Proteomics of Patient-Derived Striatal Medium Spiny Neurons in Multiple System Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Striatal GABAergic Medium Spiny Neuron (MSN) Differentiation
2.2. Immunocytochemistry (ICC)
2.3. Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.4. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
2.5. Proteomics
2.5.1. PANTHER Classification System
2.5.2. Reactome Database
2.5.3. Ingenuity Pathway Analysis (IPA)
2.6. Statistical Analysis
3. Results
3.1. Striatal GABAergic Medium Spiny Neuron (MSN) Differentiation
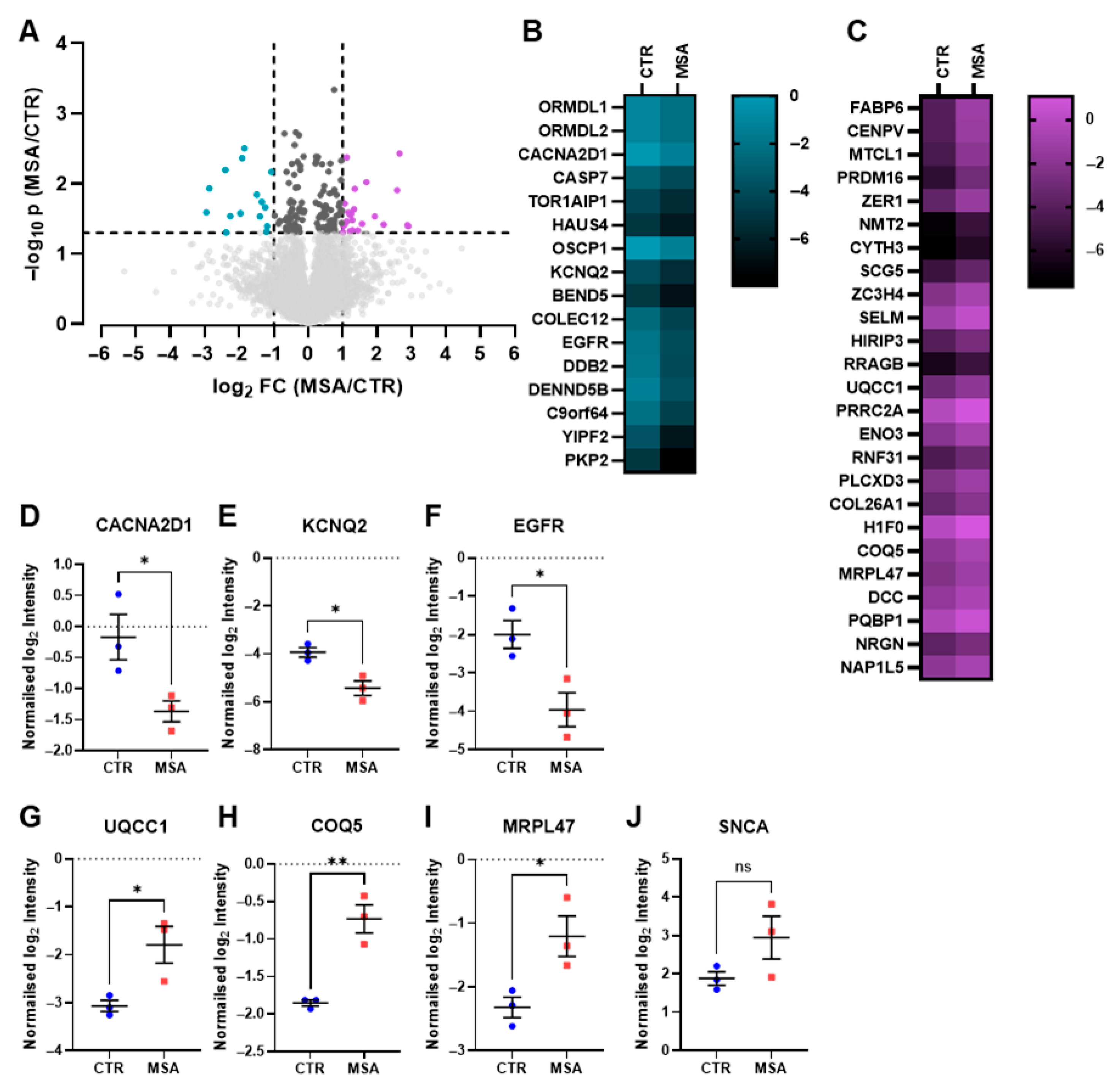
3.2. Proteome Analysis
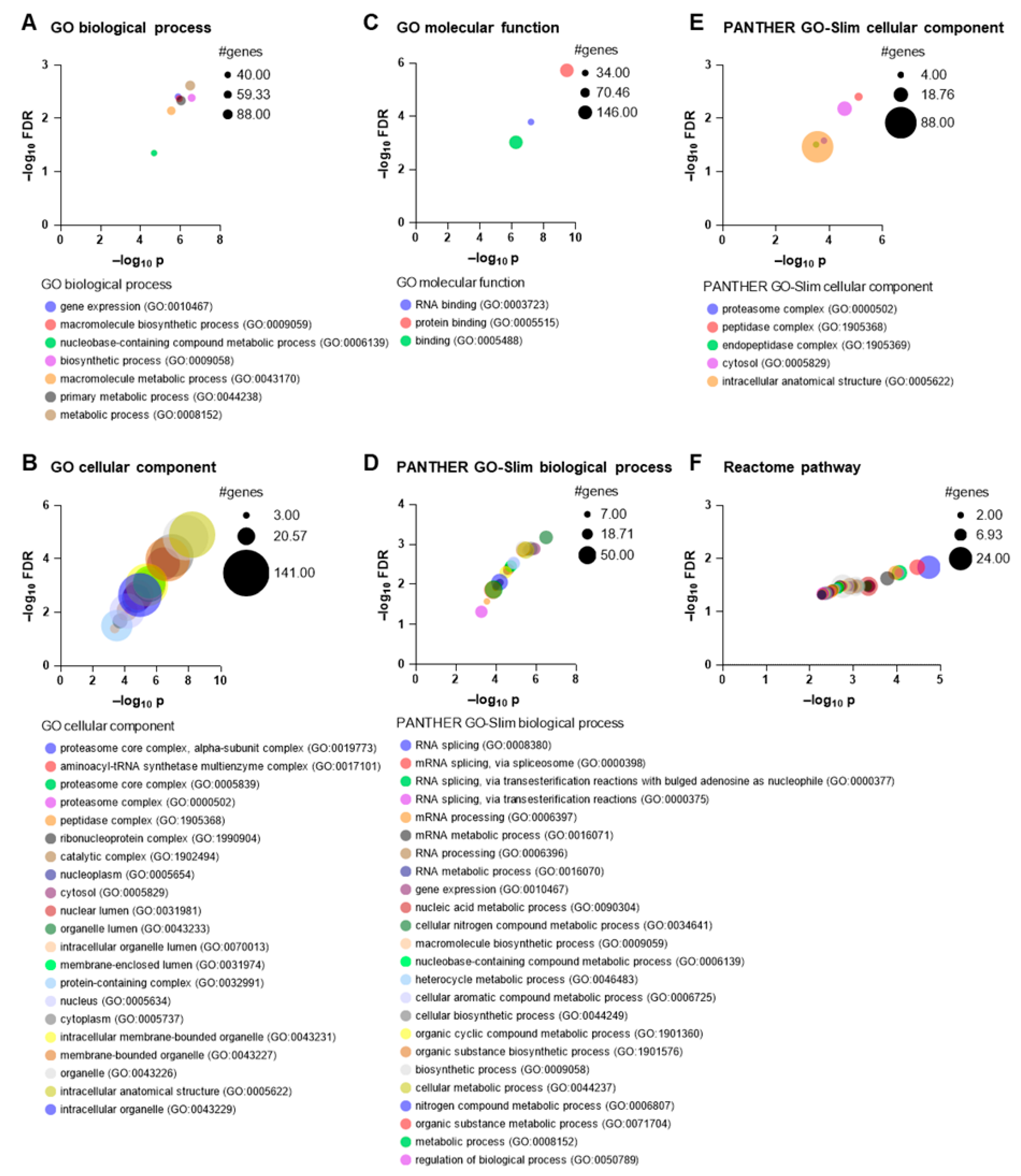
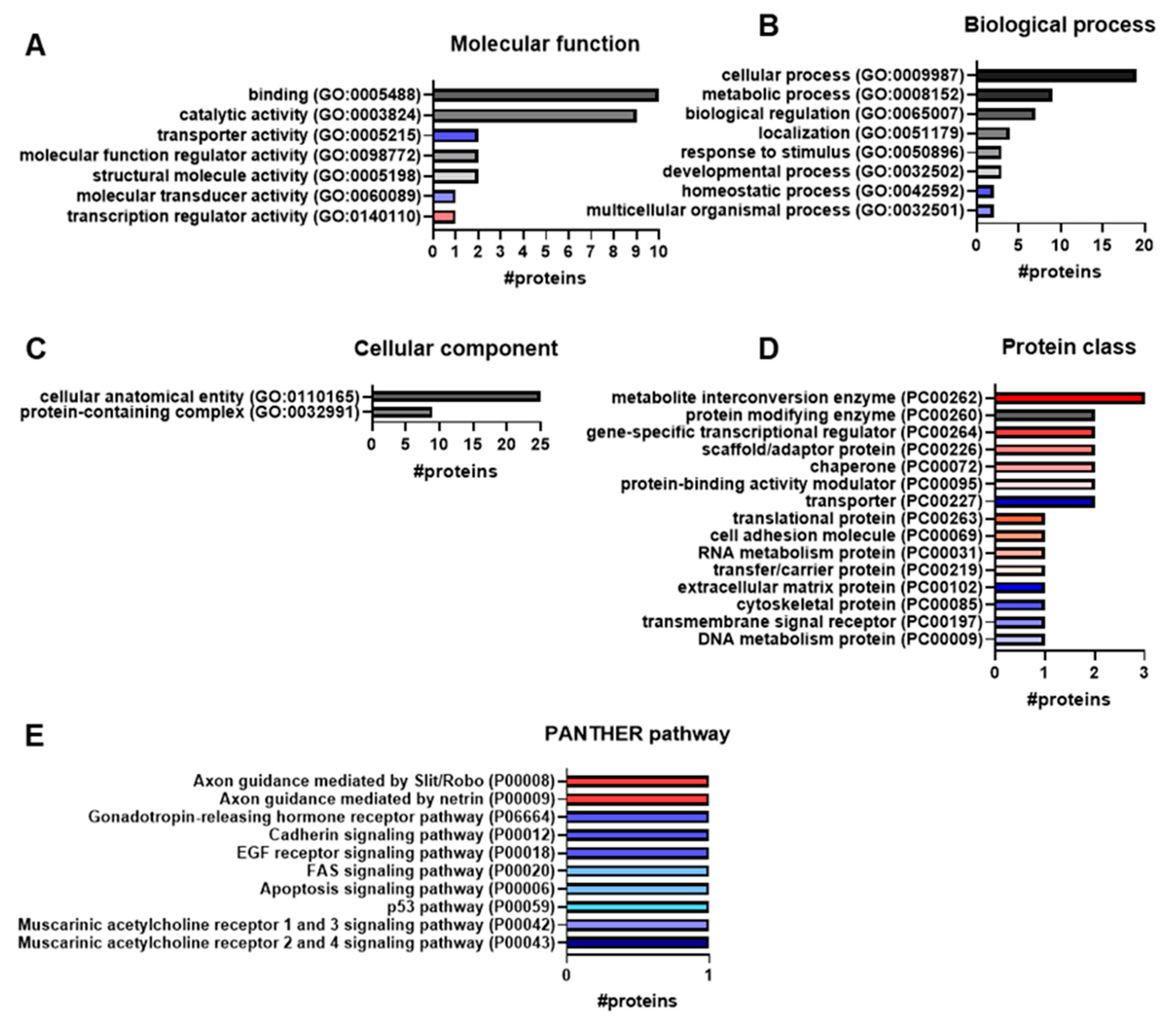
3.3. Gene Ontology Analysis
3.4. Reactome Analysis
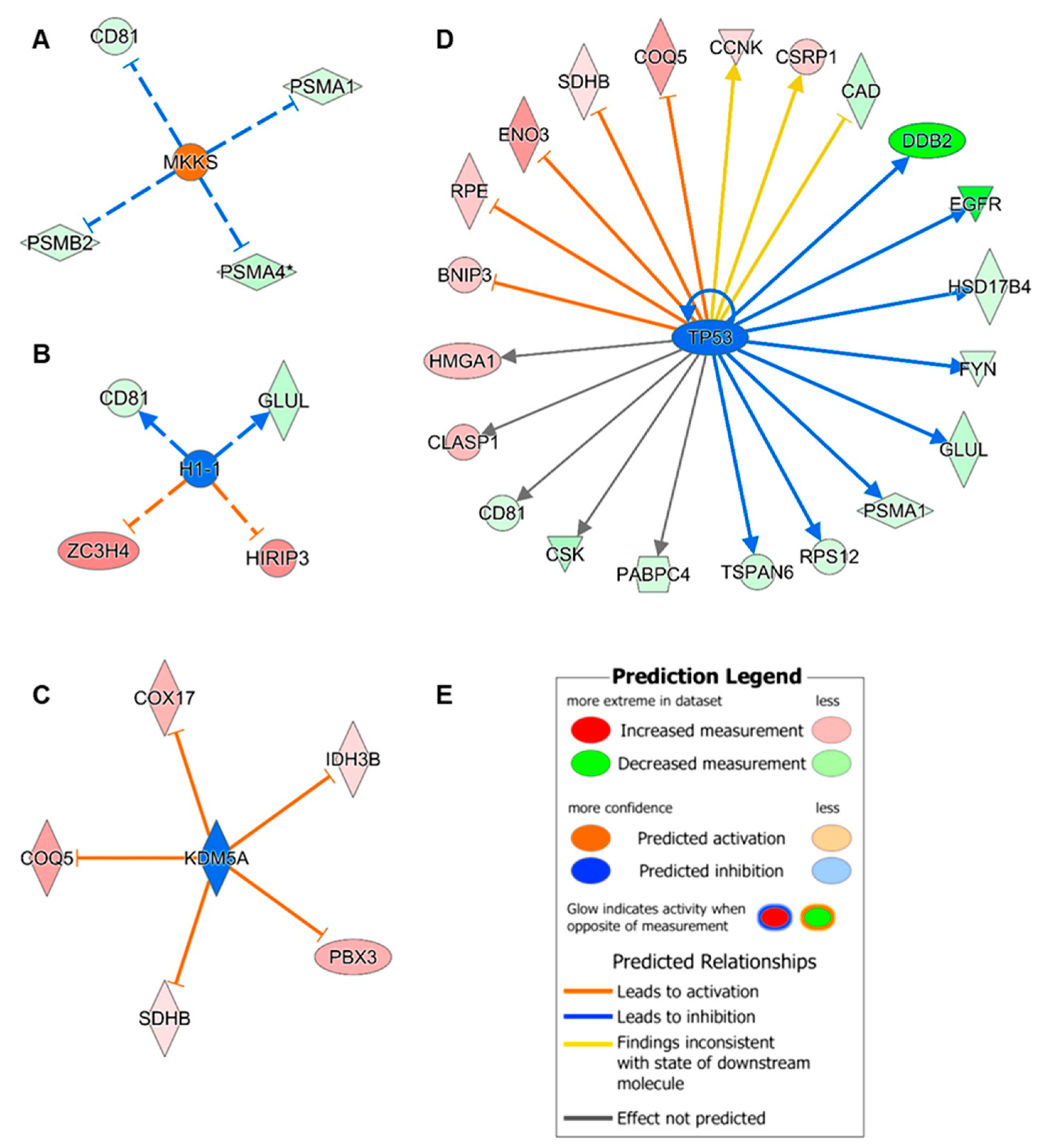
3.5. Upstream Regulator Analysis
3.6. Potential Biomarker Candidates
3.6.1. Proteins Associated with Axons and Neurites
3.6.2. Neuronal Functional Proteins
3.6.3. Neuronal Protection
3.6.4. Myelination
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jellinger, K.A. Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy. J. Alzheimer’s Dis. 2018, 62, 1141–1179. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Shang, H. Multiple System Atrophy: An Update and Emerging Directions of Biomarkers and Clinical Trials. J. Neurol. 2024, 271, 2324–2344. [Google Scholar] [CrossRef] [PubMed]
- Nandanwar, D.; Truong, D.D. Multiple System Atrophy: Diagnostic Challenges and a Proposed Diagnostic Algorithm. Clin. Park. Relat. Disord. 2024, 11, 100271. [Google Scholar] [CrossRef]
- Poewe, W.; Stankovic, I.; Halliday, G.; Meissner, W.G.; Wenning, G.K.; Pellecchia, M.T.; Seppi, K.; Palma, J.-A.; Kaufmann, H. Multiple System Atrophy. Nat. Rev. Dis. Primers 2022, 8, 56. [Google Scholar] [CrossRef]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef]
- Laferrière, F.; Claverol, S.; Bezard, E.; De Giorgi, F.; Ichas, F. Similar Neuronal Imprint and No Cross-Seeded Fibrils in α-Synuclein Aggregates from MSA and Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 10. [Google Scholar] [CrossRef]
- Henkel, L.M.; Kankowski, S.; Moellenkamp, T.M.; Smandzich, N.J.; Schwarz, S.; Di Fonzo, A.; Göhring, G.; Höglinger, G.; Wegner, F. IPSC-Derived Striatal Medium Spiny Neurons from Patients with Multiple System Atrophy Show Hypoexcitability and Elevated α-Synuclein Release. Cells 2023, 12, 223. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Mohamed Yusoff, A.A.; Mohd Khair, S.Z.N. Unraveling Mitochondrial Dysfunction: Comprehensive Perspectives on Its Impact on Neurodegenerative Diseases. Rev. Neurosci. 2025, 36, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Grossmann, D.; Delcambre, S.; Hermann, A.; Grünewald, A. Novel Insights into Parkin-Mediated Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Curr. Opin. Neurobiol. 2023, 80, 102720. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy Metabolism and Inflammation in Brain Aging and Alzheimer’s Disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef]
- Compta, Y.; Giraldo, D.M.; Muñoz, E.; Antonelli, F.; Fernández, M.; Bravo, P.; Soto, M.; Cámara, A.; Torres, F.; Martí, M.J.; et al. Cerebrospinal Fluid Levels of Coenzyme Q10 Are Reduced in Multiple System Atrophy. Park. Relat. Disord. 2018, 46, 16–23. [Google Scholar] [CrossRef]
- Monzio Compagnoni, G.; Kleiner, G.; Samarani, M.; Aureli, M.; Faustini, G.; Bellucci, A.; Ronchi, D.; Bordoni, A.; Garbellini, M.; Salani, S.; et al. Mitochondrial Dysregulation and Impaired Autophagy in IPSC-Derived Dopaminergic Neurons of Multiple System Atrophy. Stem Cell Rep. 2018, 11, 1185–1198. [Google Scholar] [CrossRef]
- Wan, L.; Zhu, S.; Chen, Z.; Qiu, R.; Tang, B.; Jiang, H. Multidimensional Biomarkers for Multiple System Atrophy: An Update and Future Directions. Transl. Neurodegener. 2023, 12, 38. [Google Scholar] [CrossRef]
- George, N.P.; Kwon, M.; Jang, Y.E.; Kim, S.G.; Hwang, J.S.; Lee, S.S.; Lee, G. Integrative Analysis of Metabolome and Proteome in the Cerebrospinal Fluid of Patients with Multiple System Atrophy. Cells 2025, 14, 265. [Google Scholar] [CrossRef]
- Marques, T.M.; van Rumund, A.; Kersten, I.; Bruinsma, I.B.; Wessels, H.J.C.T.; Gloerich, J.; Kaffa, C.; Esselink, R.A.J.; Bloem, B.R.; Kuiperij, H.B.; et al. Identification of Cerebrospinal Fluid Biomarkers for Parkinsonism Using a Proteomics Approach. NPJ Park. Dis. 2021, 7, 107. [Google Scholar] [CrossRef]
- Magdalinou, N.K.; Noyce, A.J.; Pinto, R.; Lindstrom, E.; Holmén-Larsson, J.; Holtta, M.; Blennow, K.; Morris, H.R.; Skillbäck, T.; Warner, T.T.; et al. Identification of Candidate Cerebrospinal Fluid Biomarkers in Parkinsonism Using Quantitative Proteomics. Park. Relat. Disord. 2017, 37, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rydbirk, R.; Østergaard, O.; Folke, J.; Hempel, C.; DellaValle, B.; Andresen, T.L.; Løkkegaard, A.; Hejl, A.-M.; Bode, M.; Blaabjerg, M.; et al. Brain Proteome Profiling Implicates the Complement and Coagulation Cascade in Multiple System Atrophy Brain Pathology. Cell. Mol. Life Sci. 2022, 79, 336. [Google Scholar] [CrossRef]
- Dick, F.; Johanson, G.A.S.; Tysnes, O.-B.; Alves, G.; Dölle, C.; Tzoulis, C. Brain Proteome Profiling Reveals Common and Divergent Signatures in Parkinson’s Disease, Multiple System Atrophy, and Progressive Supranuclear Palsy. Mol. Neurobiol. 2025, 62, 2801–2816. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Tittle, T.R.; Barot, R.R.; Betts, D.J.; Gallagher, J.J.; Kordower, J.H.; Chu, Y.; Killinger, B.A. Proximity Proteomics Reveals Unique and Shared Pathological Features between Multiple System Atrophy and Parkinson’s Disease. Acta Neuropathol. Commun. 2025, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Janelidze, S.; Zetterberg, H.; Brix, B.; Mattsson, N.; Surova, Y.; Blennow, K.; Hansson, O. Cerebrospinal Fluid Levels of Neurogranin in Parkinsonian Disorders. Mov. Disord. 2020, 35, 513–518. [Google Scholar] [CrossRef]
- Laurens, B.; Constantinescu, R.; Freeman, R.; Gerhard, A.; Jellinger, K.; Jeromin, A.; Krismer, F.; Mollenhauer, B.; Schlossmacher, M.G.; Shaw, L.M.; et al. Fluid Biomarkers in Multiple System Atrophy: A Review of the MSA Biomarker Initiative. Neurobiol. Dis. 2015, 80, 29–41. [Google Scholar] [CrossRef]
- Staege, S.; Kutschenko, A.; Baumann, H.; Glaß, H.; Henkel, L.; Gschwendtberger, T.; Kalmbach, N.; Klietz, M.; Hermann, A.; Lohmann, K.; et al. Reduced Expression of GABAA Receptor Alpha2 Subunit Is Associated With Disinhibition of DYT-THAP1 Dystonia Patient-Derived Striatal Medium Spiny Neurons. Front. Cell Dev. Biol. 2021, 9, 650586. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, F.; Gerhard, R.; Hönig, R.; Giehl, K.; Pich, A. TcdB of Clostridioides Difficile Mediates RAS-Dependent Necrosis in Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 4258. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for Large-Scale Genome and Gene Function Analysis with the PANTHER Classification System (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Rothfels, K.; Milacic, M.; Matthews, L.; Haw, R.; Sevilla, C.; Gillespie, M.; Stephan, R.; Gong, C.; Ragueneau, E.; May, B.; et al. Using the Reactome Database. Curr. Protoc. 2023, 3, e722. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal Analysis Approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Satake, T.; Yamashita, K.; Hayashi, K.; Miyatake, S.; Tamura-Nakano, M.; Doi, H.; Furuta, Y.; Shioi, G.; Miura, E.; Takeo, Y.H.; et al. MTCL1 Plays an Essential Role in Maintaining Purkinje Neuron Axon Initial Segment. EMBO J. 2017, 36, 1227–1242. [Google Scholar] [CrossRef]
- Meriane, M.; Tcherkezian, J.; Webber, C.A.; Danek, E.I.; Triki, I.; McFarlane, S.; Bloch-Gallego, E.; Lamarche-Vane, N. Phosphorylation of DCC by Fyn Mediates Netrin-1 Signaling in Growth Cone Guidance. J. Cell Biol. 2004, 167, 687–698. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, S.; Han, J. Polyglutamine Binding Protein 1 Regulates Neurite Outgrowth through Recruiting N-WASP. J. Biol. Chem. 2024, 300, 107537. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin: A Potential Biomarker of Neurological and Mental Diseases. Front. Aging Neurosci. 2020, 12, 584743. [Google Scholar] [CrossRef]
- Díez-Guerra, F.J. Neurogranin, a Link between Calcium/Calmodulin and Protein Kinase C Signaling in Synaptic Plasticity. IUBMB Life 2010, 62, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Dahimene, S.; von Elsner, L.; Holling, T.; Mattas, L.S.; Pickard, J.; Lessel, D.; Pilch, K.S.; Kadurin, I.; Pratt, W.S.; Zhulin, I.B.; et al. Biallelic CACNA2D1 Loss-of-Function Variants Cause Early-Onset Developmental Epileptic Encephalopathy. Brain 2022, 145, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C.; Lee, A. Presynaptic Calcium Channels: Specialized Control of Synaptic Neurotransmitter Release. Nat. Rev. Neurosci. 2020, 21, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.L.; Hoshi, N. Modulation of Kv7 Channels and Excitability in the Brain. Cell. Mol. Life Sci. 2017, 74, 495–508. [Google Scholar] [CrossRef]
- Hou, B.; Varghese, N.; Soh, H.; Santaniello, S.; Tzingounis, A.V. Loss of KCNQ2 or KCNQ3 Leads to Multifocal Time-Varying Activity in the Neonatal Forebrain Ex Vivo. eNeuro 2021, 8, ENEURO.0024-21.2021. [Google Scholar] [CrossRef]
- Liu, N.; Wu, W.-L.; Wan, X.-R.; Wang, J.; Huang, J.-N.; Jiang, Y.-Y.; Sheng, Y.-C.; Wu, J.-C.; Liang, Z.-Q.; Qin, Z.-H.; et al. Regulation of FSP1 Myristoylation by NADPH: A Novel Mechanism for Ferroptosis Inhibition. Redox Biol. 2024, 73, 103176. [Google Scholar] [CrossRef]
- Reeves, M.A.; Bellinger, F.P.; Berry, M.J. The Neuroprotective Functions of Selenoprotein M and Its Role in Cytosolic Calcium Regulation. Antioxid. Redox Signal 2010, 12, 809–818. [Google Scholar] [CrossRef]
- Figlia, G.; Müller, S.; Hagenston, A.M.; Kleber, S.; Roiuk, M.; Quast, J.-P.; ten Bosch, N.; Carvajal Ibañez, D.; Mauceri, D.; Martin-Villalba, A.; et al. Brain-Enriched RagB Isoforms Regulate the Dynamics of MTORC1 Activity through GATOR1 Inhibition. Nat. Cell Biol. 2022, 24, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Helwig, M.; Hoshino, A.; Berridge, C.; Lee, S.-N.; Lorenzen, N.; Otzen, D.E.; Eriksen, J.L.; Lindberg, I. The Neuroendocrine Protein 7B2 Suppresses the Aggregation of Neurodegenerative Disease-Related Proteins. J. Biol. Chem. 2013, 288, 1114–1124. [Google Scholar] [CrossRef]
- Clarke, B.A.; Majumder, S.; Zhu, H.; Lee, Y.T.; Kono, M.; Li, C.; Khanna, C.; Blain, H.; Schwartz, R.; Huso, V.L.; et al. The Ormdl Genes Regulate the Sphingolipid Synthesis Pathway to Ensure Proper Myelination and Neurologic Function in Mice. Elife 2019, 8, 51067. [Google Scholar] [CrossRef]
- Wu, R.; Li, A.; Sun, B.; Sun, J.-G.; Zhang, J.; Zhang, T.; Chen, Y.; Xiao, Y.; Gao, Y.; Zhang, Q.; et al. A Novel M6A Reader Prrc2a Controls Oligodendroglial Specification and Myelination. Cell Res. 2019, 29, 23–41. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Striatal Plasticity and Basal Ganglia Circuit Function. Neuron 2008, 60, 543–554. [Google Scholar] [CrossRef]
- Nilsson, J.; Constantinescu, J.; Nellgård, B.; Jakobsson, P.; Brum, W.S.; Gobom, J.; Forsgren, L.; Dalla, K.; Constantinescu, R.; Zetterberg, H.; et al. Cerebrospinal Fluid Biomarkers of Synaptic Dysfunction Are Altered in Parkinson’s Disease and Related Disorders. Mov. Disord. 2023, 38, 267–277. [Google Scholar] [CrossRef]
- Zhong, L.; Cherry, T.; Bies, C.E.; Florence, M.A.; Gerges, N.Z. Neurogranin Enhances Synaptic Strength through Its Interaction with Calmodulin. EMBO J. 2009, 28, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.; Chandrasekar, A.; Wang, X.; Putkey, J.A.; Waxham, M.N. Neurogranin Alters the Structure and Calcium Binding Properties of Calmodulin. J. Biol. Chem. 2014, 289, 14644–14655. [Google Scholar] [CrossRef]
- Wang, H. KCNQ2 and KCNQ3 Potassium Channel Subunits: Molecular Correlates of the M-Channel. Science (1979) 1998, 282, 1890–1893. [Google Scholar] [CrossRef]
- Jin, J.; Xue, L.; Bai, X.; Zhang, X.; Tian, Q.; Xie, A. Association between Epidermal Growth Factor Receptor Gene Polymorphisms and Susceptibility to Parkinson’s Disease. Neurosci. Lett. 2020, 736, 135273. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Lin, S.-J.; Chiang, W.-Y.; Chang, Y.-T.; Yang, C.-C.; Liao, C.-Y.; Chang, Y.-L.; Lin, C.-H.; Teng, S.-C. EGFR Phosphorylates DNAJB1 to Suppress α-Synuclein Aggregation in Parkinson’s Disease. NPJ Park. Dis. 2025, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Baye, L.M.; Schulz, N.P.; Beck, J.S.; Zhang, Q.; Slusarski, D.C.; Sheffield, V.C. BBS6, BBS10, and BBS12 Form a Complex with CCT/TRiC Family Chaperonins and Mediate BBSome Assembly. Proc. Natl. Acad. Sci. USA 2010, 107, 1488–1493. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, H.; Zhou, J. Organization, Functions, and Mechanisms of the BBSome in Development, Ciliopathies, and Beyond. Elife 2023, 12, 87623. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.; Schneider, R. The Role of Linker Histone H1 Modifications in the Regulation of Gene Expression and Chromatin Dynamics. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2016, 1859, 486–495. [Google Scholar] [CrossRef]
- Albig, W.; Drabent, B.; Kunz, J.; Kalff-Suske, M.; Grzeschik, K.-H.; Doenecke, D. All Known Human H1 Histone Genes Except the H10 Gene Are Clustered on Chromosome 6. Genomics 1993, 16, 649–654. [Google Scholar] [CrossRef]
- Ponte, I.; Andrés, M.; Jordan, A.; Roque, A. Towards Understanding the Regulation of Histone H1 Somatic Subtypes with OMICs. J. Mol. Biol. 2021, 433, 166734. [Google Scholar] [CrossRef]
- Goers, J.; Manning-Bog, A.B.; McCormack, A.L.; Millett, I.S.; Doniach, S.; Di Monte, D.A.; Uversky, V.N.; Fink, A.L. Nuclear Localization of α-Synuclein and Its Interaction with Histones. Biochemistry 2003, 42, 8465–8471. [Google Scholar] [CrossRef]
- Tu, S.; Teng, Y.-C.; Yuan, C.; Wu, Y.-T.; Chan, M.-Y.; Cheng, A.-N.; Lin, P.-H.; Juan, L.-J.; Tsai, M.-D. The ARID Domain of the H3K4 Demethylase RBP2 Binds to a DNA CCGCCC Motif. Nat. Struct. Mol. Biol. 2008, 15, 419–421. [Google Scholar] [CrossRef]
- Wang, H.; Guo, B.; Guo, X. Histone Demethylases in Neurodevelopment and Neurodegenerative Diseases. Int. J. Neurosci. 2024, 134, 1372–1382. [Google Scholar] [CrossRef]
- Guhathakurta, S.; Kim, J.; Adams, L.; Basu, S.; Song, M.K.; Adler, E.; Je, G.; Fiadeiro, M.B.; Kim, Y. Targeted Attenuation of Elevated Histone Marks at SNCA Alleviates A-synuclein in Parkinson’s Disease. EMBO Mol. Med. 2021, 13, e12188. [Google Scholar] [CrossRef] [PubMed]
- Hernández Borrero, L.J.; El-Deiry, W.S. Tumor Suppressor P53: Biology, Signaling Pathways, and Therapeutic Targeting. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188556. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Duplan, E.; Giordano, C.; Checler, F.; Alves da Costa, C. Direct α-Synuclein Promoter Transactivation by the Tumor Suppressor P53. Mol. Neurodegener. 2016, 11, 13. [Google Scholar] [CrossRef]
- López, K.L.R.; Simpson, J.E.; Watson, L.C.; Mortiboys, H.; Hautbergue, G.M.; Bandmann, O.; Highley, J.R. TIGAR Inclusion Pathology Is Specific for Lewy Body Diseases. Brain Res. 2019, 1706, 218–223. [Google Scholar] [CrossRef]
- Probst-Cousin, S.; Rickert, C.H.; Schmid, K.W.; Gullota, F. Cell Death Mechanisms in Multiple System Atrophy. J. Neuropathol. Exp. Neurol. 1998, 57, 814–821. [Google Scholar] [CrossRef] [PubMed]

| ID Code | Sex | Age at Biopsy | Origin/Reference |
|---|---|---|---|
| CTR1 | M | 59 | Henkel et al. [7] |
| CTR2 | F | 62 | StemBANCC consortium SFC084-03-02-01A |
| CTR3 | F | 62 | Henkel et al. [7] |
| P1 | F | 78 | Monzio Compagnoni et al. [13] |
| P2 | M | 52 | Henkel et al. [7] |
| P3 | F | 56 | Henkel et al. [7] |
| Gene Names | Protein Names | MSA vs. CTR | p-Value | Fold Change (log2) |
|---|---|---|---|---|
| MTCL1 | Microtubule cross-linking factor 1 |  | 0.0037 | 2.6503 |
| NMT2 | Glycylpeptide N-tetradecanoyltransferase 2 |  | 0.0294 | 1.9344 |
| SCG5 | Neuroendocrine protein 7B2 |  | 0.0377 | 1.5578 |
| SELM | Selenoprotein M |  | 0.0119 | 1.3475 |
| RRAGB | Ras-related GTP-binding protein B |  | 0.0463 | 1.2842 |
| PRRC2A | Proline-rich coiled coil 2A |  | 0.0259 | 1.2435 |
| DCC | Netrin receptor DCC (Deleted in Colorectal Carcinoma) |  | 0.0193 | 1.0619 |
| PQBP1 | Polyglutamine-binding protein 1 |  | 0.0388 | 1.0494 |
| NRGN | Neurogranin |  | 0.0401 | 1.017 |
| ORMDL1 | Orosomucoid 1 (ORM1)-like protein 1 |  | 0.0068 | −1.071 |
| ORMDL2 | ORM1-like protein 2 |  | 0.0068 | −1.071 |
| CACNA2D1 | Voltage-dependent calcium channel subunit alpha-2/delta-1 |  | 0.0405 | −1.1958 |
| KCNQ2 | Voltage-gated potassium channel subunit Kv7.2 |  | 0.0144 | −1.4919 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smandzich, N.J.; Pich, A.; Gschwendtberger, T.; Greten, S.; Ye, L.; Klietz, M.; Di Fonzo, A.; Henkel, L.M.; Wegner, F. Proteomics of Patient-Derived Striatal Medium Spiny Neurons in Multiple System Atrophy. Cells 2025, 14, 1394. https://doi.org/10.3390/cells14171394
Smandzich NJ, Pich A, Gschwendtberger T, Greten S, Ye L, Klietz M, Di Fonzo A, Henkel LM, Wegner F. Proteomics of Patient-Derived Striatal Medium Spiny Neurons in Multiple System Atrophy. Cells. 2025; 14(17):1394. https://doi.org/10.3390/cells14171394
Chicago/Turabian StyleSmandzich, Nadine J., Andreas Pich, Thomas Gschwendtberger, Stephan Greten, Lan Ye, Martin Klietz, Alessio Di Fonzo, Lisa M. Henkel, and Florian Wegner. 2025. "Proteomics of Patient-Derived Striatal Medium Spiny Neurons in Multiple System Atrophy" Cells 14, no. 17: 1394. https://doi.org/10.3390/cells14171394
APA StyleSmandzich, N. J., Pich, A., Gschwendtberger, T., Greten, S., Ye, L., Klietz, M., Di Fonzo, A., Henkel, L. M., & Wegner, F. (2025). Proteomics of Patient-Derived Striatal Medium Spiny Neurons in Multiple System Atrophy. Cells, 14(17), 1394. https://doi.org/10.3390/cells14171394







