Immunophenotypic Profile of Normal Hematopoietic Populations in Human Bone Marrow: Influence of Gender and Aging as a Basis for Reference Value Establishment
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Participants and Sample Obtainment
2.2. Flow Cytometry Protocol for Hematopoietic and Immune Cell Analysis
2.3. Data Analysis and Gating Strategy
2.4. Statistical Analysis
3. Results
3.1. Concordance of Single vs. Dual Platform Measures, Bone Marrow Purity Assessment, and Dysplasia Analysis
3.2. Age-Related Characterization of Hematopoietic Populations and Maturation Dynamics
3.3. Age-Associated Variations in Bone Marrow Cellularity
3.4. B Cell Maturation and Plasma Cell Distribution Across Age Groups
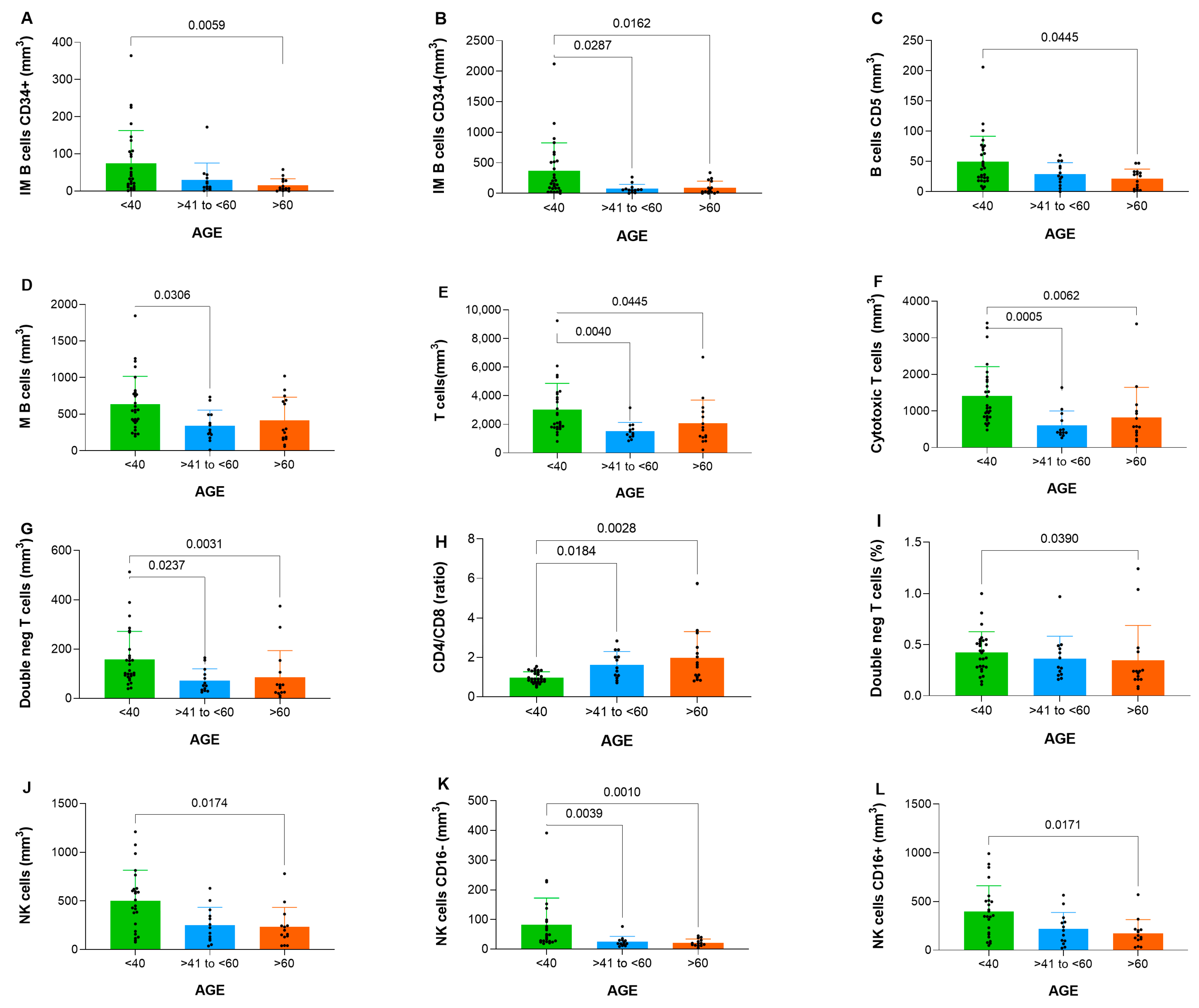
3.5. T and NK Cell Subpopulation Distribution Across Age Groups
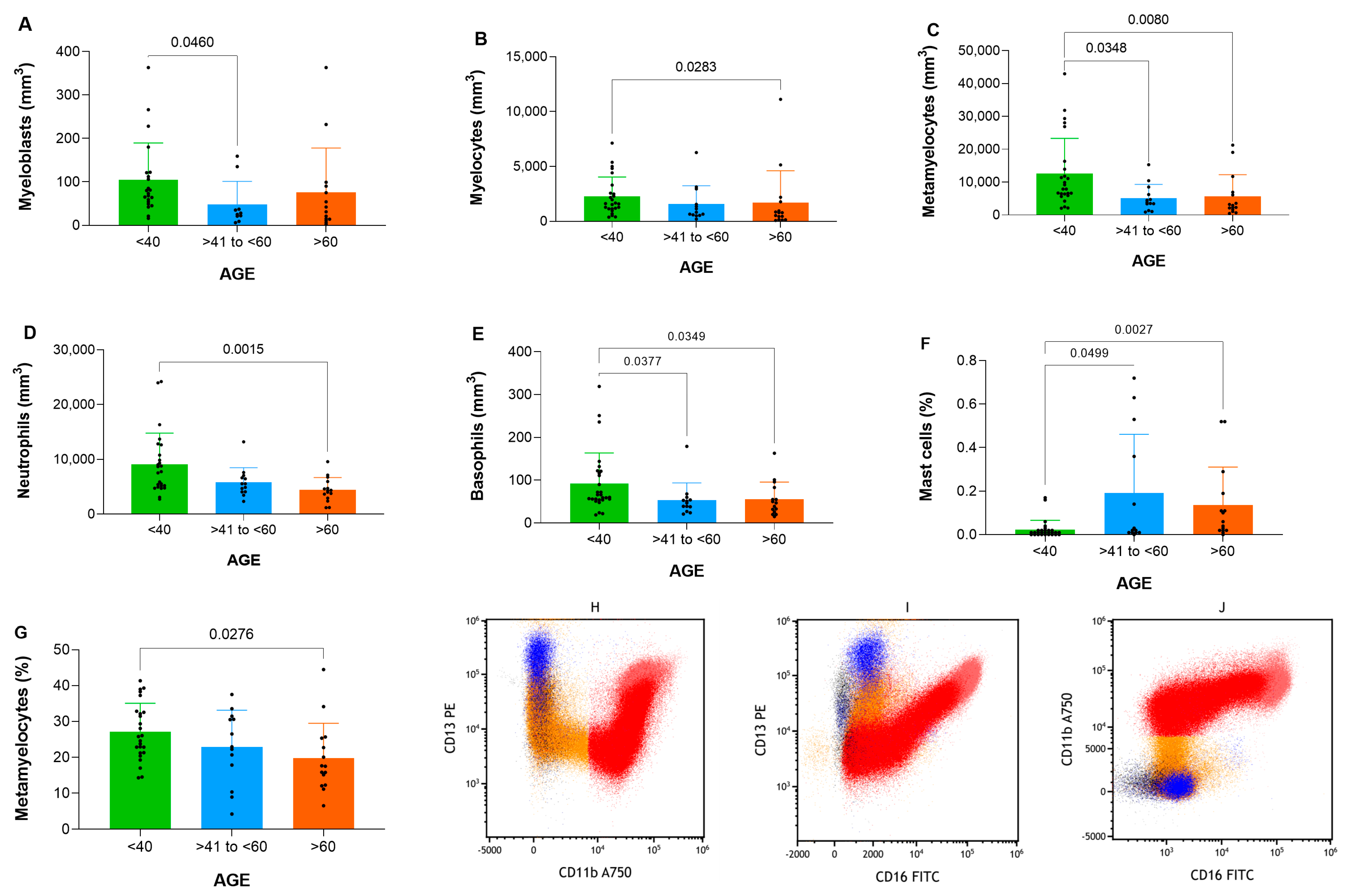
3.6. Granulocyte Maturation and Age-Related Differences
3.7. Monocytic Maturation, Dendritic Cell Subpopulations and Age-Related Differences
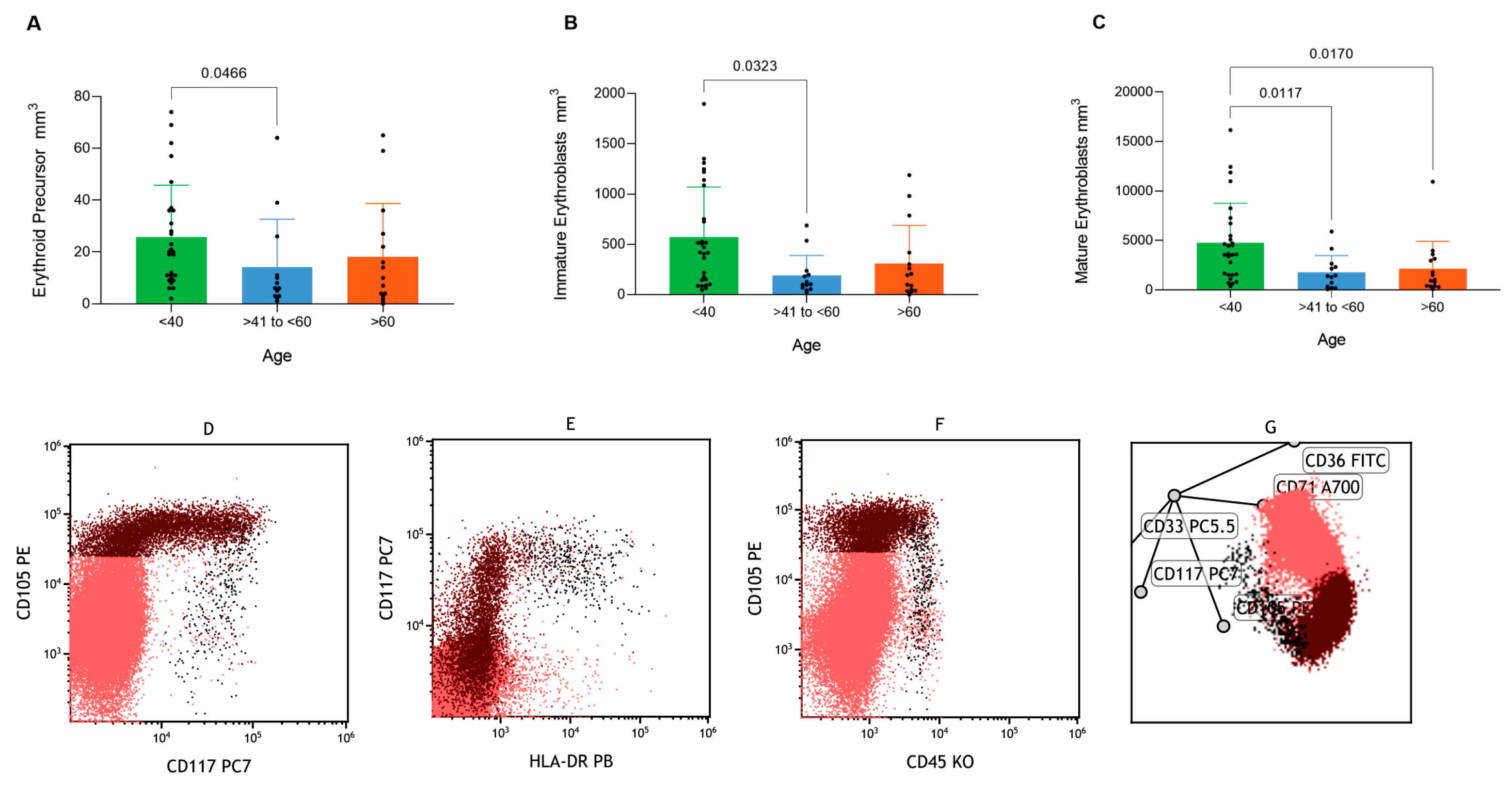
3.8. Erythroid Lineage Maturation and Age-Related Differences
3.9. Gender-Based Analysis of Immune Cell Subpopulations
3.10. Evaluation of the Kappa/Lambda Ratio in B Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Bone Marrow |
| CLSI | Clinical and Laboratorial Standard Institute |
| CAAE | Certificate of Ethical Review Presentation |
| FC | Flow Cytometry |
| FSC | Forward Scatter |
| NK | Natural Killer |
| SSC | Side Scatter |
| WHO | World Health Organization |
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A. Classification of Tumors of the Hematopoietic and Lymphoid Tissues. Discovering Diseases—Defining Their Features. J. Hematol. 2020, 1, 7–9. [Google Scholar] [CrossRef]
- Arnoulet, C.; Béné, M.C.; Durrieu, F.; Feuillard, J.; Fossat, C.; Husson, B.; Jouault, H.; Maynadié, M.; Lacombe, F. Four- and five-color flow cytometry analysis of leukocyte differentiation pathways in normal bone marrow: A reference document based on a systematic approach by the GTLLF and GEIL. Cytom. Part B Clin. Cytom. 2010, 78, 4–10. [Google Scholar] [CrossRef]
- Roa-Higuera, D.C.; Fiorentino-Gómez, S.; Rodríguez-Pardo, V.M.; Campos-Arenas, A.M.; Infante-Acosta, E.A.; Cardozo-Romero, C.C.; Quijano-Gómez, S.M. Immunophenotypic analysis of normal cell samples from bone marrow: Applications in quality control of cytometry laboratories. Univ. Sci. 2010, 15, 206–223. [Google Scholar] [CrossRef]
- Brooimans, R.A.; Kraan, J.; van Putten, W.; Cornelissen, J.J.; Löwenberg, B.; Gratama, J.W. Flow cytometric differential of leukocyte populations in normal bone marrow: Influence of peripheral blood contamination. Cytom. Part B Clin Cytom. 2009, 76, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sabattini, E.; Bacci, F.; Sagramoso, C.; Pileri, S.A. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: An overview. Pathologica 2010, 102, 83–87. [Google Scholar]
- van Lochem, E.G.; van der Velden, V.H.J.; Wind, H.K.; Marvelde, J.G.T.; Westerdaal, N.A.C.; van Dongen, J.J.M. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: Reference patterns for age-related changes and disease-induced shifts. Cytom. Part B Clin Cytom. 2004, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Pont, J.; Souvignet, A.; Campos, L.; Plesa, A.; Bulabois, B.; Pernollet, M.; Raskovalova, T.; Dumestre-Perard, C.; Cesbron, J.; Jacob, M. Accurate quantification of fourteen normal bone marrow cell subsets in infants to the elderly by flow cytometry. Cytom. Part B Clin Cytom. 2018, 94, 627–636. [Google Scholar] [CrossRef]
- Bento, L.C.; Correia, R.P.; Mangueira, C.L.P.; Barroso, R.D.S.; Rocha, F.A.; Bacal, N.S.; Marti, L.C. The Use of Flow Cytometry in Myelodysplastic Syndromes: A Review. Front. Oncol. 2017, 7, 270. [Google Scholar] [CrossRef]
- Matarraz, S.; Fernandez, C.; Albors, M.; Teodosio, C.; López, A.; Jara-Acevedo, M.; Cervero, C.; Caballero, G.; Gutierrez, O.; Orfao, A. Cell-cycle distribution of different cell compartments in normal versus reactive bone marrow: A frame of reference for the study of dysplastic hematopoiesis. Cytom. Part B Clin Cytom. 2011, 80, 354–361. [Google Scholar] [CrossRef]
- Lorand-Metze, I.; Longhini, A.L.; Oliveira-Duarte, G.; Correia, R.P.; Santos-Silva, M.C.; Yamamoto, M.; Sandes, A.F.; Oliveira, A.F.; Souto, E.X.; Ikoma, M.R.V.; et al. Normal variation of bone marrow B-cell precursors according to age–reference ranges for studies in myelodysplastic syndromes in Brazil. Cytom. Part B Clin Cytom. 2018, 94, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Nies, K.P.; Kraaijvanger, R.; Lindelauf, K.H.; Drent, R.J.; Rutten, R.M.; Ramaekers, F.C.; Leers, M.P. Determination of the proliferative fractions in differentiating hematopoietic cell lineages of normal bone marrow. Cytom. Part A 2018, 93, 1097–1105. [Google Scholar] [CrossRef]
- Rajab, A.; Porwit, A. Screening bone marrow samples for abnormal lymphoid populations and myelodysplasia-related features with one 10-color 14-antibody screening tube. Cytom. Part B Clin Cytom. 2015, 88, 253–260. [Google Scholar] [CrossRef]
- Orfao, A.; Matarraz, S.; Pérez-Andrés, M.; Almeida, J.; Teodosio, C.; Berkowska, M.A.; van Dongen, J.J. Immunophenotypic dissection of normal hematopoiesis. J. Immunol. Methods 2019, 475, 112684. [Google Scholar] [CrossRef]
- Nelson, P.N.; Reynolds, G.M.; Waldron, E.E.; Ward, E.; Giannopoulos, K.; Murray, P.G. Monoclonal antibodies. Mol. Pathol. 2000, 53, 111–117. [Google Scholar] [CrossRef]
- Davis, B.H.; Holden, J.; Bene, M.; Borowitz, M.; Braylan, R.; Cornfield, D.; Gorczyca, W.; Lee, R.; Maiese, R.; Orfao, A.; et al. 2006 Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: Medical indications. Cytom. Part B Clin Cytom. 2007, 72 (Suppl. 1), S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Loken, M.R.; Shah, V.O.; Dattilio, K.L.; Civin, C.I. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood 1987, 70, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Kalina, T.; Flores-Montero, J.; van der Velden, V.H.J.; Martin-Ayuso, M.; Böttcher, S.; Ritgen, M.; Almeida, J.; Lhermitte, L.; Asnafi, V.; Mendonça, A.; et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012, 26, 1986–2010. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Clinical Flow Cytometric Analysis of Neoplastic Hematolymphoid Cells; Approved Guideline, 2nd ed.; CLSI document H43-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- Overgaard, N.H.; Jung, J.W.; Steptoe, R.J.; Wells, J.W. CD4+/CD8+ double-positive T cells: More than just a developmental stage? J. Leukoc. Biol. 2015, 97, 31–38. [Google Scholar] [CrossRef]
- Parel, Y.; Chizzolini, C. CD4+ CD8+ double positive (DP) T cells in health and disease. Autoimmun. Rev. 2004, 3, 215–220. [Google Scholar] [CrossRef]
- Rahemtullah, A.; Reichard, K.K.; Preffer, F.I.; Harris, N.L.; Hasserjian, R.P. A double-positive CD4+CD8+ T-cell population is commonly found in nodular lymphocyte predominant Hodgkin lymphoma. Am. J. Clin. Pathol. 2006, 126, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the immune system. Mechanisms and therapeutic targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 5), S422–S428. [Google Scholar] [CrossRef]
- Ogata, K. Diagnostic flow cytometry for low-grade myelodysplastic syndromes. Hematol. Oncol. 2008, 26, 193–198. [Google Scholar] [CrossRef]
- Levy, R.; Warnke, R.; Dorfman, R.F.; Haimovich, J. The monoclonality of human B-cell lymphomas. J. Exp. Med. 1977, 145, 1014–1028. [Google Scholar] [CrossRef]
- Marti, G.E.; Rawstron, A.C.; Ghia, P.; Hillmen, P.; Houlston, R.S.; Kay, N.; Schleinitz, T.A.; Caporaso, N.; International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br. J. Haematol. 2005, 130, 325–332. [Google Scholar] [CrossRef]
- Wood, B. Multicolor immunophenotyping: Human immune system hematopoiesis. Methods Cell Biol. 2004, 75, 559–576. [Google Scholar] [PubMed]
- Westers, T.M.; Cremers, E.M.; Oelschlaegel, U.; Johansson, U.; Bettelheim, P.; Matarraz, S.; Orfao, A.; Moshaver, B.; Brodersen, L.E.; Loken, M.R.; et al. Immunophenotypic analysis of erythroid dysplasia in myelodysplastic syndromes. A report from the IMDSFlow working group. Haematologica 2017, 102, 308–319. [Google Scholar] [CrossRef]
- Groarke, E.M.; Young, N.S. Aging and Hematopoiesis. Clin. Geriatr. Med. 2019, 35, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.G.; Henry, C.J. Aging and immunotherapies: New horizons for the golden ages. Aging Cancer 2020, 1, 30–44. [Google Scholar] [CrossRef]
- Boyd, J. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guidelines; CLSI document C28-A3. 28, No. 3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]

| Healthy Donors | Median (1st and 3rd Quartiles) | Minimum-Maximum |
|---|---|---|
| Age, years | 41 (28–62) | 18–91 |
| Hemoglobin, g/dL | 13.25 (11.70–14.50) | 10.10–18.60 |
| Haematocrit, % | 38.25 (35.00–42.15) | 28.00–56.00 |
| Mean corpuscular volume, fL | 86.45 (84.00–88.42) | 79.20–98.20 |
| Platelets, mm3 | 243.000 (200.750–286.250) | 136.000–530.000 |
| Cell Population | AGE | p Value | |||
|---|---|---|---|---|---|
| <40 Years | 41–60 Years | >60 Years | |||
| Immature B-cells CD34 (positive) | |||||
| Median | (mm3) | 37 (14–107) | 12 (7–35) | 7 (3–28) | 0.006 |
| (%) | 0.14 (0.06–0.20) | 0.09 (0.03–0.17) | 0.04 (0.02–0.10) | 0.053 | |
| Min Max | (mm3) | 1–364 | 1–172 | 0–58 | |
| (%) | 0.00–0.95 | 0.00–0.73 | 0.00–0.27 | ||
| Immature B-cells CD34 (negative) | |||||
| Median | (mm3) | 182 (81–523) | 58 (48–71) | 29 (17–198) | 0.004 |
| (%) | 0.5 (0.2–1.0) | 0.3 (0.1–0.5) | 0.3 (0.1–0.6) | 0.062 | |
| Min Max | (mm3) | 2–2120 | 0–265 | 0–339 | |
| (%) | 0.0–5.5 | 0.0–1.1 | 0.0–1.6 | ||
| B-cells CD5 (positive) | |||||
| Median | (mm3) | 38 (21–71) | 26 (16–42) | 26 (4–33) | 0.043 |
| (%) | 0.11 (0.05–0.22) | 0.22 (0.06–0.23) | 0.07 (0.03–0.15) | 0.288 | |
| Min Max | (mm3) | 5–206 | 0–60 | 1–47 | |
| (%) | 0.02–0.29 | 0.00–0.37 | 0.01–0.55 | ||
| Mature B-cells | |||||
| Median | (mm3) | 552 (394–787) | 285 (220–492) | 283 (162–690) | 0.015 |
| (%) | 1.7 (1.2–2.2) | 1.7 (1.0–3.1) | 2.1 (0.9–2.9) | 0.740 | |
| Min Max | (mm3) | 199–1844 | 10–734 | 58–1021 | |
| (%) | 0.7–4.6 | 0.1–6.0 | 0.2–5.6 | ||
| Plasma cells (polyclonal) | |||||
| Median | (mm3) | 49 (32–84) | 35 (13–54) | 42 (15–119) | 0.270 |
| (%) | 0.16 (0.07–0.24) | 0.14 (0.12–0.22) | 0.21 (0.13–0.44) | 0.315 | |
| Min Max | (mm3) | 1–355 | 2–203 | 1–571 | |
| (%) | 0.00–0.82 | 0.01–0.69 | 0.01–0.74 | ||
| Plasma cells CD19 (polyclonal) | |||||
| Median | (mm3) | 41 (25–71) | 20 (9–37) | 30 (11–100) | 0.104 |
| (%) | 0.13 (0.06–0.20) | 0.11 (0.05–0.17) | 0.15 (0.10–0.37) | 0.392 | |
| Min Max | (mm3) | 1–306 | 2–186 | 1–456 | |
| (%) | 0.00–0.74 | 0.01–0.63 | 0.01–0.59 | ||
| Plasma cells CD56 (polyclonal) | |||||
| Median | (mm3) | 3 (1–7) | 3 (1–4) | 4 (1–11) | 0.780 |
| (%) | 0.01 (0.00–0.03) | 0.02 (0.01–0.02) | 0.02 (0.01–0.05) | 0.080 | |
| Min Max | (mm3) | 0–34 | 0–9 | 0–93 | |
| (%) | 0.00–0.05 | 0.00–0.03 | 0.00–0.12 | ||
| T-cells | |||||
| Median | (mm3) | 2125 (1800–4002) | 1379 (1170–1668) | 1596 (1088–2876) | 0.002 |
| (%) | 7.4 (5.7–9.6) | 8.0 (7.3–9.9) | 9.4 (11.3–5.8) | 0.485 | |
| Min Max | (mm3) | 801–9251 | 850–3160 | 211–6708 | |
| (%) | 3.6–18.0 | 3.4–14.1 | 3.3–21.4 | ||
| Helper T-cells | |||||
| Median | (mm3) | 1031 (847–1711) | 799 (542–916) | 936 (557–1721) | 0.090 |
| (%) | 3.3 (2.2–4.7) | 4.9 (3.2–5.7) | 5.3 (3.4–6.8) | 0.074 | |
| Min Max | (mm3) | 234–5240 | 526–1340 | 172–2849 | |
| (%) | 1.1–9.3 | 1.6–7.0 | 1.9–11.9 | ||
| Cytotoxic T-cells | |||||
| Median | (mm3) | 1087 (840–1827) | 419 (407–738) | 571 (339–995) | <0.001 |
| (%) | 3.4 (2.9–4.1) | 2.8 (1.9–3.3) | 2.7 (2.1–4.2) | 0.127 | |
| Min Max | (mm3) | 483–3405 | 269–1643 | 30–3381 | |
| (%) | 1.7–7.5 | 1.2–7.0 | 0.5–7.9 | ||
| Double-positive T-cells | |||||
| Median | (mm3) | 44 (29–79) | 21 (9–55) | 57 (20–83) | 0.149 |
| (%) | 0.12 (0.08–0.22) | 0.17 (0.05–0.24) | 0.21 (0.13–0.41) | 0.132 | |
| Min Max | (mm3) | 4–532 | 2–137 | 3–130 | |
| (%) | 0.02–1.48 | 0.01–0.80 | 0.04–0.59 | ||
| Double-negative T-cells | |||||
| Median | (mm3) | 108 (89–186) | 52 (34–97) | 48 (24–106) | 0.001 |
| (%) | 0.4 (0.3–0.5) | 0.3 (0.2–0.5) | 0.2 (0.4–0.2) | 0.041 | |
| Min Max | (mm3) | 39–513 | 25–165 | 5–375 | |
| (%) | 0.1–1.0 | 0.2–1.0 | 0.1–1.2 | ||
| Ratio CD4/CD8 | |||||
| Median | ratio | 0.9 (0.8–1.3) | 1.5 (1.1–2.0) | 1.6 (1.0–2.5) | 0.001 |
| Min Max | 0.5–1.5 | 0.7–2.8 | 0.8–5.7 | ||
| NK-cells | |||||
| Median | (mm3) | 481 (226–626) | 226 (111–345) | 179 (129–246) | 0.007 |
| (%) | 1.1 (0.7–1.8) | 1.1 (0.6–1.3) | 0.9 (0.4–1.6) | 0.592 | |
| Min Max | (mm3) | 77–1210 | 37–631 | 39–781 | |
| (%) | 0.2–5.4 | 0.4–4.4 | 0.3–3.8 | ||
| NK-cells CD16 (negative) | |||||
| Median | (mm3) | 47 (28–96) | 19 (17–30) | 20 (13–30) | <0.001 |
| (%) | 0.14 (0.09–0.24) | 0.12 (0.07–0.19) | 0.10 (0.09–0.13) | 0.147 | |
| Min Max | (mm3) | 19–392 | 9–77 | 4–45 | |
| (%) | 0.07–0.67 | 0.03–0.25 | 0.03–0.21 | ||
| NK-cells CD16 (positive) | |||||
| Median | (mm3) | 353 (171–507) | 203 (96–289) | 142 (106–210) | 0.011 |
| (%) | 0.95 (0.51–1.53) | 0.93 (0.45–1.00) | 0.82 (0.32–1.41) | 0.784 | |
| Min Max | (mm3) | 48–992 | 20–565 | 28–570 | |
| (%) | 0.15–4.97 | 0.20–4.14 | 0.21–2.80 | ||
| Cell Population | AGE | p Value | |||
|---|---|---|---|---|---|
| <40 Years | 41–60 Years | >60 Years | |||
| Myeloblasts | |||||
| Median | (mm3) | 77 (54–121) | 28 (21–37) | 37 (14–90) | 0.030 |
| (%) | 0.23 (0.19–0.32) | 0.14 (0.13–0.32) | 0.21 (0.12–0.31) | 0.726 | |
| Min Max | (mm3) | 16–363 | 6–159 | 2–363 | |
| (%) | 0.06–0.44 | 0.03–0.44 | 0.04–0.48 | ||
| Promyelocytes | |||||
| Median | (mm3) | 220 (114–331) | 91 (67–159) | 91 (27–245) | 0.029 |
| (%) | 0.6 (0.4–07) | 0.5 (0.4–0.7) | 0.5 (0.3–0.8) | 0.831 | |
| Min Max | (mm3) | 41–747 | 38–348 | 3–1215 | |
| (%) | 0.2–1.3 | 0.3–1.3 | 0.1–1.6 | ||
| Myelocytes | |||||
| Median | (mm3) | 1818 (1172–3867) | 987 (600–2258) | 791 (156–1777) | 0.015 |
| (%) | 5.5 (4.0–6.7) | 4.9 (4.0–6.8) | 3.5 (1.8–6.7) | 0.170 | |
| Min Max | (mm3) | 376–7127 | 206–6265 | 79–11,114 | |
| (%) | 2.4–12.2 | 1.4–29.3 | 1.4–14.8 | ||
| Metamyelocytes (band cell) | |||||
| Median | (mm3) | 9554 (6426–15,143) | 4184 (3418–7393) | 2956 (1695–6947) | 0.002 |
| (%) | 26.6 (22.1–32.7) | 24.7 (19.3–31.0) | 17.5 (12.3–25.4) | 0.016 | |
| Min Max | (mm3) | 2052–42,968 | 906–15,294 | 464–21,284 | |
| (%) | 14.3–41.4 | 4.2–37.5 | 6.5–44.5 | ||
| Neutrophils | |||||
| Median | (mm3) | 7643 (5183–10,344) | 5560 (4398–6845) | 4315 (2932–5950) | 0.001 |
| (%) | 22.3 (16.8–26.3) | 26.9 (22.8–30.4) | 21.7 (15.2–30.7) | 0.157 | |
| Min Max | (mm3) | 2725–24,203 | 2331–13,200 | 1181–9588 | |
| (%) | 8.9–43.1 | 20.4–42.6 | 9.5–43.5 | ||
| Eosinophils | |||||
| Median | (mm3) | 605 (497–933) | 338 (222–733) | 429 (85–713) | 0.030 |
| (%) | 2.0 (1.7–2.6) | 1.7 (1.2–3.4) | 2.0 (1.2–3.6) | 0.801 | |
| Min Max | (mm3) | 292–3575 | 166–1402 | 58–2694 | |
| (%) | 1.1–3.6 | 0.9–6.1 | 0.5–4.5 | ||
| Basophils | |||||
| Median | (mm3) | 63 (55–119) | 47 (38–54) | 47 (26–83) | 0.009 |
| (%) | 0.20 (0.14–0.35) | 0.24 (0.18–0.30) | 0.22 (0.15–0.32) | 0.507 | |
| Min Max | (mm3) | 19; 319 | 21; 179 | 15; 163 | |
| (%) | 0.07–0.50 | 0.10–0.61 | 0.13–0.59 | ||
| Mast cells | |||||
| Median | (mm3) | 3 (2–7) | 4 (3–52) | 19 (4–32) | |
| (%) | 0.01 (0.00–0.02) | 0.02 (0.01–0.36) | 0.06 (0.02–0.19) | 0.054 | |
| Min Max | (mm3) | 0–64 | 0–255 | 0–82 | 0.002 |
| (%) | 0.00–0.17 | 0.00–0.72 | 0.00–0.52 | ||
| Cell Population | AGE | p Value | |||
|---|---|---|---|---|---|
| <40 Years | 41–60 Years | >60 Years | |||
| Monoblasts | |||||
| Median | (mm3) | 39 (18–116) | 50 (14–122) | 25 (5–63) | 0.126 |
| (%) | 0.11 (0.05–0.31) | 0.25 (0.08–0.78) | 0.12 (0.02–0.34) | 0.350 | |
| Min Max | (mm3) | 6–280 | 3–299 | 0–127 | |
| (%) | 0.02–0.77 | 0.02–1.02 | 0.00–0.75 | ||
| Promonocytes | |||||
| Median | (mm3) | 526 (325–930) | 222 (143–273) | 207 (105–438) | 0.001 |
| (%) | 2 (1–2) | 1 (1–1) | 1 (1–2) | 0.114 | |
| Min Max | (mm3) | 107–2420 | 61–602 | 16–1095 | |
| (%) | 1–3 | 1–2 | 0–3 | ||
| Mature monocytes | |||||
| Median | (mm3) | 892 (1042–508) | 566 (725–487) | 484 (600–355) | 0.007 |
| (%) | 2 (3–1) | 3 (4–3) | 3 (4–2) | 0.162 | |
| Min Max | (mm3) | 286–1528 | 163–908 | 112–1044 | |
| (%) | 1–6 | 1–6 | 1–8 | ||
| Classical monocytes | |||||
| Median | (mm3) | 662 (443–861) | 502 (452–558) | 384 (315–440) | 0.005 |
| (%) | 1.9 (1.2–2.5) | 2.4 (2.3–3.1) | 2.1 (1.4–3.9) | 0.211 | |
| Min Max | (mm3) | 275–1328 | 131–807 | 81–876 | |
| (%) | 0.8–5.1 | 0.7–5.1 | 1.3–5.5 | ||
| Intermediate monocytes | |||||
| Median | (mm3) | 53 (19–63) | 25 (13–44) | 34 (21–62) | 0.353 |
| (%) | 0.10 (0.06–0.24) | 0.17 (0.10–0.22) | 0.20 (0.06–0.32) | 0.540 | |
| Min Max | (mm3) | 3–275 | 9–95 | 1–79 | |
| (%) | 0.01–0.91 | 0.03–0.34 | 0.01–1.07 | ||
| Non-classical monocytes | |||||
| Median | (mm3) | 29 (16–35) | 15 (7–48) | 18 (9–40) | 0.816 |
| (%) | 0.06 (0.05–0.11) | 0.08 (0.05–0.28) | 0.12 (0.02–0.16) | 0.435 | |
| Min Max | (mm3) | 0–66 | 1–134 | 1–97 | |
| (%) | 0.0–7.6 | 0.1–15.2 | 0.3–16.2 | ||
| Myeloid Dendritic cells | |||||
| Median | (mm3) | 69 (43–113) | 32 (21–43) | 22 (13–62) | 0.001 |
| (%) | 0.09 (0.04–0.12) | 0.07 (0.02–0.10) | 0.07 (0.02–0.12) | 0.827 | |
| Min Max | (mm3) | 15–314 | 5–74 | 4–206 | |
| (%) | 0.01–0.18 | 0.00–0.24 | 0.01–0.51 | ||
| Plasmacytoid Dendritic cells | |||||
| Median | (mm3) | 30 (9–53) | 13 (7–16) | 14 (3–46) | 0.108 |
| (%) | 0.20 (0.15–0.27) | 0.15 (0.12–0.18) | 0.16 (0.11–0.23) | 0.827 | |
| Min Max | (mm3) | 1–96 | 0–48 | 1–75 | |
| (%) | 0.08–0.40 | 0.04–0.36 | 0.05–0.35 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, F.A.; Correa, R.P.; Bento, L.C.; Taniguchi, L.F.P.; Bacal, N.S.; Marti, L.C. Immunophenotypic Profile of Normal Hematopoietic Populations in Human Bone Marrow: Influence of Gender and Aging as a Basis for Reference Value Establishment. Cells 2025, 14, 1392. https://doi.org/10.3390/cells14171392
de Sousa FA, Correa RP, Bento LC, Taniguchi LFP, Bacal NS, Marti LC. Immunophenotypic Profile of Normal Hematopoietic Populations in Human Bone Marrow: Influence of Gender and Aging as a Basis for Reference Value Establishment. Cells. 2025; 14(17):1392. https://doi.org/10.3390/cells14171392
Chicago/Turabian Stylede Sousa, Flavia Arandas, Rodolfo Patussi Correa, Laiz Cameirão Bento, Luiz Fabiano Presente Taniguchi, Nydia Strachman Bacal, and Luciana Cavalheiro Marti. 2025. "Immunophenotypic Profile of Normal Hematopoietic Populations in Human Bone Marrow: Influence of Gender and Aging as a Basis for Reference Value Establishment" Cells 14, no. 17: 1392. https://doi.org/10.3390/cells14171392
APA Stylede Sousa, F. A., Correa, R. P., Bento, L. C., Taniguchi, L. F. P., Bacal, N. S., & Marti, L. C. (2025). Immunophenotypic Profile of Normal Hematopoietic Populations in Human Bone Marrow: Influence of Gender and Aging as a Basis for Reference Value Establishment. Cells, 14(17), 1392. https://doi.org/10.3390/cells14171392






