Recent Advancements in the Relationship Between the Autonomic Nervous System and the Pancreas, Encompassing the Regulation of Regeneration and Apoptosis
Abstract
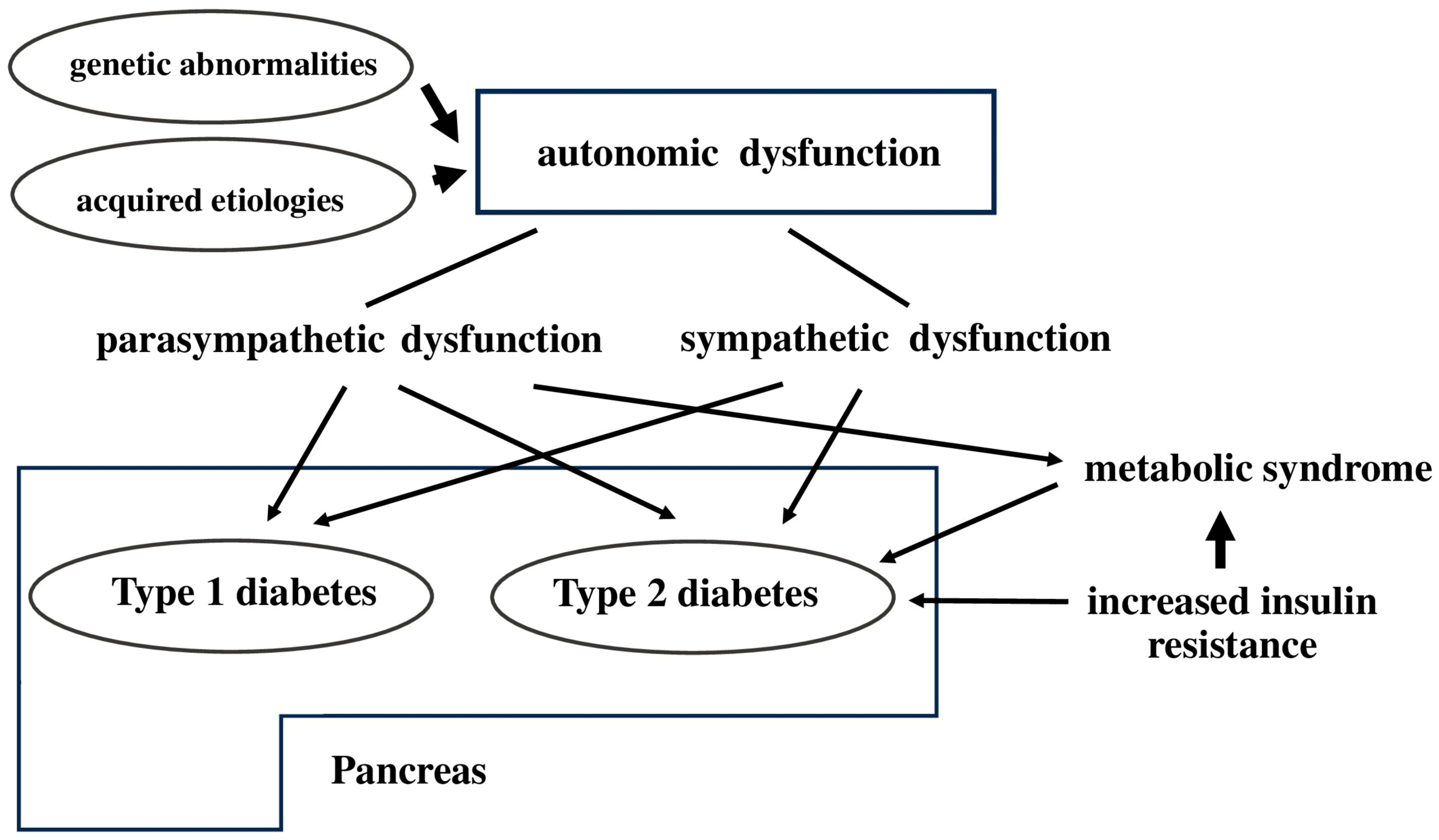
1. Introduction
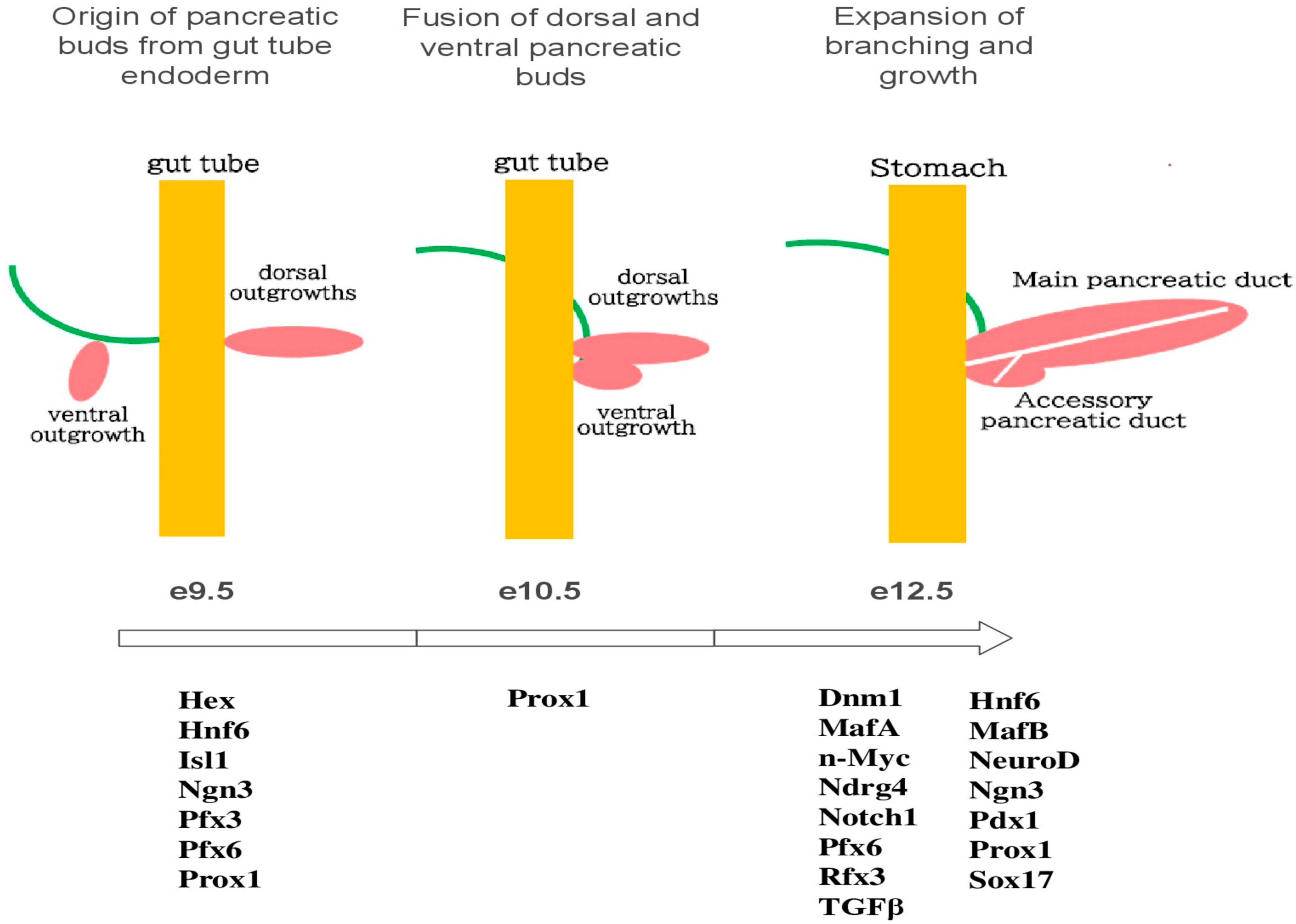
2. Gene Expression of the Pancreas
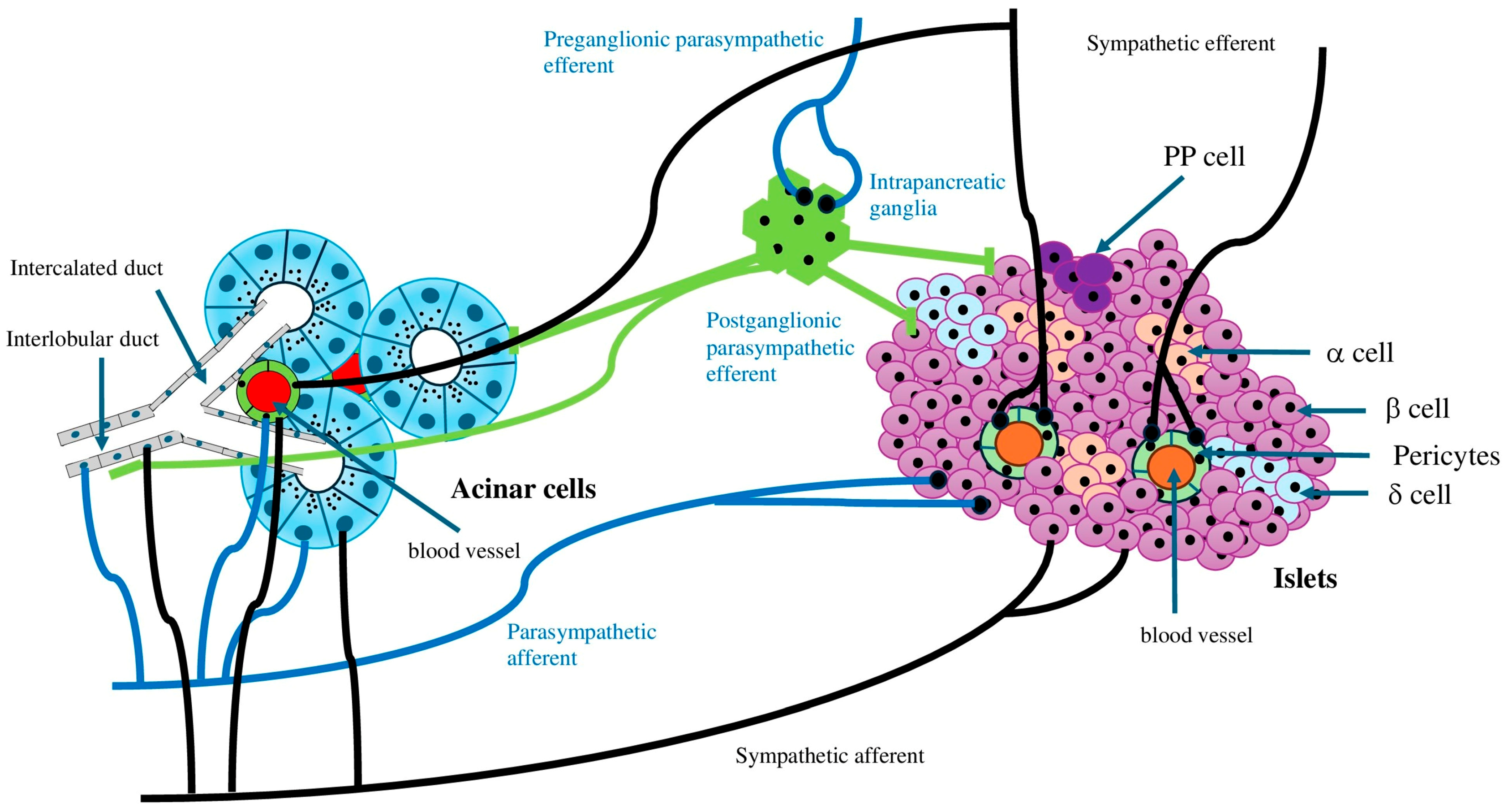
3. Autonomic Nerve Innervation and Pancreatic Function and Regeneration
3.1. Sympathetic Nerve
3.2. Parasympathetic Nerve
3.3. Other Factors
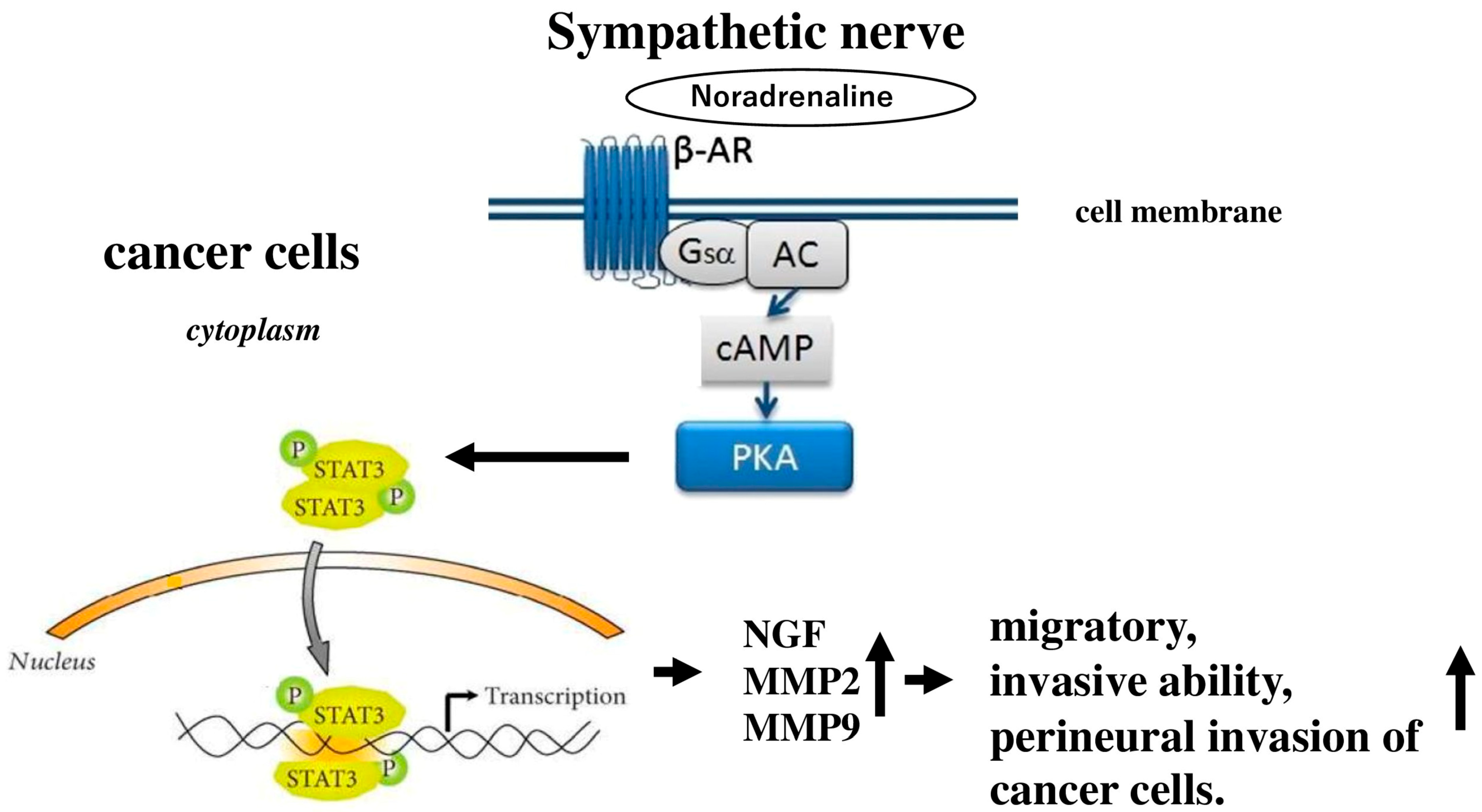
4. Autonomic Nervous System and Carcinogenesis of the Pancreas
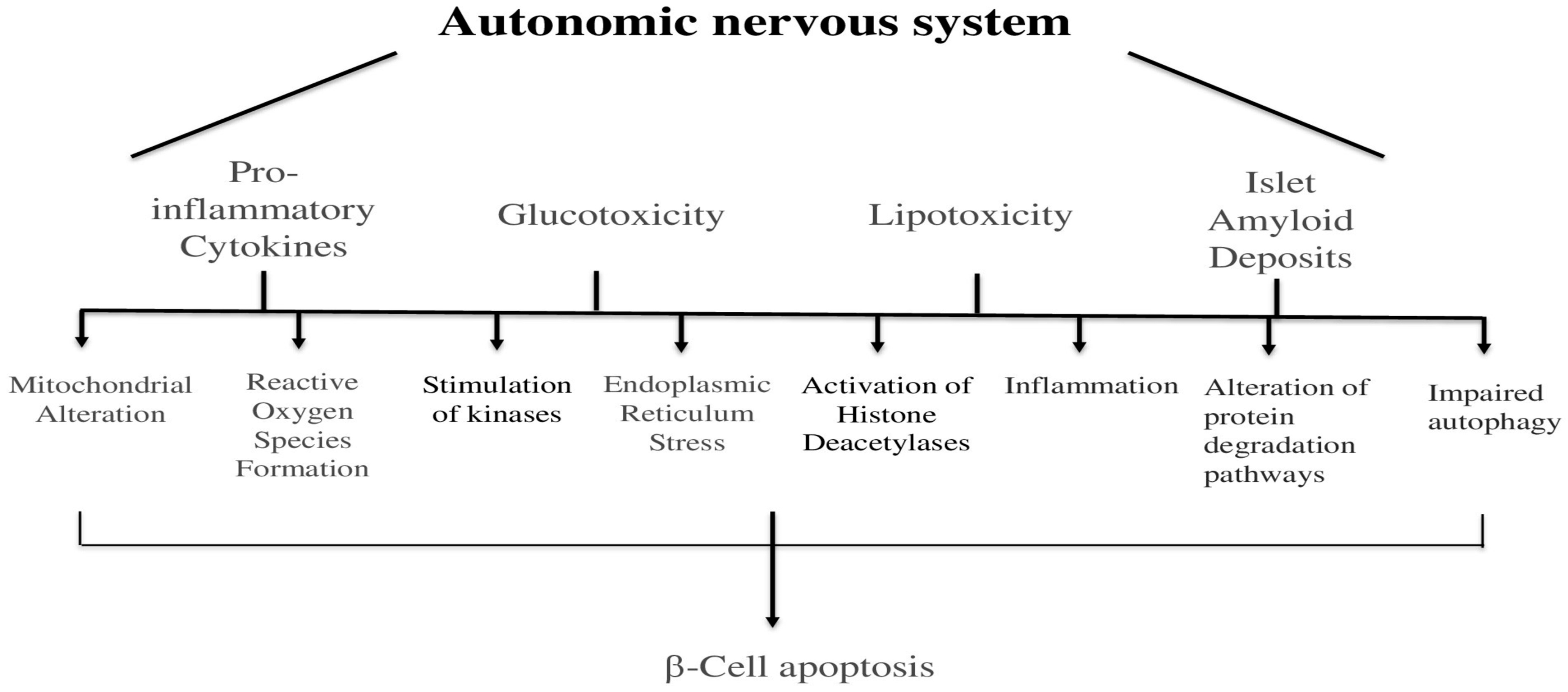
5. Autonomic Nervous System and Apoptosis of the Pancreas
6. Autonomic Nervous System and Non-Apoptotic Cell Deaths of the Pancreas
7. Autonomic Nervous System and Metabolism of the Pancreas
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kiba, T. Relationships between the autonomic nervous system and the pancreas including regulation of regeneration and apoptosis: Recent developments. Pancreas 2004, 29, e51–e58. [Google Scholar] [CrossRef] [PubMed]
- Estefanía, M.M.; Ganier, O.; Hernández, P.; Schvartzman, J.B.; Mechali, M.; Krimer, D.B. DNA replication fading as proliferating cells advance in their commitment to terminal differentiation. Sci. Rep. 2012, 2, 279. [Google Scholar] [CrossRef]
- Villasenor, A.; Chong, D.C.; Cleaver, O. Biphasic Ngn3 expression in the developing pancreas. Dev. Dyn. 2008, 237, 3270–3279. [Google Scholar] [CrossRef]
- Jørgensen, M.C.; Ahnfelt-Rønne, J.; Hald, J.; Madsen, O.D.; Serup, P.; Hecksher-Sørensen, J. An illustrated review of early pancreas development in the mouse. Endocr. Rev. 2007, 28, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Mattsson, G.; Andersson, A.; Jansson, L.; Carlsson, P.O. Islet endothelial cells and pancreatic beta-cell proliferation: Studies in vitro and during pregnancy in adult rats. Endocrinology 2006, 147, 2315–2324. [Google Scholar] [CrossRef]
- Zhang, H.; Ables, E.T.; Pope, C.F.; Washington, M.K.; Hipkens, S.; Means, A.L.; Path, G.; Seufert, J.; Costa, R.H.; Leiter, A.B.; et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech. Dev. 2009, 126, 958–973. [Google Scholar] [CrossRef]
- Bonal, C.; Herrera, P.L. Genes controlling pancreas ontogeny. Int. J. Dev. Biol. 2008, 52, 823–835. [Google Scholar] [CrossRef]
- Pavlinkova, G.; Smolik, O. NEUROD1: Transcriptional and epigenetic regulator of human and mouse neuronal and endocrine cell lineage programs. Front. Cell Dev. Biol. 2024, 12, 1435546. [Google Scholar] [CrossRef]
- Noguchi, H.; Xu, G.; Matsumoto, S.; Kaneto, H.; Kobayashi, N.; Bonner-Weir, S.; Hayashi, S. Induction of pancreatic stem/progenitor cells into insulin-producing cells by adenoviral-mediated gene transfer technology. Cell Transplant. 2006, 15, 929–938. [Google Scholar] [CrossRef]
- Fernandez-Zapico, M.E.; van Velkinburgh, J.C.; Gutiérrez-Aguilar, R.; Neve, B.; Froguel, P.; Urrutia, R.; Stein, R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J. Biol. Chem. 2009, 284, 36482–36490. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Matsuoka, T.A.; Katakami, N.; Matsuhisa, M. Combination of MafA, PDX-1 and NeuroD is a useful tool to efficiently induce insulin-producing surrogate beta-cells. Curr. Med. Chem. 2009, 16, 3144–3151. [Google Scholar] [CrossRef]
- Artner, I.; Hang, Y.; Mazur, M.; Yamamoto, T.; Guo, M.; Lindner, J.; Magnuson, M.A.; Stein, R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 2010, 59, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kilic, G.; Aydin, M.; Burke, Z.; Oliver, G.; Sosa-Pineda, B. Prox1 activity controls pancreas morphogenesis and participates in the production of “secondary transition” pancreatic endocrine cells. Dev. Biol. 2005, 286, 182–194. [Google Scholar] [CrossRef]
- Duque, M.; Amorim, J.P.; Bessa, J. Ptf1a function and transcriptional cis-regulation, a cornerstone in vertebrate pancreas development. FEBS J. 2022, 289, 5121–5136. [Google Scholar] [CrossRef]
- Aftab, S.; Semenec, L.; Chu, J.S.; Chen, N. Identification and characterization of novel human tissue-specific RFX transcription factors. BMC Evol. Biol. 2008, 8, 226. [Google Scholar] [CrossRef]
- Ait-Lounis, A.; Bonal, C.; Seguín-Estévez, Q.; Schmid, C.D.; Bucher, P.; Herrera, P.L.; Durand, B.; Meda, P.; Reith, W. The transcription factor Rfx3 regulates beta-cell differentiation, function, and glucokinase expression. Diabetes 2010, 59, 1674–1685. [Google Scholar] [CrossRef]
- Smith, S.B.; Qu, H.Q.; Taleb, N.; Kishimoto, N.Y.; Scheel, D.W.; Lu, Y.; Patch, A.-M.; Grabs, R.; Wang, J.; Lynn, F.C.; et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature 2010, 463, 775–780. [Google Scholar] [CrossRef]
- Wang, J.F.; Hill, D.J. Identification and action of N-myc downstream regulated gene 4 A2 in rat pancreas. J. Endocrinol. 2009, 201, 15–25. [Google Scholar] [CrossRef][Green Version]
- Spence, J.R.; Lange, A.W.; Lin, S.C.; Kaestner, K.H.; Lowy, A.M.; Kim, I.; Whitsett, J.A.; Wells, J.M. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell 2009, 17, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Bosch, J.A.; Goll, M.G.; Hesselson, D.; Dong, P.D.; Shin, D.; Chi, N.C.; Shin, C.H.; Schlegel, A.; Halpern, M.; et al. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev. Biol. 2009, 334, 213–223. [Google Scholar] [CrossRef] [PubMed]
- McCorry, L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007, 71, 78. [Google Scholar] [CrossRef]
- Lkhagvasuren, B.; Mee-Inta, O.; Zhao, Z.W.; Hiramoto, T.; Boldbaatar, D.; Kuo, Y.M. Pancreas-Brain Crosstalk. Front. Neuroanat. 2021, 15, 691777. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, R.D.; Bhatia, M. Substance P treatment stimulates chemokine synthesis in pancreatic acinar cells via the activation of NF-κB. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G1113–G1119. [Google Scholar] [CrossRef]
- Barreto, S.G.; Carati, C.J.; Toouli, J.; Saccone, G.T. The islet-acinar axis of the pancreas: More than just insulin. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G10–G22. [Google Scholar] [CrossRef]
- Brunicardi, F.C.; Shavelle, D.M.; Andersen, D.K. Neural regulation of the endocrine pancreas. Int. J. Pancreatol. 1995, 18, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.; Winer, S.; Chan, Y.; Truong, D.; Tang, L.; Yantha, J.; Paltser, G.; Dosch, H. Islet glia, neurons, and beta cells. Ann. N. Y. Acad. Sci. 2008, 1150, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Ahrén, B. Autonomic regulation of islet hormone secretion—Implications for health and disease. Diabetologia 2000, 43, 393–410. [Google Scholar] [CrossRef]
- Ushiki, T.; Watanabe, S. Distribution and ultrastructure of the autonomic nerves in the mouse pancreas. Microsc. Res. Tech. 1997, 37, 399–406. [Google Scholar] [CrossRef]
- Krivova, Y.S.; Proshchina, A.E.; Otlyga, D.A.; Leonova, O.G.; Saveliev, S.V. Prenatal development of sympathetic innervation of the human pancreas. Ann. Anat. 2022, 240, 151880. [Google Scholar] [CrossRef]
- Amella, C.; Cappello, F.; Kahl, P.; Fritsch, H.; Lozanoff, S.; Sergi, C. Spatial and temporal dynamics of innervation during the development of fetal human pancreas. Neuroscience 2008, 154, 1477–1487. [Google Scholar] [CrossRef]
- Love, J.A.; Yi, E.; Smith, T.G. Autonomic pathways regulating pancreatic exocrine secretion. Auton. Neurosci. 2007, 133, 19–34. [Google Scholar] [CrossRef]
- DiMagno, E.P. Regulation of interdigestive gastrointestinal motility and secretion. Digestion 1997, 58 (Suppl. 1), 53–55. [Google Scholar] [CrossRef]
- Chandra, R.; Liddle, R. Regulation of Pancreatic Secretion. Pancreapedia Exocrine Pancreas Knowl. Base 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Dando, R.; Jacques-Silva, M.C.; Fachado, A.; Molina, J.; Abdulreda, M.H.; Ricordi, C.; Roper, S.D.; Berggren, P.O.; Caicedo, A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011, 17, 888–892. [Google Scholar] [CrossRef]
- Fasanella, K.E.; Christianson, J.A.; Chanthaphavong, R.S.; Davis, B.M. Distribution and neurochemical identification of pancreatic afferents in the mouse. J. Comp. Neurol. 2008, 509, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, B.; Bovara, M.; Barbara, G.; De Ponti, F.; Stanghellini, V.; Tonini, M.; Guerrini, S.; Cremon, C.; Degli Esposti, M.; Koumandou, M.; et al. Neurology and neuropathology of the pancreatic innervation. JOP 2002, 3, 26–33. [Google Scholar] [PubMed]
- MacDonald, A.J.; Yang, Y.H.C.; Cruz, A.M.; Beall, C.; Ellacott, K.L.J. Brain-Body Control of Glucose Homeostasis—Insights from Model Organisms. Front. Endocrinol. 2021, 12, 662769. [Google Scholar] [CrossRef] [PubMed]
- Giannulis, I.; Mondini, E.; Cinti, F.; Frontini, A.; Murano, I.; Barazzoni, R.; Barbatelli, G.; Accili, D.; Cinti, S. Increased density of inhibitory noradrenergic parenchymal nerve fibers in hypertrophic islets of Langerhans of obese mice. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 384–392. [Google Scholar] [CrossRef]
- Lacey, R.J.; Chan, S.L.; Cable, H.C.; James, R.F.; Perrett, C.W.; Scarpello, J.H.; Morgan, N.G. Expression of alpha 2 and beta-adrenoceptor subtypes in human islets of Langerhans. J. Endocrinol. 1996, 148, 531–543. Available online: https://pubmed.ncbi.nlm.nih.gov/8778232 (accessed on 28 August 2025). [CrossRef]
- Thorens, B. Central control of glucose homeostasis: The brain–endocrine pancreas axis. Diabetes Metab. 2010, 36 (Suppl. S3), S45–S49. [Google Scholar] [CrossRef]
- Taborsky, G.J.; Mei, Q.; Hackney, D.J.; Figlewicz, D.P.; LeBoeuf, R.; Mundinger, T.O. Loss of islet sympathetic nerves and impairment of glucagon secretion in the NOD mouse: Relationship to invasive insulitis. Diabetologia 2009, 52, 2602–2611. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.; Butterworth, E.A.; Boatwright, J.L.; Nair, M.A.; Nasif, L.H.; Nasif, K.; Revell, A.Y.; Riva, A.; Mathews, C.E.; Gerling, I.C.; et al. Islet sympathetic innervation and islet neuropathology in patients with type 1 diabetes. Sci. Rep. 2021, 11, 6562. [Google Scholar] [CrossRef]
- Thorens, B. Neural regulation of pancreatic islet cell mass and function. Diabetes Obes. Metab. 2014, 16 (Suppl. S1), 87–95. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Bohland, M.; Sanchez-Watts, G.; Watts, A.G.; Donovan, C.M. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E96–E101. [Google Scholar] [CrossRef] [PubMed]
- Dunning, B.E.; Taborsky, G.J. Neural control of islet function by norepinephrine and sympathetic neuropeptides. Adv. Exp. Med. Biol. 1991, 291, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Benthem, L.; Mundinger, T.O.; Taborsky, G.J. Parasympathetic inhibition of sympathetic neural activity to the pancreas. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E378–E381. [Google Scholar] [CrossRef]
- Cryer, P.E.; Binder, C.; Bolli, G.B.; Cherrington, A.D.; Gale, E.A.; Gerich, J.E.; Sherwin, R.S. Hypoglycemia in IDDM. Diabetes 1989, 38, 1193–1199. [Google Scholar] [CrossRef]
- Mei, Q.; Mundinger, T.O.; Lernmark, A.; Taborsky, G.J. Early, selective, and marked loss of sympathetic nerves from the islets of BioBreeder diabetic rats. Diabetes 2002, 51, 2997–3002. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Hua, T.E.; Fu, Y.Y.; Pasricha, P.J.; Tang, S.C. 3-D imaging and illustration of the perfusive mouse islet sympathetic innervation and its remodelling in injury. Diabetologia 2012, 55, 3252–3261. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Speier, S.; Molano, R.D.; Formoso, A.; Gans, I.; Abdulreda, M.H.; Cabrera, O.; Molina, J.; Fachado, A.; Ricordi, C.; et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc. Natl. Acad. Sci. USA 2012, 109, 21456–21461. [Google Scholar] [CrossRef]
- Shimada, K.; Tachibana, T.; Fujimoto, K.; Sasaki, T.; Okabe, M. Temporal and Spatial Cellular Distribution of Neural Crest Derivatives and Alpha Cells during Islet Development. Acta Histochem. Cytochem. 2012, 45, 65–75. [Google Scholar] [CrossRef]
- Burris, R.E.; Hebrok, M. Pancreatic innervation in mouse development and beta-cell regeneration. Neuroscience 2007, 150, 592–602. [Google Scholar] [CrossRef]
- Cabrera-Vásquez, S.; Navarro-Tableros, V.; Sánchez-Soto, C.; Gutiérrez-Ospina, G.; Hiriart, M. Remodelling sympathetic innervation in rat pancreatic islets ontogeny. BMC Dev. Biol. 2009, 9, 34. [Google Scholar] [CrossRef]
- Proshchina, A.E.; Krivova, Y.S.; Barabanov, V.M.; Saveliev, S.V. Ontogeny of neuro-insular complexes and islets innervation in the human pancreas. Front. Endocrinol. 2014, 5, 57. [Google Scholar] [CrossRef]
- Cabrera-Vásquez, M.; Mendoza-Rodríguez, C.A.; Cerbón, M.; Camacho-Arroyo, I.; Domínguez, R. Nerve growth factor and its receptors in the human fetal pancreas. Pancreas 2009, 38, 465–470. [Google Scholar] [CrossRef]
- Muñoz-Bravo, J.L.; Hidalgo-Figueroa, M.; Pascual, A.; López-Barneo, J.; Leal-Cerro, A.; Cano, D.A. GDNF is required for neural colonization of the pancreas. Development 2013, 140, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Young, H.M.; Newgreen, D. Enteric neural crest-derived cells: Origin, identification, migration, and differentiation. Anat. Rec. 2001, 262, 1–15. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. Principles of Neural Science, 4th ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Borden, P.; Houtz, J.; Leach, S.D.; Kuruvilla, R. Sympathetic innervation during development is necessary for pancreatic islet architecture and functional maturation. Cell Rep. 2013, 4, 287–301. [Google Scholar] [CrossRef]
- Lausier, J.; Diaz, W.C.; Roskens, V.; LaRock, K.; Herzer, K.; Fong, C.G.; Latour, M.G.; Peshavaria, M.; Jetton, T.L. Vagal control of pancreatic β-cell proliferation. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E786–E793. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Chun, S.J.; Niijima, A.; Romijn, H.J.; Nagai, K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 2001, 431, 405–423. [Google Scholar] [CrossRef]
- Glebova, N.O.; Ginty, D.D. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J. Neurosci. 2004, 24, 743–751. [Google Scholar] [CrossRef]
- Edwards, R.H.; Rutter, W.J.; Hanahan, D. Directed expression of NGF to pancreatic beta cells in transgenic mice leads to selective hyperinnervation of the islets. Cell 1989, 58, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Marliss, E.B.; Girardier, L.; Seydoux, J.; Solheim, C.B.; Kanazawa, Y.; Orcin, L.; Renold, A.E.; Porte, D. Glucagon release induced by pancreatic nerve stimulation in the dog. J. Clin. Investig. 1973, 52, 1246–1259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Havel, P.J.; Veith, R.C.; Dunning, B.E.; Taborsky, G.J. Pancreatic noradrenergic nerves are activated by neuroglucopenia but not by hypotension or hypoxia in the dog. J. Clin. Investig. 1988, 82, 1538–1545. [Google Scholar] [CrossRef]
- Nekrep, N.; Wang, J.; Miyatsuka, T.; German, M.S. Signals from the neural crest regulate beta-cell mass in the pancreas. Development 2008, 135, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Shen, C.N.; Lin, P.Y.; Peng, S.J.; Chien, H.J.; Chou, Y.H.; Chamberlain, C.E.; Pasricha, P.J. Pancreatic neuro-insular network in young mice revealed by 3D panoramic histology. Diabetologia 2018, 61, 158–167. [Google Scholar] [CrossRef]
- Oomori, Y.; Iuchi, H.; Ishikawa, K.; Satoh, Y.; Ono, K. Immunocytochemical study of tyrosine hydroxylase and dopamine β-hydroxylase immunoreactivities in the rat pancreas. Arch. Histol. Cytol. 1994, 57, 297–306. [Google Scholar] [CrossRef]
- Yi, E.; Smith, T.G.; Love, J.A. Noradrenergic innervation of rabbit pancreatic ganglia. Auton. Neurosci. 2005, 117, 87–96. [Google Scholar] [CrossRef]
- Das, V.A.; Robinson, R.; Paulose, C.S. Enhanced beta-adrenergic receptors in the brain and pancreas during pancreatic regeneration in weanling rats. Mol. Cell Biochem. 2006, 289, 11–19. [Google Scholar] [CrossRef]
- Renuka, T.R.; Savitha, B.; Paulose, C.S. Muscarinic M1 and M3 receptor binding alterations in pancreas during pancreatic regeneration of young rats. Endocr. Res. 2005, 31, 259–270. [Google Scholar] [CrossRef][Green Version]
- Ahren, B.; Wierup, N.; Sundler, F. Neuropeptides and the Regulation of Islet Function. Diabetes 2006, 55 (Suppl. S2), S98–S107. [Google Scholar] [CrossRef][Green Version]
- Sorenson, R.L.; Garry, D.G.; Brelje, T.C. Structural and Functional Considerations of GABA in Islets of Langerhans: β-Cells and Nerves. Diabetes 1991, 40, 1365–1374. [Google Scholar] [CrossRef]
- Saravia-Fernandez, F.; Faveeuw, C.; Blasquez-Bulant, C.; Tappaz, M.; Throsby, M.; Pelletier, G.; Vaudry, H.; Dardenne, M.; Homo-Delarche, F. Localization of gamma-aminobutyric acid and glutamic acid decarboxylase in the pancreas of the nonobese diabetic mouse. Endocrinology 1996, 137, 3497–3506. [Google Scholar] [CrossRef]
- Jänig, W. Functional Anatomy of the Peripheral Sympathetic and Parasympathetic Systems. In The Integrative Action of the Autonomic Nervous System: Neurobiology of Homeostasis; Cambridge University Press: Cambridge, UK, 2022; pp. 9–33. [Google Scholar] [CrossRef]
- Gilon, P.; Henquin, J.C. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 2001, 22, 565–604. [Google Scholar] [CrossRef]
- Okumura, T.; Pappas, T.N.; Taylor, I.L. Pancreatic polypeptide microinjection into the dorsal motor nucleus inhibits pancreatic secretion in rats. Gastroenterology 1995, 108, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.C.; Almarghalani, D.A.; Gibson, H.M.; Meqdad, M.A.; Antypas, R.B.; Lingireddy, A.; AbouAlaiwi, W.A. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol. Genom. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Rodriguez-Diaz, R.; Fachado, A.; Jacques-Silva, M.C.; Berggren, P.O.; Caicedo, A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014, 63, 2714–2726. [Google Scholar] [CrossRef]
- Delgado, E.; Perez-Basterrechea, M.; Suarez-Alvarez, B.; Zhou, H.; Revuelta, E.M.; Garcia-Gala, J.M.; Perez, S.; Alvarez-Viejo, M.; Menendez, E.; Lopez-Larrea, C.; et al. Modulation of Autoimmune T-Cell Memory by Stem Cell Educator Therapy: Phase 1/2 Clinical Trial. EBioMedicine 2015, 2, 2024–2036. [Google Scholar] [CrossRef]
- Gautam, D.; Han, S.J.; Hamdan, F.F.; Jeon, J.; Li, B.; Li, J.H.; Cui, Y.; Mears, D.; Lu, H.; Deng, C.; et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006, 3, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Miyakawa, T.; Duttaroy, A.; Yamanaka, A.; Moriguchi, T.; Makita, R.; Ogawa, M.; Chou, C.J.; Xia, B.; Crawley, J.N.; et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 2001, 410, 207–212. [Google Scholar] [CrossRef]
- Arbuzova, A.; Murray, D.; McLaughlin, S. MARCKS, membranes, and calmodulin: Kinetics of their interaction. Biochim. Biophys. Acta 1998, 1376, 369–379. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Abdulreda, M.H.; Formoso, A.L.; Gans, I.; Ricordi, C.; Berggren, P.O.; Caicedo, A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011, 14, 45–54. [Google Scholar] [CrossRef]
- N’Guyen, J.M.; Magnan, C.; Laury, M.C.; Thibault, C.; Leveteau, J.; Gilbert, M.; Pénicaud, L.; Ktorza, A. Involvement of the autonomic nervous system in the in vivo memory to glucose of pancreatic beta cell in rats. J. Clin. Investig. 1994, 94, 1456–1462. [Google Scholar] [CrossRef][Green Version]
- Berthoud, H.R.; Powley, T.L. Characterization of vagal innervation of the rat pancreas using lectin horseradish peroxidase conjugates. J. Auton. Nerv. Syst. 1982, 6, 577–588. [Google Scholar] [CrossRef]
- Taborsky, G.J., Jr.; Mundinger, T.O. Minireview: The Role of the Autonomic Nervous System in Mediating the Glucagon Response to Hypoglycemia. Endocrinology 2012, 153, 1055–1062. [Google Scholar] [CrossRef]
- Edvell, A.; Lindström, P. Vagotomy in young obese hyperglycemic mice: Effects on syndrome development and islet proliferation. Am. J. Physiol. 1998, 274, E1034–E1039. [Google Scholar] [CrossRef]
- Li, Y.; Owyang, C. Musings on the wanderer: What’s new in our understanding of vago-vagal reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G461–G469. [Google Scholar] [CrossRef]
- Jansson, L.; Hellerström, C. Glucose-induced changes in pancreatic islet blood flow mediated by central nervous system. Am. J. Physiol. 1986, 251, E644–E647. [Google Scholar] [CrossRef] [PubMed]
- Imai, J.; Katagiri, H.; Yamada, T.; Ishigaki, Y.; Suzuki, T.; Kudo, H.; Uno, K.; Hasegawa, Y.; Gao, J.; Kaneko, K.; et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science 2008, 322, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Tanaka, K.; Numata, K.; Hoshino, M.; Misugi, K.; Inoue, S. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology 1996, 110, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Tarussio, D.; Metref, S.; Seyer, P.; Mounien, L.; Vallois, D.; Magnan, C.; Foretz, M.; Thorens, B. Nervous glucose sensing regulates postnatal β cell proliferation and glucose homeostasis. J. Clin. Investig. 2014, 124, 413–424. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Liu, Z.; Lai, L.; Sun, R.; Shen, R.; Li, Y.; He, L.; Pu, W.; Lv, Z.; et al. Use of a dual genetic system to decipher exocrine cell fate conversions in the adult pancreas. Cell Discov. 2023, 9, 1. [Google Scholar] [CrossRef]
- Rehfeld, J.F.; Larsson, L.I.; Goltermann, N.R.; Schwartz, T.W.; Holst, J.J.; Jensen, S.L.; Morley, J.S. Neural regulation of pancreatic hormone secretion by the C-terminal tetrapeptide of CCK. Nature 1980, 284, 33–38. [Google Scholar] [CrossRef]
- Park, S.; Ahn, I.S.; Kim, D.S. Central infusion of leptin improves insulin resistance and suppresses beta cell function, but not beta-cell mass, primarily through the sympathetic nervous system in a type 2 diabetic rat model. Life Sci. 2010, 86, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T. Gene Expression Analysis in Rat Pancreas Observed with Whole-Transcript Exon Array after Ventromedial Hypothalamic Lesions. Ann. Neurosci. 2017, 24, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef]
- McVary, K.T.; Razzaq, A.; Lee, C.; Venegas, M.F.; Rademaker, A.; McKenna, K.E. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol. Reprod. 1994, 51, 99–107. [Google Scholar] [CrossRef]
- Szpunar, M.J.; Belcher, E.K.; Dawes, R.P.; Madden, K.S. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav. Immun. 2016, 53, 223–233. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Vigna, S.R.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Villagrana, R.D.; Albores-García, D.; Cervantes-Villagrana, A.R.; García-Acevez, S.J. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Target. Ther. 2020, 5, 99. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Roger, E.; Martel, S.; Bertrand-Chapel, A.; Depollier, A.; Chuvin, N.; Pommier, R.M.; Yacoub, K.; Caligaris, C.; Cardot-Ruffino, V.; Chauvet, V.; et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFβ signaling. Cell Death Dis. 2019, 10, 886. [Google Scholar] [CrossRef]
- Ondicova, K.; Mravec, B. Role of nervous system in cancer aetiopathogenesis. Lancet Oncol. 2010, 11, 596–601. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Q.; Wang, L.; Hu, H.; Li, J.; Zhang, D.; Zhang, M. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol. Rep. 2009, 22, 825–830. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Is norepinephrine an etiological factor in some types of cancer? Int. J. Cancer 2009, 124, 257–263. [Google Scholar] [CrossRef]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; To, K.K.W.; Zhu, S.; Wang, F.; Fu, L. Tumor-associated macrophages remodel the suppressive tumor immune microenvironment and targeted therapy for immunotherapy. J. Exp. Clin. Cancer Res. 2025, 44, 145. [Google Scholar] [CrossRef]
- Sanchez, L.R.; Borriello, L.; Entenberg, D.; Condeelis, J.S.; Oktay, M.H.; Karagiannis, G.S. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019, 106, 259–274. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef] [PubMed]
- Partecke, L.I.; Käding, A.; Trung, D.N.; Diedrich, S.; Sendler, M.; Weiss, F.; Kühn, J.-P.; Mayerle, J.; Beyer, K.; von Bernstorff, W.; et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget 2017, 8, 22501–22512. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Contino, G.; Deshpande, V.; Tzatsos, A.; Conrad, C.; Benes, C.H.; Levy, D.E.; Settleman, J.; Engelman, J.A.; Bardeesy, N. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011, 71, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cao, J.; Huang, K.J.; Zhang, F.; Jiang, T.; Zhu, L.; Qiu, Z.J. Inhibition of STAT3 activity with AG490 decreases the invasion of human pancreatic cancer cells in vitro. Cancer Sci. 2006, 97, 1417–1423. [Google Scholar] [CrossRef]
- Schuller, H.M.; Al-Wadei, H.A.; Majidi, M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer 2008, 112, 767–778. [Google Scholar] [CrossRef]
- Landen, C.N.; Lin, Y.G.; Armaiz Pena, G.N.; Das, P.D.; Arevalo, J.M.; Kamat, A.A.; Han, L.Y.; Jennings, N.B.; Spannuth, W.A.; Thaker, P.H.; et al. Neuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancer. Cancer Res. 2007, 67, 10389–10396. [Google Scholar] [CrossRef]
- Guo, K.; Ma, Q.; Li, J.; Wang, Z.; Shan, T.; Li, W.; Xu, Q.; Xie, K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 2013, 12, 264–273. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef]
- Song, B.; Scheuner, D.; Ron, D.; Pennathur, S.; Kaufman, R.J. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Investig. 2008, 118, 3378–3389. [Google Scholar] [CrossRef]
- Momi, N.; Ponnusamy, M.P.; Kaur, S.; Rachagani, S.; Kunigal, S.S.; Chellappan, S.; Ouellette, M.M.; Batra, S.K. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene 2013, 32, 1384–1395. [Google Scholar] [CrossRef]
- Lin, G.; Sun, L.; Wang, R.; Guo, Y.; Xie, C. Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 170–178. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.C.; Mansour, J.; Mollaee, M.; Wagner, K.-U.; Koduru, P.; Yopp, A.; et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Valtorta, E.; Cerea, G.; Amatu, A.; Schirru, M.; Marrapese, G.; Fiorillo, V.; Recchimuzzo, P.; Cavenago, I.S.; Bonazzina, E.F.; et al. TRKA expression and NTRK1 gene copy number across solid tumours. J. Clin. Pathol. 2018, 71, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T. Mutation status in yes-associated protein 1 (YAP1) in an insulinoma cell line Rin-5F. Cancer Genet. 2025, 294–295, 76–79. [Google Scholar] [CrossRef]
- Wrona, D. Neural-immune interactions: An integrative view of the bidirectional relationship between the brain and immune systems. J. Neuroimmunol. 2006, 172, 38–58. [Google Scholar] [CrossRef]
- Dalle, S.; Abderrahmani, A.; Renard, E. Pharmacological inhibitors of β-cell dysfunction and death as therapeutics for diabetes. Front. Endocrinol. 2023, 14, 1076343. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.J.; Kim, J.S.; Rho, J.G.; Shin, J.J.; Song, W.K.; Lee, E.K.; Egan, J.M.; Kim, W.; Jo, D.-G. Cannabinoids Regulate Bcl-2 and Cyclin D2 Expression in Pancreatic β Cells. PLoS ONE 2016, 11, e0150981. [Google Scholar] [CrossRef]
- Jourdan, T.; Godlewski, G.; Cinar, R.; Bertola, A.; Szanda, G.; Liu, J.; Tam, J.; Han, T.; Mukhopadhyay, B.; Skarulis, M.C.; et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013, 19, 1132–1140. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ford, E.L.; Tran, H.; Wice, B.M.; Crosby, S.D.; Dorn, G.W., II; Polonsky, K.S. Loss of Nix in Pdx1-deficient mice prevents apoptotic and necrotic β cell death and diabetes. J. Clin. Investig. 2010, 120, 4435–4445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, X.; Yan, H.; Xu, Z.; Yang, B.; Luo, P.; He, Q. The inducible role of autophagy in cell death: Emerging evidence and future perspectives. Cell Commun. Signal. 2025, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Muralidharan, C.; May, S.C.; Tersey, S.A.; Mirmira, R.G. Inside the β Cell: Molecular Stress Response Pathways in Diabetes Pathogenesis. Endocrinology 2022, 164, bqac184. [Google Scholar] [CrossRef] [PubMed]
- Fløyel, T.; Brorsson, C.; Nielsen, L.B.; Miani, M.; Bang-Berthelsen, C.H.; Friedrichsen, M.; Overgaard, A.J.; Berchtold, L.A.; Wiberg, A.; Poulsen, P.; et al. CTSH regulates β-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc. Natl. Acad. Sci. USA 2014, 111, 10305–10310. [Google Scholar] [CrossRef]
- Belfrage, H.; Kuuliala, K.; Kuuliala, A.; Mustonen, H.; Puolakkainen, P.; Kylänpää, L.; Louhimo, J. Circulating Markers of Necroptosis in Acute Pancreatitis. Dig. Dis. Sci. 2024, 69, 3333–3343. [Google Scholar] [CrossRef]
- Shlomovitz, I.; Erlich, Z.; Speir, M.; Zargarian, S.; Baram, N.; Engler, M.; Edry-Botzer, L.; Munitz, A.; Croker, B.A.; Gerlic, M. Necroptosis directly induces the release of full-length biologically active IL-33 in vitro and in an inflammatory disease model. FEBS J. 2019, 8, 507–522. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Yu, T.Y.; Lee, M.K. Autonomic dysfunction, diabetes and metabolic syndrome. J. Diabetes Investig. 2021, 12, 2108–2111. [Google Scholar] [CrossRef]
- Cui, C.; Ohnuma, H.; Daimon, M.; Susa, S.; Yamaguchi, H.; Kameda, W.; Jimbu, Y.; Oizumi, T.; Kato, T. Ghrelin infused into the portal vein inhibits glucose-stimulated insulin secretion in Wistar rats. Peptides 2008, 29, 1241–1246. [Google Scholar] [CrossRef]
- Yada, T.; Dezaki, K.; Iwasaki, Y. GLP-1 and ghrelin inversely regulate insulin secretion and action in pancreatic islets, vagal afferents, and hypothalamus for controlling glycemia and feeding. Am. J. Physiol. Cell Physiol. 2025, 328, C1793–C1807. [Google Scholar] [CrossRef]
- Matveyenko, A.V.; Donovan, C.M. Metabolic sensors mediate hypoglycemic detection at the portal vein. Diabetes 2006, 55, 1276–1282. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C. Final answer: Ghrelin can suppress insulin secretion in humans, but is it clinically relevant? Diabetes 2010, 59, 2726–2728. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.; Mani, H.; Dovey, T.; Halford, J.; Boyland, E.; Daousi, C.; Wilding, J.P.H.; Pinkney, J. Ghrelin inhibits autonomic function in healthy controls, but has no effect on obese and vagotomized subjects. Clin. Endocrinol. 2010, 73, 678–685. Available online: https://pubmed.ncbi.nlm.nih.gov/20738316 (accessed on 28 August 2025). [CrossRef] [PubMed]
- Yu, Q.; Guo, Q.; Jin, S.; Gao, C.; Zheng, P.; Li, D.P.; Wu, Y. Melatonin suppresses sympathetic vasomotor tone through enhancing GABAA receptor activity in the hypothalamus. Front. Physiol. 2023, 14, 1166246. [Google Scholar] [CrossRef] [PubMed]
- Peschke, E.; Bähr, I.; Mühlbauer, E. Melatonin and pancreatic islets: Interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 2013, 14, 6981–7015. [Google Scholar] [CrossRef]
- Tuomi, T.; Nagorny, C.L.F.; Singh, P.; Bennet, H.; Yu, Q.; Alenkvist, I.; Isomaa, B.; Östman, B.; Söderström, J.; Pesonen, A.K.; et al. Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 2016, 23, 1067–1077. [Google Scholar] [CrossRef]
- Kiba, T. Overexpression of Pdx-1 Gene increases Ins1 gene mRNA expression, not Ins2 Gene mRNA expression, in insulionoma cell line RIN-5F. Acta Endocrinol. 2022, 18, 164–167. [Google Scholar] [CrossRef]
- Kiba, T. Overexpression of PTEN Gene increases Ins2 gene mRNA expression, not Ins1 gene mRNA expression, In insulinoma cell line RIN-5F. Acta Endocrinol. 2023, 19, 277–280. [Google Scholar] [CrossRef]
- Sha, L.; Westerlund, J.; Szurszewski, J.H.; Bergsten, P. Amplitude modulation of pulsatile insulin secretion by intrapancreatic ganglion neurons. Diabetes 2001, 50, 51–55. [Google Scholar] [CrossRef]
- Lee, B.; Song, T.; Lee, K.; Kim, J.; Han, S.; Berggren, P.O.; Ryu, S.H.; Jo, J. Phase modulation of insulin pulses enhances glucose regulation and enables inter-islet synchronization. PLoS ONE 2017, 12, e0172901. [Google Scholar] [CrossRef]
- Fendler, B.; Zhang, M.; Satin, L.; Bertram, R. Synchronization of pancreatic islet oscillations by intrapancreatic ganglia: A modeling study. Biophys. J. 2009, 97, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Owyang, C.; Logsdon, C.D. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 2004, 127, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, Y.; Yin, X.; Xu, R.; Yin, C.; Wang, C.; Zhao, Y. Pancreatic endocrine and exocrine signaling and crosstalk in physiological and pathological status. Signal Transduct. Target. Ther. 2025, 10, 39. [Google Scholar] [CrossRef] [PubMed]

| Type of Cell Death | Features | Relevance to Pancreas |
|---|---|---|
| Endocrine cells | ||
| Pyroptosis | Caspase-1 or 4/5/11 mediated, inflammatory | Linked to islet inflammation in diabetes |
| Autophagic cell death | Excessive autophagy leading to cell demise | Observed in stress-induced β-cell dysfunction |
| Exocrine cells | ||
| Necroptosis | Regulated necrosis via RIPK1/RIPK3/MLKL | Seen in pancreatitis |
| Ferroptosis | Iron-dependent lipid peroxidation | Implicated in acinar cell damage |
| Function/Target Area | Sympathetic Nervous System | Parasympathetic Nervous System |
|---|---|---|
| Endocrine secretion (islets) | Inhibits insulin release (via noradrenaline on α2AR) | Stimulates insulin release (via vagal ACh signaling) |
| Exocrine secretion (acinar cells) | Inhibitory (via vasoconstriction and reduced perfusion) | Stimulatory (via ACh acting on muscarinic receptors) |
| Pancreatic blood flow | Vasoconstriction (reduced perfusion) | Vasoconstriction (reduced perfusion) |
| Neurotransmitters involved | Noradrenaline, NPY, GABA, Galanin | Ach, Nitric oxide, VIP, GRP, PACAP |
| Receptor types | Adrenergic receptors (α, β), TRPV1 channels | Muscarinic acetylcholine receptors (M1–M5) |
| Impact on glucose homeostasis | Promotes hyperglycemia (via insulin suppression, glucagon stimulation) | Promotes normoglycemia (via insulin stimulation) |
| Cell regeneration or proliferation | Stimulates β cells (via noradrenaline on β2AR) | Stimulates β cells and acinar cells (via vagal ACh signaling) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiba, T. Recent Advancements in the Relationship Between the Autonomic Nervous System and the Pancreas, Encompassing the Regulation of Regeneration and Apoptosis. Cells 2025, 14, 1371. https://doi.org/10.3390/cells14171371
Kiba T. Recent Advancements in the Relationship Between the Autonomic Nervous System and the Pancreas, Encompassing the Regulation of Regeneration and Apoptosis. Cells. 2025; 14(17):1371. https://doi.org/10.3390/cells14171371
Chicago/Turabian StyleKiba, Takayoshi. 2025. "Recent Advancements in the Relationship Between the Autonomic Nervous System and the Pancreas, Encompassing the Regulation of Regeneration and Apoptosis" Cells 14, no. 17: 1371. https://doi.org/10.3390/cells14171371
APA StyleKiba, T. (2025). Recent Advancements in the Relationship Between the Autonomic Nervous System and the Pancreas, Encompassing the Regulation of Regeneration and Apoptosis. Cells, 14(17), 1371. https://doi.org/10.3390/cells14171371






