The Combination of Ibrutinib with BH3 Mimetics or Dichloroacetate Is Effective in B-CLL
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Cells from Patients and Cell Culture
2.2. Cell Incubation with Drugs
2.3. Flow Cytometry Assays
2.4. Cell Growth Assays
2.5. Western Blot Experiments
2.6. Immunoprecipitation
2.7. Statistical Analysis
3. Results
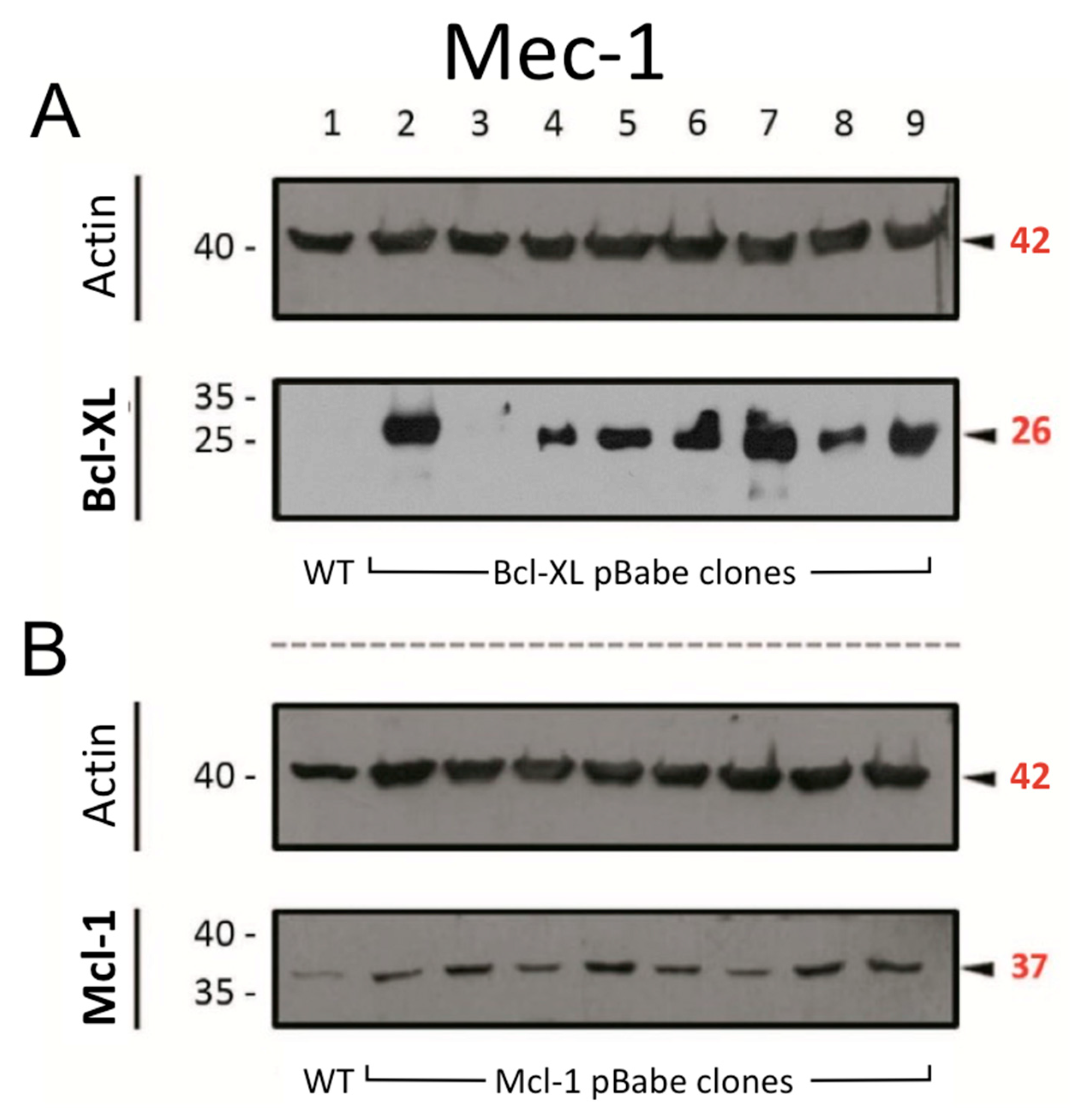
3.1. Generation of Sublines from Mec-1 Cells
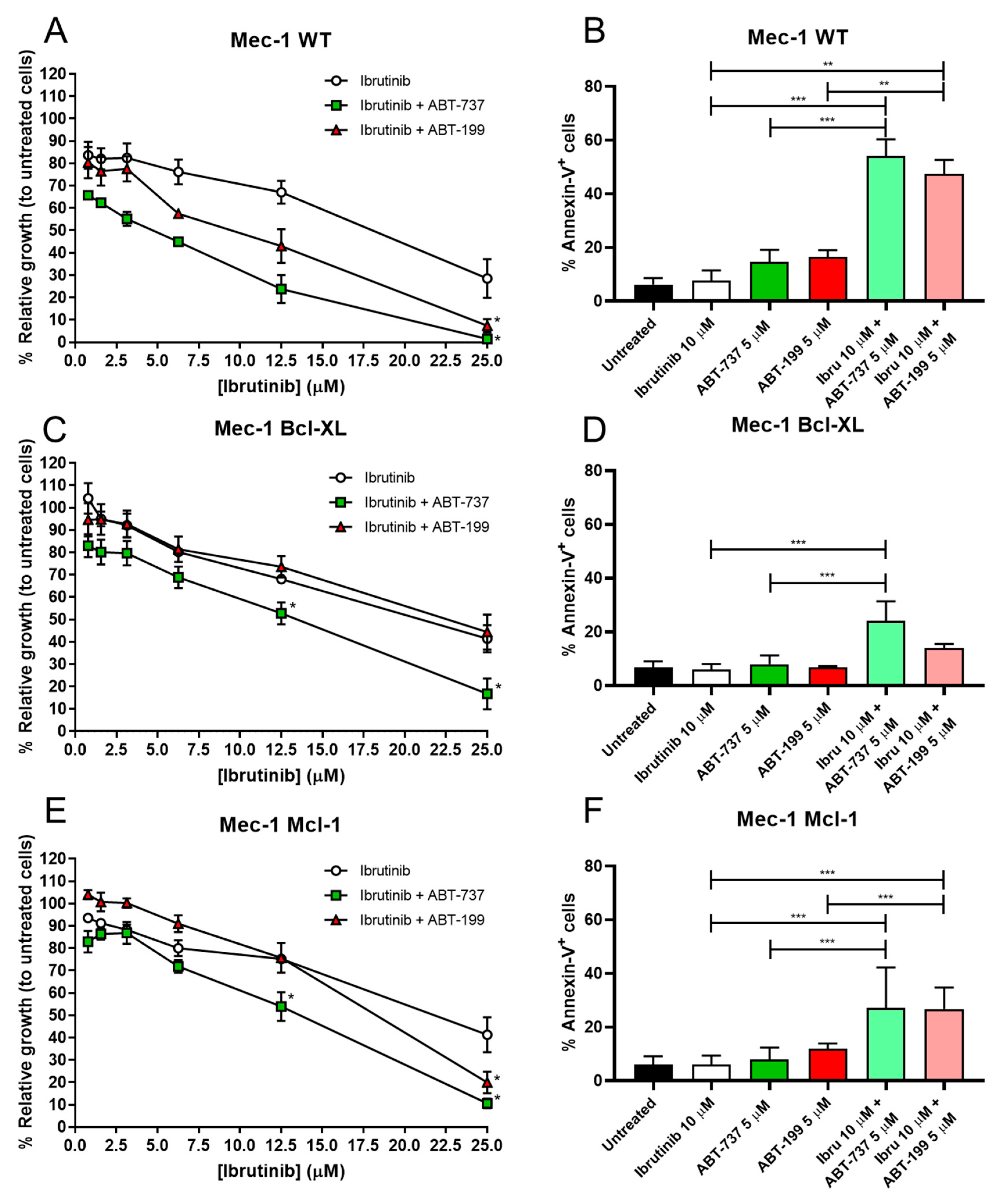
3.2. Combination of BH3 Mimetics and Ibrutinib in Cell Growth and Apoptosis in Mec-1 Cell Lines
3.3. Apoptosis Induction of BH3 Mimetics and Ibrutinib in B-CLL Samples
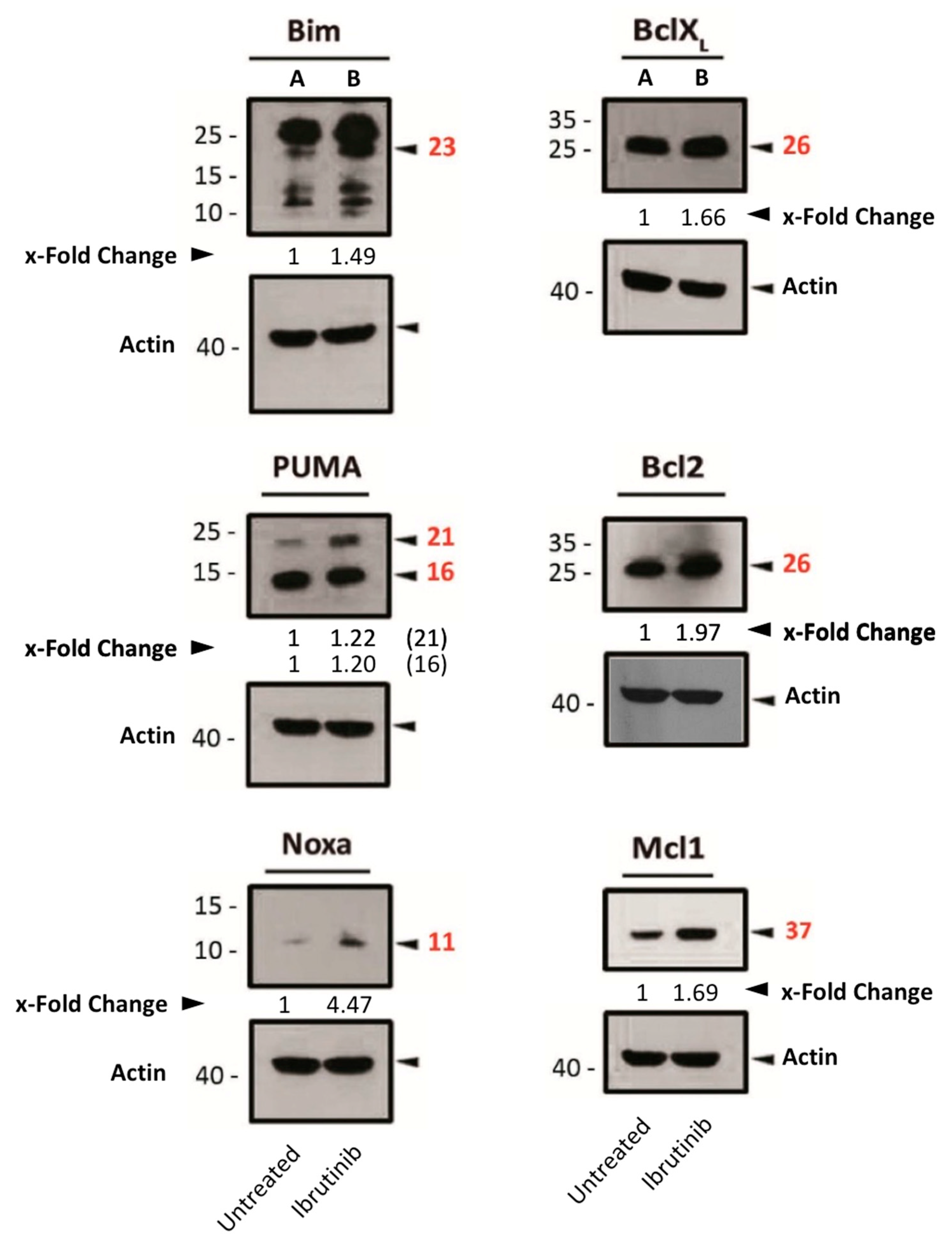
3.4. Assessment of Pro- or Anti-Apoptotic Protein Expression Following Ibrutinib Exposure
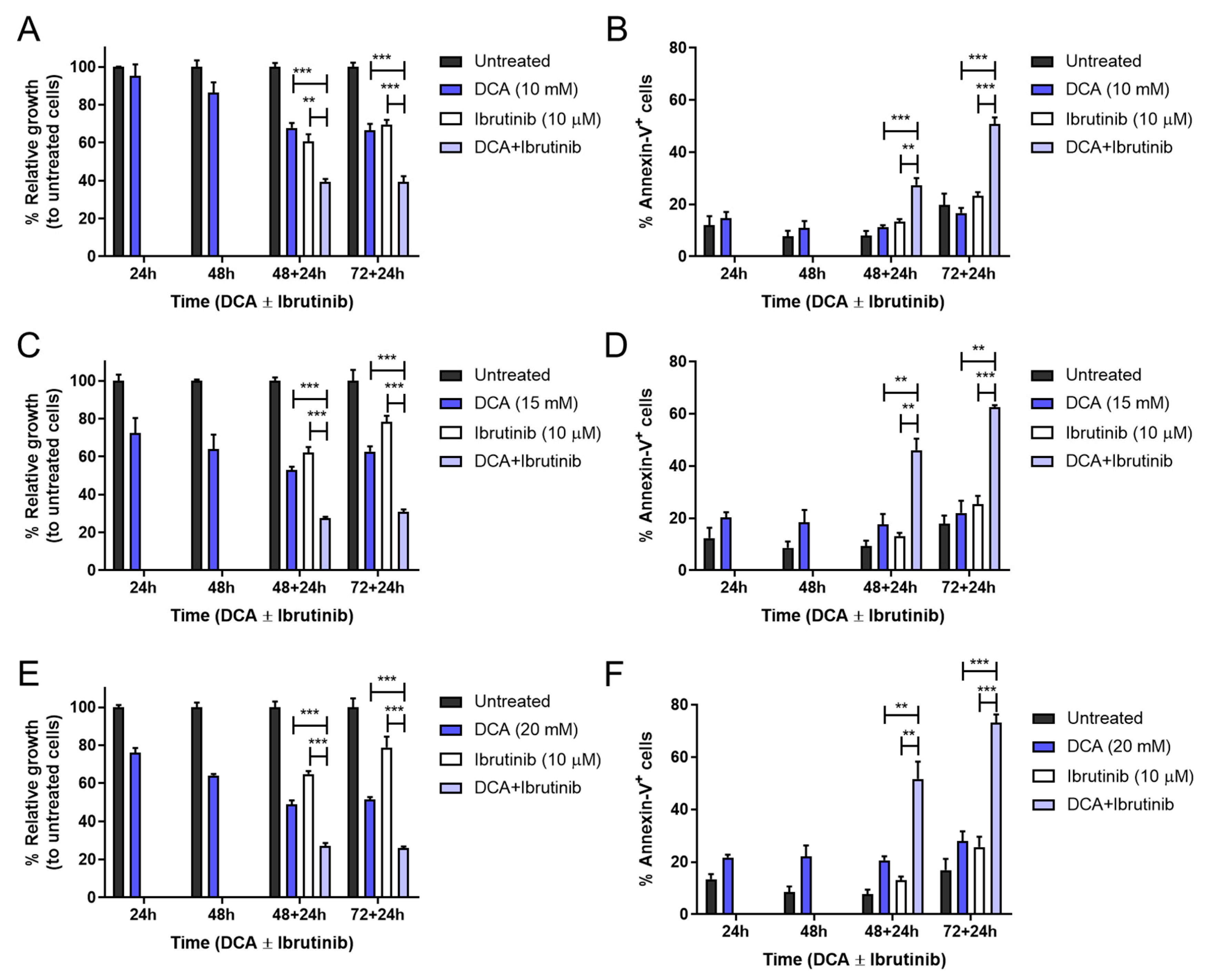
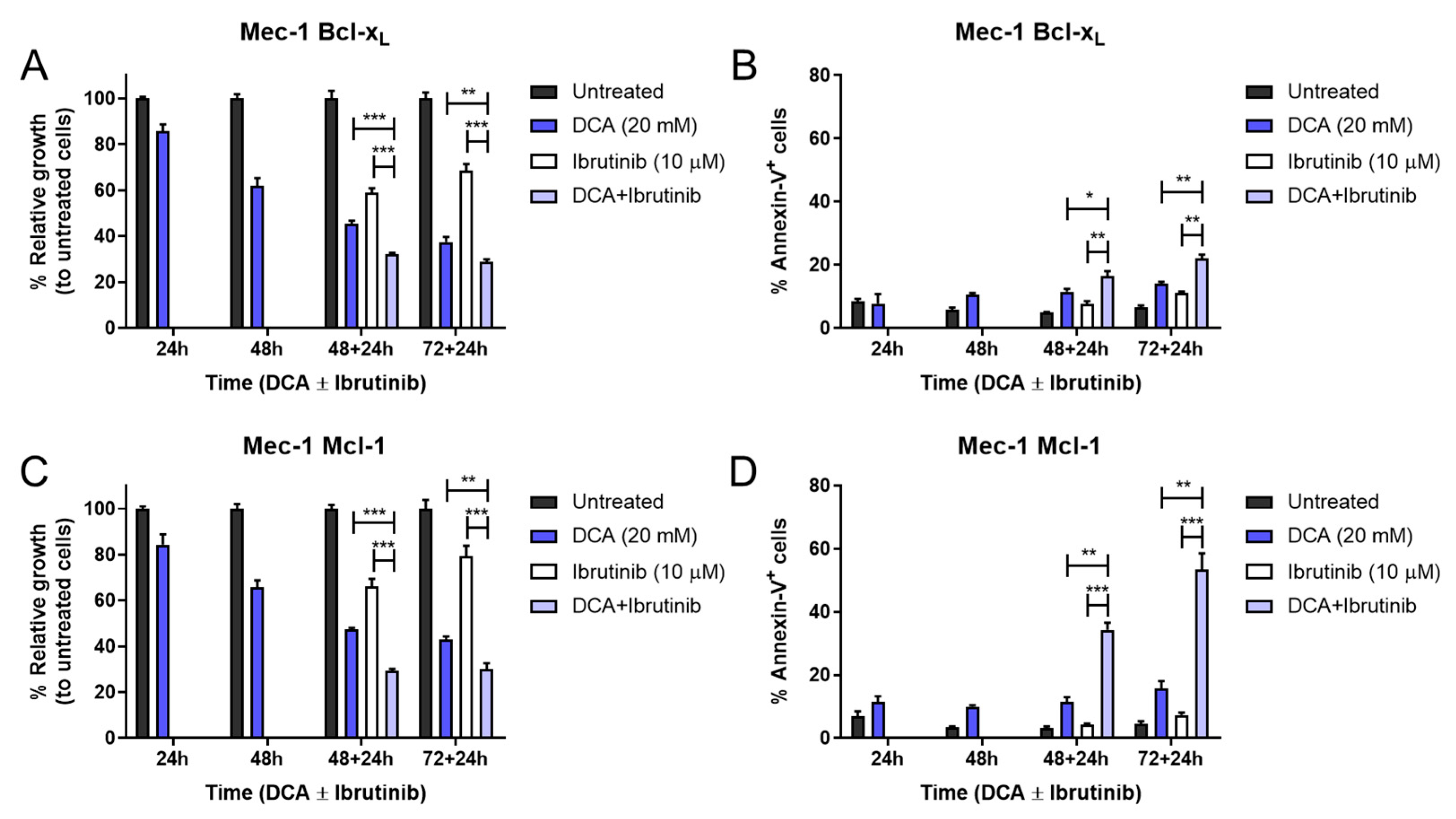
3.5. Combination of DCA and Ibrutinib in Cell Growth and Apoptosis in Mec-1 Cell Lines
3.6. Apoptosis Induction of DCA and Ibrutinib in B-CLL Samples
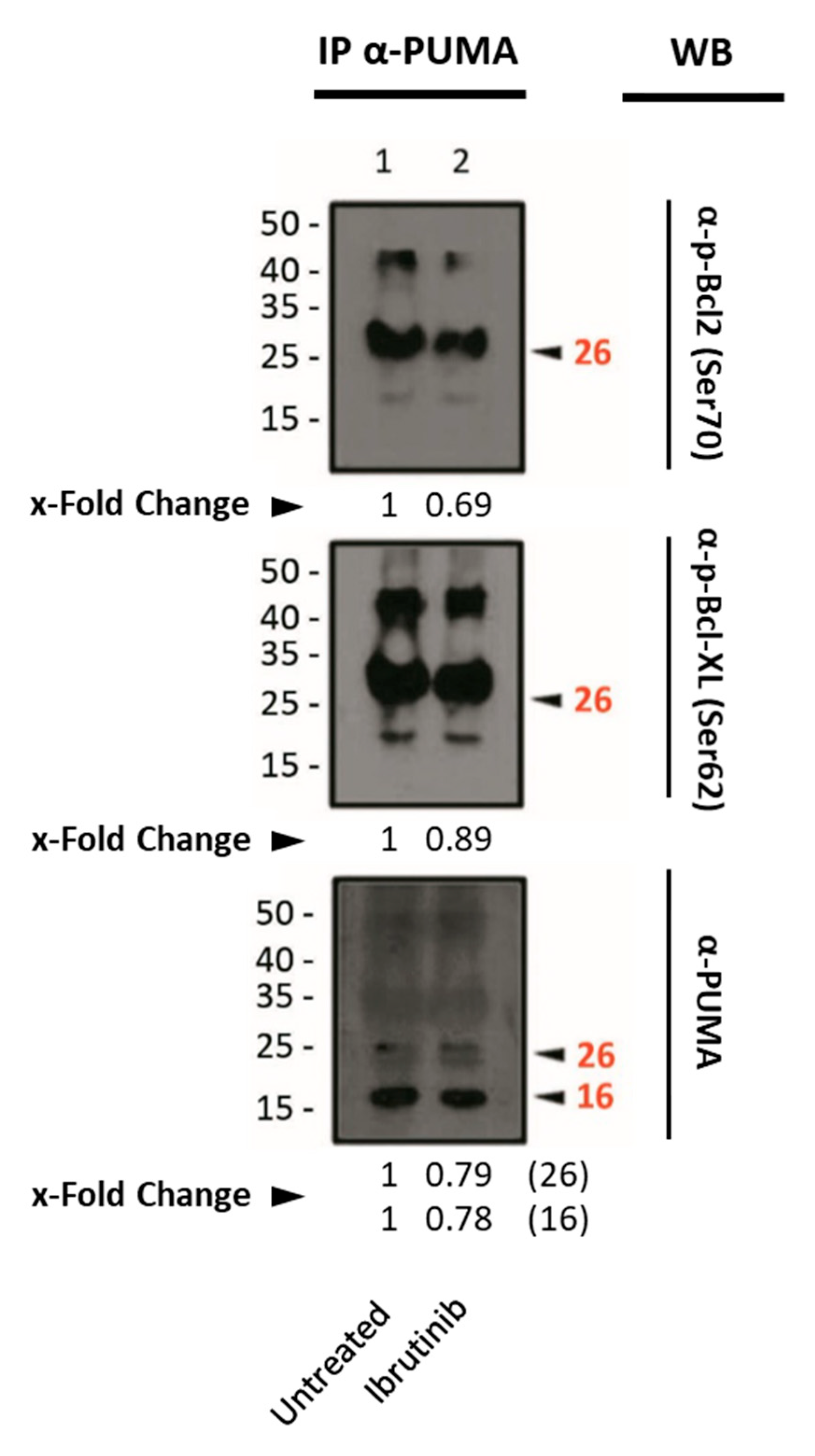
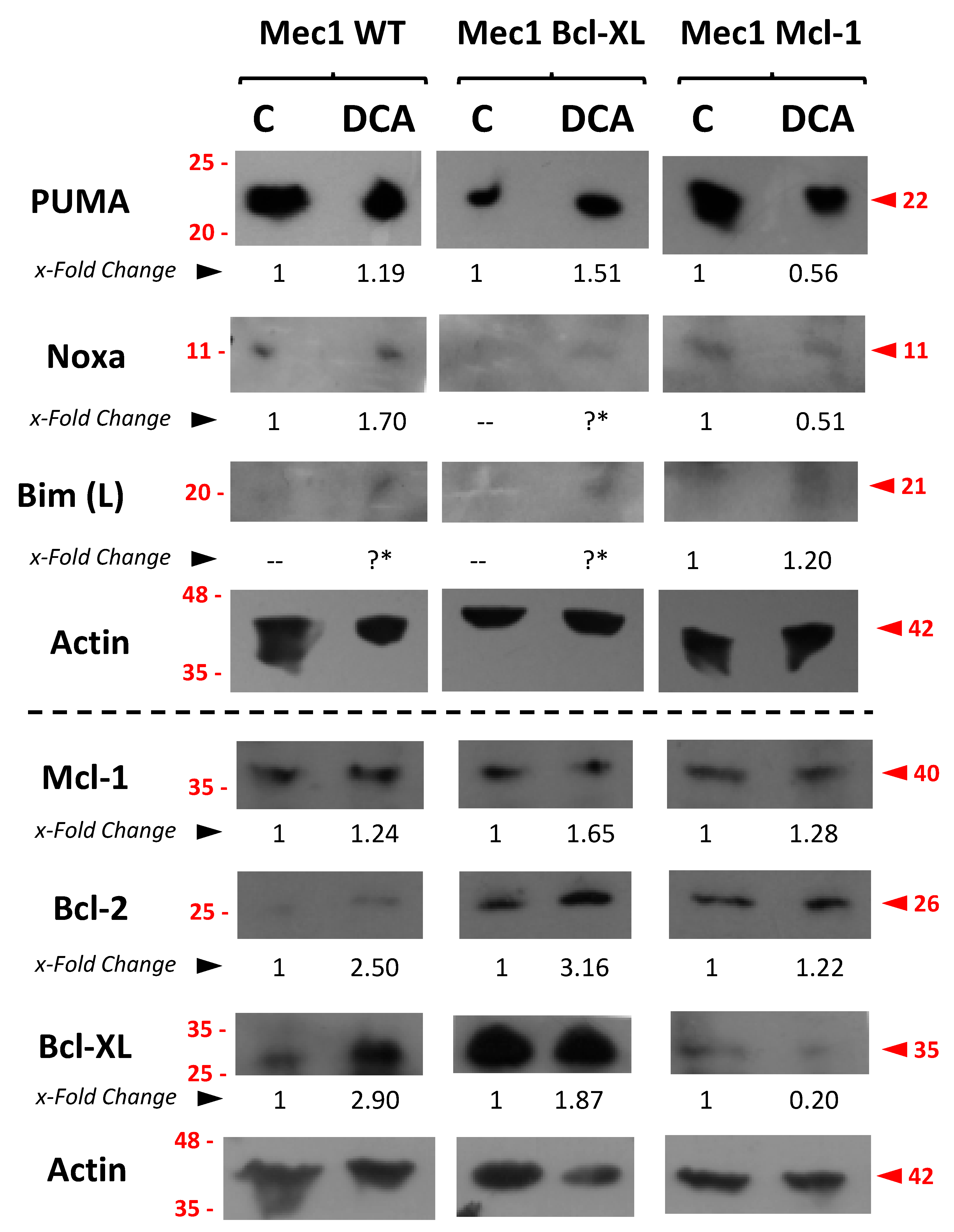
3.7. Assessment of Pro- or Anti-Apoptotic Proteins Following DCA Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallek, M. Chronic Lymphocytic Leukemia: 2025 Update on the Epidemiology, Pathogenesis, Diagnosis, and Therapy. Am. J. Hematol. 2025, 100, 450–480. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic lymphocytic leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.D.; Chan, J.K.C.; Coupland, S.E.; Du, M.Q.; Ferry, J.A.; Jong, D.D.; Gratzinger, D.; Lim, M.S.; Naresh, K.N.; Nicolae, A.; et al. The 5th edition of the World Health Organization Classification of mature lymphoid and stromal tumors-an overview and update. Leuk. Lymphoma 2024, 65, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Buggins, A.G.S.; Pepper, C.J. The role of Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk. Res. 2010, 34, 837–842. [Google Scholar] [CrossRef]
- Townsend, P.; Kozhevnikova, M.; Cexus, O.; Zamyathin, A.; Soond, S. BH3-mimetics: Recent developments in cancer therapy. J. Exp. Clin. Cancer Res. 2021, 40, 355. [Google Scholar] [CrossRef]
- Advani, R.H.; Buggy, J.J.; Sharman, J.P.; Smith, S.M.; Boyd, T.E.; Grant, B.; Kolibaba, K.S.; Furman, R.R.; Rodriguez, S.; Chang, B.Y.; et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J. Clin. Oncol. 2013, 31, 88–94. [Google Scholar] [CrossRef]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef]
- Efremov, D.G.; Turkalj, S.; Laurenti, L. Mechanisms of B Cell Receptor Activation and Responses to B Cell Receptor Inhibitors in B Cell Malignancies. Cancers 2020, 12, 1396. [Google Scholar] [CrossRef]
- Allan, J.N.; Kipps, T.J.; Bokun, A.; Maegawa, R.; O’Brien, S.M. CLL-481 Ibrutinib Efficacy Across Subgroups of Patients With High-Risk Chronic Lymphocytic Leukemia: A Systematic Literature Review. Clin. Lymphoma Myeloma Leuk. 2024, 24, 356. [Google Scholar] [CrossRef]
- Allan, J.N.; Shanafelt, T.; Wiestner, A.; Moreno, C.; O’Brien, S.M.; Li, J.; Krigsfeld, G.; Dean, J.P.; Ahn, I.E. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: A pooled analysis from four clinical trials. Br. J. Haematol. 2022, 196, 947–953. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Dartigeas, C.; Quinquenel, A.; Ysebaert, L.; Dilhuydy, M.-S.; Anglaret, B.; Slama, B.; Le Du, K.; Tardy, S.; Tchernonog, E.; Orfeuvre, H.; et al. Final results on effectiveness and safety of Ibrutinib in patients with chronic lymphocytic leukemia from the non-interventional FIRE study. Ann. Hematol. 2024, 104, 1079–1093. [Google Scholar] [CrossRef]
- Saevels, K.; Snauwaert, S.; Vanstralen, G.; Andre, M.; Offner, F.; Maes, A.; Lahaye, M.; Janssens, A. Real-World Effectiveness and Safety of Ibrutinib in Patients with Chronic Lymphocytic Leukemia (CLL) in Belgium after 4 Years. Blood 2023, 142, 6550. [Google Scholar] [CrossRef]
- Kozarac, S.; Vukovic, V.; Fradley, M.; Antic, D. BTKi-induced cardiovascular toxicity in CLL: Risk mitigation and management strategies. Blood Rev. 2025, 70, 101268. [Google Scholar] [CrossRef]
- Lipfert, C.; Myung Sun, K.; Alyson, H.; Vinay, K.P. Recall of ibrutinib and issues with therapeutic approval. BMJ Oncol. 2024, 3, 418. [Google Scholar] [CrossRef]
- Azizuddin, S.; Kazi, M.; Nadaf, A.; Hasan, N.; Husain, A.; Kesharwani, P.; Ahmad, F.J. Unlocking the potential of ibrutinib: A comprehensive review on its role in the multifaceted landscape of cancer therapy. Process Biochem. 2024, 143, 44–59. [Google Scholar] [CrossRef]
- Timofeeva, N.; Jain, N.; Gandhi, V. Ibrutinib and venetoclax in combination for chronic lymphocytic leukemia: Synergy in practice. Blood Neoplasia. 2024, 1, 100034. [Google Scholar] [CrossRef] [PubMed]
- Diepstraten, S.T.; Anderson, M.A.; Czabotar, P.E.; Lessene, G.; Strasser, A.; Kelly, G.L. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 2022, 22, 45–64. [Google Scholar] [CrossRef]
- Yalniz, F.F.; Wierda, W.G. Targeting BCL2 in Chronic Lymphocytic Leukemia and Other Hematologic Malignancies. Drugs 2019, 79, 1287–1304. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Rozovski, U.; Hazan-Halevy, I.; Barzilai, M.; Keating, M.J.; Estrov, Z. Metabolism pathways in chronic lymphocytic leukemia. Leuk. Lymphoma. 2016, 57, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Belkahla, S.; Marco-Brualla, J.; Fayd’herbe de Maudave, A.; Falvo, P.; Allende-Vega, N.; Constantinides, M.; Khan, A.U.H.; Coenon, L.; Alexia, C.; Mitola, G.; et al. The metabolism of cells regulates their sensitivity to NK cells depending on p53 status. Sci. Rep. 2022, 12, 3234. [Google Scholar] [CrossRef] [PubMed]
- Catalán, E.; Charni, S.; Jaime, P.; Aguiló, J.I.; Enríquez, J.A.; Naval, J.; Pardo, J.; Villalba, M.; Anel, A. MHC-I modulation due to changes in tumor cell metabolism regulates tumor sensitivity to CTL and NK cells. Oncoimmunology 2015, 4, 985924. [Google Scholar] [CrossRef]
- Marco-Brualla, J.; de Miguel, D.; Martínez-Lostao, L.; Anel, A. DR5 Up-Regulation Induced by Dichloroacetate Sensitizes Tumor Cells to Lipid Nanoparticles Decorated with TRAIL. J. Clin. Med. 2023, 12, 608. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Vergara, X.; Tabera, S.; Montero, J.C.; Esparís-Ogando, A.; López-Pérez, R.; Mateo, G.; Gutiérrez, N.; Parmo-Cabañas, M.; Teixidó, J.; San Miguel, J.F.; et al. Multifunctional role of Erk5 in multiple myeloma. Blood 2005, 105, 4492–4499. [Google Scholar] [CrossRef]
- Gonzalo, Ó.; Benedi, A.; Vela, L.; Anel, A.; Naval, J.; Marzo, I. Study of the Bcl-2 Interactome by BiFC Reveals Differences in the Activation Mechanism of Bax and Bak. Cells 2023, 12, 800. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Salem, A.; Menon, R. Clinical pharmacokinetics and pharmacodynamics of venetoclax, a selective Be B and cytotoxicity assays. Clin. Transl. Sci. 2024, 17, 13807. [Google Scholar] [CrossRef]
- Chipuk, J.E.; and Green, D.R. PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle 2009, 8, 2692–2696. [Google Scholar] [CrossRef]
- Nie, Y.; Yun, X.; Zhang, Y.; Wang, X. Targeting metabolic reprogramming in chronic lymphocytic leukemia. Exp. Hematol. Oncol. 2022, 11, 39. [Google Scholar] [CrossRef]
- Luo, Z.; Eichinger, K.M.; Zhang, A.; Li, S. Targeting cancer metabolic pathways for improving chemotherapy and immunotherapy. Cancer Lett. 2023, 575, 216396. [Google Scholar] [CrossRef]
- Allende-Vega, N.; Krzywinska, E.; Orecchioni, S.; Lopez-Royuela, N.; Reggiani, F.; Talarico, G.; Rossi, J.; Rossignol, R.; Hicheri, Y.; Cartron, G.; et al. The presence of wild type p53 in hematological cancers improves the efficacy of combinational therapy targeting metabolism. Oncotarget 2015, 6, 19228–19245. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer 2018, 18, 148–167. [Google Scholar] [CrossRef]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Salem, J.-E.; Clauss, S.; Hanley, A.; Bapat, A.; Hulsmans, M.; Iwamoto, Y.; Wojtkiewicz, G.; Cetinbas, M.; Schloss, M.J.; et al. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation 2020, 142, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.M.; Peer, C.J.; Figg, W.D. Ibrutinib’s off-target mechanism: Cause for dose optimization. Cancer Biol. Ther. 2021, 22, 529–531. [Google Scholar] [CrossRef]
- Tam, C.; Thompson, P.A. BTK inhibitors in CLL: Second-generation drugs and beyond. Blood Adv. 2024, 8, 2300–2309. [Google Scholar] [CrossRef]
- Maher, N.; Mouhssine, S.; Matti, B.; Alwan, A.; Gaidano, G. Molecular Mechanisms in the Transformation from Indolent to Aggressive B Cell Malignancies. Cancers 2025, 17, 907. [Google Scholar] [CrossRef]
- Cervantes-Gomez, F.; Lamothe, B.; Woyach, J.A.; Wierda, W.G.; Keating, M.J.; Balakrishnan, K.; Gandhi, V. Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as Optimal Partner with Ibrutinib in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2015, 21, 3705–3715. [Google Scholar] [CrossRef]
- Jain, N.; Keating, M.; Thompson, P.; Ferrajoli, A.; Burger, J.; Borthakur, G.; Takahashi, K.; Estrov, Z.; Fowler, N.; Kadia, T.; et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N. Engl. J. Med. 2019, 380, 2095–2103. [Google Scholar] [CrossRef]
- Haselager, M.V.; Kielbassa, K.; ter Burg, J.; Bax, D.J.C.; Fernandes, S.M.; Borst, J.; Tam, C.; Forconi, F.; Chiodin, G.; Brown, J.R.; et al. Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood 2020, 136, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, V.; Agriesti, F.; Scrima, R.; Laurenzana, I.; Perrone, D.; Tataranni, T.; Mazzoccoli, C.; Lo Muzio, L.; Capitanio, N.; Piccoli, C. Dichloroacetate, a selective mitochondria-targeting drug for oral squamous cell carcinoma: A metabolic perspective of treatment. Oncotarget 2015, 6, 1217–1230. [Google Scholar] [CrossRef]
- Cao, W.; Yacoub, S.; Shiverick, K.T.; Namiki, K.; Sakai, Y.; Porvasnik, S.; Urbanek, C.; Rosser, C.J. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate 2008, 68, 1223–1231. [Google Scholar] [CrossRef]
- Kan, P.C.; Chang, Y.J.; Chien, C.S.; Su, C.Y.; Fang, H.W. Coupling Dichloroacetate Treatment with Curcumin Significantly Enhances Anticancer Potential. Anticancer Res. 2018, 38, 6253–6261. [Google Scholar] [CrossRef]
- Bonnet, S.; Archer, S.L.; Allalunis-Turner, J.; Haromy, A.; Beaulieu, C.; Thompson, R.; Lee, C.T.; Lopaschuk, G.D.; Puttagunta, L.; Bonnet, S.; et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Chandel, N.S.; Williamson, E.K.; Schumacker, P.T.; Thompson, C.B. Bcl-xL Regulates the Membrane Potential and Volume Homeostasis of Mitochondria. Cell 1997, 91, 627–637. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marco-Brualla, J.; Gonzalo, O.; Azaceta, G.; Izquierdo, I.; Palomera, L.; Villalba, M.; Marzo, I.; Anel, A. The Combination of Ibrutinib with BH3 Mimetics or Dichloroacetate Is Effective in B-CLL. Cells 2025, 14, 1343. https://doi.org/10.3390/cells14171343
Marco-Brualla J, Gonzalo O, Azaceta G, Izquierdo I, Palomera L, Villalba M, Marzo I, Anel A. The Combination of Ibrutinib with BH3 Mimetics or Dichloroacetate Is Effective in B-CLL. Cells. 2025; 14(17):1343. https://doi.org/10.3390/cells14171343
Chicago/Turabian StyleMarco-Brualla, Joaquín, Oscar Gonzalo, Gemma Azaceta, Isabel Izquierdo, Luis Palomera, Martín Villalba, Isabel Marzo, and Alberto Anel. 2025. "The Combination of Ibrutinib with BH3 Mimetics or Dichloroacetate Is Effective in B-CLL" Cells 14, no. 17: 1343. https://doi.org/10.3390/cells14171343
APA StyleMarco-Brualla, J., Gonzalo, O., Azaceta, G., Izquierdo, I., Palomera, L., Villalba, M., Marzo, I., & Anel, A. (2025). The Combination of Ibrutinib with BH3 Mimetics or Dichloroacetate Is Effective in B-CLL. Cells, 14(17), 1343. https://doi.org/10.3390/cells14171343







