Utility and Future Perspectives of Circulating Tumor DNA Analysis in Non-Small Cell Lung Cancer Patients in the Era of Perioperative Chemo-Immunotherapy
Abstract
1. Introduction
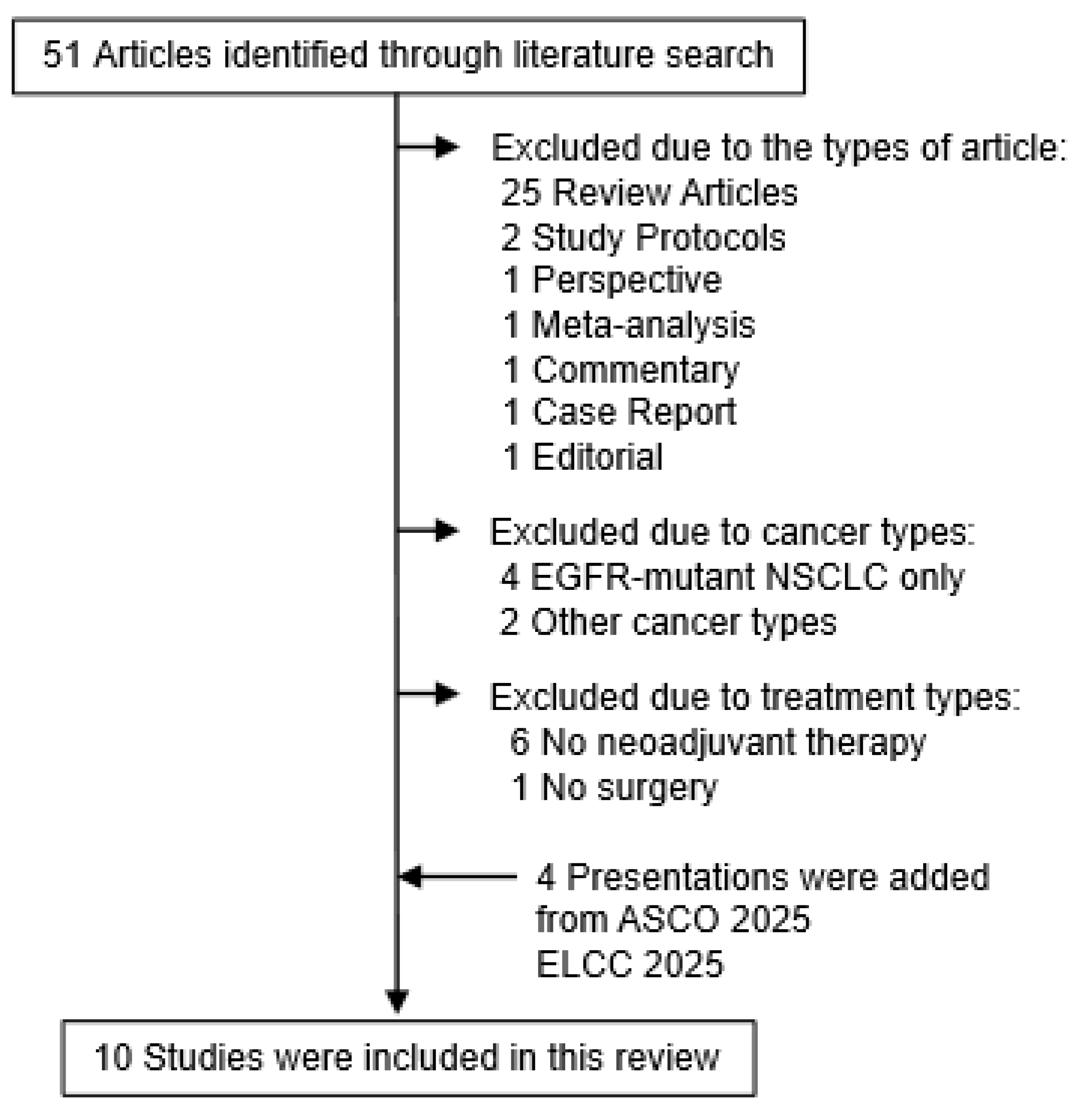
2. Literature Search
3. Current Clinical Challenges of Perioperative/Neoadjuvant Chemo-Immunotherapy
3.1. Need for Neoadjuvant Chemo-Immunotherapy
3.2. Adequate Treatment Courses of Neoadjuvant Chemo-Immunotherapy
3.3. Monitoring Tumor Response to Neoadjuvant Treatment
3.4. Recurrence Risk Stratification After Pulmonary Resection
4. Utility of ctDNA Analysis as a Tool to Solve the Above Clinical Questions
4.1. Utility of ctDNA Analysis Before and During Neoadjuvant Treatment
4.2. Utility of ctDNA Analysis Before Surgical Resection
4.3. Landmark ctDNA Status as a Tool for MRD Detection
4.4. Longitudinal ctDNA Analysis for Early Detection of Disease Recurrence
5. Ongoing Clinical Trials Involving ctDNA During Perioperative Chemo-Immunotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Felip, E.; Altorki, N.; Zhou, C.; Vallières, E.; Martínez-Martí, A.; Rittmeyer, A.; Chella, A.; Reck, M.; Goloborodko, O.; Huang, M.; et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase III trial. Ann. Oncol. 2023, 34, 907–919. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.D.; Garassino, M.C.; Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.H.; Chen, K.N.; Dooms, C.; Majem, M.; et al. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 404, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Abbosh, C.; Hodgson, D.; Doherty, G.J.; Gale, D.; Black, J.R.M.; Horn, L.; Reis-Filho, J.S.; Swanton, C. Implementing circulating tumor DNA as a prognostic biomarker in resectable non-small cell lung cancer. Trends Cancer 2024, 10, 643–654. [Google Scholar] [CrossRef]
- Peng, Y.; Mei, W.; Ma, K.; Zeng, C. Circulating Tumor DNA and Minimal Residual Disease (MRD) in Solid Tumors: Current Horizons and Future Perspectives. Front. Oncol. 2021, 11, 763790. [Google Scholar] [CrossRef]
- Pellini, B.; Chaudhuri, A.A. Circulating Tumor DNA Minimal Residual Disease Detection of Non-Small-Cell Lung Cancer Treated with Curative Intent. J. Clin. Oncol. 2022, 40, 567–575. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Qin, Z.; Ljubimov, V.A.; Zhou, C.; Tong, Y.; Liang, J. Cell-free circulating tumor DNA in cancer. Chin. J. Cancer 2016, 35, 36. [Google Scholar] [CrossRef]
- Burgener, J.M.; Rostami, A.; De Carvalho, D.D.; Bratman, S.V. Cell-free DNA as a post-treatment surveillance strategy: Current status. Semin. Oncol. 2017, 44, 330–346. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Kasi, A.K.; Parikh, A.R.; Mahipal, A. Finding Waldo: The Evolving Paradigm of Circulating Tumor DNA (ctDNA)-Guided Minimal Residual Disease (MRD) Assessment in Colorectal Cancer (CRC). Cancers 2022, 14, 3078. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Suda, K.; Sudhaman, S.; Hamada, A.; Chiba, M.; Shimoji, M.; Takemoto, T.; Kalashnikova, E.; Cheung, S.K.; Krainock, M.; et al. Clinical Significance of Perioperative Minimal Residual Disease Detected by Circulating Tumor DNA in Patients with Lung Cancer with a Long Follow-up Data: An Exploratory Study. JTO Clin. Res. Rep. 2025, 6, 100762. [Google Scholar] [CrossRef]
- Gale, D.; Heider, K.; Ruiz-Valdepenas, A.; Hackinger, S.; Perry, M.; Marsico, G.; Rundell, V.; Wulff, J.; Sharma, G.; Knock, H.; et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann. Oncol. 2022, 33, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, H.; Shi, Y.; Yang, F.; Wang, L.T.; Kang, G.; Nie, Y.; Wang, J. Perioperative Dynamic Changes in Circulating Tumor DNA in Patients with Lung Cancer (DYNAMIC). Clin. Cancer Res. 2019, 25, 7058–7067. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, F.; Shen, H.; Wang, C.; Li, X.; Chervova, O.; Wu, S.; Qiu, F.; Peng, D.; Zhu, X.; et al. Individualized tumor-informed circulating tumor DNA analysis for postoperative monitoring of non-small cell lung cancer. Cancer Cell 2023, 41, 1749–1762.e6. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early Detection of Molecular Residual Disease in Localized Lung Cancer by Circulating Tumor DNA Profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef]
- Xia, L.; Pu, Q.; Kang, R.; Mei, J.; Li, L.; Yang, Y.; Deng, S.; Feng, G.; Ma, L.; Lin, F.; et al. Dynamic ctDNA to inform the precise management of resected NSCLC: LUNGCA-2 study. J. Clin. Oncol. 2023, 41, 8528. [Google Scholar] [CrossRef]
- Chin, R.I.; Chen, K.; Usmani, A.; Chua, C.; Harris, P.K.; Binkley, M.S.; Azad, T.D.; Dudley, J.C.; Chaudhuri, A.A. Detection of Solid Tumor Molecular Residual Disease (MRD) Using Circulating Tumor DNA (ctDNA). Mol. Diagn. Ther. 2019, 23, 311–331. [Google Scholar] [CrossRef]
- Fu, R.; Huang, J.; Tian, X.; Liang, C.; Xiong, Y.; Zhang, J.T.; Jiang, B.; Dong, S.; Gong, Y.; Gao, W.; et al. Postoperative circulating tumor DNA can refine risk stratification in resectable lung cancer: Results from a multicenter study. Mol. Oncol. 2023, 17, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, B.X.; Li, J.; Shao, Y.; Li, M.T.; Li, J.J.; Kuang, P.P.; Liu, Z.; Sun, T.Y.; Wu, H.Q.; et al. Perioperative circulating tumor DNA as a potential prognostic marker for operable stage I to IIIA non-small cell lung cancer. Cancer 2022, 128, 708–718. [Google Scholar] [CrossRef]
- Tian, X.; Liu, X.; Wang, K.; Wang, R.; Li, Y.; Qian, K.; Wang, T.; Zhao, X.; Liu, L.; Zhang, P.L.; et al. Postoperative ctDNA in indicating the recurrence risk and monitoring the effect of adjuvant therapy in surgical non-small cell lung cancer. Thorac. Cancer 2024, 15, 797–807. [Google Scholar] [CrossRef]
- Tran, H.T.; Heeke, S.; Sujit, S.; Vokes, N.; Zhang, J.; Aminu, M.; Lam, V.K.; Vaporciyan, A.; Swisher, S.G.; Godoy, M.C.B.; et al. Circulating tumor DNA and radiological tumor volume identify patients at risk for relapse with resected, early-stage non-small-cell lung cancer. Ann. Oncol. 2024, 35, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, S.; Mitschke, J.; Wiesemann, S.; Rassner, M.; Andrieux, G.; Deuter, M.; Mutter, J.; Lüchtenborg, A.M.; Kottmann, D.; Titze, L.; et al. Early assessment of circulating tumor DNA after curative-intent resection predicts tumor recurrence in early-stage and locally advanced non-small-cell lung cancer. Mol. Oncol. 2022, 16, 527–537. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Zhang, J.; Xing, P.; Wu, M.; Meng, F.; Jiang, F.; Wang, J.; Bao, H.; Huang, J.; et al. Circulating tumor DNA integrating tissue clonality detects minimal residual disease in resectable non-small-cell lung cancer. J. Hematol. Oncol. 2022, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.; Reck, M.; Felip, E.; Altorki, N.K.; Vallieres, E.; Liersch, R.; Oizumi, S.; Tanaka, H.; Hamm, J.T.; McCune, S.; et al. 1211P IMpower010: ctDNA status and 5y DFS follow up in patients (pts) with resected NSCLC who received adjuvant chemotherapy (chemo) followed by atezolizumab (atezo) or best supportive care (BSC). Ann. Oncol. 2024, 35, S779–S780. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef]
- Wang, B.; Zou, B.; Xu, S.; Zhao, C.; Pei, J.; Wang, S.; Zhao, K.; Yu, J.; Liu, J. Postoperative ctDNA detection predicts relapse but has limited effects in guiding adjuvant therapy in resectable stage I NSCLC. Front. Oncol. 2023, 13, 1083417. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Wang, C.; Lu, S.; Felip, E.; Broderick, S.; Swanson, S.; et al. Overall survival with neoadjuvant nivolumab (NIVO) + chemotherapy (chemo) in patients with resectable NSCLC in CheckMate 816. J Clin Oncol. 2025, 43, LBA8000. [Google Scholar] [CrossRef]
- Cascone, T.; Awad, M.M.; Spicer, J.; He, J.; Lu, S.; Tanaka, F.; Cornelissen, R.; Petruzelka, L.B.; Ito, H.; Koch, L.D.O.; et al. Perioperative nivolumab (NIVO) vs placebo (PBO) in patients (pts) with resectable NSCLC: Updated survival and biomarker analyses from CheckMate 77T. J. Clin. Oncol. 2025, 43, LBA8010. [Google Scholar] [CrossRef]
- Reck, M.; Gale, D.; Zhu, Z.; Harpole, D.H.; Taube, J.M.; Mitsudomi, T.; Luong, D.V.; Hochmair, M.; Lee, K.-Y.; Horio, Y.; et al. Association of post-surgical MRD status with neoadjuvant ctDNA dynamics, genomic mutations, and clinical outcomes in patients with resectable NSCLC (R-NSCLC) from the phase 3 AEGEAN trial. J. Clin. Oncol. 2025, 43, 8009. [Google Scholar] [CrossRef]
- Serna, R.; Nadal, E.; Gonzalez-Larriba, J.L.; Martinez-Marti, A.; Caro, R.; Bosch-Barrera, J.; Juan, V.; Molla, M.A.; Reguart, N.; Carpeño, J.; et al. 210P: Prognostic Value of minimal residual disease in the NADIM II trial. J. Thorac. Oncol. 2025, 20, S138–S139. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.D.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Wang, C.; Lu, S.; Felip, E.; Swanson, S.J.; Brahmer, J.R.; et al. Overall Survival with Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer. N. Engl. J. Med. 2025, 393, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Liu, W.; Chen, C.; Zhang, T.; Ma, Y.; Cui, L.; Gu, Y.; Bei, T.; Zhao, X.; Zhang, B.; et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl. Lung Cancer Res. 2022, 11, 263–276. [Google Scholar] [CrossRef]
- Provencio, M.; Serna-Blasco, R.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; et al. Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J. Clin. Oncol. 2022, 40, 2924–2933. [Google Scholar] [CrossRef]
- Deutsch, J.S.; Cimino-Mathews, A.; Thompson, E.; Provencio, M.; Forde, P.M.; Spicer, J.; Girard, N.; Wang, D.; Anders, R.A.; Gabrielson, E.; et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat. Med. 2024, 30, 218–228. [Google Scholar] [CrossRef]
- Liu, S.Y.; Dong, S.; Yang, X.N.; Liao, R.Q.; Jiang, B.Y.; Wang, Q.; Ben, X.S.; Qiao, G.B.; Lin, J.T.; Yan, H.H.; et al. Neoadjuvant nivolumab with or without platinum-doublet chemotherapy based on PD-L1 expression in resectable NSCLC (CTONG1804): A multicenter open-label phase II study. Signal Transduct. Target. Ther. 2023, 8, 442. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, L.; Guo, W.; Wang, S.; Tao, X.; Li, L.; Mao, Y.; Tan, F.; Gao, Y.; Wu, N.; et al. Efficacy, Safety, and Biomarker Analysis of Neoadjuvant Camrelizumab and Apatinib in Patients with Resectable NSCLC: A Phase 2 Clinical Trial. J. Thorac. Oncol. 2023, 18, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Si, H.; Zhuang, F.; Li, C.; Zhang, L.; Zhao, Y.; Chen, T.; Dong, Y.; Wang, T.; Hou, L.; et al. Predicting therapeutic response to neoadjuvant immunotherapy based on an integration model in resectable stage IIIA (N2) non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2024, 169, 242–253.e4. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Jia, R.; Wang, Z.; Wu, J.; Chen, X.; Li, J.; Yang, D.; Li, X.; Wang, W.; et al. Neoadjuvant chemoimmunotherapy cycle number selection for non-small cell lung cancer and clinical outcomes: A real-world analysis. Front. Oncol. 2023, 13, 1200625. [Google Scholar] [CrossRef]
- Shao, M.; Yao, J.; Wang, Y.; Zhao, L.; Li, B.; Li, L.; Wu, Z.; Chen, Z.; Fan, J.; Qiu, F. Two vs three cycles of neoadjuvant sintilimab plus chemotherapy for resectable non-small-cell lung cancer: NeoSCORE trial. Signal Transduct. Target. Ther. 2023, 8, 146. [Google Scholar] [CrossRef]
- Reck, M.; Gale, D.; Harpole, D.; Taube, J.M.; Mitsudomi, T.; Hochmair, M.J.; Winder, T.; Zhu, Z.; Lai, Z.; Stewart, R.; et al. LBA59 Associations of ctDNA clearance and pathological response with neoadjuvant treatment in patients with resectable NSCLC from the phase III AEGEAN trial. Ann. Oncol. 2023, 34, S1300. [Google Scholar] [CrossRef]
- Suda, K. DNA shedding in non-small-cell lung cancer: Useful to assess? Lancet Respir. Med. 2018, 6, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Qin, C.; Wang, Q.; Tian, D.; Chen, Z. Circulating tumour DNA-Based molecular residual disease detection in resectable cancers: A systematic review and meta-analysis. eBioMedicine 2024, 103, 105109. [Google Scholar] [CrossRef]
- Saw, S.P.L.; Ong, B.-H.; Chua, K.L.M.; Takano, A.; Tan, D.S.W. Revisiting neoadjuvant therapy in non-small-cell lung cancer. Lancet Oncol. 2021, 22, e501–e516. [Google Scholar] [CrossRef]
- Pataer, A.; Kalhor, N.; Correa, A.M.; Raso, M.G.; Erasmus, J.J.; Kim, E.S.; Behrens, C.; Lee, J.J.; Roth, J.A.; Stewart, D.J.; et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J. Thorac. Oncol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Cottrell, T.R.; Thompson, E.D.; Forde, P.M.; Stein, J.E.; Duffield, A.S.; Anagnostou, V.; Rekhtman, N.; Anders, R.A.; Cuda, J.D.; Illei, P.B.; et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for quantitative immune-related pathologic response criteria (irPRC). Ann. Oncol. 2018, 29, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Dacic, S.; Wistuba, I.; Sholl, L.; Adusumilli, P.; Bubendorf, L.; Bunn, P.; Cascone, T.; Chaft, J.; Chen, G.; et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J. Thorac. Oncol. 2020, 15, 709–740. [Google Scholar] [CrossRef]
- Abbosh, C.; Frankell, A.M.; Harrison, T.; Kisistok, J.; Garnett, A.; Johnson, L.; Veeriah, S.; Moreau, M.; Chesh, A.; Chaunzwa, T.L.; et al. Tracking early lung cancer metastatic dissemination in TRACERx using ctDNA. Nature 2023, 616, 553–562. [Google Scholar] [CrossRef]

| Ref. | Year | N | Neoadjuvant Regimen | Adjuvant Regimen | ctDNA Assay |
|---|---|---|---|---|---|
| [35] | 2022 | 22 | ICI + CTx vs. ICI combo vs. CTx | - | Tumor-agnostic |
| [36] | 2022 | 46 | Nivo + CTx | Nivo vs. placebo | Tumor-agnostic |
| [37] | 2023 | 358 | Nivo + CTx vs. CTx | - | Tumor-informed |
| [38] | 2023 | 52 | Nivo + CTx vs. Nivo | - | Tumor-informed |
| [39] | 2023 | 78 | Camrelizumab + apatinib | - | Tumor-agnostic |
| [40] | 2025 | 45 | Sintilimab + CTx | - | Tumor-agnostic |
| [30] | 2025 | 358 | Nivo + CTx vs. CTx | - | Tumor-informed |
| [31] | 2025 | 461 | Nivo + CTx vs. CTx | Nivo vs. placebo | - |
| [32] | 2025 | 802 | Durval + CTx vs. CTx | Durval vs. placebo | Tumor-informed |
| [33] | 2025 | 86 | Nivo + CTx vs. CTx | Nivo vs. placebo | Tumor-agnostic |
| NCT | Primary Endpoints | Secondary Endpoints | Exploratory Analysis |
|---|---|---|---|
| NCT04638582 | ctDNA clearance (pre/post-surgery) | MPR, pCR, radiologic response | Correlation of ctDNA dynamics with DFS/OS |
| NCT05382052 | Association of ctDNA clearance with PFS | DFS, OS | Longitudinal ctDNA monitoring feasibility |
| NCT05778253 | ctDNA clearance, AI-based prediction of pCR | MPR, ORR, DFS, OS, QoL, surgical outcomes | Correlation of AI pathology with clinical outcomes |
| NCT06111807 | Sensitivity/specificity of ctDNA assays | Association of ctDNA with recurrence/DFS | ctDNA-guided risk stratification |
| NCT06123754 | MPR (pre-op), EFS (post-op) | pCR, DFS, OS, safety | Biomarker analysis, ctDNA dynamics |
| NCT06221462 | pCR/MPR, safety, surgical feasibility | DFS, OS, AEs | ctDNA clearance, radiologic-pathologic correlation |
| NCT06284317 | DFS in non-pCR patients | DFS/OS in pCR group, safety, ctDNA analysis | Correlation of ctDNA with recurrence and survival |
| NCT06743555 | Feasibility and safety of surgery omission | EFS, OS, recurrence rate | ctDNA monitoring for recurrence detection |
| NCT06902272 | Correlation between ctDNA and pCR/MPR | DFS, OS, ctDNA kinetics | ctDNA MRD detection, longitudinal profiling |
| NCT06977074 | pCR, MPR, surgical eligibility | DFS, OS, AEs | ctDNA-guided treatment optimization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohara, S.; Suda, K.; Tsutani, Y. Utility and Future Perspectives of Circulating Tumor DNA Analysis in Non-Small Cell Lung Cancer Patients in the Era of Perioperative Chemo-Immunotherapy. Cells 2025, 14, 1312. https://doi.org/10.3390/cells14171312
Ohara S, Suda K, Tsutani Y. Utility and Future Perspectives of Circulating Tumor DNA Analysis in Non-Small Cell Lung Cancer Patients in the Era of Perioperative Chemo-Immunotherapy. Cells. 2025; 14(17):1312. https://doi.org/10.3390/cells14171312
Chicago/Turabian StyleOhara, Shuta, Kenichi Suda, and Yasuhiro Tsutani. 2025. "Utility and Future Perspectives of Circulating Tumor DNA Analysis in Non-Small Cell Lung Cancer Patients in the Era of Perioperative Chemo-Immunotherapy" Cells 14, no. 17: 1312. https://doi.org/10.3390/cells14171312
APA StyleOhara, S., Suda, K., & Tsutani, Y. (2025). Utility and Future Perspectives of Circulating Tumor DNA Analysis in Non-Small Cell Lung Cancer Patients in the Era of Perioperative Chemo-Immunotherapy. Cells, 14(17), 1312. https://doi.org/10.3390/cells14171312








