TREM2 in Neurodegenerative Diseases: Mechanisms and Therapeutic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Screening Process
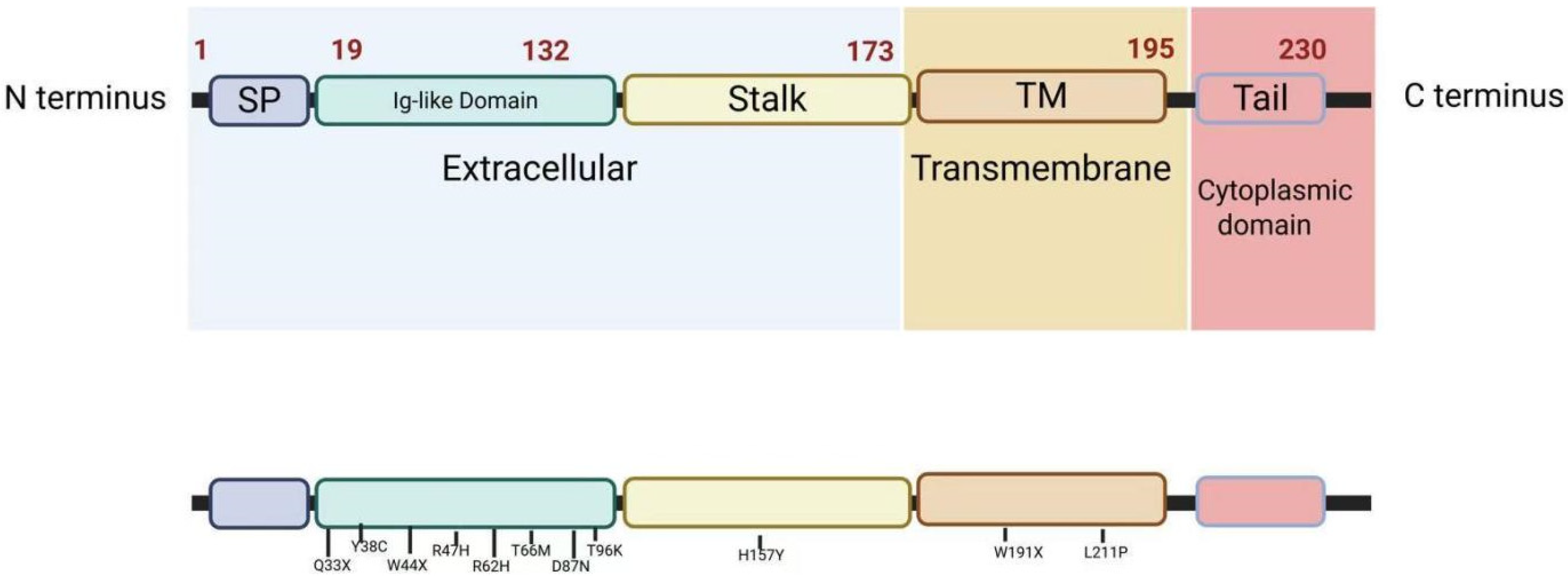
3. Structure of TREM2
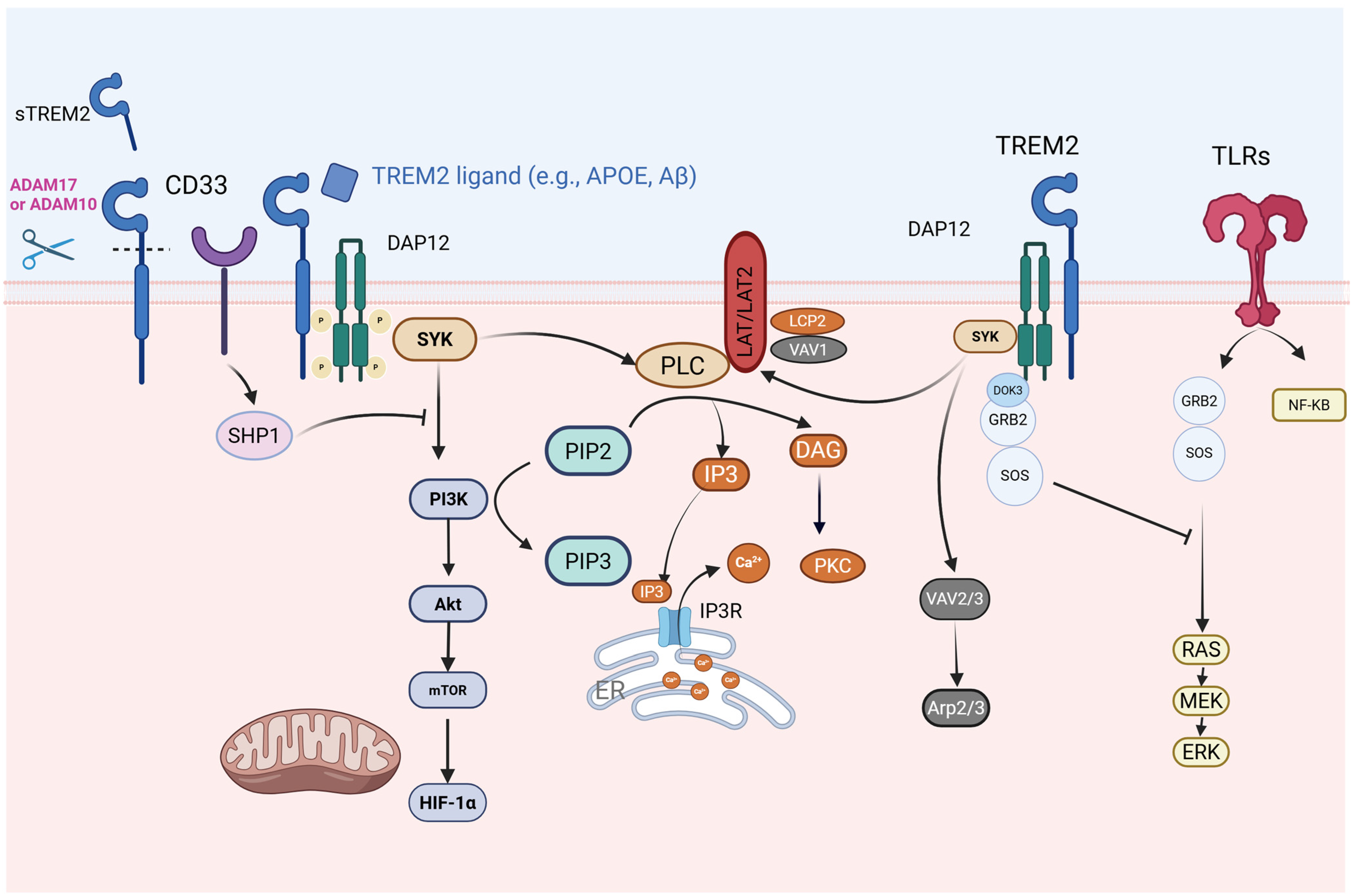
4. TREM2 Signaling Pathways
4.1. TREM2-Toll-like Receptor 4 (TLR4)/MAPK Axis in Neuroinflammatory Regulation
4.2. TREM2 and Calcium-Modulated Signaling Pathways
4.3. TREM2 and SHIP1-Related Signaling Pathways
4.4. Crosstalk Between TREM2 and CD33
5. Soluble TREM2 (sTREM2)
6. TREM2 Variants
| Variant | Mutation Type | Associated Diseases | Domain/Location | Functional Impact | Clinical Significance | References |
|---|---|---|---|---|---|---|
| R47H | Missense | AD, ALS | Coding Exon 2 | Reduces binding to APOE, Aβ, and lipoproteins; impairs SYK/PI3K signaling and phagocytosis. | Increases AD risk (OR ≈ 2–3); linked to diffuse amyloid plaques and axonal dystrophy. | [54,55] |
| R62H | Missense | AD, FTD | Coding Exon 2 | Disrupts dimerization and ligand binding; reduces signaling efficiency. | Moderately increases AD risk (OR ≈ 1.5). | [56,57] |
| T66M | Missense | FTD/FTLDNHD | Coding Exon 2 | Results in misfolding of the TREM2 protein and loss of function. | Results in early-onset dementia and bone cysts. | [58,59,60,61] |
| Y38C | Frameshift mutation | FTD/FTLDNHD | Coding Exon 2 | Alters the flanking sequence of a cysteine for the Ig V-fold interchain disulfide bond; disrupts protein function. | Severe early-onset NHD with dementia and bone lesions. | [58,62,63] |
| Q33X | Nonsense | NHD, AD | Coding Exon 2 | Produces truncated proteins, resulting in a complete loss of protein function. | Homozygous: NHD; heterozygous: increases AD risk. | [58,64] |
| D87N | Missense | AD | Coding Exon 2 | Reduces APOE binding but enhances signaling in some contexts. | Increases AD risk in controversy. | [55] |
| T96K | Missense | AD, FTD | Coding Exon 2 | - | A significant genetic risk factor for late-onset AD (LOAD) in the Tunisian population. | [65,66] |
| H157Y | Missense | AD | Coding Exon 3 | Increases shedding of sTREM2. Reduces activation in response to phospholipid ligands and decreases phagocytosis. | Increases risk of AD. | [67,68] |
| L211P | Missense | FTD | Coding Exon 4 | - | Increases risk of FTD, especially bvFTD. | [65] |
| W44X | Nonsense | NHD | Coding Exon 2 | Produces non-functional truncated proteins that fail to activate microglia. | Strongly associated with NHD phenotype. | [47] |
| W191X | Nonsense | AD | Coding Exon 4 | Stop-gain mutation. | Suggestive association with AD. | [69] |
7. Impact of TREM2 on Microglial Function
7.1. Influence on Microglial Homeostasis and Activation
7.2. TREM2 and Microglial Metabolism
7.3. TREM2 and Microglial Regulation of Inflammation
7.4. Impact of TREM2 on Microglial Phagocytosis
8. TREM2 in AD
9. TREM2 in PD
10. TREM2 in ALS
11. TREM2 in NHD
12. TREM2 in Other Neurological Disorders
12.1. Multiple Sclerosis (MS)
12.2. Neuromyelitis Optica Spectrum Disorder (NMOSD)
12.3. Cerebral Small-Vessel Disease (SVD)
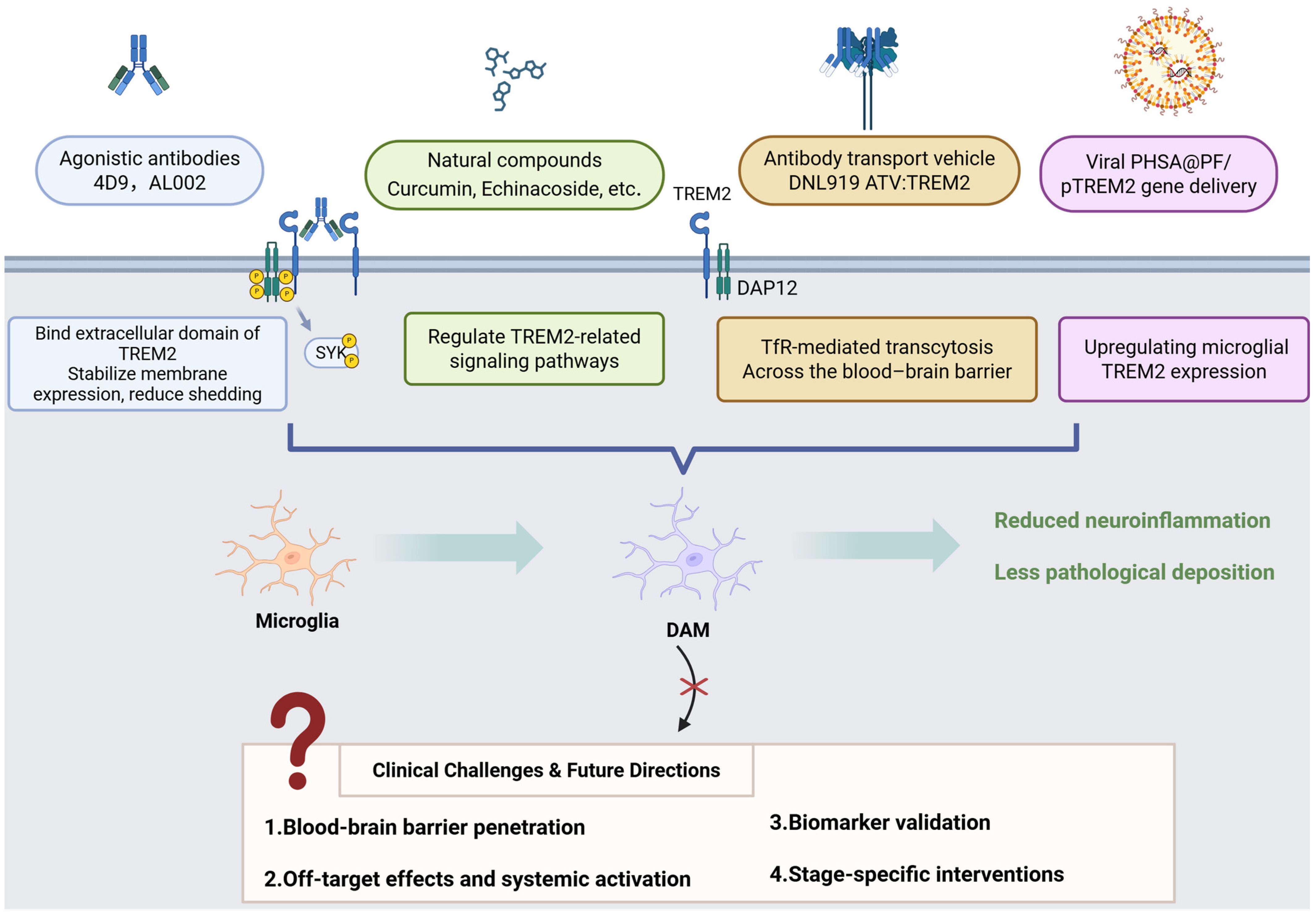
13. Therapeutic Potential of TREM2 in Future Applications
| Drug Name | Drug Type | Mechanism of Action | Indication | Development Stage | Notes | References | Registration Number |
|---|---|---|---|---|---|---|---|
| 4D9 | Monoclonal antibody (agonist) | Stabilizes TREM2 on the cell surface and inhibits shedding; activates TREM2-DAP12/SYK signaling through phospho-SYK. | AD | _ | Reduces amyloidogenesis and homeostatic markers. Enhances microglial TREM2 expression in AD models. | [156,165] | _ |
| AL002 | Humanized mAb (agonist) | Activates TREM2 signaling. | AD | Phase II completed (2024) | Enhances microglial activity to reduce Aβ plaques and neuroinflammation. | [157,158] | NCT04592874 |
| Iluzanebart (VGL101) | Humanized mAb (agonist) | Enhances the TREM2-DAP12/SYK pathway. | AD, adult-onset leukoencephalopathy (ALSP) | Phase II | Well-tolerated; monthly dosing reduces CSF sTREM2 levels. | [166] | NCT05677659 |
| PY314 | Humanized mAb (antagonist) | Blocks TREM2 and depletes tumor-associated macrophages. | Solid tumors (e.g., ovarian cancer) | Phase Ib | Combined with PD-1 inhibitors for platinum-resistant ovarian cancer. | [167,168] | NCT04691375 |
| VHB937 | Humanized mAb (agonist) | Activates TREM2 and reduces shedding. | Neurodegeneration, metabolic syndrome | Preclinical/Phase I (2024 filing) | Enhances microglial phagocytosis; neuroprotective in animal models. | [169] | NCT06643481 |
| DNL919 (TAK-920) | Monoclonal antibody (agonist) | Activates TREM2 signaling. | AD | Discontinued (2023) | Phase I showed hematologic toxicity; narrow therapeutic window. | [12] | NCT05450549 |
| Ab18 TVD-Ig/αTfR | Tetravalent bispecific antibody | Enhances TREM2 clustering and blood–brain barrier (BBB) penetration. | AD | Preclinical | Engineered antibody reduces Aβ burden and improves cognition in mice. | [170] | _ |
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| TREM2 | Triggering Receptor Expressed on Myeloid Cells 2 |
| GWASs | Genome-Wide Association Studies |
| DAM | Disease-Associated Microglia |
| ITAM | Immunoreceptor Tyrosine-based Activation Motif |
| DAP12 | DNAX-Activating Protein 12 |
| SYK | Spleen Tyrosine Kinase |
| PI3K | Phosphatidylinositol 3-Kinase |
| PLCγ | Phospholipase C gamma |
| IP3 | Inositol Trisphosphate |
| DAG | Diacylglycerol |
| mTOR | Mechanistic Target of Rapamycin |
| MAPK | Mitogen-Activated Protein Kinase |
| TLR4 | Toll-Like Receptor 4 |
| NF-κB | Nuclear Factor kappa B |
| NLRP3 | NOD-Like Receptor family Pyrin domain containing 3 |
| IL-1β | Interleukin-1 beta |
| TNF-α | Tumor Necrosis Factor alpha |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| NHD | Nasu–Hakola Disease |
| FTD | Frontotemporal Dementia |
| sTREM2 | Soluble TREM2 |
| CSF | Cerebrospinal Fluid |
| SNP | Single-Nucleotide Polymorphism |
| ADAM10/17 | A Disintegrin and Metalloproteinase 10/17 |
| BBB | Blood–Brain Barrier |
| PET | Positron Emission Tomography |
| APOE | Apolipoprotein E |
| Aβ | Amyloid-beta |
| NFTs | Neurofibrillary Tangles |
| LPS | Lipopolysaccharide |
| MOG | Myelin Oligodendrocyte Glycoprotein |
| EAE | Experimental Autoimmune Encephalomyelitis |
| MS | Multiple Sclerosis |
| NMOSD | Neuromyelitis Optica Spectrum Disorder |
| SVD | Small Vessel Disease |
| CAA | Cerebral Amyloid Angiopathy |
| BCAS | Bilateral Carotid Artery Stenosis |
| CD33 | Cluster of Differentiation 33 |
| TDP-43 | TAR DNA-binding protein 43 |
| CNS | Central Nervous System |
| HDL | High-Density Lipoprotein |
| LDL | Low-Density Lipoprotein |
| TfR | Transferrin Receptor |
| TBI | Traumatic Brain Injury |
| Th17 | T helper 17 cells |
| ZAP70 | Zeta-chain-Associated Protein Kinase 70 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| AQP4 | Aquaporin-4 |
| OPCs | Oligodendrocyte Precursor Cells |
| SVD-HTN | Hypertensive Small Vessel Disease |
| ALSP | Adult-Onset Leukoencephalopathy |
| TREM | Triggering Receptor Expressed on Myeloid Cells |
| TYROBP | TYRO Protein Tyrosine Kinase-Binding Protein |
| PLOSL | Polycystic Lipomembranous Osteodysplasia with Sclerosing Leukoencephalopathy |
| BV2 | Murine Microglial Cell Line |
| CHO | Chinese Hamster Ovary |
| iPSC | Induced Pluripotent Stem Cell |
| BMDMs | Bone Marrow-Derived Macrophages |
| CPZ | Cuprizone |
| LXR | Liver X Receptor |
| ACAT1 | Acyl-CoA Cholesterol Acyltransferase 1 |
| oxLDL | Oxidized Low-Density Lipoprotein |
| ABCA1 | ATP-Binding Cassette Sub-family A Member 1 |
| SHIP1 | Src Homology 2-containing Inositol Phosphatase 1 |
| SHIP2 | Src Homology 2-containing Inositol Phosphatase 2 |
| S1P | Sphingosine-1-phosphate |
| TREM2R47H | TREM2 R47H Variant |
| TREM2CV | TREM2 Common Variant |
| TREM2KO | TREM2 Knockout |
| TREM2−/− | TREM2-Deficient |
| SAMP8 | Senescence-Accelerated Mouse Prone 8 |
| POCD | Postoperative Cognitive Dysfunction |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| α-syn | α-Synuclein |
| TH | Tyrosine Hydroxylase |
| hTDP-43 | Human TAR DNA-binding Protein 43 |
| hTREM2 | Human TREM2 |
| CD11c | Cluster of Differentiation 11c |
| CD68 | Cluster of Differentiation 68 |
| CD4 | Cluster of Differentiation 4 |
| MHC-I | Major Histocompatibility Complex Class I |
| MHC-II | Major Histocompatibility Complex Class II |
| LAM | Lipid-Associated Macrophages |
| ATP | Adenosine Triphosphate |
| HSP70 | Heat Shock Protein 70 |
| GLUT5 | Glucose Transporter 5 |
| SPP1 | Secreted Phosphoprotein 1 |
| p-SYK | Phosphorylated SYK |
| FDG | Fluorodeoxyglucose |
References
- Liu, L.; Zhao, Y.; Zhang, F. RNA Methylation Modifications in Neurodegenerative Diseases: Focus on Their Enzyme System. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Grobler, C.; Van Tongeren, M.; Gettemans, J.; Kell, D.B.; Pretorius, E. Alzheimer’s Disease: A Systems View Provides a Unifying Explanation of Its Development. J. Alzheimer’s Dis. 2023, 91, 43–70. [Google Scholar] [CrossRef]
- Kurkinen, M.; Fułek, M.; Fułek, K.; Beszłej, J.A.; Kurpas, D.; Leszek, J. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: Should We Change Our Thinking? Biomolecules 2023, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Balkhi, S.; Di Spirito, A.; Poggi, A.; Mortara, L. Immune Modulation in Alzheimer’s Disease: From Pathogenesis to Immunotherapy. Cells 2025, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, T.; He, Q.; Ke, W.; Li, X.; Du, J.; Deng, S.; Shu, Z.; Wu, J.; Yang, B.; et al. Microglia Facilitate and Stabilize the Response to General Anesthesia via Modulating the Neuronal Network in a Brain Region-Specific Manner. eLife 2023, 12, RP92252. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, M.; Chen, M.; Li, X.; Feng, X. The Role of Macrophage Plasticity in Neurodegenerative Diseases. Biomark. Res. 2024, 12, 81. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, X.-X.; Yang, X.-X.; Gao, H.-M.; Zhang, F. Dihydroquercetin Alleviates Dopamine Neuron Loss via Regulating TREM2 Activation. Int. J. Biol. Macromol. 2024, 269, 132179. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, L.; Xin, W.; Pan, Y.; Tatenhorst, L.; Hao, Z.; Gerner, S.T.; Huber, S.; Juenemann, M.; Butz, M.; et al. TREM2 Regulates Microglial Lipid Droplet Formation and Represses Post-Ischemic Brain Injury. Biomed. Pharmacother. 2024, 170, 115962. [Google Scholar] [CrossRef]
- Piccio, L.; Deming, Y.; Del-Águila, J.L.; Ghezzi, L.; Holtzman, D.M.; Fagan, A.M.; Fenoglio, C.; Galimberti, D.; Borroni, B.; Cruchaga, C. Cerebrospinal Fluid Soluble TREM2 is Higher in Alzheimer Disease and Associated with Mutation Status. Acta Neuropathol. 2016, 131, 925–933. [Google Scholar] [CrossRef]
- Heslegrave, A.; Heywood, W.; Paterson, R.; Magdalinou, N.; Svensson, J.; Johansson, P.; Öhrfelt, A.; Blennow, K.; Hardy, J.; Schott, J.; et al. Increased Cerebrospinal Fluid Soluble TREM2 Concentration in Alzheimer’s Disease. Mol. Neurodegener. 2016, 11, 3. [Google Scholar] [CrossRef]
- Van Lengerich, B.; Zhan, L.; Xia, D.; Chan, D.; Joy, D.; Park, J.I.; Tatarakis, D.; Calvert, M.; Hummel, S.; Lianoglou, S.; et al. A TREM2-Activating Antibody with a Blood–Brain Barrier Transport Vehicle Enhances Microglial Metabolism in Alzheimer’s Disease Models. Nat. Neurosci. 2023, 26, 416–429. [Google Scholar] [CrossRef]
- Jay, T.R.; Von Saucken, V.E.; Landreth, G.E. TREM2 in Neurodegenerative Diseases. Mol. Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Wang, H.; Yang, K.; Luan, J.; Wang, S. TREM2: Potential Therapeutic Targeting of Microglia for Alzheimer’s Disease. Biomed. Pharmacother. 2023, 165, 115218. [Google Scholar] [CrossRef]
- Wunderlich, P.; Glebov, K.; Kemmerling, N.; Tien, N.T.; Neumann, H.; Walter, J. Sequential Proteolytic Processing of the Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) Protein by Ectodomain Shedding and γ-Secretase-Dependent Intramembranous Cleavage. J. Biol. Chem. 2013, 288, 33027–33036. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Qin, Q.; Yang, H.-C.; Wang, Y.-Y.; Mi, Y.-X.; Yin, Y.-S.; Wang, M.; Yu, C.-J.; Tang, Y. TREM2 in the Pathogenesis of AD: A Lipid Metabolism Regulator and Potential Metabolic Therapeutic Target. Mol. Neurodegener. 2022, 17, 40. [Google Scholar] [CrossRef]
- George, J. TREM2 as an Evolving Therapeutic Target in Alzheimer’s Disease. Neural Regen. Res. 2023, 18, 2680–2681. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Colonna, M. TREM2—A Key Player in Microglial Biology and Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Groza, Y.; Mierzwicka, J.M.; Malý, P. Current Understanding on TREM-2 Molecular Biology and Physiopathological Functions. Int. Immunopharmacol. 2024, 134, 112042. [Google Scholar] [CrossRef]
- Kleinberger, G.; Yamanishi, Y.; Suárez-Calvet, M.; Czirr, E.; Lohmann, E.; Cuyvers, E.; Struyfs, H.; Pettkus, N.; Wenninger-Weinzierl, A.; Mazaheri, F.; et al. TREM2 Mutations Implicated in Neurodegeneration Impair Cell Surface Transport and Phagocytosis. Sci. Transl. Med. 2014, 6, 243ra86. [Google Scholar] [CrossRef]
- Neumann, H.; Takahashi, K. Essential Role of the Microglial Triggering Receptor Expressed on Myeloid Cells-2 (TREM2) for Central Nervous Tissue Immune Homeostasis. J. Neuroimmunol. 2007, 184, 92–99. [Google Scholar] [CrossRef]
- Hwang, M.; Savarin, C.; Kim, J.; Powers, J.; Towne, N.; Oh, H.; Bergmann, C.C. Trem2 Deficiency Impairs Recovery and Phagocytosis and Dysregulates Myeloid Gene Expression During Virus-Induced Demyelination. J. Neuroinflamm. 2022, 19, 267. [Google Scholar] [CrossRef]
- Yu, M.; Chang, Y.; Zhai, Y.; Pang, B.; Wang, P.; Li, G.; Jiang, T.; Zeng, F. TREM2 Is Associated with Tumor Immunity and Implies Poor Prognosis in Glioma. Front. Immunol. 2023, 13, 1089266. [Google Scholar] [CrossRef] [PubMed]
- Kober, D.L.; Brett, T.J. TREM2-Ligand Interactions in Health and Disease. J. Mol. Biol. 2017, 429, 1607–1629. [Google Scholar] [CrossRef]
- Colonna, M. The Biology of TREM Receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Nugent, A.A.; Lin, K.; Van Lengerich, B.; Lianoglou, S.; Przybyla, L.; Davis, S.S.; Llapashtica, C.; Wang, J.; Kim, D.J.; Xia, D.; et al. TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron 2020, 105, 837–854.e9. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Song, W.M.; Huang, S.C.-C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663.e13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Y.; Hunter, S.; Kim, M.-K.; Indik, Z.K.; Schreiber, A.D. The Effect of Phosphatases SHP-1 and SHIP-1 on Signaling by the ITIM- and ITAM-Containing Fcγ Receptors FcγRIIB and FcγRIIA. J. Leukoc. Biol. 2003, 73, 823–829. [Google Scholar] [CrossRef]
- Peng, Q.; Malhotra, S.; Torchia, J.A.; Kerr, W.G.; Coggeshall, K.M.; Humphrey, M.B. TREM2- and DAP12-Dependent Activation of PI3K Requires DAP10 and Is Inhibited by SHIP1. Sci. Signal. 2010, 3, ra38. [Google Scholar] [CrossRef]
- Ruganzu, J.B.; Peng, X.; He, Y.; Wu, X.; Zheng, Q.; Ding, B.; Lin, C.; Guo, H.; Yang, Z.; Zhang, X.; et al. Downregulation of TREM2 Expression Exacerbates Neuroinflammatory Responses through TLR4-Mediated MAPK Signaling Pathway in a Transgenic Mouse Model of Alzheimer’s Disease. Mol. Immunol. 2022, 142, 22–36. [Google Scholar] [CrossRef]
- Chen, S.; Peng, J.; Sherchan, P.; Ma, Y.; Xiang, S.; Yan, F.; Zhao, H.; Jiang, Y.; Wang, N.; Zhang, J.H.; et al. TREM2 Activation Attenuates Neuroinflammation and Neuronal Apoptosis via PI3K/Akt Pathway After Intracerebral Hemorrhage in Mice. J. Neuroinflamm. 2020, 17, 168. [Google Scholar] [CrossRef]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schöneberg, T.; Schaefer, M.; Krügel, U.; et al. Extracellular Ca2+ Is a Danger Signal Activating the NLRP3 Inflammasome through G Protein-Coupled Calcium Sensing Receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Obst, J.; Hall-Roberts, H.L.; Smith, T.B.; Kreuzer, M.; Magno, L.; Di Daniel, E.; Davis, J.B.; Mead, E. PLCγ2 Regulates TREM2 Signalling and Integrin-Mediated Adhesion and Migration of Human iPSC-Derived Macrophages. Sci. Rep. 2021, 11, 19842. [Google Scholar] [CrossRef] [PubMed]
- Jairaman, A.; McQuade, A.; Granzotto, A.; Kang, Y.J.; Chadarevian, J.P.; Gandhi, S.; Parker, I.; Smith, I.; Cho, H.; Sensi, S.L.; et al. TREM2 Regulates Purinergic Receptor-Mediated Calcium Signaling and Motility in Human iPSC-Derived Microglia. eLife 2022, 11, e73021. [Google Scholar] [CrossRef] [PubMed]
- Terzioglu, G.; Young-Pearse, T.L. Microglial Function, INPP5D/SHIP1 Signaling, and NLRP3 Inflammasome Activation: Implications for Alzheimer’s Disease. Mol. Neurodegener. 2023, 18, 89. [Google Scholar] [CrossRef]
- Ramakrishnan, G.S.; Berry, W.L.; Pacherille, A.; Kerr, W.G.; Chisholm, J.D.; Pedicone, C.; Humphrey, M.B. SHIP Inhibition Mediates Select TREM2-Induced Microglial Functions. Mol. Immunol. 2024, 170, 35–45. [Google Scholar] [CrossRef]
- Griciuc, A.; Patel, S.; Federico, A.N.; Choi, S.H.; Innes, B.J.; Oram, M.K.; Cereghetti, G.; McGinty, D.; Anselmo, A.; Sadreyev, R.I.; et al. TREM2 Acts Downstream of CD33 in Modulating Microglial Pathology in Alzheimer’s Disease. Neuron 2019, 103, 820–835.e7. [Google Scholar] [CrossRef]
- Wißfeld, J.; Mathews, M.; Mossad, O.; Picardi, P.; Cinti, A.; Redaelli, L.; Pradier, L.; Brüstle, O.; Neumann, H. Reporter Cell Assay for Human CD33 Validated by Specific Antibodies and Human iPSC-Derived Microglia. Sci. Rep. 2021, 11, 13462. [Google Scholar] [CrossRef] [PubMed]
- Wißfeld, J.; Nozaki, I.; Mathews, M.; Raschka, T.; Ebeling, C.; Hornung, V.; Brüstle, O.; Neumann, H. Deletion of Alzheimer’s Disease-associated CD33 Results in an Inflammatory Human Microglia Phenotype. Glia 2021, 69, 1393–1412. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Z.; Zhang, X.; Xiong, M.; Meng, L.; Zhang, Z. TREM2 Ectodomain and Its Soluble Form in Alzheimer’s Disease. J. Neuroinflamm. 2020, 17, 204. [Google Scholar] [CrossRef]
- Dong, M.-H.; Zhou, L.-Q.; Tang, Y.; Chen, M.; Xiao, J.; Shang, K.; Deng, G.; Qin, C.; Tian, D.-S. CSF sTREM2 in Neurological Diseases: A Two-Sample Mendelian Randomization Study. J. Neuroinflamm. 2022, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Jericó, I.; Vicuña-Urriza, J.; Blanco-Luquin, I.; Macias, M.; Martinez-Merino, L.; Roldán, M.; Rojas-Garcia, R.; Pagola-Lorz, I.; Carbayo, A.; De Luna, N.; et al. Profiling TREM2 Expression in Amyotrophic Lateral Sclerosis. Brain Behav. Immun. 2023, 109, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, Y.; Zhuo, R.; Wang, T.; Wang, K.; Huang, R.; Wang, D.; Gao, Y.; Zhu, Y.; Sheng, X.; et al. Soluble TREM2 Ameliorates Pathological Phenotypes by Modulating Microglial Functions in an Alzheimer’s Disease Model. Nat. Commun. 2019, 10, 1365. [Google Scholar] [CrossRef]
- Belsare, K.D.; Wu, H.; Mondal, D.; Bond, A.; Castillo, E.; Jin, J.; Jo, H.; Roush, A.E.; Pilla, K.B.; Sali, A.; et al. Soluble TREM2 Inhibits Secondary Nucleation of Aβ Fibrillization and Enhances Cellular Uptake of Fibrillar Aβ. Proc. Natl. Acad. Sci. USA 2022, 119, e2114486119. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, X.-F.; Wang, T.; Wang, Z.; Liao, C.; Wang, Z.; Huang, R.; Wang, D.; Li, X.; Wu, L.; et al. Soluble TREM2 Induces Inflammatory Responses and Enhances Microglial Survival. J. Exp. Med. 2017, 214, 597–607. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, X.; Li, Y.; Bu, G.; Chen, X.-F. TREM2 and sTREM2 in Alzheimer’s Disease: From Mechanisms to Therapies. Mol. Neurodegener. 2025, 20, 43. [Google Scholar] [CrossRef]
- Paloneva, J.; Manninen, T.; Christman, G.; Hovanes, K.; Mandelin, J.; Adolfsson, R.; Bianchin, M.; Bird, T.; Miranda, R.; Salmaggi, A.; et al. Mutations in Two Genes Encoding Different Subunits of a Receptor Signaling Complex Result in an Identical Disease Phenotype. Am. J. Hum. Genet. 2002, 71, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Paloneva Bm, J.; Autti, T.; Raininko, R.; Partanen, J.; Salonen, O.; Puranen, M.; Hakola, P.; Haltia, M. CNS Manifestations of Nasu–Hakola Disease: A Frontal Dementia with Bone Cysts. Neurology 2001, 56, 1552–1558. [Google Scholar] [CrossRef]
- Klünemann, H.H.; Ridha, B.H.; Magy, L.; Wherrett, J.R.; Hemelsoet, D.M.; Keen, R.W.; De Bleecker, J.L.; Rossor, M.N.; Marienhagen, J.; Klein, H.E.; et al. The Genetic Causes of Basal Ganglia Calcification, Dementia, and Bone Cysts: DAP12 and TREM2. Neurology 2005, 64, 1502–1507. [Google Scholar] [CrossRef]
- Filipello, F.; You, S.-F.; Mirfakhar, F.S.; Mahali, S.; Bollman, B.; Acquarone, M.; Korvatska, O.; Marsh, J.A.; Sivaraman, A.; Martinez, R.; et al. Defects in Lysosomal Function and Lipid Metabolism in Human Microglia Harboring a TREM2 Loss of Function Mutation. Acta Neuropathol. 2023, 145, 749–772. [Google Scholar] [CrossRef]
- Yuan, P.; Condello, C.; Keene, C.D.; Wang, Y.; Bird, T.D.; Paul, S.M.; Luo, W.; Colonna, M.; Baddeley, D.; Grutzendler, J. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 2016, 90, 724–739. [Google Scholar] [CrossRef]
- Sayed, F.A.; Kodama, L.; Fan, L.; Carling, G.K.; Udeochu, J.C.; Le, D.; Li, Q.; Zhou, L.; Wong, M.Y.; Horowitz, R.; et al. AD-Linked R47H-TREM2 Mutation Induces Disease-Enhancing Microglial States via AKT Hyperactivation. Sci. Transl. Med. 2021, 13, eabe3947. [Google Scholar] [CrossRef]
- Rayaprolu, S.; Mullen, B.; Baker, M.; Lynch, T.; Finger, E.; Seeley, W.W.; Hatanpaa, K.J.; Lomen-Hoerth, C.; Kertesz, A.; Bigio, E.H.; et al. TREM2 in Neurodegeneration: Evidence for Association of the p.R47H Variant with Frontotemporal Dementia and Parkinson’s Disease. Mol. Neurodegener. 2013, 8, 19. [Google Scholar] [CrossRef]
- Cady, J.; Koval, E.D.; Benitez, B.A.; Zaidman, C.; Jockel-Balsarotti, J.; Allred, P.; Baloh, R.H.; Ravits, J.; Simpson, E.; Appel, S.H.; et al. TREM2 Variant p.R47H as a Risk Factor for Sporadic Amyotrophic Lateral Sclerosis. JAMA Neurol. 2014, 71, 449. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kober, D.L.; Alexander-Brett, J.M.; Karch, C.M.; Cruchaga, C.; Colonna, M.; Holtzman, M.J.; Brett, T.J. Neurodegenerative Disease Mutations in TREM2 Reveal a Functional Surface and Distinct Loss-of-Function Mechanisms. eLife 2016, 5, e20391. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Lohmann, E.; Brás, J.M.; Gibbs, J.R.; Rohrer, J.D.; Gurunlian, N.; Dursun, B.; Bilgic, B.; Hanagasi, H.; Gurvit, H.; et al. Using Exome Sequencing to Reveal Mutations in TREM2 Presenting as a Frontotemporal Dementia-like Syndrome without Bone Involvement. JAMA Neurol. 2013, 70, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kleinberger, G.; Brendel, M.; Mracsko, E.; Wefers, B.; Groeneweg, L.; Xiang, X.; Focke, C.; Deußing, M.; Suárez-Calvet, M.; Mazaheri, F.; et al. The FTD-like Syndrome Causing TREM 2 T66M Mutation Impairs Microglia Function, Brain Perfusion, and Glucose Metabolism. EMBO J. 2017, 36, 1837–1853. [Google Scholar] [CrossRef]
- Le Ber, I.; De Septenville, A.; Guerreiro, R.; Bras, J.; Camuzat, A.; Caroppo, P.; Lattante, S.; Couarch, P.; Kabashi, E.; Bouya-Ahmed, K.; et al. Homozygous TREM2 Mutation in a Family with Atypical Frontotemporal Dementia. Neurobiol. Aging 2014, 35, 2419.e23–2419.e25. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ji, I.J.; An, H.J.; Kang, M.; Kang, S.; Kim, D.; Yoon, S. Disease-Associated Mutations of TREM2 Alter the Processing of N-Linked Oligosaccharides in the Golgi Apparatus. Traffic 2015, 16, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.; Sung, K.; Wu, C. Predicting the Impact of Missense Mutations on an Unresolved Protein’s Stability, Structure, and Function: A Case Study of Alzheimer’s Disease-Associated TREM2 R47H Variant. Comput. Struct. Biotechnol. J. 2025, 27, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.S.; Lin, P.B.C.; Pennington, T.; Di Prisco, G.V.; Jannu, A.J.; Xu, G.; Moutinho, M.; Zhang, J.; Atwood, B.K.; Puntambekar, S.S.; et al. Trem2 Y38C Mutation and Loss of Trem2 Impairs Neuronal Synapses in Adult Mice. Mol. Neurodegener. 2020, 15, 62. [Google Scholar] [CrossRef]
- Schuler, O. A Case of Possible Autoimmune Bilateral Vestibulopathy Treated with Steroids. J. Neurol. Neurosurg. Psychiatry 2003, 74, 825. [Google Scholar] [CrossRef]
- Thelen, M.; Razquin, C.; Hernández, I.; Gorostidi, A.; Sánchez-Valle, R.; Ortega-Cubero, S.; Wolfsgruber, S.; Drichel, D.; Fliessbach, K.; Duenkel, T.; et al. Investigation of the Role of Rare TREM2 Variants in Frontotemporal Dementia Subtypes. Neurobiol. Aging 2014, 35, 2657.e13–2657.e19. [Google Scholar] [CrossRef]
- Achouri-Rassas, A.; Fray, S.; Said, Z.; Ben Sassi, S.; Ben Ali, N.; Baraket, G. Genetic Association Study between Rs2234253 (p.T96K) Variant of TREM2 and Alzheimer’s Disease in a Tunisian Population. Neurol. Res. 2025, 47, 290–295. [Google Scholar] [CrossRef]
- Jiang, T.; Tan, L.; Chen, Q.; Tan, M.-S.; Zhou, J.-S.; Zhu, X.-C.; Lu, H.; Wang, H.-F.; Zhang, Y.-D.; Yu, J.-T. A Rare Coding Variant in TREM2 Increases Risk for Alzheimer’s Disease in Han Chinese. Neurobiol. Aging 2016, 42, 217.e1–217.e3. [Google Scholar] [CrossRef]
- Qiao, W.; Chen, Y.; Zhong, J.; Madden, B.J.; Charlesworth, C.M.; Martens, Y.A.; Liu, C.-C.; Knight, J.; Ikezu, T.C.; Kurti, A.; et al. Trem2 H157Y Increases Soluble TREM2 Production and Reduces Amyloid Pathology. Mol. Neurodegener. 2023, 18, 8. [Google Scholar] [CrossRef]
- Jin, S.C.; Carrasquillo, M.M.; Benitez, B.A.; Skorupa, T.; Carrell, D.; Patel, D.; Lincoln, S.; Krishnan, S.; Kachadoorian, M.; Reitz, C.; et al. TREM2 Is Associated with Increased Risk for Alzheimer’s Disease in African Americans. Mol. Neurodegener. 2015, 10, 19. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sudan, R.; Peng, V.; Zhou, Y.; Du, S.; Yuede, C.M.; Lei, T.; Hou, J.; Cai, Z.; Cella, M.; et al. TREM2 Drives Microglia Response to Amyloid-β via SYK-Dependent and -Independent Pathways. Cell 2022, 185, 4153–4169.e19. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, W.M.; Andhey, P.S.; Swain, A.; Levy, T.; Miller, K.R.; Poliani, P.L.; Cominelli, M.; Grover, S.; Gilfillan, S.; et al. Human and Mouse Single-Nucleus Transcriptomics Reveal TREM2-Dependent and TREM2-Independent Cellular Responses in Alzheimer’s Disease. Nat. Med. 2020, 26, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.-P.; York, E.M.; MacVicar, B.A. Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends Neurosci. 2020, 43, 854–869. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic Control of Autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Piollet, M.; Porsch, F.; Rizzo, G.; Kapser, F.; Schulz, D.J.J.; Kiss, M.G.; Schlepckow, K.; Morenas-Rodriguez, E.; Sen, M.O.; Gropper, J.; et al. TREM2 Protects from Atherosclerosis by Limiting Necrotic Core Formation. Nat. Cardiovasc. Res. 2024, 3, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Tagliatti, E.; Desiato, G.; Mancinelli, S.; Bizzotto, M.; Gagliani, M.C.; Faggiani, E.; Hernández-Soto, R.; Cugurra, A.; Poliseno, P.; Miotto, M.; et al. Trem2 Expression in Microglia Is Required to Maintain Normal Neuronal Bioenergetics during Development. Immunity 2024, 57, 86–105.e9. [Google Scholar] [CrossRef]
- Graham, L.C.; Harder, J.M.; Soto, I.; De Vries, W.N.; John, S.W.M.; Howell, G.R. Chronic Consumption of a Western Diet Induces Robust Glial Activation in Aging Mice and in a Mouse Model of Alzheimer’s Disease. Sci. Rep. 2016, 6, 21568. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Peng, Y.; Zhang, L.; Chao, F.; Jiang, L.; Xiao, Q.; Liang, X.; Tang, J.; Yang, H.; et al. Long-Term Running Exercise Improves Cognitive Function and Promotes Microglial Glucose Metabolism and Morphological Plasticity in the Hippocampus of APP/PS1 Mice. J. Neuroinflamm. 2022, 19, 34. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e14. [Google Scholar] [CrossRef]
- Patterson, M.T.; Firulyova, M.M.; Xu, Y.; Hillman, H.; Bishop, C.; Zhu, A.; Hickok, G.H.; Schrank, P.R.; Ronayne, C.E.; Caillot, Z.; et al. Trem2 Promotes Foamy Macrophage Lipid Uptake and Survival in Atherosclerosis. Nat. Cardiovasc. Res. 2023, 2, 1015–1031. [Google Scholar] [CrossRef]
- Patterson, M.T.; Xu, Y.; Hillman, H.; Osinski, V.; Schrank, P.R.; Kennedy, A.E.; Barrow, F.; Zhu, A.; Tollison, S.; Shekhar, S.; et al. Trem2 Agonist Reprograms Foamy Macrophages to Promote Atherosclerotic Plaque Stability—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1646–1657. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.-T.; Zhu, X.-C.; Tan, M.-S.; Gu, L.-Z.; Zhang, Y.-D.; Tan, L. Triggering Receptor Expressed on Myeloid Cells 2 Knockdown Exacerbates Aging-Related Neuroinflammation and Cognitive Deficiency in Senescence-Accelerated Mouse Prone 8 Mice. Neurobiol. Aging 2014, 35, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Cheng, X.; Xu, J.; Liu, Y.; Zhou, J.; Jiang, L.; Gu, X.; Xia, T. Activation of TREM2 Attenuates Neuroinflammation via PI3K/Akt Signaling Pathway to Improve Postoperative Cognitive Dysfunction in Mice. Neuropharmacology 2022, 219, 109231. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, Z.; Zhang, P.; Feng, J.; Xie, J.; Zheng, Y.; Liang, X.; Zhu, B.; Chen, Z.; Feng, S.; et al. TREM2 Deficiency Aggravates NLRP3 Inflammasome Activation and Pyroptosis in MPTP-Induced Parkinson’s Disease Mice and LPS-Induced BV2 Cells. Mol. Neurobiol. 2024, 61, 2590–2605. [Google Scholar] [CrossRef]
- Forabosco, P.; Ramasamy, A.; Trabzuni, D.; Walker, R.; Smith, C.; Bras, J.; Levine, A.P.; Hardy, J.; Pocock, J.M.; Guerreiro, R.; et al. Insights into TREM2 Biology by Network Analysis of Human Brain Gene Expression Data. Neurobiol. Aging 2013, 34, 2699–2714. [Google Scholar] [CrossRef]
- Jiang, T.; Tan, L.; Zhu, X.-C.; Zhang, Q.-Q.; Cao, L.; Tan, M.-S.; Gu, L.-Z.; Wang, H.-F.; Ding, Z.-Z.; Zhang, Y.-D.; et al. Upregulation of TREM2 Ameliorates Neuropathology and Rescues Spatial Cognitive Impairment in a Transgenic Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2014, 39, 2949–2962. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Konishi, H.; Sayo, A.; Takai, T.; Kiyama, H. TREM2/DAP12 Signal Elicits Proinflammatory Response in Microglia and Exacerbates Neuropathic Pain. J. Neurosci. 2016, 36, 11138–11150. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New Insights into the Role of TREM2 in Alzheimer’s Disease. Mol. Neurodegener. 2018, 13, 66. [Google Scholar] [CrossRef]
- Popescu, A.S.; Butler, C.A.; Allendorf, D.H.; Piers, T.M.; Mallach, A.; Roewe, J.; Reinhardt, P.; Cinti, A.; Redaelli, L.; Boudesco, C.; et al. Alzheimer’s Disease-associated R47H TREM2 Increases, but Wild-type TREM2 Decreases, Microglial Phagocytosis of Synaptosomes and Neuronal Loss. Glia 2023, 71, 974–990. [Google Scholar] [CrossRef]
- Schoch, K.M.; Ezerskiy, L.A.; Morhaus, M.M.; Bannon, R.N.; Sauerbeck, A.D.; Shabsovich, M.; Jafar-nejad, P.; Rigo, F.; Miller, T.M. Acute Trem2 Reduction Triggers Increased Microglial Phagocytosis, Slowing Amyloid Deposition in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2100356118. [Google Scholar] [CrossRef]
- Yin, S.; Chi, X.; Wan, F.; Li, Y.; Zhou, Q.; Kou, L.; Sun, Y.; Wu, J.; Zou, W.; Wang, Y.; et al. TREM2 Signaling in Parkinson’s Disease: Regulation of Microglial Function and α-Synuclein Pathology. Int. Immunopharmacol. 2024, 143, 113446. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Liu, Y.U.; Zhao, S.; Zhang, L.; Bosco, D.B.; Pang, Y.-P.; Zhong, J.; Sheth, U.; Martens, Y.A.; Zhao, N.; et al. TREM2 Interacts with TDP-43 and Mediates Microglial Neuroprotection against TDP-43-Related Neurodegeneration. Nat. Neurosci. 2022, 25, 26–38. [Google Scholar] [CrossRef]
- Pang, X.; Chu, Y.; Zhou, L.; Chen, M.; You, Y.; Tang, Y.; Yang, S.; Zhang, H.; Xiao, J.; Deng, G.; et al. Trem2 Deficiency Attenuates Microglial Phagocytosis and Autophagic-lysosomal Activation in White Matter Hypoperfusion. J. Neurochem. 2023, 167, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Ji, J.; Sun, Y.; Huang, X.; Cai, Z.; Yang, J.; Guo, W.; Guo, R.; Cheng, H.; Sun, X. Sphingosine-1-Phosphate, a Novel TREM2 Ligand, Promotes Microglial Phagocytosis to Protect against Ischemic Brain Injury. Acta Pharm. Sin. B 2022, 12, 1885–1898. [Google Scholar] [CrossRef]
- Cignarella, F.; Filipello, F.; Bollman, B.; Cantoni, C.; Locca, A.; Mikesell, R.; Manis, M.; Ibrahim, A.; Deng, L.; Benitez, B.A.; et al. TREM2 Activation on Microglia Promotes Myelin Debris Clearance and Remyelination in a Model of Multiple Sclerosis. Acta Neuropathol. 2020, 140, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Hou, J.-K.; Gao, Q.; Yu, J.-T.; Zhou, J.-S.; Zhao, H.-D.; Zhang, Y.-D. TREM2 p.H157Y Variant and the Risk of Alzheimer’s Disease: A Meta-Analysis Involving 14,510 Subjects. Curr. Neurovascular Res. 2016, 13, 318–320. [Google Scholar] [CrossRef]
- Song, W.; Hooli, B.; Mullin, K.; Jin, S.C.; Cella, M.; Ulland, T.K.; Wang, Y.; Tanzi, R.E.; Colonna, M. Alzheimer’s Disease-Associated TREM2 Variants Exhibit Either Decreased or Increased Ligand-Dependent Activation. Alzheimer’s Dement. 2017, 13, 381–387. [Google Scholar] [CrossRef]
- Benitez, B.A.; Cooper, B.; Pastor, P.; Jin, S.-C.; Lorenzo, E.; Cervantes, S.; Cruchaga, C. TREM2 Is Associated with the Risk of Alzheimer’s Disease in Spanish Population. Neurobiol. Aging 2013, 34, 1711.e15–1711.e17. [Google Scholar] [CrossRef]
- Ghani, M.; Sato, C.; Kakhki, E.G.; Gibbs, J.R.; Traynor, B.; St George-Hyslop, P.; Rogaeva, E. Mutation Analysis of the MS4A and TREM Gene Clusters in a Case-Control Alzheimer’s Disease Data Set. Neurobiol. Aging 2016, 42, 217.e7–217.e13. [Google Scholar] [CrossRef]
- Jin, S.C.; Benitez, B.A.; Karch, C.M.; Cooper, B.; Skorupa, T.; Carrell, D.; Norton, J.B.; Hsu, S.; Harari, O.; Cai, Y.; et al. Coding Variants in TREM2 Increase Risk for Alzheimer’s Disease. Hum. Mol. Genet. 2014, 23, 5838–5846. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.; Katsel, P.; Fam, P.; Tan, W.; Purohit, D.P.; Haroutunian, V. The Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) Is Associated with Enhanced Inflammation, Neuropathological Lesions and Increased Risk for Alzheimer’s Dementia. Alzheimer’s Dement. 2015, 11, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Carmona, S.; Zahs, K.; Wu, E.; Dakin, K.; Bras, J.; Guerreiro, R. The Role of TREM2 in Alzheimer’s Disease and Other Neurodegenerative Disorders. Lancet Neurol. 2018, 17, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Tan, L.; Zhu, X.-C.; Zhou, J.-S.; Cao, L.; Tan, M.-S.; Wang, H.-F.; Chen, Q.; Zhang, Y.-D.; Yu, J.-T. Silencing of TREM2 Exacerbates Tau Pathology, Neurodegenerative Changes, and Spatial Learning Deficits in P301S Tau Transgenic Mice. Neurobiol. Aging 2015, 36, 3176–3186. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Wang, L.; Zhan, H.; Luo, X.; Zeng, Y.; Wu, W.; Zhang, X.; Wang, F. TREM2 Ameliorates Neuroinflammatory Response and Cognitive Impairment via PI3K/AKT/FoxO3a Signaling Pathway in Alzheimer’s Disease Mice. Aging 2020, 12, 20862–20879. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.-D.; Chen, Q.; Gao, Q.; Zhu, X.-C.; Zhou, J.-S.; Shi, J.-Q.; Lu, H.; Tan, L.; Yu, J.-T. TREM2 Modifies Microglial Phenotype and Provides Neuroprotection in P301S Tau Transgenic Mice. Neuropharmacology 2016, 105, 196–206. [Google Scholar] [CrossRef]
- Bouchon, A.; Hernández-Munain, C.; Cella, M.; Colonna, M. A DAP12-Mediated Pathway Regulates Expression of CC Chemokine Receptor 7 and Maturation of Human Dendritic Cells. J. Exp. Med. 2001, 194, 1111–1122. [Google Scholar] [CrossRef]
- Jay, T.R.; Hirsch, A.M.; Broihier, M.L.; Miller, C.M.; Neilson, L.E.; Ransohoff, R.M.; Lamb, B.T.; Landreth, G.E. Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2017, 37, 637–647. [Google Scholar] [CrossRef]
- Wang, Y.; Ulland, T.K.; Ulrich, J.D.; Song, W.; Tzaferis, J.A.; Hole, J.T.; Yuan, P.; Mahan, T.E.; Shi, Y.; Gilfillan, S.; et al. TREM2-Mediated Early Microglial Response Limits Diffusion and Toxicity of Amyloid Plaques. J. Exp. Med. 2016, 213, 667–675. [Google Scholar] [CrossRef]
- Meilandt, W.J.; Ngu, H.; Gogineni, A.; Lalehzadeh, G.; Lee, S.-H.; Srinivasan, K.; Imperio, J.; Wu, T.; Weber, M.; Kruse, A.J.; et al. Trem2 Deletion Reduces Late-Stage Amyloid Plaque Accumulation, Elevates the Aβ42:Aβ40 Ratio, and Exacerbates Axonal Dystrophy and Dendritic Spine Loss in the PS2APP Alzheimer’s Mouse Model. J. Neurosci. 2020, 40, 1956–1974. [Google Scholar] [CrossRef]
- Parhizkar, S.; Arzberger, T.; Brendel, M.; Kleinberger, G.; Deussing, M.; Focke, C.; Nuscher, B.; Xiong, M.; Ghasemigharagoz, A.; Katzmarski, N.; et al. Loss of TREM2 Function Increases Amyloid Seeding but Reduces Plaque-Associated ApoE. Nat. Neurosci. 2019, 22, 191–204. [Google Scholar] [CrossRef]
- Leyns, C.E.G.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Remolina Serrano, J.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 Deficiency Attenuates Neuroinflammation and Protects against Neurodegeneration in a Mouse Model of Tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Leyns, C.E.G.; Sauerbeck, A.D.; St-Pierre, M.-K.; Xiong, M.; Kim, N.; Serrano, J.R.; Tremblay, M.-È.; Kummer, T.T.; Colonna, M.; et al. Impact of TREM2R47H Variant on Tau Pathology–Induced Gliosis and Neurodegeneration. J. Clin. Investig. 2020, 130, 4954–4968. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Meilandt, W.J.; Xie, L.; Gandham, V.D.; Ngu, H.; Barck, K.H.; Rezzonico, M.G.; Imperio, J.; Lalehzadeh, G.; Huntley, M.A.; et al. Trem2 Restrains the Enhancement of Tau Accumulation and Neurodegeneration by β-Amyloid Pathology. Neuron 2021, 109, 1283–1301.e6. [Google Scholar] [CrossRef]
- Shirotani, K.; Hori, Y.; Yoshizaki, R.; Higuchi, E.; Colonna, M.; Saito, T.; Hashimoto, S.; Saito, T.; Saido, T.C.; Iwata, N. Aminophospholipids Are Signal-Transducing TREM2 Ligands on Apoptotic Cells. Sci. Rep. 2019, 9, 7508. [Google Scholar] [CrossRef]
- Zhao, N.; Qiao, W.; Li, F.; Ren, Y.; Zheng, J.; Martens, Y.A.; Wang, X.; Li, L.; Liu, C.-C.; Chen, K.; et al. Elevating Microglia TREM2 Reduces Amyloid Seeding and Suppresses Disease-Associated Microglia. J. Exp. Med. 2022, 219, e20212479. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, B.; Liu, Y.; Zhang, X.; Yin, J.; Li, X.; Jiang, L.; Hodges, A.P.; Rosenthal, S.B.; Zhou, L.; et al. Multi-Omic Comparison of Alzheimer’s Variants in Human ESC-Derived Microglia Reveals Convergence at APOE. J. Exp. Med. 2020, 217, e20200474. [Google Scholar] [CrossRef]
- Kober, D.L.; Stuchell-Brereton, M.D.; Kluender, C.E.; Dean, H.B.; Strickland, M.R.; Steinberg, D.F.; Nelson, S.S.; Baban, B.; Holtzman, D.M.; Frieden, C.; et al. Functional Insights from Biophysical Study of TREM2 Interactions with apoE and Aβ1-42. Alzheimer’s Dement. 2021, 17, 475–488. [Google Scholar] [CrossRef]
- Fitz, N.F.; Wolfe, C.M.; Playso, B.E.; Biedrzycki, R.J.; Lu, Y.; Nam, K.N.; Lefterov, I.; Koldamova, R. Trem2 Deficiency Differentially Affects Phenotype and Transcriptome of Human APOE3 and APOE4 Mice. Mol. Neurodegener. 2020, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Xiong, C.; Jasielec, M.S.; Bateman, R.J.; Goate, A.M.; Benzinger, T.L.S.; Ghetti, B.; Martins, R.N.; Masters, C.L.; Mayeux, R.; et al. Longitudinal Change in CSF Biomarkers in Autosomal-Dominant Alzheimer’s Disease. Sci. Transl. Med. 2014, 6, 226ra30. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J.; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; et al. Identification of Soluble TREM-2 in the Cerebrospinal Fluid and Its Association with Multiple Sclerosis and CNS Inflammation. Brain 2008, 131, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Araque Caballero, M.Á.; Kleinberger, G.; Bateman, R.J.; Fagan, A.M.; Morris, J.C.; Levin, J.; Danek, A.; Ewers, M.; Haass, C.; et al. Early Changes in CSF sTREM2 in Dominantly Inherited Alzheimer’s Disease Occur after Amyloid Deposition and Neuronal Injury. Sci. Transl. Med. 2016, 8, 369ra178. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Kleinberger, G.; Araque Caballero, M.Á.; Brendel, M.; Rominger, A.; Alcolea, D.; Fortea, J.; Lleó, A.; Blesa, R.; Gispert, J.D.; et al. sTREM 2 Cerebrospinal Fluid Levels Are a Potential Biomarker for Microglia Activity in Early-stage Alzheimer’s Disease and Associate with Neuronal Injury Markers. EMBO Mol. Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Hammond, T.R.; Marsh, S.E.; Stevens, B. Immune Signaling in Neurodegeneration. Immunity 2019, 50, 955–974. [Google Scholar] [CrossRef]
- Ouchi, Y.; Yoshikawa, E.; Sekine, Y.; Futatsubashi, M.; Kanno, T.; Ogusu, T.; Torizuka, T. Microglial Activation and Dopamine Terminal Loss in Early Parkinson’s Disease. Ann. Neurol. 2005, 57, 168–175. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F. Targeting TREM2 for Parkinson’s Disease: Where to Go? Front. Immunol. 2021, 12, 795036. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, X.; Yan, H.; Qin, Y.; Yan, S.; Liu, J.; Zhao, Y.; Jiang, F.; Lou, H. TREM2 Deficiency Aggravates A-synuclein–Induced Neurodegeneration and Neuroinflammation in Parkinson’s Disease Models. FASEB J. 2019, 33, 12164–12174. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Jiang, Q.; Jiang, Y.; Feng, R.; Zhang, L.; Chen, Z.; Li, K.; Liu, J. Convergent Genetic and Expression Datasets Highlight TREM2 in Parkinson’s Disease Susceptibility. Mol. Neurobiol. 2016, 53, 4931–4938. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Gu, L.; Huang, W.; Shi, X.; Zhang, X.; An, X.; Lin, Q.; Tzeng, C.-M. Association Study of TREM2 Polymorphism Rs75932628 with Leucoaraiosis or Parkinson’s Disease in the Han Chinese Population. BMJ Open 2016, 6, e009499. [Google Scholar] [CrossRef]
- Dardiotis, E.; Rikos, D.; Siokas, V.; Aloizou, A.-M.; Tsouris, Z.; Sakalakis, E.; Brotis, A.G.; Bogdanos, D.P.; Hadjigeorgiou, G.M. Assessment of TREM2 Rs75932628 Variant’s Association with Parkinson’s Disease in a Greek Population and Meta-Analysis of Current Data. Int. J. Neurosci. 2021, 131, 544–548. [Google Scholar] [CrossRef]
- Peng, G.; Qiu, J.; Liu, H.; Zhou, M.; Huang, S.; Guo, W.; Lin, Y.; Chen, X.; Li, Z.; Li, G.; et al. Analysis of Cerebrospinal Fluid Soluble TREM2 and Polymorphisms in Sporadic Parkinson’s Disease in a Chinese Population. J. Mol. Neurosci. 2020, 70, 294–301. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, X.; Wang, L.; Li, H.; Yang, L.; Li, X.; Yu, X.; Xie, A. Effects of Soluble TREM2 on Motor Progression in Parkinson’s Disease. Neurosci. Lett. 2023, 807, 137277. [Google Scholar] [CrossRef]
- Ortega-Cubero, S.; Lorenzo-Betancor, O.; Lorenzo, E.; Agúndez, J.A.G.; Jiménez-Jiménez, F.J.; Ross, O.A.; Wurster, I.; Mielke, C.; Lin, J.-J.; Coria, F.; et al. TREM2 R47H Variant and Risk of Essential Tremor: A Cross-Sectional International Multicenter Study. Park. Relat. Disord. 2015, 21, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Guo, Y.; Wei, X.; Yan, S.; Qin, Y.; Zhang, X.; Jiang, F.; Lou, H. TREM2 Overexpression Attenuates Neuroinflammation and Protects Dopaminergic Neurons in Experimental Models of Parkinson’s Disease. Exp. Neurol. 2018, 302, 205–213. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From Genes to Mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Appel, S.H. Immune Dysregulation in Amyotrophic Lateral Sclerosis: Mechanisms and Emerging Therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- Shu, X.; Wei, C.; Tu, W.-Y.; Zhong, K.; Qi, S.; Wang, A.; Bai, L.; Zhang, S.-X.; Luo, B.; Xu, Z.-Z.; et al. Negative Regulation of TREM2-Mediated C9orf72 Poly-GA Clearance by the NLRP3 Inflammasome. Cell Rep. 2023, 42, 112133. [Google Scholar] [CrossRef]
- Siokas, V.; Aloizou, A.-M.; Liampas, I.; Tsouris, Z.; Mentis, A.-F.A.; Nasios, G.; Papadimitriou, D.; Bogdanos, D.P.; Hadjigeorgiou, G.M.; Dardiotis, E. Lack of Association between TREM2 Rs75932628 Variant and Amyotrophic Lateral Sclerosis. Mol. Biol. Rep. 2021, 48, 2601–2610. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Wei, Q.; Guo, X.; Cao, B.; Ou, R.; Zhao, B.; Shang, H.-F. Assessment of TREM2 Rs75932628 Association with Amyotrophic Lateral Sclerosis in a Chinese Population. J. Neurol. Sci. 2015, 355, 193–195. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Green, C.; Altschuler, G.; Wei, W.; Bury, J.J.; Heath, P.R.; Wyles, M.; Gelsthorpe, C.; Highley, J.R.; Lorente-Pons, A.; et al. A Data-Driven Approach Links Microglia to Pathology and Prognosis in Amyotrophic Lateral Sclerosis. Acta Neuropathol. Commun. 2017, 5, 23. [Google Scholar] [CrossRef]
- Paloneva, J.; Kestilä, M.; Wu, J.; Salminen, A.; Böhling, T.; Ruotsalainen, V.; Hakola, P.; Bakker, A.B.H.; Phillips, J.H.; Pekkarinen, P.; et al. Loss-of-Function Mutations in TYROBP (DAP12) Result in a Presenile Dementia with Bone Cysts. Nat. Genet. 2000, 25, 357–361. [Google Scholar] [CrossRef]
- Guerreiro, R.; Bilgic, B.; Guven, G.; Brás, J.; Rohrer, J.; Lohmann, E.; Hanagasi, H.; Gurvit, H.; Emre, M. Novel Compound Heterozygous Mutation in TREM2 Found in a Turkish Frontotemporal Dementia-like Family. Neurobiol. Aging 2013, 34, 2890.e1–2890.e5. [Google Scholar] [CrossRef]
- Dardiotis, E.; Siokas, V.; Pantazi, E.; Dardioti, M.; Rikos, D.; Xiromerisiou, G.; Markou, A.; Papadimitriou, D.; Speletas, M.; Hadjigeorgiou, G.M. A Novel Mutation in TREM2 Gene Causing Nasu-Hakola Disease and Review of the Literature. Neurobiol. Aging 2017, 53, 194.e13–194.e22. [Google Scholar] [CrossRef]
- Numasawa, Y.; Yamaura, C.; Ishihara, S.; Shintani, S.; Yamazaki, M.; Tabunoki, H.; Satoh, J.-I. Nasu-Hakola Disease with a Splicing Mutation of TREM2 in a Japanese Family: Japanese NHD Family with TREM2 Mutation. Eur. J. Neurol. 2011, 18, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Prinz, M.; Van Loo, G. Microglia in Central Nervous System Inflammation and Multiple Sclerosis Pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Piccio, L.; Buonsanti, C.; Mariani, M.; Cella, M.; Gilfillan, S.; Cross, A.H.; Colonna, M.; Panina-Bordignon, P. Blockade of TREM-2 Exacerbates Experimental Autoimmune Encephalomyelitis. Eur. J. Immunol. 2007, 37, 1290–1301. [Google Scholar] [CrossRef]
- Qu, S.; Hu, S.; Xu, H.; Wu, Y.; Ming, S.; Zhan, X.; Wang, C.; Huang, X. TREM-2 Drives Development of Multiple Sclerosis by Promoting Pathogenic Th17 Polarization. Neurosci. Bull. 2024, 40, 17–34. [Google Scholar] [CrossRef]
- You, Y.-F.; Chen, M.; Tang, Y.; Yu, W.-X.; Pang, X.-W.; Chu, Y.-H.; Zhang, H.; Shang, K.; Deng, G.; Zhou, L.-Q.; et al. TREM2 Deficiency Inhibits Microglial Activation and Aggravates Demyelinating Injury in Neuromyelitis Optica Spectrum Disorder. J. Neuroinflamm. 2023, 20, 89. [Google Scholar] [CrossRef]
- Qin, C.; Chen, M.; Dong, M.-H.; Yang, S.; Zhang, H.; You, Y.-F.; Zhou, L.-Q.; Chu, Y.-H.; Tang, Y.; Pang, X.-W.; et al. Soluble TREM2 Triggers Microglial Dysfunction in Neuromyelitis Optica Spectrum Disorders. Brain 2024, 147, 163–176. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Chen, Y.-F.; Yen, R.-F.; Lo, Y.-L.; Yang, K.-C.; Jeng, J.-S.; Tsai, L.-K.; Chang, C.-F. Plasma Soluble TREM2 Is Associated with White Matter Lesions Independent of Amyloid and Tau. Brain 2021, 144, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hatfield, J.; Sullivan, J.K.; Xu, F.; Van Nostrand, W.E. Robust Neuroinflammation and Perivascular Pathology in rTg-DI Rats, a Novel Model of Microvascular Cerebral Amyloid Angiopathy. J. Neuroinflamm. 2020, 17, 78. [Google Scholar] [CrossRef]
- Li, Z.; Yu, S.; Li, L.; Zhou, C.; Wang, L.; Tang, S.; Gu, N.; Zhang, Z.; Huang, Z.; Chen, H.; et al. TREM2 Alleviates White Matter Injury after Traumatic Brain Injury in Mice Might Be Mediated by Regulation of DHCR24/LXR Pathway in Microglia. Clin. Transl. Med. 2024, 14, e1665. [Google Scholar] [CrossRef]
- Schlepckow, K.; Monroe, K.M.; Kleinberger, G.; Cantuti-Castelvetri, L.; Parhizkar, S.; Xia, D.; Willem, M.; Werner, G.; Pettkus, N.; Brunner, B.; et al. Enhancing Protective Microglial Activities with a Dual Function TREM2 Antibody to the Stalk Region. EMBO Mol. Med. 2020, 12, e11227. [Google Scholar] [CrossRef]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-Human TREM2 Induces Microglia Proliferation and Reduces Pathology in an Alzheimer’s Disease Model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Simmons, A.; Mayorga, A.; Burgess, B.; Nguyen, T.; Budda, B.; Rychkova, A.; Rhinn, H.; Tassi, I.; Ward, M.; et al. Preclinical and First-in-Human Evaluation of AL002, a Novel TREM2 Agonistic Antibody for Alzheimer’s Disease. Alzheimer’s Res. Ther. 2024, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Fassler, M.; Rappaport, M.S.; Cuño, C.B.; George, J. Engagement of TREM2 by a Novel Monoclonal Antibody Induces Activation of Microglia and Improves Cognitive Function in Alzheimer’s Disease Models. J. Neuroinflamm. 2021, 18, 19. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; Zhao, Y.; Zhang, Y.; Zhang, X.; Guan, J.; Liu, Y.; Fu, J. Curcumin Protects against Cognitive Impairments in a Rat Model of Chronic Cerebral Hypoperfusion Combined with Diabetes Mellitus by Suppressing Neuroinflammation, Apoptosis, and Pyroptosis. Int. Immunopharmacol. 2021, 93, 107422. [Google Scholar] [CrossRef]
- Teter, B.; Morihara, T.; Lim, G.P.; Chu, T.; Jones, M.R.; Zuo, X.; Paul, R.M.; Frautschy, S.A.; Cole, G.M. Curcumin Restores Innate Immune Alzheimer’s Disease Risk Gene Expression to Ameliorate Alzheimer Pathogenesis. Neurobiol. Dis. 2019, 127, 432–448. [Google Scholar] [CrossRef]
- Wang, J.; Du, L.; Zhang, T.; Chu, Y.; Wang, Y.; Wang, Y.; Ji, X.; Kang, Y.; Cui, R.; Zhang, G.; et al. Edaravone Dexborneol Ameliorates the Cognitive Deficits of APP/PS1 Mice by Inhibiting TLR4/MAPK Signaling Pathway via Upregulating TREM2. Neuropharmacology 2024, 255, 110006. [Google Scholar] [CrossRef]
- Wang, K.; Zan, S.; Xu, J.; Sun, W.; Li, C.; Zhang, W.; Ni, D.; Cheng, R.; Li, L.; Yu, Z.; et al. Yishen Huazhuo Decoction Regulates Microglial Polarization to Reduce Alzheimer’s Disease-Related Neuroinflammation through TREM2. Heliyon 2024, 10, e35800. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, M.; Zhang, X.; Wang, C.; Zheng, Y.; Hu, Y. Hydroxysafflor Yellow A Inhibits Aβ1–42-Induced Neuroinflammation by Modulating the Phenotypic Transformation of Microglia via TREM2/TLR4/NF-κB Pathway in BV-2 Cells. Neurochem. Res. 2022, 47, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, M.; Schaefer, R.; Schlepckow, K.; Kunze, L.H.; Struebing, F.L.; Brunner, B.; Willem, M.; Bartos, L.M.; Feiten, A.; Palumbo, G.; et al. PET Imaging of Microglia in Alzheimer’s Disease Using Copper-64 Labeled TREM2 Antibodies. Theranostics 2024, 14, 6319–6336. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.C.; Gergits, F.W.; Renoux, A.J.; Weisman, E.J.; Dejanovic, B.; Huang, L.; Pandya, B.; McLaren, D.G.; Lynch, B.A.; Fisher, R.; et al. Rescue of in Vitro Models of CSF1R-Related Adult-Onset Leukodystrophy by Iluzanebart: Mechanisms and Therapeutic Implications of TREM2 Agonism. J. Neuroinflamm. 2025, 22, 26. [Google Scholar] [CrossRef]
- Beckermann, K.E.; Patnaik, A.; Winer, I.; Tan, W.; Bashir, B.; Kyriakopoulos, C.E.; Sweis, R.F.; Chamberlain, M.; Rini, B.I. A Phase 1b Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Py314 in Combination with Pembrolizumab in Patients with Advanced Renal Cell Carcinoma. Investig. New Drugs 2024, 42, 179–184. [Google Scholar] [CrossRef]
- Yeku, O.O.; Barve, M.; Tan, W.W.; Wang, J.; Patnaik, A.; LoRusso, P.; Richardson, D.L.; Naqash, A.R.; Lynam, S.K.; Fu, S.; et al. Myeloid Targeting Antibodies PY159 and PY314 for Platinum-Resistant Ovarian Cancer. J. Immunother. Cancer 2025, 13, e010959. [Google Scholar] [CrossRef] [PubMed]
- Stangel, M.; Feuerbach, D.; Shimshek, D.; Gasparini, F.; Galimberti, I.; George, N.; Peraus, G.; Sovago, J. VHB937, a TREM2 Stabilizing and Activating Antibody Strongly Reduces Pathology After Peripheral Administration in a Broad Range of Animal Models for Neuroinflammation and Neurodegeneration (P4-4.004). Neurology 2024, 102, 5160. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Jiang, L.; Fan, X.; Li, L.; Li, X.; Arase, H.; Zhao, Y.; Cao, W.; Zheng, H.; et al. A Tetravalent TREM2 Agonistic Antibody Reduced Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Sci. Transl. Med. 2022, 14, eabq0095. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zheng, X.; Ma, H.; Zhu, M.; Li, X.; Sun, X.; Feng, X. TREM2 in Neurodegenerative Diseases: Mechanisms and Therapeutic Potential. Cells 2025, 14, 1387. https://doi.org/10.3390/cells14171387
Li L, Zheng X, Ma H, Zhu M, Li X, Sun X, Feng X. TREM2 in Neurodegenerative Diseases: Mechanisms and Therapeutic Potential. Cells. 2025; 14(17):1387. https://doi.org/10.3390/cells14171387
Chicago/Turabian StyleLi, Ling, Xiaoxiao Zheng, Hongyue Ma, Mingxia Zhu, Xiuli Li, Xiaodan Sun, and Xinhong Feng. 2025. "TREM2 in Neurodegenerative Diseases: Mechanisms and Therapeutic Potential" Cells 14, no. 17: 1387. https://doi.org/10.3390/cells14171387
APA StyleLi, L., Zheng, X., Ma, H., Zhu, M., Li, X., Sun, X., & Feng, X. (2025). TREM2 in Neurodegenerative Diseases: Mechanisms and Therapeutic Potential. Cells, 14(17), 1387. https://doi.org/10.3390/cells14171387







