Acute Shear Stress Induces TWIST-Mediated EndMT in Venous Endothelial Cells and Human Long Saphenous Veins
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Comparative Reverse-Transcriptase Polymerase Chain Reaction
2.3. Silencing of TWIST1 and TWIST2
2.4. EC Culture and Shear Stress
2.5. Ex Vivo Perfusion of Veins
2.6. Detection of Proteins in Cultured Cells
2.7. Immunohistochemistry
2.8. RNAscope In Situ Hybridisation
2.9. LSV Culture Well
2.10. Spatial Cell Sequencing
2.11. Statistics
3. Results
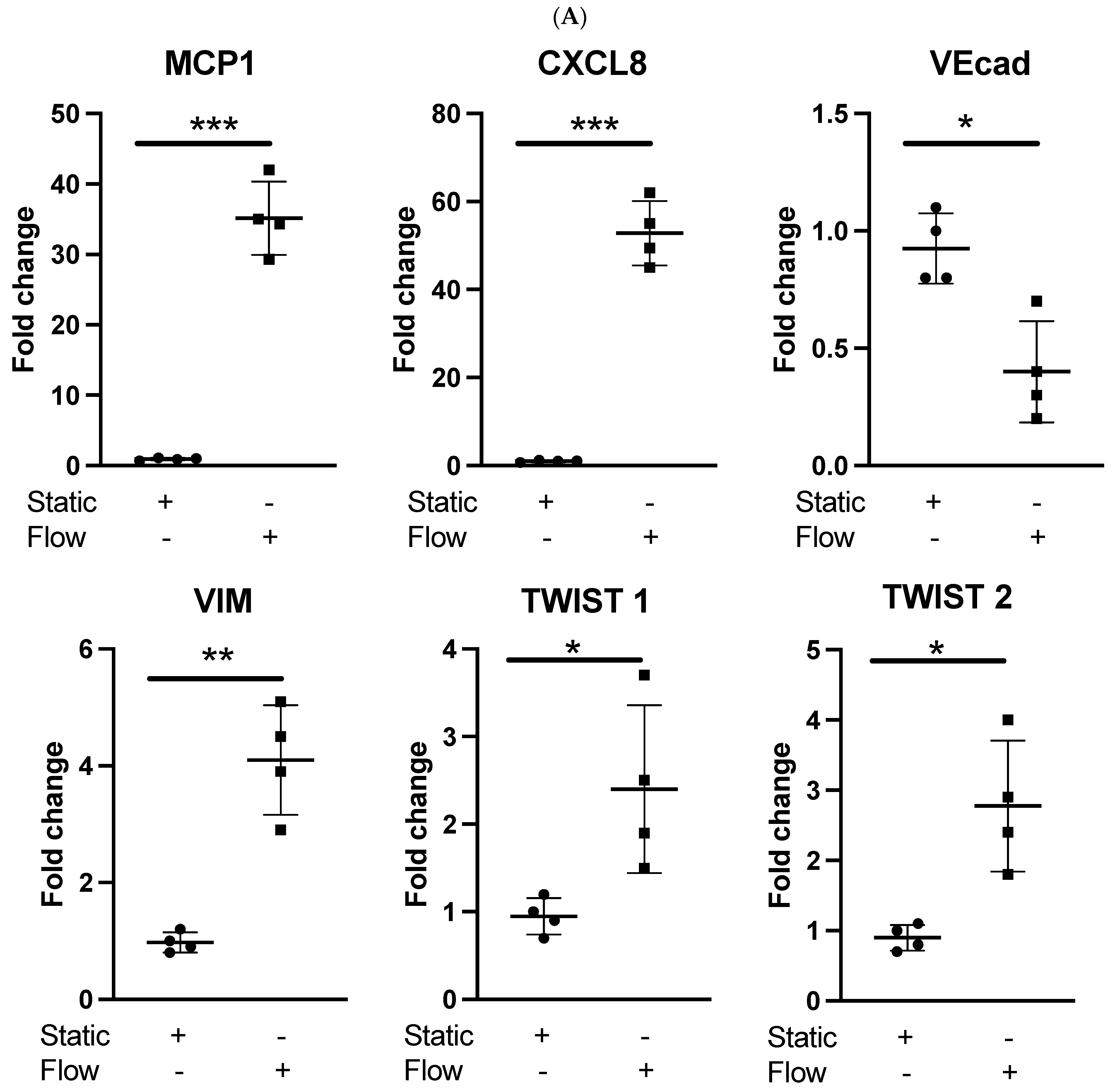
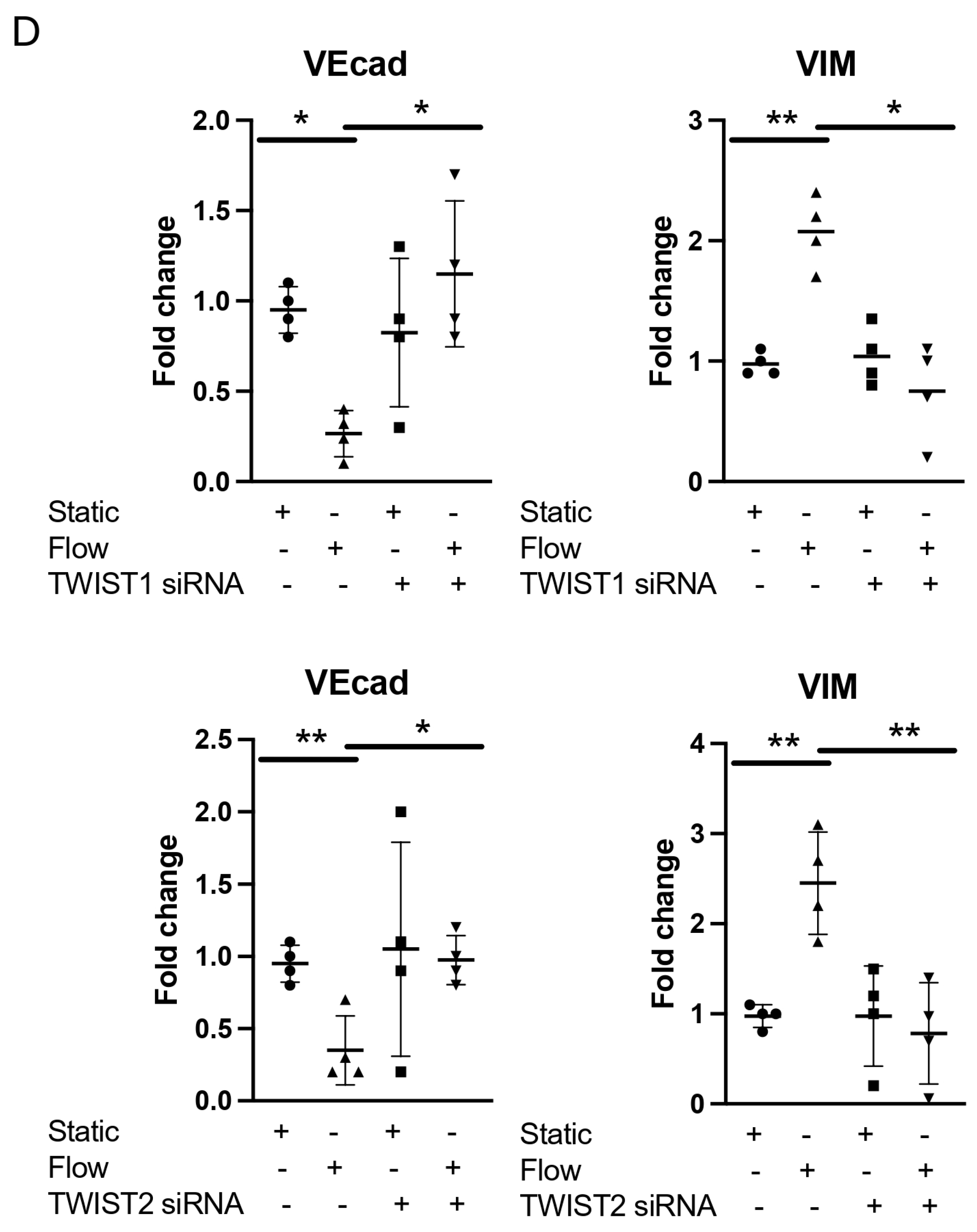
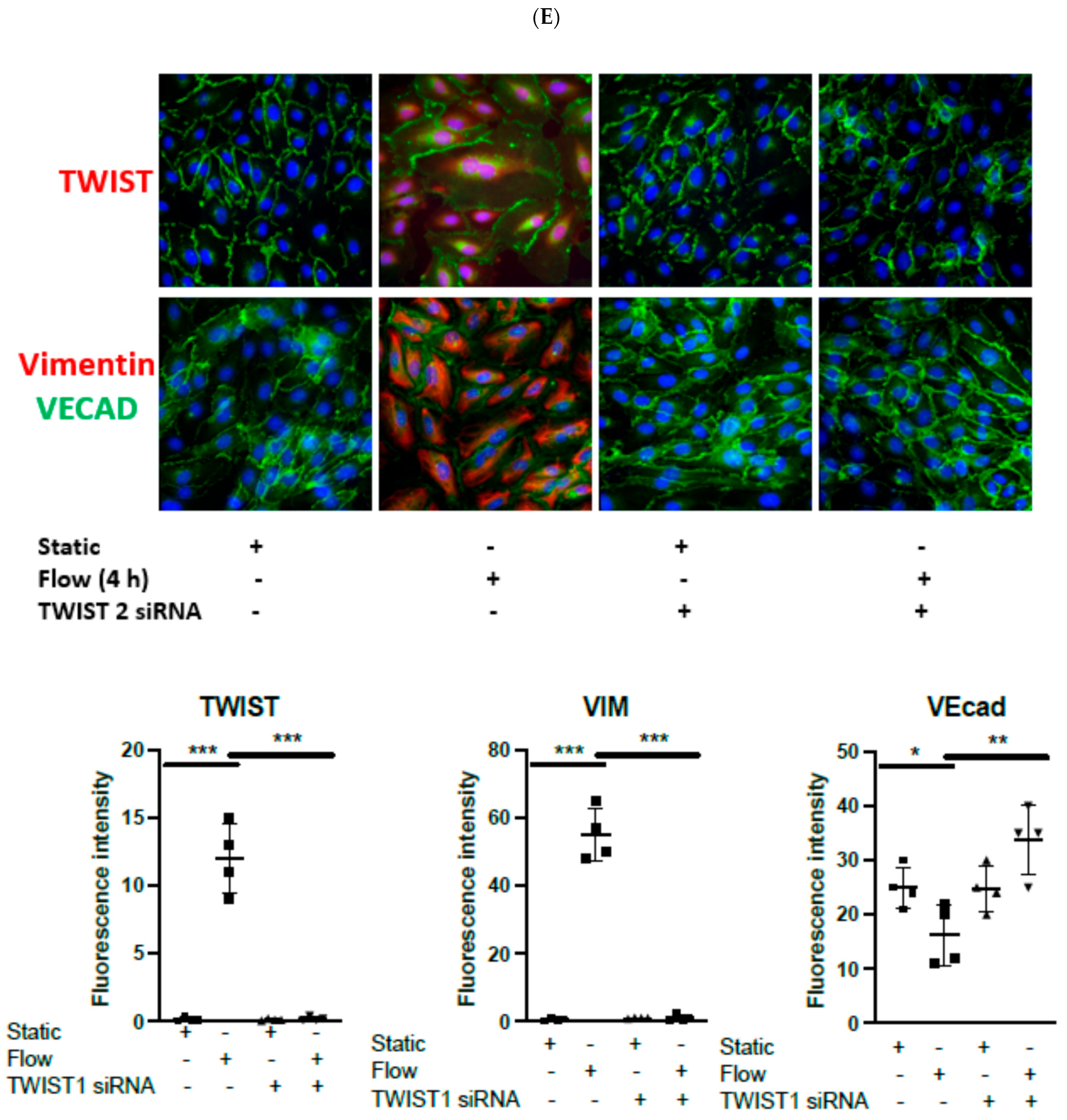
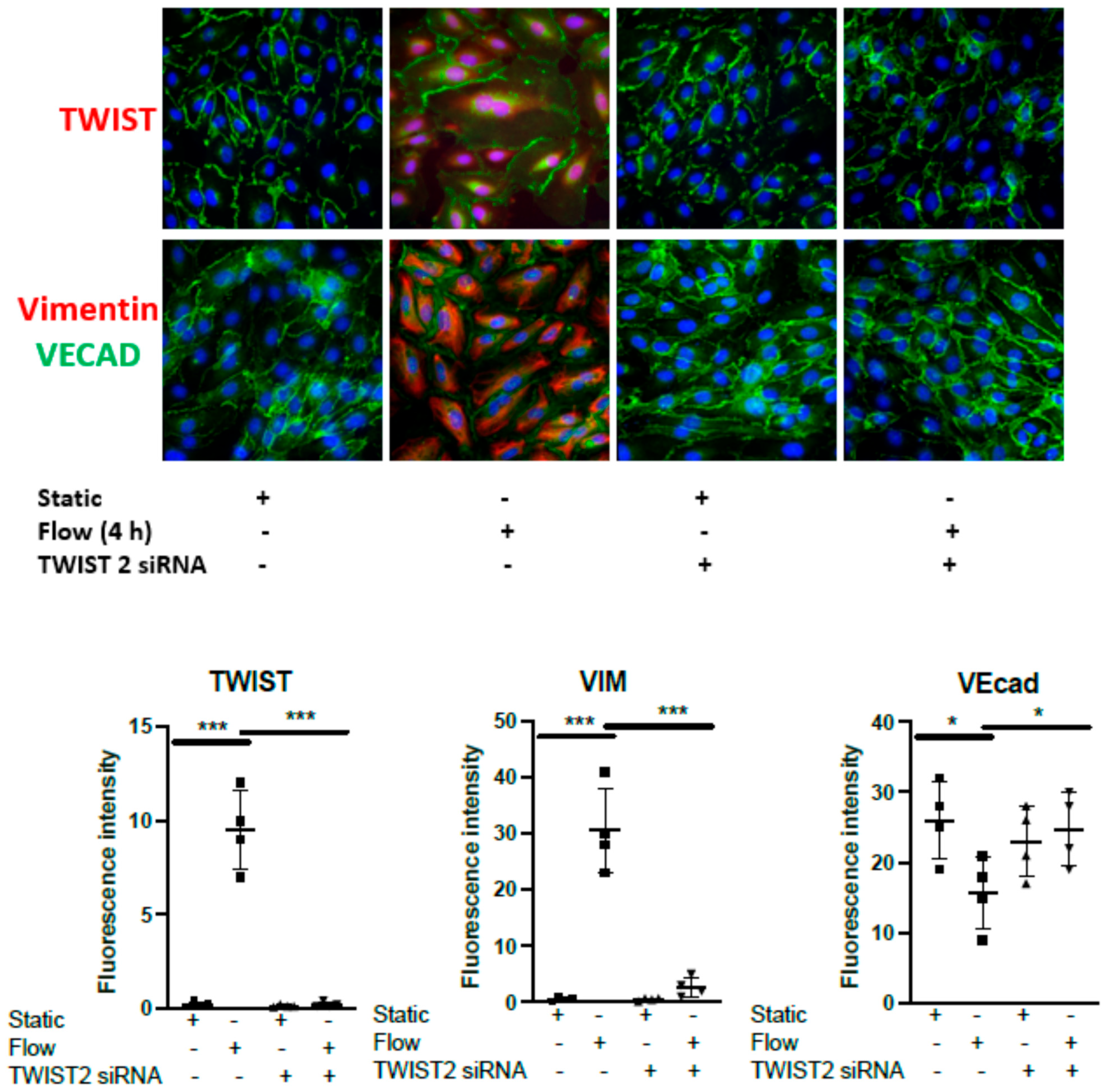
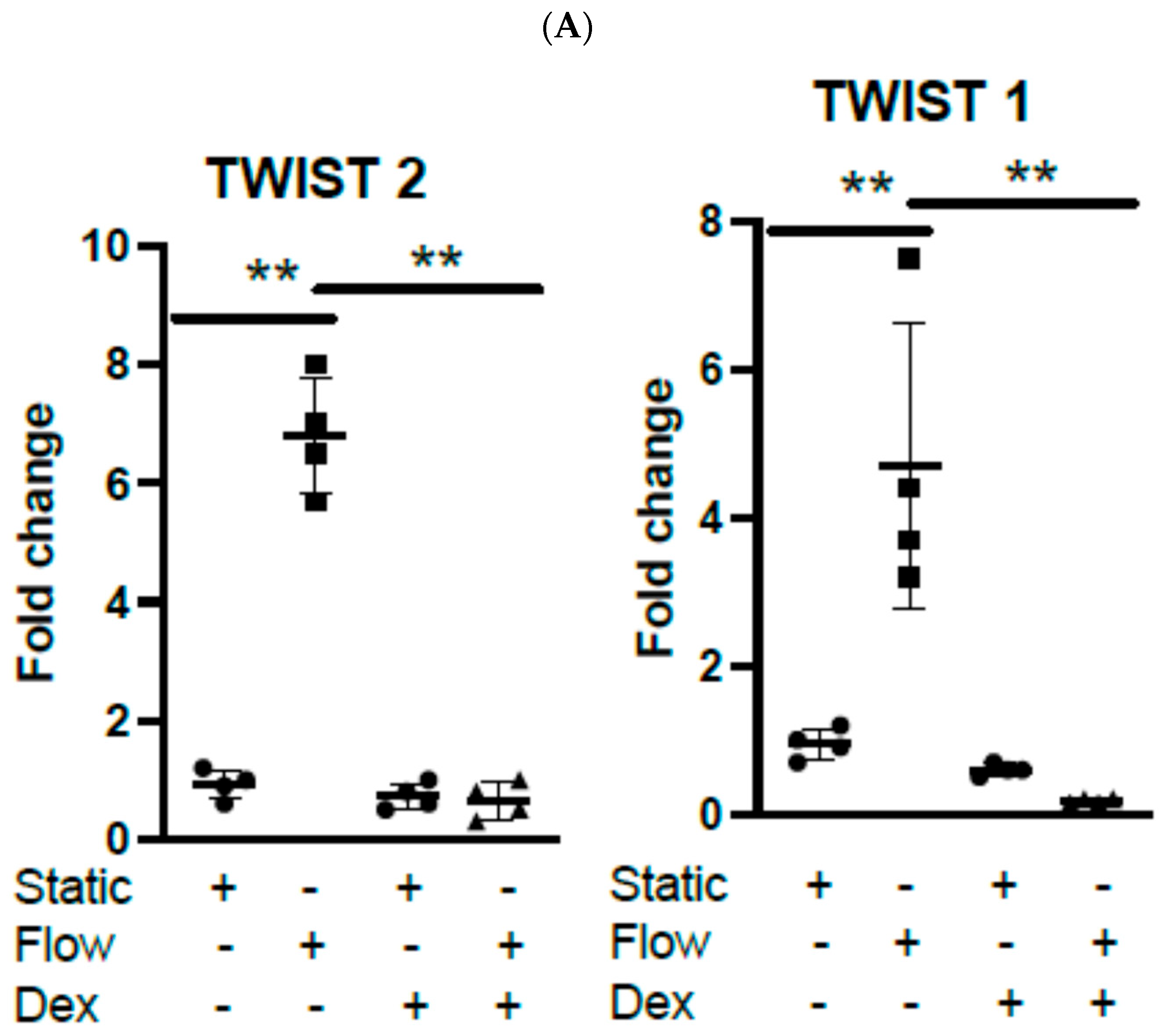
3.1. Acute Arterial Shear Stress Promotes EndMT in Venous ECs In Vitro
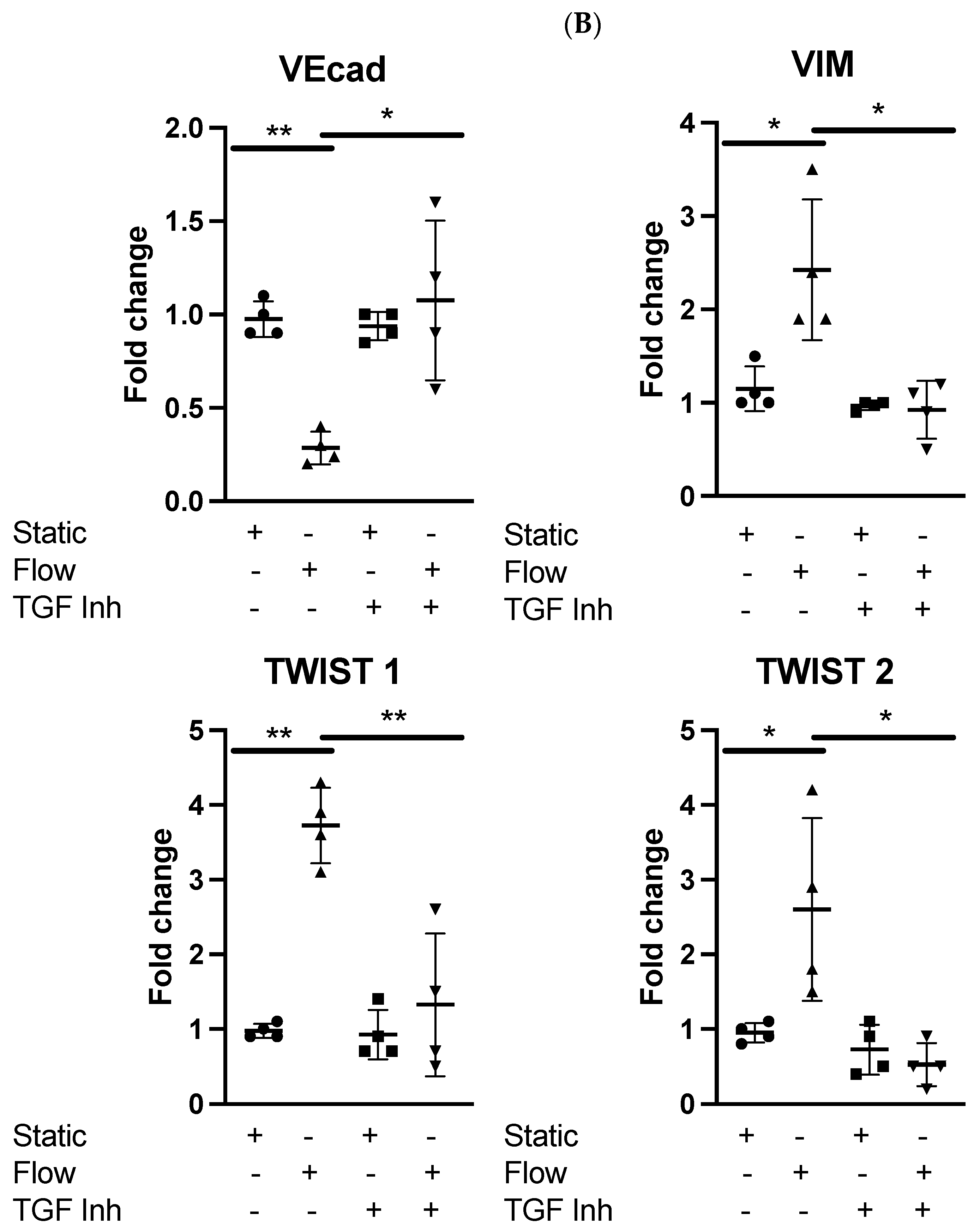
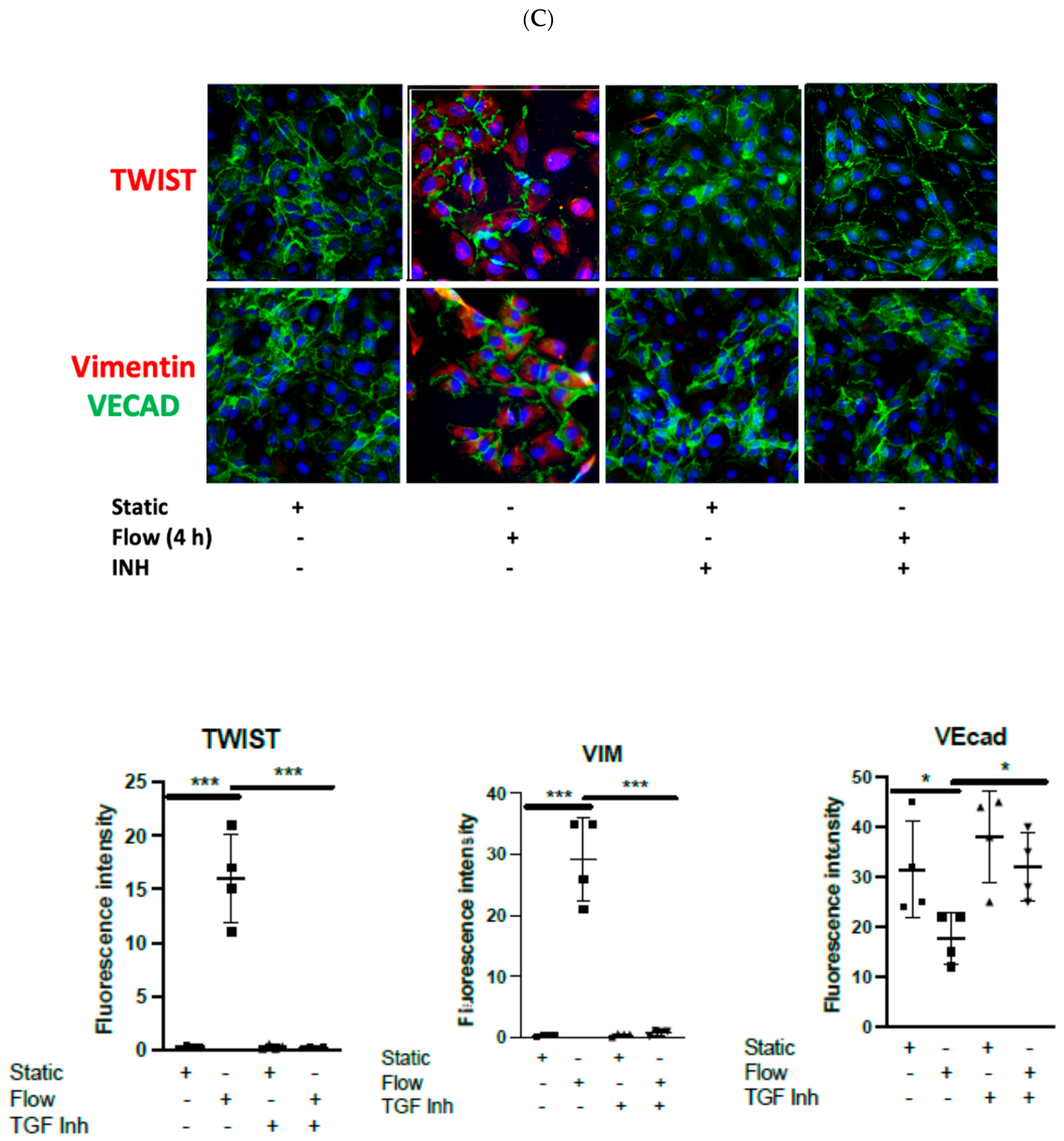
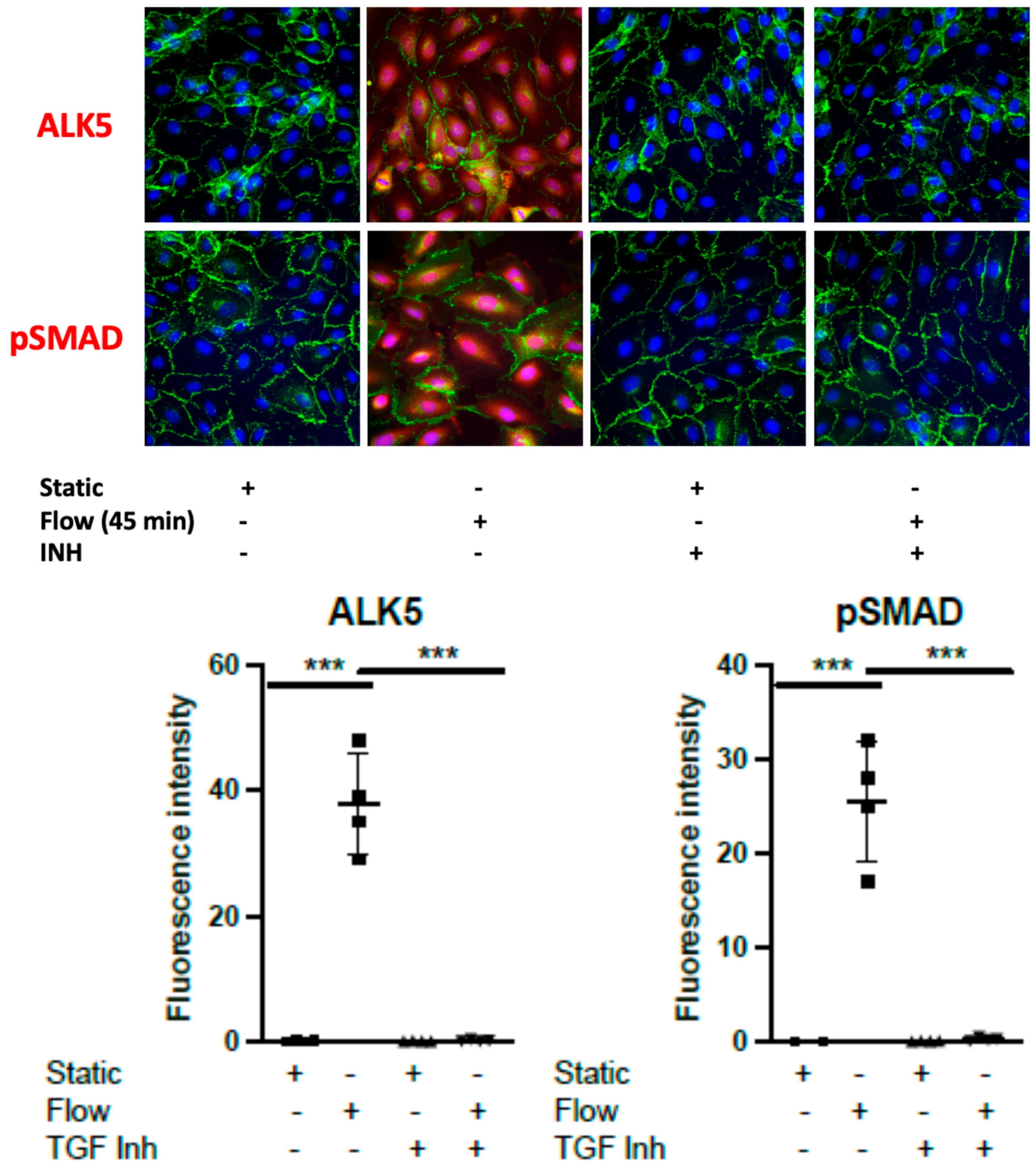
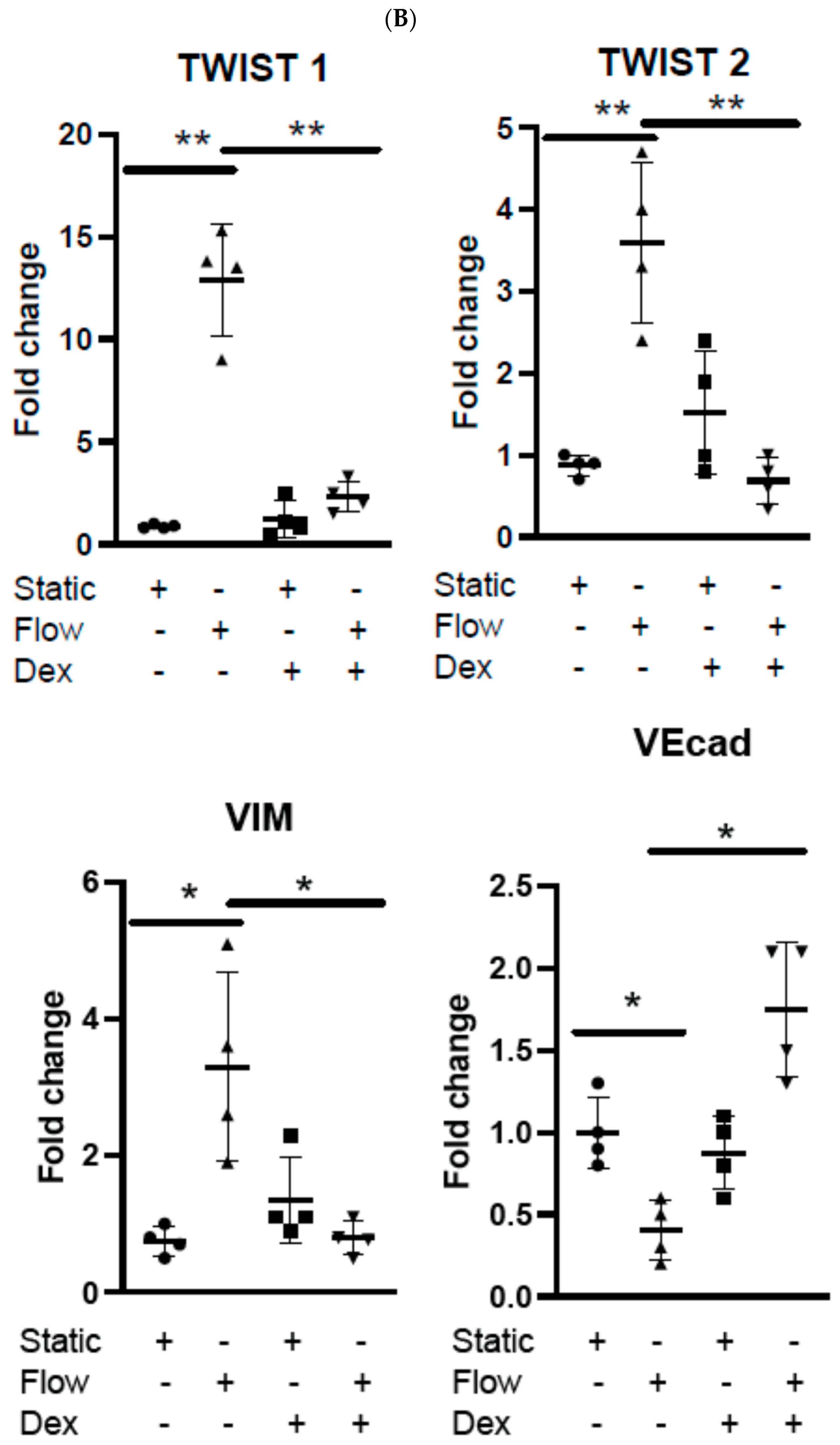
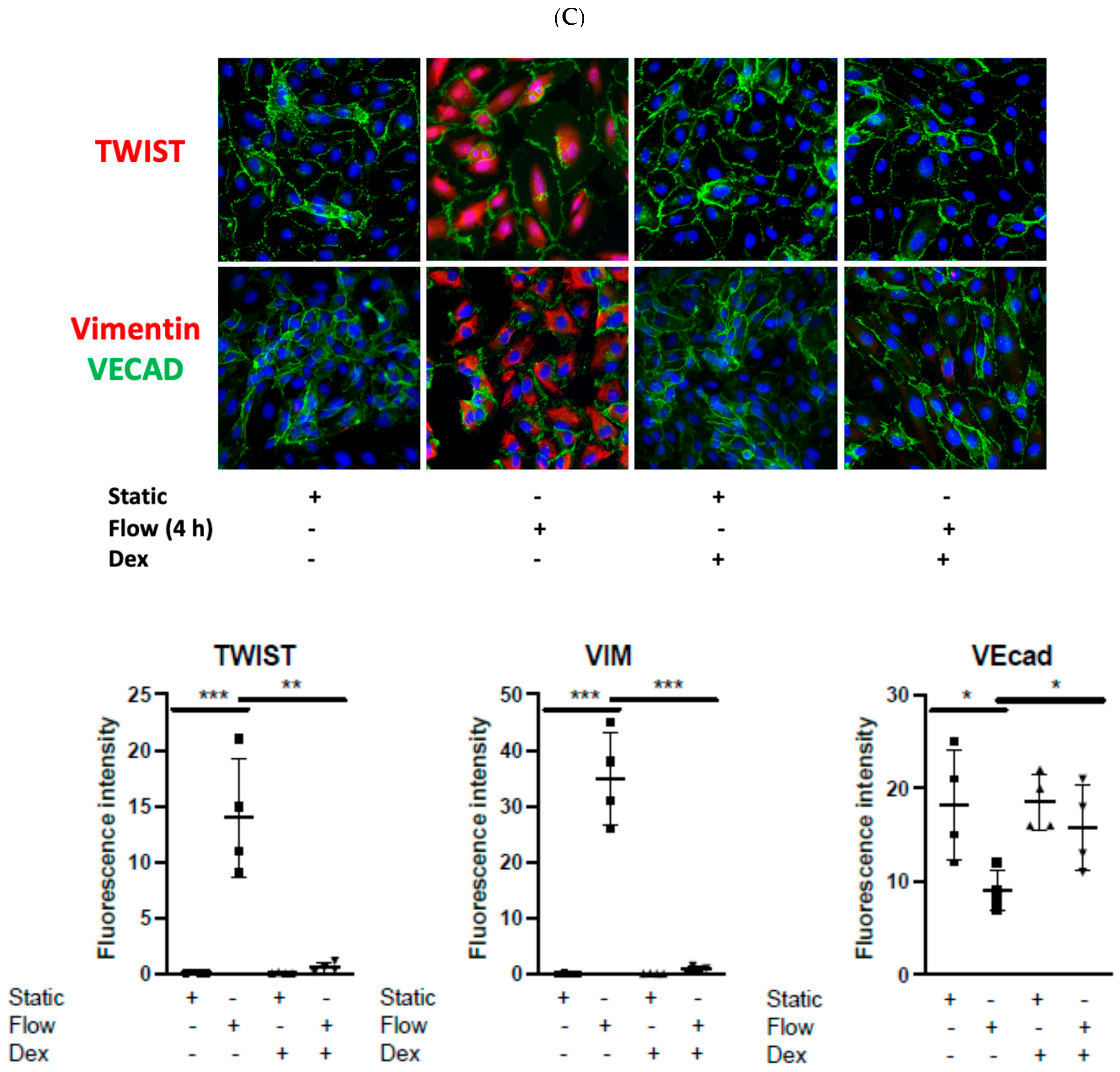
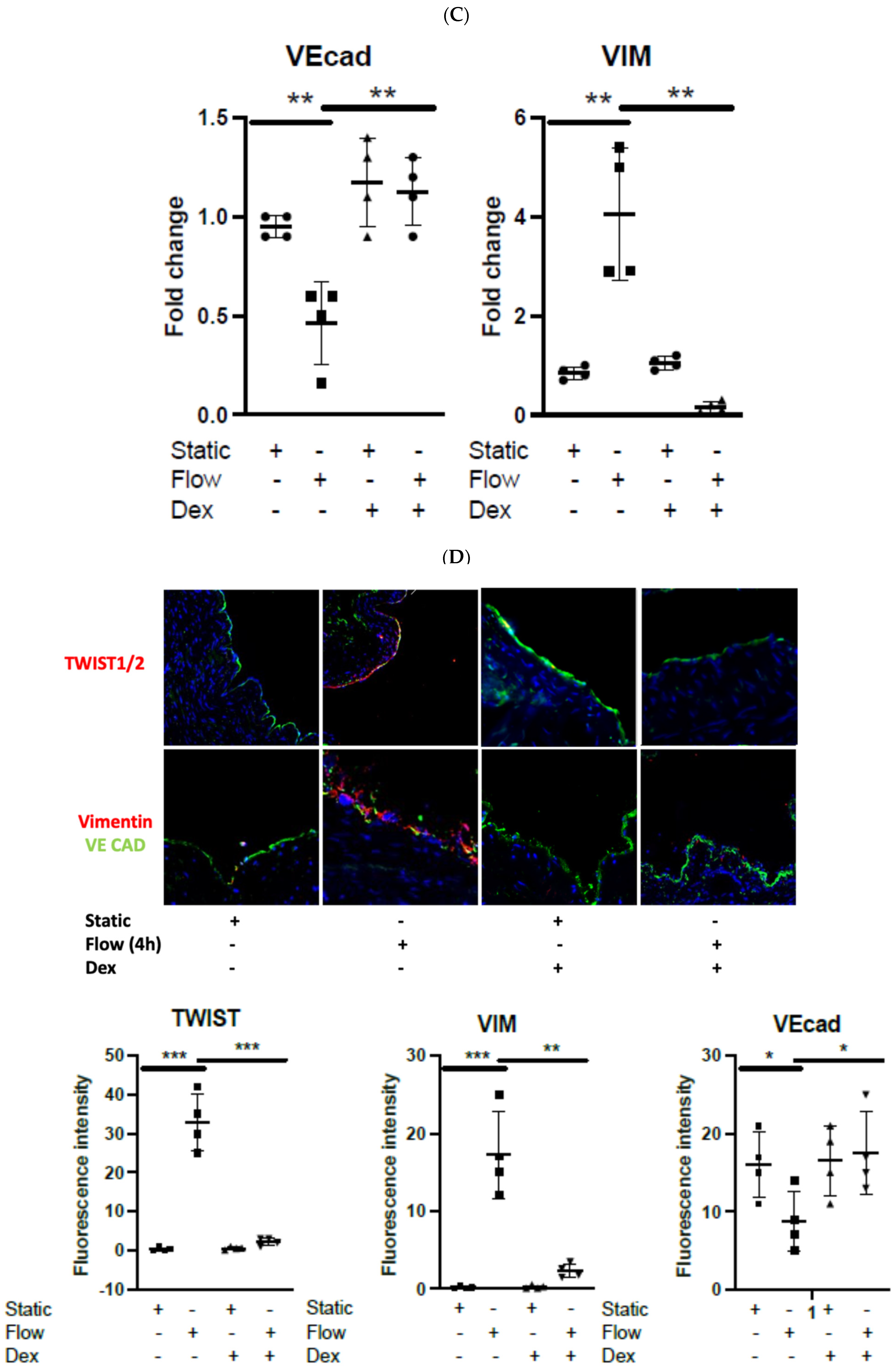
3.2. Dexamethasone Pretreatment Suppressed EndMT in Venous Ecs
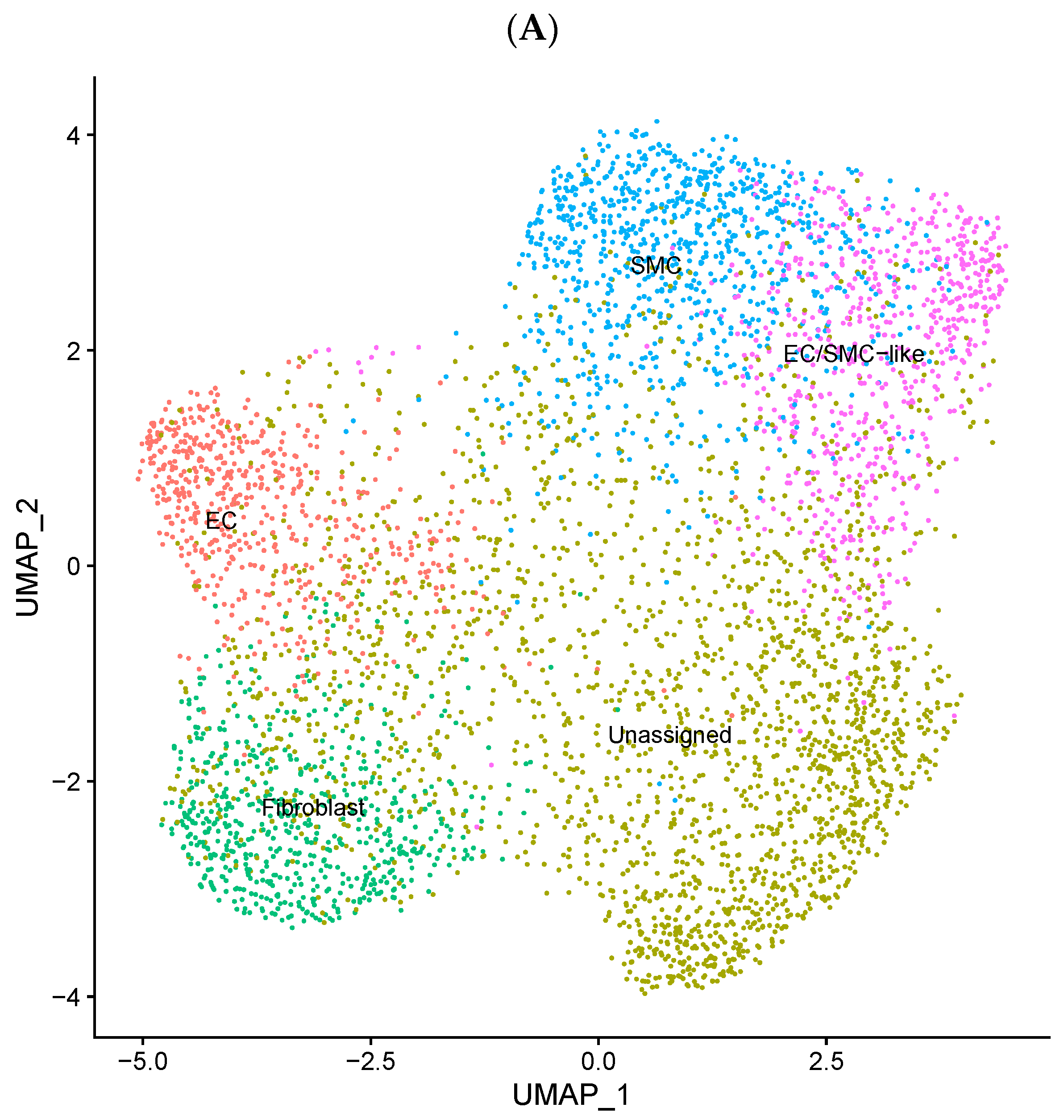
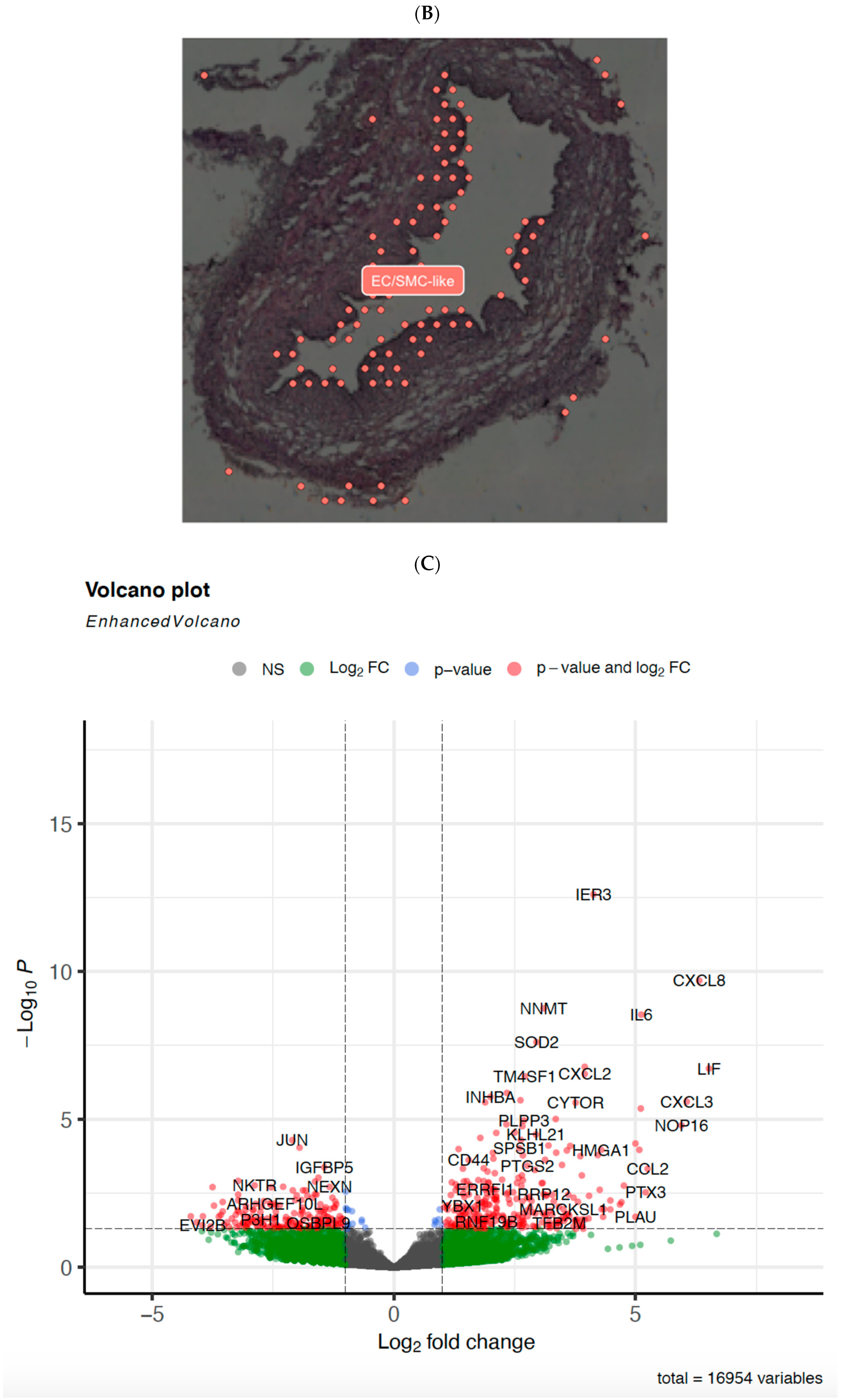
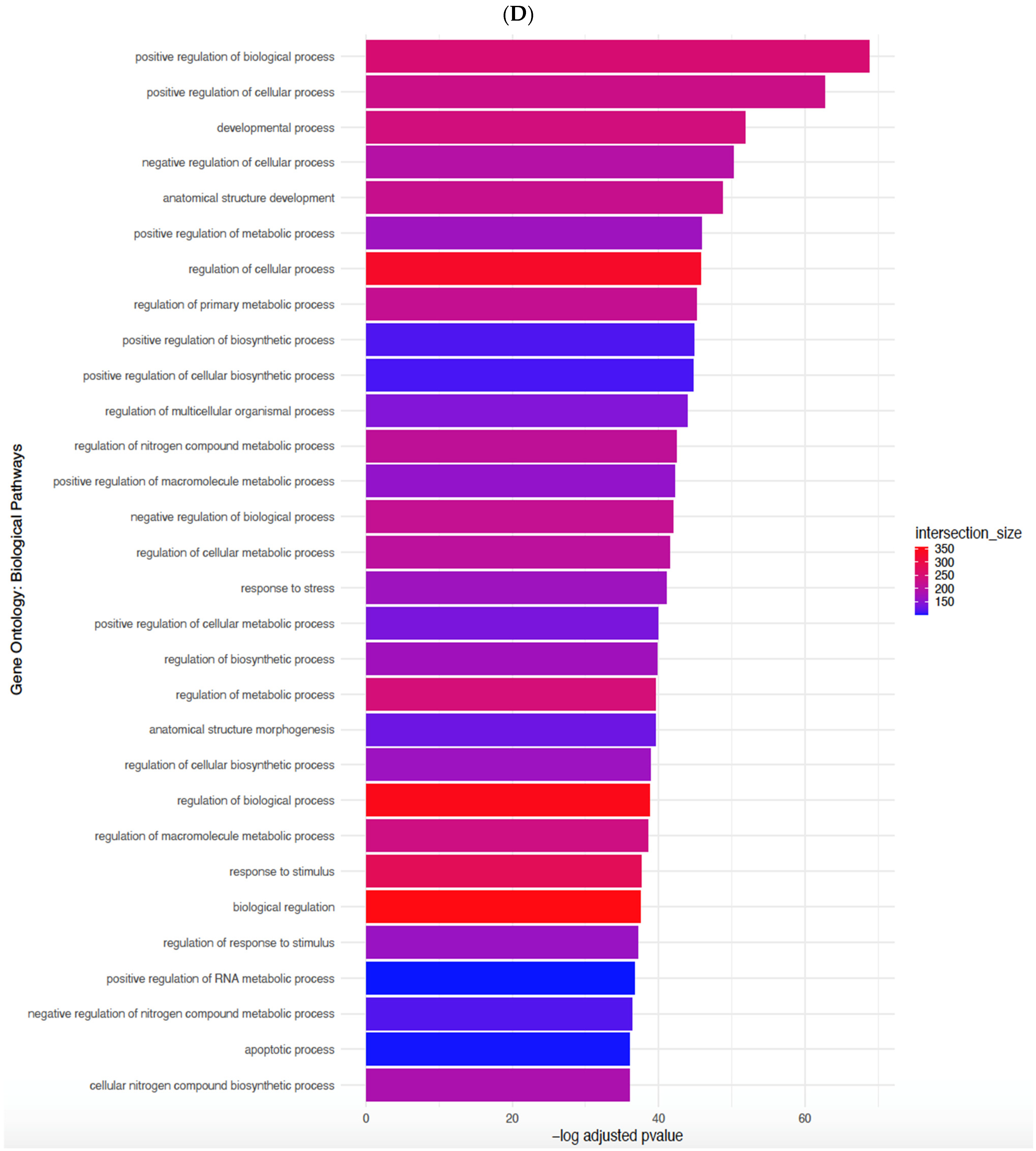
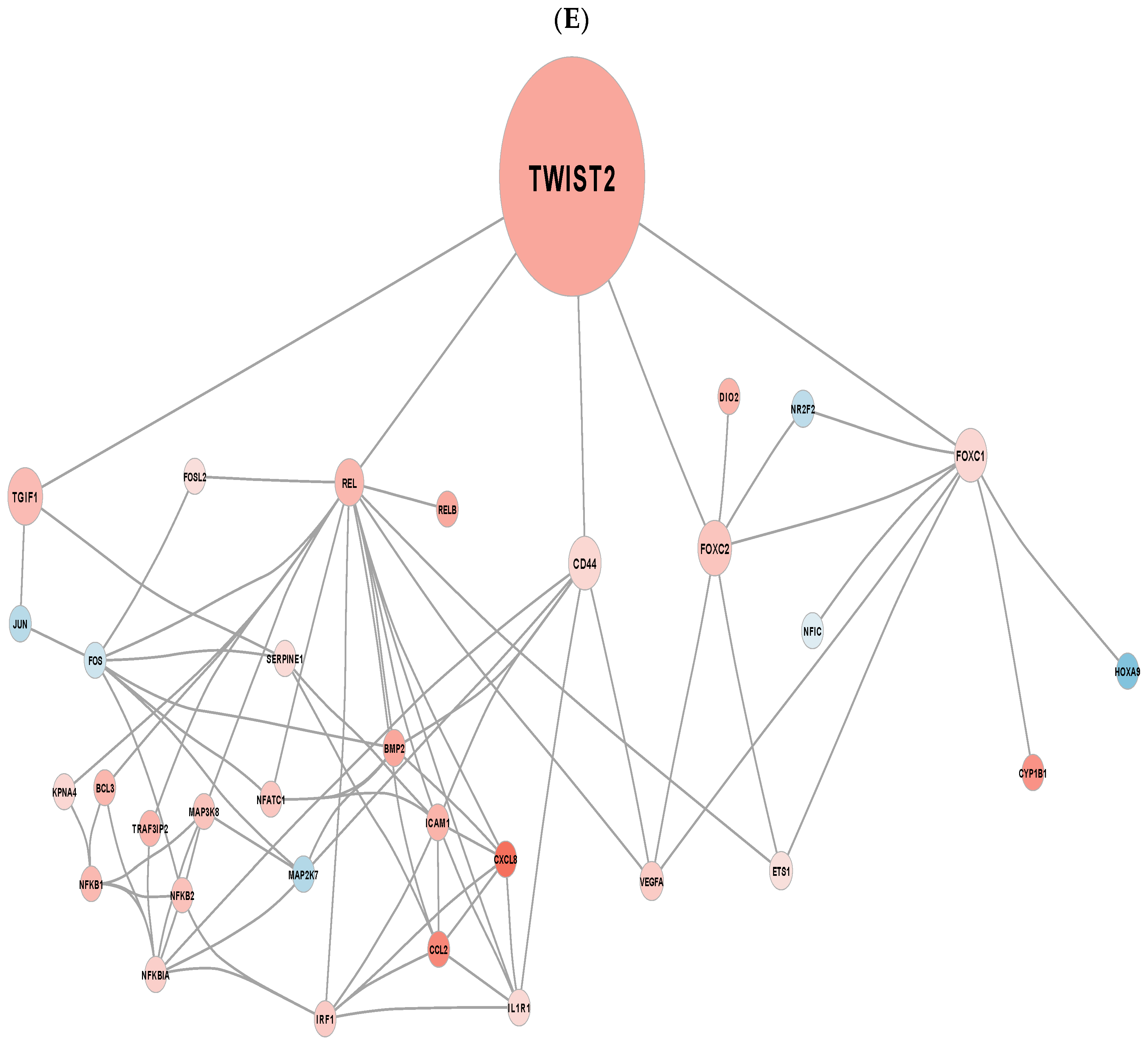
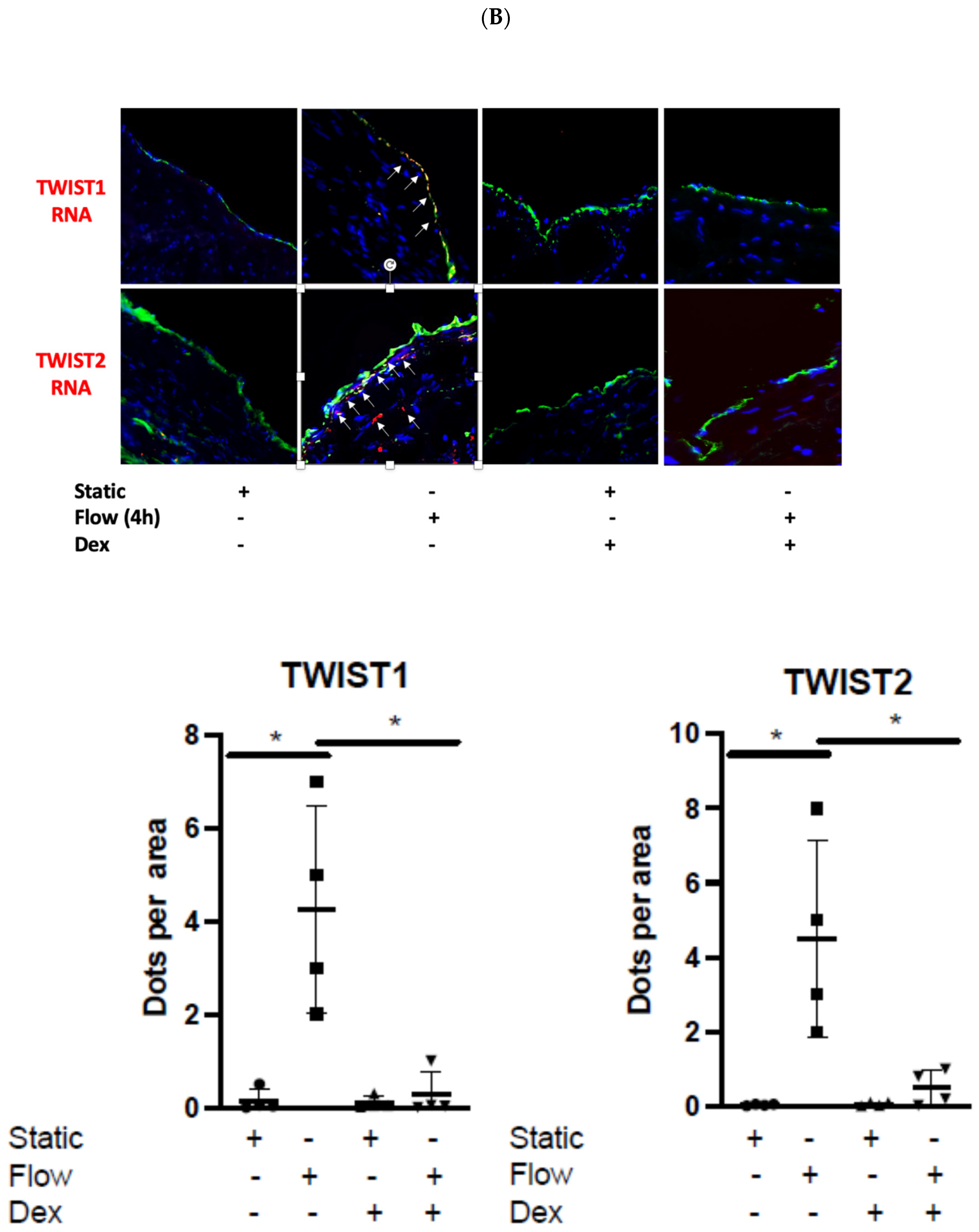
3.3. EndMT Activation in Veins Exposed to Arterial Flow Ex Vivo Using Untargeted Spatial Cell Sequencing
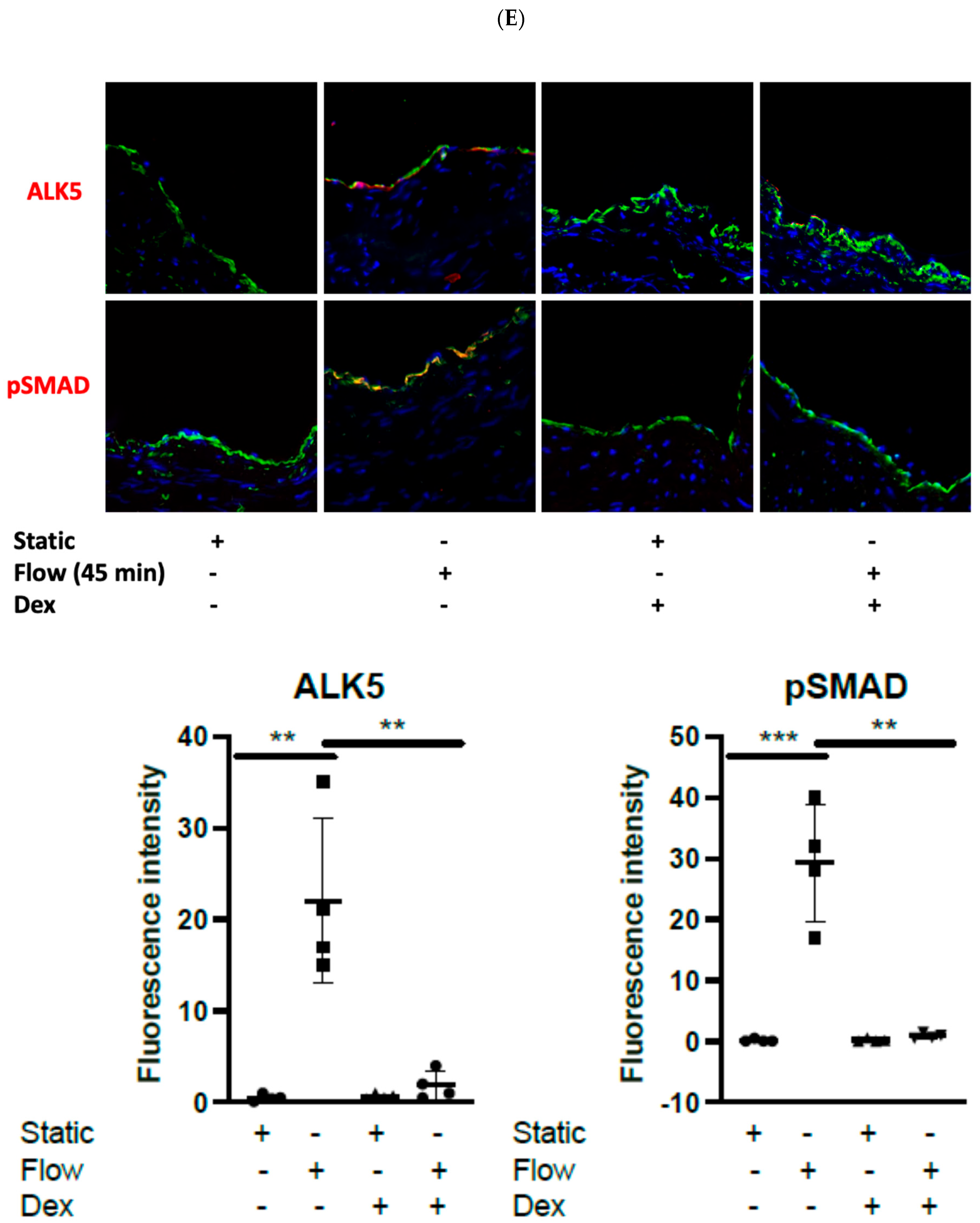
3.4. Dexamethasone Pretreatment Suppressed EndMT in Veins Exposed to Arterial Flow Ex Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaudino, M.; Sandner, S.; An, K.R.; Dimagli, A.; Di Franco, A.; Audisio, K.; Harik, L.; Perezgrovas-Olaria, R.; Soletti, G.; Fremes, S.E.; et al. Graft Failure After Coronary Artery Bypass Grafting and Its Association With Patient Characteristics and Clinical Events: A Pooled Individual Patient Data Analysis of Clinical Trials With Imaging Follow-Up. Circulation 2023, 148, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Layton, G.R.; Sinha, S.; Caputo, M.; Angelini, G.D.; Fudulu, D.P.; Zakkar, M. Two Decades of CABG in the UK: A Propensity Matched Analysis of Outcomes by Conduit Choice. J. Clin. Med. 2024, 13, 4717. [Google Scholar] [CrossRef] [PubMed]
- Guida, G.; Ward, A.O.; Bruno, V.D.; George, S.J.; Caputo, M.; Angelini, G.D.; Zakkar, M. Saphenous vein graft disease, pathophysiology, prevention, and treatment. A review of the literature. J. Card. Surg. 2020, 35, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Ladak, S.S.; McQueen, L.W.; Layton, G.R.; Aujla, H.; Adebayo, A.; Zakkar, M. The Role of Endothelial Cells in the Onset, Development and Modulation of Vein Graft Disease. Cells 2022, 11, 3066. [Google Scholar] [CrossRef]
- McQueen, L.W.; Ladak, S.S.; Zakkar, M. Acute shear stress and vein graft disease. Int. J. Biochem. Cell Biol. 2022, 144, 106173. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- He, C.; Ye, P.; Zhang, X.; Esmaeili, E.; Li, Y.; Lü, P.; Cai, C. The Role of TGF-β Signaling in Saphenous Vein Graft Failure after Peripheral Arterial Disease Bypass Surgery. Int. J. Mol. Sci. 2023, 24, 10381. [Google Scholar] [CrossRef]
- Cooley, B.C.; Nevado, J.; Mellad, J.; Yang, D.; St Hilaire, C.; Negro, A.; Fang, F.; Chen, G.; San, H.; Walts, A.D.; et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 2014, 6, 227ra34. [Google Scholar] [CrossRef]
- Zakkar, M.; Luong, L.A.; Chaudhury, H.; Ruud, O.; Punjabi, P.P.; Anderson, J.R.; Mullholand, J.W.; Clements, A.T.; Krams, R.; Foin, N.; et al. Dexamethasone arterializes venous endothelial cells by inducing mitogen-activated protein kinase phosphatase-1: A novel antiinflammatory treatment for vein grafts? Circulation 2011, 123, 524–532. [Google Scholar] [CrossRef]
- McQueen, L.W.; Ladak, S.S.; Layton, G.R.; Wadey, K.; George, S.J.; Angelini, G.D.; Murphy, G.J.; Zakkar, M. Osteopontin Activation and Microcalcification in Venous Grafts Can Be Modulated by Dexamethasone. Cells 2023, 12, 2627. [Google Scholar] [CrossRef]
- Ward, A.O.; Angelini, G.D.; Caputo, M.; Evans, P.C.; Johnson, J.L.; Suleiman, M.S.; Tulloh, R.M.; George, S.J.; Zakkar, M. NF-κB inhibition prevents acute shear stress-induced inflammation in the saphenous vein graft endothelium. Sci. Rep. 2020, 10, 15133. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef]
- Abraham, S.M.; Lawrence, T.; Kleiman, A.; Warden, P.; Medghalchi, M.; Tuckermann, J.; Saklatvala, J.; Clark, A.R. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 2006, 203, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Hoppstädter, J.; Ammit, A.J. Role of Dual-Specificity Phosphatase 1 in Glucocorticoid-Driven Anti-inflammatory Responses. Front. Immunol. 2019, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Auwardt, R.B.; Mudge, S.J.; Chen, C.G.; Power, D.A. Regulation of nuclear factor kappaB by corticosteroids in rat mesangial cells. J. Am. Soc. Nephrol. 1998, 9, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, W.; Schulze, M.; Engels, C.; Klasmeier, E.; Pfeilschifter, J. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: Involvement of nuclear factor-kappaB and Ets transcription factors. Mol. Endocrinol. 2002, 16, 1752–1766. [Google Scholar] [CrossRef]
- Gerber, A.N.; Newton, R.; Sasse, S.K. Repression of transcription by the glucocorticoid receptor: A parsimonious model for the genomics era. J. Biol. Chem. 2021, 296, 100687. [Google Scholar] [CrossRef]
- Schwartze, J.T.; Becker, S.; Sakkas, E.; Wujak, Ł.A.; Niess, G.; Usemann, J.; Reichenberger, F.; Herold, S.; Vadász, I.; Mayer, K.; et al. Glucocorticoids recruit Tgfbr3 and Smad1 to shift transforming growth factor-β signaling from the Tgfbr1/Smad2/3 axis to the Acvrl1/Smad1 axis in lung fibroblasts. J. Biol. Chem. 2014, 289, 3262–3275. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Zhou, H.; Setia, O.; Liu, B.; Kanasaki, K.; Koya, D.; Dardik, A.; Fernandez-Hernando, C.; Goodwin, J. Loss of endothelial glucocorticoid receptor accelerates diabetic nephropathy. Nat. Commun. 2021, 12, 2368. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, M.; Wei, H.; Arslan Ahmad, M.; Ma, Y.; Mao, X.; Hao, L.; Ao, Q. Poly (ɛ-Caprolactone-co-l,l-Lactide) Vascular External Sheath Carrying Prednisone for Improving Patency Rate of the Vein Graft. Tissue Eng. Part A 2022, 28, 394–404. [Google Scholar] [CrossRef]
- Schepers, A.; Pires, N.M.; Eefting, D.; de Vries, M.R.; van Bockel, J.H.; Quax, P.H. Short-term dexamethasone treatment inhibits vein graft thickening in hypercholesterolemic ApoE3Leiden transgenic mice. J. Vasc. Surg. 2006, 43, 809–815. [Google Scholar] [CrossRef]
- Zakkar, M.; Chaudhury, H.; Sandvik, G.; Enesa, K.; Luong, L.A.; Cuhlmann, S.; Mason, J.C.; Krams, R.; Clark, A.R.; Haskard, D.O.; et al. Increased endothelial mitogen-activated protein kinase phosphatase-1 expression suppresses proinflammatory activation at sites that are resistant to atherosclerosis. Circ. Res. 2008, 103, 726–732. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Anderson, C.M.; Zhang, B.; Miller, M.; Butko, E.; Wu, X.; Laver, T.; Kernag, C.; Kim, J.; Luo, Y.; Lamparski, H.; et al. Fully Automated RNAscope In Situ Hybridization Assays for Formalin-Fixed Paraffin-Embedded Cells and Tissues. J. Cell Biochem. 2016, 117, 2201–2208. [Google Scholar] [CrossRef] [PubMed]
- McQueen, L.W.; Ladak, S.S.; Layton, G.R.; Wozniak, M.; Solomon, C.; El-Dean, Z.; Murphy, G.J.; Zakkar, M. Spatial Transcriptomic Profiling of Human Saphenous Vein Exposed to Ex Vivo Arterial Haemodynamics-Implications for Coronary Artery Bypass Graft Patency and Vein Graft Disease. Int. J. Mol. Sci. 2024, 25, 10368. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.10xgenomics.com/ (accessed on 1 February 2025).
- Stuart, T.; Butler, A.; Hoffman, P.; Hafemeister, C.; Papalexi, E.; Mauck, W.M., III; Hao, Y.; Stoeckius, M.; Smibert, P.; Satija, R. Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902.e21. [Google Scholar] [CrossRef] [PubMed]
- Crowell, H.L.; Soneson, C.; Germain, P.-L.; Calini, D.; Collin, L.; Raposo, C.; Malhotra, D.; Robinson, M.D. muscat detects subpopulation-specific state transitions from multi-sample multi-condition single-cell transcriptomics data. Nat. Commun. 2020, 11, 6077. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2021, 50, D687–D692. [Google Scholar] [CrossRef]
- Pico, A.R.; Kelder, T.; van Iersel, M.P.; Hanspers, K.; Conklin, B.R.; Evelo, C. WikiPathways: Pathway Editing for the People. PLoS Biol. 2008, 6, e184. [Google Scholar] [CrossRef]
- Carlin, D.E.; Demchak, B.; Pratt, D.; Sage, E.; Ideker, T. Network propagation in the cytoscape cyberinfrastructure. PLoS Comput. Biol. 2017, 13, e1005598. [Google Scholar] [CrossRef]
- Sapitro, J.; Dunmire, J.J.; Scott, S.E.; Sutariya, V.; Geldenhuys, W.J.; Hewit, M.; Yue, B.Y.; Nakamura, H. Suppression of transforming growth factor-β effects in rabbit subconjunctival fibroblasts by activin receptor-like kinase 5 inhibitor. Mol. Vis. 2010, 16, 1880–1892. [Google Scholar]
- Zheng, S.; Hedl, M.; Abraham, C. Twist1 and Twist2 Contribute to Cytokine Downregulation following Chronic NOD2 Stimulation of Human Macrophages through the Coordinated Regulation of Transcriptional Repressors and Activators. J. Immunol. 2015, 195, 217–226. [Google Scholar] [CrossRef]
- Zhou, Y.; Kato, H.; Asanoma, K.; Kondo, H.; Arima, T.; Kato, K.; Matsuda, T.; Wake, N. Identification of FOXC1 as a TGF-beta1 responsive gene and its involvement in negative regulation of cell growth. Genomics 2002, 80, 465–472. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Ding, S.M.; Zhou, L.; Xie, H.Y.; Chen, K.J.; Zhang, W.; Xing, C.Y.; Guo, H.J.; Zheng, S.S. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int. J. Biol. Sci. 2012, 8, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Ruben, S.M.; Klement, J.F.; Coleman, T.A.; Maher, M.; Chen, C.H.; Rosen, C.A. I-Rel: A novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992, 6, 745–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conway, D.E.; Sakurai, Y.; Weiss, D.; Vega, J.D.; Taylor, W.R.; Jo, H.; Eskin, S.G.; Marcus, C.B.; McIntire, L.V. Expression of CYP1A1 and CYP1B1 in human endothelial cells: Regulation by fluid shear stress. Cardiovasc. Res. 2009, 81, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Sahan-Firat, S.; Jennings, B.L.; Yaghini, F.A.; Song, C.Y.; Estes, A.M.; Fang, X.R.; Farjana, N.; Khan, A.I.; Malik, K.U. 2,3′,4,5′-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: Contribution of cytochrome P-450 1B1. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1891–H1901. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, Q.; Gong, Q.; Li, A.; Sun, M.; Jiang, S.; Jin, Y.; Zhang, Z.; He, J.; Zhuang, L. Bioinformatics and Experimental Analyses Reveal NFIC as an Upstream Transcriptional Regulator for Ischemic Cardiomyopathy. Genes 2022, 13, 1051. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Yang, G.; Lan, Y.; Han, J.; Wang, J.; Yin, D.; Song, R.; Zheng, T.; Zhang, S.; et al. Tetraspanin 1 promotes epithelial-to-mesenchymal transition and metastasis of cholangiocarcinoma via PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 300. [Google Scholar] [CrossRef]
- Chen, F.; Kook, H.; Milewski, R.; Gitler, A.D.; Lu, M.M.; Li, J.; Nazarian, R.; Schnepp, R.; Jen, K.; Biben, C.; et al. Hop is an unusual homeobox gene that modulates cardiac development. Cell 2002, 110, 713–723. [Google Scholar] [CrossRef]
- van Meeteren, L.A.; ten Dijke, P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012, 347, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Derynck, R. TGF-beta signaling in cancer—A double-edged sword. Trends Cell Biol. 2001, 11, S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L.; Attisano, L.; Wieser, R.; Ventura, F.; Massagué, J. Mechanism of activation of the TGF-beta receptor. Nature 1994, 370, 341–347. [Google Scholar] [CrossRef]
- Medici, D.; Kalluri, R. Endothelial-mesenchymal transition and its contribution to the emergence of stem cell phenotype. Semin. Cancer Biol. 2012, 22, 379–384. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef]
- Madri, J.A.; Reidy, M.A.; Kocher, O.; Bell, L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab. Investig. 1989, 60, 755–765. [Google Scholar]
- Nabel, E.G.; Shum, L.; Pompili, V.J.; Yang, Z.Y.; San, H.; Shu, H.B.; Liptay, S.; Gold, L.; Gordon, D.; Derynck, R.; et al. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc. Natl. Acad. Sci. USA 1993, 90, 10759–10763. [Google Scholar] [CrossRef]
- Kanzaki, T.; Tamura, K.; Takahashi, K.; Saito, Y.; Akikusa, B.; Oohashi, H.; Kasayuki, N.; Ueda, M.; Morisaki, N. In vivo effect of TGF- beta 1. Enhanced intimal thickening by administration of TGF- beta 1 in rabbit arteries injured with a balloon catheter. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.G.; Rasmussen, L.M.; Ruoslahti, E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J. Clin. Investig. 1994, 93, 1172–1178. [Google Scholar] [CrossRef]
- Majesky, M.W.; Lindner, V.; Twardzik, D.R.; Schwartz, S.M.; Reidy, M.A. Production of transforming growth factor beta 1 during repair of arterial injury. J. Clin. Investig. 1991, 88, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.; Gunn, J.; Francis, S.E.; Holt, C.M.; Arnold, N.D.; Cumberland, D.C.; Ferguson, M.W.; Crossman, D.C. TGFbeta is active, and correlates with activators of TGFbeta, following porcine coronary angioplasty. Cardiovasc. Res. 2001, 50, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.R.; Stark, V.K.; Turnipseed, W.D. The temporal relationship between the development of vein graft intimal hyperplasia and growth factor gene expression. J. Vasc. Surg. 1995, 22, 51–58. [Google Scholar] [CrossRef]
- Low, E.L.; Baker, A.H.; Bradshaw, A.C. TGFβ, smooth muscle cells and coronary artery disease: A review. Cell. Signal. 2019, 53, 90–101. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Savage, M.; LaBlanche, J.M.; Grip, L.; Serruys, P.W.; Fitzgerald, P.; Fischman, D.; Goldberg, S.; Brinker, J.A.; Zeiher, A.M.; et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2002, 106, 1243–1250. [Google Scholar] [CrossRef]
- Wen, F.Q.; Kohyama, T.; Sköld, C.M.; Zhu, Y.K.; Liu, X.; Romberger, D.J.; Stoner, J.; Rennard, S.I. Glucocorticoids modulate TGF-beta production. Inflammation 2002, 26, 279–290. [Google Scholar] [CrossRef]
- Song, C.Z.; Tian, X.; Gelehrter, T.D. Glucocorticoid receptor inhibits transforming growth factor-beta signaling by directly targeting the transcriptional activation function of Smad3. Proc. Natl. Acad. Sci. USA 1999, 96, 11776–11781. [Google Scholar] [CrossRef]
- Luppen, C.A.; Chandler, R.L.; Noh, T.; Mortlock, D.P.; Frenkel, B. BMP-2 vs. BMP-4 expression and activity in glucocorticoid-arrested MC3T3-E1 osteoblasts: Smad signaling, not alkaline phosphatase activity, predicts rescue of mineralization. Growth Factors 2008, 26, 226–237. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladak, S.S.; McQueen, L.W.; Tomkova, K.; Layton, G.R.; Murphy, G.J.; Zakkar, M. Acute Shear Stress Induces TWIST-Mediated EndMT in Venous Endothelial Cells and Human Long Saphenous Veins. Cells 2025, 14, 1369. https://doi.org/10.3390/cells14171369
Ladak SS, McQueen LW, Tomkova K, Layton GR, Murphy GJ, Zakkar M. Acute Shear Stress Induces TWIST-Mediated EndMT in Venous Endothelial Cells and Human Long Saphenous Veins. Cells. 2025; 14(17):1369. https://doi.org/10.3390/cells14171369
Chicago/Turabian StyleLadak, Shameem S., Liam W. McQueen, Kristina Tomkova, Georgia R. Layton, Gavin J. Murphy, and Mustafa Zakkar. 2025. "Acute Shear Stress Induces TWIST-Mediated EndMT in Venous Endothelial Cells and Human Long Saphenous Veins" Cells 14, no. 17: 1369. https://doi.org/10.3390/cells14171369
APA StyleLadak, S. S., McQueen, L. W., Tomkova, K., Layton, G. R., Murphy, G. J., & Zakkar, M. (2025). Acute Shear Stress Induces TWIST-Mediated EndMT in Venous Endothelial Cells and Human Long Saphenous Veins. Cells, 14(17), 1369. https://doi.org/10.3390/cells14171369







