Heparanase-Neutralizing Monoclonal Antibody (mAb A54) Attenuates Tumor Growth and Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Monoclonal Antibodies (mAb)
2.2. Cells and Cell Culture
2.3. Heparanase Enzymatic Activity—ECM Degradation Assay
2.4. Matrigel Invasion Assay
2.5. Tumor Models
2.6. U87 Glioma
2.7. CAG Myeloma
2.8. MPC-11 Myeloma
2.9. Pancreatic Cancer
2.10. Crysalization of the Enzyme–Antibody Complex
2.11. Statistics
3. Results
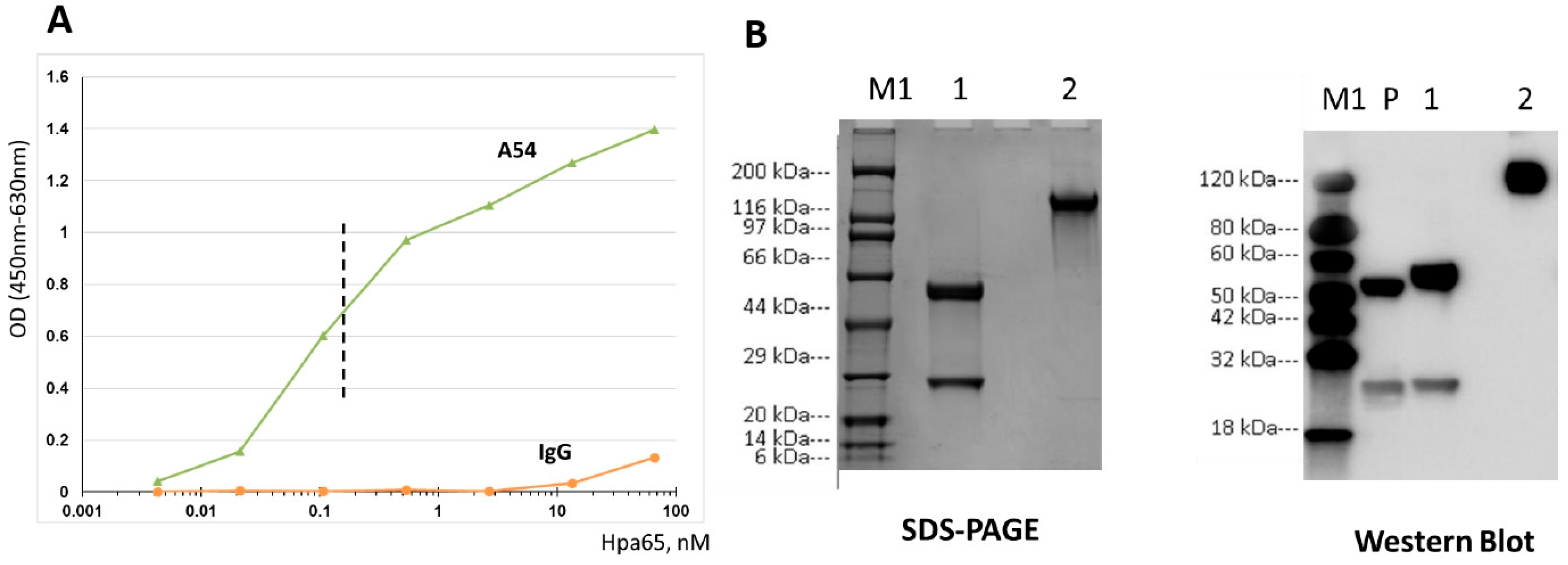
3.1. Generation and Characterization of Heparanase-Neutralizing mAb
3.2. A54 mAb Inhibits Heparanase Enzymatic Activity, Cellular Uptake, and Cell Invasion
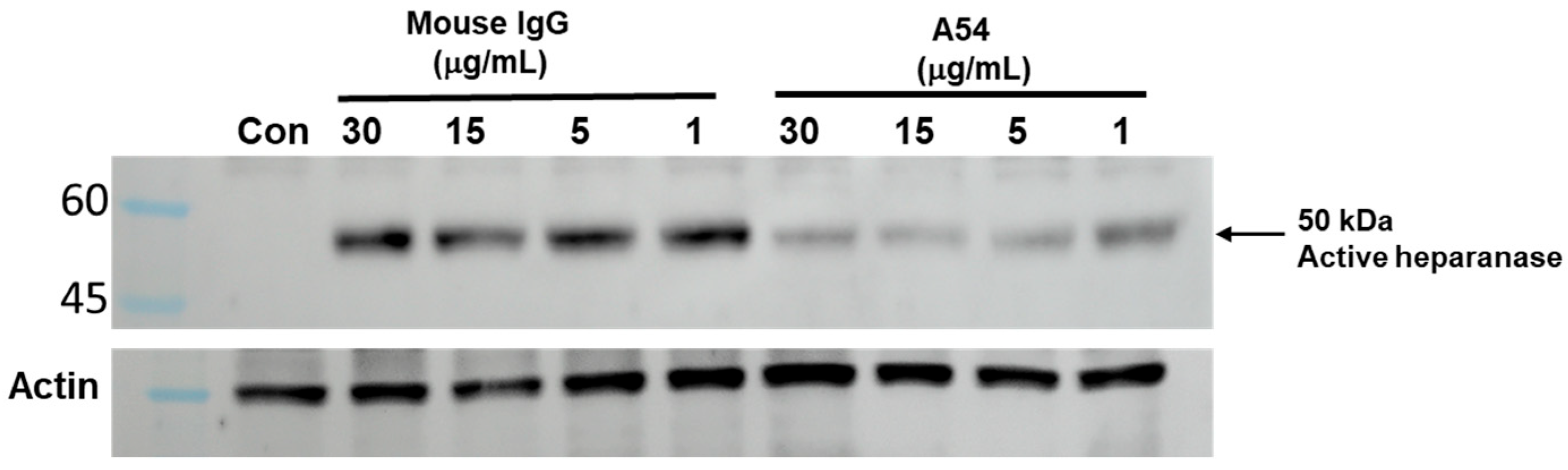
3.2.1. Enzymatic Activity
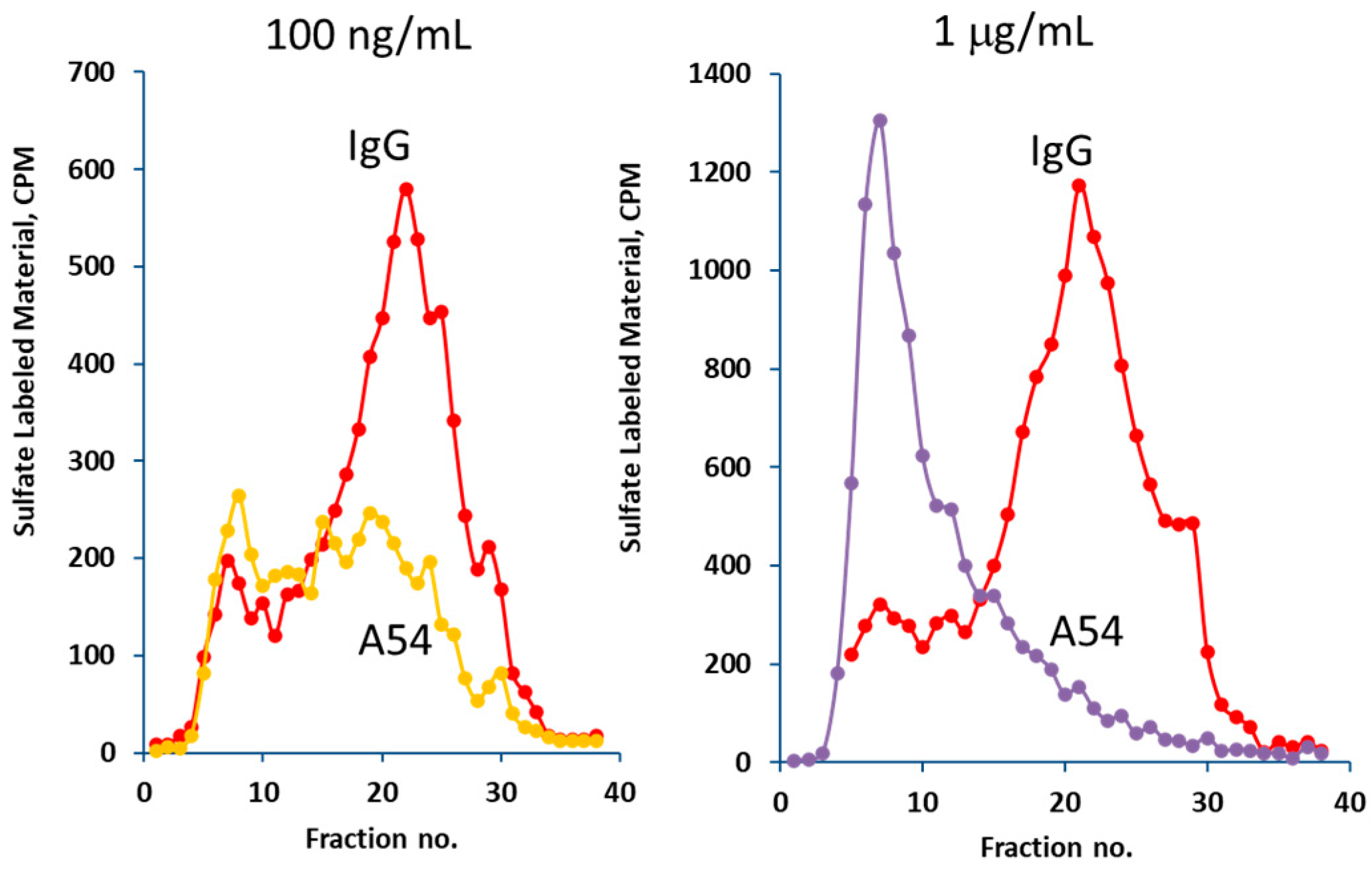
3.2.2. Heparanase Uptake and Processing
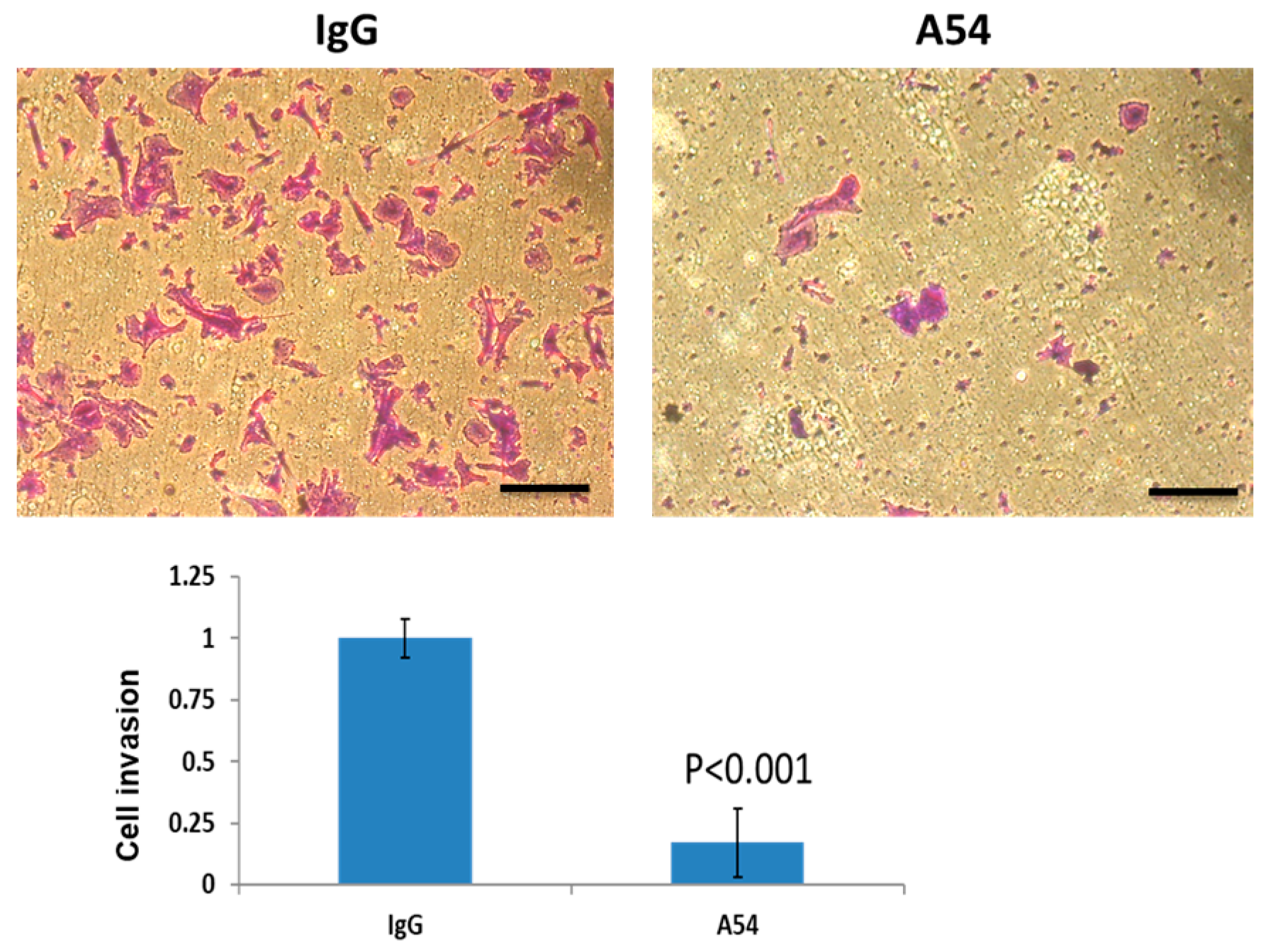
3.2.3. Cell Invasion
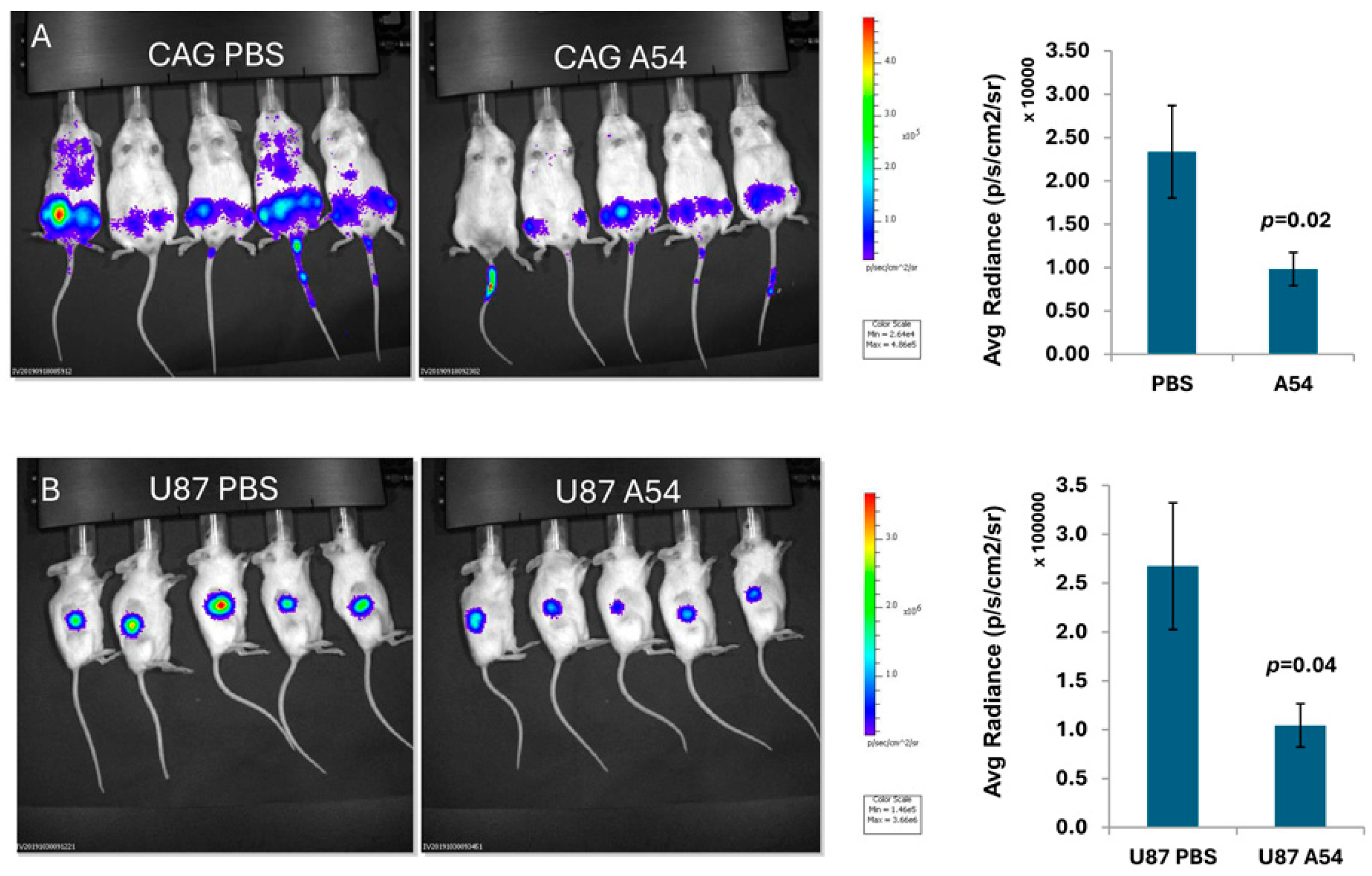
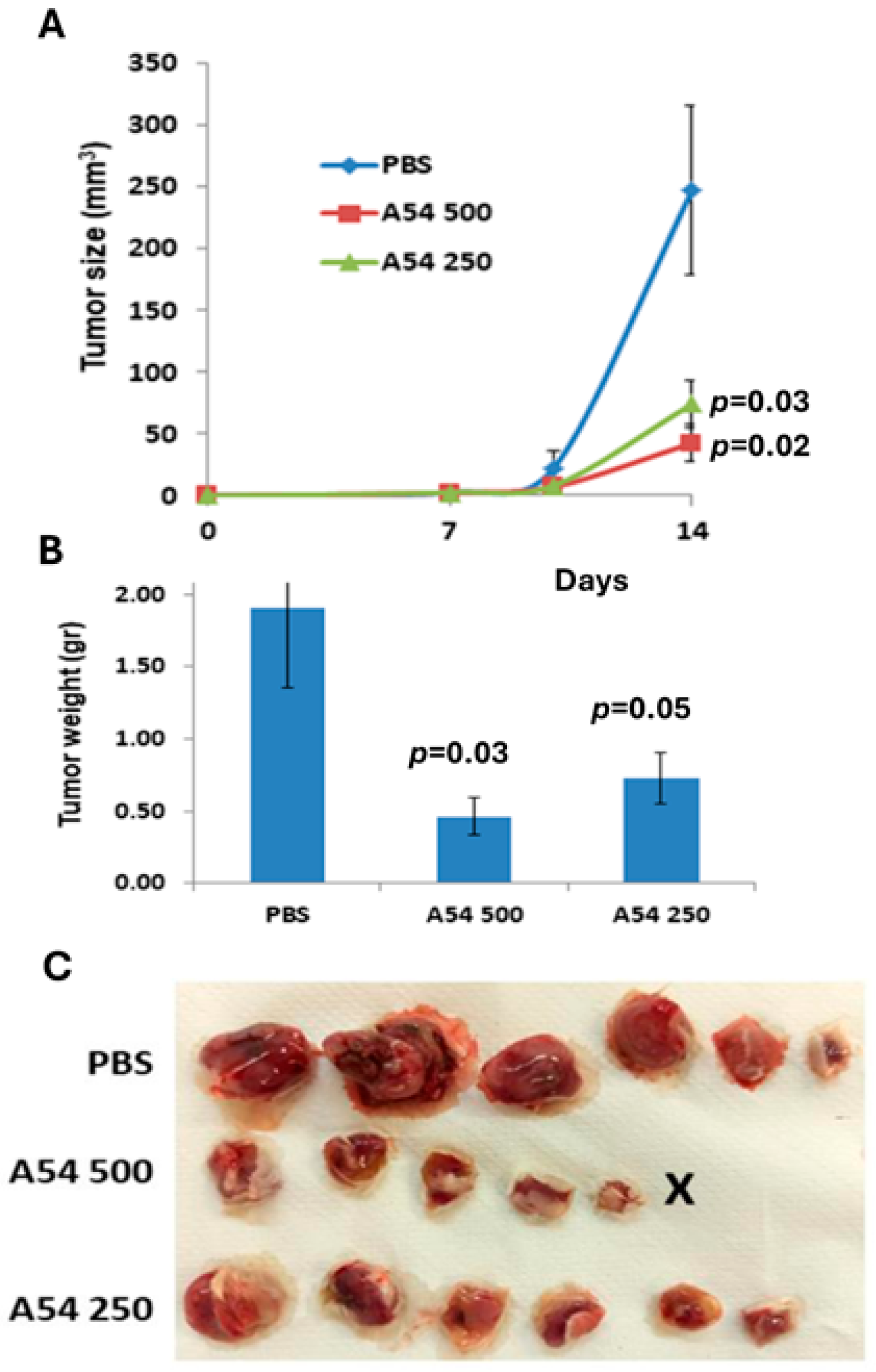
3.2.4. Anti-Heparanase A54 mAb Attenuates Myeloma and Glioma Tumor Growth
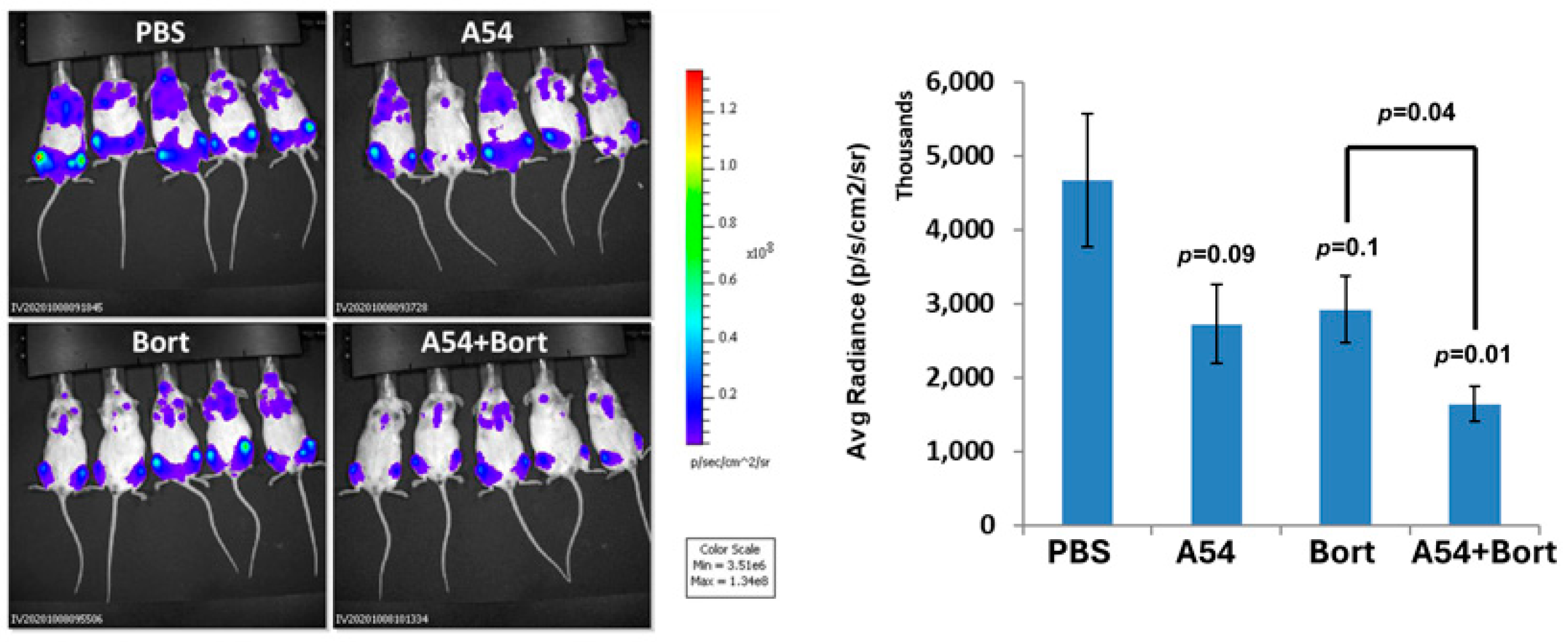
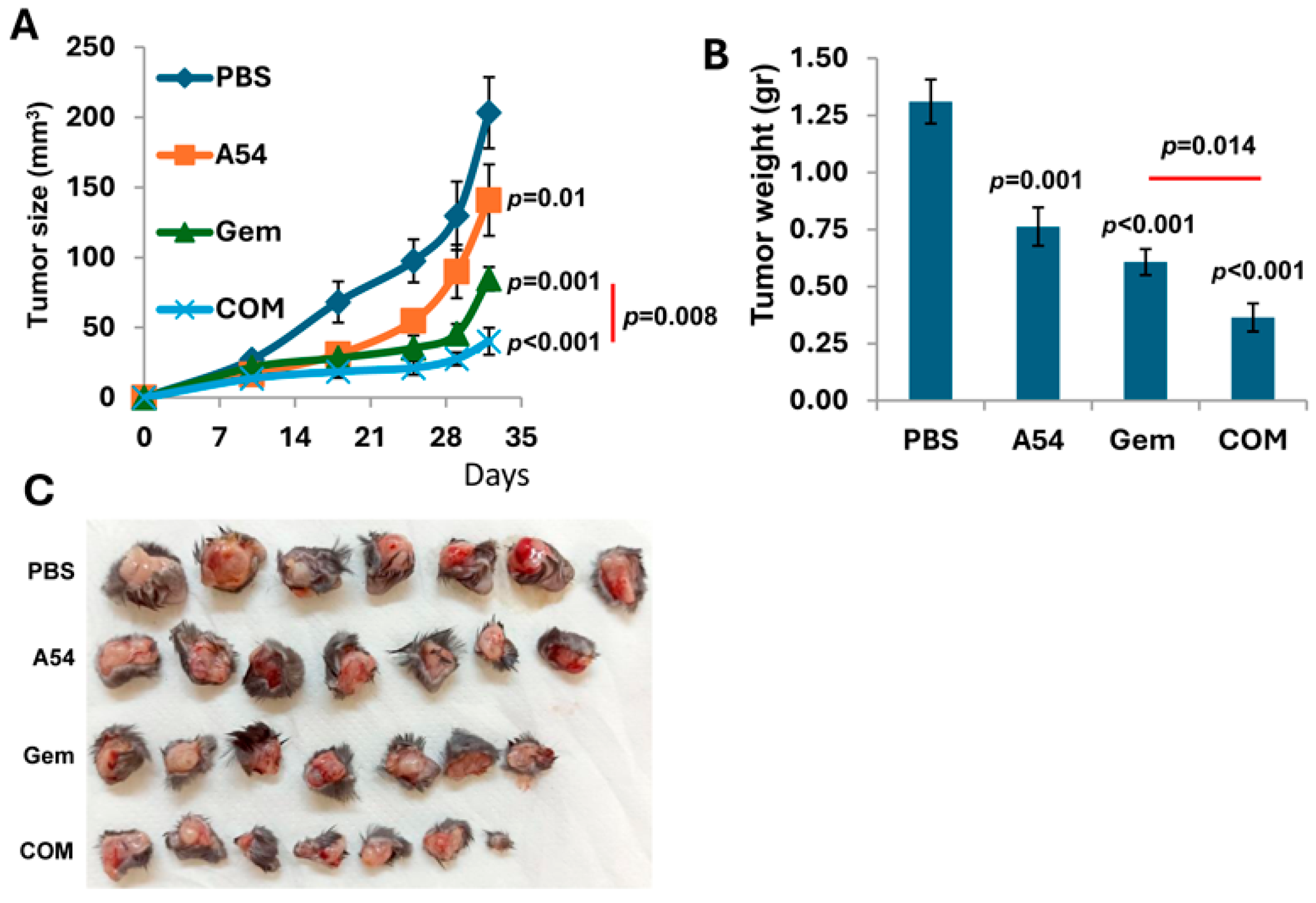
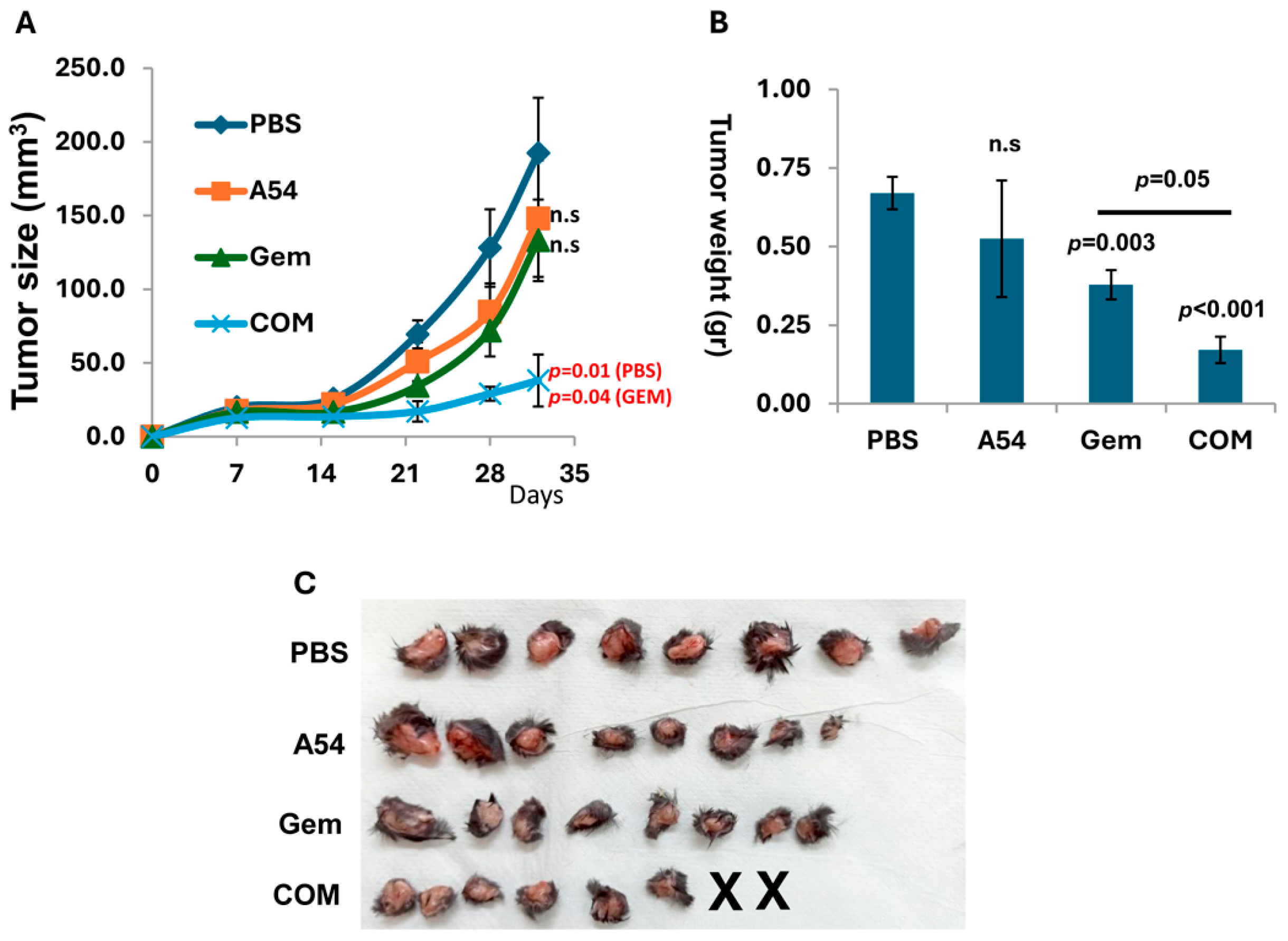
3.2.5. Combination of A54 mAb and Gemcitabine Attenuates Pancreatic Tumor Growth
3.2.6. Humanized A54 Antibody
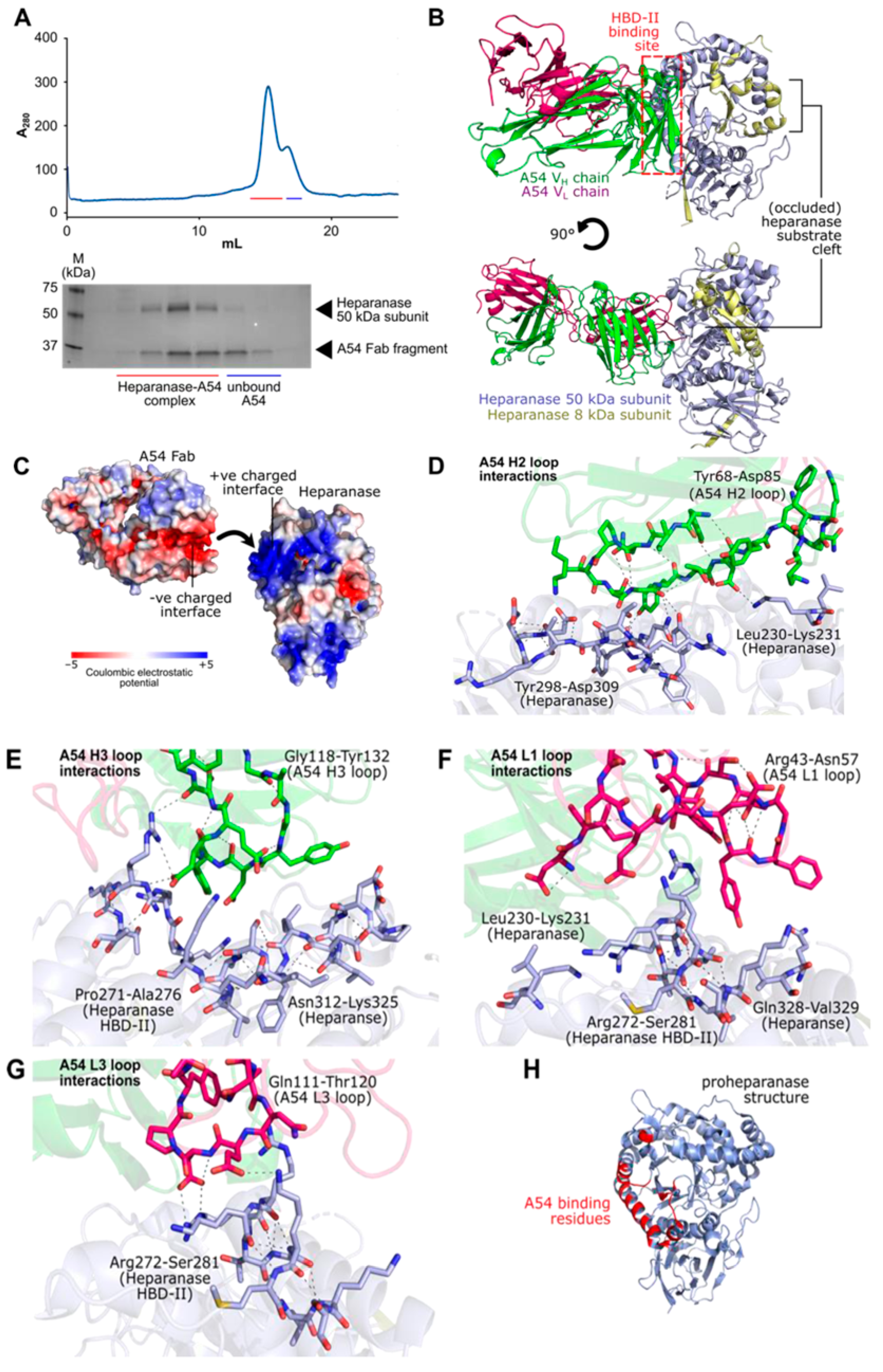
3.2.7. Structural Features of the Interaction Between A54 and Heparanase Unravel HBD-II as the Predominant Epitope
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extra Cellular Matrix |
| FCS | Fetal Calf Serum |
| kDa | Kilodalton |
| HS | Heparan Sulfate |
| Hpa | Heparanase |
| IgG | Immunoglobulin |
| IVIS | In Vivo Imaging System |
| HBD | Heparin Binding Domain |
| mAb | Monoclonal Antibody |
| PDB | Protein Data Bank |
| PBS | Phosphate-Buffered Saline |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PF4 | Platelet Factor 4 |
| SEM | Standard Error of the Mean |
References
- Yang, Y.; Yuan, F.; Zhou, H.; Quan, J.; Liu, C.; Wang, Y.; Xiao, F.; Liu, Q.; Liu, J.; Zhang, Y.; et al. Potential roles of heparanase in cancer therapy: Current trends and future direction. J. Cell. Physiol. 2023, 238, 896–917. [Google Scholar] [CrossRef]
- Mayfosh, A.J.; Nguyen, T.K.; Hulett, M.D. The Heparanase Regulatory Network in Health and Disease. Int. J. Mol. Sci. 2021, 22, 11096. [Google Scholar] [CrossRef]
- Wu, L.; Viola, C.M.; Brzozowski, A.M.; Davies, G.J. Structural characterization of human heparanase reveals insights into substrate recognition. Nat. Struct. Mol. Biol. 2015, 22, 1016–1022. [Google Scholar] [CrossRef]
- Wu, L.; Davies, G.J. An Overview of the Structure, Mechanism and Specificity of Human Heparanase. Adv. Exp. Med. Biol. 2020, 1221, 139–167. [Google Scholar]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the hallmarks of cancer. J. Trans. Med. 2020, 18, 453. [Google Scholar] [CrossRef]
- Rabelink, T.J.; Berg, B.M.V.D.; Garsen, M.; Wang, G.; Elkin, M.; Vlag, J.V.D. Heparanase: Roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat. Rev. Nephrol. 2017, 13, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.; Meirovitz, A.; Hirshoren, N.; Bulvik, R.; Binder, A.; Rubinstein, A.M.; Elkin, M. Versatile role of heparanase in inflammation. Matrix Biol. 2013, 32, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Koganti, R.; Suryawanshi, R.; Shukla, D. Heparanase, cell signaling, and viral infections. Cell. Mol. Life Sci. (CMLS) 2020, 77, 5059–5077. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Gambaro, G.; Onisto, M. Impact of Heparanse on Organ Fibrosis. Adv. Exp. Med. Biol. 2020, 1221, 669–684. [Google Scholar]
- Nguyen, T.K.; Paone, S.; Chan, E.; Poon, I.K.; Baxter, A.A.; Thomas, S.R.; Hulett, M.D. Heparanase: A Novel Therapeutic Target for the Treatment of Atherosclerosis. Cells 2022, 12, 3198. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Folkman, J.; Sullivan, R.; Fridman, R.; Ishai-Michaeli, R.; Sasse, J.; Klagsbrun, M. Endothelial cell-derived basic fibroblast growth factor: Synthesis and deposition into subendothelial extracellular matrix. Proc. Natl. Acad. Sci. USA 1987, 84, 2292–2296. [Google Scholar] [CrossRef]
- Pinhal, M.A.; Melo, C.M.; Nader, H.B. The Good and Bad Sides of Heparanase-1 and Heparanase-2. Adv. Exp. Med. Biol. 2020, 1221, 821–845. [Google Scholar] [PubMed]
- Ramani, V.C.; Yang, Y.; Ren, Y.; Nan, L.; Sanderson, R.D. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J. Biol. Chem. 2011, 286, 6490–6499. [Google Scholar] [CrossRef] [PubMed]
- Khanna, M.; Parish, C.R. Heparanase: Historical Aspects and Future Perspectives. Adv. Exp. Med. Biol. 2020, 1221, 71–96. [Google Scholar]
- Rivara, S.; Milazzo, F.M.; Giannini, G. Heparanase: A rainbow pharmacological target associated to multiple pathologies including rare diseases. Future Med. Chem. 2016, 8, 647–680. [Google Scholar] [CrossRef]
- Giannini, G.; Battistuzzi, G.; Rivara, S. The Control of Heparanase Through the Use of Small Molecules. Adv. Exp. Med. Biol. 2020, 1221, 567–603. [Google Scholar]
- He, P.; Zhang, X.; Xia, K.; Green, D.E.; Baytas, S.; Xu, Y.; Pham, T.; Liu, J.; Zhang, F.; Almond, A.; et al. Chemoenzymatic synthesis of sulfur-linked sugar polymers as heparanase inhibitors. Nat. Commun. 2022, 13, 7438. [Google Scholar] [CrossRef]
- Noseda, A.; Barbieri, P. Roneparstat: Development, Preclinical and Clinical Studies. Adv. Exp. Med. Biol. 2020, 1221, 523–538. [Google Scholar] [PubMed]
- Dredge, K.; Brennan, T.V.; Hammond, E.; Lickliter, J.D.; Lin, L.; Bampton, D.; Handley, P.; Lankesheer, F.; Morrish, G.; Yang, Y.; et al. A Phase I study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours. Br. J. Cancer 2018, 118, 1035–1041. [Google Scholar] [CrossRef]
- Zhou, H.; Roy, S.; Cochran, E.; Zouaoui, R.; Chu, C.L.; Duffner, J.; Zhao, G.; Smith, S.; Galcheva-Gargova, Z.; Karlgren, J.; et al. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PLoS ONE 2011, 6, e21106. [Google Scholar] [CrossRef]
- Abdulsalam, H.; Hix, M.A.; Philip, L.; Singh, K.; Walker, A.R.; Nguyen, H.M. From Docking and Mo-lecular Dynamics to Experimental Discovery: Exploring the Hydrophobic Landscapes of Heparanase to Design Potent Inhibitors. J. Chem. Inf. Model. 2025, 65, 6899–6912. [Google Scholar] [CrossRef] [PubMed]
- Galli, M.; Chatterjee, M.; Grasso, M.; Specchia, G.; Magen, H.; Einsele, H.; Celeghini, I.; Barbieri, P.; Paoletti, D.; Pace, S.; et al. Phase I study of the heparanase inhibitor roneparstat: An innovative approach for multiple myeloma therapy. Haematologica 2018, 103, e469–e472. [Google Scholar] [CrossRef]
- Hammond, E.; Haynes, N.M.; Cullinane, C.; Brennan, T.V.; Bampton, D.; Handley, P.; Karoli, T.; Lanksheer, F.; Lin, L.; Yang, Y.; et al. Immunomodulatory activities of pixatimod: Emerging nonclinical and clinical data, and its potential utility in combination with PD-1 inhibitors. J. Immunother. Cancer 2018, 6, 54. [Google Scholar] [CrossRef]
- Lemech, C.; Dredge, K.; Bampton, D.; Hammond, E.; Clouston, A.; Waterhouse, N.J.; Stanley, A.C.; Mouttie, L.L.-E.; Chojnowski, G.M.; Haydon, A.; et al. Phase Ib open-label, multicenter study of pixatimod, an activator of TLR9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J. Immunother. Cancer 2023, 11, e006136. [Google Scholar] [CrossRef]
- Liu, C.J.; Chang, J.; Lee, P.H.; Lin, D.Y.; Wu, C.C.; Jeng, L.B.; Lin, Y.J.; Mok, K.T.; Lee, W.C.; Yeh, H.Z.; et al. Adjuvant heparanase inhibitor PI-88 therapy for hepatocellular carcinoma recurrence. World J. Gastroenterol. 2014, 20, 11384–11393. [Google Scholar] [CrossRef]
- Philip, L.; Abdulsalam, H.; Singh, K.; Nguyen, H.M. Investigation into the binding domains of platelet factor 4 unlocks new avenues for the design and synthesis of selective sulfated pseudo-tetrasaccharide aminoglycoside ligands. Eur. J. Med. Chem. 2025, 295, 117792. [Google Scholar] [CrossRef]
- Arepally, G.M. Heparin-induced thrombocytopenia. Blood J. Am. Soc. Hematol. 2017, 129, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.D.; Turnbull, J.E.; Gallagher, J.T.; Lander, A.D. Fine structure of heparan sulfate regulates syndecan-1 function and cell behavior. J. Biol. Chem. 1994, 269, 13100–13106. [Google Scholar] [CrossRef]
- Ghoti, H.; Ackerman, S.; Rivella, S.; Casu, C.; Nadir, Y. Heparanase Level and Procoagulant Activity Are Increased in Thalassemia and Attenuated by Janus Kinase 2 Inhibition. Am. J. Pathol. 2020, 190, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, M.; Arvatz, G.; Horowitz, N.; Feld, S.; Naroditsky, I.; Zhang, Y.; Ng, M.; Hammond, E.; Nevo, E.; Vlodavsky, I.; et al. Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I. Preparation of extracellular matrices produced by cultured corneal endothelial and PF-HR9 endodermal cells. Cur. Prot. Cell. Biol. 2001, 1, 10–14. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Friedmann, Y.; Elkin, M.; Aingorn, H.; Atzmon, R.; Ishai-Michaeli, R.; Bitan, M.; Pappo, O.; Peretz, T.; Michal, I.; et al. Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 1999, 5, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Bandari, S.K.; Sanderson, R.D. Extracellular vesicles released during hypoxia transport heparanase and enhance macrophage migration, endothelial tube formation and cancer cell stemness. Proteoglycan Res. 2023, 1, e1. [Google Scholar] [CrossRef]
- Barash, U.; Spyrou, A.; Liu, P.; Vlodavsky, E.; Zhu, C.; Luo, J.; Su, D.; Ilan, N.; Forsberg-Nilsson, K.; Vlodavsky, I.; et al. Heparanase promotes glioma progression via enhancing CD24 expression. Int. J. Cancer 2019, 145, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, T.; Nishida, D.; Horibe, K. Primary tumor-induced immunity suppresses bone metastases of breast cancer in syngeneic immunocompetent mouse models. Bone 2024, 178, 116944. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Benelli, R. The chemoinvasion assay: A method to assess tumor and endothelial cell invasion and its modulation. Nat. Protoc. 2007, 2, 504–511. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug. Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Tickle, I.J.; Flensburg, C.; Keller, P.; Paciorek, W.; Sharff, A.; Vonrhein, C.; Bricogne, G. STARANISO; Global Phasing Ltd.: Cambridge, UK, 2016; Available online: https://staraniso.globalphasing.org/cgi-bin/staraniso.cgi (accessed on 13 August 2025).
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Skubak, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef]
- Kovalevskiy, O.; Nicholls, R.A.; Murshudov, G.N. Automated refinement of macromolecular structures at low resolution using prior information. Acta Crystallogr. Sect. D Struct. Biol. 2016, 72, 1149–1161. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Zetser, A.; Levy-Adam, F.; Kaplan, V.; Gingis-Velitski, S.; Bashenko, Y.; Schubert, S.; Flugelman, M.Y.; Vlodavsky, I.; Ilan, N. Processing and activation of latent heparanase occurs in lysosomes. J. Cell Sci. 2004, 117, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Gingis-Velitski, S.; Zetser, A.; Kaplan, V.; Ben-Zaken, O.; Cohen, E.; Levy-Adam, F.; Bashenko, Y.; Flugelman, M.Y.; Vlodavsky, I.; Ilan, N. Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J. Biol. Chem. 2004, 279, 44084–44092. [Google Scholar] [CrossRef]
- Levy-Adam, F.; Abboud-Jarrous, G.; Guerrini, M.; Beccati, D.; Vlodavsky, I.; Ilan, N. Identification and characterization of heparin/heparan sulfate binding domains of the endoglycosidase heparanase. J. Biol. Chem. 2005, 280, 20457–20466. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Levidiotis, V.; Freeman, C.; Tikellis, C.; Cooper, M.E.; Power, D.A. Heparanase Is Involved in the Pathogenesis of Proteinuria as a Result of Glomerulonephritis. J. Am. Soc. Nephrol. 2004, 15, 68–78. [Google Scholar] [CrossRef]
- Myler, H.A.; Lipke, E.A.; Rice, E.E.; West, J.L. Novel heparanase-inhibiting antibody reduces neointima formation. J. Biochem. 2006, 139, 339–345. [Google Scholar] [CrossRef]
- Fux, L.; Feibish, N.; Cohen-Kaplan, V.; Gingis-Velitski, S.; Feld, S.; Geffen, C.; Vlodavsky, I.; Ilan, N. Structure-function approach identifies a COOH-terminal domain that mediates heparanase signaling. Cancer Res. 2009, 69, 1758–1767. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Doweck, I.; Naroditsky, I.; Vlodavsky, I.; Ilan, N. Heparanase augments epidermal growth factor receptor phosphorylation: Correlation with head and neck tumor progression. Cancer Res. 2008, 68, 10077–10085. [Google Scholar] [CrossRef]
- Barash, U.; Cohen-Kaplan, V.; Arvatz, G.; Gingis-Velitski, S.; Levy-Adam, F.; Nativ, O.; Shemesh, R.; Ayalon-Sofer, M.; Ilan, N.; Vlodavsky, I. A novel human heparanase splice variant, T5, endowed with protumorigenic characteristics. FASEB J. 2010, 24, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barash, U.; Farhoud, M.; Odeh, M.; Huberman, E.; Wu, L.; Vlodavsky, I. Heparanase-Neutralizing Monoclonal Antibody (mAb A54) Attenuates Tumor Growth and Metastasis. Cells 2025, 14, 1379. https://doi.org/10.3390/cells14171379
Barash U, Farhoud M, Odeh M, Huberman E, Wu L, Vlodavsky I. Heparanase-Neutralizing Monoclonal Antibody (mAb A54) Attenuates Tumor Growth and Metastasis. Cells. 2025; 14(17):1379. https://doi.org/10.3390/cells14171379
Chicago/Turabian StyleBarash, Uri, Malik Farhoud, Maali Odeh, Eliezer Huberman, Liang Wu, and Israel Vlodavsky. 2025. "Heparanase-Neutralizing Monoclonal Antibody (mAb A54) Attenuates Tumor Growth and Metastasis" Cells 14, no. 17: 1379. https://doi.org/10.3390/cells14171379
APA StyleBarash, U., Farhoud, M., Odeh, M., Huberman, E., Wu, L., & Vlodavsky, I. (2025). Heparanase-Neutralizing Monoclonal Antibody (mAb A54) Attenuates Tumor Growth and Metastasis. Cells, 14(17), 1379. https://doi.org/10.3390/cells14171379






