The Role of Tumor Microenvironment in Triple-Negative Breast Cancer and Its Therapeutic Targeting
Abstract
1. Introduction
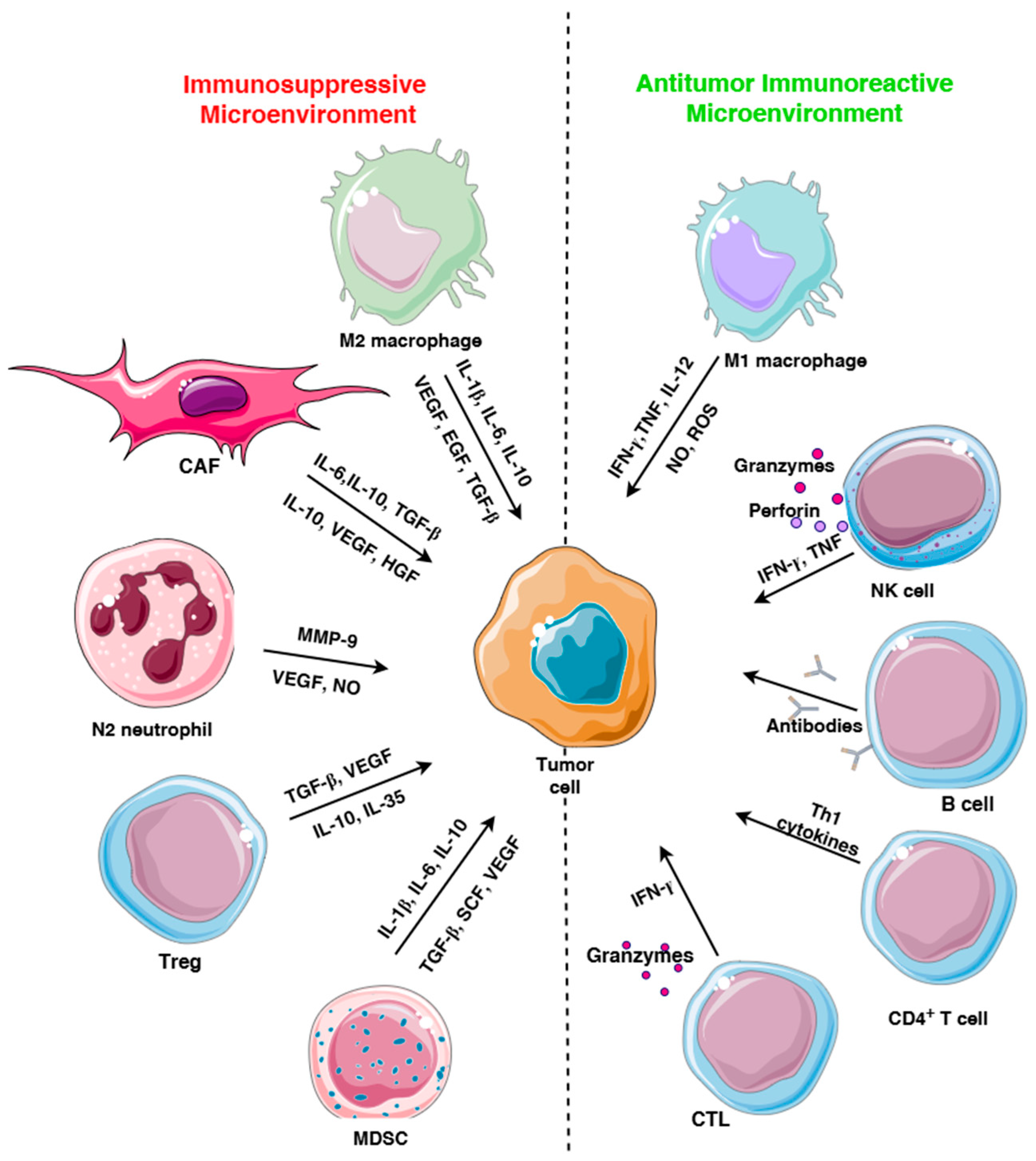
2. Immune Cells in TME
2.1. Immune Checkpoint Receptors
2.2. Suppressive Immune Cells in TME
2.2.1. Regulatory T Cells
2.2.2. Tumor-Associated Macrophages
2.2.3. MDSC
2.2.4. Tumor-Associated Neutrophils
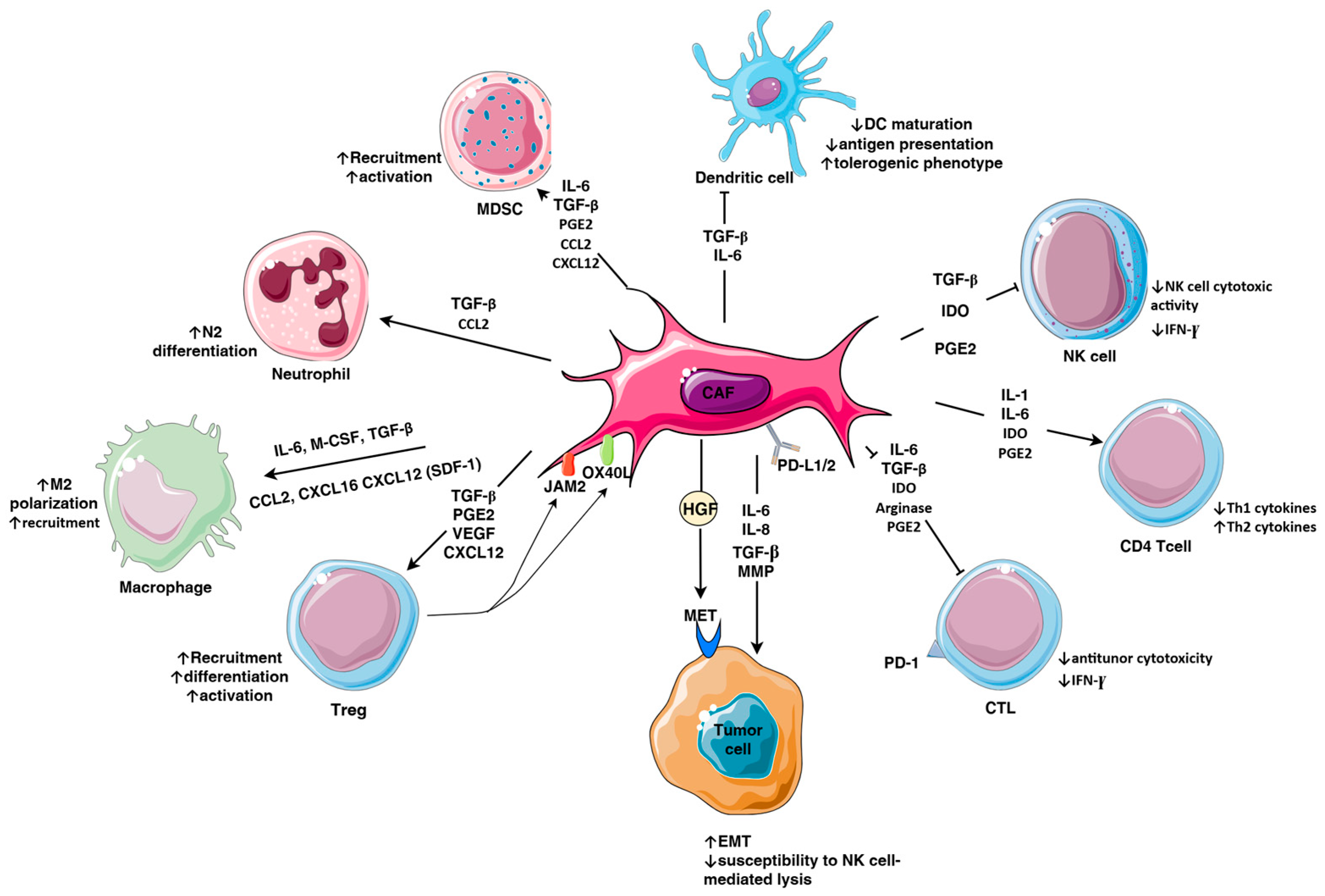
3. Cancer-Associated Fibroblasts
3.1. Subpopulations of Fibroblasts in TNBC
3.2. Immunosuppressive Factors Secreted by CAFs
3.3. ECM Remodeling
3.4. CAFs and Tumor Metabolism
4. Cancer-Associated Adipocytes
5. Therapies Targeting TME in TNBC
5.1. Targeting Cytokines, Enzymes, Metabolites, Chemokines, and Immune Cell Recruitment
5.1.1. Targeting IL-6
5.1.2. Targeting IL-8
5.1.3. CXCR4 Antagonist
5.1.4. IDO1 Inhibition
5.1.5. Targeting PGE2
5.1.6. PI3Kγ Inhibition
5.2. CAF-Directed Therapies
5.2.1. Reverting CAFs into Quiescent State
5.2.2. Targeting ECM
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furukawa, N.; Stearns, V.; Santa-Maria, C.A.; Popel, A.S. The tumor microenvironment and triple-negative breast cancer aggressiveness: Shedding light on mechanisms and targeting. Expert Opin. Ther. Targets 2022, 26, 1041–1056. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Shyr, Y.; Pietenpol, J.A.; Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanovi’c, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.W.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-NegativeBreast Cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Z.; Ma, D.; Suo, C.; Shi, J.; Xue, M.; Hu, X.; Xiao, Y.; Yu, K.-D.; Liu, Y.-R.; Yu, Y.; et al. Genomic and Transcriptomic Landscape of Triple-Negative Breast Cancers: Subtypes and Treatment Strategies. Cancer Cell 2019, 35, 428–440.e5. [Google Scholar] [CrossRef]
- Nedeljković, M.; Vuletić, A.; Mirjačić Martinović, K. Divide and Conquer-Targeted Therapy for Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 1396. [Google Scholar] [CrossRef]
- He, Y.; Jiang, Z.; Chen, C.; Wang, X. Classification of Triple-Negative Breast Cancers Based on Immunogenomic Profiling. J. Exp. Clin. Cancer Res. 2018, 37, 327. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Caillat-Zucman, S. Control of NK Cell Activation by Immune Checkpoint Molecules. Int. J. Mol. Sci. 2017, 18, 2129. [Google Scholar] [CrossRef]
- Kwa, M.J.; Adams, S. Checkpoint Inhibitors in Triple-negative Breast Cancer (TNBC): Where to Go from Here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, F.; Liu, Z.; Fan, Z. Immunotherapy for Triple-Negative Breast Cancer: Combination Strategies to Improve Outcome. Cancers 2023, 15, 321. [Google Scholar] [CrossRef]
- Chung, W.; Eum, H.H.; Lee, H.-O.; Lee, K.-M.; Lee, H.-B.; Kim, K.-T.; Ryu, H.S.; Kim, S.; Lee, J.E.; Park, Y.H.; et al. Single-Cell RNA-Seq Enables Comprehensive Tumour and Immune Cell Profiling in Primary Breast Cancer. Nat. Commun. 2017, 8, 15081. [Google Scholar] [CrossRef]
- Coleman, C.; Selvakumar, T.; Thurlapati, A.; Graf, K.; Pavuluri, S.; Mehrotra, S.; Sahin, O.; Sivapiragasam, A. Harnessing Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Opportunities and Barriers to Clinical Integration. Int. J. Mol. Sci. 2025, 26, 4292. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers From Two Phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Mathieu, M.C.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 2015, 26, 1698–1704. [Google Scholar] [CrossRef]
- Solinas, C.; Carbognin, L.; De Silva, P.; Criscitiello, C.; Lambertini, M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast 2017, 35, 142–150. [Google Scholar] [CrossRef]
- Gao, Z.H.; Li, C.X.; Liu, M.; Jiang, J.Y. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: A meta-analysis. BMC Cancer 2020, 20, 1150. [Google Scholar] [CrossRef]
- Vihervuori, H.; Autere, T.A.; Repo, H.; Kurki, S.; Kallio, L.; Lintunen, M.M.; Talvinen, K.; Kronqvist, P. Tumor-infiltrating lymphocytes and CD8+ T cells predict survival of triple-negative breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 3105–3114. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Tsvetkova, V.; Orvieto, E.; Piacentini, F.; Ficarra, G.; Griguolo, G.; Miglietta, F.; Giarratano, T.; Omarini, C.; Bonaguro, S.; et al. Immune characterization of breast cancer metastases: Prognostic implications. Breast Cancer Res. 2018, 20, 62. [Google Scholar] [CrossRef]
- Ciarka, A.; Piątek, M.; Pęksa, R.; Kunc, M.; Senkus, E. Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Prognostic and Predictive Significance Across Molecular Subtypes. Biomedicines 2024, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Ding, J.; Chen, Y. Role of CD8+ T lymphocyte cells: Interplay with stromal cells in tumor microenvironment. Acta Pharm. Sin. B 2021, 11, 1365–1378. [Google Scholar] [CrossRef]
- Schmitz, J.; Assenmacher, M.; Radbruch, A. Regulation of T helper cell cytokine expression: Functional dichotomy of antigen-presenting cells. Eur. J. Immunol. 1993, 23, 191–199. [Google Scholar] [CrossRef]
- Karpisheh, V.; Ahmadi, M.; Abbaszadeh-Goudarzi, K.; Mohammadpour Saray, M.; Barshidi, A.; Mohammadi, H.; Yousefi, M.; Jadidi-Niaragh, F. The role of Th17 cells in the pathogenesis and treatment of breast cancer. Cancer Cell Int. 2022, 22, 108. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Lan, Y.; Wan, Y.; Wang, Q.; Wang, C.; Xu, L.; Chen, Y.; Liu, W.; Zhang, X.; Li, Y.; et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19+ B lymphocytes and T cells in Invasive breast cancer. Oncoimmunology 2015, 5, e1075112. [Google Scholar] [CrossRef]
- Miligy, I.; Mohan, P.; Gaber, A.; Aleskandarany, M.A.; Nolan, C.C.; Diez-Rodriguez, M.; Mukherjee, A.; Chapman, C.; Ellis, I.O.; Green, A.R.; et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 2017, 71, 258–268. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, F.; Ai, L.; Mu, S.; Li, Y.; Yang, C.; Hu, Y. Tumor-infiltrating B cells as a favorable prognostic biomarker in breast cancer: A systematic review and meta-analysis. Cancer Cell Int. 2021, 21, 310. [Google Scholar] [CrossRef]
- Toney, N.J.; Opdenaker, L.M.; Frerichs, L.; Modarai, S.R.; Ma, A.; Archinal, H.; Ajayi, G.O.; Sims-Mourtada, J. B cells enhance IL-1 beta driven invasiveness in triple negative breast cancer. Sci. Rep. 2025, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Toney, N.J.; Opdenaker, L.M.; Cicek, K.; Frerichs, L.; Kennington, C.R.; Oberly, S.; Archinal, H.; Somasundaram, R.; Sims-Mourtada, J. Tumor-B-cell interactions promote isotype switching to an immunosuppressive IgG4 antibody response through upregulation of IL-10 in triple negative breast cancers. J. Transl. Med. 2022, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, E.; Sakakibara, M.; Sakakibara, J.; Masuda, T.; Fujimoto, H.; Hayama, S.; Nagashima, T.; Sangai, T.; Nakagawa, A.; Nakatani, Y.; et al. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer 2019, 26, 180–189. [Google Scholar] [CrossRef]
- Cooper, M.A.; Colonna, M.; Yokoyama, W.M. Hidden talents of natural killers: NK cells in innate and adaptive immunity. EMBO Rep. 2009, 10, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.S.; Purdy, A.K. Structure/function of human killer cell immunoglobulin-like receptors: Lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 2011, 132, 315–325. [Google Scholar] [CrossRef]
- Konjevic, G.; Jurisic, V.; Jovic, V.; Vuletic, A.; Mirjacic Martinovic, K.; Radenkovic, S.; Spuzic, I. Investigation of NK cell function and their modulation in different malignancies. Immunol. Res. 2012, 52, 139–156. [Google Scholar] [CrossRef]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef]
- Mamessier, E.; Sylvain, A.; Thibult, M.L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- Batlle, E.; Massagu’e, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.; Vuletić, A.; Mirjačić Martinović, K. Natural killer cell receptors: Alterations and therapeutic targeting in malignancies. Immunol. Res. 2016, 64, 25–35. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The Role of Cytokines in the Regulation of NK Cells in the Tumor Environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Chitadze, G.; Bhat, J.; Lettau, M.; Janssen, O.; Kabelitz, D. Generation of soluble NKG2D ligands: Proteolytic cleavage, exosome secretion and functional implications. Scand. J. Immunol. 2013, 78, 120–129. [Google Scholar] [CrossRef]
- Hofman, T.; Ng, S.W.; Garcés-Lázaro, I.; Heigwer, F.; Boutros, M.; Cerwenka, A. IFNγ mediates the resistance of tumor cells to distinct NK cell subsets. J. Immunother. Cancer 2024, 12, e009410. [Google Scholar] [CrossRef]
- Ben-Shmuel, A.; Gruper, Y.; Halperin, C.; Levi-Galibov, O.; Rosenberg-Fogler, H.; Barki, D.; Carradori, G.; Stein, Y.; Yagel, G.; Naumova, M.; et al. Cancer-associated fibroblasts serve as decoys to suppress NK cell anti-cancer cytotoxicity in breast cancer. Cancer Discov. 2025, 15, 1247–1269. [Google Scholar] [CrossRef]
- Stojanovic, A.; Correia, M.P.; Cerwenka, A. Shaping of NK cell responses by the tumor microenvironment. Cancer Microenviron. 2013, 6, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Balzasch, B.M.; Cerwenka, A. Microenvironmental signals shaping NK-cell reactivity in cancer. Eur. J. Immunol. 2023, 53, e2250103. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Si, F.; Huang, L.; Zhao, Y.; Zhang, C.; Hoft, D.F.; Peng, G. NK and NKT cells have distinct properties and functions in cancer. Oncogene 2021, 40, 4521–4537. [Google Scholar] [CrossRef] [PubMed]
- Thacker, G.; Henry, S.; Nandi, A.; Debnath, R.; Singh, S.; Nayak, A.; Susnik, B.; Boone, M.M.; Zhang, Q.; Kesmodel, S.B.; et al. Immature natural killer cells promote progression of triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eabl4414. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Ding, F.; Lu, J.; Huang, T.; Zhong, G.; Zhu, P.; Ma, Y.; Li, J.; Wang, X.; et al. Phenotypes and cytokines of NK cells in triple-negative breast cancer resistant to checkpoint blockade immunotherapy. Breast Cancer Res. 2025, 27, 51. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Philips, A.V.; Meric-Bernstam, F.; Qiao, N.; Wu, Y.; Harrington, S.; Su, X.; Wang, Y.; Gonzalez-Angulo, A.M.; Akcakanat, A.; et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Chen, L.; Gibbons, D.L.; Goswami, S.; Cortez, M.A.; Ahn, Y.H.; Byers, L.A.; Zhang, X.; Yi, X.; Dwyer, D.; Lin, W.; et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 2014, 5, 5241. [Google Scholar] [CrossRef]
- Castagnoli, L.; Cancila, V.; Cordoba-Romero, S.L.; Faraci, S.; Talarico, G.; Belmonte, B.; Iorio, M.V.; Milani, M.; Volpari, T.; Chiodoni, C.; et al. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene 2019, 38, 4047–4060. [Google Scholar] [CrossRef]
- Maeda, T.; Baram, T.; Oren, N.; Erlichman, N.; Meshel, T.; Ben-Baruch, A. Inflammation-Driven Regulation of PD-L1 and PD-L2, and Their Cross-Interactions with Protective Soluble TNFα Receptors in Human Triple-Negative Breast Cancer. Cancers 2022, 14, 3513. [Google Scholar] [CrossRef]
- Ju, X.; Zhang, H.; Zhou, Z.; Chen, M.; Wang, Q. Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-a signaling. Exp. Cell Res. 2020, 396, 112315. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J., Jr.; Yokota, S.J.; Bankert, R.B. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J. Immunol. 2007, 178, 5552–5562. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yao, H.; Li, C.; Fang, J.Y.; Xu, J. Regulation of PD-L1: Emerging routes for targeting tumor immune evasion. Front. Pharmacol. 2018, 9, 536. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Saeki, H.; Nakashima, Y.; Ito, S.; Oki, E.; Morita, M.; Oda, Y.; Okano, S.; Maehara, Y. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci. 2017, 108, 1119–1127. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; Xue, J.; Qi, M.; Liu, X.; Huang, Y.; Hu, J.; Dong, H.; Ling, K. PD-L1 tumor-intrinsic signaling and its therapeutic implication in triple-negative breast cancer. JCI Insight 2021, 6, e131458. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Vasudevaraju, P.; Vempati, R.K.; Rakshmitha, M.; Merchant, N.; Nagaraju, G.P. Regulatory T cells: Their role in triple-negative breast cancer progression and metastasis. Cancer 2022, 128, 1171–1183. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, X.; Zheng, M.; Yu, Y.; Jin, G.; Zhang, S. Regulatory T cells are associated with the tumor immune microenvironment and immunotherapy response in triple-negative breast cancer. Front. Immunol. 2023, 14, 1263537. [Google Scholar] [CrossRef] [PubMed]
- Nunez, N.G.; Tosello Boari, J.; Ramos, R.N.; Richer, W.; Cagnard, N.; Anderfuhren, C.D.; Niborski, L.L.; Bigot, J.; Meseure, D.; De La Rochere, P.; et al. Tumor invasion in draining lymph nodes is associated with Treg accumulation in breast cancer patients. Nat. Commun. 2020, 11, 3272. [Google Scholar] [CrossRef]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef]
- Cai, J.; Wang, D.; Zhang, G.; Guo, X. The Role Of PD-1/PD-L1 Axis In Treg Development And Function: Implications For Cancer Immunotherapy. OncoTargets Ther. 2019, 12, 8437–8445. [Google Scholar] [CrossRef]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical relevance of host immunity in breast cancer: From TILs to the clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef]
- Papaioannou, E.; Sakellakis, M.; Melachrinou, M.; Tzoracoleftherakis, E.; Kalofonos, H.; Kourea, E. A Standardized Evaluation Method for FOXP3+ Tregs and CD8+ T-cells in Breast Carcinoma: Association with Breast Carcinoma Subtypes, Stage and Prognosis. Anticancer Res. 2019, 39, 1217–1232. [Google Scholar] [CrossRef]
- Stenström, J.; Hedenfalk, I.; Hagerling, C. Regulatory T lymphocyte infiltration in metastatic breast cancer-an independent prognostic factor that changes with tumor progression. Breast Cancer Res. 2021, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, L.; Yuan, L.; Duan, W.; Zhao, W.; Wang, S.; Zhang, Q. A prognostic risk model for patients with triple negative breast cancer based on stromal natural killer cells, tumor-associated macrophages and growth-arrest specific protein 6. Cancer Sci. 2016, 107, 882–889. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Zu, X. Tumor-associated macrophages: An important player in breast cancer progression. Thorac Cancer 2022, 13, 269–276, Erratum in Thorac Cancer 2022, 13, 3437. [Google Scholar] [CrossRef]
- Ma, H.; Gao, L.; Chang, R.; Zhai, L.; Zhao, Y. Crosstalk between macrophages and immunometabolism and their potential roles in tissue repair and regeneration. Heliyon 2024, 10, e38018. [Google Scholar] [CrossRef]
- Holbert, C.E.; Casero, R.A., Jr.; Stewart, T.M. Polyamines: The pivotal amines in influencing the tumor microenvironment. Discov. Oncol. 2024, 15, 173. [Google Scholar] [CrossRef]
- de Boniface, J.; Mao, Y.; Schmidt-Mende, J.; Kiessling, R.; Poschke, I. Expression patterns of the immunomodulatory enzyme arginase 1 in blood, lymph nodes and tumor tissue of early-stage breast cancer patients. Oncoimmunology 2012, 1, 1305–1312. [Google Scholar] [CrossRef]
- Linde, N.; Casanova-Acebes, M.; Sosa, M.S.; Mortha, A.; Rahman, A.; Farias, E.; Harper, K.; Tardio, E.; Reyes Torres, I.; Jones, J.; et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat. Commun. 2018, 9, 21. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, X.H.; Gao, S.T.; Chen, C.; Xu, X.Y.; Sun, Q.; Zhou, Z.H.; Wu, G.Z.; Yu, Q.; Xu, G.; et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J. Surg. Res. 2018, 222, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Groth, C.; Hu, X.; Weer, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vetsika, E.K.; Koukos, A.; Kotsakis, A. Myeloid-Derived Suppressor Cells: Major Figures that Shape the Immunosuppressive and Angiogenic Network in Cancer. Cells 2019, 8, 1647. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C.A. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef]
- Kumar, S.; Wilkes, D.W.; Samuel, N.; Blanco, M.A.; Nayak, A.; Alicea-Torres, K.; Gluck, C.; Sinha, S.; Gabrilovich, D.; Chakrabarti, R. ΔNp63-driven recruitment of myeloid-derived suppressor cells promotes metastasis in triple-negative breast cancer. J. Clin. Investig. 2018, 128, 5095–5109. [Google Scholar] [CrossRef]
- Zheng, H.; Siddharth, S.; Parida, S.; Wu, X.; Sharma, D. Tumor Microenvironment: Key Players in Triple Negative Breast Cancer Immunomodulation. Cancers 2021, 13, 3357. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xu, X.; Wu, M.; Xue, L.; Zhu, J.; Xia, H.; Ding, S.; Fu, S.; Wang, X.; Wang, Y.; et al. Neutrophils in triple-negative breast cancer: An underestimated player with increasingly recognized importance. Breast Cancer Res. 2023, 25, 88. [Google Scholar] [CrossRef]
- Ganguly, D.; Chandra, R.; Karalis, J.; Teke, M.; Aguilera, T.; Maddipati, R.; Wachsmann, M.B.; Ghersi, D.; Siravegna, G.; Zeh, H.J., 3rd; et al. Cancer-Associated Fibroblasts: Versatile Players in the Tumor Microenvironment. Cancers 2020, 12, 2652. [Google Scholar] [CrossRef]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Imparato, V.; Masone, S.; Masullo, M.; Nasso, R.; Montagnani, S.; Arcucci, A. Involvement of Breast Cancer-Associated Fibroblasts in Tumor Development, Therapy Resistance and Evaluation of Potential Therapeutic Strategies. Curr. Med. Chem. 2018, 25, 3414–3434. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Dzobo, K.; Vogelsang, M.; Thomford, N.E.; Dandara, C.; Kallmeyer, K.; Pepper, M.S.; Parker, M.I. Wharton’s jelly-derived mesenchymal stromal cells and fibroblast-derived extracellular matrix synergistically activate apoptosis in a p21-dependent mechanism in WHCO1 and MDA MB 231 cancer cells in vitro. Stem Cells Int. 2016, 2016, 4842134. [Google Scholar] [CrossRef]
- Hosaka, K.; Yang, Y.; Seki, T.; Fischer, C.; Dubey, O.; Fredlund, E.; Hartman, J.; Religa, P.; Morikawa, H.; Ishii, Y.; et al. Pericyte–fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, E5618–E5627. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuede, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Weber, C.E.; Kothari, A.N.; Wai, P.Y.; Li, N.Y.; Driver, J.; Zapf, M.A.C.; Franzen, C.A.; Gupta, G.N.; Osipo, C.; Zlobin, A.; et al. Osteopontin mediates an MZF1-TGF-beta 1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene 2015, 34, 4821–4833. [Google Scholar] [CrossRef]
- Sarkar, T.R.; Nguyen, T.; Gundre, E.; Ogunlusi, O.; El-Sobky, M.; Giri, B.; Sarkar, T.R. Cancer-associated fibroblasts: The chief architect in the tumor microenvironment. Front. Cell Dev. Biol. 2023, 11, 1089068. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Sharon, Y.; Raz, Y.; Cohen, N.; Ben-Shmuel, A.; Schwartz, H.; Geiger, T.; Erez, N. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015, 75, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Athavale, D.; Balch, C.; Zhang, Y.; Yao, X.; Song, S. The role of Hippo/YAP1 in cancer-associated fibroblasts: Literature review and future perspectives. Cancer Lett. 2024, 604, 217244. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018, 33, 463. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, M.; Zou, X.; Chen, Z.; Shen, L.; Hu, J.; Kong, M.; Huang, J.; Ni, C.; Xia, W. Fibroblast Activation Protein (FAP)+ cancer-associated fibroblasts induce macrophage M2-like polarization via the Fibronectin 1-Integrin α5β1 axis in breast cancer. Oncogene 2025, 44, 2396–2412. [Google Scholar] [CrossRef]
- Lobba, A.R.M.; Carreira, A.C.O.; Cerqueira, O.L.D.; Fujita, A.; DeOcesano-Pereira, C.; Osorio, C.A.B.; Soares, F.A.; Rameshwar, P.; Sogayar, M.C. High CD90 (THY-1) expression positively correlates with cell transformation and worse prognosis in basal-like breast cancer tumors. PLoS ONE 2018, 13, e0199254. [Google Scholar] [CrossRef]
- Primac, I.; Maquoi, E.; Blacher, S.; Heljasvaara, R.; Van Deun, J.; Smeland, H.Y.H.; Canale, A.; Louis, T.; Stuhr, L.; Sounni, N.E.; et al. Stromal integrin alpha 11 regulates PDGFR beta signaling and promotes breast cancer progression. J. Clin. Investig. 2019, 129, 4509–4528. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Hansen, B.; Ornås, D.; Grigorian, M.; Klingelhöfer, J.; Tulchinsky, E.; Lukanidin, E.; Ambartsumian, N. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene 2004, 23, 5487–5495. [Google Scholar] [CrossRef]
- Lee, W.Y.; Su, W.C.; Lin, P.W.; Guo, H.R.; Chang, T.W.; Chen, H.H. Expression of S100A4 and Met: Potential predictors for metastasis and survival in early-stage breast cancer. Oncology 2004, 66, 429–438. [Google Scholar] [CrossRef]
- Rudland, P.S.; Platt-Higgins, A.; Renshaw, C.; West, C.R.; Winstanley, J.H.; Robertson, L.; Barraclough, R. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000, 60, 1595–1603. [Google Scholar] [PubMed]
- Kamarudin, N.A.; Abd Shukor, N.; Farouk, W.I.; Hanapi, N.A.M.; Mohammed, F. Stromal expression of CD10 in invasive breast carcinoma and its association with tumour stage, grade, ER, PR and HER2 status. Malay. J. Pathol. 2021, 43, 389–396. [Google Scholar]
- Su, S.C.; Chen, J.N.; Yao, H.R.; Liu, J.; Yu, S.B.; Lao, L.Y.; Wang, M.H.; Luo, M.L.; Xing, Y.; Chen, F.; et al. CD10(+) GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018, 172, 841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, T.; Yuan, Y.; Zhu, Y. What is new in cancer-associated fibroblast biomarkers? Cell Commun. Signal 2023, 21, 96. [Google Scholar] [CrossRef]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675. [Google Scholar] [CrossRef]
- Sloan, E.K.; Ciocca, D.R.; Pouliot, N.; Natoli, A.; Restall, C.; Henderson, M.A.; Fanelli, M.A.; Cuello-Carrión, F.D.; Gago, F.E.; Anderson, R.L. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am. J. Pathol. 2009, 174, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally disinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Wu, S.Z.; Al-Eryani, G.; Roden, D.L.; Junankar, S.; Harvey, K.; Andersson, A.; Thennavan, A.; Wang, C.; Torpy, J.R.; Bartonicek, N.; et al. A single-cell and spatially resolved atlas of human breast cancers. Nat. Genet. 2021, 53, 1334–1347. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Pelon, F.; Bourachot, B.; Kieffer, Y.; Magagna, I.; Mermet-Meillon, F.; Bonnet, I.; Costa, A.; Givel, A.M.; Attieh, Y.; Barbazan, J.; et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020, 11, 404. [Google Scholar] [CrossRef]
- Friedman, G.; Levi-Galibov, O.; David, E.; Bornstein, C.; Giladi, A.; Dadiani, M.; Mayo, A.; Halperin, C.; Pevsner-Fischer, M.; Lavon, H.; et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4+ and PDPN+ CAFs to clinical outcome. Nat. Cancer 2020, 1, 692–708. [Google Scholar] [CrossRef] [PubMed]
- Vuletić, A.; Mirjačić Martinović, K.; Tišma Miletić, N.; Zoidakis, J.; Castellvi-Bel, S.; Čavić, M. Cross-Talk Between Tumor Cells Undergoing Epithelial to Mesenchymal Transition and Natural Killer Cells in Tumor Microenvironment in Colorectal Cancer. Front. Cell Dev. Biol. 2021, 9, 750022. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeong, J.; Lee, D.S.; Hyeon, D.Y.; Park, G.W.; Jeon, S.; Lee, K.B.; Jang, J.Y.; Hwang, D.; Kim, H.M.; et al. PD-L1-directed PlGF/VEGF blockade synergizes with chemotherapy by targeting CD141+ cancer-associated fibroblasts in pancreatic cancer. Nat. Commun. 2022, 13, 6292. [Google Scholar] [CrossRef]
- Jia, H.; Chen, X.; Zhang, L.; Chen, M. Cancer associated fibroblasts in cancer development and therapy. J. Hematol. Oncol. 2025, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wu, Y.; Li, J.; Liu, J.; Yi, Z.; Wang, X.; Sun, J.; Li, L.; Wu, Q.; Chen, Y.; et al. Insulin-like growth factor 2 drives fibroblast-mediated tumor immunoevasion and confers resistance to immunotherapy. J. Clin. Investig. 2024, 134, e183366. [Google Scholar] [CrossRef]
- Cheng, J.T.; Deng, Y.N.; Yi, H.M.; Wang, G.Y.; Fu, B.S.; Chen, W.J.; Liu, W.; Tai, Y.; Peng, Y.W.; Zhang, Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5, e198. [Google Scholar] [CrossRef]
- Ielpo, S.; Barberini, F.; Dabbagh Moghaddam, F.; Pesce, S.; Cencioni, C.; Spallotta, F.; De Ninno, A.; Businaro, L.; Marcenaro, E.; Bei, R.; et al. Crosstalk and communication of cancer-associated fibroblasts with natural killer and dendritic cells: New frontiers and unveiled opportunities for cancer immunotherapy. Cancer Treat. Rev. 2024, 131, 102843. [Google Scholar] [CrossRef]
- Gok Yavuz, B.; Gunaydin, G.; Gedik, M.E.; Kosemehmetoglu, K.; Karakoc, D.; Ozgur, F.; Guc, D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs. Sci. Rep. 2019, 9, 3172. [Google Scholar] [CrossRef]
- Allaoui, R.; Bergenfelz, C.; Mohlin, S.; Hagerling, C.; Salari, K.; Werb, Z.; Anderson, R.L.; Ethier, S.P.; Jirström, K.; Påhlman, S.; et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat. Commun. 2016, 7, 13050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.H.; Zhao, Y.X.; Chen, C.; Xu, X.Y.; Sun, Q.; Wu, H.Y.; Chen, M.; Sang, J.F.; Su, L.; et al. Cancer-Associated Fibroblasts Correlate with Tumor-Associated Macrophages Infiltration and Lymphatic Metastasis in Triple Negative Breast Cancer Patients. J. Cancer 2018, 9, 4635–4641. [Google Scholar] [CrossRef] [PubMed]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Du, R.; Zhang, X.; Lu, X.; Ma, X.; Guo, X.; Shi, C.; Ren, X.; Ma, X.; He, Y.-; Gao, Y.; et al. PDPN positive CAFs contribute to HER2 positive breast cancer resistance to trastuzumab by inhibiting antibody-dependent NK cell-mediated cytotoxicity. Drug Resist. Updates 2023, 68, 100947. [Google Scholar] [CrossRef]
- Lu, G.; Qiu, Y.; Su, X. Targeting CXCL12-CXCR4 Signaling Enhances Immune Checkpoint Blockade Therapy Against TripleNegative Breast Cancer. Eur. J. Pharm. Sci. 2021, 157, 105606. [Google Scholar] [CrossRef]
- Soto-Perez-de-Celis, E.; Chavarri-Guerra, Y.; Leon-Rodriguez, E.; Gamboa-Dominguez, A. Tumor-Associated Neutrophils in Breast Cancer Subtypes. Asian Pac. J. Cancer Prev. 2017, 18, 2689–2693. [Google Scholar] [CrossRef]
- Kwantwi, L.B.; Wang, S.; Zhang, W.; Peng, W.; Cai, Z.; Sheng, Y.; Xiao, H.; Wang, X.; Wu, Q. Tumor-associated neutrophils activated by tumor-derived CCL20 (C-C motif chemokine ligand 20) promote T cell immunosuppression via programmed death-ligand 1 (PD-L1) in breast cancer. Bioengineered 2021, 12, 6996–7006. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, Y.; Zhang, H.; Nan, F. Breast cancer stromal fibroblasts promote the generation of CD44+CD24-cells through SDF-1/CXCR4 interaction. J. Exp. Clin. Cancer Res. 2010, 29, 80. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promotetumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Breaking Barriers: The Promise and Challenges of Immune Checkpoint Inhibitors in Triple-Negative Breast Cancer. Biomedicines 2024, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Graveel, C.R.; DeGroot, J.D.; Su, Y.; Koeman, J.; Dykema, K.; Leung, S.; Snider, J.; Davies, S.R.; Swiatek, P.J.; Cottingham, S.; et al. Met induces diverse mammary carcinomas in mice and is associated with human basal breast cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 12909–12914. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Lamichhane, A.; Rafsanjani Nejad, P.; Heiss, J.; Baumann, H.; Gudneppanavar, R.; Leipzig, N.D.; Konopka, M.; Luker, G.D.; Tavana, H. Therapeutic Targeting of Stromal-Tumor HGF-MET Signaling in an Organotypic Triple-Negative Breast Tumor Model. Mol. Cancer Res. 2022, 20, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Robertson, C. The extracellular matrix in breast cancer predicts prognosis through composition, splicing, and crosslinking. Exp. Cell Res. 2016, 343, 73–81. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.X.; Wu, H.T.; Li, X.L.; Wen, X.F.; Du, C.W.; Zhang, G.J. Collagen1A1 (COL1A1) promotes metastasis of breast cancer and is a potential therapeutic target. Discov. Med. 2018, 25, 211–223. [Google Scholar]
- Kim, S.H.; Lee, H.Y.; Jung, S.P.; Kim, S.; Lee, J.E.; Nam, S.J.; Bae, J.W. Role of secreted type I collagen derived from stromal cells in two breast cancer cell lines. Oncol. Lett. 2014, 8, 507–512. [Google Scholar] [CrossRef]
- Sethi, A.; Mao, W.; Wordinger, R.J.; Clark, A.F. Transforming growth factor-beta induces extracellular matr.ix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5240–5250. [Google Scholar] [CrossRef]
- Heydari, S.; Tajik, F.; Safaei, S.; Kamani, F.; Karami, B.; Dorafshan, S.; Madjd, Z.; Ghods, R. The association between tumor-stromal collagen features and the clinical outcomes of patients with breast cancer: A systematic review. Breast Cancer Res. 2025, 27, 69. [Google Scholar] [CrossRef]
- Yao, E.S.; Zhang, H.; Chen, Y.Y.; Lee, B.; Chew, K.; Moore, D.; Park, C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007, 67, 659–664. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Lappano, R.; Santolla, M.F.; Marsico, S.; Caruso, A.; Maggiolini, M. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs). Breast Cancer Res. 2013, 15, R64. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.K.; Mohammad, K.S.; Fournier, P.G.; McKenna, C.R.; Davis, H.W.; Niewolna, M.; Peng, X.H.; Chirgwin, J.M.; Guise, T.A. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS ONE 2009, 4, e6896. [Google Scholar] [CrossRef] [PubMed]
- Al-Jomah, N.; Al-Mohanna, F.H.; Aboussekhra, A. Tocilizumab suppresses the pro-carcinogenic effects of breast cancer-associated fibroblasts through inhibition of the STAT3/AUF1 pathway. Carcinogenesis 2021, 42, 1439–1448. [Google Scholar] [CrossRef]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Whitaker-Menezes, D.; Dasgupta, A.; Philp, N.J.; Lin, Z.; Gandara, R.; Sneddon, S.F.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Using the “reverse Warburg e_ect” to identify high-risk breast cancer patients: Stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle 2012, 11, 1108–1117. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, L.; Baddour, J.; Achreja, A.; Bernard, V.; Moss, T.; Marini, J.C.; Tudawe, T.; Seviour, E.G.; San Lucas, F.A.; et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 2016, 5, e10250. [Google Scholar] [CrossRef]
- Fazzari, J.; Lin, H.; Murphy, C.; Ungard, R.; Singh, G. Inhibitors of glutamate release from breast cancer cells; new targets for cancer-induced bone-pain. Sci. Rep. 2015, 5, 8380. [Google Scholar] [CrossRef]
- Qin, W.; Chen, B.; Li, X.; Zhao, W.; Wang, L.; Zhang, N.; Wang, X.; Luo, D.; Liang, Y.; Li, Y.; et al. Cancer-associated fibroblasts secrete CSF3 to promote TNBC progression via enhancing PGM2L1-dependent glycolysis reprogramming. Cell Death Dis. 2025, 16, 249. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, H.; Li, J.; Zeng, N.; Zhang, Q.; Hou, K.; Li, J.; Yu, J.; Wu, Y. Dissecting the emerging role of cancer-associated adipocyte-derived cytokines in remodeling breast cancer progression. Heliyon 2024, 10, e35200. [Google Scholar] [CrossRef]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; Macdougald, O.A. Inhibition of Adipogenesis by Wnt Signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Wu, C.; Dong, S.; Huang, R.; Chen, X. Cancer-Associated Adipocytes and Breast Cancer: Intertwining in the Tumor Microenvironment and Challenges for Cancer Therapy. Cancers 2023, 15, 726. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Attané, C.; Milhas, D.; Dirat, B.; Dauvillier, S.; Guerard, A.; Gilhodes, J.; Lazar, I.; Alet, N.; Laurent, V.; et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2017, 2, e87489. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Zhang, Q.; Fares, H.M.; Wang, Y.; Han, Y.; Sun, L. Contribution of adipocytes in the tumor microenvironment to breast cancer metabolism. Cancer Lett. 2022, 534, 215616. [Google Scholar] [CrossRef]
- Juárez-Cruz, J.C.; Zuñiga-Eulogio, M.D.; Olea-Flores, M.; Castañeda-Saucedo, E.; Mendoza-Catalán, M.Á.; Ortuño-Pineda, C.; Moreno-Godínez, M.E.; Villegas-Comonfort, S.; Padilla-Benavides, T.; Navarro-Tito, N. Leptin induces cell migration and invasion in a FAK-Src-dependent manner in breast cancer cells. Endocr. Connect. 2019, 8, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Espina, V.; Williams, T.W.; Lin, Y.; Berry, D.; Jelicks, L.A.; Lee, H.; Temple, K.; Graves, R.; Pollard, J.; et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Investig. 2005, 115, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Simpson, E.R.; Brown, K.A. Inflammation, dysregulated metabolism and aromatase in obesity and breast cancer. Curr. Opin. Pharmacol. 2016, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ren, J.; Ten Dijke, P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Choudhury, A.D.; Hamilton, E.; Rosen, L.S.; Stratton, K.L.; Gordon, M.S.; Schaer, D.; Liu, L.; Zhang, L.; Mittapalli, R.K.; et al. PF-06952229, a selective TGF-β-R1 inhibitor: Preclinical development and a first-in-human, phase I, dose-escalation study in advanced solid tumors. ESMO Open 2024, 9, 103653. [Google Scholar] [CrossRef]
- Yoon, J.; Jung, S.M.; Park, S.H.; Kato, M.; Yamashita, T.; Lee, I.; Sudo, K.; Nakae, S.; Han, J.S.; Kim, O.H.; et al. Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Mol. Med. 2013, 5, 1720–1739, Erratum in EMBO Mol. Med. 2014, 6, 703. [Google Scholar] [CrossRef] [PubMed]
- Goff, L.W.; De Braud, F.G.; Cohen, R.B.; Berlin, J.; Noberasco, C.; Borghaei, H.; Wang, E.; Lowe, D.H.; Levin, W.J.; Gallo-Stampino, C. Phase i study of pf-03446962, a fully human mab against alk 1, a TGFbeta receptor involved in tumor angiogenesis. J. Clin. Oncol. 2010, 28 (Suppl. 15), 3034. [Google Scholar] [CrossRef]
- Yap, T.A.; Vieito, M.; Baldini, C.; Sepúlveda-Sánchez, J.M.; Kondo, S.; Simonelli, M.; Cosman, R.; van der Westhuizen, A.; Atkinson, V.; Carpentier, A.F.; et al. In-Human Phase I Study of a Next-Generation, Oral, TGFβ Receptor 1 Inhibitor, LY3200882, in Patients with Advanced Cancer. Clin. Cancer Res. 2021, 27, 6666–6676. [Google Scholar] [CrossRef]
- Nadal, E.; Saleh, M.; Aix, S.P.; Ochoa-de-Olza, M.; Patel, S.P.; Antonia, S.; Zhao, Y.; Gueorguieva, I.; Man, M.; Estrem, S.T.; et al. A phase Ib/II study of galunisertib in combination with nivolumab in solid tumors and non-small cell lung cancer. BMC Cancer 2023, 23, 708. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, J.; Wang, Y.; Li, M.; Jiang, H.; Liu, Y.; Yin, X.; Ge, M.; Xiang, X.; Ying, J.; et al. Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: A dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC Med. 2022, 20, 408. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.M.; Kaufman, P.A.; Wilks, S.T.; Andreopoulou, E.; et al. Eribulin Plus Pembrolizumab in Patients with Metastatic Triple-Negative Breast Cancer (ENHANCE 1): A Phase Ib/II Study. Clin. Cancer Res. 2021, 27, 3061–3068. [Google Scholar] [CrossRef]
- George, V.; Chaturvedi, P.; Shrestha, N.; Kanakraj, L.; Gilkes, C.; Encalada, N.; Wang, M.; Zhu, X.; Liu, B.; Rhode, P.; et al. Bifunctional immunotherapeutic HCW9218 facilitates recruitment of immune cells from tumor draining lymph nodes to promote antitumor activity and enhance checkpoint blockade efficacy in solid tumors. Cancer Res. 2023, 83, 4441. [Google Scholar] [CrossRef]
- Fu, S.; Lin, J. Blocking Interleukin-6 and Interleukin-8 Signaling Inhibits Cell Viability, Colony-forming Activity, and Cell Migration in Human Triple-negative Breast Cancer and Pancreatic Cancer Cells. Anticancer Res. 2018, 38, 6271–6279. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Stroud, C.R.G.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Shih, P.C. Revisiting the development of small molecular inhibitors that directly target the signal transducer and activator of transcription 3 (STAT3) domains. Life Sci. 2020, 242, 117241. [Google Scholar] [CrossRef]
- Nishina, T.; Fujita, T.; Yoshizuka, N.; Sugibayashi, K.; Murayama, K.; Kuboki, Y. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: A phase 1 study. BMJ Open 2022, 12, e055718. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Tian, S.; Li, H. Discovery of STAT3 Inhibitors: Recent Advances and Future Perspectives. Curr. Med. Chem. 2023, 30, 1824–1847. [Google Scholar] [CrossRef]
- Dominguez, C.; McCampbell, K.K.; David, J.M.; Palena, C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2017, 2, e94296. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Collins, J.M.; Donahue, R.N.; Palena, C.; Madan, R.A.; Karzai, F.; Marté, J.L.; Strauss, J.; Gatti-Mays, M.E.; et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer 2019, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Martín, M.; Kaufman, P.A.; Gil-Martin, M.; Pardo, P.G.; Lopez-Tarruella, S.; Manso, L.; Ciruelos, E.M.; Perez-Fidalgo, J.A.; Hernando, C.; et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol. 2018, 19, 812–824. [Google Scholar] [CrossRef]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2020, 9, 1777625. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Wu, Y.H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef]
- Luke, J.J.; Bever, K.; Hodi, F.S.; Taube, J.; Massey, A.; Yao, D.; Neely, J.; Tam, R.; Lee, G.; Gupta, A.; et al. Rationale and feasibility of a rapid integral biomarker program that informs immune-oncology clinical trials: The ADVISE trial. J. Immunother. Cancer 2025, 13, e011170. [Google Scholar] [CrossRef]
- Powderly, J.D.; Klempner, S.J.; Naing, A.; Bendell, J.; Garrido-Laguna, I.; Catenacci, D.V.T.; Taylor, M.H.; Lee, J.J.; Zheng, F.; Zhou, F.; et al. Epacadostat plus pembrolizumab and chemotherapy for advanced solid tumors: Results from the phase I/II ECHO-207/KEYNOTE-723 study. Oncologist 2022, 27, 905-e848. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhao, Z.; Liu, L.; Bai, L.; Tong, R.; Yang, H.; Zhong, L. Targeting Indoleamine Dioxygenase and Tryptophan Dioxygenase in Cancer Immunotherapy: Clinical Progress and Challenges. Drug Des. Devel Ther. 2022, 16, 2639–2657. [Google Scholar] [CrossRef] [PubMed]
- Vleeshouwers, W.; van den Dries, K.; de Keijzer, S.; Joosten, B.; Lidke, D.S.; Cambi, A. Characterization of the Signaling Modalities of Prostaglandin E2 Receptors EP2 and EP4 Reveals Crosstalk and a Role for Microtubules. Front. Immunol. 2021, 11, 613286. [Google Scholar] [CrossRef]
- Ma, X.; Holt, D.; Kundu, N.; Reader, J.; Goloubeva, O.; Take, Y.; Fulton, A.M. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology 2013, 2, e22647. [Google Scholar] [CrossRef]
- Ma, X.; Kundu, N.; Collin, P.D.; Goloubeva, O.; Fulton, A.M. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res. Treat. 2012, 132, 1001–1008. [Google Scholar] [CrossRef]
- Hong, D.S.; Parikh, A.; Shapiro, G.I.; Varga, A.; Naing, A.; Meric-Bernstam, F.; Ataman, Ö.; Reyderman, L.; Binder, T.A.; Ren, M.; et al. First-in-human phase I study of immunomodulatory E7046, an antagonist of PGE2-receptor E-type 4 (EP4), in patients with advanced cancers. J. Immunother. Cancer 2020, 8, e000222. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.; Cook, N.; Leung, C.; Aktas, B.Y.; Dawson, R.C.; Demeris, N.; Mozgunov, P.; Howe, D.; Austin, N.; Swain, N.; et al. 679TiP Cancer Research UK phase I/IIa trial of the prostaglandin E2 (PGE2) receptor 4 (EP4) antagonist HTL0039732 as monotherapy and in combination with immunotherapy in patients (pts) with advanced solid tumors. Ann. Oncol. 2024, 35, S529–S530. [Google Scholar] [CrossRef]
- Trigueiros, B.A.F.D.S.; Santos, I.J.S.; Pimenta, F.P.; Ávila, A.R. A Long Way to Go: A Scenario for Clinical Trials of PI3K Inhibitors in Treating Cancer. Cancer Control 2024, 31, 10732748241238047. [Google Scholar] [CrossRef]
- O’Connell, B.C.; Hubbard, C.; Zizlsperger, N.; Fitzgerald, D.; Kutok, J.L.; Varner, J.; Ilaria, R., Jr.; Cobleigh, M.A.; Juric, D.; Tkaczuk, K.H.R.; et al. Eganelisib combined with immune checkpoint inhibitor therapy and chemotherapy in frontline metastatic triple-negative breast cancer triggers macrophage reprogramming, immune activation and extracellular matrix reorganization in the tumor microenvironment. J. Immunother. Cancer 2024, 12, e009160. [Google Scholar] [CrossRef]
- Hong, D.S.; Postow, M.; Chmielowski, B.; Sullivan, R.; Patnaik, A.; Cohen, E.E.W.; Shapiro, G.; Steuer, C.; Gutierrez, M.; Yeckes-Rodin, H.; et al. Eganelisib, a First-in-Class PI3Kγ Inhibitor, in Patients with Advanced Solid Tumors: Results of the Phase 1/1b MARIO-1 Trial. Clin. Cancer Res. 2023, 29, 2210–2219. [Google Scholar] [CrossRef]
- Melero, I.; Lostes Baradji, M.; Spanggaard, I.; Lee, D.; Spicer, J.; Thistlethwaite, C.F.; Symeonides, S.; Oh, D.; Hollebecque, A.; Rusterholz, C. A Phase I study of a tumor-targeted, fibroblast activation protein (FAP)-CD40 agonist (RO7300490) in patients with advanced solid tumors. J. Immunother. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Solek, J.; Braun, M.; Sadej, R.; Romanska, H.M. FGFR related phenotypic and functional profile of CAFs in prognostication of breast cancer (Review). Int. J. Oncol. 2024, 65, 94. [Google Scholar] [CrossRef]
- Pant, S.; Schuler, M.; Iyer, G.; Witt, O.; Doi, T.; Qin, S.; Tabernero, J.; Reardon, D.A.; Massard, C.; Minchom, A.; et al. RAGNAR Investigators. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): An international, single-arm, phase 2 study. Lancet Oncol. 2023, 24, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Ruiz-Borrego, M.; Jimenez, B.; Antolín, S.; García-Saenz, J.A.; Corral, J.; Jerez, Y.; Trigo, J.; Urruticoechea, A.; Colom, H.; Gonzalo, N.; et al. A phase Ib study of sonidegib (LDE225), an oral small molecule inhibitor of smoothened or Hedgehog pathway, in combination with docetaxel in triple negative advanced breast cancer patients: GEICAM/2012–12 (EDALINE) study. Investig. New Drugs 2019, 37, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, H.; Li, X.; Han, L.; Xu, N.; Shi, A. Signaling pathway inhibitors target breast cancer stem cells in triple-negative breast cancer. Oncol. Rep. 2019, 41, 437–446. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Akman, S.A.; Melin, S.A.; Case, L.D.; Schwartz, G.G. Oral paricalcitol (19-nor-1,25-dihydroxyvitamin D2) in women receiving chemotherapy for metastatic breast cancer: A feasibility trial. Cancer Biol. Ther. 2013, 14, 476–480. [Google Scholar] [CrossRef]
- Chronopoulos, A.; Robinson, B.; Sarper, M.; Cortes, E.; Auernheimer, V.; Lachowski, D.; Attwood, S.; García, R.; Ghassemi, S.; Fabry, B.; et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun. 2016, 7, 12630. [Google Scholar] [CrossRef] [PubMed]
- Coulson, R.; Liew, S.H.; Connelly, A.A.; Yee, N.S.; Deb, S.; Kumar, B.; Vargas, A.C.; O’Toole, S.A.; Parslow, A.C.; Poh, A.; et al. The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget 2017, 8, 18640–18656. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic Target | Agent | Therapy Design | Group of Patients | Clinical Trial Number and Phase | Status |
|---|---|---|---|---|---|
| TGF-β pathway | |||||
| TGF-β RI (ALK5) | PF-06952229 | Monotherapy | Advanced solid tumors | NCT03685591 Phase I | Terminated |
| TGF-β RI (ALK4/ALK5) | Vactosertib (TEW-197) | Monotherapy | Advanced solid tumors | NCT02160106 Phase I | Completed |
| TGF-β RI (ALK1) | PF-03446962 | Monotherapy PF-03446962 +bavacizumab | Advanced solid tumors | NCT00557856 Phase I | Completed |
| TGF-β RI (ALK5) | LY3200882 | Monotherapy LY3200882+ nab-Paclitaxel LY3200882+anti-PD-L1 (LY3300054) | Patients with solid tumors | NCT02937272 Phase I | Active, not recruiting |
| TGF-β RI (ALK5) | galunisertib monohydrate (LY2157299) | Monotherapy | Advanced refractory solid tumors | NCT02423343 Phase Ib | Completed |
| TGF-β RII and PD-L-1 | bifunctional antibody SHR-1701 | Monotherapy | Metastatic or locally advanced solid tumors | NCT03710265 Phase II/III | Unknown status |
| TGF-β RII and PD-L1 | bifunctional antibody bintrafusp alfa | Monotherapy | Patients with Metastatic TNBC | NCT03579472 Phase Ib/II | Terminated |
| TGF-β RII and IL-15Rα | bifunctional antibody HCW9218 | Monotherapy | Advanced solid tumors | NCT05322408 Phase I | Active, not recruiting |
| IL-6/JAK/STAT-3 pathway | |||||
| IL-6 R | tocilizumab | Monotherapy | Steroid-dependent immune-related adverse events (irAEs) | NCT04375228 Phase II | Active, not recruiting |
| STAT3 | OPB-51602 | Monotherapy | Advanced Cancer | NCT01423903 Phase I | Completed |
| STAT3 | VVD-130850 | Monotherapy | Advanced solid and hematologic tumors | NCT06188208 Phase I | Recruiting |
| STAT3 | IMX-110 | IMX-110 + tislelizumab (anti-PD-1) | Advanced solid tumors | NCT05840835 Phase I/IIa | Recruiting |
| STAT3 | AZD9150 | AZD9150 + durvalumab | Advanced solid tumors | NCT02499328 Phase Ib/II | Active, not recruiting |
| AZD9150 + durvalumab | Advanced solid tumors | NCT03394144 Phase I | Completed | ||
| IL-8 | |||||
| IL-8 | BMS-986253 (HuMax-IL-8) | Monotherapy | Advanced solid tumors | NCT02536469 Phase Ib | Completed |
| CXCL12/CXCR4 pathway | |||||
| CXCR4 | Bilixafortide (POL6326) | Bilixafortide + eribulin | Relapsed TNBC and hormone refractory ER-positive metastatic BC | NCT01837095 Phase I | Completed |
| Agent | Therapy Design | Group of Patients | Clinical Trial Number and Phase | Status |
|---|---|---|---|---|
| BMS-986205 (Linrodostat) | BMS-986205+ nivolumab BMS-986205 + nivolumab + ipilimumab BMS-986205 + ipilimumab | Advanced malignant tumors | NCT02658890 Phase I/IIa | Completed |
| BMS-986205 | BMS-986205+ nivolumab/relatlimab/ipilimumab | Advanced malignant tumors | NCT03335540 (ADVISE) Phase I | Completed |
| BMS-986205 | BMS-986205 + relatlimab (anti-LAG-3) + nivolumab | Advanced malignant tumors | NCT03459222 Phase I/II | Completed |
| Epacadostat | Epacadostat + pembrolizumab + chemotherapy | Advanced or metastatic solid tumors | NCT03085914 (ECHO-207/KEYNOTE-723) Phase I/II | Completed |
| LY3381916 | LY3381916 + antiPD-L1 (LY3300054) | Solid tumors | NCT03343613 Phase I | Terminated |
| E7046 | Monotherapy | Selected advanced malignancies | NCT02540291 Phase I | Terminated |
| HTL0039732 | Monotherapy HTL0039732 + atezolizumab | Advanced solid tumors | NCT05944237 Phase Ib/IIa | Recruiting |
| Buparlisib (AN0025) | Buparlisib (AN2025) + AN0025 AN2025 + AN0025 + atezolizumab | Advanced solid tumors | NCT04975958 Phase Ia | Completed |
| eganelisib (IPI-549) | Monotherapy PI-549 + nivolumab | Advanced solid tumors | NCT02637531 Phase I/Ib | Unknown status |
| IPI-549 | IPI-549+ tecentriq (atezolizumab) + nab-paclitaxel | Front-line TNBC | NCT03961698 MARIO-3 Phase II | Active, not recruiting |
| Agent | Therapy Design | Group of Patients | Clinical Trial Number and Phase | Status |
|---|---|---|---|---|
| E7046 | Monotherapy | Advanced malignancies | NCT02540291 Phase I | Terminated |
| HTL0039732 | Monotherapy HTL003973+ atezolizumab | Advanced solid tumors | NCT05944237 Phase I/IIa | Recruiting |
| AN0025 | AN0025 + AN2025 (pan-class I PI3K inhibitor) AN0025 +atezolizumab | Advanced malignant tumors | NCT04975958 Phase Ia | Completed |
| Agent | Therapy Design | Group of Patients | Clinical Trial Number and Phase | Status |
|---|---|---|---|---|
| Eganelisib (IPI-549) | Monotherapy | Advanced solid tumors | NCT02637531 Phase I/Ib | Terminated |
| IPI-549 | IPI-549 + nabpaclitaxel + atzolizumab | Front-line triple TNBC | NCT03961698 Phase II | Unknown status |
| Activity/Mechanism | Agent | Therapy Design | Group of Patients | Clinical Trial | Status |
|---|---|---|---|---|---|
| Targeting FAPα | |||||
| FAPα- targeted CD40 agonist antibody | RO7300490 | Monotherapy RO7300490 + atezolizumab | Advanced and/or metastatic solid tumors | NCT04857138 Phase I | Completed |
| Targeting FGFR signaling | |||||
| Pan FGFR 1-4 inhibitor | Erdafitinib | Monotherapy | Advanced solid tumors | NCT04083976 RAGNAR Phase II | Completed |
| Erdafitinib + Fulvestrant+ Palbociclib | ER+/HER2-/FGFR-amplified BC | NCT03238196 Phase I | Active, not recruiting | ||
| Targeting Hedgehog signaling | |||||
| Smoothened receptor (SMO) antagonist | Sonidegib (LDE225) | Sonidegib + docitaxel | Advanced TNBC | NCT02027376 EDALINE Phase Ib | Completed |
| Inhibitor of SMO | Vismodegib (GDC-0449) | Vismodegib + standard neoadjuvant chemotherapy | TNBC | NCT02694224 Phase II | Unknown status |
| Reverting CAFs into quiescent state | |||||
| Vitamin D receptor agonist | 19-nor-1,25-dihydroxyvitamin D2 | 19-nor-1,25-dihydroxyvitamin D2 + standard Neoadjuvant Chemotherapy | Metastatic Breast Cancer | NCT00637897 Phase I | Completed |
| RARβ agonist | All trans retinoic acid (ATRA) | ATRA + non-steroidal aromatase inhibitor anastrozole | Hormonal receptor+/HER2- early BC | NCT04113863 Phase II | Recruiting |
| Targeting ECM | |||||
| Inhibition of hyaluronidase activity | PEGPH20 PEGylated Recombinant Human hylaronidaze | PEGPH20 + eribulin mesylate | HER2-, high-hyaluronan metastatic BC | NCT02753595 Phase Ib/II | Terminated |
| Inhibition of colagen and hyaluron production | Losartan (angiotensin receptor blocker) | Losartan + camrelizumab +doxorubicin | TNBC treated with no more than 1 Prior line of chemotherapy | NCT05097248 Phase II | Unknown status |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuletic, A.; Mirjacic Martinovic, K.; Jurisic, V. The Role of Tumor Microenvironment in Triple-Negative Breast Cancer and Its Therapeutic Targeting. Cells 2025, 14, 1353. https://doi.org/10.3390/cells14171353
Vuletic A, Mirjacic Martinovic K, Jurisic V. The Role of Tumor Microenvironment in Triple-Negative Breast Cancer and Its Therapeutic Targeting. Cells. 2025; 14(17):1353. https://doi.org/10.3390/cells14171353
Chicago/Turabian StyleVuletic, Ana, Katarina Mirjacic Martinovic, and Vladimir Jurisic. 2025. "The Role of Tumor Microenvironment in Triple-Negative Breast Cancer and Its Therapeutic Targeting" Cells 14, no. 17: 1353. https://doi.org/10.3390/cells14171353
APA StyleVuletic, A., Mirjacic Martinovic, K., & Jurisic, V. (2025). The Role of Tumor Microenvironment in Triple-Negative Breast Cancer and Its Therapeutic Targeting. Cells, 14(17), 1353. https://doi.org/10.3390/cells14171353







