Standardized Workflow and Analytical Validation of Cell-Free DNA Extraction for Liquid Biopsy Using a Magnetic Bead-Based Cartridge System
Abstract
1. Introduction
2. Materials and Methods
2.1. cfDNA Extraction and Its Components
2.2. cfDNA Reference Standards
2.3. Multi-Analyte ctDNA Plasma Control
2.4. ctDNA Complete Reference Material
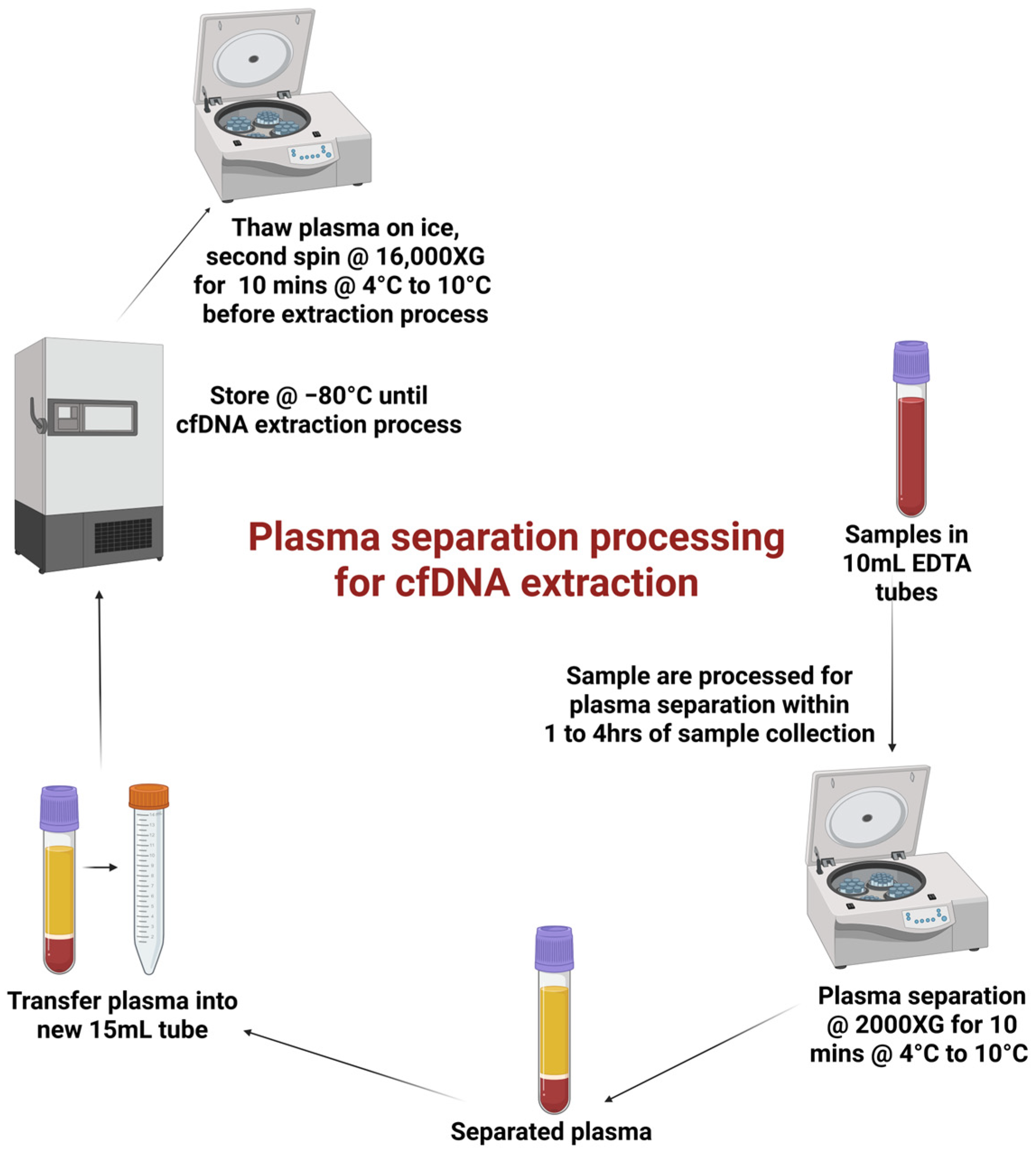
2.5. Clinical Specimen Processing and Storage
2.6. Extraction Specificity Controls
2.7. cfDNA Extraction and Quantification
3. Results
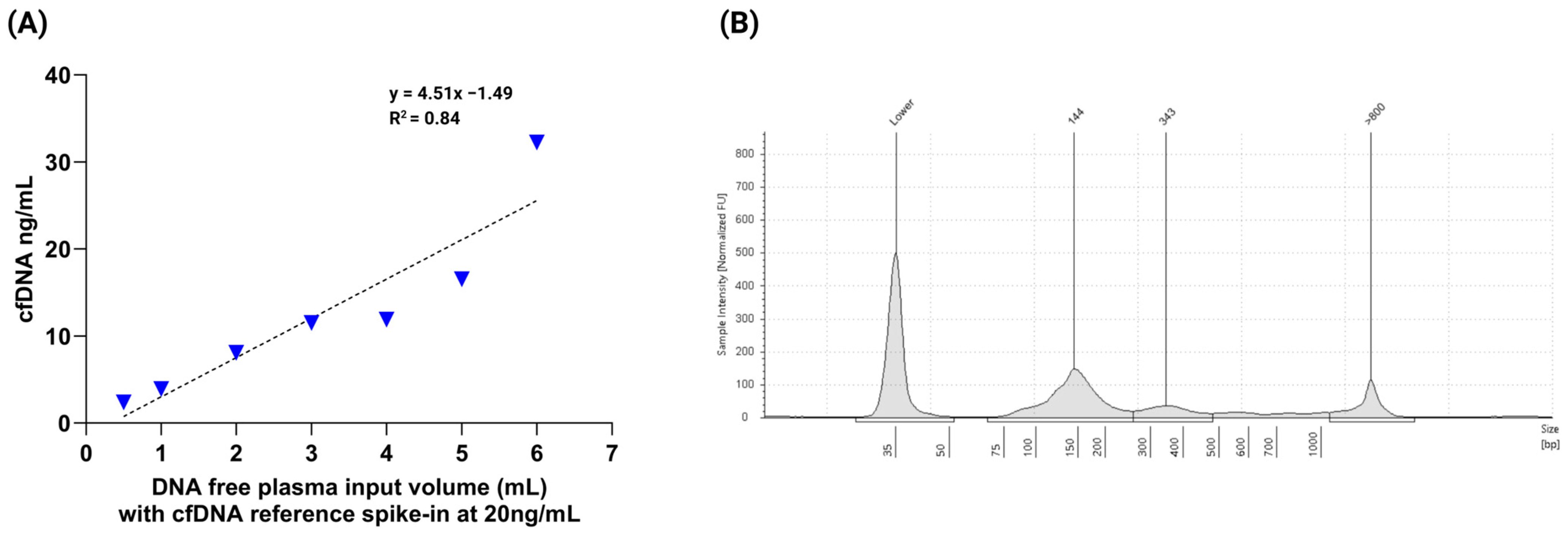
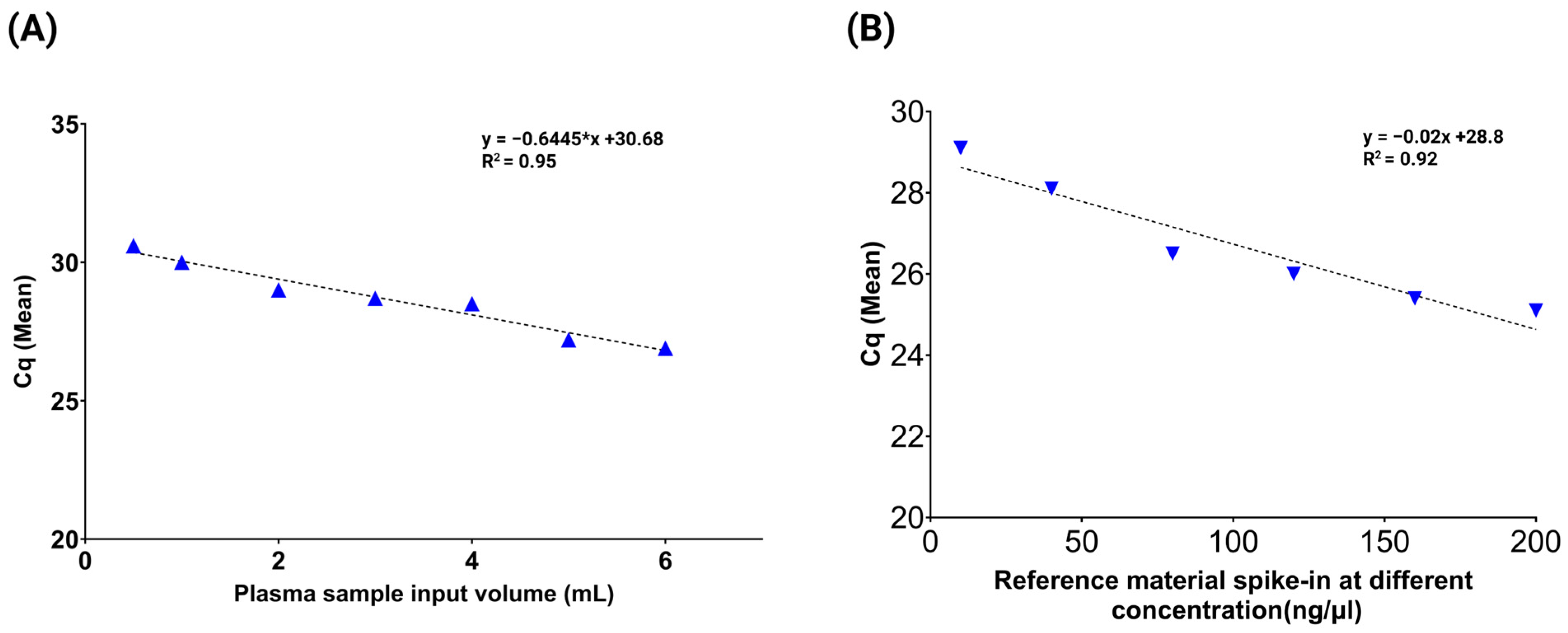
3.1. Evaluation of cfDNA Reference Standards
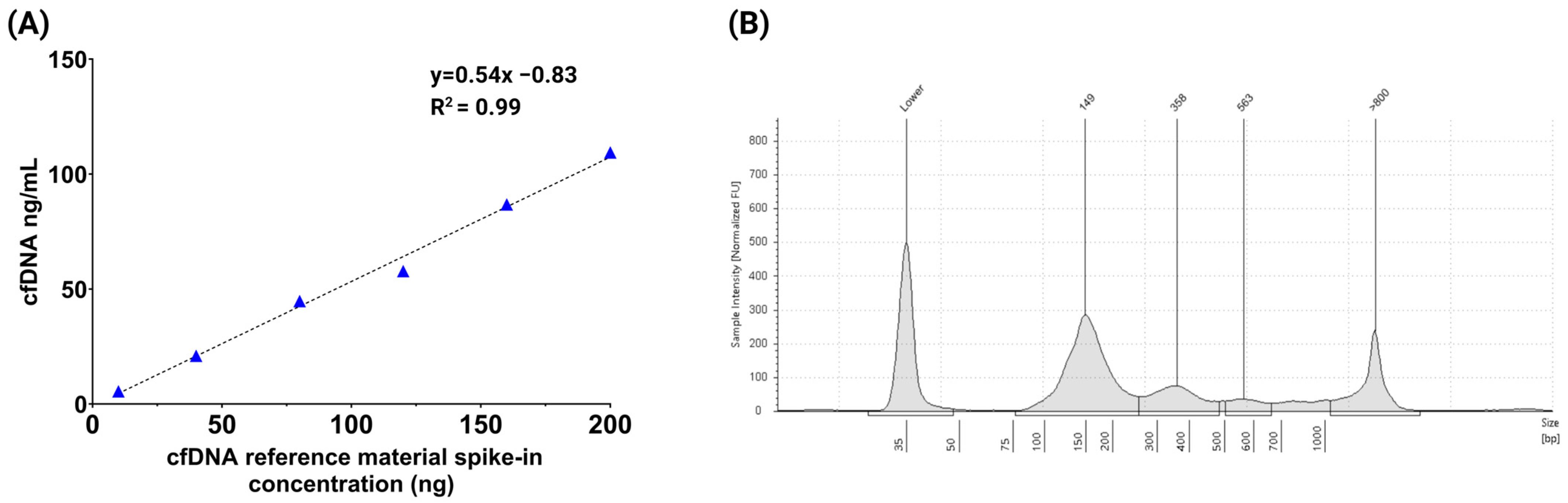
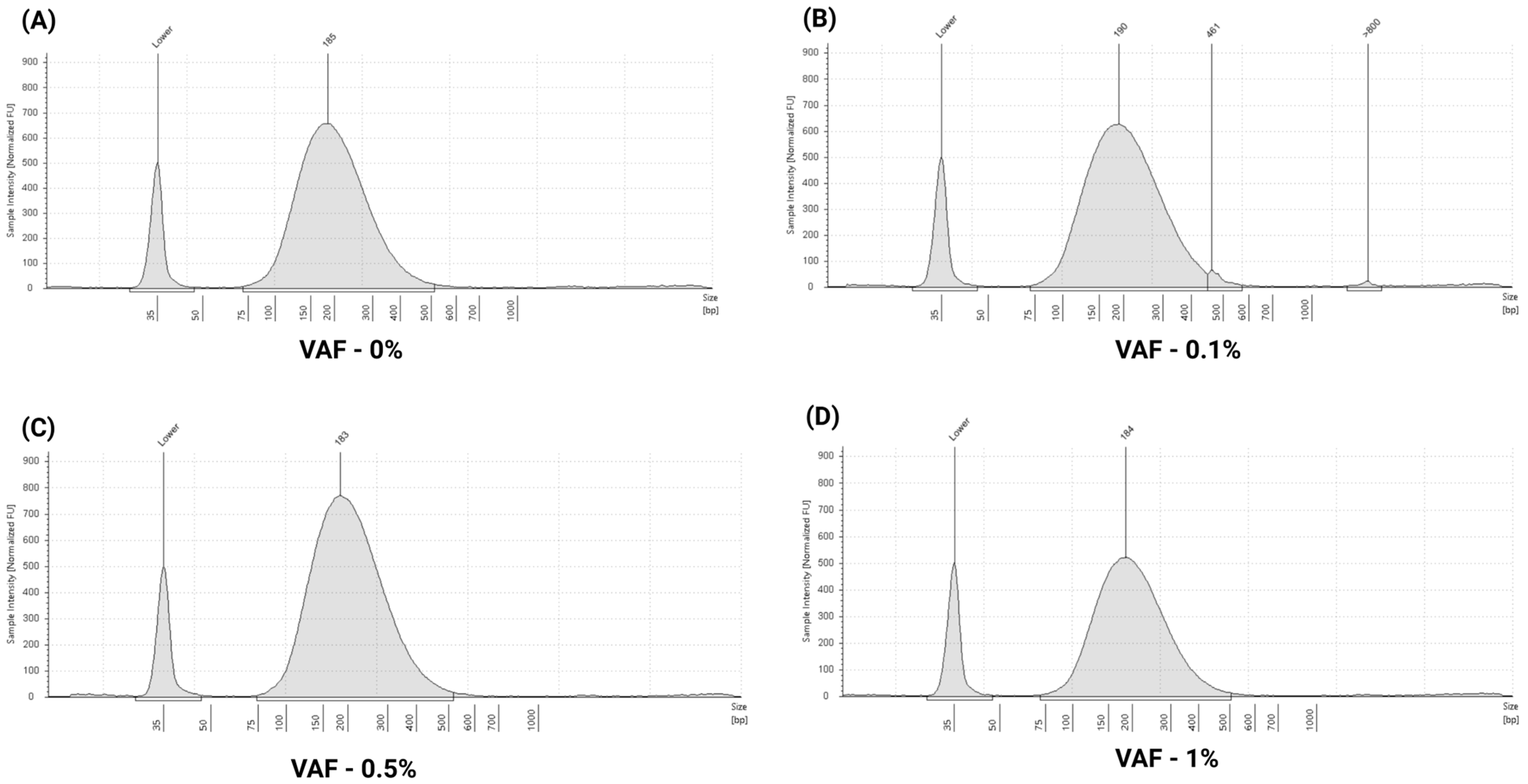
3.2. Assessment of Multi-Analyte ctDNA Plasma Control
3.3. Performance Evaluation of ctDNA Complete Reference Material
3.4. Precision and Reproducibility
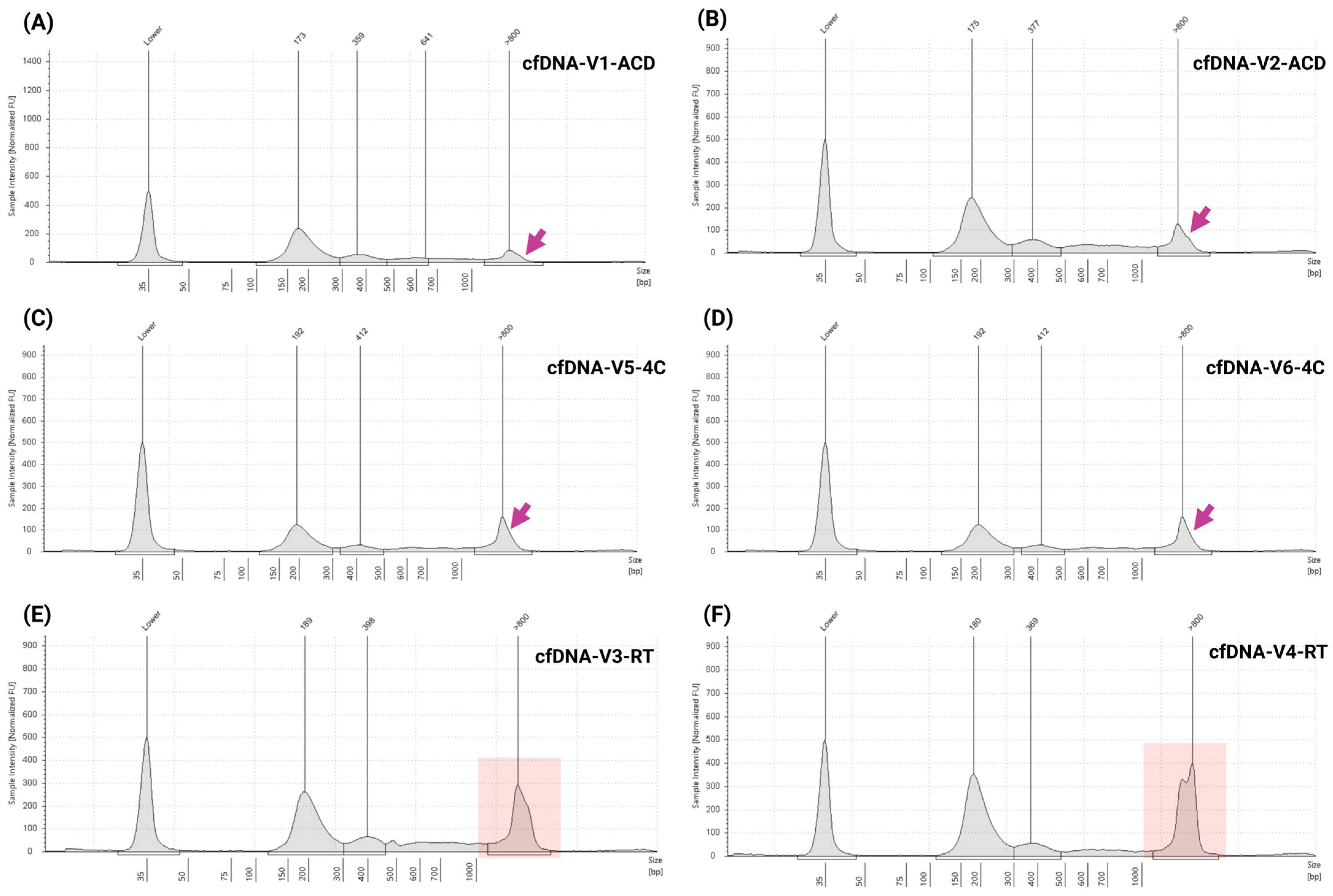
3.5. Assessment of Specificity, Sample Storage Conditions, and Stability
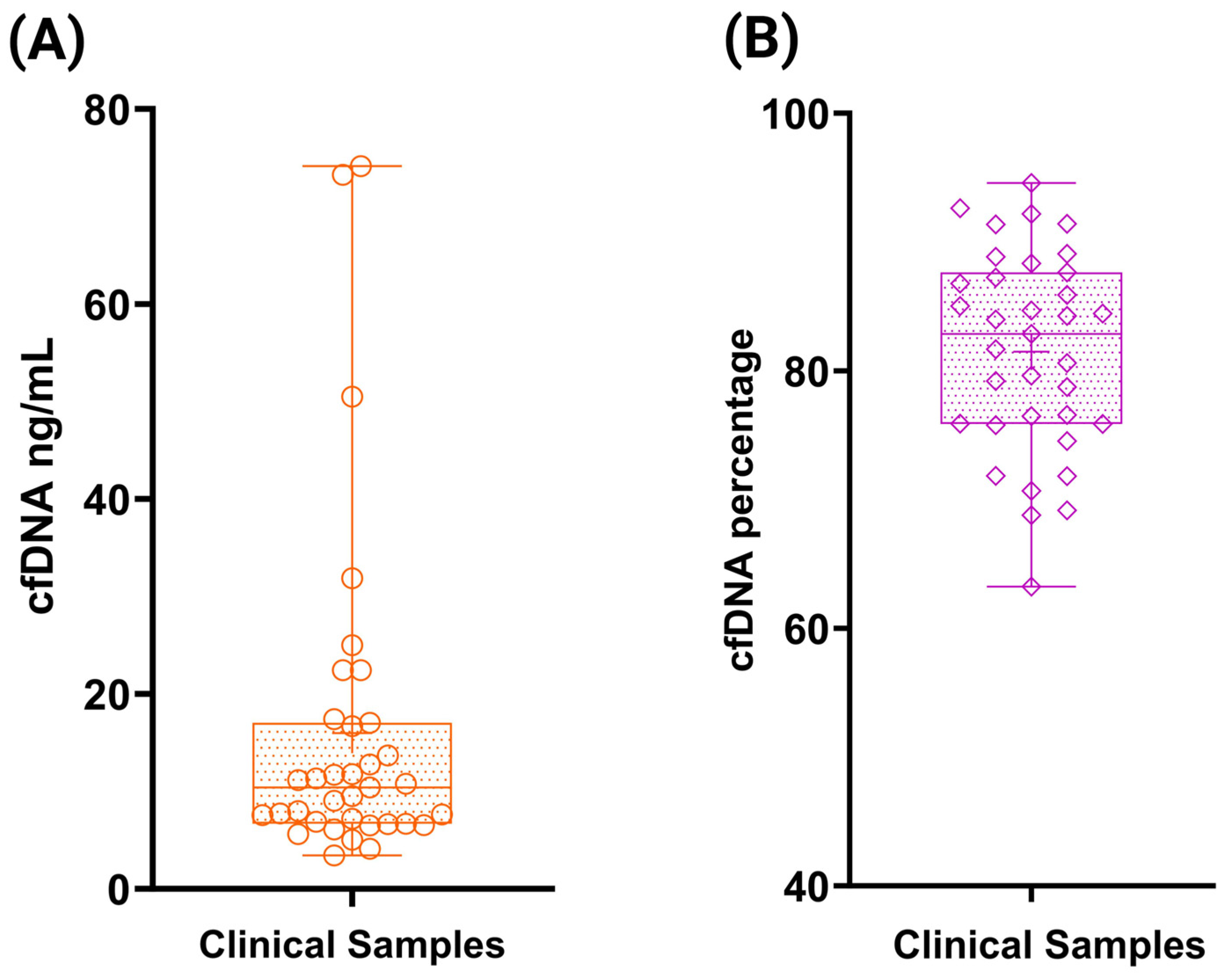
3.6. Accuracy Assessment Using ctDNA Reference Standards and Clinical Samples
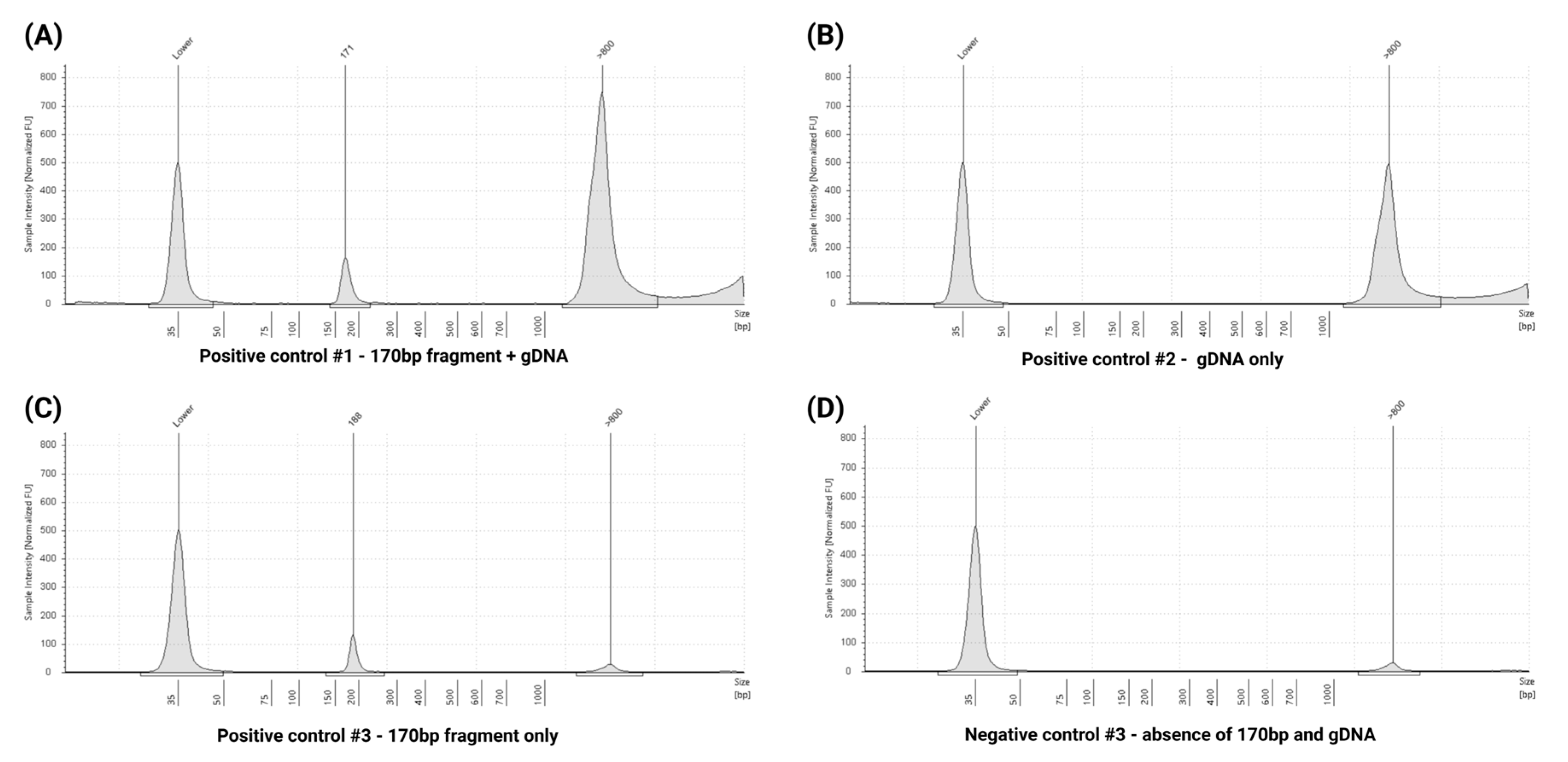
3.7. Quality Control Assessment Using Extraction Specificity Controls
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Vaart, M.; Pretorius, P.J. The Origin of Circulating Free DNA. Clin. Chem. 2007, 53, 2215. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ljubimov, V.A.; Zhou, C.; Tong, Y.; Liang, J. Cell-Free Circulating Tumor DNA in Cancer. Chin. J. Cancer 2016, 35, 36. [Google Scholar] [CrossRef] [PubMed]
- Volckmar, A.; Sültmann, H.; Riediger, A.; Fioretos, T.; Schirmacher, P.; Endris, V.; Stenzinger, A.; Dietz, S. A Field Guide for Cancer Diagnostics Using Cell-free DNA: From Principles to Practice and Clinical Applications. Genes. Chromosomes Cancer 2018, 57, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Udomruk, S.; Orrapin, S.; Pruksakorn, D.; Chaiyawat, P. Size Distribution of Cell-Free DNA in Oncology. Crit. Rev. Oncol./Hematol. 2021, 166, 103455. [Google Scholar] [CrossRef]
- Vellanki, P.J.; Ghosh, S.; Pathak, A.; Fusco, M.J.; Bloomquist, E.W.; Tang, S.; Singh, H.; Philip, R.; Pazdur, R.; Beaver, J.A. Regulatory Implications of ctDNA in Immuno-Oncology for Solid Tumors. J. Immunother. Cancer 2023, 11, e005344. [Google Scholar] [CrossRef]
- Febbo, P.G.; Allo, M.; Alme, E.B.; Cuyun Carter, G.; Dumanois, R.; Essig, A.; Kiernan, E.; Kubler, C.B.; Martin, N.; Popescu, M.C.; et al. Recommendations for the Equitable and Widespread Implementation of Liquid Biopsy for Cancer Care. JCO Precis. Oncol. 2024, 8, e2300382. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The Emerging Role of Cell-Free DNA as a Molecular Marker for Cancer Management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef]
- Elazezy, M.; Joosse, S.A. Techniques of Using Circulating Tumor DNA as a Liquid Biopsy Component in Cancer Management. Comput. Struct. Biotechnol. J. 2018, 16, 370–378. [Google Scholar] [CrossRef]
- Babayan, A.; Pantel, K. Advances in Liquid Biopsy Approaches for Early Detection and Monitoring of Cancer. Genome Med. 2018, 10, 21. [Google Scholar] [CrossRef]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
- Chu, D.; Park, B.H. Liquid Biopsy: Unlocking the Potentials of Cell-Free DNA. Virchows Arch. 2017, 471, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.; Yap, T.A.; Pope, L.; Cassidy, A.M.; Dukes, J.P.; Riisnaes, R.; Massard, C.; Cassier, P.A.; Miranda, S.; Clark, J.; et al. Multi-Purpose Utility of Circulating Plasma DNA Testing in Patients with Advanced Cancers. PLoS ONE 2012, 7, e47020. [Google Scholar] [CrossRef]
- Fleischhacker, M.; Schmidt, B. Circulating Nucleic Acids (CNAs) and Cancer--a Survey. Biochim. Biophys. Acta 2007, 1775, 181–232. [Google Scholar] [CrossRef]
- Markus, H.; Contente-Cuomo, T.; Farooq, M.; Liang, W.S.; Borad, M.J.; Sivakumar, S.; Gollins, S.; Tran, N.L.; Dhruv, H.D.; Berens, M.E.; et al. Evaluation of Pre-Analytical Factors Affecting Plasma DNA Analysis. Sci. Rep. 2018, 8, 7375. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Aucamp, J.; Pretorius, P.J. Cell-Free DNA: Preanalytical Variables. Clin. Chim. Acta 2015, 450, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; van den Broek, D.; Denis, M.G.; Hofman, P.; Hubank, M.; Mouliere, F.; Paz-Ares, L.; Schuuring, E.; Sültmann, H.; Vainer, G.; et al. Recommendations for a Practical Implementation of Circulating Tumor DNA Mutation Testing in Metastatic Non-Small-Cell Lung Cancer. ESMO Open 2022, 7, 100399. [Google Scholar] [CrossRef]
- Kang, Q.; Henry, N.L.; Paoletti, C.; Jiang, H.; Vats, P.; Chinnaiyan, A.M.; Hayes, D.F.; Merajver, S.D.; Rae, J.M.; Tewari, M. Comparative Analysis of Circulating Tumor DNA Stability In K3EDTA, Streck, and CellSave Blood Collection Tubes. Clin. Biochem. 2016, 49, 1354–1360. [Google Scholar] [CrossRef]
- Meddeb, R.; Pisareva, E.; Thierry, A.R. Guidelines for the Preanalytical Conditions for Analyzing Circulating Cell-Free DNA. Clin. Chem. 2019, 65, 623–633. [Google Scholar] [CrossRef]
- Lockwood, C.M.; Borsu, L.; Cankovic, M.; Earle, J.S.L.; Gocke, C.D.; Hameed, M.; Jordan, D.; Lopategui, J.R.; Pullambhatla, M.; Reuther, J.; et al. Recommendations for Cell-Free DNA Assay Validations. J. Mol. Diagn. 2023, 25, 876–897. [Google Scholar] [CrossRef]
- Van Der Leest, P.; Schuuring, E. Critical Factors in the Analytical Work Flow of Circulating Tumor DNA-Based Molecular Profiling. Clin. Chem. 2024, 70, 220–233. [Google Scholar] [CrossRef]
- Fusco, N.; Venetis, K.; Pepe, F.; Shetty, O.; Farinas, S.C.; Heeke, S.; Burnier, J.V.; Patton, S.J.; Heitzer, E.; Nigita, G.; et al. International Society of Liquid Biopsy (ISLB) Perspective on Minimal Requirements for ctDNA Testing in Solid Tumors. J. Liq. Biopsy 2025, 8, 100301. [Google Scholar] [CrossRef]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct Detection of Early-Stage Cancers Using Circulating Tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef] [PubMed]
- Underhill, H.R.; Kitzman, J.O.; Hellwig, S.; Welker, N.C.; Daza, R.; Baker, D.N.; Gligorich, K.M.; Rostomily, R.C.; Bronner, M.P.; Shendure, J. Fragment Length of Circulating Tumor DNA. PLoS Genet. 2016, 12, e1006162. [Google Scholar] [CrossRef]
- Terp, S.K.; Pedersen, I.S.; Stoico, M.P. Extraction of Cell-Free DNA: Evaluation of Efficiency, Quantity, and Quality. J. Mol. Diagn. 2024, 26, 310–319. [Google Scholar] [CrossRef]
- Deans, Z.C.; Butler, R.; Cheetham, M.; Dequeker, E.M.C.; Fairley, J.A.; Fenizia, F.; Hall, J.A.; Keppens, C.; Normanno, N.; Schuuring, E.; et al. IQN Path ASBL Report from the First European cfDNA Consensus Meeting: Expert Opinion on the Minimal Requirements for Clinical ctDNA Testing. Virchows Arch. 2019, 474, 681–689. [Google Scholar] [CrossRef]
- Xue, X.; Teare, M.D.; Holen, I.; Zhu, Y.M.; Woll, P.J. Optimizing the Yield and Utility of Circulating Cell-Free DNA from Plasma and Serum. Clin. Chim. Acta 2009, 404, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Mojtabanezhad Shariatpanahi, A.; Rokni, P.; Shahabi, E.; Varshoee Tabrizi, F.; Kerachian, M.A. Simple and Cost-Effective Laboratory Methods to Evaluate and Validate Cell-Free DNA Isolation. BMC Res. Notes 2018, 11, 757. [Google Scholar] [CrossRef]
- Deharvengt, S.J.; Green, D.C.; Spracklin, S.B.; Oilivia, D.-H.; Galllagher, T.L.; Sathyanarayana, S.H.; Tsongalis, G.J.; Shah, P.S. Implementation of Liquid Biopsy for Clinical Somatic Variant Testing of Solid Tumors. J. Mol. Diagn. 2024, 26, S127. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO Recommendations on the Use of Circulating Tumour DNA Assays for Patients with Cancer: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; et al. Switch to Fulvestrant and Palbociclib versus No Switch in Advanced Breast Cancer with Rising ESR1 Mutation during Aromatase Inhibitor and Palbociclib Therapy (PADA-1): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Mo, S.; Ye, L.; Wang, D.; Han, L.; Zhou, S.; Wang, H.; Dai, W.; Wang, Y.; Luo, W.; Wang, R.; et al. Early Detection of Molecular Residual Disease and Risk Stratification for Stage I to III Colorectal Cancer via Circulating Tumor DNA Methylation. JAMA Oncol. 2023, 9, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Hallermayr, A.; Neuhann, T.M.; Steinke-Lange, V.; Scharf, F.; Laner, A.; Ewald, R.; Liesfeld, B.; Holinski-Feder, E.; Pickl, J.M.A. Highly Sensitive Liquid Biopsy Duplex Sequencing Complements Tissue Biopsy to Enhance Detection of Clinically Relevant Genetic Variants. Front. Oncol. 2022, 12, 1014592. [Google Scholar] [CrossRef]
- Serio, V.B.; Palmieri, M.; Loberti, L.; Granata, S.; Fallerini, C.; Vaghi, M.; Renieri, A.; Pinto, A.M. Nosological and Theranostic Approach to Vascular Malformation through cfDNA NGS Liquid Biopsy. J. Clin. Med. 2022, 11, 3740. [Google Scholar] [CrossRef]
- D’Gama, A.M.; Poduri, A. Brain Somatic Mosaicism in Epilepsy: Bringing Results Back to the Clinic. Neurobiol. Dis. 2023, 181, 106104. [Google Scholar] [CrossRef]
- Hallermayr, A.; Keßler, T.; Fujera, M.; Liesfeld, B.; Bernstein, S.; von Ameln, S.; Schanze, D.; Steinke-Lange, V.; Pickl, J.M.A.; Neuhann, T.M.; et al. Impact of cfDNA Reference Materials on Clinical Performance of Liquid Biopsy NGS Assays. Cancers 2023, 15, 5024. [Google Scholar] [CrossRef]
- Wang, M.; Huang, X.; Li, X.; Guo, Q.; Xu, W.; Zhao, M.; Wang, X.; Wang, L.; Lou, J. Performance Comparison of Commercial Kits for Isolating and Detecting Circulating Tumor DNA. Scand. J. Clin. Lab. Investig. 2021, 81, 276–281. [Google Scholar] [CrossRef]
- Warton, K.; Graham, L.-J.; Yuwono, N.; Samimi, G. Comparison of 4 Commercial Kits for the Extraction of Circulating DNA from Plasma. Cancer Genet. 2018, 228–229, 143–150. [Google Scholar] [CrossRef]
- Polatoglou, E.; Mayer, Z.; Ungerer, V.; Bronkhorst, A.J.; Holdenrieder, S. Isolation and Quantification of Plasma Cell-Free DNA Using Different Manual and Automated Methods. Diagnostics 2022, 12, 2550. [Google Scholar] [CrossRef]
- Van Der Leest, P.; Boonstra, P.A.; Ter Elst, A.; Van Kempen, L.C.; Tibbesma, M.; Koopmans, J.; Miedema, A.; Tamminga, M.; Groen, H.J.M.; Reyners, A.K.L.; et al. Comparison of Circulating Cell-Free DNA Extraction Methods for Downstream Analysis in Cancer Patients. Cancers 2020, 12, 1222. [Google Scholar] [CrossRef]
- Pandoh, P.K.; Corbett, R.D.; McDonald, H.; Alcaide, M.; Kirk, H.; Trinh, E.; Haile, S.; MacLeod, T.; Smailus, D.; Bilobram, S.; et al. A High-Throughput Protocol for Isolating Cell-Free Circulating Tumor DNA from Peripheral Blood. Biotechniques 2019, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Pallisgaard, N.; Spindler, K.-L.G.; Andersen, R.F.; Brandslund, I.; Jakobsen, A. Controls to Validate Plasma Samples for Cell Free DNA Quantification. Clin. Chim. Acta 2015, 446, 141–146. [Google Scholar] [CrossRef]
- Goh, S.K.; Cox, D.R.A.; Wong, B.K.L.; Musafer, A.; Witkowski, T.; Do, H.; Muralidharan, V.; Dobrovic, A. A Synthetic DNA Construct to Evaluate the Recovery Efficiency of Cell-Free DNA Extraction and Bisulfite Modification. Clin. Chem. 2021, 67, 1201–1209. [Google Scholar] [CrossRef]
- Lampignano, R.; Neumann, M.H.D.; Weber, S.; Kloten, V.; Herdean, A.; Voss, T.; Groelz, D.; Babayan, A.; Tibbesma, M.; Schlumpberger, M.; et al. Multicenter Evaluation of Circulating Cell-Free DNA Extraction and Downstream Analyses for the Development of Standardized (Pre)Analytical Work Flows. Clin. Chem. 2020, 66, 149–160. [Google Scholar] [CrossRef]
- van Ginkel, J.H.; van den Broek, D.A.; van Kuik, J.; Linders, D.; de Weger, R.; Willems, S.M.; Huibers, M.M.H. Preanalytical Blood Sample Workup for Cell-Free DNA Analysis Using Droplet Digital PCR for Future Molecular Cancer Diagnostics. Cancer Med. 2017, 6, 2297–2307. [Google Scholar] [CrossRef]
- El Messaoudi, S.; Rolet, F.; Mouliere, F.; Thierry, A.R. Circulating Cell Free DNA: Preanalytical Considerations. Clin. Chim. Acta 2013, 424, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.M.; Nocon, A.; Held, S.A.E.; Kobilay, M.; Skowasch, D.; Bronkhorst, A.J.; Ungerer, V.; Fredebohm, J.; Diehl, F.; Holdenrieder, S.; et al. Pre-Analytical Evaluation of Streck Cell-Free DNA Blood Collection Tubes for Liquid Profiling in Oncology. Diagnostics 2023, 13, 1288. [Google Scholar] [CrossRef]
- van Wijk, I.J.; de Hoon, A.C.; Jurhawan, R.; Tjoa, M.L.; Griffioen, S.; Mulders, M.A.; van Vugt, J.M.; Oudejans, C.B. Detection of Apoptotic Fetal Cells in Plasma of Pregnant Women. Clin. Chem. 2000, 46, 729–731. [Google Scholar]
- Ungerer, V.; Bronkhorst, A.J.; Holdenrieder, S. Preanalytical Variables That Affect the Outcome of Cell-Free DNA Measurements. Crit. Rev. Clin. Lab. Sci. 2020, 57, 484–507. [Google Scholar] [CrossRef]
- Chiu, R.W.; Poon, L.L.; Lau, T.K.; Leung, T.N.; Wong, E.M.; Lo, Y.M. Effects of Blood-Processing Protocols on Fetal and Total DNA Quantification in Maternal Plasma. Clin. Chem. 2001, 47, 1607–1613. [Google Scholar]
- Sorber, L.; Zwaenepoel, K.; Jacobs, J.; De Winne, K.; Goethals, S.; Reclusa, P.; Van Casteren, K.; Augustus, E.; Lardon, F.; Roeyen, G.; et al. Circulating Cell-Free DNA and RNA Analysis as Liquid Biopsy: Optimal Centrifugation Protocol. Cancers 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Barrios, C.; Nieto-Alcolado, I.; Torrente, M.; Jiménez-Sánchez, C.; Calvo, V.; Gutierrez-Sanz, L.; Palka, M.; Donoso-Navarro, E.; Provencio, M.; Romero, A. Comparison of Methods for Circulating Cell-Free DNA Isolation Using Blood from Cancer Patients: Impact on Biomarker Testing. Transl. Lung Cancer Res. 2016, 5, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Lehle, S.; Emons, J.; Hack, C.C.; Heindl, F.; Hein, A.; Preuß, C.; Seitz, K.; Zahn, A.L.; Beckmann, M.W.; Fasching, P.A.; et al. Evaluation of Automated Techniques for Extraction of Circulating Cell-Free DNA for Implementation in Standardized High-Throughput Workflows. Sci. Rep. 2023, 13, 373. [Google Scholar] [CrossRef]
- Beynon, R.A.; Ingle, S.M.; Langdon, R.; May, M.; Ness, A.; Martin, R.M.; Suderman, M.; Ingarfield, K.; Marioni, R.E.; McCartney, D.L.; et al. Epigenetic Biomarkers of Ageing Are Predictive of Mortality Risk in a Longitudinal Clinical Cohort of Individuals Diagnosed with Oropharyngeal Cancer. Clin. Epigenet 2022, 14, 1. [Google Scholar] [CrossRef]
- Van Der Meer, A.J.; Kroeze, A.; Hoogendijk, A.J.; Soussan, A.A.; Ellen Van Der Schoot, C.; Wuillemin, W.A.; Voermans, C.; Van Der Poll, T.; Zeerleder, S. Systemic Inflammation Induces Release of Cell-Free DNA from Hematopoietic and Parenchymal Cells in Mice and Humans. Blood Adv. 2019, 3, 724–728. [Google Scholar] [CrossRef]
- Pessoa, L.S.; Heringer, M.; Ferrer, V.P. ctDNA as a Cancer Biomarker: A Broad Overview. Crit. Rev. Oncol./Hematol. 2020, 155, 103109. [Google Scholar] [CrossRef]
- Bryzgunova, O.E.; Konoshenko, M.Y.; Laktionov, P.P. Concentration of Cell-Free DNA in Different Tumor Types. Expert. Rev. Mol. Diagn. 2021, 21, 63–75. [Google Scholar] [CrossRef]
- van der Leest, P.; Ketelaar, E.M.; van Noesel, C.J.M.; van den Broek, D.; van Boerdonk, R.A.A.; Deiman, B.; Rifaela, N.; van der Geize, R.; Huijsmans, C.J.J.; Speel, E.J.M.; et al. Dutch National Round Robin Trial on Plasma-Derived Circulating Cell-Free DNA Extraction Methods Routinely Used in Clinical Pathology for Molecular Tumor Profiling. Clin. Chem. 2022, 68, 963–972. [Google Scholar] [CrossRef]
- Van Casteren, K.; Keppens, C.; Schuuring, E.; Deans, Z.C.; Normanno, N.; Patton, S.J.; Dequeker, E.M.C.; International Quality Network for Pathology ctDNA Working Group and the European Society of Pathology Foundation Assessors. External Quality Assessment Schemes for Biomarker Testing in Oncology: Comparison of Performance between Formalin-Fixed, Paraffin-Embedded-Tissue and Cell-Free Tumor DNA in Plasma. J. Mol. Diagn. 2020, 22, 736–747. [Google Scholar] [CrossRef]



| Gene | COSMIC ID | Seracare-Listed HGVS-c | Seracare-Listed HGVS-p | Transcript | Alteration Type | ctDNA-Seq Result | ctDNA-Seq Observed HGVS-c | ctDNA-Seq Observed HGVS-p |
|---|---|---|---|---|---|---|---|---|
| AKT1 | COSM33765 | c.49G>A | p.E17K | NM_001382430.1 | SNV | Detected | c.49G>A | p.E17K |
| ALK | COSM28055 | c.3522C>A | p.F1174L | NM_004304.5 | SNV | Detected | c.3522C>A | p.F1174L |
| ALK | COSM144250 | c.3604G>A | p.G1202R | NM_004304.5 | SNV | Detected | c.3604G>A | p.G1202R |
| BRAF | COSM476 | c.1799T>A | p.V600E | NM_004333.6 | SNV | Detected | c.1799T>A | p.V600E |
| BRCA1 | COSM219054 | c.1961del | p.K654fs | NM_007294.4 | Deletion | Detected | c.1961del | p.K654Sfs*47 |
| BRCA2 | COSM1738241 | c.7934del | p.R2645fs | NM_000059.4 | Deletion | Detected | c.7934del | p.R2645Nfs*3 |
| EGFR | COSM6223 | c.2235_2249del | p.E746_A750del | NM_005228.5 | Deletion | Detected | c.2100_2114del | p.E701_A705del |
| EGFR | COSM12370 | c.2240_2257del | p.L747_P753delinsS | NM_005228.5 | Deletion | Detected | c.2105_2122del | p.L702_p708delinsS |
| EGFR | COSM6256 | c.2254_2277del | p.S752_1759del | NM_005228.5 | Deletion | Detected | c.2254_2277del | p.S752_1759del |
| EGFR | COSM6240 | c.2369C>T | p.T790M | NM_005228.5 | SNV | Detected | c.2369C>T | p.T790M |
| EGFR | COSM6224 | c.2573T>G | p.L858R | NM_005228.5 | SNV | Detected | c.2573T>G | p.L858R |
| ERBB2 | COSM20959 | c.2313_2324dup | p.Y772_A775dup | NM_004448.4 | Insertion | Detected | c.2223_2234dup | p.Y742_A745dup |
| KIT | COSM1314 | c.2447A>T | p.D816V | NM_000222.3 | SNV | Detected | c.2435A.T | p.D812V |
| KRAS | COSM554 | c.183A>C | p.Q61H | NM_004985.5 | SNV | Detected | c.183A>C | p.Q61H |
| KRAS | COSM516 | c.34G>T | p.G12C | NM_004985.5 | SNV | Detected | c.34G>T | p.G12C |
| KRAS | COSM521 | c.35G>A | p.G12D | NM_004985.5 | SNV | Detected | c.35G>A | p.G12D |
| NRAS | COSM584 | c.182A>G | p.Q61R | NM_002524.5 | SNV | Detected | c.182A>G | p.Q61R |
| PIK3CA | COSM775 | c.3140A>G | p.H1047R | NM_006218.4 | SNV | Detected | c.3140A>G | p.H1047R |
| PIK3CA | NA | c.3204_3205insA | p.*1069Mext*3 | NM_006218.4 | Insertion | Detected | c.3204_3205insA | p.*1069Mext*3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathyanarayana, S.H.; Spracklin, S.B.; Deharvengt, S.J.; Green, D.C.; Instasi, M.D.; Gallagher, T.L.; Shah, P.S.; Tsongalis, G.J. Standardized Workflow and Analytical Validation of Cell-Free DNA Extraction for Liquid Biopsy Using a Magnetic Bead-Based Cartridge System. Cells 2025, 14, 1062. https://doi.org/10.3390/cells14141062
Sathyanarayana SH, Spracklin SB, Deharvengt SJ, Green DC, Instasi MD, Gallagher TL, Shah PS, Tsongalis GJ. Standardized Workflow and Analytical Validation of Cell-Free DNA Extraction for Liquid Biopsy Using a Magnetic Bead-Based Cartridge System. Cells. 2025; 14(14):1062. https://doi.org/10.3390/cells14141062
Chicago/Turabian StyleSathyanarayana, Shivaprasad H., Sarah B. Spracklin, Sophie J. Deharvengt, Donald C. Green, Margery D. Instasi, Torrey L. Gallagher, Parth S. Shah, and Gregory J. Tsongalis. 2025. "Standardized Workflow and Analytical Validation of Cell-Free DNA Extraction for Liquid Biopsy Using a Magnetic Bead-Based Cartridge System" Cells 14, no. 14: 1062. https://doi.org/10.3390/cells14141062
APA StyleSathyanarayana, S. H., Spracklin, S. B., Deharvengt, S. J., Green, D. C., Instasi, M. D., Gallagher, T. L., Shah, P. S., & Tsongalis, G. J. (2025). Standardized Workflow and Analytical Validation of Cell-Free DNA Extraction for Liquid Biopsy Using a Magnetic Bead-Based Cartridge System. Cells, 14(14), 1062. https://doi.org/10.3390/cells14141062







