Prepubertal Diabetes Stagnates Testicular Development by Skewing Autophagy Homeostasis in Leydig Cells
Abstract
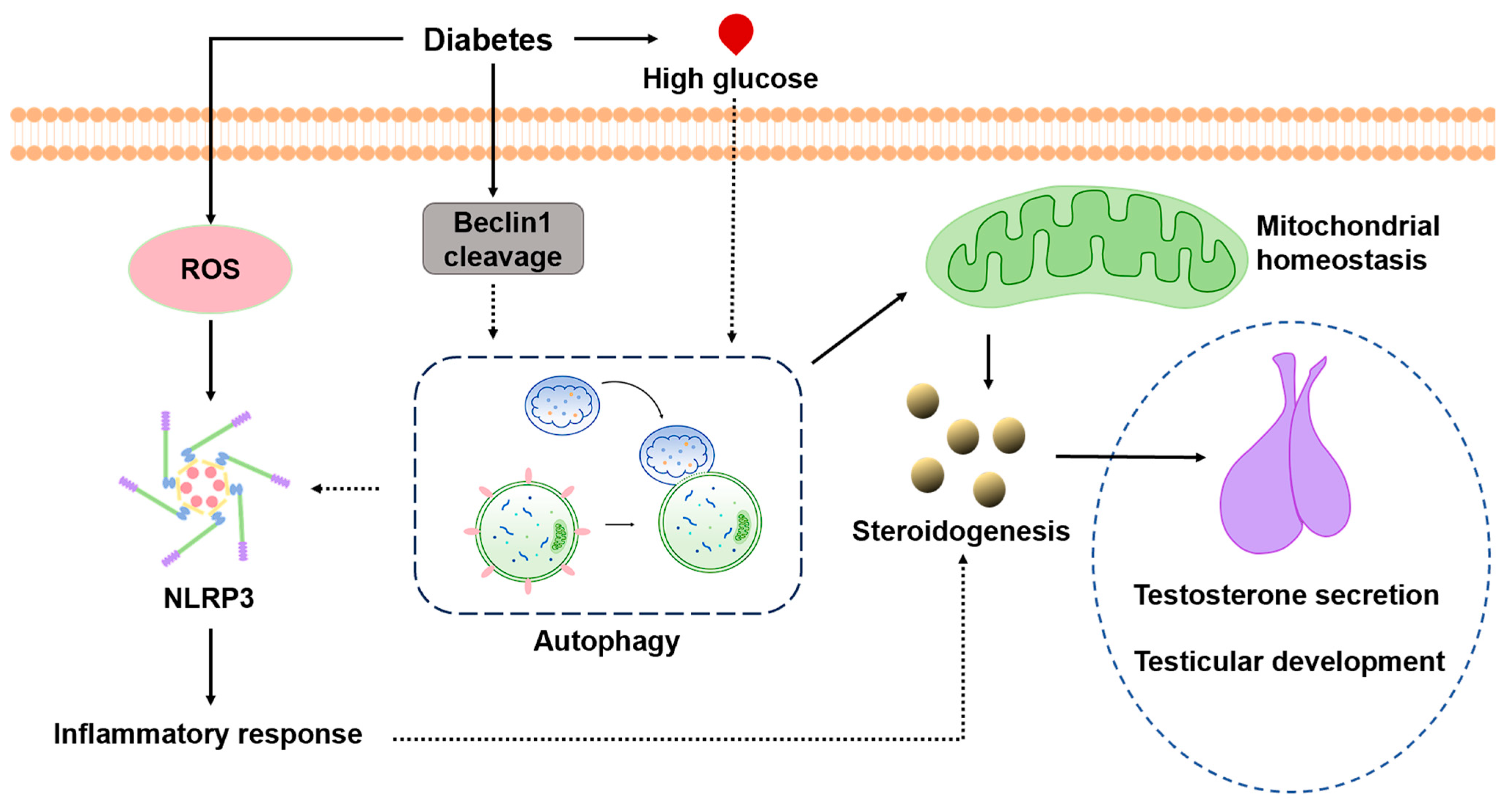
1. Introduction
2. Materials and Methods
2.1. The Animals and Treatment
2.2. Blood Glucose Measurements and Serum Testosterone Detection
2.3. Hematoxylin and Eosin (H&E) and Immunofluorescence
2.4. Leydig Cell Isolation and In Vitro Culture
2.5. Western Blot Analysis
2.6. Intracellular ROS Level Measurements
2.7. JC-1 Staining
2.8. ELISA
2.9. Mitochondrial Isolation
2.10. Transmission Electron Microscopy
2.11. Cell NLRP3 Transfection
2.12. The Statistical Analysis
3. Results
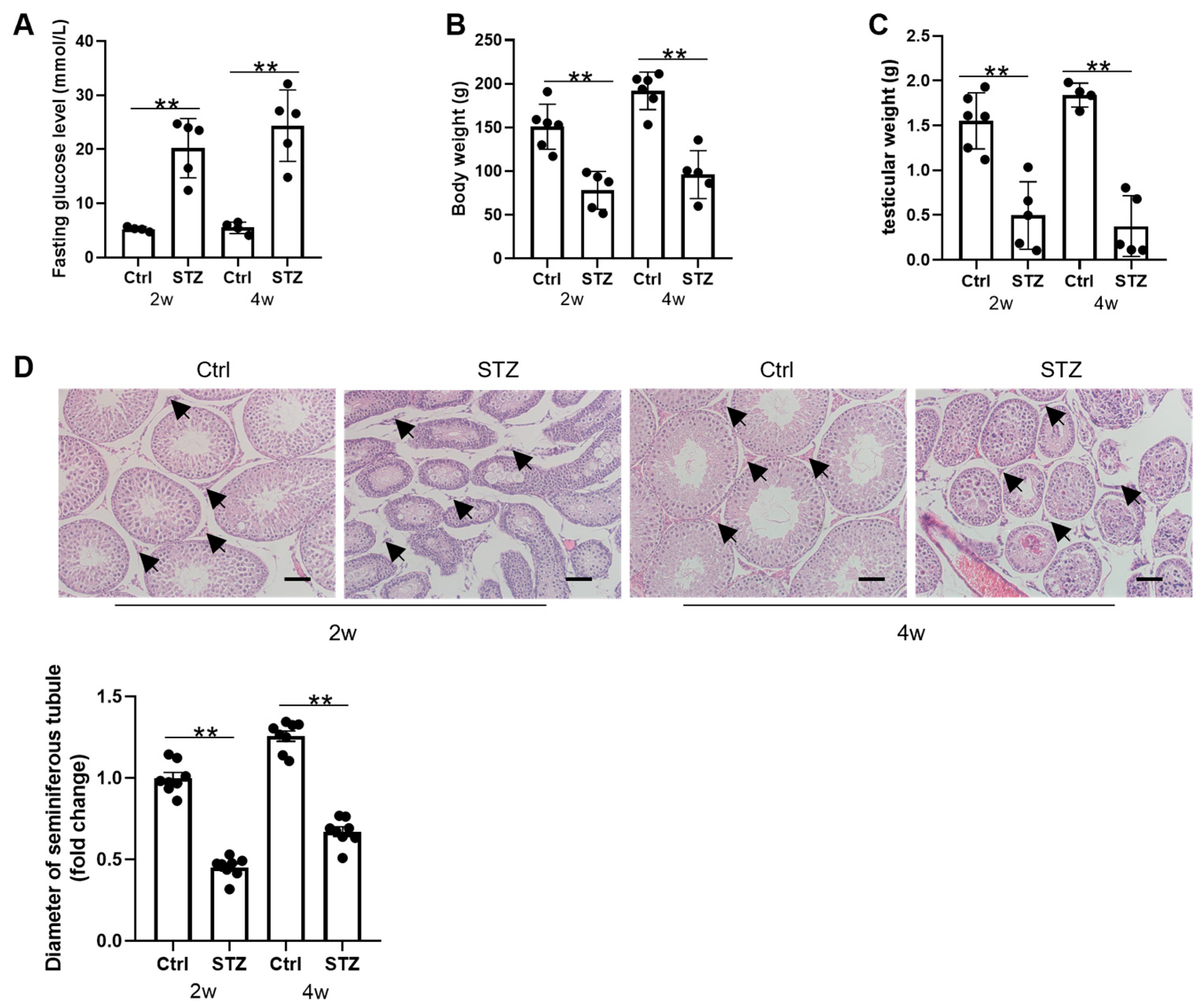
3.1. Prepubertal Diabetes Is Deleterious to Testicular Development
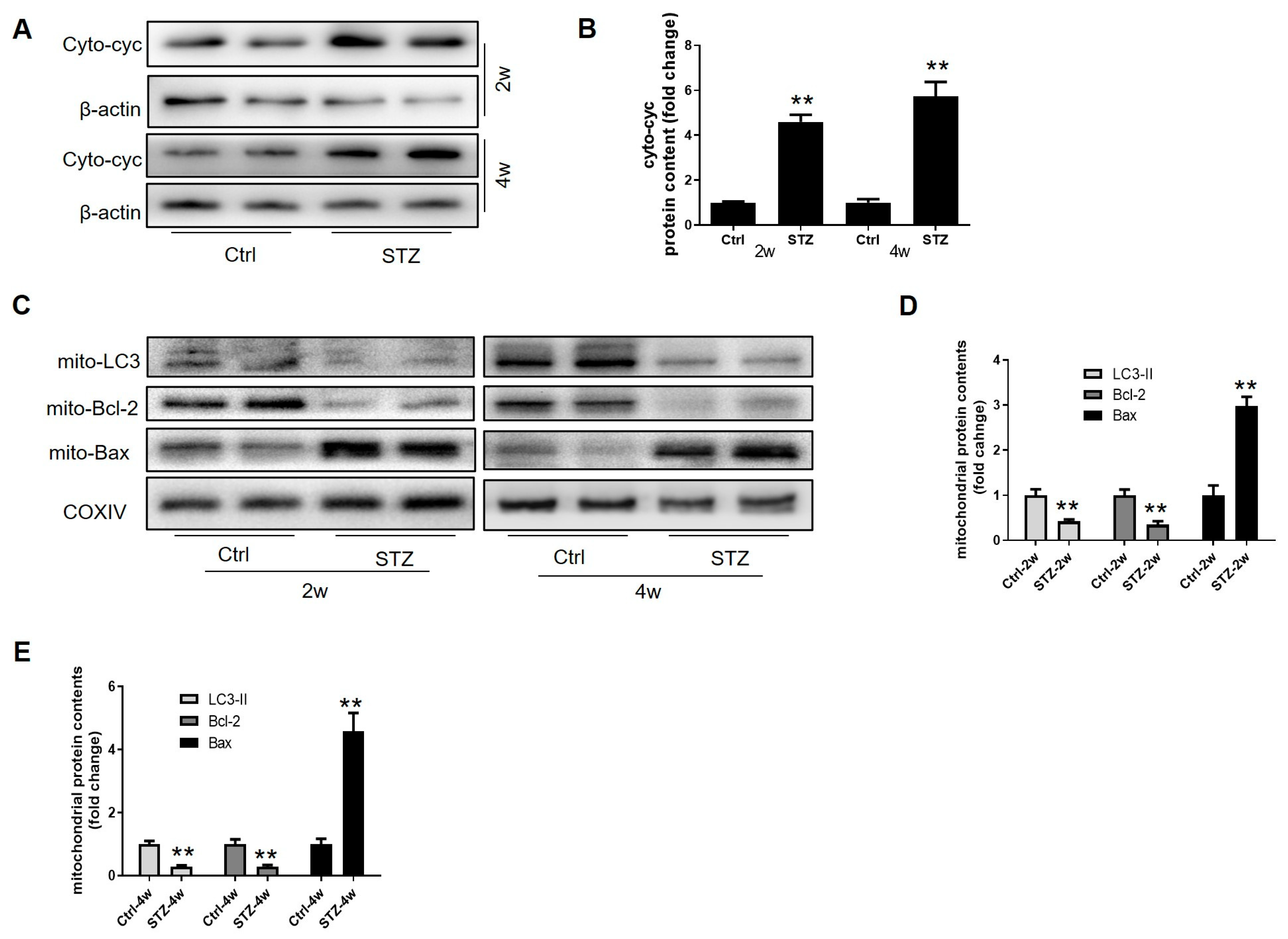
3.2. Prepubertal Diabetes Attenuates Leydig Cell Differentiation and Improves Cell Apoptosis
3.3. Prepubertal Diabetes Induces NLRP3 Expression and an Inflammatory Response in Leydig Cells
3.4. Beclin1 Cleavage Is Involved in Diabetes-Induced Autophagy Inhibition in Leydig Cells
3.5. Prepubertal Diabetes Drives a Skew in Mitochondrial Homeostasis in the Leydig Cells by Stagnating Mitophagy
3.6. Autophagy Is Required for Leydig Cell Steroidogenesis and Maturation
3.7. High Glucose Stagnates Leydig Cell Maturation by Inhibiting Autophagy


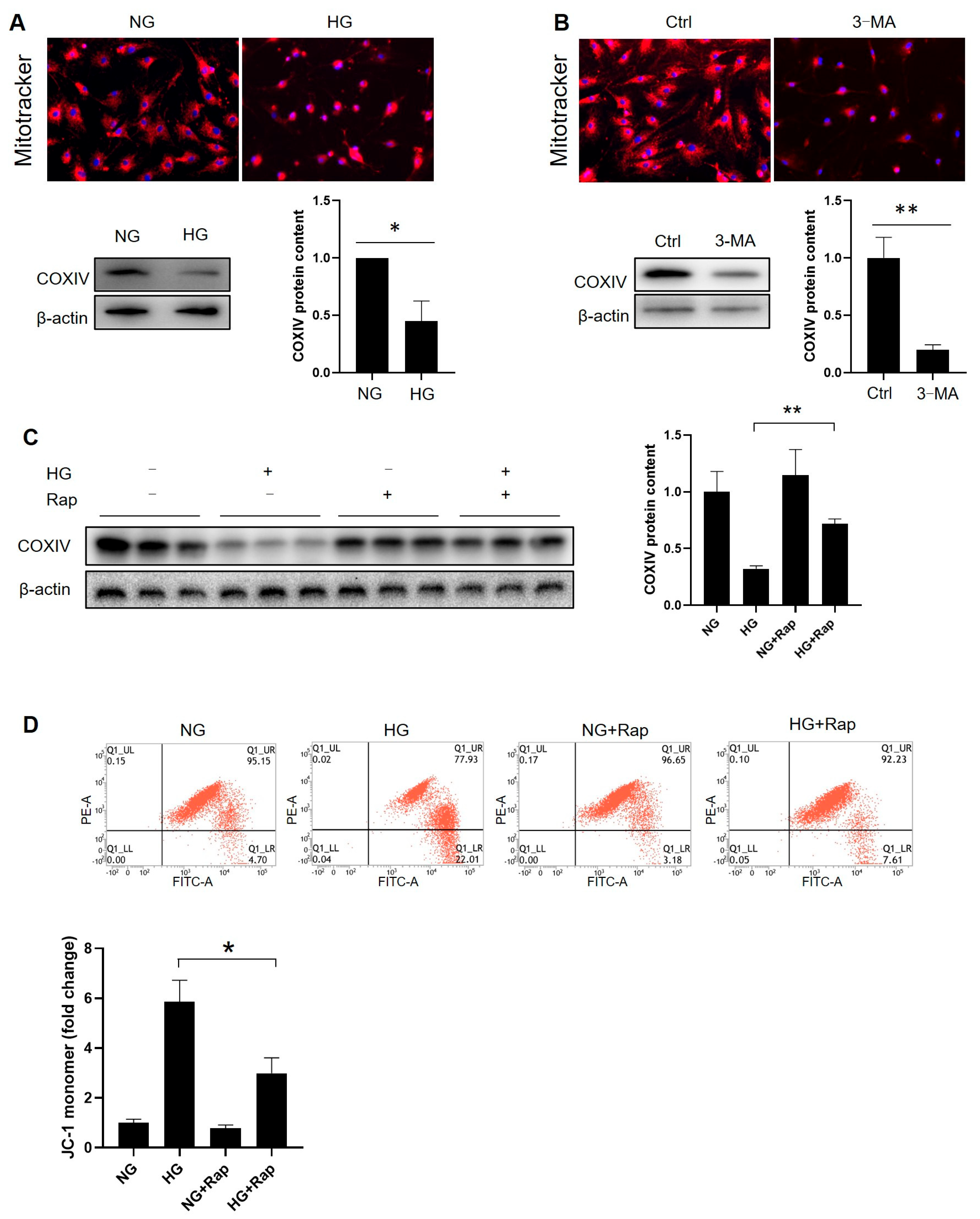
3.8. Facilitating Autophagy Reverses High-Glucose-Induced Mitochondrial Alteration in Immature Leydig Cells
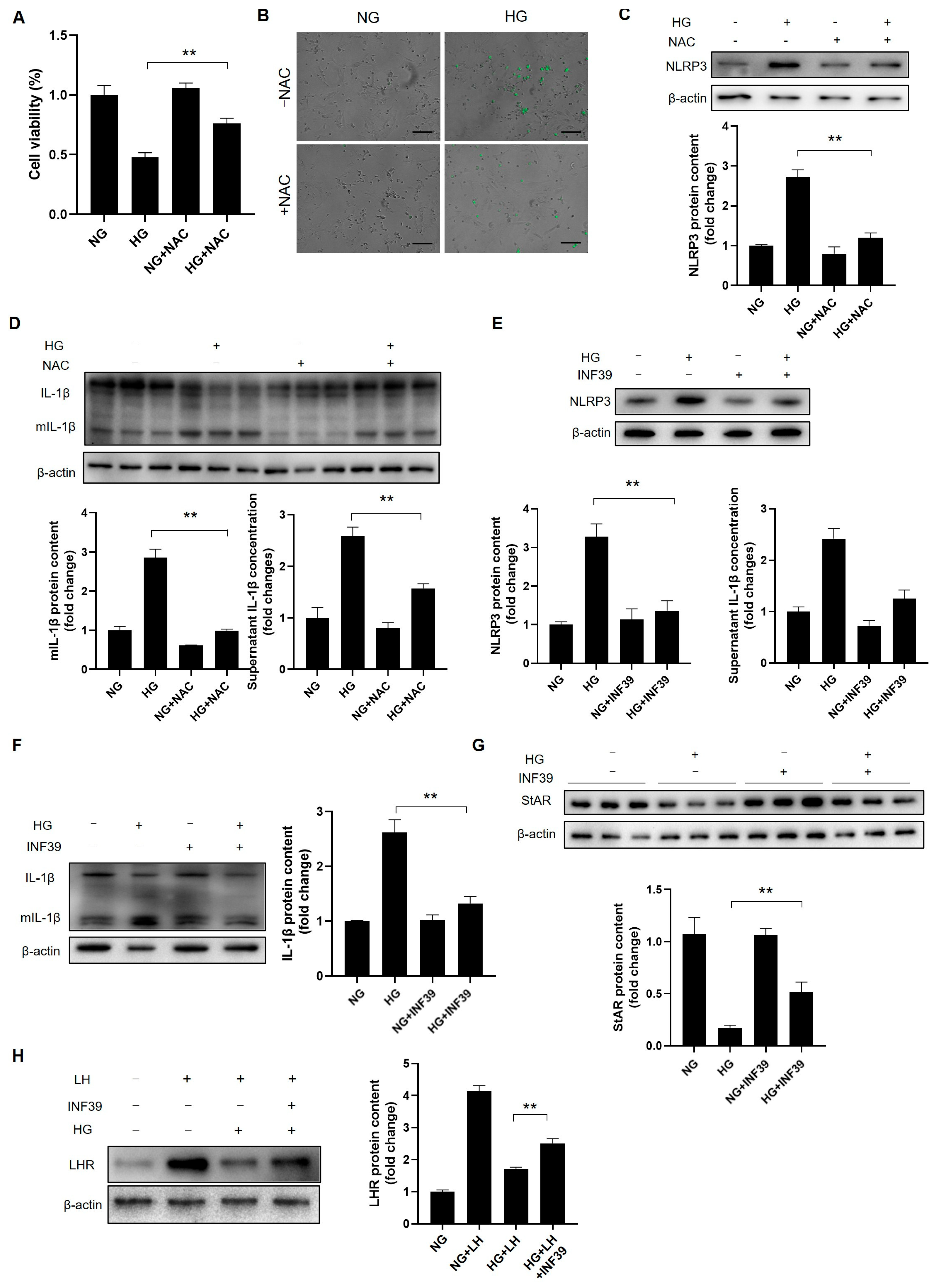
3.9. The Induction of Autophagy Abrogates High-Glucose-Induced NLRP3 Activation in Leydig Cells
3.10. ROS Hamper Leydig Cell Steroidogenesis and Maturation by Fortifying NLRP3-Mediated Inflammation

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lohela, T.J.; Lilius, T.O.; Nedergaard, M. The glymphatic system: Implications for drugs for central nervous system diseases. Nat. Rev. Drug Discov. 2022, 21, 763–779. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Nellaiappan, K.; Preeti, K.; Khatri, D.K.; Singh, S.B. Diabetic complications: An update on pathobiology and therapeutic strategies. Curr. Diabetes Rev. 2022, 18, 31–44. [Google Scholar] [CrossRef]
- Huang, R.; Chen, J.; Guo, B.; Jiang, C.; Sun, W. Diabetes-induced male infertility: Potential mechanisms and treatment options. Mol. Med. 2024, 30, 11. [Google Scholar] [CrossRef]
- Jiao, S.; Zhao, D.; Yi, H. Testosterone biosynthesis and spermatogenesis disruption by PM exposure: The hidden role of bitter taste transduction. J. Steroid Biochem. Mol. Biol. 2025, 247, 106670. [Google Scholar] [CrossRef]
- Luo, D.; Qi, X.; Xu, X.; Yang, L.; Yu, C.; Guan, Q. Involvement of p38 MAPK in Leydig cell aging and age-related decline in testosterone. Front. Endocrinol. 2023, 14, 1088249. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, K.L.; Chang, G.Q.; Pamy, P.S.; Hill, J.O.; Gayles, E.C.; Leibowitz, S.F. Weight gain model in prepubertal rats: Prediction and phenotyping of obesity-prone animals at normal body weight. Int. J. Obes. 2007, 31, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, H.; Chen, Y.; Hua, L.; Wu, X.; Gao, X.; Mao, L. Unveiling Leydig cell heterogeneity and its role in male infertility: A single-cell transcriptomic study of human testicular tissue. Reprod. Biol. 2025, 25, 100972. [Google Scholar] [CrossRef]
- Badejogbin, O.C.; Chijioke-Agu, O.E.; Olubiyi, M.V.; Agunloye, M.O. Pathogenesis of testicular dysfunction in diabetes: Exploring the mechanism and therapeutic interventions. J. Assist. Reprod. Gen. 2025, 42, 367–379. [Google Scholar] [CrossRef]
- Nandi, A.; Poretsky, L. Diabetes and the female reproductive system. Endocrinol. Metab. Clin. 2013, 42, 915–946. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.; Zeng, Q.; Duan, J. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Chandrashekara, K.; Srinag, B.; Krishnamurthy, H. Experimental diabetes-induced testicular damage in prepubertal rats. J. Diabetes 2014, 6, 48–59. [Google Scholar]
- Nna, V.U.; Bakar, A.B.A.; Ahmad, A.; Mohamed, M. Diabetes-induced testicular oxidative stress, inflammation, and caspase-dependent apoptosis: The protective role of metformin. Arch. Physiol. Biochem. 2020, 126, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Green, D.R. Autophagy-independent functions of the autophagy machinery. Cell 2019, 177, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Yamamoto, H.; Zhang, S.; Mizushima, N. Autophagy genes in biology and disease. Nat. Rev. Gen. 2023, 24, 382–400. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Y.; Gao, D.; Lin, D.; Ma, W.; Chang, D. Metabolic pathways and male fertility: Exploring the role of Sertoli cells in energy homeostasis and spermatogenesis. Am. J. Physiol. Endocrinol. Metab. 2025, 329, E160–E178. [Google Scholar] [CrossRef]
- Bassi, G.; Sidhu, S.K.; Mishra, S. The expanding role of mitochondria, autophagy and lipophagy in steroidogenesis. Cells 2021, 10, 1851. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Chang, Z.; Xin, H.; Liu, T.; Zhang, Y.; Li, G.; Zhou, F.; Gong, Y.; Gao, Z.; et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J. Androl. 2010, 13, 881–888. [Google Scholar] [CrossRef]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. Biotechniques 2007, 43, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Klinefelter, G.R.; Hall, P.F.; Ewing, L.L. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol. Reprod. 1987, 36, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Chen, D.; Zhao, X.; Huang, F.; Guan, X.; Wen, X.; Wang, J.; Shao, J.; Xie, J.; Shan, D.; et al. Isolation of Leydig cells from adult rat testes by magnetic-activated cell sorting protocol based on prolactin receptor expression. Andrology 2022, 10, 1197–1207. [Google Scholar] [CrossRef]
- Gui, W.; Zhou, C.; Wu, D.; Lin, X. 62-PUB: The mechanism of IGF2-IGF2R signaling in regulating testosterone synthesis in testicular Leydig cells. Diabetes 2024, 74, 62-PUB. [Google Scholar] [CrossRef]
- Kirat, D.; Alahwany, A.M.; Arisha, A.H.; Abdelkhalek, A.; Miyasho, T. Role of Macroautophagy in Mammalian Male Reproductive Physiology. Cells 2023, 12, 1322. [Google Scholar] [CrossRef]
- Cao, Z.; Tian, K.; Ran, Y.; Zhou, H.; Zhou, L.; Ding, Y.; Tang, X. Beclin-1: A therapeutic target at the intersection of autophagy, immunotherapy, and cancer treatment. Front. Immunol. 2024, 15, 1506426. [Google Scholar] [CrossRef]
- Afzal, A.; Zhang, Y.; Afzal, H.; Saddozai, U.A.K.; Zhang, L.; Ji, X.Y.; Khawar, M.B. Functional role of autophagy in testicular and ovarian steroidogenesis. Front. Cell Develop. Biol. 2024, 12, 1384047. [Google Scholar] [CrossRef]
- Pekkurnaz, G.; Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 2022, 4, 802–812. [Google Scholar] [CrossRef]
- Gupta, S.; Cassel, S.L.; Sutterwala, F.S.; Dagvadorj, J. Regulation of the NLRP3 inflammasome by autophagy and mitophagy. Immunol. Rev. 2025, 329, e13410. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.; Wan, S.; Ma, X.; Long, Y.; Xu, Y.; Jiang, Z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of inflammatory associated impairment of sperm function, spermatogenesis and steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, J.; Qiu, D. Causal relationship between genetically predicted type 2 diabetes mellitus and male infertility. Front. Endocrinol. 2024, 15, 1357279. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Dey, S. Emerging concepts on Leydig cell development in fetal and adult testis. Front. Endocrinol. 2023, 13, 1086276. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.R.D.L.; Mucida, Y.M.; Xavier, J.D.C.; Fernandes, L.N.; Silva, R.D.O.; Bandeira, F. Bone mineral density, trabecular bone score and muscle strength in transgender men receiving testosterone therapy versus cisgender men. Steroids 2022, 178, 108951. [Google Scholar] [CrossRef] [PubMed]
- Latifi, Z.; Nikanfar, S.; Khodavirdilou, R.; Beirami, S.M.; Khodavirdilou, L.; Fattahi, A.; Oghbaei, F. MicroRNAs as diagnostic biomarkers in diabetes male infertility: A systematic review. Mol. Biol. Rep. 2025, 52, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, X.; Gao, L.; Zhang, W.; Zhong, F.; Liu, S.; Huang, Y.; Wang, Y.; Wei, W.; Xu, D. Mitochondrial malfunction-initiated Leydig cell premature senescence partially participates in 1-nitropyrene-evoked downregulation of steroidogenic synthases in testes. Free Rad. Biol. Med. 2024, 225, 456–468. [Google Scholar] [CrossRef]
- Li, S.; Susztak, K. Mitochondrial dysfunction has a central role in diabetic kidney disease. Nat. Rev. Nephrol. 2025, 21, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Swaminathan, K. Hyperglycemia-induced mitochondrial alterations in liver. Life Sci. 2010, 87, 197–214. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Ellidag, H.Y.; Aslankoç, R.; Kök, M.; Aykal, G.; Aydın, Ö.; Özmen, Ö.; Çakır, R.C.; Doğan, U. Serum testosterone levels and oxidative stress in type 1 diabetes, type 2 diabetes, and obesity. Endokrynol. Pol. 2024, 75, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, F.; Zhang, Z.; Zhang, Y.; Tang, Y.; Bi, J.; Shi, C.; Wang, D.; Yang, H.; Wang, Z.; et al. Diabetes-induced autophagy dysregulation engenders testicular impairment via oxidative stress. Oxidative Med. Cell. Longev. 2023, 2023, 4365895. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Zhu, Y.; Wang, S.; Ping, P.; Chen, X. Lipophagy contributes to testosterone biosynthesis in male rat Leydig cells. Endocrinology 2018, 159, 1119–1129. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Zheng, Y. Prepubertal Diabetes Stagnates Testicular Development by Skewing Autophagy Homeostasis in Leydig Cells. Cells 2025, 14, 1376. https://doi.org/10.3390/cells14171376
Tang Z, Zheng Y. Prepubertal Diabetes Stagnates Testicular Development by Skewing Autophagy Homeostasis in Leydig Cells. Cells. 2025; 14(17):1376. https://doi.org/10.3390/cells14171376
Chicago/Turabian StyleTang, Zonghao, and Youkun Zheng. 2025. "Prepubertal Diabetes Stagnates Testicular Development by Skewing Autophagy Homeostasis in Leydig Cells" Cells 14, no. 17: 1376. https://doi.org/10.3390/cells14171376
APA StyleTang, Z., & Zheng, Y. (2025). Prepubertal Diabetes Stagnates Testicular Development by Skewing Autophagy Homeostasis in Leydig Cells. Cells, 14(17), 1376. https://doi.org/10.3390/cells14171376





