Profibrotic Molecules Are Reduced in CRISPR-Edited Emery–Dreifuss Muscular Dystrophy Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transduction of Fibroblasts and Myoblasts by Lentiviral Vector for EMD Editing
2.3. Nucleofection of EDMD1 and EDMD2 Cells
2.4. Analysis of CRISPR/Cas9 on- and Off-Target Editing
2.5. microRNA Profiling from EDMD1 Fibroblast Cell Cultures
2.5.1. RNA Extraction
2.5.2. Library Preparation and Sequencing
2.5.3. microRNA RT-qPCR Analysis
2.5.4. Cytokine Quantification
2.5.5. Immunofluorescence Staining
2.5.6. Antibodies
2.5.7. Statistical Analysis
3. Results
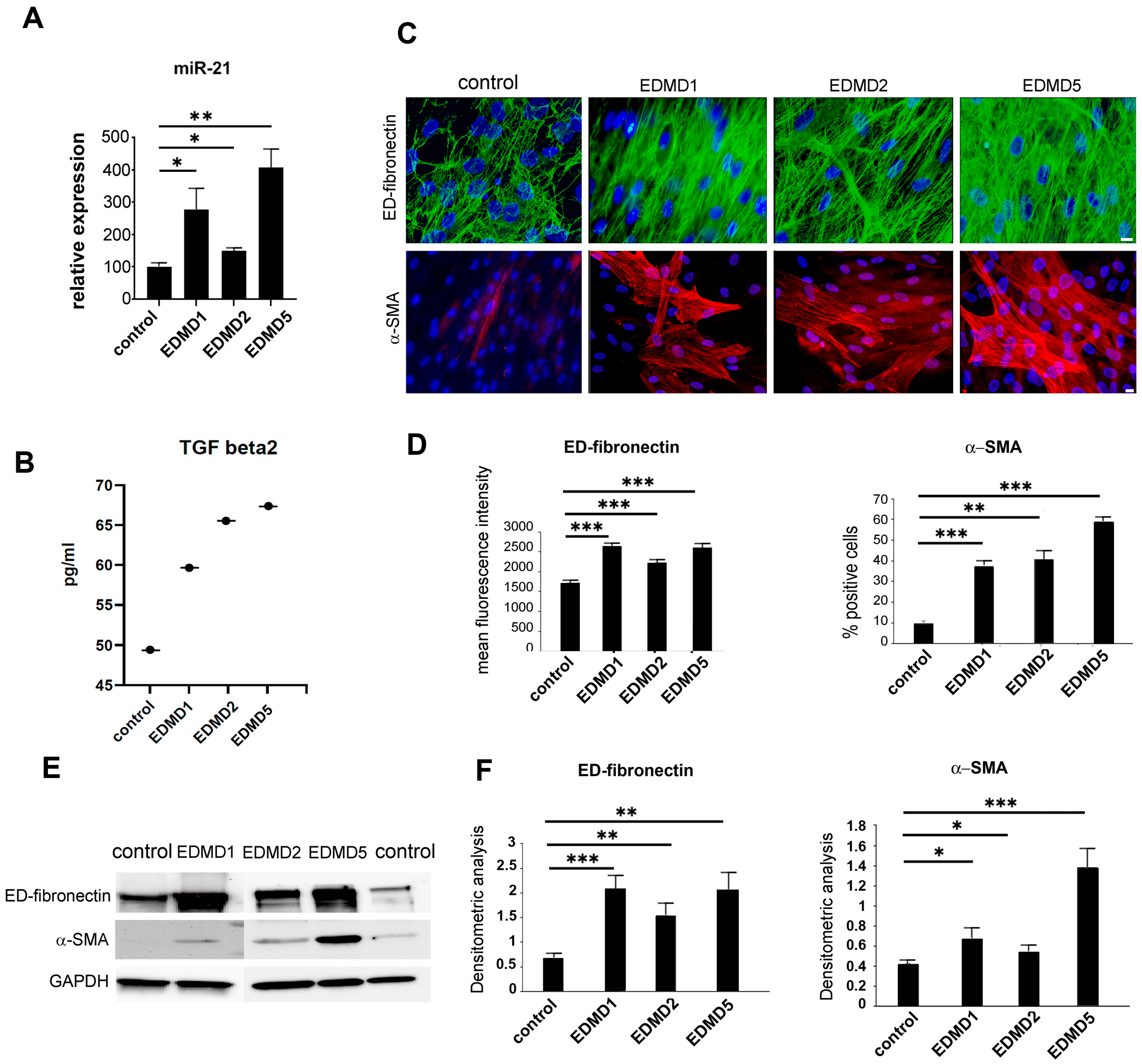
3.1. Profibrotic Markers in EDMD
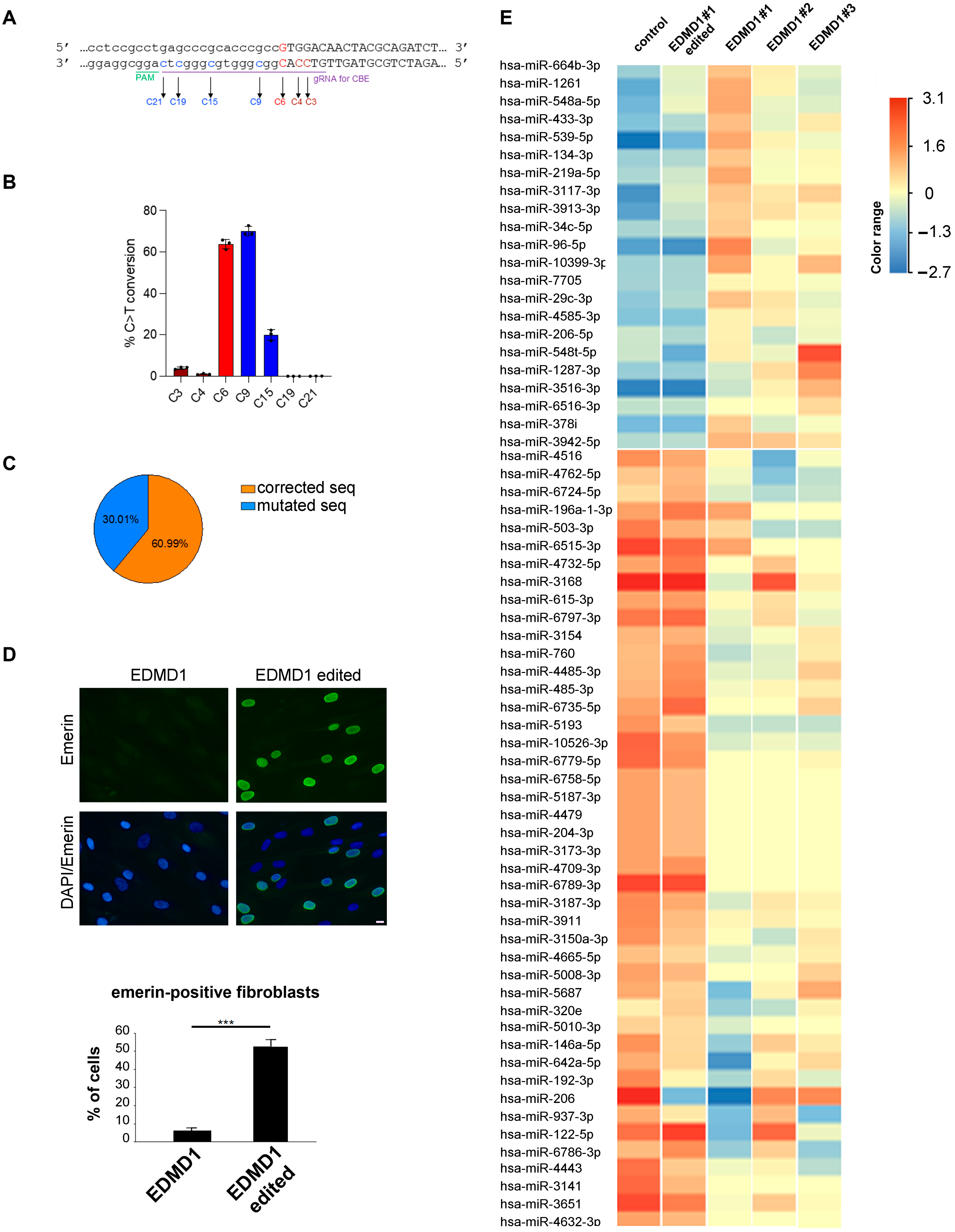
3.2. Generation of Isogenic EDMD1 Fibroblast Cultures
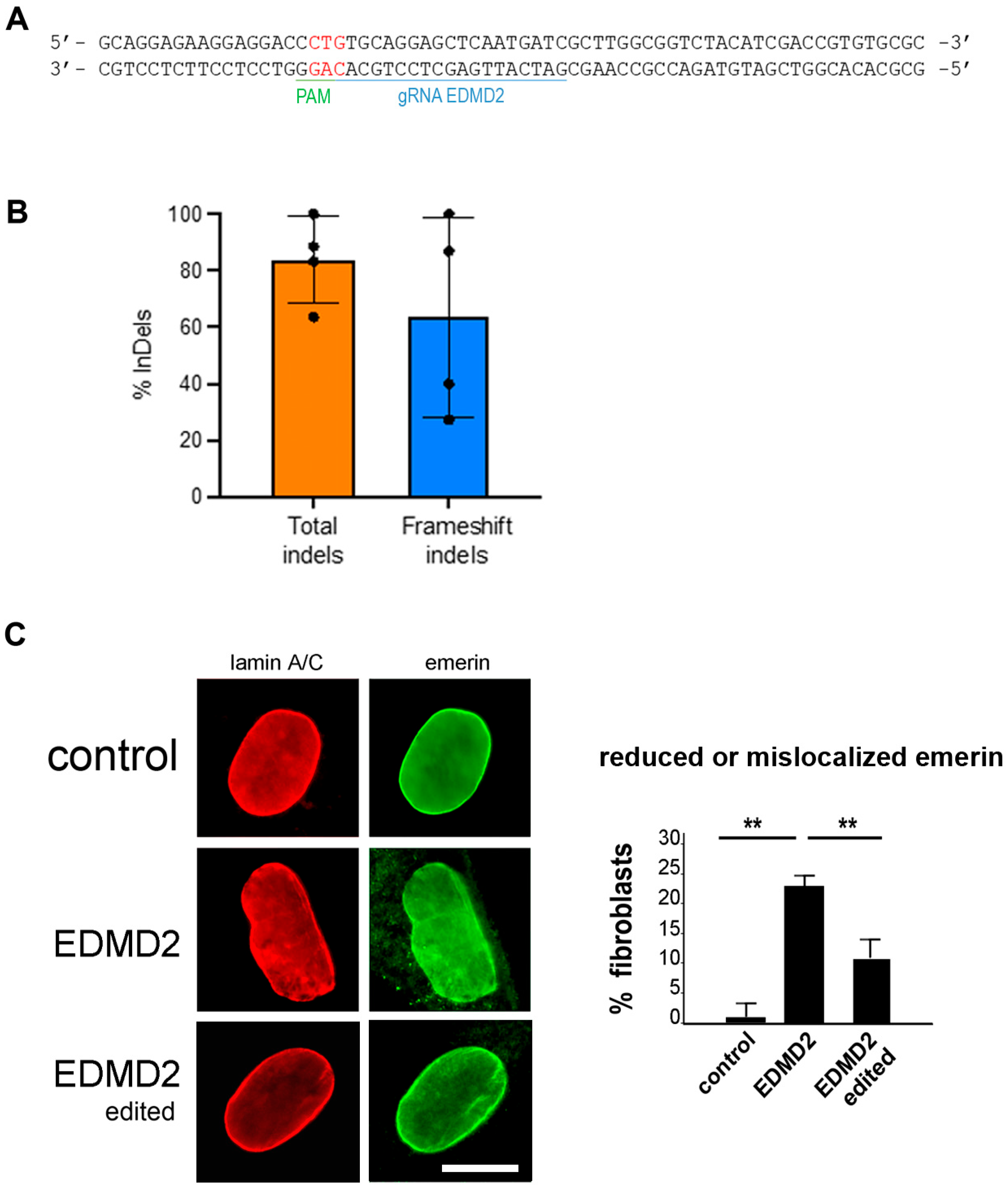
3.3. CRISPR/Cas Editing of EDMD2 Fibroblast Cultures
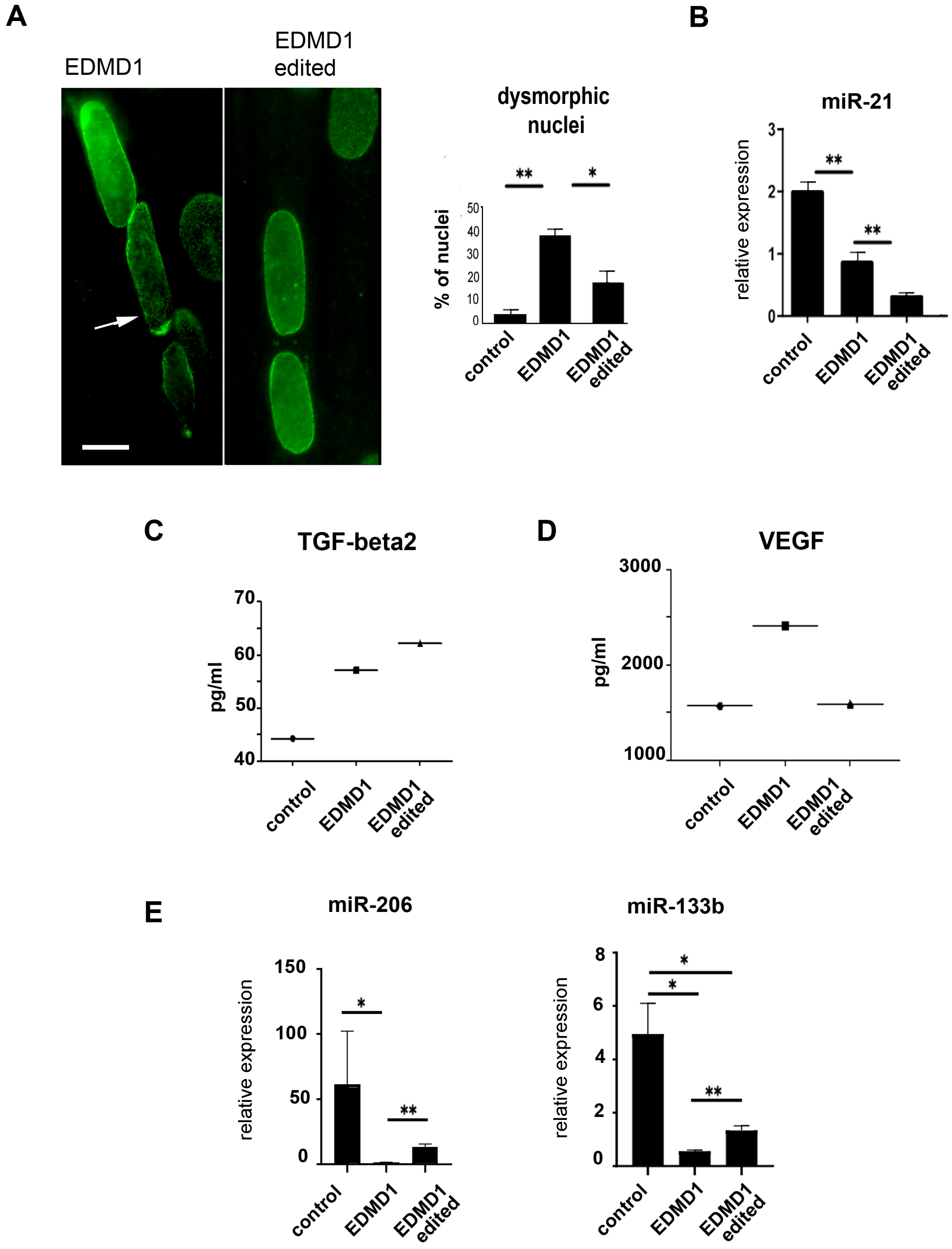
3.4. Characterization of Corrected EDMD1 and EDMD2 Fibroblasts
3.5. Establishment of Isogenic EDMD1 Myoblasts
3.6. Characterization of Isogenic EDMD1 Myoblasts
| miRNA | Change in EDMD1 Fibroblasts (F) or Myoblasts (M) | Rescue in CRISPR-Edited EDMD1 Cells | Suggested miRNA Pathway | Target | EDMD Studies | Myology or Fibrosis Studies |
|---|---|---|---|---|---|---|
| miR-21 | Up (F, M) | yes | fibrosis | Smad7 YAP | - | [18,33] |
| miR34c-5p | Up (F) | yes | muscle homeostasis | nNOS | - | [34] |
| miR-133b | Down (M) | yes | muscle homeostasis fibrosis | CTGF | [16] | [19,35] |
| miR134-3p | Up (F) | yes | apoptosis | AKT | - | [36] |
| miR146a-5p | Down (F) | yes | fibrosis | FGF2 | [15] *** | [37] |
| miR192-3p | Down (F) | yes | regeneration | NR3C1 PIM1 | [17] **** | [38] |
| miR-204-3p | Down (F) | yes | proliferation autophagy | IGFBP2 | - | [39] |
| miR-206 | Up/Down (F) * Down (M) | Yes | muscle homeostasis fibrosis | HDAC4 | - | [19] |
| miR-320 | Down (F) | yes | fibrosis | IFITM1 | - | [40] |
| miR-5193 | Down (F) | yes | aging | TP53 | - | [41] |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brull, A.; Morales Rodriguez, B.; Bonne, G.; Muchir, A.; Bertrand, A.T. The Pathogenesis and Therapies of Striated Muscle Laminopathies. Front. Physiol. 2018, 9, 1533. [Google Scholar] [CrossRef]
- Bonne, G.; Di Barletta, M.R.; Varnous, S.; Becane, H.M.; Hammouda, E.H.; Merlini, L.; Muntoni, F.; Greenberg, C.R.; Gary, F.; Urtizberea, J.A.; et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999, 21, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Bione, S.; Maestrini, E.; Rivella, S.; Mancini, M.; Regis, S.; Romeo, G.; Toniolo, D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 1994, 8, 323–327. [Google Scholar] [CrossRef]
- Zhang, Q.; Bethmann, C.; Worth, N.F.; Davies, J.D.; Wasner, C.; Feuer, A.; Ragnauth, C.D.; Yi, Q.; Mellad, J.A.; Warren, D.T.; et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 2007, 16, 2816–2833. [Google Scholar] [CrossRef]
- Gueneau, L.; Bertrand, A.T.; Jais, J.P.; Salih, M.A.; Stojkovic, T.; Wehnert, M.; Hoeltzenbein, M.; Spuler, S.; Saitoh, S.; Verschueren, A.; et al. Mutations of the FHL1 gene cause Emery-Dreifuss muscular dystrophy. Am. J. Hum. Genet. 2009, 85, 338–353. [Google Scholar] [CrossRef]
- Liang, W.C.; Mitsuhashi, H.; Keduka, E.; Nonaka, I.; Noguchi, S.; Nishino, I.; Hayashi, Y.K. TMEM43 mutations in Emery-Dreifuss muscular dystrophy-related myopathy. Ann. Neurol. 2011, 69, 1005–1013. [Google Scholar] [CrossRef]
- Meinke, P.; Mattioli, E.; Haque, F.; Antoku, S.; Columbaro, M.; Straatman, K.R.; Worman, H.J.; Gundersen, G.G.; Lattanzi, G.; Wehnert, M.; et al. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 2014, 10, e1004605. [Google Scholar] [CrossRef]
- Ditaranto, R.; Boriani, G.; Biffi, M.; Lorenzini, M.; Graziosi, M.; Ziacchi, M.; Pasquale, F.; Vitale, G.; Berardini, A.; Rinaldi, R.; et al. Differences in cardiac phenotype and natural history of laminopathies with and without neuromuscular onset. Orphanet J. Rare Dis. 2019, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, P.; Carboni, N.; Ricci, G.; Siciliano, G.; Politano, L.; Maggi, L.; Mongini, T.; Vercelli, L.; Rodolico, C.; Biagini, E.; et al. Elevated TGF beta2 serum levels in Emery-Dreifuss Muscular Dystrophy: Implications for myocyte and tenocyte differentiation and fibrogenic processes. Nucleus 2018, 9, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Chatzifrangkeskou, M.; Le Dour, C.; Wu, W.; Morrow, J.P.; Joseph, L.C.; Beuvin, M.; Sera, F.; Homma, S.; Vignier, N.; Mougenot, N.; et al. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum. Mol. Genet. 2016, 25, 2220–2233. [Google Scholar] [CrossRef]
- Cenni, V.; Evangelisti, C.; Santi, S.; Sabatelli, P.; Neri, S.; Cavallo, M.; Lattanzi, G.; Mattioli, E. Desmin and Plectin Recruitment to the Nucleus and Nuclei Orientation Are Lost in Emery-Dreifuss Muscular Dystrophy Myoblasts Subjected to Mechanical Stimulation. Cells 2024, 13, 162. [Google Scholar] [CrossRef]
- Mattioli, E.; Columbaro, M.; Capanni, C.; Maraldi, N.M.; Cenni, V.; Scotlandi, K.; Marino, M.T.; Merlini, L.; Squarzoni, S.; Lattanzi, G. Prelamin A-mediated recruitment of SUN1 to the nuclear envelope directs nuclear positioning in human muscle. Cell Death Differ. 2011, 18, 1305–1315. [Google Scholar] [CrossRef]
- Mattioli, E.; Columbaro, M.; Jafferali, M.H.; Schena, E.; Hallberg, E.; Lattanzi, G. Samp1 Mislocalization in Emery-Dreifuss Muscular Dystrophy. Cells 2018, 7, 170. [Google Scholar] [CrossRef]
- Chai, R.J.; Werner, H.; Li, P.Y.; Lee, Y.L.; Nyein, K.T.; Solovei, I.; Luu, T.D.A.; Sharma, B.; Navasankari, R.; Maric, M.; et al. Disrupting the LINC complex by AAV mediated gene transduction prevents progression of Lamin induced cardiomyopathy. Nat. Commun. 2021, 12, 4722. [Google Scholar] [CrossRef] [PubMed]
- de Las Heras, J.I.; Todorow, V.; Krecinic-Balic, L.; Hintze, S.; Czapiewski, R.; Webb, S.; Schoser, B.; Meinke, P.; Schirmer, E.C. Metabolic, fibrotic and splicing pathways are all altered in Emery-Dreifuss muscular dystrophy spectrum patients to differing degrees. Hum. Mol. Genet. 2023, 32, 1010–1031. [Google Scholar] [CrossRef]
- Vignier, N.; Amor, F.; Fogel, P.; Duvallet, A.; Poupiot, J.; Charrier, S.; Arock, M.; Montus, M.; Nelson, I.; Richard, I.; et al. Distinctive serum miRNA profile in mouse models of striated muscular pathologies. PLoS ONE 2013, 8, e55281. [Google Scholar] [CrossRef]
- Sylvius, N.; Bonne, G.; Straatman, K.; Reddy, T.; Gant, T.W.; Shackleton, S. MicroRNA expression profiling in patients with lamin A/C-associated muscular dystrophy. FASEB J. 2011, 25, 3966–3978. [Google Scholar] [CrossRef]
- Song, X.; Liu, F.; Chen, M.; Zhu, M.; Zheng, H.; Wang, W.; Chen, D.; Li, M.; Chen, S. MiR-21 regulates skeletal muscle atrophy and fibrosis by targeting TGF-beta/SMAD7-SMAD2/3 signaling pathway. Heliyon 2024, 10, e33062. [Google Scholar] [CrossRef]
- Bonanno, S.; Marcuzzo, S.; Malacarne, C.; Giagnorio, E.; Masson, R.; Zanin, R.; Arnoldi, M.T.; Andreetta, F.; Simoncini, O.; Venerando, A.; et al. Circulating MyomiRs as Potential Biomarkers to Monitor Response to Nusinersen in Pediatric SMA Patients. Biomedicines 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Veltrop, R.J.A.; Kukk, M.M.; Topouzidou, K.; Didden, L.; Muchir, A.; van Steenbeek, F.G.; Schurgers, L.J.; Harakalova, M. From gene to mechanics: A comprehensive insight into the mechanobiology of LMNA mutations in cardiomyopathy. Cell Commun. Signal. 2024, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Winbanks, C.E.; Wang, B.; Beyer, C.; Koh, P.; White, L.; Kantharidis, P.; Gregorevic, P. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J. Biol. Chem. 2011, 286, 13805–13814. [Google Scholar] [CrossRef]
- Jia, H.; Kaster, N.; Khan, R.; Ayari-Akkari, A. The Roles of myomiRs in the Pathogenesis of Sarcopenia: From Literature to In Silico Analysis. Mol. Biotechnol. 2025. [Google Scholar] [CrossRef]
- Patrizi, C.; Llado, M.; Benati, D.; Iodice, C.; Marrocco, E.; Guarascio, R.; Surace, E.M.; Cheetham, M.E.; Auricchio, A.; Recchia, A. Allele-specific editing ameliorates dominant retinitis pigmentosa in a transgenic mouse model. Am. J. Hum. Genet. 2021, 108, 295–308. [Google Scholar] [CrossRef]
- Benati, D.; Cattin, E.; Corradi, F.; Ferrari, T.; Pedrazzoli, E.; Patrizi, C.; Marchionni, M.; Bertorelli, R.; De Sanctis, V.; Merlini, L.; et al. Restored Collagen VI Microfilaments Network in the Extracellular Matrix of CRISPR-Edited Ullrich Congenital Muscular Dystrophy Fibroblasts. Biomolecules 2024, 14, 1412. [Google Scholar] [CrossRef]
- Recchia, A.; Bonini, C.; Magnani, Z.; Urbinati, F.; Sartori, D.; Muraro, S.; Tagliafico, E.; Bondanza, A.; Stanghellini, M.T.; Bernardi, M.; et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Gluba-Brzozka, A.; Olszewski, R.; Parolczyk, M.; Rysz-Gorzynska, M.; Rysz, J. miRNA biomarkers in renal disease. Int. Urol. Nephrol. 2022, 54, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, C.; Tramacere, I.; Cavalcante, P.; Schena, E.; Politano, L.; Carboni, N.; Gambineri, A.; D’Amico, A.; Ruggiero, L.; Ricci, G.; et al. Cytokine Profile in Striated Muscle Laminopathies: New Promising Biomarkers for Disease Prediction. Cells 2020, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Vita, G.L.; Polito, F.; Oteri, R.; Arrigo, R.; Ciranni, A.M.; Musumeci, O.; Messina, S.; Rodolico, C.; Di Giorgio, R.M.; Vita, G.; et al. Hippo signaling pathway is altered in Duchenne muscular dystrophy. PLoS ONE 2018, 13, e0205514. [Google Scholar] [CrossRef]
- Yu, W.; Yang, M.K.; Sung, D.J.; Park, T.J.; Kim, M.; Ntigura, E.; Kim, S.H.; Kim, B.; Park, S.W.; Bae, Y.M. Differential expression profiles of miRNA in the serum of sarcopenic rats. Biochem. Biophys. Rep. 2022, 30, 101251. [Google Scholar] [CrossRef]
- Girardi, F.; Taleb, A.; Ebrahimi, M.; Datye, A.; Gamage, D.G.; Peccate, C.; Giordani, L.; Millay, D.P.; Gilbert, P.M.; Cadot, B.; et al. TGFbeta signaling curbs cell fusion and muscle regeneration. Nat Commun. 2021, 12, 750. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Zhu, B.; Wang, L.; Chen, C.; Hong, M.; Huang, Y.; Li, H.; Han, H.; Cai, B.; et al. Increasing the efficiency and targeting range of cytidine base editors through fusion of a single-stranded DNA-binding protein domain. Nat. Cell Biol. 2020, 22, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Camozzi, D.; Capanni, C.; Cenni, V.; Mattioli, E.; Columbaro, M.; Squarzoni, S.; Lattanzi, G. Diverse lamin-dependent mechanisms interact to control chromatin dynamics. Focus on laminopathies. Nucleus 2014, 5, 427–440. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Zhan, W.; Deng, M.; Wu, X.; Chen, Z.; Xie, J.; Ye, W.; Zhao, M.; Chu, J. miR-21-5p Enriched Exosomes from Human Embryonic Stem Cells Promote Osteogenesis via YAP1 Modulation. Int. J. Nanomed. 2024, 19, 13095–13112. [Google Scholar] [CrossRef]
- Guilbaud, M.; Gentil, C.; Peccate, C.; Gargaun, E.; Holtzmann, I.; Gruszczynski, C.; Falcone, S.; Mamchaoui, K.; Ben Yaou, R.; Leturcq, F.; et al. miR-708-5p and miR-34c-5p are involved in nNOS regulation in dystrophic context. Skelet. Muscle 2018, 8, 15. [Google Scholar] [CrossRef]
- Cao, D.; Wang, Y.; Zhang, Y.; Zhang, Y.; Huang, Q.; Yin, Z.; Cai, G.; Chen, X.; Sun, X. Regulation of connective tissue growth factor expression by miR-133b for the treatment of renal interstitial fibrosis in aged mice with unilateral ureteral obstruction. Stem Cell Res. Ther. 2021, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bao, Y.; Yang, F.; Li, X.; Wang, F.; Zhang, C. miR-134-3p Regulates Cell Proliferation and Apoptosis by Targeting INHBA via Inhibiting the TGF-beta/PI3K/AKT Pathway in Sheep Granulosa Cells. Biology 2024, 14, 24. [Google Scholar] [CrossRef]
- Mehjabin, A.; Kabir, M.; Micolucci, L.; Akhtar, M.M.; Mollah, A.; Islam, M.S. MicroRNA in Fibrotic Disorders: A Potential Target for Future Therapeutics. Front. Biosci. 2023, 28, 317. [Google Scholar] [CrossRef]
- Lucchini, M.; De Arcangelis, V.; Santoro, M.; Morosetti, R.; Broccolini, A.; Mirabella, M. Serum-Circulating microRNAs in Sporadic Inclusion Body Myositis. Int. J. Mol. Sci. 2023, 24, 11139. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Dong, B.; Fang, Z.F.; Hu, X.Q.; Tang, L.; Zhou, S.H. Knockdown of lncRNA AK139328 alleviates myocardial ischaemia/reperfusion injury in diabetic mice via modulating miR-204-3p and inhibiting autophagy. J. Cell Mol. Med. 2018, 22, 4886–4898. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, S.; Li, H.; Zhan, J.; Wang, F.; Fan, J.; Nie, X.; Wang, Y.; Wen, Z.; Chen, Y.; et al. The double face of miR-320: Cardiomyocytes-derived miR-320 deteriorated while fibroblasts-derived miR-320 protected against heart failure induced by transverse aortic constriction. Signal Transduct. Target. Ther. 2021, 6, 69. [Google Scholar] [CrossRef]
- Wei, H.; Yi, T.; Li, Q.; Guo, Y.; Shen, C.; Jin, P. Application of lncRNA-miRNA-mRNA ceRNA network analysis in the treatment of androgenic alopecia. J. Clin. Lab. Anal. 2023, 37, e24791. [Google Scholar] [CrossRef]
- Xu, Q.; Miao, Y.; Ren, J.; Sun, Y.; Li, C.; Cai, X.; Wang, Z. Silencing of Nesprin-2 inhibits the differentiation of myofibroblasts from fibroblasts induced by mechanical stretch. Int. Wound J. 2022, 19, 978–986. [Google Scholar] [CrossRef]
- Li, C.; Warren, D.T.; Zhou, C.; De Silva, S.; Wilson, D.G.S.; Garcia-Maya, M.; Wheeler, M.A.; Meinke, P.; Sawyer, G.; Ehler, E.; et al. Nesprin-2 is a novel scaffold protein for telethonin and FHL-2 in the cardiomyocyte sarcomere. J. Biol. Chem. 2024, 300, 107254. [Google Scholar] [CrossRef]
- Li, C.X.; Talele, N.P.; Boo, S.; Koehler, A.; Knee-Walden, E.; Balestrini, J.L.; Speight, P.; Kapus, A.; Hinz, B. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat. Mater. 2017, 16, 379–389. [Google Scholar] [CrossRef]
- Owens, D.J.; Fischer, M.; Jabre, S.; Moog, S.; Mamchaoui, K.; Butler-Browne, G.; Coirault, C. Lamin Mutations Cause Increased YAP Nuclear Entry in Muscle Stem Cells. Cells 2020, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Messeant, J.; Moog, S.; Viggars, M.; Ferry, A.; Mamchaoui, K.; Lacene, E.; Romero, N.; Brull, A.; Bonne, G.; et al. Lamin-Related Congenital Muscular Dystrophy Alters Mechanical Signaling and Skeletal Muscle Growth. Int. J. Mol. Sci. 2020, 22, 306. [Google Scholar] [CrossRef]
- Machowska, M.; Bearzi, C.; Piekarowicz, K.; Laczmanska, I.; Rzepecki, R. Generation of one control and four iPSCs clones from patients with Emery-Dreifuss muscular dystrophy type 1. Stem Cell Res. 2021, 55, 102487. [Google Scholar] [CrossRef]
- Boettger, T.; Wust, S.; Nolte, H.; Braun, T. The miR-206/133b cluster is dispensable for development, survival and regeneration of skeletal muscle. Skelet. Muscle 2014, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006, 38, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, C.; Suarez, Y.; Lu, J.; Mishima, Y.; Giraldez, A.J. miR-1 and miR-206 regulate angiogenesis by modulating VegfA expression in zebrafish. Development 2012, 139, 4356–4364. [Google Scholar] [CrossRef]
- Verma, M.; Asakura, Y.; Wang, X.; Zhou, K.; Unverdi, M.; Kann, A.P.; Krauss, R.S.; Asakura, A. Endothelial cell signature in muscle stem cells validated by VEGFA-FLT1-AKT1 axis promoting survival of muscle stem cell. eLife 2024, 13, e73592. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2015, 11, 345–352. [Google Scholar] [CrossRef] [PubMed]


| Chromosome | Gene | Exon/ Intron | Mutation (Gene) | Mutation (Protein) | Disease | Cell Type |
|---|---|---|---|---|---|---|
| Chr.X | EMD | Exon 1 | c.1A>G | p.0 | EDMD1 | Dermal Fibroblasts/ Myoblasts |
| Chr.X | EMD | Exon 6 | c.650_654dup | p.Gln219TrpfsX20 | EDMD1 | Myoblasts |
| Chr.1 | LMNA | Exon 1 | c.103_104insCTG | p.L35PinsV | EDMD2 | Dermal Fibroblasts |
| Cytokine | Function | Trend | Cell type | Changed in EDMD2 Serum [27] |
|---|---|---|---|---|
| G-CSF | Anti-inflammatory cytokine [27] | unchanged | EDMD1 fibroblasts and myoblasts | Up |
| IL-6 | Pro-inflammatory cytokine, required for myogenesis, drives LMNA-dependent senescence pathways [28,29] | Up | EDMD1 fibroblasts | Up |
| IL-8 | Anti-inflammatory and pro-aging cytokine [27] | Down | EDMD1 fibroblasts and myoblasts | Unaffected |
| IL-9 | Pro-inflammatory cytokine | Down | EDMD1 fibroblasts | Up |
| MCP-1 (CCL2) | Pro-inflammatory cytokine [27] | Up | EDMD1 myoblasts | Unaffected |
| MIP-1b (CCL4) | Inflammatory chemokine [27] | Up | EDMD1 myoblasts | Unaffected |
| VEGF | Regulates myoblast survival, is a miR-206 target [21] | Up | EDMD1 myoblasts | Unaffected |
| TGFbeta 1 | Pro-fibrotic factor [30] | Up | EDMD1 fibroblasts | Unaffected |
| TGFbeta 2 | Pro-fibrotic factor—Promotes the alternative activation of macrophages into the M2 subtype, which are anti-inflammatory cells and profibrotic [9,27] | Up | EDMD1 myoblasts and fibroblasts | Up |
| TGFbeta 3 | Involved in adult myogenesis, limits cell fusion [30] | Unaffected | EDMD1 myoblasts and fibroblasts | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattin, E.; Schena, E.; Mattioli, E.; Marcuzzo, S.; Bonanno, S.; Cavalcante, P.; Corradi, F.; Benati, D.; Farinazzo, G.; Cattaneo, M.; et al. Profibrotic Molecules Are Reduced in CRISPR-Edited Emery–Dreifuss Muscular Dystrophy Fibroblasts. Cells 2025, 14, 1321. https://doi.org/10.3390/cells14171321
Cattin E, Schena E, Mattioli E, Marcuzzo S, Bonanno S, Cavalcante P, Corradi F, Benati D, Farinazzo G, Cattaneo M, et al. Profibrotic Molecules Are Reduced in CRISPR-Edited Emery–Dreifuss Muscular Dystrophy Fibroblasts. Cells. 2025; 14(17):1321. https://doi.org/10.3390/cells14171321
Chicago/Turabian StyleCattin, Eleonora, Elisa Schena, Elisabetta Mattioli, Stefania Marcuzzo, Silvia Bonanno, Paola Cavalcante, Federico Corradi, Daniela Benati, Giorgia Farinazzo, Marco Cattaneo, and et al. 2025. "Profibrotic Molecules Are Reduced in CRISPR-Edited Emery–Dreifuss Muscular Dystrophy Fibroblasts" Cells 14, no. 17: 1321. https://doi.org/10.3390/cells14171321
APA StyleCattin, E., Schena, E., Mattioli, E., Marcuzzo, S., Bonanno, S., Cavalcante, P., Corradi, F., Benati, D., Farinazzo, G., Cattaneo, M., De Sanctis, V., Bertorelli, R., Maggi, L., Giannotta, M., Pini, A., Vattemi, G., Cassandrini, D., Cavallo, M., Manferdini, C., ... Lattanzi, G. (2025). Profibrotic Molecules Are Reduced in CRISPR-Edited Emery–Dreifuss Muscular Dystrophy Fibroblasts. Cells, 14(17), 1321. https://doi.org/10.3390/cells14171321








