Integrating Macrophages into Human-Engineered Cardiac Tissue
Abstract
1. Introduction
2. Myocardial Injury and the Immune System
2.1. Inflammation Phase
2.2. Reparative and Proliferative Phase
3. Cardiac Resident Macrophages
4. Generation of hPSC-Derived Macrophages
4.1. General Methods to Achieve Mesoderm Commitment and Hemogenic Endothelium Specification
4.1.1. Monolayer 2D Cell Culture
4.1.2. Co-Culture with Stromal Cells
4.1.3. Embryoid Body (EB) Formation
4.2. Polarization of hPSC-Derived Macrophages (hPSC-Ms)
4.3. Challenges and Future Directions
5. Methods to Generate hECTs
5.1. Scaffold-Based and Biohybrid Approaches
5.2. Electrical and Mechanical Conditioning Models
5.3. Miniaturized and Scalable Models
5.4. Disease Modeling with hPSC-CMs and hECTs
5.5. Integration of Artificial Intelligence for Functional Phenotyping
6. Inclusion of Resident Macrophages in hPSC-Derived hECTs
7. Future Directions
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef]
- Lyadova, I.; Gerasimova, T.; Nenasheva, T. Macrophages Derived From Human Induced Pluripotent Stem Cells: The Diversity of Protocols, Future Prospects, and Outstanding Questions. Front. Cell Dev. Biol. 2021, 9, 640703. [Google Scholar] [CrossRef]
- Lyadova, I.; Vasiliev, A. Macrophages derived from pluripotent stem cells: Prospective applications and research gaps. Cell Biosci. 2022, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Wong, W.J.; Moreira, M.; Pasqualini, C.; Ginhoux, F. Induced pluripotent stem cell-derived macrophages as a platform for modelling human disease. Nat. Rev. Immunol. 2025, 25, 108–124. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef]
- Pluijmert, N.J.; Atsma, D.E.; Quax, P.H.A. Post-ischemic Myocardial Inflammatory Response: A Complex and Dynamic Process Susceptible to Immunomodulatory Therapies. Front. Cardiovasc. Med. 2021, 8, 647785. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Mestril, R.; Hilal-Dandan, R.; Brunton, L.L.; Dillmann, W.H. Small Heat Shock Proteins and Protection Against Ischemic Injury in Cardiac Myocytes. Circulation 1997, 96, 4343–4348. [Google Scholar] [CrossRef] [PubMed]
- Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury, Nature. (n.d.). Available online: https://www.nature.com/articles/nature08780 (accessed on 23 February 2025).
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.-Y.; Strassheim, D.; Ishizaka, A.; Abraham, E. Involvement of Toll-like Receptors 2 and 4 in Cellular Activation by High Mobility Group Box 1 Protein*. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef]
- Camous, L.; Roumenina, L.; Bigot, S.; Brachemi, S.; Frémeaux-Bacchi, V.; Lesavre, P.; Halbwachs-Mecarelli, L. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 2011, 117, 1340–1349. [Google Scholar] [CrossRef]
- Yan, X.; Anzai, A.; Katsumata, Y.; Matsuhashi, T.; Ito, K.; Endo, J.; Yamamoto, T.; Takeshima, A.; Shinmura, K.; Shen, W.; et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013, 62, 24–35. [Google Scholar] [CrossRef]
- Ma, Y.; Yabluchanskiy, A.; Lindsey, M.L. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Dewald, O.; Zymek, P.; Winkelmann, K.; Koerting, A.; Ren, G.; Abou-Khamis, T.; Michael, L.H.; Rollins, B.J.; Entman, M.L.; Frangogiannis, N.G. CCL2/Monocyte Chemoattractant Protein-1 Regulates Inflammatory Responses Critical to Healing Myocardial Infarcts. Circ. Res. 2005, 96, 881–889. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Monocyte and Macrophage Heterogeneity in the Heart. Circ. Res. 2013, 112, 1624–1633. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Dunne, A.; Monaghan, M.G. The Role of Macrophages in the Infarcted Myocardium: Orchestrators of ECM Remodeling. Front. Cardiovasc. Med. 2019, 6, 101. [Google Scholar] [CrossRef]
- Rolski, F.; Błyszczuk, P. Complexity of TNF-α Signaling in Heart Disease. J. Clin. Med. 2020, 9, 3267. [Google Scholar] [CrossRef]
- Maekawa, Y.; Anzai, T.; Yoshikawa, T.; Asakura, Y.; Takahashi, T.; Ishikawa, S.; Mitamura, H.; Ogawa, S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J. Am. Coll. Cardiol. 2002, 39, 241–246. [Google Scholar] [CrossRef]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol. 2017, 112, 33. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Chen, W.; Frangogiannis, N.G. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 2011, 51, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef]
- Glinton, K.E.; Ma, W.; Lantz, C.; Grigoryeva, L.S.; DeBerge, M.; Liu, X.; Febbraio, M.; Kahn, M.; Oliver, G.; Thorp, E.B. Macrophage-produced VEGFC is induced by efferocytosis to ameliorate cardiac injury and inflammation. J. Clin. Investig. 2022, 132, e140685. [Google Scholar] [CrossRef]
- Rosenkranz, S. TGF-β1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef]
- He, L.; Huang, X.; Kanisicak, O.; Li, Y.; Wang, Y.; Li, Y.; Pu, W.; Liu, Q.; Zhang, H.; Tian, X.; et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J. Clin. Investig. 2017, 127, 2968–2981. [Google Scholar] [CrossRef] [PubMed]
- Coultas, L.; Chawengsaksophak, K.; Rossant, J. Endothelial cells and VEGF in vascular development. Nature 2005, 438, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, C.; Maliba, R.; Favier, J.; Théorêt, J.-F.; Merhi, Y.; Sirois, M.G. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 2005, 105, 1523–1530. [Google Scholar] [CrossRef]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Saxena, A.; Dobaczewski, M.; Rai, V.; Haque, Z.; Chen, W.; Li, N.; Frangogiannis, N.G. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1233–H1242. [Google Scholar] [CrossRef]
- Chaudhry, A.; Samstein, R.M.; Treuting, P.; Liang, Y.; Pils, M.C.; Heinrich, J.-M.; Jack, R.S.; Wunderlich, F.T.; Brüning, J.C.; Müller, W.; et al. Interleukin-10 Signaling in Regulatory T Cells Is Required for Suppression of Th17 Cell-Mediated Inflammation. Immunity 2011, 34, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, N.; Yopp, A.C.; Chen, D.; Mao, M.; Chen, D.; Zhang, H.; Ding, Y.; Bromberg, J.S. TGF-β Induces Foxp3 + T-Regulatory Cells from CD4 + CD25—Precursors. Am. J. Transplant. 2004, 4, 1614–1627. [Google Scholar] [CrossRef]
- Santos-Zas, I.; Lemarié, J.; Tedgui, A.; Ait-Oufella, H. Adaptive Immune Responses Contribute to Post-ischemic Cardiac Remodeling. Front. Cardiovasc. Med. 2019, 5, 198. [Google Scholar] [CrossRef]
- Revelo, X.S.; Parthiban, P.; Chen, C.; Barrow, F.; Fredrickson, G.; Wang, H.; Yücel, D.; Herman, A.; van Berlo, J.H. Cardiac Resident Macrophages Prevent Fibrosis and Stimulate Angiogenesis. Circ. Res. 2021, 129, 1086–1101. [Google Scholar] [CrossRef]
- Suku, M.; Forrester, L.; Biggs, M.; Monaghan, M.G. Resident Macrophages and Their Potential in Cardiac Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 579–591. [Google Scholar] [CrossRef]
- Long, C.; Guo, R.; Han, R.; Li, K.; Wan, Y.; Xu, J.; Gong, X.; Zhao, Y.; Yao, X.; Liu, J. Effects of macrophages on the proliferation and cardiac differentiation of human induced pluripotent stem cells. Cell Commun. Signal. 2022, 20, 108. [Google Scholar] [CrossRef]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef]
- DeBerge, M.; Yeap, X.Y.; Dehn, S.; Zhang, S.; Grigoryeva, L.; Misener, S.; Procissi, D.; Zhou, X.; Lee, D.C.; Muller, W.A.; et al. MerTK Cleavage on Resident Cardiac Macrophages Compromises Repair After Myocardial Ischemia Reperfusion Injury. Circ. Res. 2017, 121, 930–940. [Google Scholar] [CrossRef]
- DeBerge, M.; Glinton, K.; Subramanian, M.; Wilsbacher, L.D.; Rothlin, C.V.; Tabas, I.; Thorp, E.B. Macrophage AXL receptor tyrosine kinase inflames the heart after reperfused myocardial infarction. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Klepikova, A.; Nenasheva, T.; Sheveleva, O.; Protasova, E.; Antonov, D.; Gainullina, A.; Chikina, E.; Sakovnich, O.; Gerasimova, T.; Nikitina, I.; et al. iPSC-Derived Macrophages: The Differentiation Protocol Affects Cell Immune Characteristics and Differentiation Trajectories. Int. J. Mol. Sci. 2022, 23, 6087. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Z.W.; Kozaki, T.; Ginhoux, F. Studying tissue macrophages in vitro: Are iPSC-derived cells the answer? Nat. Rev. Immunol. 2018, 18, 716–725. [Google Scholar] [CrossRef]
- Douvaras, P.; Sun, B.; Wang, M.; Kruglikov, I.; Lallos, G.; Zimmer, M.; Terrenoire, C.; Zhang, B.; Gandy, S.; Schadt, E.; et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Rep. 2017, 8, 1516–1524. [Google Scholar] [CrossRef]
- Konttinen, H.; Cabral-da-Silva, M.E.C.; Ohtonen, S.; Wojciechowski, S.; Shakirzyanova, A.; Caligola, S.; Giugno, R.; Ishchenko, Y.; Hernández, D.; Fazaludeen, M.F.; et al. PSEN1ΔE9, APPswe, and APOE4 Confer Disparate Phenotypes in Human iPSC-Derived Microglia. Stem Cell Rep. 2019, 13, 669–683. [Google Scholar] [CrossRef]
- Cao, X.; Yakala, G.K.; Van Den Hil, F.E.; Cochrane, A.; Mummery, C.L.; Orlova, V.V. Differentiation and Functional Comparison of Monocytes and Macrophages from hiPSCs with Peripheral Blood Derivatives. Stem Cell Rep. 2019, 12, 1282–1297. [Google Scholar] [CrossRef]
- Cui, D.; Franz, A.; Fillon, S.A.; Jannetti, L.; Isambert, T.; Fundel-Clemens, K.; Huber, H.J.; Viollet, C.; Ghanem, A.; Niwa, A.; et al. High-Yield Human Induced Pluripotent Stem Cell-Derived Monocytes and Macrophages Are Functionally Comparable With Primary Cells. Front. Cell Dev. Biol. 2021, 9, 656867. [Google Scholar] [CrossRef] [PubMed]
- Takata, K.; Kozaki, T.; Lee, C.Z.W.; Thion, M.S.; Otsuka, M.; Lim, S.; Utami, K.H.; Fidan, K.; Park, D.S.; Malleret, B.; et al. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity 2017, 47, 183–198.e6. [Google Scholar] [CrossRef]
- Abud, E.M.; Ramirez, R.N.; Martinez, E.S.; Healy, L.M.; Nguyen, C.H.H.; Newman, S.A.; Yeromin, A.V.; Scarfone, V.M.; Marsh, S.E.; Fimbres, C.; et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron 2017, 94, 278–293.e9. [Google Scholar] [CrossRef]
- Vodyanik, M.A.; Bork, J.A.; Thomson, J.A.; Slukvin, I.I. Human embryonic stem cell–derived CD34+ cells: Efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 2005, 105, 617–626. [Google Scholar] [CrossRef]
- Lynch, M.R.; Gasson, J.C.; Paz, H. Modified ES/OP9 Co-Culture Protocol Provides Enhanced Characterization of Hematopoietic Progeny. J. Vis. Exp. (JoVE) 2011, 52, e2559. [Google Scholar] [CrossRef]
- Kambal, A.; Mitchell, G.; Cary, W.; Gruenloh, W.; Jung, Y.; Kalomoiris, S.; Nacey, C.; McGee, J.; Lindsey, M.; Fury, B.; et al. Generation of HIV-1 Resistant and Functional Macrophages From Hematopoietic Stem Cell–derived Induced Pluripotent Stem Cells. Mol. Ther. 2011, 19, 584–593. [Google Scholar] [CrossRef]
- Brault, J.; Goutagny, E.; Telugu, N.; Shao, K.; Baquié, M.; Satre, V.; Coutton, C.; Grunwald, D.; Brion, J.-P.; Barlogis, V.; et al. Optimized Generation of Functional Neutrophils and Macrophages from Patient-Specific Induced Pluripotent Stem Cells: Ex Vivo Models of X0 -Linked, AR220- and AR470- Chronic Granulomatous Diseases. BioRes. Open Access 2014, 3, 311–326. [Google Scholar] [CrossRef]
- Choi, K.-D.; Yu, J.; Smuga-Otto, K.; Salvagiotto, G.; Rehrauer, W.; Vodyanik, M.; Thomson, J.; Slukvin, I. Hematopoietic and Endothelial Differentiation of Human Induced Pluripotent Stem Cells. Stem Cells 2009, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-D.; Vodyanik, M.; Slukvin, I.I. Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat. Protoc. 2011, 6, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Clanchy, F.I.L.; Hamilton, J.A. The development of macrophages from human CD34+ haematopoietic stem cells in serum-free cultures is optimized by IL-3 and SCF. Cytokine 2013, 61, 33–37. [Google Scholar] [CrossRef]
- Buchrieser, J.; James, W.; Moore, M.D. Human Induced Pluripotent Stem Cell-Derived Macrophages Share Ontogeny with MYB-Independent Tissue-Resident Macrophages. Stem Cell Rep. 2017, 8, 334–345. [Google Scholar] [CrossRef]
- Wilgenburg Bvan Browne, C.; Vowles, J.; Cowley, S.A. Efficient, Long Term Production of Monocyte-Derived Macrophages from Human Pluripotent Stem Cells under Partly-Defined and Fully-Defined Conditions. PLoS ONE 2013, 8, e71098. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Hale, C.; Mukhopadhyay, S. A Simple Multistep Protocol for Differentiating Human Induced Pluripotent Stem Cells into Functional Macrophages. In Macrophages: Methods and Protocols; Rousselet, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 13–28. [Google Scholar] [CrossRef]
- Lopez-Yrigoyen, M.; May, A.; Ventura, T.; Taylor, H.; Fidanza, A.; Cassetta, L.; Pollard, J.W.; Forrester, L.M.; Lopez-Yrigoyen, M. Production and Characterization of Human Macrophages from Pluripotent Stem Cells. J. Vis. Exp. (JoVE) 2020, 158, e61038. [Google Scholar] [CrossRef]
- Park, T.S.; Hirday, R.; Quinn, R.; Jacob, S.P.; Feldman, R.A.; Bose, D.; Sharma, R.; Bharti, K. Differentiation of monocytes and polarized M1/M2 macrophages from human induced pluripotent stem cells. STAR Protoc. 2024, 5, 102827. [Google Scholar] [CrossRef]
- Shi, J.; Xue, C.; Liu, W.; Zhang, H. Differentiation of Human-Induced Pluripotent Stem Cells to Macrophages for Disease Modeling and Functional Genomics. Curr. Protoc. Stem Cell Biol. 2019, 48, e74. [Google Scholar] [CrossRef]
- Hong, D.; Ding, J.; Li, O.; He, Q.; Ke, M.; Zhu, M.; Liu, L.; Ou, W.-B.; He, Y.; Wu, Y. Human-induced pluripotent stem cell-derived macrophages and their immunological function in response to tuberculosis infection. Stem Cell Res. Ther. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Haake, K.; Neehus, A.-L.; Buchegger, T.; Kühnel, M.P.; Blank, P.; Philipp, F.; Oleaga-Quintas, C.; Schulz, A.; Grimley, M.; Goethe, R.; et al. Patient iPSC-Derived Macrophages to Study Inborn Errors of the IFN-? Responsive Pathway. Cells 2020, 9, 483. [Google Scholar] [CrossRef]
- Atkins, M.H.; Scarfò, R.; McGrath, K.E.; Yang, D.; Palis, J.; Ditadi, A.; Keller, G.M. Modeling human yolk sac hematopoiesis with pluripotent stem cells. J. Exp. Med. 2021, 219, e20211924. [Google Scholar] [CrossRef]
- Shen, J.; Lyu, S.; Xu, Y.; Zhang, S.; Li, L.; Li, J.; Mou, J.; Xie, L.; Tang, K.; Wen, W.; et al. Activating innate immune responses repolarizes hPSC-derived CAR macrophages to improve anti-tumor activity. Cell Stem Cell 2024, 31, 1003–1019.e9. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, C.; Shah, R.; Bermingham, K.; Hinkle, C.C.; Li, W.; Rodrigues, A.; Tabita-Martinez, J.; Millar, J.S.; Cuchel, M.; et al. Functional Analysis and Transcriptomic Profiling of iPSC-Derived Macrophages and Their Application in Modeling Mendelian Disease. Circ. Res. 2015, 117, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Alasoo, K.; Martinez, F.O.; Hale, C.; Gordon, S.; Powrie, F.; Dougan, G.; Mukhopadhyay, S.; Gaffney, D.J. Transcriptional profiling of macrophages derived from monocytes and iPS cells identifies a conserved response to LPS and novel alternative transcription. Sci. Rep. 2015, 5, 12524. [Google Scholar] [CrossRef]
- Hagemeyer, N.; Kierdorf, K.; Frenzel, K.; Xue, J.; Ringelhan, M.; Abdullah, Z.; Godin, I.; Wieghofer, P.; Costa Jordão, M.J.; Ulas, T.; et al. Transcriptome-based profiling of yolk sac-derived macrophages reveals a role for Irf8 in macrophage maturation. EMBO J. 2016, 35, 1730–1744. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Lachmann, N. From macrophage biology to macrophage-based cellular immunotherapies. Gene Ther. 2021, 28, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, F.; Xing, J.; Huang, X.; Mo, S.; Wei, X.; Tan, M.-H.; Yu, H. Generation of mature kupffer cells from human induced pluripotent stem cells. Biomaterials 2019, 192, 377–391. [Google Scholar] [CrossRef]
- Luque-Martin, R.; Mander, P.K.; Leenen, P.J.M.; Winther, M.P.J. Classic and new mediators for in vitro modelling of human macrophages. J. Leukoc. Biol. 2021, 109, 549–560. [Google Scholar] [CrossRef]
- Park, D.S.; Kozaki, T.; Tiwari, S.K.; Moreira, M.; Khalilnezhad, A.; Torta, F.; Olivié, N.; Thiam, C.H.; Liani, O.; Silvin, A.; et al. iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature 2023, 623, 397–405. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef]
- Kawai, Y.; Tohyama, S.; Arai, K.; Tamura, T.; Soma, Y.; Fukuda, K.; Shimizu, H.; Nakayama, K.; Kobayashi, E. Scaffold-Free Tubular Engineered Heart Tissue From Human Induced Pluripotent Stem Cells Using Bio-3D Printing Technology in vivo. Front. Cardiovasc. Med. 2022, 8, 806215. [Google Scholar] [CrossRef]
- Hoekstra, M.; Mummery, C.L.; Wilde, A.A.M.; Bezzina, C.R.; Verkerk, A.O. Induced pluripotent stem cell derived cardiomyocytes as models for cardiac arrhythmias. Front. Physiol. 2012, 3, 346. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef]
- Richards, D.J.; Li, Y.; Kerr, C.M.; Yao, J.; Beeson, G.C.; Coyle, R.C.; Chen, X.; Jia, J.; Damon, B.; Wilson, R.; et al. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng. 2020, 4, 446–462. [Google Scholar] [CrossRef]
- Fleischer, S.; Nash, T.R.; Tamargo, M.A.; Lock, R.I.; Venturini, G.; Morsink, M.; Graney, P.L.; Li, V.; Lamberti, M.J.; Liberman, M.; et al. An engineered human cardiac tissue model reveals contributions of systemic lupus erythematosus autoantibodies to myocardial injury. Nat. Cardiovasc. Res. 2024, 3, 1123–1139. [Google Scholar] [CrossRef]
- Chen, T.; Vunjak-Novakovic, G. Human Tissue-Engineered Model of Myocardial Ischemia–Reperfusion Injury. Tissue Eng. Part A 2019, 25, 711–724. [Google Scholar] [CrossRef]
- Wu, Q.; Xue, R.; Zhao, Y.; Ramsay, K.; Wang, E.Y.; Savoji, H.; Veres, T.; Cartmell, S.H.; Radisic, M. Automated fabrication of a scalable heart-on-a-chip device by 3D printing of thermoplastic elastomer nanocomposite and hot embossing. Bioact. Mater. 2024, 33, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Loskill, P.; Deveshwar, N.; Spencer, C.I.; Judge, L.M.; Mandegar, M.A.; BFox, C.; Mohamed, T.M.A.; Ma, Z.; Mathur, A.; et al. Miniaturized iPS-Cell-Derived Cardiac Muscles for Physiologically Relevant Drug Response Analyses. Sci. Rep. 2016, 6, 24726. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Wang, G.; McCain, M.L.; Yang, L.; He, A.; Pasqualini, F.S.; Agarwal, A.; Yuan, H.; Jiang, D.; Zhang, D.; Zangi, L.; et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014, 20, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Massé, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef]
- Sidorov, V.Y.; Samson, P.C.; Sidorova, T.N.; Davidson, J.M.; Lim, C.C.; Wikswo, J.P. I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater. 2017, 48, 68–78. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Rivera-Arbeláez, J.M.; Cofiño-Fabres, C.; Schwach, V.; Slaats, R.H.; ten Den, S.A.; Vermeul, K.; van den Berg, A.; Pérez-Pomares, J.M.; Segerink, L.I.; et al. A New Versatile Platform for Assessment of Improved Cardiac Performance in Human-Engineered Heart Tissues. J. Pers. Med. 2022, 12, 214. [Google Scholar] [CrossRef]
- Goldfracht, I.; Efraim, Y.; Shinnawi, R.; Kovalev, E.; Huber, I.; Gepstein, A.; Arbel, G.; Shaheen, N.; Tiburcy, M.; Zimmermann, W.H.; et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019, 92, 145–159. [Google Scholar] [CrossRef]
- Hamidzada, H.; Pascual-Gil, S.; Wu, Q.; Kent, G.M.; Massé, S.; Kantores, C.; Kuzmanov, U.; Gomez-Garcia, M.J.; Rafatian, N.; Gorman, R.A.; et al. Primitive macrophages induce sarcomeric maturation and functional enhancement of developing human cardiac microtissues via efferocytic pathways. Nat. Cardiovasc. Res. 2024, 3, 567–593. [Google Scholar] [CrossRef]
- Landau, S.; Zhao, Y.; Hamidzada, H.; Kent, G.M.; Okhovatian, S.; Lu, R.X.Z.; Liu, C.; Wagner, K.T.; Cheung, K.; Shawky, S.A.; et al. Primitive macrophages enable long-term vascularization of human heart-on-a-chip platforms. Cell Stem Cell 2024, 31, 1222–1238.e10. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Yazawa, M.; Liu, J.; Han, L.; Sanchez-Freire, V.; Abilez, O.J.; Navarrete, E.G.; Hu, S.; Wang, L.; Lee, A.; et al. Patient-Specific Induced Pluripotent Stem Cells as a Model for Familial Dilated Cardiomyopathy. Sci. Transl. Med. 2012, 4, ra47–ra130. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Lee, A.S.; Liang, P.; Sanchez-Freire, V.; Nguyen, P.K.; Wang, L.; Han, L.; Yen, M.; Wang, Y.; Sun, N.; et al. Abnormal Calcium Handling Properties Underlie Familial Hypertrophic Cardiomyopathy Pathology in Patient-Specific Induced Pluripotent Stem Cells. Cell Stem Cell 2013, 12, 101–113. [Google Scholar] [CrossRef]
- Wang, K.; Schriver, B.J.; Aschar-Sobbi, R.; Yi, A.Y.; Feric, N.T.; Graziano, M.P. Human engineered cardiac tissue model of hypertrophic cardiomyopathy recapitulates key hallmarks of the disease and the effect of chronic mavacamten treatment. Front. Bioeng. Biotechnol. 2023, 11, 1227184. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Nash, T.R.; Zhang, X.; Rao, J.; Abriola, L.; Kim, Y.; Zakharov, S.; Kim, M.; Luo, L.J.; Morsink, M.; et al. Engineered cardiac tissue model of restrictive cardiomyopathy for drug discovery. Cell Rep. Med. 2023, 4, 100976. [Google Scholar] [CrossRef]
- Moretti, A.; Bellin, M.; Welling, A.; Jung, C.B.; Lam, J.T.; Bott-Flügel, L.; Dorn, T.; Goedel, A.; Höhnke, C.; Hofmann, F.; et al. Patient-Specific Induced Pluripotent Stem-Cell Models for Long-QT Syndrome. New Engl. J. Med. 2010, 363, 1397–1409. [Google Scholar] [CrossRef]
- Lahti, A.L.; Kujala, V.J.; Chapman, H.; Koivisto, A.-P.; Pekkanen-Mattila, M.; Kerkelä, E.; Hyttinen, J.; Kontula, K.; Swan, H.; Conklin, B.R.; et al. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis. Models Mech. 2012, 5, 220–230. [Google Scholar] [CrossRef]
- Veldhuizen, J.; Mann, H.F.; Karamanova, N.; Van Horn, W.D.; Migrino, R.Q.; Brafman, D.; Nikkhah, M. Modeling long QT syndrome type 2 on-a-chip via in-depth assessment of isogenic gene-edited 3D cardiac tissues. Sci. Adv. 2022, 8, eabq6720. [Google Scholar] [CrossRef]
- Lemoine, M.D.; Krause, T.; Koivumäki, J.T.; Prondzynski, M.; Schulze, M.L.; Girdauskas, E.; Willems, S.; Hansen, A.; Eschenhagen, T.; Christ, T. Human Induced Pluripotent Stem Cell–Derived Engineered Heart Tissue as a Sensitive Test System for QT Prolongation and Arrhythmic Triggers. Circ. Arrhythmia Electrophysiol. 2018, 11, e006035. [Google Scholar] [CrossRef]
- Malan, D.; Zhang, M.; Stallmeyer, B.; Müller, J.; Fleischmann, B.K.; Schulze-Bahr, E.; Sasse, P.; Greber, B. Human iPS cell model of type 3 long QT syndrome recapitulates drug-based phenotype correction. Basic Res. Cardiol. 2016, 111, 14. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, M.; Hsueh, B.; Jia, X.; Pasca, A.M.; Bernstein, J.A.; Hallmayer, J.; Dolmetsch, R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 2011, 471, 230–234. [Google Scholar] [CrossRef]
- Kim, Y.; Wang, K.; Lock, R.I.; Nash, T.R.; Fleischer, S.; Wang, B.Z.; Fine, B.M.; Vunjak-Novakovic, G. BeatProfiler: Multimodal In Vitro Analysis of Cardiac Function Enables Machine Learning Classification of Diseases and Drugs. IEEE Open J. Eng. Med. Biol. 2024, 5, 238–249. [Google Scholar] [CrossRef]
- Lock, R.I.; Graney, P.L.; Tavakol, D.N.; Nash, T.R.; Kim, Y.; Sanchez, E.; Morsink, M.; Ning, D.; Chen, C.; Fleischer, S.; et al. Macrophages enhance contractile force in iPSC-derived human engineered cardiac tissue. Cell Rep. 2024, 43, 114302. [Google Scholar] [CrossRef]
- Xia, N.; Lu, Y.; Gu, M.; Li, N.; Liu, M.; Jiao, J.; Zhu, Z.; Li, J.; Li, D.; Tang, T.; et al. A Unique Population of Regulatory T Cells in Heart Potentiates Cardiac Protection From Myocardial Infarction. Circulation 2020, 142, 1956–1973. [Google Scholar] [CrossRef] [PubMed]
- Albany, C.J.; Trevelin, S.C.; Giganti, G.; Lombardi, G.; Scottà, C. Getting to the Heart of the Matter: The Role of Regulatory T-Cells (Tregs) in Cardiovascular Disease (CVD) and Atherosclerosis. Front. Immunol. 2019, 10, 2795. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; McKenzie, B.S.; Beilke, J.; Ramsdell, F. Opportunities for Treg cell therapy for the treatment of human disease. Front. Immunol. 2023, 14, 1166135. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Koga, K.; Sato, T.; Shinohara, T.; Iriguchi, S.; Matsuda, A.; Nakazono, K.; Shioiri, M.; Miyake, Y.; Kassai, Y.; et al. Human iPSC-derived CD4+ Treg-like cells engineered with chimeric antigen receptors control GvHD in a xenograft model. Cell Stem Cell 2024, 31, 795–802.e6. [Google Scholar] [CrossRef]
- Drutman, S.B.; Trombetta, E.S. Dendritic Cells Continue To Capture and Present Antigens after Maturation In Vivo. J. Immunol. 2010, 185, 2140–2146. [Google Scholar] [CrossRef]
- Yip, S.; Wang, N.; Sugimura, R. Give Them Vasculature and Immune Cells: How to Fill the Gap of Organoids. Cells Tissues Organs 2023, 212, 369–382. [Google Scholar] [CrossRef]
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017, 8, 1764. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Tohyama, S. Human Engineered Heart Tissue Models for Disease Modeling and Drug Discovery. Front. Cell Dev. Biol. 2022, 10, 855763. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, X.; Li, G.; Gokulnath, P.; Xiao, J. Generating 3D human cardiac constructs from pluripotent stem cells. eBioMedicine 2022, 76. [Google Scholar] [CrossRef]
- Acun, A.; Nguyen, T.D.; Zorlutuna, P. In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction. Acta Biomater. 2019, 94, 372–391. [Google Scholar] [CrossRef]


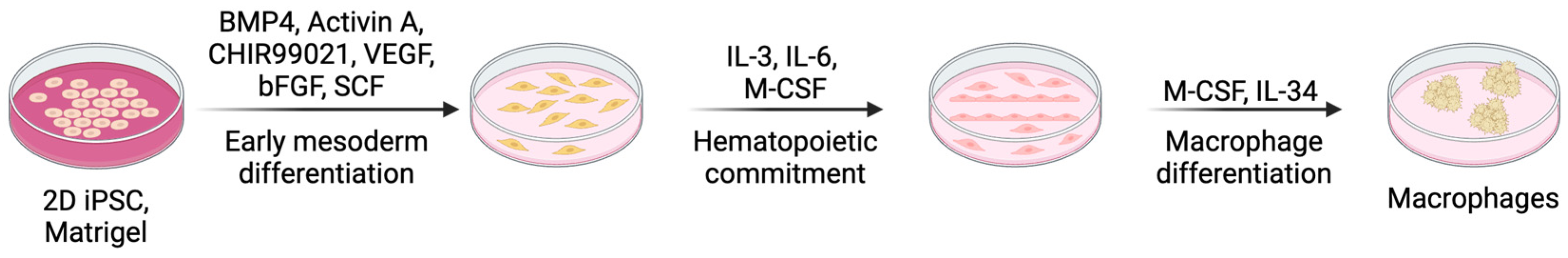
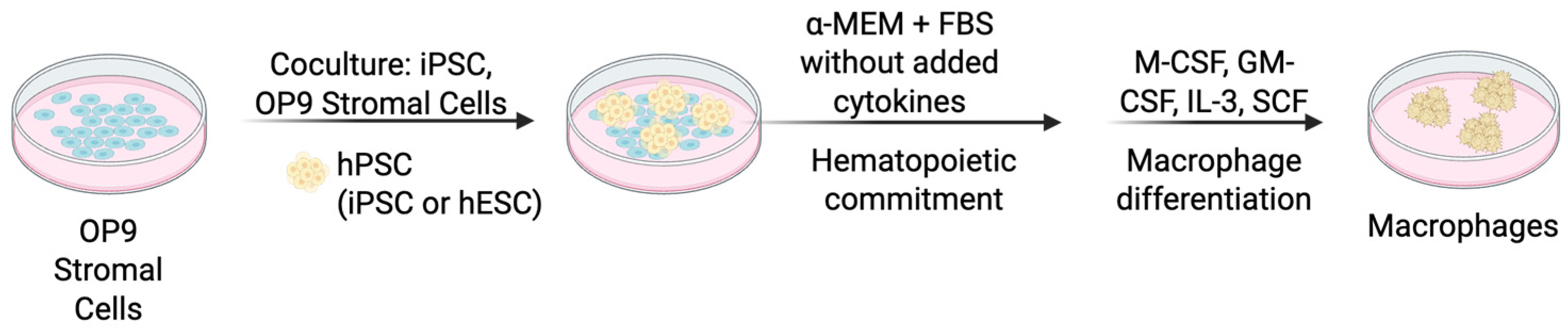
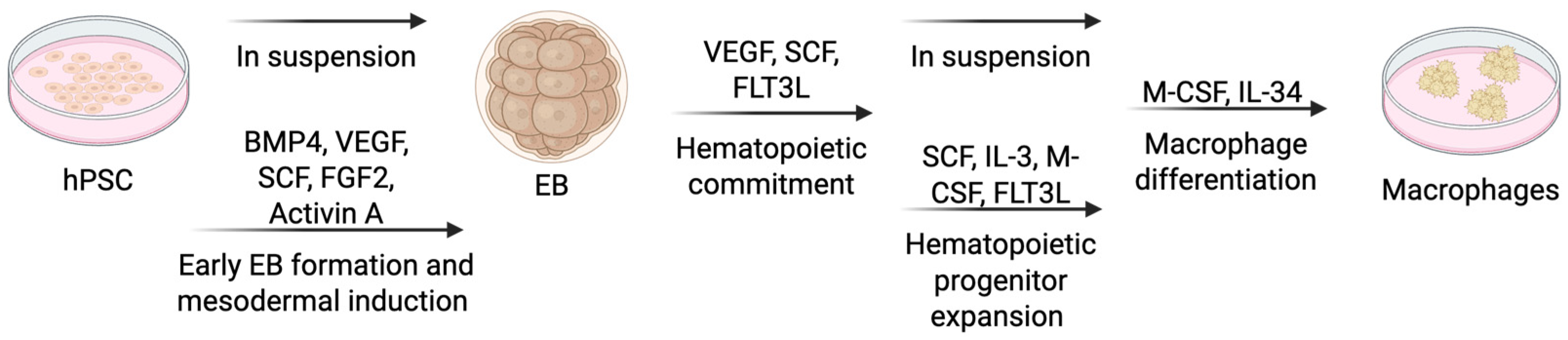
| Differentiation Method | Key Characteristics | Advantages | Limitations |
|---|---|---|---|
| Monolayer (2D) Culture | Stepwise differentiation using defined media; cytokines (BMP4, VEGF, SCF, IL-3, IL-6, M-CSF, IL-34) | Precise control, reproducible, efficient | Requires optimization, variability among iPSC lines, limited maturation |
| Co-Culture with Stromal Cells (OP9) | Stromal cell interactions drive differentiation; minimal cytokine use initially | High yield, functional macrophages, mimics niche signaling | Batch variability, xenogeneic concerns (mouse cells), undefined factors |
| Embryoid Body (EB) Formation | Mimics yolk sac hematopoiesis; cytokines (BMP4, VEGF, SCF, FGF2, FLT3L, IL-3, M-CSF, IL-34) used | Resembles embryonic development, scalable, facilitates genetic editing | Variable EB size, inconsistent yields, undefined components (e.g., serum) |
| Study | Cell Types Included | Key Findings | Implications |
|---|---|---|---|
| Lock et al. (2024) | iPSC-derived cardiomyocytes, fibroblasts, macrophages (hematopoietic origin) | Enhanced contractile force; improved calcium handling and beta-adrenergic signaling | Physiologically relevant cardiac models; useful in regenerative medicine and drug testing |
| Landau et al. (2024) | Human embryonic stem cell (hESC)-derived cardiomyocytes, endothelial cells, stromal cells, primitive macrophages (by EB Formation; yolk sac-like origin) | Improved vascularization and vessel stability; increased pro-angiogenic signaling | Effective modeling of cardiac vascular networks; potential for improving tissue integration post-transplantation |
| Hamidzada et al. (2024) | hESC-derived cardiomyocytes, fibroblasts, primitive macrophages (by EB Formation; yolk sac-like origin) | Enhanced contractility and relaxation; promoted sarcomeric maturation via efferocytosis | Improved cardiac tissue maturation; valuable for modeling developmental processes and regenerative therapies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.P.; Fine, B.M. Integrating Macrophages into Human-Engineered Cardiac Tissue. Cells 2025, 14, 1393. https://doi.org/10.3390/cells14171393
Zhao YP, Fine BM. Integrating Macrophages into Human-Engineered Cardiac Tissue. Cells. 2025; 14(17):1393. https://doi.org/10.3390/cells14171393
Chicago/Turabian StyleZhao, Yi Peng, and Barry M. Fine. 2025. "Integrating Macrophages into Human-Engineered Cardiac Tissue" Cells 14, no. 17: 1393. https://doi.org/10.3390/cells14171393
APA StyleZhao, Y. P., & Fine, B. M. (2025). Integrating Macrophages into Human-Engineered Cardiac Tissue. Cells, 14(17), 1393. https://doi.org/10.3390/cells14171393






