HDACs in the Brain: From Chromatin Remodeling to Neurodegenerative Disease
Abstract
1. Introduction
2. Classification and Functional Roles of Histone Deacetylases
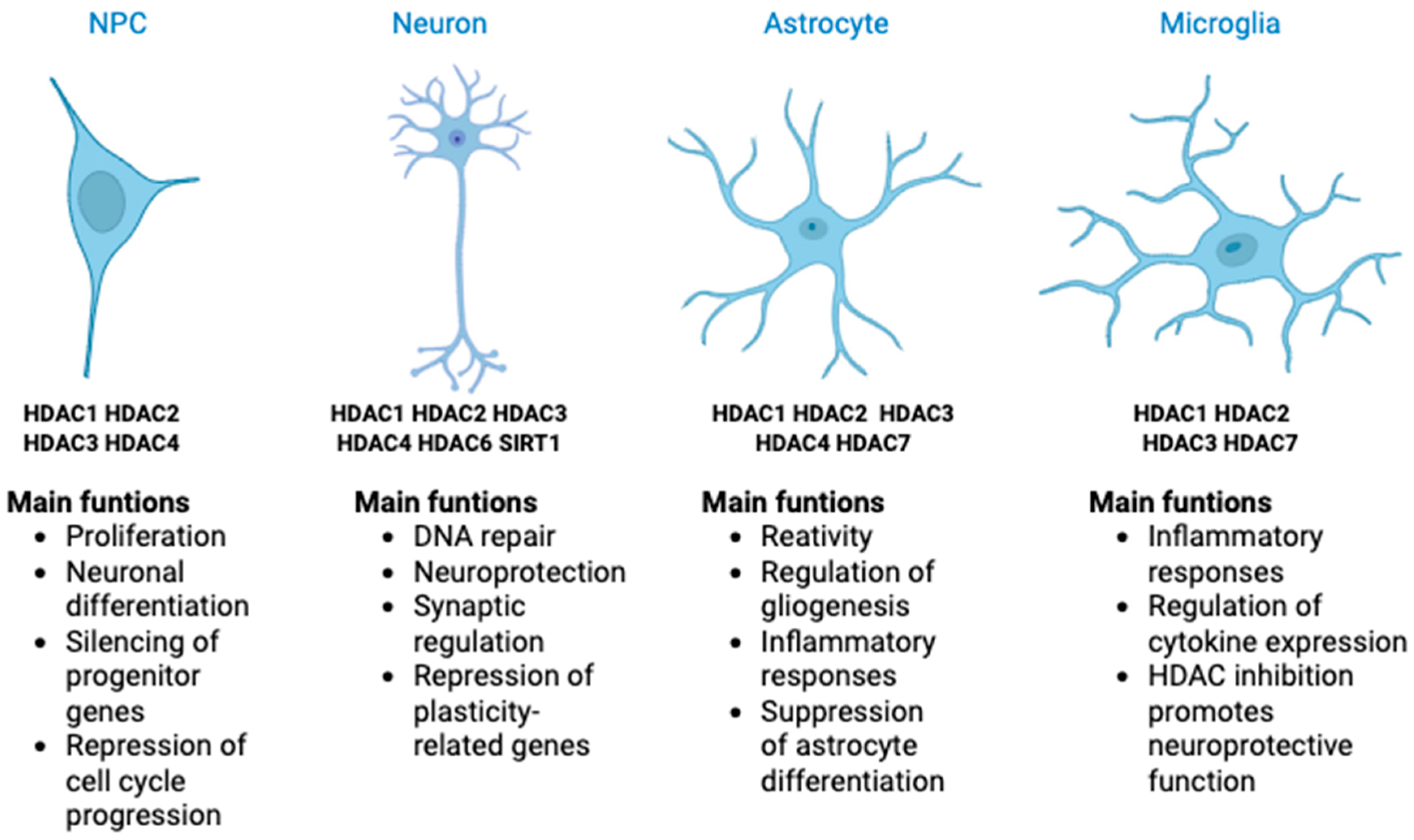
3. HDAC in the Central Nervous System
3.1. Role of HDACs in Neural Progenitor Cells
3.2. Role of HDAC in Neuron Cells
3.3. Role of HDAC in Glial Cells
4. Implications of HDAC/HDACi in Neurodegenerative Diseases
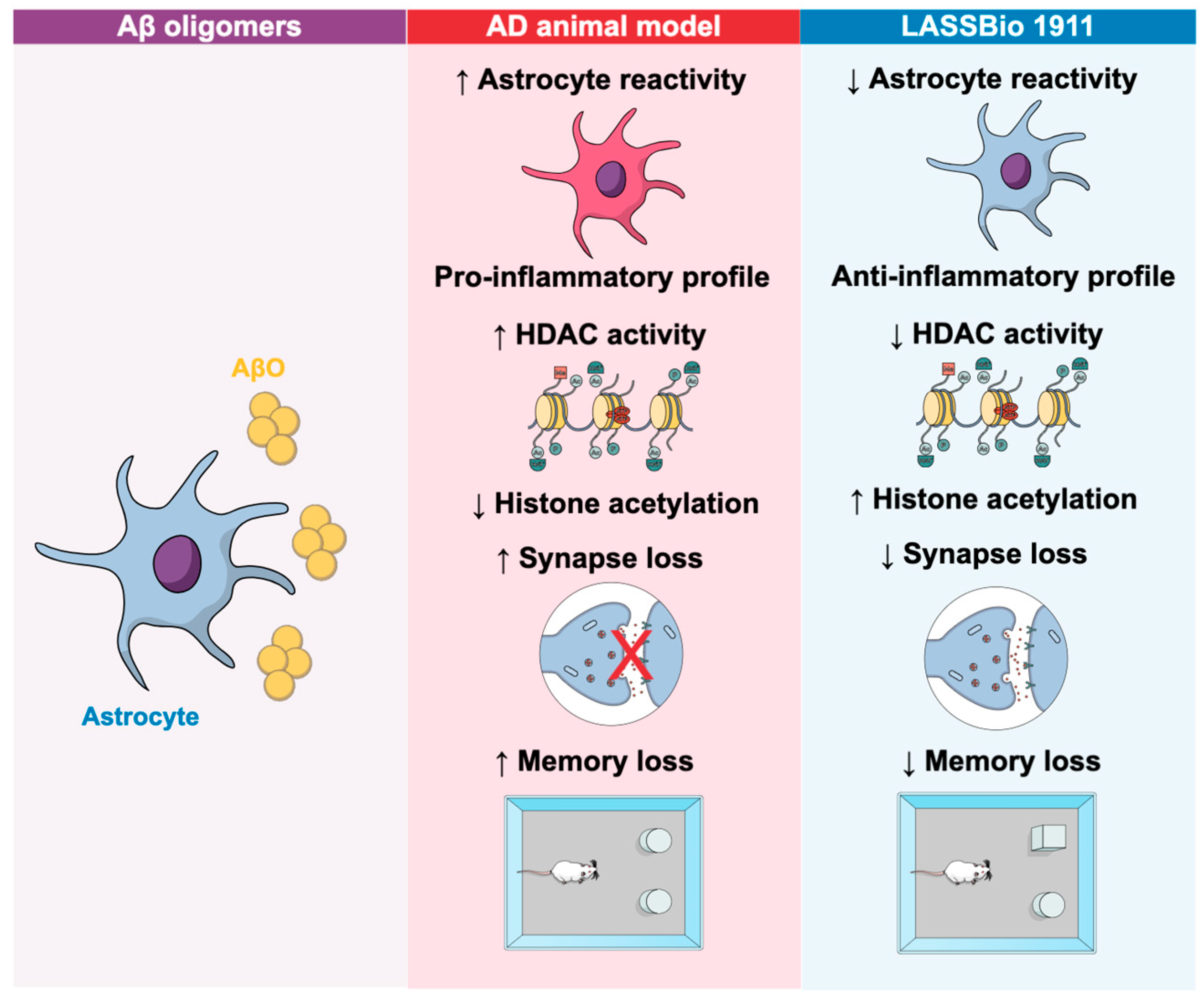
4.1. Implications of HDACs and HDACi in Alzheimer’s Disease
| HDAC | Changes in AD | Observed Effects | Brain Region | Reference |
|---|---|---|---|---|
| HDAC1 | ↓ Reduced | Correlation with ↑ Aβ and tau, brain atrophy, cognitive decline | General | [51] |
| HDAC2 | ↑ Elevated | Tau hyperphosphorylation, aggregation, dendritic abnormalities | General | [53,54,55] |
| HDAC3 | ↑ Elevated | Reduced dendritic spine density, neuroinflammation | Hippocampus | [56,58] |
| HDAC4 | ↑ Elevated | Associated with amyloid pathology, synaptic dysfunction | Multiple regions | [59,60] |
| HDAC5 | Variable | Loss impairs memory without impacting AD pathogenesis | - | [61] |
| HDAC6 | ↑ Elevated | Contributes to neurodegeneration, possible beneficial effects | Cortex, Hippocampus | [62,63,64,65] |
| Inhibitor | Class/Target | Mechanism of Action | Effects in AD | Model/Study | Reference |
|---|---|---|---|---|---|
| Tacedinaline | Class I | Restores ER-mitochondria crosstalk | ↓ ER-Ca2+ retention, ↓ mitochondrial depolarization | AD models | [56] |
| SAHA | Pan-HDAC | Restores interneuron activity | Rescues gamma oscillation deficits in hippocampus | PSAPP mice | [74] |
| BG45 | Class I | Modulates GRIP1/AMPA receptor pathway | ↑ AMPA subunit expression, ↓ neuronal loss | APP/PS1, APP cells | [75] |
| TSA | Pan-HDAC | Restores H4 acetylation | Improves memory performance and LTP | APP/PS1 | [73] |
| Sodium butyrate | Pan-HDAC | ↑ Hippocampal histone acetylation | Enhances associative memory, ↑ synaptic plasticity | 5XFAD, AD models | [71,72] |
| T-518 | HDAC6 selective | Crosses blood/brain barrier | Behavioral and pathological improvements | Tauopathy model | [66] |
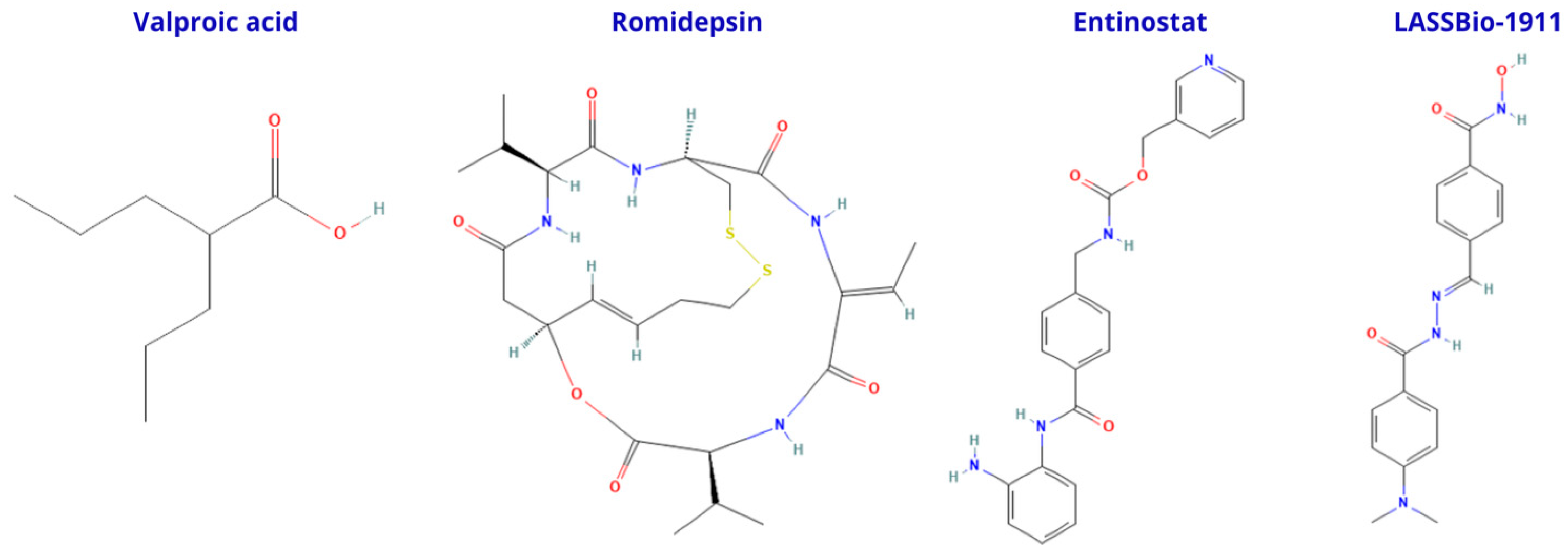
| LASSBio-1911 | HDAC6 | ↑ Histone acetylation | ↓ Inflammatory cytokines, ↓ astrocyte reactivity, cognitive rescue | Aβ oligomers | [57] |
| Compounds 11b/6a | HDAC6/Class IIa | Selective isoform inhibition | ↓ Aβ aggregation, ↓ oligomers, AChE inhibition | In Vitro | [67] |
| W2 and I2 | HDACi | ↓ Aβ-producing enzymes, ↑ degrading enzymes | ↓ Aβ levels, improved learning deficits | AD model | [69] |
4.2. Implications of HDACs and HDACi in Parkinson’s Disease
| Inhibitor | Class/Target | Mechanism of Action | Effects in PD | Model/Study | Reference |
|---|---|---|---|---|---|
| Entinostat | Pan-HDAC | Downregulates HDAC enzymes | ↓ PD markers, ↑ neuroprotective genes, normalized neurotransmitters | Rat PD model | [84] |
| NMJ-2/NMJ-3 | HDACi (novel) | Reduces oxidative stress and inflammation | ↓ Motor/non-motor deficits, restored dopamine levels | MPTP rat model | [85] |
| Valproic acid | Pan-HDAC | Anti-inflammatory, antioxidant | ↓ Motor/non-motor deficits, restored dopamine levels | MPTP rat model | [85] |
| HGC | HDAC6 selective | Acetylation of NDUFV1 protein | Protected dopaminergic neurons, improved behavior, maintained mitochondrial function | MPTP mouse model | [86] |
| MC1568 | HDAC6 selective | Neuroprotective, anti-inflammatory | Protected dopaminergic neurons, ↓ microglial activation, ↓ forelimb akinesia | 6-OHDA rat model | [87] |
| Tubastatin A | HDAC6 selective | Activates chaperone-mediated autophagy | ↑ α-tubulin acetylation, ↓ phosphorylated α-synuclein | Rat PD model | [82] |
5. Epigenetic Alterations in Human Brain Tissue: HDAC Dysregulation in NDDs
6. Clinical Trials with HDACi
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid-beta peptides |
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| GCIs | glial cytoplasmic inclusions |
| H4 | histone 4 |
| HD | Huntington’s disease |
| HDACs | histone deacetylases |
| HDACi | histone deacetylase inhibitors |
| HATs | histone acetyltransferases |
| LBs | Lewy bodies |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NDDs | neurodegenerative diseases |
| PD | Parkinson’s disease |
| PET | positron emission tomography |
References
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.Y.; Chen, X.; Liu, G.H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J.; et al. Antiageing strategy for neurodegenerative diseases: From mechanisms to clinical advances. Signal Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef]
- Xiao, T.; Chen, Z.; Xie, Y.; Yang, C.; Wu, J.; Gao, L. Histone deacetylase inhibitors: Targeting epigenetic regulation in the treatment of acute leukemia. Ther. Adv. Hematol. 2024, 15, 20406207241283277. [Google Scholar] [CrossRef]
- Chuang, D.M.; Leng, Y.; Marinova, Z.; Kim, H.J.; Chiu, C.T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009, 32, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, G.; Mercatelli, D.; Di Muzio, G.; Triboli, L.; De Rosa, P.; Perini, G.; Giorgi, F.M. Histone Deacetylases (HDACs): Evolution, Specificity, Role in Transcriptional Complexes, and Pharmacological Actionability. Genes 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kundu, S.; Singh, A.; Singh, S. Understanding the Role of Histone Deacetylase and their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr. Neuropharmacol. 2022, 20, 158–178. [Google Scholar] [CrossRef]
- Bonomi, R.E.; Riordan, W.; Gelovani, J.G. The Structures, Functions, and Roles of Class III HDACs (Sirtuins) in Neuropsychiatric Diseases. Cells 2024, 13, 1644. [Google Scholar] [CrossRef]
- Han, B.; Wang, M.; Li, J.; Chen, Q.; Sun, N.; Yang, X.; Zhang, Q. Perspectives and new aspects of histone deacetylase inhibitors in the therapy of CNS diseases. Eur. J. Med. Chem. 2023, 258, 115613. [Google Scholar] [CrossRef]
- MacDonald, J.L.; Roskams, A.J. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev. Dyn. 2008, 237, 2256–2267. [Google Scholar] [CrossRef]
- Nieto-Estevez, V.; Changarathil, G.; Adeyeye, A.O.; Coppin, M.O.; Kassim, R.S.; Zhu, J.; Hsieh, J. HDAC1 Regulates Neuronal Differentiation. Front. Mol. Neurosci. 2021, 14, 815808. [Google Scholar] [CrossRef]
- Wang, H.; Matise, M.P. Tcf7l2/Tcf4 Transcriptional Repressor Function Requires HDAC Activity in the Developing Vertebrate CNS. PLoS ONE 2016, 11, e0163267. [Google Scholar] [CrossRef]
- Foti, S.B.; Chou, A.; Moll, A.D.; Roskams, A.J. HDAC inhibitors dysregulate neural stem cell activity in the postnatal mouse brain. Int. J. Dev. Neurosci. 2013, 31, 434–447. [Google Scholar] [CrossRef]
- Montgomery, R.L.; Hsieh, J.; Barbosa, A.C.; Richardson, J.A.; Olson, E.N. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc. Natl. Acad. Sci. USA 2009, 106, 7876–7881. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Y.; Wang, Y.; Cai, Z.; Lu, Z.; Li, L.; Huang, R.; Hagelkruys, A.; Matthias, P.; Zhang, H.; et al. HDAC1 and HDAC2 Regulate Intermediate Progenitor Positioning to Safeguard Neocortical Development. Neuron 2019, 101, 1117–1133.e5. [Google Scholar] [CrossRef]
- Hagelkruys, A.; Lagger, S.; Krahmer, J.; Leopoldi, A.; Artaker, M.; Pusch, O.; Zezula, J.; Weissmann, S.; Xie, Y.; Schofer, C.; et al. A single allele of Hdac2 but not Hdac1 is sufficient for normal mouse brain development in the absence of its paralog. Development 2014, 141, 604–616. [Google Scholar] [CrossRef]
- Jawerka, M.; Colak, D.; Dimou, L.; Spiller, C.; Lagger, S.; Montgomery, R.L.; Olson, E.N.; Wurst, W.; Gottlicher, M.; Gotz, M. The specific role of histone deacetylase 2 in adult neurogenesis. Neuron Glia Biol. 2010, 6, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, G.; Lilja, T.; Wallenborg, K.; Falcao, A.M.; Marques, S.C.; Gracias, A.; Solum, D.; Paap, R.; Walfridsson, J.; Teixeira, A.I.; et al. Neural stem cell differentiation is dictated by distinct actions of nuclear receptor corepressors and histone deacetylases. Stem Cell Rep. 2014, 3, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ruan, H.; Guo, X.; Li, L.; Shen, W. HDAC1 regulates the proliferation of radial glial cells in the developing Xenopus tectum. PLoS ONE 2015, 10, e0120118. [Google Scholar] [CrossRef]
- Cunliffe, V.T.; Casaccia-Bonnefil, P. Histone deacetylase 1 is essential for oligodendrocyte specification in the zebrafish CNS. Mech. Dev. 2006, 123, 24–30. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R. Histone deacetylase-3: Friend and foe of the brain. Exp. Biol. Med. 2020, 245, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hsieh, J. HDAC3 controls gap 2/mitosis progression in adult neural stem/progenitor cells by regulating CDK1 levels. Proc. Natl. Acad. Sci. USA 2014, 111, 13541–13546. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, J.; Yang, X.J. Histone Deacetylase 3 Governs Perinatal Cerebral Development via Neural Stem and Progenitor Cells. iScience 2019, 20, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Shaked, M.; Weissmuller, K.; Svoboda, H.; Hortschansky, P.; Nishino, N.; Wolfl, S.; Tucker, K.L. Histone deacetylases control neurogenesis in embryonic brain by inhibition of BMP2/4 signaling. PLoS ONE 2008, 3, e2668. [Google Scholar] [CrossRef]
- Liu, H.; Wu, H.; Wang, Y.; Wang, Y.; Wu, X.; Ju, S.; Wang, X. Inhibition of class II histone deacetylase blocks proliferation and promotes neuronal differentiation of the embryonic rat neural progenitor cells. Acta Neurobiol. Exp. 2012, 72, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Cavalli, V. HDAC signaling in neuronal development and axon regeneration. Curr. Opin. Neurobiol. 2014, 27, 118–126. [Google Scholar] [CrossRef]
- Bornelov, S.; Reynolds, N.; Xenophontos, M.; Gharbi, S.; Johnstone, E.; Floyd, R.; Ralser, M.; Signolet, J.; Loos, R.; Dietmann, S.; et al. The Nucleosome Remodeling and Deacetylation Complex Modulates Chromatin Structure at Sites of Active Transcription to Fine-Tune Gene Expression. Mol. Cell 2018, 71, 56–72 e54. [Google Scholar] [CrossRef]
- Sun, Y.E.; Cheng, L.; Hu, K. With NuRD, HDACs Go “Nerdy”. Dev. Cell 2014, 30, 9–10. [Google Scholar] [CrossRef]
- Kim, D.; Frank, C.L.; Dobbin, M.M.; Tsunemoto, R.K.; Tu, W.; Peng, P.L.; Guan, J.S.; Lee, B.H.; Moy, L.Y.; Giusti, P.; et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 2008, 60, 803–817. [Google Scholar] [CrossRef]
- D’Mello, S.R. Histone deacetylases as targets for the treatment of human neurodegenerative diseases. Drug News Perspect. 2009, 22, 513–524. [Google Scholar] [CrossRef]
- Perry, S.; Kiragasi, B.; Dickman, D.; Ray, A. The Role of Histone Deacetylase 6 in Synaptic Plasticity and Memory. Cell Rep. 2017, 18, 1337–1345. [Google Scholar] [CrossRef]
- LoPresti, P. HDAC6 in Diseases of Cognition and of Neurons. Cells 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, M.M.; Madabhushi, R.; Pan, L.; Chen, Y.; Kim, D.; Gao, J.; Ahanonu, B.; Pao, P.C.; Qiu, Y.; Zhao, Y.; et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 2013, 16, 1008–1015. [Google Scholar] [CrossRef]
- Majdzadeh, N.; Wang, L.; Morrison, B.E.; Bassel-Duby, R.; Olson, E.N.; D’Mello, S.R. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev. Neurobiol. 2008, 68, 1076–1092. [Google Scholar] [CrossRef]
- Dai, Y.; Wei, T.; Shen, Z.; Bei, Y.; Lin, H.; Dai, H. Classical HDACs in the regulation of neuroinflammation. Neurochem. Int. 2021, 150, 105182. [Google Scholar] [CrossRef]
- Niu, H.; Song, W.; Pei, D.; Ma, C.; Liu, F.; Li, Y.; Han, S. Depleted histone deacetylase 3 or restored microRNA-19b-1-5p facilitates recovery of spinal cord injury via inactivating JAK2/STAT3 signaling pathway. Genomics 2022, 114, 110262. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Liu, L.; Jiang, M.; Zhao, C.; Wang, H.; He, D.; Zheng, T.; Zhou, X.; Hassan, A.; et al. Hdac3 Interaction with p300 Histone Acetyltransferase Regulates the Oligodendrocyte and Astrocyte Lineage Fate Switch. Dev. Cell 2016, 36, 316–330. [Google Scholar] [CrossRef]
- Ye, J.; Zhong, S.; Deng, Y.; Yao, X.; Liu, Q.; Wang, J.Z.; Xiao, S. HDAC7 Activates IKK/NF-kappaB Signaling to Regulate Astrocyte-Mediated Inflammation. Mol. Neurobiol. 2022, 59, 6141–6157. [Google Scholar] [CrossRef]
- Campos, B.; Bermejo, J.L.; Han, L.; Felsberg, J.; Ahmadi, R.; Grabe, N.; Reifenberger, G.; Unterberg, A.; Herold-Mende, C. Expression of nuclear receptor corepressors and class I histone deacetylases in astrocytic gliomas. Cancer Sci. 2011, 102, 387–392. [Google Scholar] [CrossRef]
- Lucio-Eterovic, A.K.; Cortez, M.A.; Valera, E.T.; Motta, F.J.; Queiroz, R.G.; Machado, H.R.; Carlotti, C.G., Jr.; Neder, L.; Scrideli, C.A.; Tone, L.G. Differential expression of 12 histone deacetylase (HDAC) genes in astrocytomas and normal brain tissue: Class II and IV are hypoexpressed in glioblastomas. BMC Cancer 2008, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.J.; Amano, I.; Imai, N.; Soares De Oliveira, L.; Vella, K.R.; Hollenberg, A.N. Nuclear Receptor CoRepressors, NCOR1 and SMRT, are required for maintaining systemic metabolic homeostasis. Mol. Metab. 2021, 53, 101315. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.S.; Choi, S.; Khattar, P.; Choi, N.; Lee, S.C. Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J. Neuroimmune Pharmacol. 2010, 5, 521–532. [Google Scholar] [CrossRef]
- Kannan, V.; Brouwer, N.; Hanisch, U.K.; Regen, T.; Eggen, B.J.; Boddeke, H.W. Histone deacetylase inhibitors suppress immune activation in primary mouse microglia. J. Neurosci. Res. 2013, 91, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Kanski, R.; Sneeboer, M.A.; van Bodegraven, E.J.; Sluijs, J.A.; Kropff, W.; Vermunt, M.W.; Creyghton, M.P.; De Filippis, L.; Vescovi, A.; Aronica, E.; et al. Histone acetylation in astrocytes suppresses GFAP and stimulates a reorganization of the intermediate filament network. J. Cell Sci. 2014, 127, 4368–4380. [Google Scholar] [CrossRef]
- Auzmendi-Iriarte, J.; Moreno-Cugnon, L.; Saenz-Antonanzas, A.; Grassi, D.; de Pancorbo, M.M.; Arevalo, M.A.; Wood, I.C.; Matheu, A. High levels of HDAC expression correlate with microglial aging. Expert. Opin. Ther. Targets 2022, 26, 911–922. [Google Scholar] [CrossRef]
- Haage, V.; Elmadany, N.; Roll, L.; Faissner, A.; Gutmann, D.H.; Semtner, M.; Kettenmann, H. Tenascin C regulates multiple microglial functions involving TLR4 signaling and HDAC1. Brain Behav. Immun. 2019, 81, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.S.; Grigg, R.; Wood, I.C. Inhibition of histone deacetylase 1 or 2 reduces induced cytokine expression in microglia through a protein synthesis independent mechanism. J. Neurochem. 2017, 143, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Meleady, L.; Towriss, M.; Kim, J.; Bacarac, V.; Dang, V.; Rowland, M.E.; Ciernia, A.V. Histone deacetylase 3 regulates microglial function through histone deacetylation. Epigenetics 2023, 18, 2241008. [Google Scholar] [CrossRef]
- Datta, M.; Staszewski, O.; Raschi, E.; Frosch, M.; Hagemeyer, N.; Tay, T.L.; Blank, T.; Kreutzfeldt, M.; Merkler, D.; Ziegler-Waldkirch, S.; et al. Histone Deacetylases 1 and 2 Regulate Microglia Function during Development, Homeostasis, and Neurodegeneration in a Context-Dependent Manner. Immunity 2018, 48, 514–529.e6. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, L.; Yu, C.; Yu, D.; Yu, G. Histone Acetylation Modifiers in the Pathogenesis of Alzheimer’s Disease. Front. Cell Neurosci. 2015, 9, 226. [Google Scholar] [CrossRef]
- Schueller, E.; Paiva, I.; Blanc, F.; Wang, X.L.; Cassel, J.C.; Boutillier, A.L.; Bousiges, O. Dysregulation of histone acetylation pathways in hippocampus and frontal cortex of Alzheimer’s disease patients. Eur. Neuropsychopharmacol. 2020, 33, 101–116. [Google Scholar] [CrossRef]
- Pascoal, T.A.; Chamoun, M.; Lax, E.; Wey, H.Y.; Shin, M.; Ng, K.P.; Kang, M.S.; Mathotaarachchi, S.; Benedet, A.L.; Therriault, J.; et al. [(11)C]Martinostat PET analysis reveals reduced HDAC I availability in Alzheimer’s disease. Nat. Commun. 2022, 13, 4171. [Google Scholar] [CrossRef]
- Pao, P.C.; Patnaik, D.; Watson, L.A.; Gao, F.; Pan, L.; Wang, J.; Adaikkan, C.; Penney, J.; Cam, H.P.; Huang, W.C.; et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 2020, 11, 2484. [Google Scholar] [CrossRef]
- Hu, C.; Hu, J.; Meng, X.; Zhang, H.; Shen, H.; Huang, P.; Schachner, M.; Zhao, W. L1CAM Beneficially Inhibits Histone Deacetylase 2 Expression under Conditions of Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 382–392. [Google Scholar] [CrossRef]
- Liu, D.; Tang, H.; Li, X.Y.; Deng, M.F.; Wei, N.; Wang, X.; Zhou, Y.F.; Wang, D.Q.; Fu, P.; Wang, J.Z.; et al. Targeting the HDAC2/HNF-4A/miR-101b/AMPK Pathway Rescues Tauopathy and Dendritic Abnormalities in Alzheimer’s Disease. Mol. Ther. 2017, 25, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Cheng, J.; Penney, J.; Gao, F.; Rueda, R.; Wang, J.; Yamakawa, S.; Kritskiy, O.; Gjoneska, E.; Tsai, L.H. The Transcription Factor Sp3 Cooperates with HDAC2 to Regulate Synaptic Function and Plasticity in Neurons. Cell Rep. 2017, 20, 1319–1334. [Google Scholar] [CrossRef]
- Marinho, D.; Ferreira, I.L.; Lorenzoni, R.; Cardoso, S.M.; Santana, I.; Rego, A.C. Reduction of class I histone deacetylases ameliorates ER-mitochondria cross-talk in Alzheimer’s disease. Aging Cell 2023, 22, e13895. [Google Scholar] [CrossRef]
- Diniz, L.P.; Morgado, J.; Bergamo Araujo, A.P.; da Silva Antonio, L.M.; Mota-Araujo, H.P.; de Sena Murteira Pinheiro, P.; Sagrillo, F.S.; Cesar, G.V.; Ferreira, S.T.; Figueiredo, C.P.; et al. Histone deacetylase inhibition mitigates cognitive deficits and astrocyte dysfunction induced by amyloid-beta (Abeta) oligomers. Br. J. Pharmacol. 2024, 181, 4028–4049. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, S.; Yu, L.; Jin, J.; Ye, X.; Liu, Y.; Xu, Y. HDAC3 negatively regulates spatial memory in a mouse model of Alzheimer’s disease. Aging Cell 2017, 16, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Chang, C.W.; Yang, B.H.; Gelovani, J.G.; Liu, R.S. Evaluation of Class IIa Histone Deacetylases Expression and In Vivo Epigenetic Imaging in a Transgenic Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 8633. [Google Scholar] [CrossRef] [PubMed]
- Colussi, C.; Aceto, G.; Ripoli, C.; Bertozzi, A.; Li Puma, D.D.; Paccosi, E.; D’Ascenzo, M.; Grassi, C. Cytoplasmic HDAC4 recovers synaptic function in the 3xTg mouse model of Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2023, 49, e12861. [Google Scholar] [CrossRef]
- Agis-Balboa, R.C.; Pavelka, Z.; Kerimoglu, C.; Fischer, A. Loss of HDAC5 impairs memory function: Implications for Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sheng, S.; Qin, C. The role of HDAC6 in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Mondal, P.; Bagdasarian, F.A.; Rani, N.; Liu, Y.; Gomm, A.; Tocci, D.R.; Choi, S.H.; Wey, H.Y.; Tanzi, R.E.; et al. Development of a potential PET probe for HDAC6 imaging in Alzheimer’s disease. Acta Pharm. Sin. B 2022, 12, 3891–3904. [Google Scholar] [CrossRef]
- Govindarajan, N.; Rao, P.; Burkhardt, S.; Sananbenesi, F.; Schluter, O.M.; Bradke, F.; Lu, J.; Fischer, A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 52–63. [Google Scholar] [CrossRef]
- Mondal, P.; Bai, P.; Gomm, A.; Bakiasi, G.; Lin, C.J.; Wang, Y.; Choi, S.H.; Tanzi, R.E.; Wang, C.; Zhang, C. Structure-Based Discovery of A Small Molecule Inhibitor of Histone Deacetylase 6 (HDAC6) that Significantly Reduces Alzheimer’s Disease Neuropathology. Adv. Sci. 2024, 11, e2304545. [Google Scholar] [CrossRef]
- Onishi, T.; Maeda, R.; Terada, M.; Sato, S.; Fujii, T.; Ito, M.; Hashikami, K.; Kawamoto, T.; Tanaka, M. A novel orally active HDAC6 inhibitor T-518 shows a therapeutic potential for Alzheimer’s disease and tauopathy in mice. Sci. Rep. 2021, 11, 15423. [Google Scholar] [CrossRef]
- Tseng, H.J.; Lin, M.H.; Shiao, Y.J.; Yang, Y.C.; Chu, J.C.; Chen, C.Y.; Chen, Y.Y.; Lin, T.E.; Su, C.J.; Pan, S.L.; et al. Synthesis and biological evaluation of acridine-based histone deacetylase inhibitors as multitarget agents against Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112193. [Google Scholar] [CrossRef]
- Langley, B.; Gensert, J.M.; Beal, M.F.; Ratan, R.R. Remodeling chromatin and stress resistance in the central nervous system: Histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.M.; Lee, T.; Yoon, H.; DiBattista, A.M.; Song, J.M.; Sohn, Y.; Moffat, E.I.; Turner, R.S.; Jung, M.; Kim, J.; et al. Mercaptoacetamide-based class II HDAC inhibitor lowers Abeta levels and improves learning and memory in a mouse model of Alzheimer’s disease. Exp. Neurol. 2013, 239, 192–201. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimers Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, K.; Li, X.; Xu, L.; Yang, Z. Sodium butyrate ameliorates the impairment of synaptic plasticity by inhibiting the neuroinflammation in 5XFAD mice. Chem. Biol. Interact. 2021, 341, 109452. [Google Scholar] [CrossRef]
- Francis, Y.I.; Fa, M.; Ashraf, H.; Zhang, H.; Staniszewski, A.; Latchman, D.S.; Arancio, O. Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2009, 18, 131–139. [Google Scholar] [CrossRef]
- Takasu, K.; Niidome, K.; Hasegawa, M.; Ogawa, K. Histone Deacetylase Inhibitor Improves the Dysfunction of Hippocampal Gamma Oscillations and Fast Spiking Interneurons in Alzheimer’s Disease Model Mice. Front. Mol. Neurosci. 2021, 14, 782206. [Google Scholar] [CrossRef]
- Han, Y.; Chen, L.; Liu, J.; Chen, J.; Wang, C.; Guo, Y.; Yu, X.; Zhang, C.; Chu, H.; Ma, H. A Class I HDAC Inhibitor Rescues Synaptic Damage and Neuron Loss in APP-Transfected Cells and APP/PS1 Mice through the GRIP1/AMPA Pathway. Molecules 2022, 27, 4160. [Google Scholar] [CrossRef]
- Kontopoulos, E.; Parvin, J.D.; Feany, M.B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006, 15, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.; Jo, A.; Khang, R.; Park, C.H.; Park, S.J.; Kwag, E.; Shin, J.H. alpha-Synuclein A53T Binds to Transcriptional Adapter 2-Alpha and Blocks Histone H3 Acetylation. Int. J. Mol. Sci. 2021, 22, 5392. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Collins, L.M.; Sullivan, A.M.; O’Keeffe, G.W. The class II histone deacetylases as therapeutic targets for Parkinson’s disease. Neuronal Signal. 2020, 4, NS20200001. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kovacs, J.J.; McLaurin, A.; Vance, J.M.; Ito, A.; Yao, T.P. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 2003, 115, 727–738. [Google Scholar] [CrossRef]
- Miki, Y.; Mori, F.; Tanji, K.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Accumulation of histone deacetylase 6, an aggresome-related protein, is specific to Lewy bodies and glial cytoplasmic inclusions. Neuropathology 2011, 31, 561–568. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Z.; Lin, S.; Chen, L.; Dzreyan, V.; Eid, M.; Demyanenko, S.; He, B. Histone Deacetylases as Epigenetic Targets for Treating Parkinson’s Disease. Brain Sci. 2022, 12, 672. [Google Scholar] [CrossRef]
- Francelle, L.; Outeiro, T.F.; Rappold, G.A. Inhibition of HDAC6 activity protects dopaminergic neurons from alpha-synuclein toxicity. Sci. Rep. 2020, 10, 6064. [Google Scholar] [CrossRef]
- Lemos, M.; Stefanova, N. Histone Deacetylase 6 and the Disease Mechanisms of alpha-Synucleinopathies. Front. Synaptic Neurosci. 2020, 12, 586453. [Google Scholar] [CrossRef]
- Li, H.; Shi, G.; Zha, H.; Zheng, L.; Luo, Z.; Wang, Y. Inhibition of histone deacetylase promotes a neuroprotective mechanism in an experimental model of Parkinson’s disease. Arch. Med. Sci. 2024, 20, 664–674. [Google Scholar] [CrossRef]
- Meka, S.T.; Bojja, S.L.; Kumar, G.; Birangal, S.R.; Rao, C.M. Novel HDAC inhibitors provide neuroprotection in MPTP-induced Parkinson’s disease model of rats. Eur. J. Pharmacol. 2023, 959, 176067. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Wang, Y.; Zhang, J.; Ding, J.; Liu, X.; Jin, Y.; Lian, B.; Ling, Y.; Sun, C. Acetylation of NDUFV1 induced by a newly synthesized HDAC6 inhibitor HGC rescues dopaminergic neuron loss in Parkinson models. iScience 2021, 24, 102302. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Goulding, S.R.; Morales-Prieto, N.; Foley, T.; Collins, L.M.; Sullivan, A.M.; O’Keeffe, G.W. Peripheral administration of the Class-IIa HDAC inhibitor MC1568 partially protects against nigrostriatal neurodegeneration in the striatal 6-OHDA rat model of Parkinson’s disease. Brain Behav. Immun. 2022, 102, 151–160. [Google Scholar] [CrossRef]
- Alsadany, M.A.; Shehata, H.H.; Mohamad, M.I.; Mahfouz, R.G. Histone deacetylases enzyme, copper, and IL-8 levels in patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2013, 28, 54–61. [Google Scholar] [CrossRef]
- Marzi, S.J.; Leung, S.K.; Ribarska, T.; Hannon, E.; Smith, A.R.; Pishva, E.; Poschmann, J.; Moore, K.; Troakes, C.; Al-Sarraj, S.; et al. A histone acetylome-wide association study of Alzheimer’s disease identifies disease-associated H3K27ac differences in the entorhinal cortex. Nat. Neurosci. 2018, 21, 1618–1627. [Google Scholar] [CrossRef]
- Santana, D.A.; Smith, M.A.C.; Chen, E.S. Histone Modifications in Alzheimer’s Disease. Genes 2023, 14, 347. [Google Scholar] [CrossRef]
- Graff, J.; Rei, D.; Guan, J.S.; Wang, W.Y.; Seo, J.; Hennig, K.M.; Nieland, T.J.; Fass, D.M.; Kao, P.F.; Kahn, M.; et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 2012, 483, 222–226. [Google Scholar] [CrossRef]
- Toker, L.; Tran, G.T.; Sundaresan, J.; Tysnes, O.B.; Alves, G.; Haugarvoll, K.; Nido, G.S.; Dolle, C.; Tzoulis, C. Genome-wide histone acetylation analysis reveals altered transcriptional regulation in the Parkinson’s disease brain. Mol. Neurodegener. 2021, 16, 31. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subbanna, S. Unlocking the epigenetic symphony: Histone acetylation’s impact on neurobehavioral change in neurodegenerative disorders. Epigenomics 2024, 16, 331–358. [Google Scholar] [CrossRef]
- Yeh, H.H.; Young, D.; Gelovani, J.G.; Robinson, A.; Davidson, Y.; Herholz, K.; Mann, D.M. Histone deacetylase class II and acetylated core histone immunohistochemistry in human brains with Huntington’s disease. Brain Res. 2013, 1504, 16–24. [Google Scholar] [CrossRef]
- Narayan, P.; Reid, S.; Scotter, E.L.; McGregor, A.L.; Mehrabi, N.F.; Singh-Bains, M.K.; Glass, M.; Faull, R.L.M.; Snell, R.G.; Dragunow, M. Inconsistencies in histone acetylation patterns among different HD model systems and HD post-mortem brains. Neurobiol. Dis. 2020, 146, 105092. [Google Scholar] [CrossRef]
- Dios, A.M.; Babu, S.; Granucci, E.J.; Mueller, K.A.; Mills, A.N.; Alshikho, M.J.; Zurcher, N.R.; Cernasov, P.; Gilbert, T.M.; Glass, J.D.; et al. Class I and II histone deacetylase expression is not altered in human amyotrophic lateral sclerosis: Neuropathological and positron emission tomography molecular neuroimaging evidence. Muscle Nerve 2019, 60, 443–452. [Google Scholar] [CrossRef]
- Guo, W.; Naujock, M.; Fumagalli, L.; Vandoorne, T.; Baatsen, P.; Boon, R.; Ordovas, L.; Patel, A.; Welters, M.; Vanwelden, T.; et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017, 8, 861. [Google Scholar] [CrossRef]
- Saleem, A.; Safdar, A. Exercise-induced histone acetylation—Playing tag with the genome. J. Physiol. 2010, 588, 905–906. [Google Scholar] [CrossRef]
- Kaliman, P.; Alvarez-Lopez, M.J.; Cosin-Tomas, M.; Rosenkranz, M.A.; Lutz, A.; Davidson, R.J. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology 2014, 40, 96–107. [Google Scholar] [CrossRef]
- Sborov, D.W.; Canella, A.; Hade, E.M.; Mo, X.; Khountham, S.; Wang, J.; Ni, W.; Poi, M.; Coss, C.; Liu, Z.; et al. A phase 1 trial of the HDAC inhibitor AR-42 in patients with multiple myeloma and T- and B-cell lymphomas. Leuk. Lymphoma 2017, 58, 2310–2318. [Google Scholar] [CrossRef]
- Kelly, W.K.; Richon, V.M.; O’Connor, O.; Curley, T.; MacGregor-Curtelli, B.; Tong, W.; Klang, M.; Schwartz, L.; Richardson, S.; Rosa, E.; et al. Phase I clinical trial of histone deacetylase inhibitor: Suberoylanilide hydroxamic acid administered intravenously. Clin. Cancer Res. 2003, 9, 3578–3588. [Google Scholar]
- Krug, L.M.; Curley, T.; Schwartz, L.; Richardson, S.; Marks, P.; Chiao, J.; Kelly, W.K. Potential role of histone deacetylase inhibitors in mesothelioma: Clinical experience with suberoylanilide hydroxamic acid. Clin. Lung Cancer 2006, 7, 257–261. [Google Scholar] [CrossRef]
- Munster, P.N.; Marchion, D.; Thomas, S.; Egorin, M.; Minton, S.; Springett, G.; Lee, J.H.; Simon, G.; Chiappori, A.; Sullivan, D.; et al. Phase I trial of vorinostat and doxorubicin in solid tumours: Histone deacetylase 2 expression as a predictive marker. Br. J. Cancer 2009, 101, 1044–1050. [Google Scholar] [CrossRef]
- Munster, P.N.; Marchion, D.C.; Bicaku, E.; Sullivan, P.; Beam, C.; Mahany, J.J.; Lush, R.; Sullivan, D.M.; Daud, A. Phase I trial of the histone deacetylase inhibitor, valproic acid and the topoisomerase II inhibitor, epirubicin: A clinical and translational Study. J. Clin. Oncol. 2005, 23 (Suppl. 16), 3084. [Google Scholar] [CrossRef]
- Sandor, V.; Bakke, S.; Robey, R.W.; Kang, M.H.; Blagosklonny, M.V.; Bender, J.; Brooks, R.; Piekarz, R.L.; Tucker, E.; Figg, W.D.; et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res. 2002, 8, 718–728. [Google Scholar]
- Beck, J.; Fischer, T.; Rowinsky, E.; Huber, C.; Mita, M.; Atadja, P.; Peng, B.; Kwong, C.; Dugan, M.; Patnaik, A. Phase I pharmacokinetic and pharmacodynamic study of LBH589: A novel histone deacetylase inhibitor. J. Clin. Oncol. 2004, 22, 3025. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Kaźmierczak, M.; Fong, C.Y.; Montesinos, P.; Venditti, A.; Mappa, S.; Spezia, R.; Ades, L. A Phase 3 Randomized Study (PRIMULA) of the Epigenetic Combination of Pracinostat, a Pan-Histone Deacetylase (HDAC) Inhibitor, with Azacitidine (AZA) in Patients with Newly Diagnosed Acute Myeloid Leukemia (AML) Unfit for Standard Intensive Chemotherapy (IC). Blood 2019, 134, 2652. [Google Scholar] [CrossRef]
- Bukowinski, A.; Chang, B.; Reid, J.M.; Liu, X.; Minard, C.G.; Trepel, J.B.; Lee, M.J.; Fox, E.; Weigel, B.J. A phase 1 study of entinostat in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1513, Pediatric Early Phase-Clinical Trial Network (PEP-CTN). Pediatr. Blood Cancer 2021, 68, e28892. [Google Scholar] [CrossRef]
- Piekarz, R.L.; Frye, R.; Turner, M.; Wright, J.J.; Allen, S.L.; Kirschbaum, M.H.; Zain, J.; Prince, H.M.; Leonard, J.P.; Geskin, L.J.; et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 2009, 27, 5410–5417. [Google Scholar] [CrossRef]
- Chu, Q.S.; Nielsen, T.O.; Alcindor, T.; Gupta, A.; Endo, M.; Goytain, A.; Xu, H.; Verma, S.; Tozer, R.; Knowling, M.; et al. A phase II study of SB939, a novel pan-histone deacetylase inhibitor, in patients with translocation-associated recurrent/metastatic sarcomas-NCIC-CTG IND 200dagger. Ann. Oncol. 2015, 26, 973–981. [Google Scholar] [CrossRef]
- Iwamoto, F.M.; Lamborn, K.R.; Kuhn, J.G.; Wen, P.Y.; Yung, W.K.; Gilbert, M.R.; Chang, S.M.; Lieberman, F.S.; Prados, M.D.; Fine, H.A. A phase I/II trial of the histone deacetylase inhibitor romidepsin for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro Oncol. 2011, 13, 509–516. [Google Scholar] [CrossRef] [PubMed]
| HDACi/Combination | Phase | Cancer Type | Key Findings | Reference |
|---|---|---|---|---|
| AR-42 | Phase I | Lymphoma, Multiple Myeloma | Safe, CD44 reduction | [100] |
| SAHA (i.v.) | Phase I | Solid and hematologic tumors | Well tolerated, increased histone acetylation | [101] |
| SAHA (oral) | Phase I | Mesothelioma | Two partial responses; manageable side effects | [102] |
| Vorinostat + Doxorubicin | Phase I | Breast, Prostate, Melanoma | HDAC2 expression predicts response | [103] |
| Valproic Acid + Epirubicin | Phase I | Not specified | Preclinical synergy explored | [104] |
| Depsipeptide | Phase I | Refractory neoplasms | Dose-limiting toxicity observed | [105] |
| LBH589A | Phase I | Various cancers | Grade 3 neutropenia and hypoglycemia | [106] |
| Pracinostat + Azacitidine | Phase III | Acute myeloid leukemia | No added toxicity; OS as primary endpoint | [107] |
| Entinostat | Phase I | Pediatric solid tumors (included CNS) | Maximum tolerated dose determined | [108] |
| Romidepsin | Phase II | Cutaneous T-cell lymphoma | 34% overall response (4 CR, 20 PR); durable benefit | [109] |
| SB939 | Phase II | Sarcomas with translocations | Stable disease in 8/14 patients; ↑ HDAC2 expression | [110] |
| Romidepsin | Phase I/II | Recurrent glioblastoma | No efficacy; median PFS 8 weeks | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diniz, L.P.; Pinheiro, P.d.S.M.; Franco, L.S.; Gomes, F.C.A. HDACs in the Brain: From Chromatin Remodeling to Neurodegenerative Disease. Cells 2025, 14, 1338. https://doi.org/10.3390/cells14171338
Diniz LP, Pinheiro PdSM, Franco LS, Gomes FCA. HDACs in the Brain: From Chromatin Remodeling to Neurodegenerative Disease. Cells. 2025; 14(17):1338. https://doi.org/10.3390/cells14171338
Chicago/Turabian StyleDiniz, Luan Pereira, Pedro de Sena Murteira Pinheiro, Lucas S. Franco, and Flávia Carvalho Alcantara Gomes. 2025. "HDACs in the Brain: From Chromatin Remodeling to Neurodegenerative Disease" Cells 14, no. 17: 1338. https://doi.org/10.3390/cells14171338
APA StyleDiniz, L. P., Pinheiro, P. d. S. M., Franco, L. S., & Gomes, F. C. A. (2025). HDACs in the Brain: From Chromatin Remodeling to Neurodegenerative Disease. Cells, 14(17), 1338. https://doi.org/10.3390/cells14171338


_Zhang.png)




