Effect of Anticoagulants on Blood Cell Counts, Cell Morphology, and Leukocyte Immune Functions of Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Rainbow Trout Farming Conditions and Peripheral Blood Sampling
2.3. Evaluation of Blood Cell Count and Cell Morphology
2.4. Evaluation of Viability in Rainbow Trout Peripheral Blood Leukocytes (PBLs)
2.5. Evaluation of Phagocytosis and Reactive Oxygen Species (ROS) Production in Rainbow Trout Peripheral Blood Leukocytes
2.6. Statistical Analysis
3. Results
3.1. Anticoagulants’ Effects on Blood Cell Counts and Cell Morphology
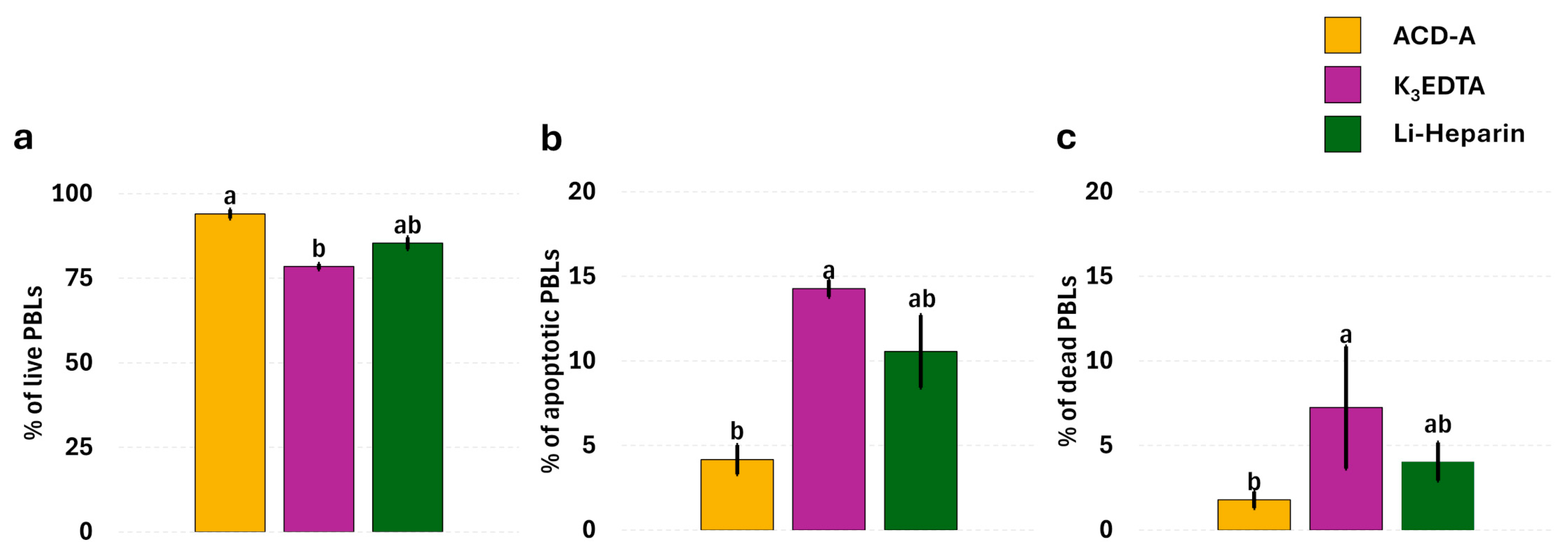
3.2. Anticoagulants’ Effects on Leukocyte Viability
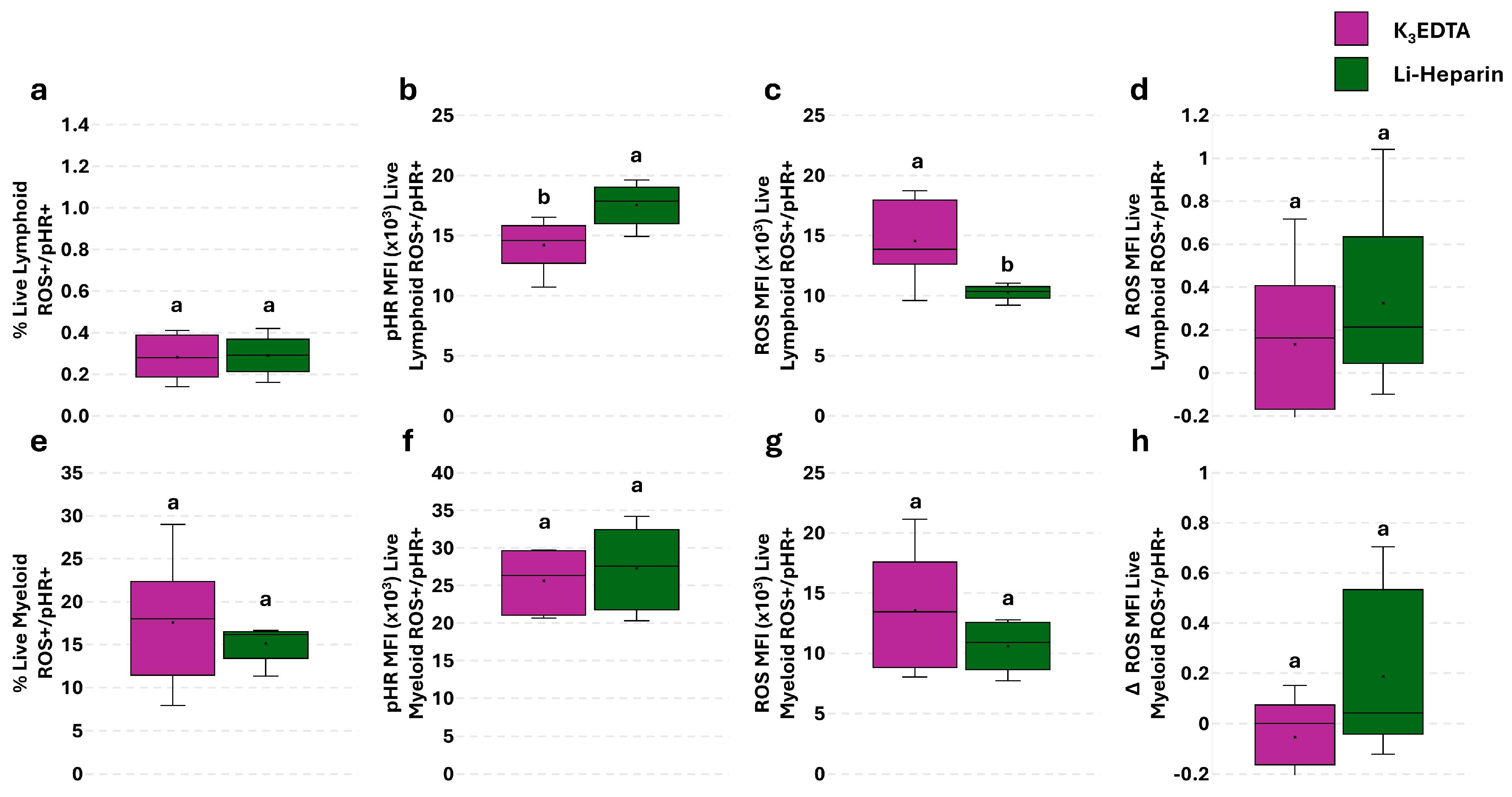
3.3. Anticoagulant Effects’ on Basal ROS Levels in PBLs: Single Assay
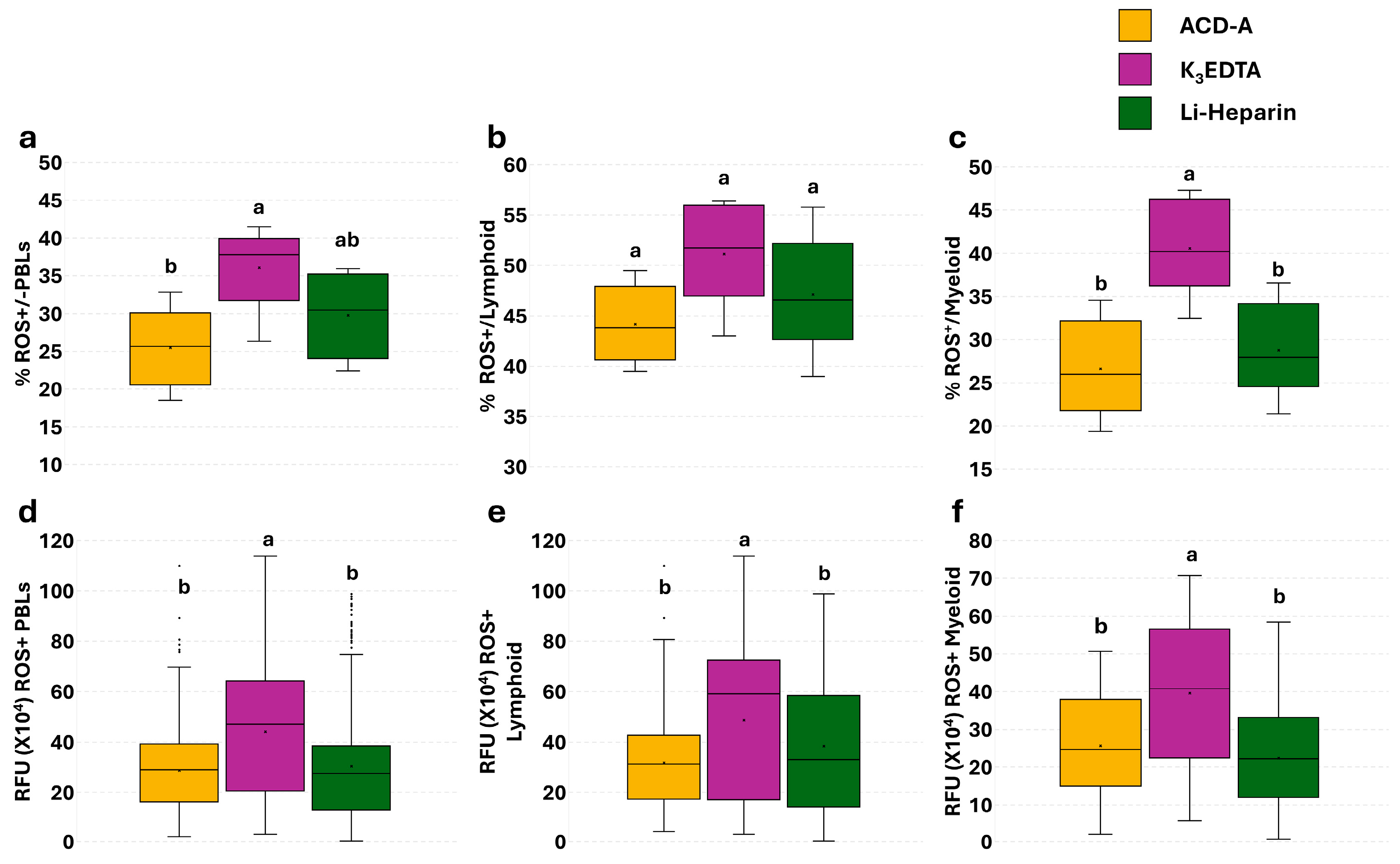
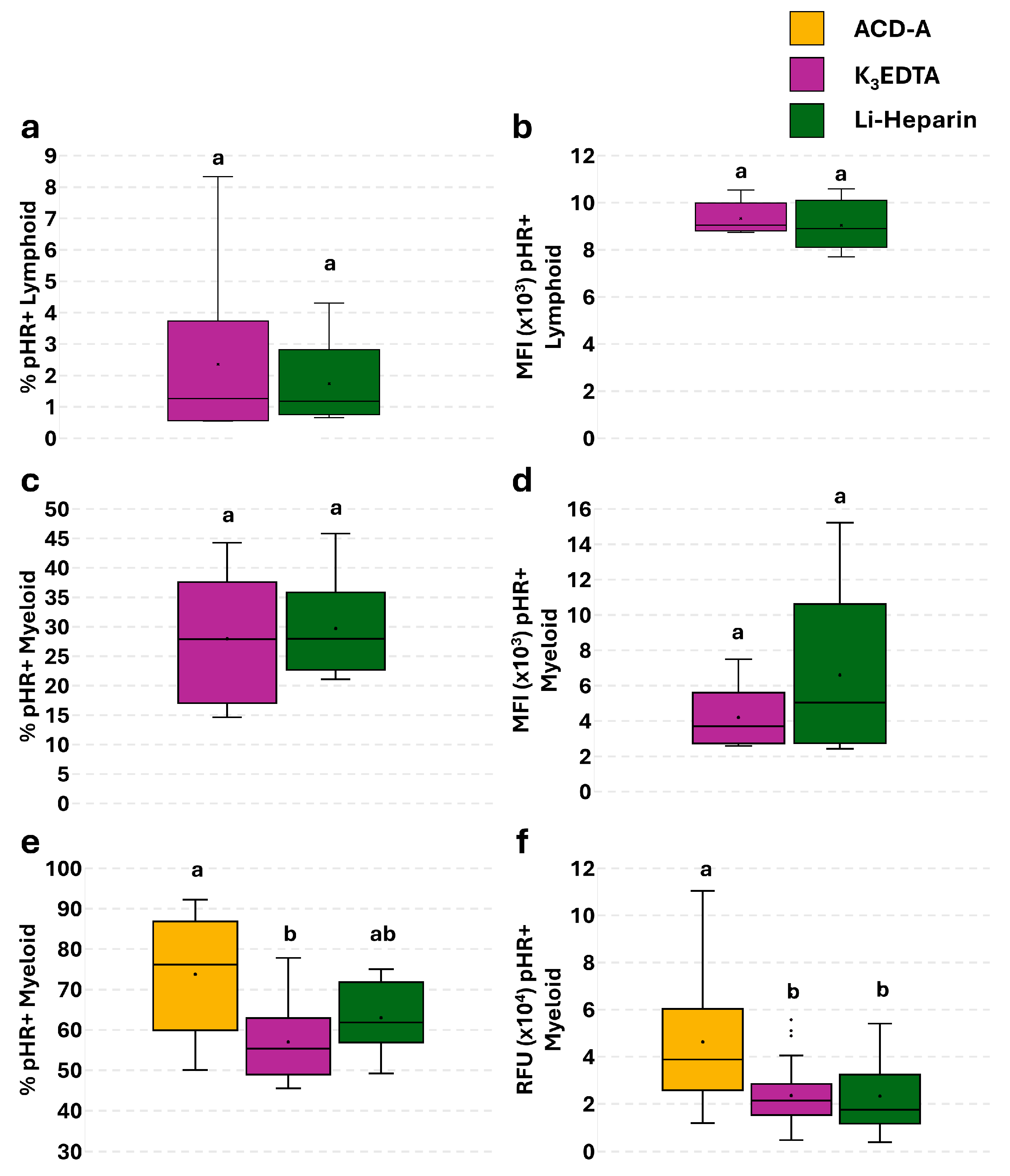
3.4. Effect of Anticoagulants on Phagocytosis in PBLs: Single Assay
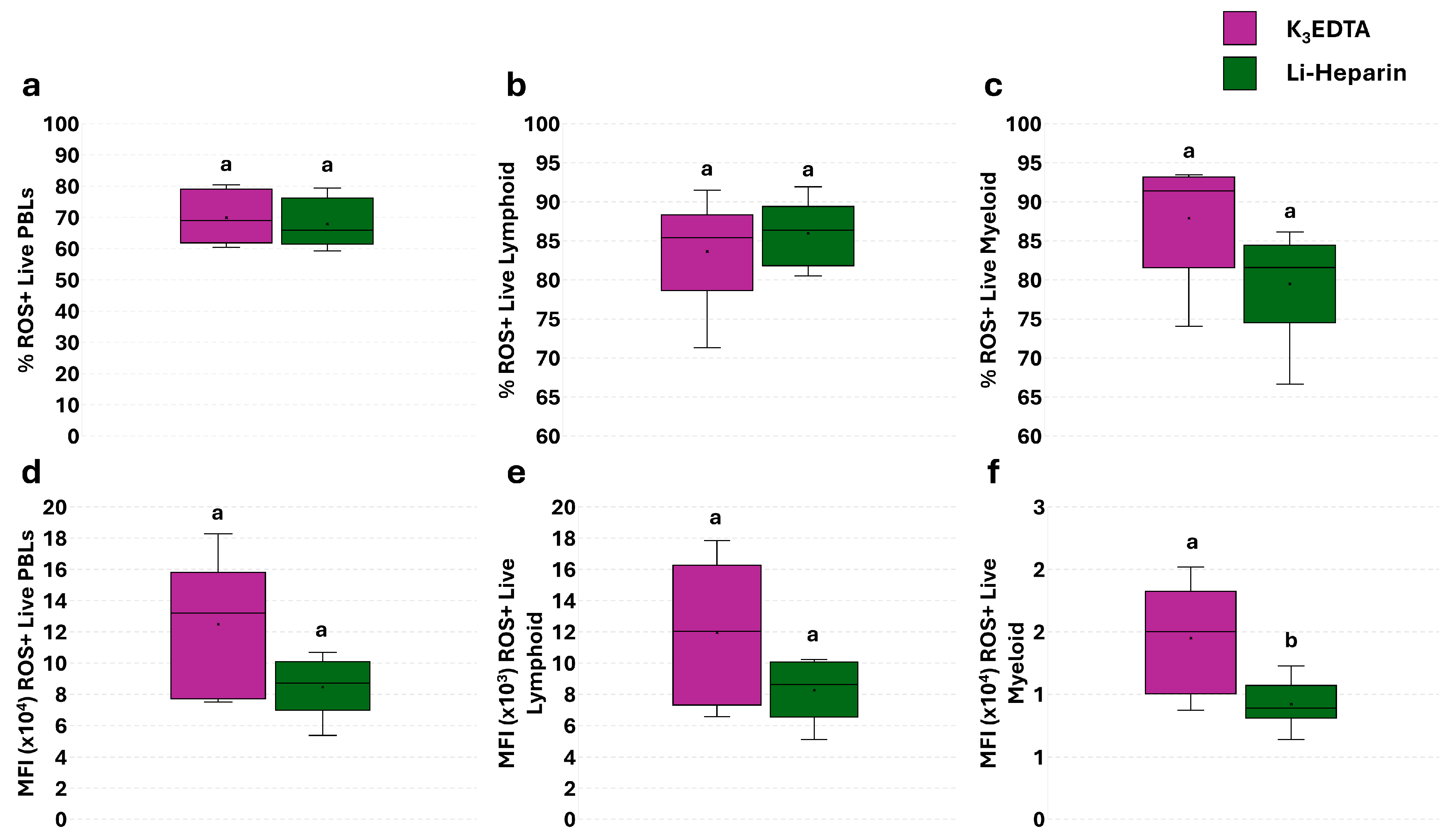
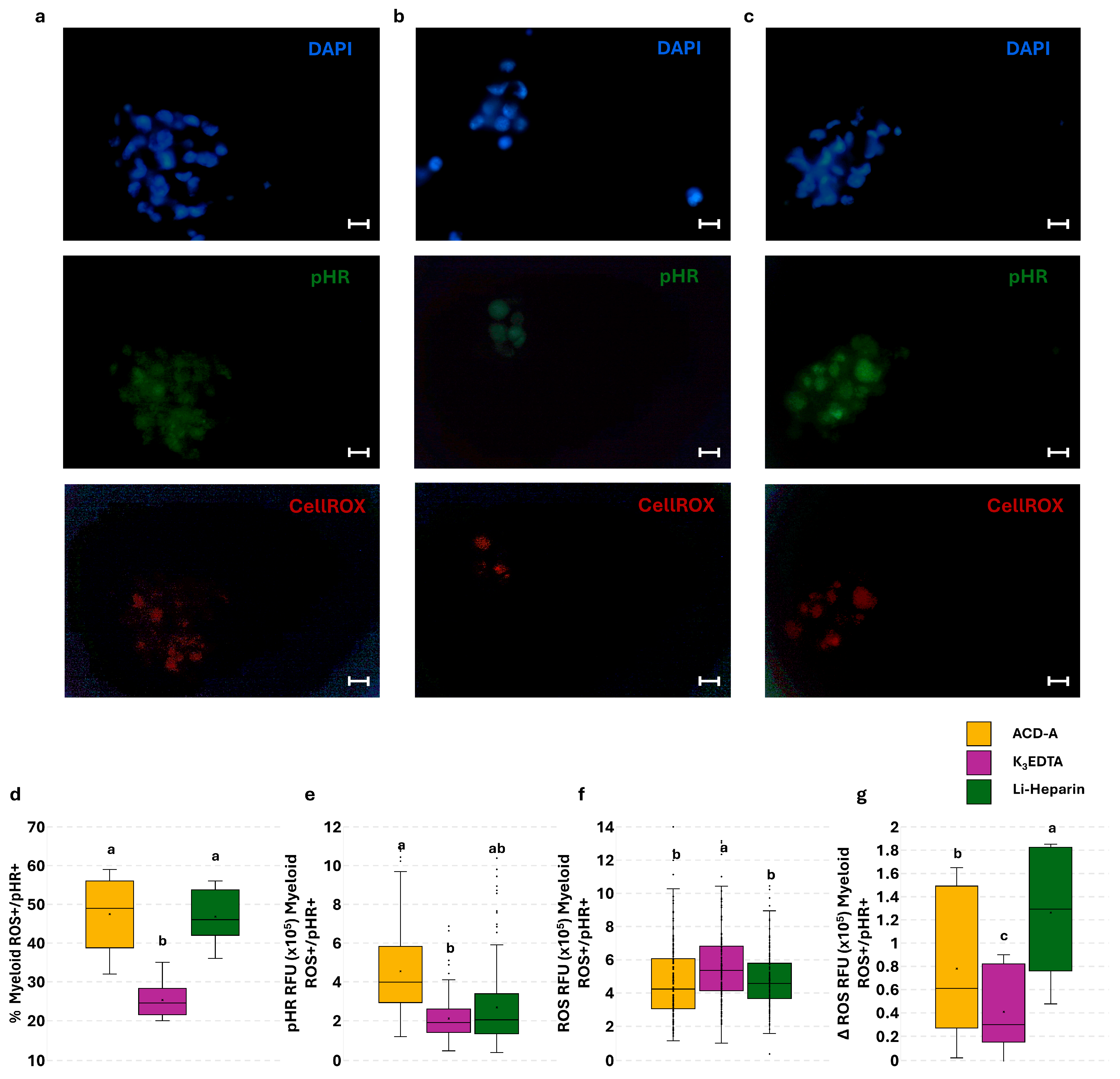
3.5. Effect of Anticoagulants on Phagocytosis and Phagocytosis-Related ROS Production in PBLs: The Flow Cytometry and Fluorescence Microscopy Combined Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Li-Heparin | Lithium Heparin |
| K3EDTA | Tripotassium Ethylenediaminetetraacetic Acid |
| ACD-A | Acid Citrate Dextrose Formula A |
| ROS | Reactive Oxygen Species |
| FDA | Food and Drugs Administration |
| PBLs | Peripheral Blood Leukocytes |
| diH2O | Deionized Water |
| L15 | Leibovitz’s L15 |
| FCS | Fetal Calf Serum |
| FITC | Fluorescein Isothiocyanate |
| APC | Allophycocyanin |
| pHR | pHrodo |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| RFU | Relative Fluorescence Unit |
| IQR | Interquartile Range |
| FSC | Forward Scatter |
| SSC | Side Scatter |
| MFI | Mean Fluorescence Intensity |
References
- Seibel, H.; Baßmann, B.; Rebl, A. Blood Will Tell: What Hematological Analyses Can Reveal About Fish Welfare. Front. Vet. Sci. 2021, 8, 616955. [Google Scholar] [CrossRef]
- Fazio, F. Fish Hematology Analysis as an Important Tool of Aquaculture: A Review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological Methods in Fish—Not Only for Beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Nabi, N.; Ahmed, I.; Wani, G.B. Hematological and Serum Biochemical Reference Intervals of Rainbow Trout, Oncorhynchus mykiss Cultured in Himalayan Aquaculture: Morphology, Morphometrics and Quantification of Peripheral Blood Cells. Saudi J. Biol. Sci. 2022, 29, 2942–2957. [Google Scholar] [CrossRef]
- Saha, N.R.; Usami, T.; Saito, T.; Suetake, H.; Suzuki, Y. Relationships between Immune and Reproductive Endocrine Systems in Fish. Fish. Sci. 2002, 68, 1135–1138. [Google Scholar] [CrossRef][Green Version]
- Řehulka, J.; Adamec, V. Red Blood Cell Indices for Rainbow Trout (Oncorhynchus mykiss Walbaum) Reared in Cage and Raceway Culture. Acta Vet. Brno 2004, 73, 105–114. [Google Scholar] [CrossRef]
- Ates, B.; Orun, I.; Talas, Z.S.; Durmaz, G.; Yilmaz, I. Effects of Sodium Selenite on Some Biochemical and Hematological Parameters of Rainbow Trout (Oncorhynchus mykiss Walbaum, 1792) Exposed to Pb2+ and Cu2+. Fish Physiol. Biochem. 2008, 34, 53–59. [Google Scholar] [CrossRef]
- Kiron, V. Fish Immune System and Its Nutritional Modulation for Preventive Health Care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Segner, H.; Rehberger, K.; Bailey, C.; Bo, J. Assessing Fish Immunotoxicity by Means of In Vitro Assays: Are We There Yet? Front. Immunol. 2022, 13, 6–11. [Google Scholar] [CrossRef]
- Neaga, A.; Lefor, J.; Lich, K.E.; Liparoto, S.F.; Xiao, Y.Q. Development and Validation of a Flow Cytometric Method to Evaluate Phagocytosis of pHrodoTM Bioparticles® by Granulocytes in Multiple Species. J. Immunol. Methods 2013, 390, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Parv, K.; Westerlund, N.; Merchant, K.; Komijani, M.; Lindsay, R.S.; Christoffersson, G. Phagocytosis and Efferocytosis by Resident Macrophages in the Mouse Pancreas. Front. Endocrinol. 2021, 12, 606175. [Google Scholar] [CrossRef]
- Scatà, M.C.; Alhussien, M.N.; Grandoni, F.; Reale, A.; Zampieri, M.; Hussen, J.; De Matteis, G. Hyperthermia-Induced Changes in Leukocyte Survival and Phagocytosis: A Comparative Study in Bovine and Buffalo Leukocytes. Front. Vet. Sci. 2023, 10, 1327148. [Google Scholar] [CrossRef]
- Clark, T.D.; Donaldson, M.R.; Drenner, S.M.; Hinch, S.G.; Patterson, D.A.; Hills, J.; Ives, V.; Carter, J.J.; Cooke, S.J.; Farrell, A.P. The Efficacy of Field Techniques for Obtaining and Storing Blood Samples from Fishes. J. Fish Biol. 2011, 79, 1322–1333. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andrä, I.; Andreata, F.; Annunziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef]
- Lulijwa, R.; Alfaro, A.C.; Merien, F.; Meyer, J.; Young, T. Advances in Salmonid Fish Immunology: A Review of Methods and Techniques for Lymphoid Tissue and Peripheral Blood Leucocyte Isolation and Application. Fish Shellfish Immunol. 2019, 95, 44–80. [Google Scholar] [CrossRef]
- Faggio, C.; Arfuso, F.; Piccione, G.; Zumbo, A.; Fazio, F. Effect of Three Different Anticoagulants and Storage Time on Haematological Parameters of Mugil cephalus (Linneaus, 1758). Turk. J. Fish. Aquat. Sci. 2014, 14, 615–621. [Google Scholar] [CrossRef]
- Sheikh, Z.A.; Ahmed, I. Comparative Evaluation of Two Anticoagulants Used for the Analysis of Haematological, Biochemical Parameters and Blood Cell Morphology of Himalayan Snow Trout, Schizopyge plagiostomus. Tissue Cell 2020, 67, 101398. [Google Scholar] [CrossRef] [PubMed]
- Ciepliński, M.; Kasprzak, M.; Grandtke, M.; Steliga, A.; Kamiński, P.; Jerzak, L. The Effect of Dipotassium EDTA and Lithium Heparin on Hematologic Values of Farmed Brown Trout Salmo trutta (L.) Spawners. Aquac. Int. 2019, 27, 79–87. [Google Scholar] [CrossRef]
- Maqbool, A.; Ahmed, I.; Sheikh, Z.A. Effects of Two Commonly Used Anticoagulants on Haematology and Erythrocyte Morphology of Rainbow Trout (Oncorhynchus mykiss). Int. J. Fish. Aquat. Stud. 2014, 2, 239–243. [Google Scholar]
- Witeska, M.; Wargocka, W. Disodium EDTA Used as Anticoagulant Causes Hemolysis in Common Carp Blood. Turk. J. Vet. Anim. Sci. 2011, 35, 99–104. [Google Scholar] [CrossRef]
- Gilor, S.; Gilor, C. Common Laboratory Artifacts Caused by Inappropriate Sample Collection and Transport: How to Get the Most out of a Sample. Top. Companion Anim. Med. 2011, 26, 109–118. [Google Scholar] [CrossRef]
- Mainwaring, G.; Rowley, A.F. The Effect of Anticoagulants on Blennius pholis L. Leucocytes. Comp. Biochem. Physiol.—Part A Physiol. 1985, 80, 85–91. [Google Scholar] [CrossRef]
- Walencik, J.; Witeska, M. The Effects of Anticoagulants on Hematological Indices and Blood Cell Morphology of Common Carp (Cyprinus carpio L.). Comp. Biochem. Physiol.—C Toxicol. Pharmacol. 2007, 146, 331–335. [Google Scholar] [CrossRef]
- Korcock, D.E.; Houston, A.H.; Gray, J.D. Effects of Sampling Conditions on Selected Blood Variables of Rainbow Trout, Salmo gairdneri Richardson. J. Fish Biol. 1988, 33, 319–330. [Google Scholar] [CrossRef]
- Smit, G.L.; Hattingh, J.; Schoonbee, H.J. Observations on Some Effects of Disodium Ethylenediamine Tetra-Acetate and Heparin on Fish Blood. Comp. Biochem. Physiol. Part C Comp. 1977, 57, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Sussman, L.N.; Camacho, D.; Rosen, E. Use of Adenine-ACD Solution in Long Term Storage of Blood. Am. J. Clin. Pathol. 1971, 55, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Betsou, F.; Gaignaux, A.; Ammerlaan, W.; Norris, P.J.; Stone, M. Biospecimen Science of Blood for Peripheral Blood Mononuclear Cell (PBMC) Functional Applications. Curr. Pathobiol. Rep. 2019, 7, 17–27. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Santillán-Araneda, M.J.; Goldstein, M.; Solarte-Murillo, L.V.; Maisey, K.; Reyes-Cerpa, S.; Vidal, M.; Reyes-Lopez, F.E. Comparison of anticoagulant vacutainer blood collection tubes on rainbow trout (Oncorhynchus mykiss) leukocyte viability during long-term storage. Fish Shellfish Immunol. 2025, 161, 110291. [Google Scholar] [CrossRef]
- Hu, Y.; Maisey, K.; Subramani, P.A.; Liu, F.; Flores-Kossack, C.; Imarai, M.; Secombes, C.J.; Wang, T. Characterisation of Rainbow Trout Peripheral Blood Leucocytes Prepared by Hypotonic Lysis of Erythrocytes, and Analysis of Their Phagocytic Activity, Proliferation and Response to PAMPs and Proinflammatory Cytokines. Dev. Comp. Immunol. 2018, 88, 104–113. [Google Scholar] [CrossRef]
- Aizawa, H.; Kawabata, H.; Sato, A.; Masuki, H.; Watanabe, T.; Tsujino, T.; Isobe, K.; Nakamura, M.; Nakata, K.; Kawase, T. A Comparative Study of the Effects of Anticoagulants on Pure Platelet-Rich Plasma Quality and Potency. Biomedicines 2020, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Kalb, M.L.; Potura, L.; Scharbert, G.; Kozek-Langenecker, S.A. The Effect of Ex Vivo Anticoagulants on Whole Blood Platelet Aggregation. Platelets 2009, 20, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.; Wesche, J.; Lenkeit, L.; Greinacher, A.; Bender, M.; Otto, O.; Palankar, R. Ex Vivo Anticoagulants Affect Human Blood Platelet Biomechanics with Implications for High-Throughput Functional Mechanophenotyping. Commun. Biol. 2022, 5, 1–14. [Google Scholar] [CrossRef]
- Carter, P.H.; Resto-Ruiz, S.; Washington, G.C.; Ethridge, S.; Palini, A.; Vogt, R.; Waxdal, M.; Fleisher, T.; Noguchi, P.D.; Marti, G.E. Flow Cytometric Analysis of Whole Blood Lysis, Three Anticoagulants, and Five Cell Preparations. Cytometry 1992, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Minarova, H.; Palikova, M.; Mares, J.; Syrova, E.; Blahova, J.; Faldyna, M.; Ondrackova, P. Optimisation of the Lymphocyte Proliferation Assay in Rainbow Trout (Oncorhynchus mykiss). Vet. Med. 2019, 64, 547–557. [Google Scholar] [CrossRef]
- Ramsdorf, W.A.; de Guimarães, F.S.F.; Ferraro, M.V.M.; Gabardo, J.; da Trindade, E.S.; Cestari, M.M. Establishment of Experimental Conditions for Preserving Samples of Fish Blood for Analysis with Both Comet Assay and Flow Cytometry. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2009, 673, 78–81. [Google Scholar] [CrossRef]
- Farías, J.G.; Herrera, E.A.; Carrasco-Pozo, C.; Sotomayor-Zárate, R.; Cruz, G.; Morales, P.; Castillo, R.L. Pharmacological Models and Approaches for Pathophysiological Conditions Associated with Hypoxia and Oxidative Stress. Pharmacol. Ther. 2016, 158, 1–23. [Google Scholar] [CrossRef]
- Wan, X.S.; Zhou, Z.; Ware, J.H.; Kennedy, A.R. Standardization of a Fluorometric Assay for Measuring Oxidative Stress in Irradiated Cells. Radiat. Res. 2005, 163, 232–240. [Google Scholar] [CrossRef]
- Wang, J.; Lei, P.; Gamil, A.A.A.; Lagos, L.; Yue, Y.; Schirmer, K.; Mydland, L.T.; Øverland, M.; Krogdahl, Å.; Kortner, T.M. Rainbow Trout (Oncorhynchus mykiss) Intestinal Epithelial Cells as a Model for Studying Gut Immune Function and Effects of Functional Feed Ingredients. Front. Immunol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Park, Y.; Abihssira-García, I.S.; Thalmann, S.; Wiegertjes, G.F.; Barreda, D.R.; Olsvik, P.A.; Kiron, V. Imaging Flow Cytometry Protocols for Examining Phagocytosis of Microplastics and Bioparticles by Immune Cells of Aquatic Animals. Front. Immunol. 2020, 11, 203. [Google Scholar] [CrossRef]
- Al-Amoudi, S.M. Anticoagulant Induced Leukoagglutination. Saudi Med. J. 2008, 29, 1192–1193. [Google Scholar]
- Deol, I.; Hernandez, A.M.; Pierre, R.V. Ethylenediamine Tetraacetic Acid-Associated Leukoagglutination. Am. J. Clin. Pathol. 1995, 103, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Daves, M. Hematology, Transfusion and Cell Therapy Unusual Leukoagglutination: A Rare Haematological Finding. Hematol. Transfus. Cell Ther. 2020, 4, 426–428. [Google Scholar]
- Haranath, P. Patient Communication in Intensive Care Unit. Indian J. Crit. Care Med. 2012, 16, 112–113. [Google Scholar] [CrossRef]
- Minarova, H.; Ondrackova, P.; Palikova, M.; Mares, J.; Blahova, J.; Jarova, K.; Faldyna, M. Optimisation of Phagocytosis Assay in Rainbow Trout (Oncorhynchus mykiss). Vet. Med. 2021, 66, 298–304. [Google Scholar] [CrossRef] [PubMed]

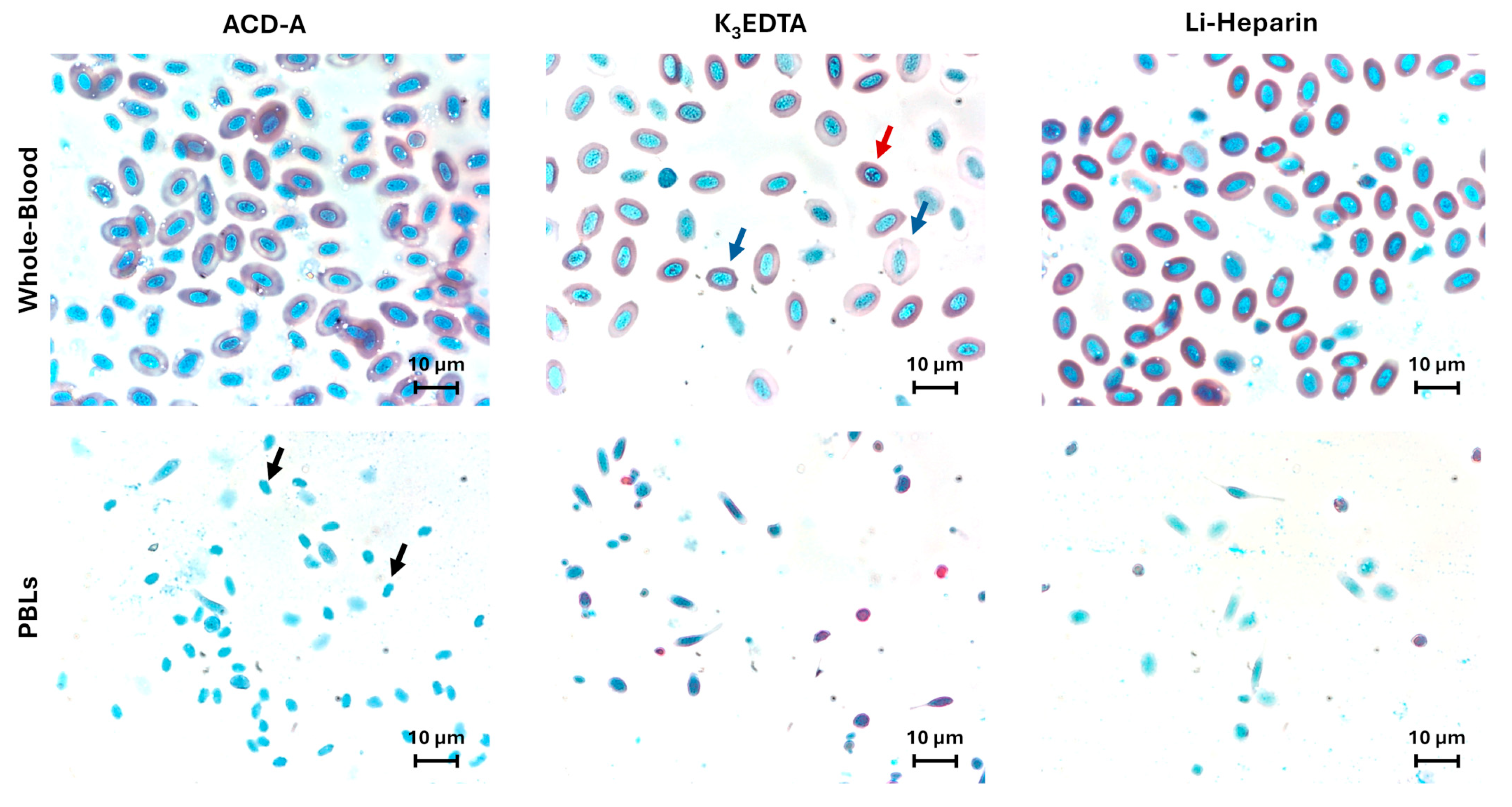
| ACD-A | K3EDTA | Li-Heparin | p-Value | |
|---|---|---|---|---|
| Mean ± SD | ||||
| RBC (×106/mm3) | 1.07 ± 0.04 b | 1.13 ± 0.06 b | 1.64 ± 0.06 a | 0.0003 |
| TBC (×104/mm3) | 3.78 ± 0.33 b | 4.48 ± 0.30 a | 4.51 ± 0.34 a | 0.008 |
| WBC (×104/mm3) | 6.08 ± 0.14 a | 6.15 ± 0.19 a | 6.21 ± 0.15 a | 0.402 |
| LC (×104/mm3) | 4.52 ± 0.2 a | 4.48 ± 0.34 a | 4.52 ± 0.16 a | 0.996 |
| SL (×104/mm3) | 2.39 ± 0.17 a | 2.46 ± 0.13 a | 2.42 ± 0.09 a | 0.633 |
| LL (×104/mm3) | 2.20 ± 0.12 a | 2.21 ± 0.10 a | 2.22 ± 0.11 a | 0.884 |
| MON (×103/mm3) | 4.10 ± 0.07 a | 4.09 ± 0.13 a | 4.11 ± 0.09 a | 0.849 |
| NEU (×104/mm3) | 1.12 ± 0.08 a | 1.11 ± 0.09 a | 1.07 ± 0.11 a | 0.738 |
| EOS (×102/mm3) | 0.80 ± 0.01 a | 1.01 ± 0.01 a | 0.70 ± 0.01 a | 0.663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Iorio, T.; Scatà, M.C.; Grandoni, F.; De Matteis, G.; Martini, A.; Tonachella, N.; Pulcini, D.; Capoccioni, F. Effect of Anticoagulants on Blood Cell Counts, Cell Morphology, and Leukocyte Immune Functions of Rainbow Trout (Oncorhynchus mykiss). Cells 2025, 14, 1372. https://doi.org/10.3390/cells14171372
De Iorio T, Scatà MC, Grandoni F, De Matteis G, Martini A, Tonachella N, Pulcini D, Capoccioni F. Effect of Anticoagulants on Blood Cell Counts, Cell Morphology, and Leukocyte Immune Functions of Rainbow Trout (Oncorhynchus mykiss). Cells. 2025; 14(17):1372. https://doi.org/10.3390/cells14171372
Chicago/Turabian StyleDe Iorio, Teresina, Maria Carmela Scatà, Francesco Grandoni, Giovanna De Matteis, Arianna Martini, Nicolò Tonachella, Domitilla Pulcini, and Fabrizio Capoccioni. 2025. "Effect of Anticoagulants on Blood Cell Counts, Cell Morphology, and Leukocyte Immune Functions of Rainbow Trout (Oncorhynchus mykiss)" Cells 14, no. 17: 1372. https://doi.org/10.3390/cells14171372
APA StyleDe Iorio, T., Scatà, M. C., Grandoni, F., De Matteis, G., Martini, A., Tonachella, N., Pulcini, D., & Capoccioni, F. (2025). Effect of Anticoagulants on Blood Cell Counts, Cell Morphology, and Leukocyte Immune Functions of Rainbow Trout (Oncorhynchus mykiss). Cells, 14(17), 1372. https://doi.org/10.3390/cells14171372








