marsche, G.; Lippe, I.T.; Peskar, B.A.; Heinemann, A.; Schuligoi, R. The Prostaglandin E2Receptor EP4 Is Expressed by Human Platelets and Potently Inhibits Platelet Aggregation and Thrombus Formation. Arter. Thromb. Vasc. Biol. 2010, 30, 2416–2423. [Google Scholar] [CrossRef]
- Jong, M.C.; Hofker, M.H.; Havekes, L.M. Role of ApoCs in Lipoprotein Metabolism: Functional Differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 472–484. [Google Scholar] [CrossRef]
- Savage, B.; Cattaneo, M.; Ruggeri, Z.M. Mechanisms of platelet aggregation. Curr. Opin. Hematol. 2001, 8, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Merten, M.; Thiagarajan, P. P-Selectin Expression on Platelets Determines Size and Stability of Platelet Aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar] [CrossRef]
- Hosseini, E.; Taherabadi, E.; Rajabi, A.; Ghasemzadeh, M. Reduction of ristocetin-induced platelet aggregation (RIPA) during storage despite plasma renewal: Evidence for hemostatic importance of GPIbα shedding. Expert Rev. Hematol. 2024, 17, 391–403. [Google Scholar] [CrossRef]
- Khetarpal, S.A.; Qamar, A.; Millar, J.S.; Rader, D.J. Targeting ApoC-III to Reduce Coronary Disease Risk. Curr. Atheroscler. Rep. 2016, 18, 54. [Google Scholar] [CrossRef]
- Kraaijenhof, J.M.; Peletier, M.C.; Nurmohamed, N.S.; Hovingh, G.K.; Alexander, V.J.; Tsimikas, S.; Stroes, E.S.G.; Kroon, J. Plasma reduction of apolipoprotein C-III with olezarsen leads to significant reductions in postprandial triglyceride levels: Results from a randomized trial. Eur. J. Prev. Cardiol. 2025, zwaf478. [Google Scholar] [CrossRef]
- Digenio, A.; Dunbar, R.L.; Alexander, V.J.; Hompesch, M.; Morrow, L.; Lee, R.G.; Graham, M.J.; Hughes, S.G.; Yu, R.; Singleton, W.; et al. Antisense-Mediated Lowering of Plasma Apolipoprotein C-III by Volanesorsen Improves Dyslipidemia and Insulin Sensitivity in Type 2 Diabetes. Diabetes Care 2016, 39, 1408–1415. [Google Scholar] [CrossRef]
- Qamar, A.; Khetarpal, S.A.; Khera, A.V.; Qasim, A.; Rader, D.J.; Reilly, M.P. Plasma Apolipoprotein C-III Levels, Triglycerides, and Coronary Artery Calcification in Type 2 Diabetics. Arter. Thromb. Vasc. Biol. 2015, 35, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, O.; Martinelli, N.; Girelli, D.; Pizzolo, F.; Friso, S.; Beltrame, F.; Lotto, V.; Annarumma, L.; Corrocher, R. Apolipoprotein C-III predicts cardiovascular mortality in severe coronary artery disease and is associated with an enhanced plasma thrombin generation. J. Thromb. Haemost. 2010, 8, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, Y.; Qi, R.; Wang, Y.; Zhang, X.; Yu, M.; Tang, Y.; Wang, M.; Shu, Y.-N.; Huang, W.; et al. Aggravated restenosis and atherogenesis in ApoCIII transgenic mice but lack of protection in ApoCIII knockouts: The effect of authentic triglyceride-rich lipoproteins with and without ApoCIII. Cardiovasc. Res. 2015, 107, 579–589. [Google Scholar] [CrossRef]
- Hosseini, E.; Beyranvand, Z.; Schoenwaelder, S.M.; Farhid, F.; Ghasemzadeh, M.; Milkovic, L. The Reactive Oxygen Species Scavenger N-Acetyl-L-Cysteine Reduces Storage-Dependent Decline in Integrin αIIbβ3-Mediated Platelet Function, Inhibiting Pre-Activation of Integrin and Its β3 Subunit Cleavage. Oxidative Med. Cell. Longev. 2025, 2025, 7499648. [Google Scholar] [CrossRef]
- Xu, X.R.; Wang, Y.; Adili, R.; Ju, L.; Spring, C.M.; Jin, J.W.; Yang, H.; Neves, M.A.D.; Chen, P.; Yang, Y.; et al. Apolipoprotein A-IV binds αIIbβ3 integrin and inhibits thrombosis. Nat. Commun. 2018, 9, 3608. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soffer, G.; Sztalryd, C.; Horenstein, R.B.; Holleran, S.; Matveyenko, A.; Thomas, T.; Nandakumar, R.; Ngai, C.; Karmally, W.; Ginsberg, H.N.; et al. Effects of APOC3 Heterozygous Deficiency on Plasma Lipid and Lipoprotein Metabolism. Arter. Thromb. Vasc. Biol. 2019, 39, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rout, M.; Lerner, M.; Blackett, P.R.; Peyton, M.D.; Stavrakis, S.; Sidorov, E.; Sanghera, D.K. Ethnic differences in ApoC-III concentration and the risk of cardiovascular disease: No evidence for the cardioprotective role of rare/loss of function APOC3 variants in non-Europeans. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 13, 100128. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Tanigawa, Y.; Zhang, W.; Chai, J.-F.; Almeida, M.; Sim, X.; Lerner, M.; Chainakul, J.; Ramiu, J.G.; Seraphin, C.; et al. APOC3 genetic variation, serum triglycerides, and risk of coronary artery disease in Asian Indians, Europeans, and other ethnic groups. Lipids Health Dis. 2021, 20, 113. [Google Scholar] [CrossRef]
- Witztum, J.L.; Geary, R.S.; O’Dea, L. Volanesorsen, Familial Chylomicronemia Syndrome, and Thrombocytopenia. N. Engl. J. Med. 2019, 381, 2582–2584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzer, M.; Gruden, E.; Curcic, S.; Cvirn, G.; Marsche, G. ApoC3 Attenuates Platelet Activation Through GPIIb/IIIa Receptor Interaction. Cells 2025, 14, 1411. https://doi.org/10.3390/cells14181411
Holzer M, Gruden E, Curcic S, Cvirn G, Marsche G. ApoC3 Attenuates Platelet Activation Through GPIIb/IIIa Receptor Interaction. Cells. 2025; 14(18):1411. https://doi.org/10.3390/cells14181411
Chicago/Turabian StyleHolzer, Michael, Eva Gruden, Sanja Curcic, Gerhard Cvirn, and Gunther Marsche. 2025. "ApoC3 Attenuates Platelet Activation Through GPIIb/IIIa Receptor Interaction" Cells 14, no. 18: 1411. https://doi.org/10.3390/cells14181411
APA StyleHolzer, M., Gruden, E., Curcic, S., Cvirn, G., & Marsche, G. (2025). ApoC3 Attenuates Platelet Activation Through GPIIb/IIIa Receptor Interaction. Cells, 14(18), 1411. https://doi.org/10.3390/cells14181411







