White Matter in Crisis: Oligodendrocytes and the Pathophysiology of Multiple Sclerosis
Abstract
1. Introduction
2. Pathophysiology of Multiple Sclerosis
2.1. Peripheral Immune Activation and CNS Infiltration
2.1.1. Activation of Autoreactive T Cells
2.1.2. Role of B Cells and Autoantibodies
2.1.3. Disruption of the Blood–Brain Barrier
2.2. Inflammatory Cascade Within the CNS
3. The Role of Oligodendrocytes in Multiple Sclerosis
3.1. Oligodendrocyte Apoptosis and Necroptosis
3.2. Impaired Oligodendrocyte Precursor Cell Differentiation
3.3. Mitochondrial Dysfunction and Energy Failure
3.4. Disruption in the Formation of Myelin Proteins
4. Therapeutic Strategies Targeting Oligodendrocytes
4.1. Promoting OPC Differentiation
| Signaling Pathway | Compound | Target | Effects on Oligodendrocytes | References |
|---|---|---|---|---|
| Wnt/β-catenin | XAV939 | Tankyrase (β-catenin) | Promotes OPC differentiation Anti-apoptotic effects on mature oligodendrocytes | [181,182,183,184,185] |
| ICG-001 | β-catenin | Promotes OPC differentiation | [186] | |
| Shh | Purmorphamine | SMO | [189,190] | |
| Clobetasol | [191,192] | |||
| Notch | DAPT | γ-secretase | [197] | |
| MW167 | γ-secretase | [198] |
4.2. Enhancing Myelination via Neurotrophic Factors and Growth Molecules
| Signaling Pathway | Compound | Target | Effects on Oligodendrocytes | References |
|---|---|---|---|---|
| BDNF/TrkB | 7,8-DHF | TrkB | Reduces demyelination and axonal loss | [201] |
| IGF-1/ IGF-1R | IGF | IGF-1R | Reduces demyelination and upregulates mRNA encoding myelin proteins | [205,206] |
| Induces remyelination | [207] | |||
| NRG1 | NRG1β1 | ErbB4 | Inhibits OPC apoptosis in vitro | [211] |
| Erb2/ErbB4 | NRG-1 reduces CSPGs and increases IL-10 in those demyelinated areas | [212] |
4.3. Epigenetic Modulation of Oligodendrocyte Fate
| Drug Family | Compound | Target | Effects on Oligodendrocytes | References |
|---|---|---|---|---|
| HDAC inhibitors | Valproic acid | HDAC1/2 | Increases endogenous myelin repair by recruiting OPCs | [215] |
| Promotes expression of associated myelin genes and oligodendrocyte function | [216] | |||
| α-linolenic acid-valproic acid | HDAC1/2 | Promote oligodendrocyte function | [217] | |
| LY294002 | PI3K/HDAC inhibitor | [218] | ||
| DNMT activators | Curcumin | GPR97 agonist DNMT activator | [219,220] | |
| Vitamin C | DNMT activator | [221] |
4.4. Cell-Based Therapies and Transplantation Approaches
4.5. Regulation of the Inflammatory Milieu
| Compound | Activity | Effects on MS | References |
|---|---|---|---|
| Pioglitazone | PPARγ agonist | Promotes the conversion of OPCs into mature oligodendrocytes | [243] |
| Minocycline | Targets various microglial activation pathways | Allows remyelination by inhibiting microglial activity | [244] |
| PLX3397 | CSF1R antagonist | Prevents demyelination, oligodendrocyte loss, and reactive astrocytosis induced by CUP treatment | [245] |
| Enhances oligodendrocyte density and remyelination in CUP-treated mice | [246] | ||
| AZD4547 | Blockade of FGFR, VEGFR2, and CSF1R | Increases the abundance of OPCs and mature oligodendrocytes in MS lesions | [247] |
| MCC950 | Selective NLRP3 inflammasome inhibitor | Mitigates neuronal damage, demyelination, and oligodendrocyte loss in EAE mouse brains | [250] |
4.6. Other Pharmacological Modulators of OPC Development and Myelin Repair
| Compound | Activity | Effects on MS | References |
|---|---|---|---|
| Anti-Nogo-A | Nogo-A blockade | Improves remyelination in EAE preclinical MS model | [253] |
| Anti-LINGO-1 | LINGO-1 blockade | Improves remyelination in EAE preclinical MS model | [254] |
| Improves remyelination in CUP-induced demyelination | [255] | ||
| Opicinumab improves remyelination | [256] | ||
| Clemastine | H1 antagonist M1/M3 antagonist | Promotes oligodendrocyte function in EAE preclinical MS model | [258,259] |
| PIPE-307 | M1 antagonists | [262] | |
| PIPE-791 | [263] | ||
| Thyroid hormone | TR agonists | Activates OPCs and enables remyelination in EAE model | [265] |
| TG68/IS25 | Induce OPCs differentiation and maturation in vitro | [266] | |
| Sobetirome/Sob-AM2 | Activates OPCs and enables remyelination in EAE model | [267] | |
| Anti-SEMA4D | SEMA4D blockade | Reduces apoptosis of OPCs and promotes their differentiation in vitro | [269] |
| Guanabenz | α2 adrenergic receptor agonist | Enhances oligodendrocyte survival in vitro | [270] |
| Trametinib | MEK inhibitor | Promotes remyelination and increases the formation of mature oligodendrocytes in the EAE preclinical MS model | [271] |
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OH | Hydroxyl radical |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| ADCC | Antibody-dependent cellular cytotoxicity |
| ADP | Adenosine diphosphate |
| aFn | Aggregated fibronectin |
| Akt | Protein kinase B (PKB) |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| APC | Antigen-presenting cell |
| ATF6 | Activating transcription factor 6 |
| ATP | Adenosine triphosphate |
| BBB | Blood–brain barrier |
| BCR | B cell receptor |
| BDNF | Brain-derived neurotrophic factor |
| bHLH | Basic helix-loop-helix |
| BiP | Binding immunoglobulin protein |
| BMP | Bone morphogenetic protein |
| BMP4 | Bone morphogenetic protein 4 |
| Breg | Regulatory B cell |
| C1q | Complement component 1q |
| C3 | Complement component 3 |
| C3b | Complement component 3b |
| C5b-9 | Complement components 5b to 9 |
| Ca2+ | Calcium ion |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL20 | C-C motif chemokine ligand 20 |
| CCL5 | C-C motif chemokine ligand 5 |
| CD28 | Cluster of differentiation 28 |
| CD4 | Cluster of differentiation 4 |
| CD44 | Cluster of differentiation 44 |
| CD8 | Cluster of differentiation 8 |
| CD80 | Cluster of differentiation 80 |
| CD86 | Cluster of differentiation 86 |
| CIITA | Class II major histocompatibility complex transactivator |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CSF1R | Colony stimulating factor 1 receptor |
| CSPG | Chondroitin sulfate proteoglycan |
| CUP | Cuprizone |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| CXCL2 | C-X-C motif chemokine ligand 2 |
| DAMP | Damage-associated molecular pattern |
| DAPT | N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester |
| DC | Dendritic cell |
| DISC | Death-inducing signaling complex |
| DMT | Disease-modifying therapy |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| EAE | Experimental autoimmune encephalomyelitis |
| EBV | Epstein–Barr virus |
| eIF2B | Eukaryotic initiation factor 2B |
| eIF2α | Eukaryotic initiation factor 2 alpha |
| ER | Endoplasmic reticulum |
| ERAD | Endoplasmic reticulum-associated degradation |
| ErbB2 | Erythroblastic leukemia viral oncogene homolog 2 |
| ErbB3 | Erythroblastic leukemia viral oncogene homolog 3 |
| ErbB4 | Erythroblastic leukemia viral oncogene homolog 4 |
| ERK | Extracellular signal-regulated kinase |
| ETC | Electron transport chain |
| EZH2 | Enhancer of zeste homolog 2 |
| FADD | Fas-associated protein with death domain |
| FADH2 | Flavin adenine dinucleotide (reduced form) |
| Fas | Fas receptor |
| FasL | Fas ligand |
| Fcγ | Fc gamma |
| Fe | Iron |
| Fe2+ | Ferrous iron |
| FGF-2 | Fibroblast growth factor 2 |
| FGFR | Fibroblast growth factor receptor |
| GFAP | Glial fibrillary acidic protein |
| Gli1 | GLI family zinc finger 1 |
| GPR97 | G protein-coupled receptor 97 |
| GRP78 | 78 kDa glucose-regulated protein |
| GRP94 | 94 kDa glucose-regulated protein |
| H+ | Proton |
| H1 | Histamine receptor 1 |
| H2O2 | Hydrogen peroxide |
| H3K27me3 | Trimethylation of lysine 27 on histone H3 |
| HDAC | Histone deacetylase |
| HDAC1 | Histone deacetylase 1 |
| HDAC2 | Histone deacetylase 2 |
| Hes1 | Hairy and enhancer of split 1 |
| Hes5 | Hairy and enhancer of split 5 |
| hESC-OPC | Human embryonic stem cell-oligodendrocyte precursor cell |
| ICAM-1 | Intercellular adhesion molecule 1 |
| ID2 | Inhibitor of DNA binding 2 |
| ID4 | Inhibitor of DNA binding 4 |
| IFN-γ | Interferon gamma |
| IGF-1 | Insulin-like growth factor 1 |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IgG | Immunoglobulin G |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IL-17 | Interleukin 17 |
| IL-17A | Interleukin 17A |
| IL-17F | Interleukin 17F |
| IL-1β | Interleukin 1 beta |
| IL-21 | Interleukin 21 |
| IL-22 | Interleukin 22 |
| IL-23 | Interleukin 23 |
| IL-33 | Interleukin 33 |
| IL-6 | Interleukin 6 |
| iNOS | Inducible nitric oxide synthase |
| iPSC-OL | Induced pluripotent stem cell-oligodendrocyte |
| IRE1α | Inositol requiring enzyme 1 alpha |
| IRF3 | Interferon regulatory factor 3 |
| ISR | Integrated stress response |
| JAM | Junctional adhesion molecule |
| K+ | Potassium ion |
| LAR | Leukocyte common antigen-related receptor |
| LFA-1 | Lymphocyte function-associated antigen 1 |
| LINGO-1 | Leucine-rich repeat and immunoglobulin-like domain-containing Nogo receptor-interacting protein 1 |
| LRP5/6 | Low-density lipoprotein receptor-related protein 5/6 |
| LT-α | Lymphotoxin alpha |
| M1 | Muscarinic acetylcholine receptor 1 |
| M3 | Muscarinic acetylcholine receptor 3 |
| mAb | Monoclonal antibody |
| MAC | Membrane attack complex |
| MAG | Myelin-associated glycoprotein |
| MAPK | Mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| MEK | Mitogen-activated protein kinase kinase |
| MHC-I | Major histocompatibility complex class I |
| MHC-II | Major histocompatibility complex class II |
| miRNA | MicroRNA |
| MLKL | Mixed lineage kinase domain-like protein |
| MMP | Matrix metalloproteinase |
| MnSOD | Manganese superoxide dismutase |
| MOG | Myelin oligodendrocyte glycoprotein |
| MOMP | Mitochondrial outer membrane permeabilization |
| mRNA | Messenger ribonucleic acid |
| MS | Multiple sclerosis |
| mtDNA | Mitochondrial DNA |
| mTOR | Mechanistic target of rapamycin |
| mTOR1 | Mechanistic target of rapamycin complex 1 |
| MyD88 | Myeloid differentiation primary response 88 |
| MYRF | Myelin regulatory factor |
| Na+ | Sodium ion |
| Na+/K+-ATPase | Sodium/potassium-ATPase |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| NADPH | Nicotinamide adenine dinucleotide phosphate (reduced form) |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NICD | Notch intracellular domain |
| NK | Natural killer |
| NKX2.2 | NK2 homeobox 2 |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NO | Nitric oxide |
| Nogo-A | Neurite outgrowth inhibitor A |
| NOX2 | NADPH oxidase 2 |
| NRG1 | Neuregulin 1 |
| NRG1β1 | Neuregulin 1 beta 1 |
| O2 | Molecular oxygen |
| O2− | Superoxide anion |
| OLIG1 | Oligodendrocyte transcription factor 1 |
| OLIG2 | Oligodendrocyte transcription factor 2 |
| ONOO− | Peroxynitrite |
| OPC | Oligodendrocyte progenitor cell |
| OXPHOS | Oxidative phosphorylation |
| PAMP | Pathogen-associated molecular pattern |
| PDGF-A | Platelet-derived growth factor A |
| PECAM-1 | Platelet endothelial cell adhesion molecule 1 |
| PERK | Protein kinase R (PKR)-like endoplasmic reticulum kinase |
| PI3K | Phosphoinositide 3-kinase |
| PLP | Proteolipid protein |
| PLP1 | Proteolipid protein 1 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PPMS | Primary-progressive multiple sclerosis |
| PRMS | Progressive-relapsing multiple sclerosis |
| PRC2 | Polycomb repressive complex 2 |
| PRR | Pattern recognition receptor |
| PTCH1 | Patched 1 |
| PTPσ | Protein tyrosine phosphatase sigma |
| RAGE | Receptor for advanced glycation endproduct |
| RBP-Jκ | Recombination signal binding protein for immunoglobulin kappa J region |
| RhoA | RhoA GTPase |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| RNS | Reactive nitrogen species |
| ROCK | Rho-associated coiled-coil containing protein kinase |
| ROS | Reactive oxygen species |
| RRMS | Relapsing-remitting multiple sclerosis |
| S100β | S100 protein beta |
| SEMA4D | Semaphorin 4D |
| Shh | Sonic hedgehog |
| SMO | Smoothened |
| Sob-AM2 | Sobetirome-AM2 |
| SOX10 | SRY-box transcription factor 10 |
| Sox6 | SRY-box transcription factor 6 |
| SPMS | Secondary-progressive multiple sclerosis |
| STAT1 | Signal transducer and activator of transcription 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| sTNF | Soluble tumor necrosis factor |
| TCF7L2 | Transcription factor 7 like 2 |
| TCR | T cell receptor |
| Tfh | T follicular helper |
| TGF-β | Transforming growth factor beta |
| TLR | Toll-like receptor |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNF-α | Tumor necrosis factor alpha |
| TRIF | TIR-domain-containing adapter-inducing interferon beta |
| TrkB | Tropomyosin receptor kinase B |
| UPR | Unfolded protein response |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| VLA-4 | Very late antigen 4 |
| Wnt | Wingless/integrated 1 |
| XBP1 | X-box binding protein 1 |
| ZO-1 | Zonula occludens 1 |
| α2 | Alpha 2 adrenergic receptor |
References
- Haki, M.; Al-Biati, H.A.; Al-Tameemi, Z.S.; Ali, I.S.; Al-Hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, 37297. [Google Scholar] [CrossRef]
- Coyle, P.K. What Can We Learn from Sex Differences in MS? J. Pers. Med. 2021, 11, 1006. [Google Scholar] [CrossRef]
- Prosperini, L.; Lucchini, M.; Ruggieri, S.; Tortorella, C.; Haggiag, S.; Mirabella, M.; Pozzilli, C.; Gasperini, C. Shift of multiple sclerosis onset towards older age. J. Neurol. Neurosurg. Psychiatry 2022, 2022, 329049. [Google Scholar] [CrossRef]
- Sabel, C.E.; Pearson, J.F.; Mason, D.F.; Willoughby, E.; Abernethy, D.A.; Taylor, B.V. The latitude gradient for multiple sclerosis prevalence is established in the early life course. Brain 2021, 144, 2038–2046. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and genetic risk factors for MS: An integrated review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, E.; Luchicchi, A.; Strijbis, E.M.M.; Geurts, J.J.G.; van Dam, A.M. Is MS affecting the CNS only? Lessons from clinic to myelin pathophysiology. Neurol. Neuroimmunol. Neuroinflamm. 2020, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou De Lorenzo, S.; Bakirtzis, C.; Konstantinidou, N.; Kesidou, E.; Parissis, D.; Evangelopoulos, M.E.; Elsayed, D.; Hamdy, E.; Said, S.; Grigoriadis, N. How Early Is Early Multiple Sclerosis? J. Clin. Med. 2023, 13, 214. [Google Scholar] [CrossRef]
- Portaccio, E.; Magyari, M.; Havrdova, E.K.; Ruet, A.; Brochet, B.; Scalfari, A.; Di Filippo, M.; Tur, C.; Montalban, X.; Amato, M.P. Multiple sclerosis: Emerging epidemiological trends and redefining the clinical course. Lancet Reg. Health Eur. 2024, 44, 100977. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Pintér, A.; Cseh, D.; Sárközi, A.; Illigens, B.M.; Siepmann, T. Autonomic Dysregulation in Multiple Sclerosis. Int. J. Mol. Sci. 2015, 16, 16920–16952. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, Q.; Wen, H.; Zhang, Y. Disease modifying therapies in relapsing-remitting multiple sclerosis: A systematic review and network meta-analysis. Autoimmun. Rev. 2021, 20, 102826. [Google Scholar] [CrossRef]
- Ziemssen, T.; Bhan, V.; Chataway, J.; Chitnis, T.; Campbell Cree, B.A.; Havrdova, E.K.; Kappos, L.; Labauge, P.; Miller, A.; Nakahara, J.; et al. Secondary Progressive Multiple Sclerosis: A Review of Clinical Characteristics, Definition, Prognostic Tools, and Disease-Modifying Therapies. Neurol. Neuroimmunol. Neuroinflamm. 2022, 10, 200064. [Google Scholar] [CrossRef]
- Sempik, I.; Dziadkowiak, E.; Moreira, H.; Zimny, A.; Pokryszko-Dragan, A. Primary Progressive Multiple Sclerosis-A Key to Understanding and Managing Disease Progression. Int. J. Mol. Sci. 2024, 25, 8751. [Google Scholar] [CrossRef]
- Tullman, M.J.; Oshinsky, R.J.; Lublin, F.D.; Cutter, G.R. Clinical characteristics of progressive relapsing multiple sclerosis. Mult. Scler. 2004, 10, 451–454. [Google Scholar] [CrossRef]
- Arredondo-Robles, A.V.; Rodríguez-López, K.P.; Ávila-Avilés, R.D. Clinical Management in Multiple Sclerosis. Neuroglia 2025, 6, 6. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef] [PubMed]
- Multz, R.A.; Jamshidi, P.; Ahrendsen, J.T. Multiple sclerosis: A practical review for pathologists. J. Pathol. Transl. Med. 2025, 59, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Barcutean, L.; Mosora, O.; Manu, D. Reviewing the Significance of Blood–Brain Barrier Disruption in Multiple Sclerosis Pathology and Treatment. Int. J. Mol. Sci. 2021, 22, 8370. [Google Scholar] [CrossRef] [PubMed]
- Zierfuss, B.; Larochelle, C.; Prat, A. Blood–brain barrier dysfunction in multiple sclerosis: Causes, consequences, and potential effects of therapies. Lancet Neurol. 2024, 23, 95–109. [Google Scholar] [CrossRef]
- Suknjaja, V.; Sakalaš, L.; Ilin, M. EARLY ONSET OF MULTIPLE SCLEROSIS–CLINICAL FEATURES. Acta Clin. Croat. 2024, 63, 23–28. [Google Scholar] [CrossRef]
- Murtonen, A.; Lehto, J.T.; Sumelahti, M.L. End of life in multiple sclerosis: Disability, causes and place of death among cases diagnosed from 1981 to 2010 in Pirkanmaa hospital district in Western Finland. Mult. Scler. Relat. Disord. 2021, 54, 103139. [Google Scholar] [CrossRef]
- Miyata, S.; Wake, H. Editorial: Oligodendrocytes: From their development to function and dysfunction. Front. Cell Neurosci. 2024, 18, 1376931. [Google Scholar] [CrossRef]
- Wellman, S.M.; Cambi, F.; Kozai, T.D. The role of oligodendrocytes and their progenitors on neural interface technology: A novel perspective on tissue regeneration and repair. Biomaterials 2018, 183, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Nave, K.A.; Asadollahi, E.; Sasmita, A. Expanding the function of oligodendrocytes to brain energy metabolism. Curr. Opin. Neurobiol. 2023, 83, 102782. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Arndt, L.; Kerkering, J.; Kuehl, T.; Infante, A.G.; Paul, F.; Rosiewicz, K.S.; Siffrin, V.; Alisch, M. Inflammatory Cytokines Associated with Multiple Sclerosis Directly Induce Alterations of Neuronal Cytoarchitecture in Human Neurons. J. Neuroimmune Pharmacol. 2023, 18, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.D.; Viero, F.T.; Rodrigues, P.; Trevisan, G. Nitric oxide involvement in the disability and active disease of multiple sclerosis: Systematic review and meta-analysis. Nitric Oxide 2024, 145, 8–20. [Google Scholar] [CrossRef]
- Piñar-Morales, R.; Durán, R.; Bautista-García, A.; García-Mansilla, M.J.; Aliaga-Gaspar, P.; Vives-Montero, F.; Barrero-Hernández, F.J. The impact of oxidative stress on symptoms associated with multiple sclerosis. Sci. Rep. 2025, 15, 22983. [Google Scholar] [CrossRef]
- Lulu, S.; Waubant, E. Humoral-targeted immunotherapies in multiple sclerosis. Neurotherapeutics 2013, 10, 34–43. [Google Scholar] [CrossRef]
- Akay, L.A.; Effenberger, A.H.; Tsai, L.H. Cell of all trades: Oligodendrocyte precursor cells in synaptic, vascular, and immune function. Genes. Dev. 2021, 35, 180–198. [Google Scholar] [CrossRef]
- Tepavčević, V.; Lubetzki, C. Oligodendrocyte progenitor cell recruitment and remyelination in multiple sclerosis: The more, the merrier? Brain 2022, 145, 4178–4192. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shams, H.; Didonna, A.; Baranzini, S.E.; Cree, B.A.C.; Hauser, S.L.; Henry, R.G.; Oksenberg, J.R. Integration of epigenetic and genetic profiles identifies multiple sclerosis disease-critical cell types and genes. Commun. Biol. 2023, 6, 342. [Google Scholar] [CrossRef] [PubMed]
- Koutsoudaki, P.N.; Papadopoulos, D.; Passias, P.G.; Koutsoudaki, P.; Gorgoulis, V.G. Cellular senescence and failure of myelin repair in multiple sclerosis. Mech. Ageing Dev. 2020, 192, 111366. [Google Scholar] [CrossRef]
- Jäkel, S.; Agirre, E.; Mendanha Falcão, A.; van Bruggen, D.; Lee, K.W.; Knuesel, I.; Malhotra, D.; Ffrench-Constant, C.; Williams, A.; Castelo-Branco, G. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 2019, 566, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Zveik, O.; Rechtman, A.; Ganz, T.; Vaknin-Dembinsky, A. The interplay of inflammation and remyelination: Rethinking MS treatment with a focus on oligodendrocyte progenitor cells. Mol. Neurodegener. 2024, 19, 53. [Google Scholar] [CrossRef]
- You, Y.; Gupta, V. The Extracellular Matrix and Remyelination Strategies in Multiple Sclerosis. eNeuro 2018, 5, e0435-17.2018. [Google Scholar] [CrossRef]
- Gaesser, J.M.; Fyffe-Maricich, S.L. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol. 2016, 283, 501–511. [Google Scholar] [CrossRef]
- Simkins, T.J.; Duncan, G.J.; Bourdette, D. Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications. Curr. Neurol. Neurosci. Rep. 2021, 21, 26. [Google Scholar] [CrossRef]
- Looser, Z.J.; Faik, Z.; Ravotto, L.; Zanker, H.S.; Jung, R.B.; Werner, H.B.; Ruhwedel, T.; Möbius, W.; Bergles, D.E.; Barros, L.F.; et al. Oligodendrocyte-axon metabolic coupling is mediated by extracellular K+ and maintains axonal health. Nat. Neurosci. 2024, 27, 433–448. [Google Scholar] [CrossRef]
- Li, S.; Sheng, Z.H. Oligodendrocyte-derived transcellular signaling regulates axonal energy metabolism. Curr. Opin. Neurobiol. 2023, 80, 102722. [Google Scholar] [CrossRef]
- Schäffner, E.; Bosch-Queralt, M.; Edgar, J.M.; Lehning, M.; Strauß, J.; Fleischer, N.; Kungl, T.; Wieghofer, P.; Berghoff, S.A.; Reinert, T.; et al. Myelin insulation as a risk factor for axonal degeneration in autoimmune demyelinating disease. Nat. Neurosci. 2023, 26, 1218–1228. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Xue, T.; Tan, X.; Li, J.; Chen, Z.; Wang, Z. Disease-modifying therapy in progressive multiple sclerosis: A systematic review and network meta-analysis of randomized controlled trials. Front. Neurol. 2024, 15, 1295770. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.Q.; Yong, V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017, 16, 617–634. [Google Scholar] [CrossRef]
- Sharma, T.; Mehan, S.; Tiwari, A.; Khan, Z.; Gupta, G.D.; Narula, A.S. Targeting Oligodendrocyte Dynamics and Remyelination: Emerging Therapies and Personalized Approaches in Multiple Sclerosis Management. Curr. Neurovasc. Res. 2025, 21, 359–417. [Google Scholar] [CrossRef] [PubMed]
- Manousi, A.; Göttle, P.; Reiche, L.; Cui, Q.L.; Healy, L.M.; Akkermann, R.; Gruchot, J.; Schira-Heinen, J.; Antel, J.P.; Hartung, H.P.; et al. Identification of novel myelin repair drugs by modulation of oligodendroglial differentiation competence. eBioMedicine 2021, 65, 103276. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodríguez, E.M.; Bribián, A.; Boyd, A.; Palomo, V.; Pastor, J.; Lagares, A.; Gil, C.; Martínez, A.; Williams, A.; de Castro, F. Promoting in vivo remyelination with small molecules: A neuroreparative pharmacological treatment for Multiple Sclerosis. Sci. Rep. 2017, 7, 43545. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.V.; Petkou, E.; Atzemoglou, N.; Gkorla, E.; Karamitrou, A.; Simos, Y.V.; Bellos, S.; Bekiari, C.; Kouklis, P.; Konitsiotis, S.; et al. Cell replacement therapy with stem cells in multiple sclerosis, a systematic review. Hum. Cell 2024, 37, 9–53. [Google Scholar] [CrossRef]
- Paroni, M.; Maltese, V.; De Simone, M.; Ranzani, V.; Larghi, P.; Fenoglio, C.; Pietroboni, A.M.; De Riz, M.A.; Crosti, M.C.; Maglie, S.; et al. Recognition of viral and self-antigens by TH1 and TH1/TH17 central memory cells in patients with multiple sclerosis reveals distinct roles in immune surveillance and relapses. J. Allergy Clin. Immunol. 2017, 140, 797–808. [Google Scholar] [CrossRef]
- Lai, S.; Wu, X.; Liu, Y.; Liu, B.; Wu, H.; Ma, K. Interaction between Th17 and central nervous system in multiple sclerosis. Brain Behav. Immun. Health 2024, 43, 100928. [Google Scholar] [CrossRef]
- Chastain, E.M.; Duncan, D.S.; Rodgers, J.M.; Miller, S.D. The role of antigen presenting cells in multiple sclerosis. Biochim. Biophys. Acta 2011, 1812, 265–274. [Google Scholar] [CrossRef]
- Sonar, S.A.; Lal, G. Differentiation and Transmigration of CD4 T Cells in Neuroinflammation and Autoimmunity. Front. Immunol. 2017, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Yang, X.; Hu, H.; Liu, P.; Liu, H. Crosstalk between dendritic cells and regulatory T cells: Protective effect and therapeutic potential in multiple sclerosis. Front. Immunol. 2022, 13, 970508. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Ransohoff, R.M. The CD4-Th1 model for multiple sclerosis: A critical re-appraisal. Trends Immunol. 2004, 25, 132–137. [Google Scholar] [CrossRef]
- Catenacci, R.B.; Galleguillos, D.; Rhodes, A.; Phillips, S.; Calabresi, P.A. MHC class I and II expression and induction in oligodendrocytes varies with age. Sci. Rep. 2025, 15, 18309. [Google Scholar] [CrossRef]
- Moser, T.; Akgün, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef]
- Milovanovic, J.; Arsenijevic, A.; Stojanovic, B.; Kanjevac, T.; Arsenijevic, D.; Radosavljevic, G.; Milovanovic, M.; Arsenijevic, N. Interleukin-17 in Chronic Inflammatory Neurological Diseases. Front. Immunol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Setiadi, A.F.; Abbas, A.R.; Jeet, S.; Wong, K.; Bischof, A.; Peng, I.; Lee, J.; Bremer, M.; Eggers, E.L.; DeVoss, J.; et al. IL-17A is associated with the breakdown of the blood-brain barrier in relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2019, 332, 147–154. [Google Scholar] [CrossRef]
- Behrens, M.; Comabella, M.; Lünemann, J.D. EBV-specific T-cell immunity: Relevance for multiple sclerosis. Front. Immunol. 2024, 15, 1509927. [Google Scholar] [CrossRef]
- Thomas, O.G.; Olsson, T. Mimicking the brain: Epstein-Barr virus and foreign agents as drivers of neuroimmune attack in multiple sclerosis. Front. Immunol. 2023, 14, 1304281. [Google Scholar] [CrossRef]
- Comi, G.; Bar-Or, A.; Lassmann, H.; Uccelli, A.; Hartung, H.P.; Montalban, X.; Sørensen, P.S.; Hohlfeld, R.; Hauser, S.L. Expert Panel of the 27th Annual Meeting of the European Charcot Foundation. Role of B Cells in Multiple Sclerosis and Related Disorders. Ann. Neurol. 2021, 89, 13–23. [Google Scholar] [CrossRef]
- Wekerle, H. B cells in multiple sclerosis. Autoimmunity 2017, 50, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.M. Impact of B cells to the pathophysiology of multiple sclerosis. J. Neuroinflamm. 2019, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Negron, A.; Stüve, O.; Forsthuber, T.G. Ectopic Lymphoid Follicles in Multiple Sclerosis: Centers for Disease Control? Front. Neurol. 2020, 11, 607766. [Google Scholar] [CrossRef] [PubMed]
- Bonnan, M. Intrathecal IgG synthesis: A resistant and valuable target for future multiple sclerosis treatments. Mult. Scler. Int. 2015, 2015, 296184. [Google Scholar] [CrossRef]
- Saez-Calveras, N.; Stuve, O. The role of the complement system in Multiple Sclerosis: A review. Front. Immunol. 2022, 13, 970486. [Google Scholar] [CrossRef]
- Ingram, G.; Loveless, S.; Howell, O.W.; Hakobyan, S.; Dancey, B.; Harris, C.L.; Robertson, N.P.; Neal, J.W.; Morgan, B.P. Complement activation in multiple sclerosis plaques: An immunohistochemical analysis. Acta Neuropathol. Commun. 2014, 2, 53. [Google Scholar] [CrossRef]
- Morille, J.; Mandon, M.; Rodriguez, S.; Roulois, D.; Leonard, S.; Garcia, A.; Wiertlewski, S.; Le Page, E.; Berthelot, L.; Nicot, A.; et al. Multiple Sclerosis CSF Is Enriched With Follicular T Cells Displaying a Th1/Eomes Signature. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, 200033. [Google Scholar] [CrossRef]
- Jones, B.E.; Maerz, M.D.; Buckner, J.H. IL-6: A cytokine at the crossroads of autoimmunity. Curr. Opin. Immunol. 2018, 55, 9–14. [Google Scholar] [CrossRef]
- Varghese, J.F.; Kaskow, B.J.; von Glehn, F.; Case, J.; Li, Z.; Julé, A.M.; Berdan, E.; Ho Sui, S.J.; Hu, Y.; Krishnan, R.; et al. Human regulatory memory B cells defined by expression of TIM-1 and TIGIT are dysfunctional in multiple sclerosis. Front. Immunol. 2024, 15, 1360219. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef] [PubMed]
- Cannella, B.; Raine, C.S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 1995, 37, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Elovaara, I.; Ukkonen, M.; Leppäkynnäs, M.; Lehtimäki, T.; Luomala, M.; Peltola, J.; Dastidar, P. Adhesion molecules in multiple sclerosis: Relation to subtypes of disease and methylprednisolone therapy. Arch. Neurol. 2000, 57, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Schwab, N.; Schneider-Hohendorf, T.; Wiendl, H. Therapeutic uses of anti-α4-integrin (anti-VLA-4) antibodies in multiple sclerosis. Int. Immunol. 2015, 27, 47–53. [Google Scholar] [CrossRef]
- Bö, L.; Peterson, J.W.; Mørk, S.; Hoffman, P.A.; Gallatin, W.M.; Ransohoff, R.M.; Trapp, B.D. Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the beta 2 integrin LFA-1 in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 1996, 55, 1060–1072. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef]
- Larochelle, C.; Alvarez, J.I.; Prat, A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011, 585, 3770–3780. [Google Scholar] [CrossRef]
- Cui, L.Y.; Chu, S.F.; Chen, N.H. The role of chemokines and chemokine receptors in multiple sclerosis. Int. Immunopharmacol. 2020, 83, 106314. [Google Scholar] [CrossRef]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef]
- Tietz, S.; Périnat, T.; Greene, G.; Enzmann, G.; Deutsch, U.; Adams, R.; Imhof, B.; Aurrand-Lions, M.; Engelhardt, B. Lack of junctional adhesion molecule (JAM)-B ameliorates experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2018, 73, 3–20. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood-Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, H.; Guan, Y. Glia Connect Inflammation and Neurodegeneration in Multiple Sclerosis. Neurosci. Bull. 2023, 39, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, F.; Sun, M.; Wu, N.; Liu, B.; Yi, X.; Ge, R.; Fan, X. Microglia in the context of multiple sclerosis. Front. Neurol. 2023, 14, 1157287. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh Manjili, F.; Yousefi-Ahmadipour, A.; Kazemi Arababadi, M. The roles played by TLR4 in the pathogenesis of multiple sclerosis; A systematic review article. Immunol. Lett. 2020, 220, 63–70. [Google Scholar] [CrossRef]
- Fan, H.; Fu, Q.; Du, G.; Qin, L.; Shi, X.; Wang, D.; Yang, Y. Microglial Mayhem NLRP3 Inflammasome’s Role in Multiple Sclerosis Pathology. CNS Neurosci. Ther. 2024, 30, 70135. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.; Chu, F.; Zhu, J.; Jin, T. Inflammatory Role of TLR-MyD88 Signaling in Multiple Sclerosis. Front. Mol. Neurosci. 2020, 12, 314. [Google Scholar] [CrossRef]
- Timmerman, R.; Zuiderwijk-Sick, E.A.; Bajramovic, J.J. P2Y6 receptor-mediated signaling amplifies TLR-induced pro-inflammatory responses in microglia. Front. Immunol. 2022, 13, 967951. [Google Scholar] [CrossRef]
- di Penta, A.; Moreno, B.; Reix, S.; Fernandez-Diez, B.; Villanueva, M.; Errea, O.; Escala, N.; Vandenbroeck, K.; Comella, J.X.; Villoslada, P. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS ONE 2013, 8, 54722. [Google Scholar] [CrossRef]
- Smith, A.N.; Shaughness, M.; Collier, S.; Hopkins, D.; Byrnes, K.R. Therapeutic targeting of microglia mediated oxidative stress after neurotrauma. Front. Med. 2022, 9, 1034692. [Google Scholar] [CrossRef]
- Edison, P. Astroglial activation: Current concepts and future directions. Alzheimers Dement. 2024, 20, 3034–3053. [Google Scholar]
- García-Domínguez, M. Relationship of S100 Proteins with Neuroinflammation. Biomolecules 2025, 15, 1125. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Hua, F.; Zhang, L.; Lin, Y.; Fang, P.; Chen, S.; Ying, J.; Wang, X. Dual roles of interleukin-33 in cognitive function by regulating central nervous system inflammation. J. Transl. Med. 2022, 20, 369. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Karus, M.; Faissner, A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front. Pharmacol. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.R.; Gardner, R.T.; Jin, H.; Staffenson, M.A.; Rueb, N.J.; Barrios, A.M.; Dudley, G.B.; Cohen, M.S.; Habecker, B.A. Small Molecules Targeting PTPσ-Trk Interactions Promote Sympathetic Nerve Regeneration. ACS Chem. Neurosci. 2022, 13, 688–699. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Alizadeh, A.; Shahsavani, N.; Chopek, J.; Ahlfors, J.E.; Karimi-Abdolrezaee, S. Suppressing CSPG/LAR/PTPσ Axis Facilitates Neuronal Replacement and Synaptogenesis by Human Neural Precursor Grafts and Improves Recovery after Spinal Cord Injury. J. Neurosci. 2022, 42, 3096–3121. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar]
- Miron, V.E.; Franklin, R.J. Macrophages and CNS remyelination. J. Neurochem. 2014, 130, 165–171. [Google Scholar] [CrossRef]
- Lei, Z.; Lin, W. Mechanisms Governing Oligodendrocyte Viability in Multiple Sclerosis and Its Animal Models. Cells 2024, 13, 116. [Google Scholar] [CrossRef]
- Denic, A.; Wootla, B.; Rodriguez, M. CD8+ T cells in multiple sclerosis. Expert. Opin. Ther. Targets 2013, 17, 1053–1066. [Google Scholar] [CrossRef]
- Saxena, A.; Bauer, J.; Scheikl, T.; Zappulla, J.; Audebert, M.; Desbois, S.; Waisman, A.; Lassmann, H.; Liblau, R.S.; Mars, L.T. Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J. Immunol. 2008, 181, 1617–1621. [Google Scholar] [CrossRef]
- Traka, M.; Podojil, J.R.; McCarthy, D.P.; Miller, S.D.; Popko, B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat. Neurosci. 2016, 19, 65–74. [Google Scholar] [CrossRef]
- Caprariello, A.V.; Mangla, S.; Miller, R.H.; Selkirk, S.M. Apoptosis of oligodendrocytes in the central nervous system results in rapid focal demyelination. Ann. Neurol. 2012, 72, 395–405. [Google Scholar] [CrossRef]
- Prineas, J.W.; Parratt, J.D. Oligodendrocytes and the early multiple sclerosis lesion. Ann. Neurol. 2012, 72, 18–31. [Google Scholar] [CrossRef]
- Caprariello, A.V.; Batt, C.E.; Zippe, I.; Romito-DiGiacomo, R.R.; Karl, M.; Miller, R.H. Apoptosis of Oligodendrocytes during Early Development Delays Myelination and Impairs Subsequent Responses to Demyelination. J. Neurosci. 2015, 35, 14031–14041. [Google Scholar] [CrossRef] [PubMed]
- Hövelmeyer, N.; Hao, Z.; Kranidioti, K.; Kassiotis, G.; Buch, T.; Frommer, F.; von Hoch, L.; Kramer, D.; Minichiello, L.; Kollias, G.; et al. Apoptosis of oligodendrocytes via Fas and TNF-R1 is a key event in the induction of experimental autoimmune encephalomyelitis. J. Immunol. 2005, 175, 5875–5884. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, A.; Matysiak, M.; Tybor, K.; Kilianek, L.; Raine, C.S.; Selmaj, K. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain 2005, 128, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Seil, F.J. Myelin Antigens and Antimyelin Antibodies. Antibodies 2018, 7, 2. [Google Scholar] [CrossRef]
- Eliseeva, D.D.; Zakharova, M.N. Myelin Oligodendrocyte Glycoprotein as an Autoantigen in Inflammatory Demyelinating Diseases of the Central Nervous System. Biochemistry 2023, 88, 551–563. [Google Scholar] [CrossRef]
- Hedegaard, C.J.; Chen, N.; Sellebjerg, F.; Sørensen, P.S.; Leslie, R.G.; Bendtzen, K.; Nielsen, C.H. Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: A role in regulating cytokine responses to MBP. Immunology 2009, 128, e451–e461. [Google Scholar] [CrossRef]
- Bittner, S.; Meuth, S.G. Targeting ion channels for the treatment of autoimmune neuroinflammation. Ther. Adv. Neurol. Disord. 2013, 6, 322–336. [Google Scholar] [CrossRef]
- Nicaise, C.; Marneffe, C.; Bouchat, J.; Gilloteaux, J. Osmotic Demyelination: From an Oligodendrocyte to an Astrocyte Perspective. Int. J. Mol. Sci. 2019, 20, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Ito, Y.; Najafov, A.; Zhang, Y.; Shan, B.; DeWitt, J.P.; Ye, J.; Zhang, X.; Chang, A.; Vakifahmetoglu-Norberg, H.; et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015, 10, 1836–1849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, Y.; Liu, S.; Li, C.; Dong, J.; Kong, X.; Ji, X.; Cheng, X.; Zhang, L. RIPK3 signaling and its role in regulated cell death and diseases. Cell Death Discov. 2024, 10, 200. [Google Scholar] [CrossRef]
- Xiao, M.; Gao, G.; Mu, J.; Sun, Q.; Zhao, Y.; Fan, X. MLKL Modulates Necroptosis and Neuroinflammation in a Mouse Model of MS. Inflammation 2025, 48. [Google Scholar] [CrossRef]
- Faergeman, S.L.; Evans, H.; Attfield, K.E.; Desel, C.; Kuttikkatte, S.B.; Sommerlund, M.; Jensen, L.T.; Frokiaer, J.; Friese, M.A.; Matthews, P.M.; et al. A novel neurodegenerative spectrum disorder in patients with MLKL deficiency. Cell Death Dis. 2020, 11, 303. [Google Scholar] [CrossRef]
- Huntemer-Silveira, A.; Patil, N.; Brickner, M.A.; Parr, A.M. Strategies for Oligodendrocyte and Myelin Repair in Traumatic CNS Injury. Front. Cell Neurosci. 2021, 14, 619707. [Google Scholar] [CrossRef]
- Duncan, I.D.; Radcliff, A.B.; Heidari, M.; Kidd, G.; August, B.K.; Wierenga, L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA 2018, 115, 11807–11816. [Google Scholar] [CrossRef]
- Mezydlo, A.; Treiber, N.; Ullrich Gavilanes, E.M.; Eichenseer, K.; Ancău, M.; Wens, A.; Ares Carral, C.; Schifferer, M.; Snaidero, N.; Misgeld, T.; et al. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron 2023, 111, 1748–1759.e8. [Google Scholar] [CrossRef]
- Gharagozloo, M.; Bannon, R.; Calabresi, P.A. Breaking the barriers to remyelination in multiple sclerosis. Curr. Opin. Pharmacol. 2022, 63, 102194. [Google Scholar] [CrossRef] [PubMed]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Emery, B.; Lu, Q.R. Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb. Perspect. Biol. 2015, 7, 020461. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Casaccia, P. Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. GLIA 2015, 63, 1357–1375. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Chen, Y.; Hoang, T.; Montgomery, R.L.; Zhao, X.H.; Bu, H.; Hu, T.; Taketo, M.M.; van Es, J.H.; Clevers, H.; et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 2009, 12, 829–838. [Google Scholar] [CrossRef]
- Castro, K.; Casaccia, P. Epigenetic modifications in brain and immune cells of multiple sclerosis patients. Mult. Scler. 2018, 24, 69–74. [Google Scholar] [CrossRef]
- John, G.R.; Shankar, S.L.; Shafit-Zagardo, B.; Massimi, A.; Lee, S.C.; Raine, C.S.; Brosnan, C.F. Multiple sclerosis: Re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat. Med. 2002, 8, 1115–1121. [Google Scholar] [CrossRef]
- Allan, K.C.; Miller, T.E.; Morton, A.R.; Scavuzzo, M.A.; Elitt, M.S.; Clayton, B.L.L.; Hu, L.R.; Vrabic, J.K.; Olsen, H.E.; Factor, D.C.; et al. Cellular Maturation of Oligodendrocytes is Governed by Transient Gene Melting. bioRxiv 2022. [Google Scholar] [CrossRef]
- Dai, Z.M.; Sun, S.; Wang, C.; Huang, H.; Hu, X.; Zhang, Z.; Lu, Q.R.; Qiu, M. Stage-specific regulation of oligodendrocyte development by Wnt/β-catenin signaling. J. Neurosci. 2014, 34, 8467–8473. [Google Scholar] [CrossRef]
- Hammond, E.; Lang, J.; Maeda, Y.; Pleasure, D.; Angus-Hill, M.; Xu, J.; Horiuchi, M.; Deng, W.; Guo, F. The Wnt effector transcription factor 7-like 2 positively regulates oligodendrocyte differentiation in a manner independent of Wnt/β-catenin signaling. J. Neurosci. 2015, 35, 5007–5022. [Google Scholar] [CrossRef]
- Yu, Y.; Casaccia, P.; Lu, Q.R. Shaping the oligodendrocyte identity by epigenetic control. Epigenetics 2010, 5, 124–128. [Google Scholar] [CrossRef]
- Juryńczyk, M.; Selmaj, K. Notch: A new player in MS mechanisms. J. Neuroimmunol. 2010, 218, 3–11. [Google Scholar] [CrossRef]
- Wu, M.; Hernandez, M.; Shen, S.; Sabo, J.K.; Kelkar, D.; Wang, J.; O’Leary, R.; Phillips, G.R.; Cate, H.S.; Casaccia, P. Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation. J. Neurosci. 2012, 32, 6651–6664. [Google Scholar] [CrossRef]
- Haase, S.; Linker, R.A. Inflammation in multiple sclerosis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211007687. [Google Scholar] [CrossRef]
- Itoh, T.; Horiuchi, M.; Itoh, A. Interferon-triggered transcriptional cascades in the oligodendroglial lineage: A comparison of induction of MHC class II antigen between oligodendroglial progenitor cells and mature oligodendrocytes. J. Neuroimmunol. 2009, 212, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Ashbaugh, J.J.; Magliozzi, R.; Dellarole, A.; Karmally, S.; Szymkowski, D.E.; Bethea, J.R. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain 2011, 134, 2736–2754. [Google Scholar] [CrossRef] [PubMed]
- Raasch, J.; Zeller, N.; van Loo, G.; Merkler, D.; Mildner, A.; Erny, D.; Knobeloch, K.P.; Bethea, J.R.; Waisman, A.; Knust, M.; et al. IkappaB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-kappaB in the central nervous system. Brain 2011, 134, 1184–1198. [Google Scholar] [PubMed]
- Horellou, P.; Wang, M.; Keo, V.; Chrétien, P.; Serguera, C.; Waters, P.; Deiva, K. Increased interleukin-6 correlates with myelin oligodendrocyte glycoprotein antibodies in pediatric monophasic demyelinating diseases and multiple sclerosis. J. Neuroimmunol. 2015, 289, 1–7. [Google Scholar] [CrossRef]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef]
- Ghorbani, S.; Yong, V.W. The extracellular matrix as modifier of neuroinflammation and remyelination in multiple sclerosis. Brain 2021, 144, 1958–1973. [Google Scholar] [CrossRef]
- Wei, S.S.; Chen, L.; Yang, F.Y.; Wang, S.Q.; Wang, P. The role of fibronectin in multiple sclerosis and the effect of drug delivery across the blood-brain barrier. Neural Regen. Res. 2023, 18, 2147–2155. [Google Scholar] [CrossRef]
- Back, S.A.; Tuohy, T.M.; Chen, H.; Wallingford, N.; Craig, A.; Struve, J.; Luo, N.L.; Banine, F.; Liu, Y.; Chang, A.; et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005, 11, 966–972. [Google Scholar] [CrossRef]
- Bauch, J.; Faissner, A. The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model. Cells 2022, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- López-Muguruza, E.; Matute, C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Song, S.J.; Tian, M.Y.; Wang, L.B.; Zhang, Y.; Li, X. Myelin debris phagocytosis in demyelinating disease. GLIA 2024, 72, 1934–1954. [Google Scholar] [CrossRef] [PubMed]
- Rosko, L.; Smith, V.N.; Yamazaki, R.; Huang, J.K. Oligodendrocyte Bioenergetics in Health and Disease. Neuroscientist 2019, 25, 334–343. [Google Scholar] [CrossRef]
- Narine, M.; Colognato, H. Current Insights Into Oligodendrocyte Metabolism and Its Power to Sculpt the Myelin Landscape. Front. Cell Neurosci. 2022, 16, 892968. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Bonora, M.; De Marchi, E.; Patergnani, S.; Suski, J.M.; Celsi, F.; Bononi, A.; Giorgi, C.; Marchi, S.; Rimessi, A.; Duszyński, J.; et al. Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 2014, 21, 1198–1208. [Google Scholar] [CrossRef]
- Ziabreva, I.; Campbell, G.; Rist, J.; Zambonin, J.; Rorbach, J.; Wydro, M.M.; Lassmann, H.; Franklin, R.J.; Mahad, D. Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. GLIA 2010, 58, 1827–1837. [Google Scholar] [CrossRef]
- Duncan, G.J.; Simkins, T.J.; Emery, B. Neuron-Oligodendrocyte Interactions in the Structure and Integrity of Axons. Front. Cell Dev. Biol. 2021, 9, 653101. [Google Scholar] [CrossRef]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Baud, O.; Haynes, R.F.; Wang, H.; Folkerth, R.D.; Li, J.; Volpe, J.J.; Rosenberg, P.A. Developmental up-regulation of MnSOD in rat oligodendrocytes confers protection against oxidative injury. Eur. J. Neurosci. 2004, 20, 29–40. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [PubMed]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018, 592, 28–742. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef]
- Campbell, G.; Mahad, D.J. Mitochondrial dysfunction and axon degeneration in progressive multiple sclerosis. FEBS Lett. 2018, 592, 1113–1121. [Google Scholar] [CrossRef]
- van den Berg, R.; Hoogenraad, C.C.; Hintzen, R.Q. Axonal transport deficits in multiple sclerosis: Spiraling into the abyss. Acta Neuropathol. 2017, 134, 1–14. [Google Scholar] [CrossRef]
- Zhai, D.; Yan, S.; Samsom, J.; Wang, L.; Su, P.; Jiang, A.; Zhang, H.; Jia, Z.; Wallach, I.; Heifets, A.; et al. Small-molecule targeting AMPA-mediated excitotoxicity has therapeutic effects in mouse models for multiple sclerosis. Sci. Adv. 2023, 9, 6187. [Google Scholar] [CrossRef]
- Hill, K.E.; Zollinger, L.V.; Watt, H.E.; Carlson, N.G.; Rose, J.W. Inducible nitric oxide synthase in chronic active multiple sclerosis plaques: Distribution, cellular expression and association with myelin damage. J. Neuroimmunol. 2004, 151, 171–179. [Google Scholar] [CrossRef]
- Li, S.; Lin, W.; Tchantchou, F.; Lai, R.; Wen, J.; Zhang, Y. Protein kinase C mediates peroxynitrite toxicity to oligodendrocytes. Mol. Cell. Neurosci. 2011, 48, 62–71. [Google Scholar] [CrossRef]
- Kukanja, P.; Langseth, C.M.; Rubio Rodríguez-Kirby, L.A.; Agirre, E.; Zheng, C.; Raman, A.; Yokota, C.; Avenel, C.; Tiklová, K.; Guerreiro-Cacais, A.O.; et al. Cellular architecture of evolving neuroinflammatory lesions and multiple sclerosis pathology. Cell 2024, 187, 1990–2009.e19. [Google Scholar] [CrossRef]
- Lerma-Martin, C.; Badia-I.-Mompel, P.; Ramirez Flores, R.O.; Sekol, P.; Schäfer, P.S.L.; Riedl, C.J.; Hofmann, A.; Thäwel, T.; Wünnemann, F.; Ibarra-Arellano, M.A.; et al. Cell type mapping reveals tissue niches and interactions in subcortical multiple sclerosis lesions. Nat. Neurosci. 2024, 27, 2354–2365. [Google Scholar] [CrossRef]
- Hendrickx, D.A.E.; van Scheppingen, J.; van der Poel, M.; Bossers, K.; Schuurman, K.G.; van Eden, C.G.; Hol, E.M.; Hamann, J.; Huitinga, I. Gene Expression Profiling of Multiple Sclerosis Pathology Identifies Early Patterns of Demyelination Surrounding Chronic Active Lesions. Front. Immunol. 2017, 8, 1810. [Google Scholar] [CrossRef] [PubMed]
- Riekkinen, P.J.; Palo, J.; Arstila, A.U.; Savolainen, H.J.; Rinne, U.K.; Kivalo, E.K.; Frey, H. Protein composition of multiple sclerosis myelin. Arch. Neurol. 1971, 24, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Raasakka, A.; Kursula, P. Flexible Players within the Sheaths: The Intrinsically Disordered Proteins of Myelin in Health and Disease. Cells 2020, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kunjamma, R.B.; Weiner, M.; Chan, J.R.; Popko, B. Prolonging the integrated stress response enhances CNS remyelination in an inflammatory environment. eLife 2021, 10, e65469. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sayahpour, F.A.; Varzandi, T.; Masoumi, M.R.; Sahraian, M.A. The interplay between endoplasmic reticulum stress and inflammation in multiple sclerosis. Sci. Rep. 2025, 15, 22916. [Google Scholar] [CrossRef]
- Stone, S.; Lin, W. The unfolded protein response in multiple sclerosis. Front. Neurosci. 2015, 9, 264. [Google Scholar] [CrossRef]
- Lin, W. Impaired eIF2B activity in ligodendrocytes contributes to VWMD pathogenesis. Neural Regen. Res. 2015, 10, 195–197. [Google Scholar] [CrossRef]
- Junjappa, R.P.; Patil, P.; Bhattarai, K.R.; Kim, H.R.; Chae, H.J. IRE1α Implications in Endoplasmic Reticulum Stress-Mediated Development and Pathogenesis of Autoimmune Diseases. Front. Immunol. 2018, 9, 1289. [Google Scholar] [CrossRef]
- Stone, S.; Wu, S.; Jamison, S.; Durose, W.; Pallais, J.P.; Lin, W. Activating transcription factor 6α deficiency exacerbates oligodendrocyte death and myelin damage in immune-mediated demyelinating diseases. GLIA 2018, 66, 1331–1345. [Google Scholar] [CrossRef]
- Mháille, A.N.; McQuaid, S.; Windebank, A.; Cunnea, P.; McMahon, J.; Samali, A.; FitzGerald, U. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2008, 67, 200–211. [Google Scholar] [CrossRef]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yu, H.; Ding, S.; Liu, H.; Liu, C.; Fu, R. Molecular mechanism of ATF6 in unfolded protein response and its role in disease. Heliyon 2024, 10, 25937. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Stone, S. Unfolded protein response in myelin disorders. Neural Regen. Res. 2020, 15, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Gopinath, S.; Lakshmi Narasimhan, R. Exploring the Molecular Aspects of Glycosylation in MOG Antibody Disease (MOGAD). Curr. Protein Pept. Sci. 2022, 23, 384–394. [Google Scholar] [CrossRef]
- Feigenson, K.; Reid, M.; See, J.; Crenshaw, E.B.; Grinspan, J.B. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol. Cell. Neurosci. 2009, 42, 255–265. [Google Scholar] [CrossRef]
- Fancy, S.P.; Baranzini, S.E.; Zhao, C.; Yuk, D.I.; Irvine, K.A.; Kaing, S.; Sanai, N.; Franklin, R.J.; Rowitch, D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes. Dev. 2009, 23, 1571–1585. [Google Scholar] [CrossRef]
- Shimizu, T.; Kagawa, T.; Wada, T.; Muroyama, Y.; Takada, S.; Ikenaka, K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev. Biol. 2005, 282, 397–410. [Google Scholar] [CrossRef]
- Huang, S.M.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Fancy, S.P.; Harrington, E.P.; Yuen, T.J.; Silbereis, J.C.; Zhao, C.; Baranzini, S.E.; Bruce, C.C.; Otero, J.J.; Huang, E.J.; Nusse, R.; et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat. Neurosci. 2011, 14, 1009–1016. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Miao, Z.; Xu, X.; Liu, C.F. XAV939, a small molecular inhibitor, provides neuroprotective effects on oligodentrocytes. J. Neurosci. Res. 2014, 92, 1252–1258. [Google Scholar] [CrossRef]
- Shih, Y.; Ly, P.T.T.; Wang, J.; Pallen, C.J. Glial and Neuronal Protein Tyrosine Phosphatase Alpha (PTPα) Regulate Oligodendrocyte Differentiation and Myelination. J. Mol. Neurosci. 2017, 62, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Ding, X.; Liu, Y.; Yang, Q.; Ren, B. Interleukin-1β attenuates the proliferation and differentiation of oligodendrocyte precursor cells through regulation of the microRNA-202-3p/β-catenin/Gli1 axis. Int. J. Mol. Med. 2020, 46, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, S.; Liu, X.; Zuo, Y.Y.; Cui, Y.G.; Wang, F.; Zhang, J.H.; Chang, Y.Z.; Yu, P. Oligodendrocyte-specific knockout of FPN1 affects CNS myelination defects and depression-like behavior in mice. Free Radic. Biol. Med. 2025, 238, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Zahaf, A.; Kassoussi, A.; Sharif, A.; Faure, H.; Traiffort, E.; Ruat, M. Sonic Hedgehog Is an Early Oligodendrocyte Marker During Remyelination. Cells 2024, 13, 1808. [Google Scholar] [CrossRef]
- Cohen, M.; Kicheva, A.; Ribeiro, A.; Blassberg, R.; Page, K.M.; Barnes, C.P.; Briscoe, J. Ptch1 and Gli regulate Shh signalling dynamics via multiple mechanisms. Nat. Commun. 2015, 6, 6709. [Google Scholar] [CrossRef]
- Prajapati, A.; Mehan, S.; Khan, Z.; Chhabra, S.; Das Gupta, G. Purmorphamine, a Smo-Shh/Gli Activator, Promotes Sonic Hedgehog-Mediated Neurogenesis and Restores Behavioural and Neurochemical Deficits in Experimental Model of Multiple Sclerosis. Neurochem. Res. 2024, 49, 1556–1576. [Google Scholar] [CrossRef]
- Namchaiw, P.; Wen, H.; Mayrhofer, F.; Chechneva, O.; Biswas, S.; Deng, W. Temporal and partial inhibition of GLI1 in neural stem cells (NSCs) results in the early maturation of NSC derived oligodendrocytes in vitro. Stem Cell Res. Ther. 2019, 10, 272. [Google Scholar] [CrossRef]
- Porcu, G.; Serone, E.; De Nardis, V.; Di Giandomenico, D.; Lucisano, G.; Scardapane, M.; Poma, A.; Ragnini-Wilson, A. Clobetasol and Halcinonide Act as Smoothened Agonists to Promote Myelin Gene Expression and RxRγ Receptor Activation. PLoS ONE. 2015, 10, 0144550. [Google Scholar] [CrossRef]
- Yao, X.; Su, T.; Verkman, A.S. Clobetasol promotes remyelination in a mouse model of neuromyelitis optica. Acta Neuropathol. Commun. 2016, 4, 42. [Google Scholar] [CrossRef]
- Wang, S.; Sdrulla, A.D.; diSibio, G.; Bush, G.; Nofziger, D.; Hicks, C.; Weinmaster, G.; Barres, B.A. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 1998, 21, 63–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Argaw, A.T.; Gurfein, B.T.; Zameer, A.; Snyder, B.J.; Ge, C.; Lu, Q.R.; Rowitch, D.H.; Raine, C.S.; Brosnan, C.F.; et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl. Acad. Sci. USA 2009, 106, 19162–19167. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Gadea, A.; Dupree, J.; Kerninon, C.; Nait-Oumesmar, B.; Aguirre, A.; Gallo, V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron 2014, 81, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, C.F.; John, G.R. Revisiting Notch in remyelination of multiple sclerosis lesions. J. Clin. Investig. 2009, 119, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.J.; Martin, B.N.; Bulek, K.; Kang, Z.; Zhao, J.; Bian, G.; Carman, J.A.; Gao, J.; Dongre, A.; et al. IL-17 induced NOTCH1 activation in oligodendrocyte progenitor cells enhances proliferation and inflammatory gene expression. Nat. Commun. 2017, 8, 15508. [Google Scholar] [CrossRef]
- Jurynczyk, M.; Jurewicz, A.; Bielecki, B.; Raine, C.S.; Selmaj, K. Inhibition of Notch signaling enhances tissue repair in an animal model of multiple sclerosis. J. Neuroimmunol. 2005, 170, 3–10. [Google Scholar] [CrossRef]
- Fletcher, J.L.; Wood, R.J.; Nguyen, J.; Norman, E.M.L.; Jun, C.M.K.; Prawdiuk, A.R.; Biemond, M.; Nguyen, H.T.H.; Northfield, S.E.; Hughes, R.A.; et al. Targeting TrkB with a Brain-Derived Neurotrophic Factor Mimetic Promotes Myelin Repair in the Brain. J. Neurosci. 2018, 38, 7088–7099. [Google Scholar] [CrossRef]
- Xiao, J. Thirty years of BDNF study in central myelination: From biology to therapy. J. Neurochem. 2023, 167, 321–336. [Google Scholar] [CrossRef]
- Makar, T.K.; Nimmagadda, V.K.; Singh, I.S.; Lam, K.; Mubariz, F.; Judge, S.I.; Trisler, D.; Bever, C.T., Jr. TrkB agonist, 7,8-dihydroxyflavone, reduces the clinical and pathological severity of a murine model of multiple sclerosis. J. Neuroimmunol. 2016, 292, 9–20. [Google Scholar] [CrossRef]
- Al-Samerria, S.; Radovick, S. The Role of Insulin-like Growth Factor-1 (IGF-1) in the Control of Neuroendocrine Regulation of Growth. Cells 2021, 10, 2664. [Google Scholar] [CrossRef]
- Zeger, M.; Popken, G.; Zhang, J.; Xuan, S.; Lu, Q.R.; Schwab, M.H.; Nave, K.A.; Rowitch, D.; D’Ercole, A.J.; Ye, P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. GLIA 2007, 55, 400–411. [Google Scholar] [CrossRef]
- Bibollet-Bahena, O.; Almazan, G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J. Neurochem. 2009, 109, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.L.; Liu, X.; Hudson, L.D.; Webster, H.D. Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 1995, 92, 6190–6194. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; D’Ercole, A.J. Insulin-like growth factor I protects oligodendrocytes from tumor necrosis factor-alpha-induced injury. Endocrinology 1999, 140, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Hlavica, M.; Delparente, A.; Good, A.; Good, N.; Plattner, P.S.; Seyedsadr, M.S.; Schwab, M.E.; Figlewicz, D.P.; Ineichen, B.V. Intrathecal insulin-like growth factor 1 but not insulin enhances myelin repair in young and aged rats. Neurosci. Lett. 2017, 648, 41–46. [Google Scholar] [CrossRef]
- Brinkmann, B.G.; Agarwal, A.; Sereda, M.W.; Garratt, A.N.; Müller, T.; Wende, H.; Stassart, R.M.; Nawaz, S.; Humml, C.; Velanac, V.; et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 2008, 59, 581–595. [Google Scholar]
- Galvez-Contreras, A.Y.; Quiñones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef]
- Mei, L.; Nave, K.A. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 2014, 83, 27–49. [Google Scholar] [CrossRef]
- Xu, C.; Lv, L.; Zheng, G.; Li, B.; Gao, L.; Sun, Y. Neuregulin1β1 protects oligodendrocyte progenitor cells from oxygen glucose deprivation injury induced apoptosis via ErbB4-dependent activation of PI3-kinase/Akt. Brain Res. 2012, 1467, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kataria, H.; Alizadeh, A.; Shahriary, G.M.; Saboktakin Rizi, S.; Henrie, R.; Santhosh, K.T.; Thliveris, J.A.; Karimi-Abdolrezaee, S. Neuregulin-1 promotes remyelination and fosters a pro-regenerative inflammatory response in focal demyelinating lesions of the spinal cord. GLIA 2018, 66, 538–561. [Google Scholar] [CrossRef] [PubMed]
- Gregath, A.; Lu, Q.R. Epigenetic modifications-insight into oligodendrocyte lineage progression, regeneration, and disease. FEBS Lett. 2018, 592, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Tekwani, B.L. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front. Pharmacol. 2020, 11, 537. [Google Scholar] [CrossRef]
- Pazhoohan, S.; Satarian, L.; Asghari, A.A.; Salimi, M.; Kiani, S.; Mani, A.R.; Javan, M. Valproic Acid attenuates disease symptoms and increases endogenous myelin repair by recruiting neural stem cells and oligodendrocyte progenitors in experimental autoimmune encephalomyelitis. Neurodegener. Dis. 2014, 13, 45–52. [Google Scholar] [CrossRef]
- Castelo-Branco, G.; Stridh, P.; Guerreiro-Cacais, A.O.; Adzemovic, M.Z.; Falcão, A.M.; Marta, M.; Berglund, R.; Gillett, A.; Hamza, K.H.; Lassmann, H.; et al. Acute treatment with valproic acid and l-thyroxine ameliorates clinical signs of experimental autoimmune encephalomyelitis and prevents brain pathology in DA rats. Neurobiol. Dis. 2014, 71, 220–233. [Google Scholar] [CrossRef]
- Rossi, M.; Petralla, S.; Protti, M.; Baiula, M.; Kobrlova, T.; Soukup, O.; Spampinato, S.M.; Mercolini, L.; Monti, B.; Bolognesi, M.L. α-Linolenic Acid-Valproic Acid Conjugates: Toward Single-Molecule Polypharmacology for Multiple Sclerosis. ACS Med. Chem. Lett. 2020, 11, 2406–2413. [Google Scholar] [CrossRef]
- Rivera, A.D.; Pieropan, F.; Williams, G.; Calzolari, F.; Butt, A.M.; Azim, K. Drug connectivity mapping and functional analysis reveal therapeutic small molecules that differentially modulate myelination. Biomed. Pharmacother. 2022, 145, 112436. [Google Scholar] [CrossRef]
- Motavaf, M.; Sadeghizadeh, M.; Babashah, S.; Zare, L.; Javan, M. Protective Effects of a Nano-Formulation of Curcumin against Cuprizone-Induced Demyelination in the Mouse Corpus Callosum. Iran. J. Pharm. Res. 2020, 19, 310–320. [Google Scholar]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin promotes oligodendrocyte differentiation and their protection against TNF-α through the activation of the nuclear receptor PPAR-γ. Sci. Rep. 2021, 11, 4952. [Google Scholar] [CrossRef]
- Ren, X.; Yang, Y.; Wang, M.; Yuan, Q.; Suo, N.; Xie, X. Vitamin C and MEK Inhibitor PD0325901 Synergistically Promote Oligodendrocytes Generation by Promoting DNA Demethylation. Molecules 2024, 29, 5939. [Google Scholar] [CrossRef]
- Ngo, C.; Kothary, R. MicroRNAs in oligodendrocyte development and remyelination. J. Neurochem. 2022, 162, 310–321. [Google Scholar] [CrossRef]
- Afrang, N.; Tavakoli, R.; Tasharrofi, N.; Alian, A.; Naderi Sohi, A.; Kabiri, M.; Fathi-Roudsari, M.; Soufizomorrod, M.; Rajaei, F.; Soleimani, M.; et al. A critical role for miR-184 in the fate determination of oligodendrocytes. Stem Cell Res. Ther. 2019, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Perdaens, O.; Bottemanne, P.; van Pesch, V. MicroRNAs dysregulated in multiple sclerosis affect the differentiation of CG-4 cells, an oligodendrocyte progenitor cell line. Front. Cell Neurosci. 2024, 18, 1336439. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, Y.Q.; Guan, Y.T.; Zhang, G.X. Induced Stem Cells as a Novel Multiple Sclerosis Therapy. Curr. Stem Cell Res. Ther. 2016, 11, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Fortune, A.J.; Fletcher, J.L.; Blackburn, N.B.; Young, K.M. Using MS induced pluripotent stem cells to investigate MS aetiology. Mult. Scler. Relat. Disord. 2022, 63, 103839. [Google Scholar] [CrossRef]
- Morales Pantoja, I.E.; Smith, M.D.; Rajbhandari, L.; Cheng, L.; Gao, Y.; Mahairaki, V.; Venkatesan, A.; Calabresi, P.A.; Fitzgerald, K.C.; Whartenby, K.A. iPSCs from people with MS can differentiate into oligodendrocytes in a homeostatic but not an inflammatory milieu. PLoS ONE 2020, 15, 233980. [Google Scholar] [CrossRef]
- Martinez-Curiel, R.; Jansson, L.; Tsupykov, O.; Avaliani, N.; Aretio-Medina, C.; Hidalgo, I.; Monni, E.; Bengzon, J.; Skibo, G.; Lindvall, O.; et al. Oligodendrocytes in human induced pluripotent stem cell-derived cortical grafts remyelinate adult rat and human cortical neurons. Stem Cell Rep. 2023, 18, 1643–1656. [Google Scholar] [CrossRef]
- Sharp, J.; Keirstead, H.S. Therapeutic applications of oligodendrocyte precursors derived from human embryonic stem cells. Curr. Opin. Biotechnol. 2007, 18, 434–440. [Google Scholar] [CrossRef]
- Alsanie, W.F.; Niclis, J.C.; Petratos, S. Human embryonic stem cell-derived oligodendrocytes: Protocols and perspectives. Stem Cells Dev. 2013, 22, 2459–2476. [Google Scholar] [CrossRef]
- Douvaras, P.; Wang, J.; Zimmer, M.; Hanchuk, S.; O’Bara, M.A.; Sadiq, S.; Sim, F.J.; Goldman, J.; Fossati, V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Rep. 2014, 3, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Miyamoto, Y.; Bando, Y.; Ono, T.; Kobayashi, S.; Doi, A.; Araki, T.; Kato, Y.; Shirakawa, T.; Suzuki, Y.; et al. Differentiation of oligodendrocyte progenitor cells from dissociated monolayer and feeder-free cultured pluripotent stem cells. PLoS ONE 2017, 12, 171947. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, T.; Zheng, Z.; Su, Y.; Wu, Z.; Zeng, C.; Yu, G.; Liu, Y.; Wang, X.; Li, H.; et al. Oligodendroglial precursor cells modulate immune response and early demyelination in a murine model of multiple sclerosis. Sci. Transl. Med. 2025, 17, 9980. [Google Scholar] [CrossRef] [PubMed]
- Meco, E.; Lampe, K.J. Microscale Architecture in Biomaterial Scaffolds for Spatial Control of Neural Cell Behavior. Front. Mater. 2018, 5, 2. [Google Scholar] [CrossRef]
- Mazur, R.A.; Lampe, K.J. Guiding Oligodendrocyte Progenitor Cell Maturation Using Electrospun Fiber Cues in a 3D Hyaluronic Acid Hydrogel Culture System. ACS Biomater. Sci. Eng. 2025, 11, 1025–1037. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A Review on the Design of Hydrogels With Different Stiffness and Their Effects on Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, H.; Hui, X. Biomaterial Scaffolds in Regenerative Therapy of the Central Nervous System. Biomed. Res. Int. 2018, 2018, 7848901. [Google Scholar] [CrossRef]
- Lager, A.M.; Corradin, O.G.; Cregg, J.M.; Elitt, M.S.; Shick, H.E.; Clayton, B.L.L.; Allan, K.C.; Olsen, H.E.; Madhavan, M.; Tesar, P.J. Rapid functional genetics of the oligodendrocyte lineage using pluripotent stem cells. Nat. Commun. 2018, 9, 3708. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Bestard-Cuche, N.; Kaczmarek, M.; Fidanza, A.; McNeil, L.; Franklin, R.J.M.; Williams, A.C. CRISPR-edited human ES-derived oligodendrocyte progenitor cells improve remyelination in rodents. Nat. Commun. 2024, 15, 8570. [Google Scholar] [CrossRef]
- Romero, J.C.; Berlinicke, C.; Chow, S.; Duan, Y.; Wang, Y.; Chamling, X.; Smirnova, L. Oligodendrogenesis and myelination tracing in a CRISPR/Cas9-engineered brain microphysiological system. Front. Cell Neurosci. 2023, 16, 1094291. [Google Scholar] [CrossRef]
- Feng, L.; Chao, J.; Ye, P.; Luong, Q.; Sun, G.; Liu, W.; Cui, Q.; Flores, S.; Jackson, N.; Shayento, A.N.H.; et al. Developing Hypoimmunogenic Human iPSC-Derived Oligodendrocyte Progenitor Cells as an Off-The-Shelf Cell Therapy for Myelin Disorders. Adv. Sci. 2023, 10, 2206910. [Google Scholar] [CrossRef]
- Blaszczyk, G.J.; Mohammadnia, A.; Piscopo, V.E.C.; Sirois, J.; Cui, Q.L.; Yaqubi, M.; Durcan, T.M.; Schneider, R.; Antel, J.P. Pro-Inflammatory Molecules Implicated in Multiple Sclerosis Divert the Development of Human Oligodendrocyte Lineage Cells. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, 200407. [Google Scholar] [CrossRef]
- Bernardo, A.; Bianchi, D.; Magnaghi, V.; Minghetti, L. Peroxisome proliferator-activated receptor-gamma agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J. Neuropathol. Exp. Neurol. 2009, 68, 797–808. [Google Scholar] [CrossRef]
- Maier, K.; Merkler, D.; Gerber, J.; Taheri, N.; Kuhnert, A.V.; Williams, S.K.; Neusch, C.; Bähr, M.; Diem, R. Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol. Dis. 2007, 25, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated microglia drive demyelination via CSF1R signaling. GLIA 2021, 69, 1583–1604. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, F.; Barati, S.; Kashani, I.R. Effect of CSF1R inhibitor on glial cells population and remyelination in the cuprizone model. Neuropeptides 2021, 89, 102179. [Google Scholar] [CrossRef] [PubMed]
- Shirvanchi, K.; Gurski, F.; Rajendran, V.; Rajendran, R.; Megalofonou, F.F.; Stadelmann-Nessler, C.; Karnati, S.; Berghoff, M. The Multi-kinase Inhibitor AZD4547 Reduces Inflammation and Neurodegeneration, and Enhances Remyelination in a Mouse Model of Multiple Sclerosis (P7-6.008). Neurology 2024, 102. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, H.; Bu, Z.; Wen, L.; Yan, L.; Feng, J. Focus on the Role of the NLRP3 Inflammasome in Multiple Sclerosis: Pathogenesis, Diagnosis, and Therapeutics. Front. Mol. Neurosci. 2022, 15, 894298. [Google Scholar] [CrossRef]
- Zhang, W.G.; Zheng, X.R.; Yao, Y.; Sun, W.J.; Shao, B.Z. The role of NLRP3 inflammasome in multiple sclerosis: Pathogenesis and pharmacological application. Front. Immunol. 2025, 16, 1572140. [Google Scholar] [CrossRef]
- Hou, B.; Yin, J.; Liu, S.; Guo, J.; Zhang, B.; Zhang, Z.; Yang, L.; Tan, X.; Long, Y.; Feng, S.; et al. Inhibiting the NLRP3 Inflammasome with MCC950 Alleviates Neurological Impairment in the Brain of EAE Mice. Mol. Neurobiol. 2024, 61, 1318–1330. [Google Scholar] [CrossRef]
- Rashidbenam, Z.; Ozturk, E.; Pagnin, M.; Theotokis, P.; Grigoriadis, N.; Petratos, S. How does Nogo receptor influence demyelination and remyelination in the context of multiple sclerosis? Front. Cell Neurosci. 2023, 17, 1197492. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, I.; Papagianni, F.; Theodorakis, K.; Karagogeos, D. Nogo-A and LINGO-1: Two Important Targets for Remyelination and Regeneration. Int. J. Mol. Sci. 2023, 24, 4479. [Google Scholar] [CrossRef] [PubMed]
- Pernet, V.; Joly, S.; Spiegel, S.; Meli, I.; Idriss, S.; Maigler, F.; Mdzomba, J.B.; Roenneke, A.K.; Franceschini, A.; Silvestri, L.; et al. Nogo-A antibody delivery through the olfactory mucosa mitigates experimental autoimmune encephalomyelitis in the mouse CNS. Cell Death Discov. 2023, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Ren, Q.G.; Xu, L.; Zhang, Z.J. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in experimental autoimmune encephalomyelitis mice. Sci. Rep. 2015, 5, 14235. [Google Scholar] [CrossRef]
- Moradbeygi, K.; Parviz, M.; Rezaeizadeh, H.; Zargaran, A.; Sahraian, M.A.; Mehrabadi, S.; Nikbakhtzadeh, M.; Zahedi, E. Anti-LINGO-1 improved remyelination and neurobehavioral deficit in cuprizone-induced demyelination. Iran. J. Basic. Med. Sci. 2021, 24, 900–907. [Google Scholar]
- Aktas, O.; Ziemssen, F.; Ziemssen, T.; Klistorner, A.; Butzkueven, H.; Izquierdo, G.; Leocani, L.; Balcer, L.J.; Galetta, S.L.; Castrillo-Viguera, C.; et al. RENEWED: A follow-up study of the opicinumab phase 2 RENEW study in participants with acute optic neuritis. Mult. Scler. Relat. Disord. 2025, 93, 106185. [Google Scholar]
- Amadio, S.; Conte, F.; Esposito, G.; Fiscon, G.; Paci, P.; Volonté, C. Repurposing Histaminergic Drugs in Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 6347. [Google Scholar] [CrossRef]
- Motawi, T.K.; El-Maraghy, S.A.; Kamel, A.S.; Said, S.E.; Kortam, M.A. Modulation of p38 MAPK and Nrf2/HO-1/NLRP3 inflammasome signaling and pyroptosis outline the anti-neuroinflammatory and remyelinating characters of Clemastine in EAE rat model. Biochem. Pharmacol. 2023, 209, 115435. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Kamel, A.S.; Ahmed, K.A.; Mohammed, R.A.; Essam, R.M. The preferential effect of Clemastine on F3/Contactin-1/Notch-1 compared to Jagged-1/Notch-1 justifies its remyelinating effect in an experimental model of multiple sclerosis in rats. Int. Immunopharmacol. 2024, 128, 111481. [Google Scholar] [CrossRef]
- Cui, Q.L.; Fogle, E.; Almazan, G. Muscarinic acetylcholine receptors mediate oligodendrocyte progenitor survival through Src-like tyrosine kinases and PI3K/Akt pathways. Neurochem. Int. 2006, 48, 383–393. [Google Scholar] [CrossRef]
- Chen, K.; Park, E.; Abd-Elrahman, K.S. Enhancing remyelination in multiple sclerosis via M1 muscarinic acetylcholine receptor. Mol. Pharmacol. 2025, 107, 100027. [Google Scholar] [CrossRef]
- Poon, M.M.; Lorrain, K.I.; Stebbins, K.J.; Edu, G.C.; Broadhead, A.R.; Lorenzana, A.J.; Roppe, J.R.; Baccei, J.M.; Baccei, C.S.; Chen, A.C.; et al. Targeting the muscarinic M1 receptor with a selective, brain-penetrant antagonist to promote remyelination in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2024, 121, 2407974121. [Google Scholar] [CrossRef]
- Poon, M.M.; Lorrain, K.I.; Stebbins, K.J.; Edu, G.C.; Broadhead, A.R.; Lorenzana, A.O.; Paulson, B.E.; Baccei, C.S.; Roppe, J.R.; Schrader, T.O.; et al. Discovery of a brain penetrant small molecule antagonist targeting LPA1 receptors to reduce neuroinflammation and promote remyelination in multiple sclerosis. Sci. Rep. 2024, 14, 10573. [Google Scholar] [CrossRef]
- Hartley, M.D.; Banerji, T.; Tagge, I.J.; Kirkemo, L.L.; Chaudhary, P.; Calkins, E.; Galipeau, D.; Shokat, M.D.; DeBell, M.J.; Van Leuven, S.; et al. Myelin repair stimulated by CNS-selective thyroid hormone action. JCI Insight 2019, 4, 126329. [Google Scholar] [CrossRef] [PubMed]
- Calza, L.; Fernandez, M.; Giuliani, A.; Aloe, L.; Giardino, L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc. Natl. Acad. Sci. USA 2002, 99, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Baldassarro, V.A.; Quadalti, C.; Runfola, M.; Manera, C.; Rapposelli, S.; Calzà, L. Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges. Pharmaceuticals 2023, 16, 1207. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Marracci, G.H.; Calkins, E.; Pocius, E.; Bensen, A.L.; Scanlan, T.S.; Emery, B.; Bourdette, D.N. Thyroid hormone and thyromimetics inhibit myelin and axonal degeneration and oligodendrocyte loss in EAE. J. Neuroimmunol. 2021, 352, 577468. [Google Scholar] [CrossRef]
- Rajabinejad, M.; Asadi, G.; Ranjbar, S.; Afshar Hezarkhani, L.; Salari, F.; Gorgin Karaji, A.; Rezaiemanesh, A. Semaphorin 4A, 4C, and 4D: Function comparison in the autoimmunity, allergy, and cancer. Gene 2020, 746, 144637. [Google Scholar] [CrossRef]
- Smith, E.S.; Jonason, A.; Reilly, C.; Veeraraghavan, J.; Fisher, T.; Doherty, M.; Klimatcheva, E.; Mallow, C.; Cornelius, C.; Leonard, J.E.; et al. SEMA4D compromises blood-brain barrier, activates microglia, and inhibits remyelination in neurodegenerative disease. Neurobiol. Dis. 2015, 73, 254–268. [Google Scholar] [CrossRef]
- Way, S.W.; Podojil, J.R.; Clayton, B.L.; Zaremba, A.; Collins, T.L.; Kunjamma, R.B.; Robinson, A.P.; Brugarolas, P.; Miller, R.H.; Miller, S.D.; et al. Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat. Commun. 2015, 6, 6532. [Google Scholar] [CrossRef]
- Yang, Y.; Suo, N.; Cui, S.H.; Wu, X.; Ren, X.Y.; Liu, Y.; Guo, R.; Xie, X. Trametinib, an anti-tumor drug, promotes oligodendrocytes generation and myelin formation. Acta Pharmacol. Sin. 2024, 45, 2527–2539. [Google Scholar] [CrossRef]
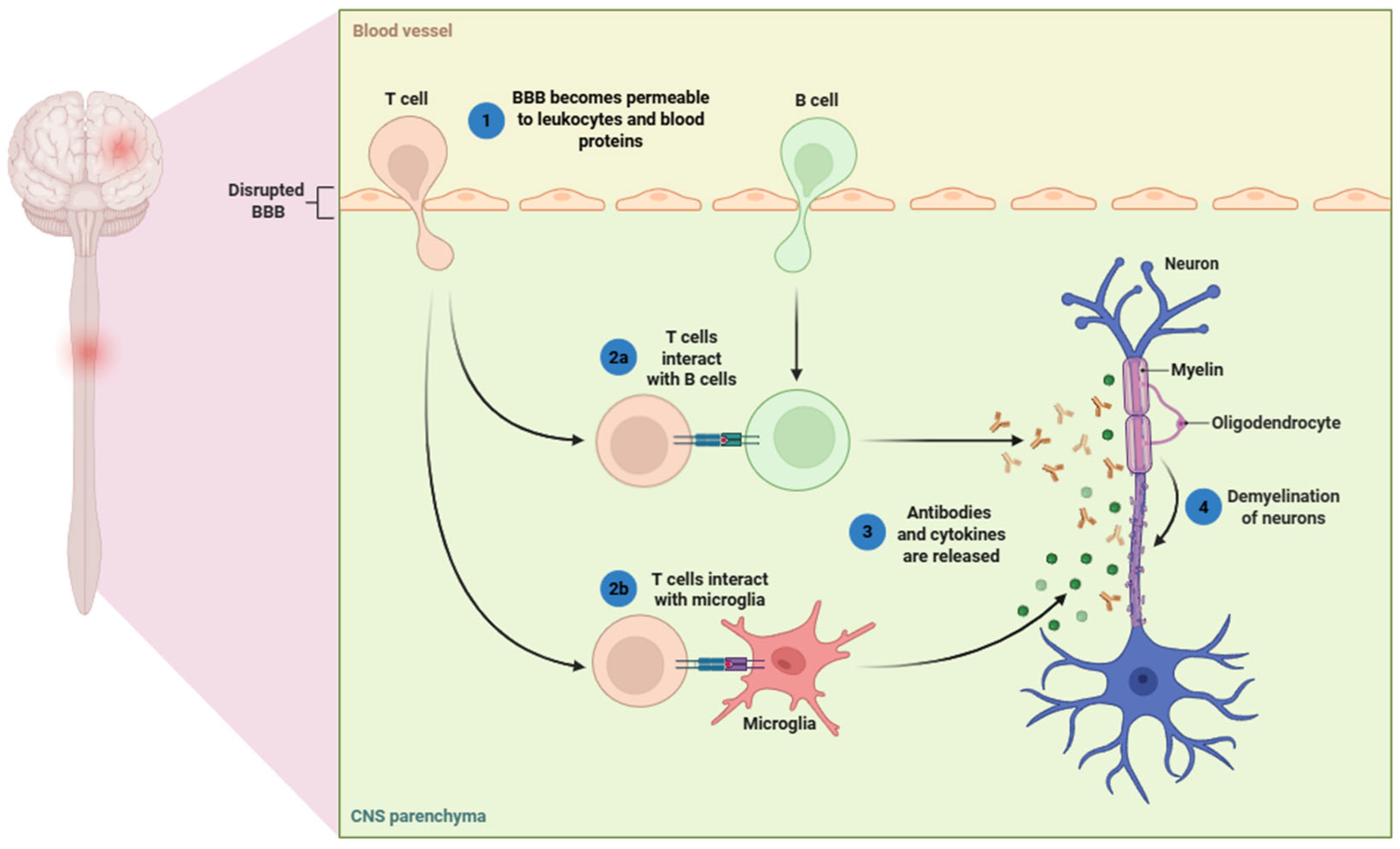

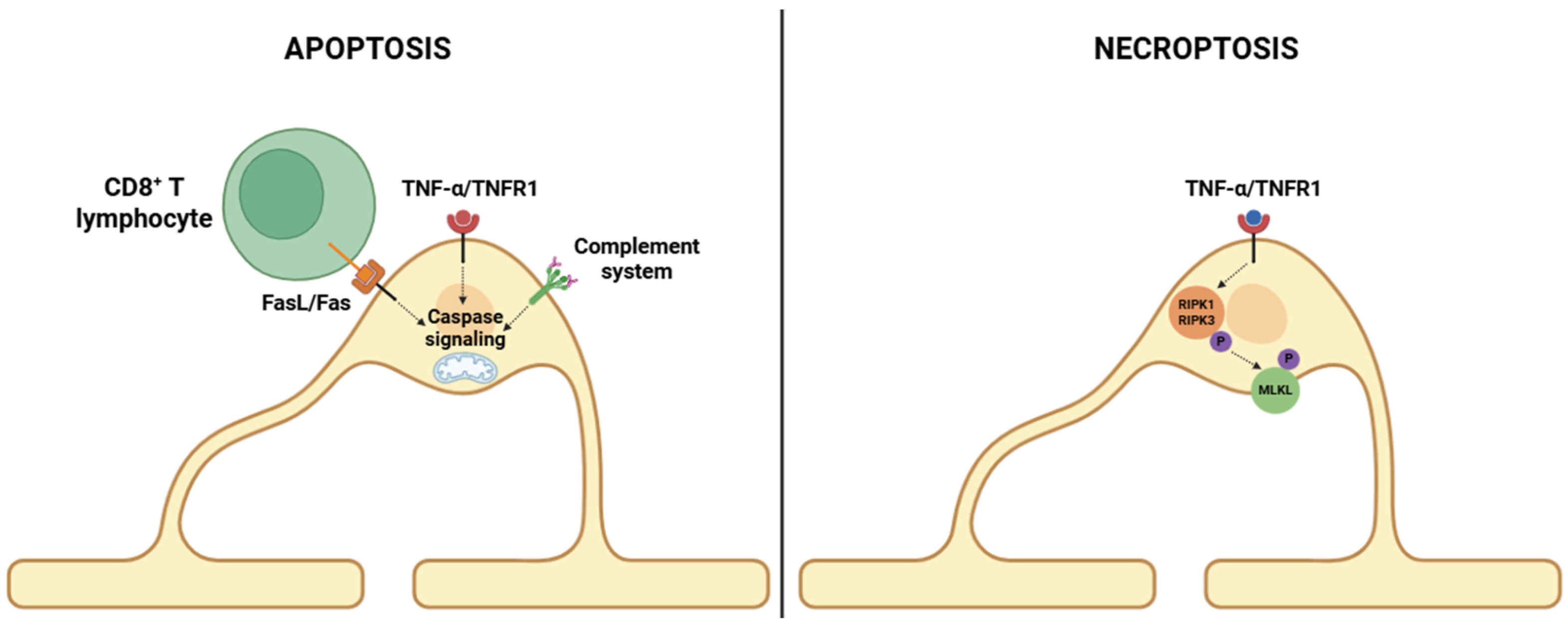
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. White Matter in Crisis: Oligodendrocytes and the Pathophysiology of Multiple Sclerosis. Cells 2025, 14, 1408. https://doi.org/10.3390/cells14181408
García-Domínguez M. White Matter in Crisis: Oligodendrocytes and the Pathophysiology of Multiple Sclerosis. Cells. 2025; 14(18):1408. https://doi.org/10.3390/cells14181408
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "White Matter in Crisis: Oligodendrocytes and the Pathophysiology of Multiple Sclerosis" Cells 14, no. 18: 1408. https://doi.org/10.3390/cells14181408
APA StyleGarcía-Domínguez, M. (2025). White Matter in Crisis: Oligodendrocytes and the Pathophysiology of Multiple Sclerosis. Cells, 14(18), 1408. https://doi.org/10.3390/cells14181408





