Generation and Characterization of Cisplatin-Resistant Oral Squamous Cell Carcinoma Cells Displaying an Epithelial–Mesenchymal Transition Signature
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. 3D Spheroid Model
2.3. Drug
2.4. Induction of Cisplatin-Resistance in OSCC Cells
2.5. Drug Sensitivity Assay
2.6. Morphology and Organization of Cytoskeleton
2.7. RNA Extraction, Library Preparation, and Sequencing
2.8. Data Processing and Identification of Differentially Expressed Genes (DEGs)
2.9. Quantitative RT-PCR (RT-qPCR) and Western Blot
2.10. Immunofluorescence
2.11. Gelatin Zymography
2.12. Migration and Invasion Assays
2.13. Statistical Analysis
3. Results
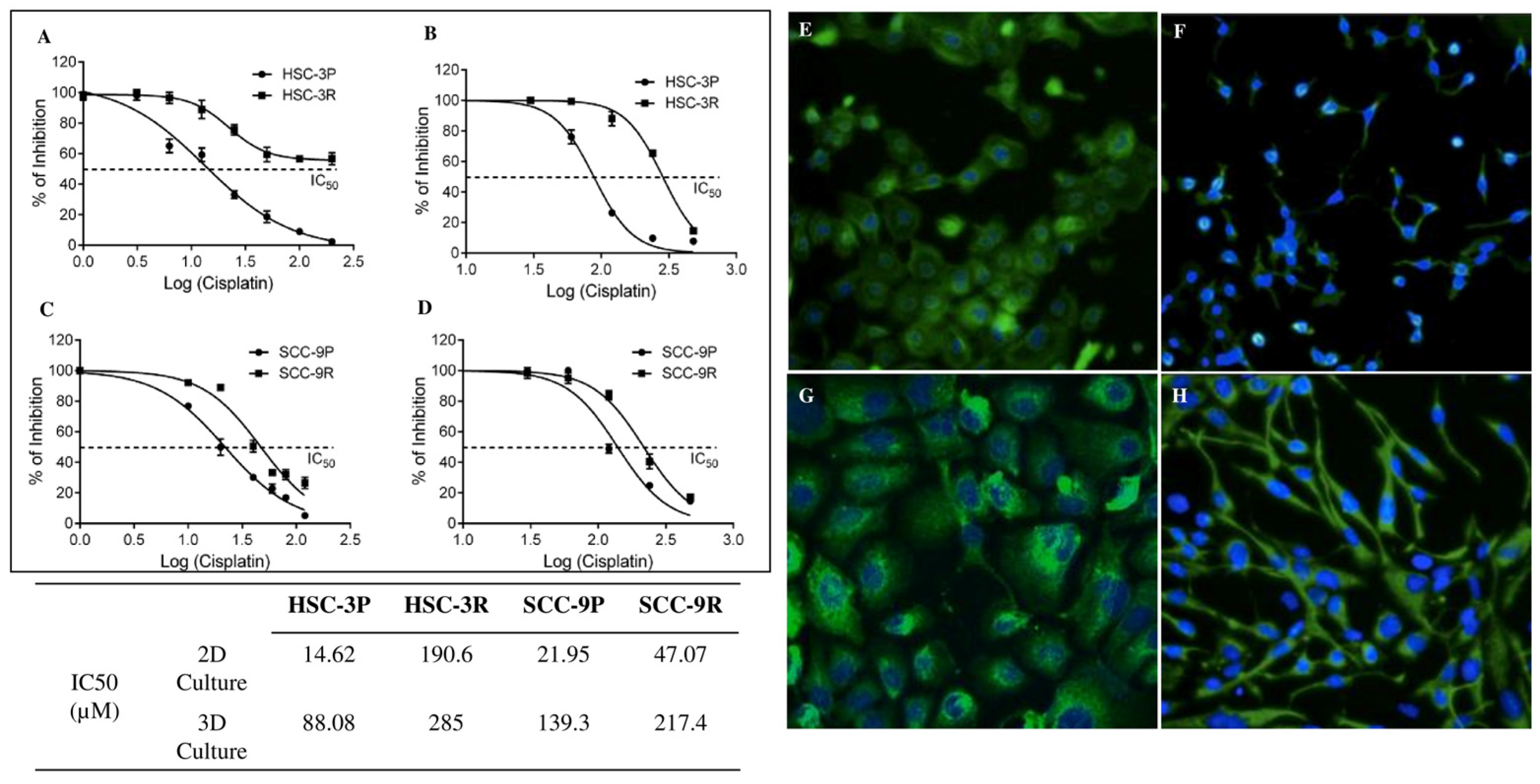
3.1. Establishment of Cisplatin-Resistant Cells
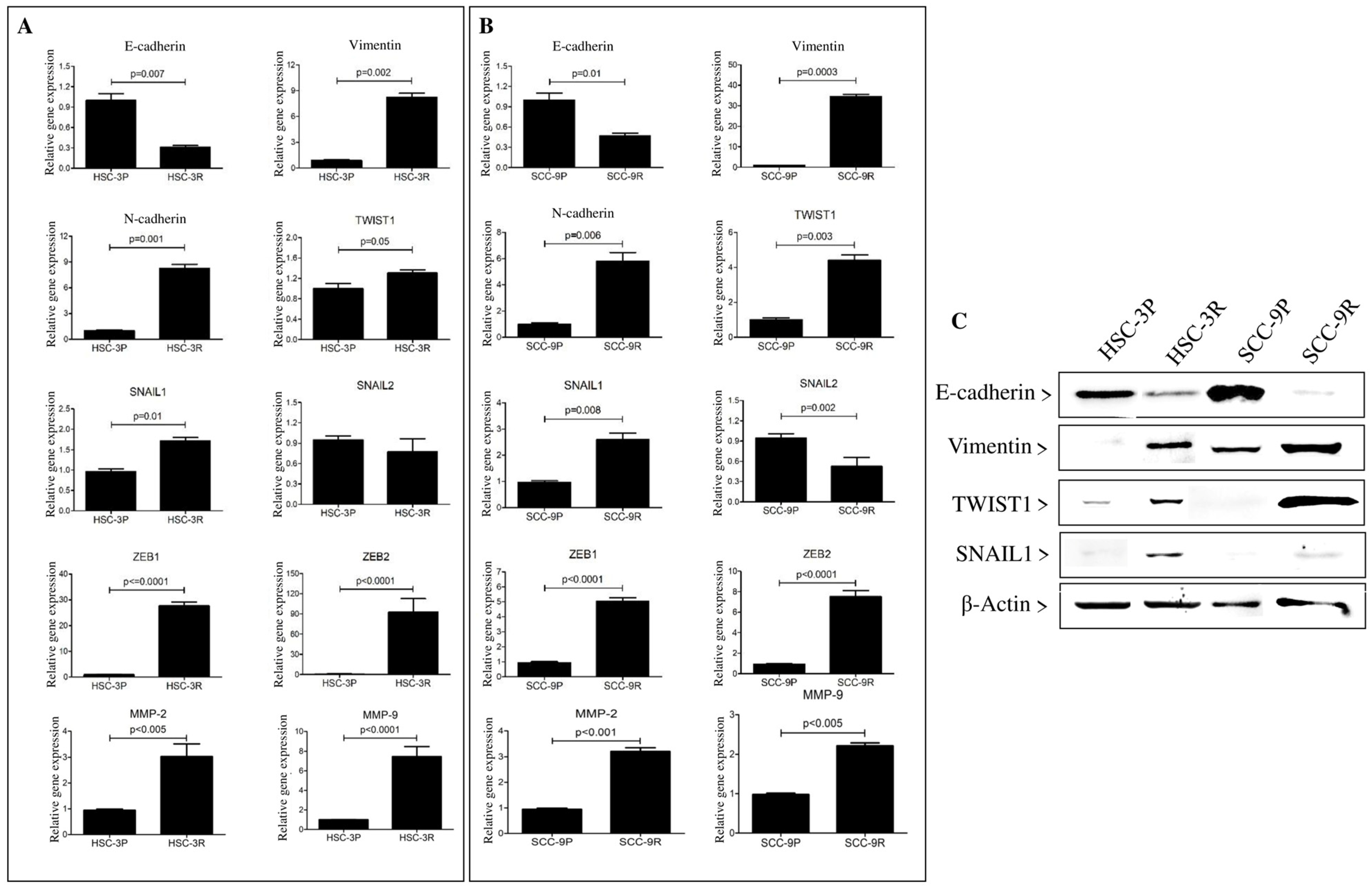
3.2. Pathway Enrichment and EMT-Related Gene Expression in Cisplatin-Resistant Cells
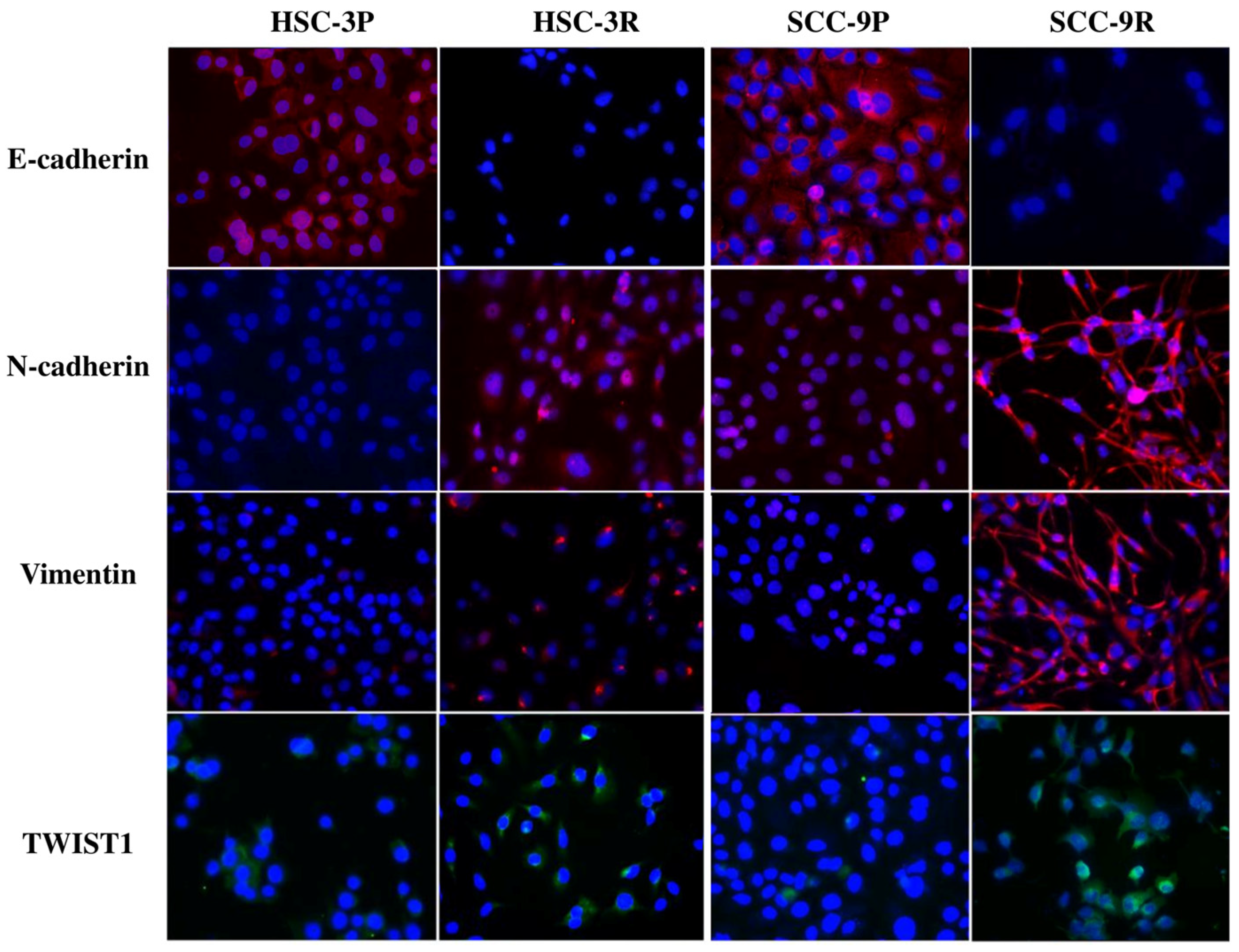
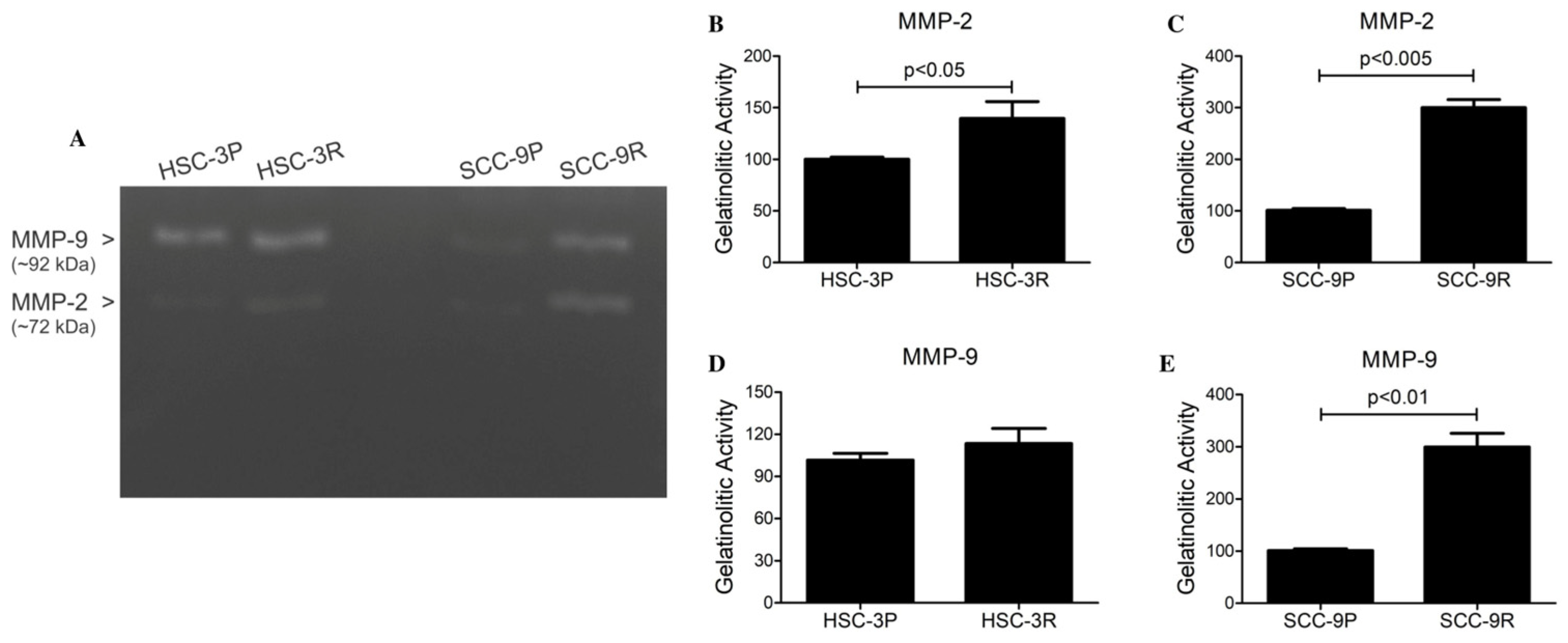
3.3. Validation of the EMT Profile of Cisplatin-Resistant Cells
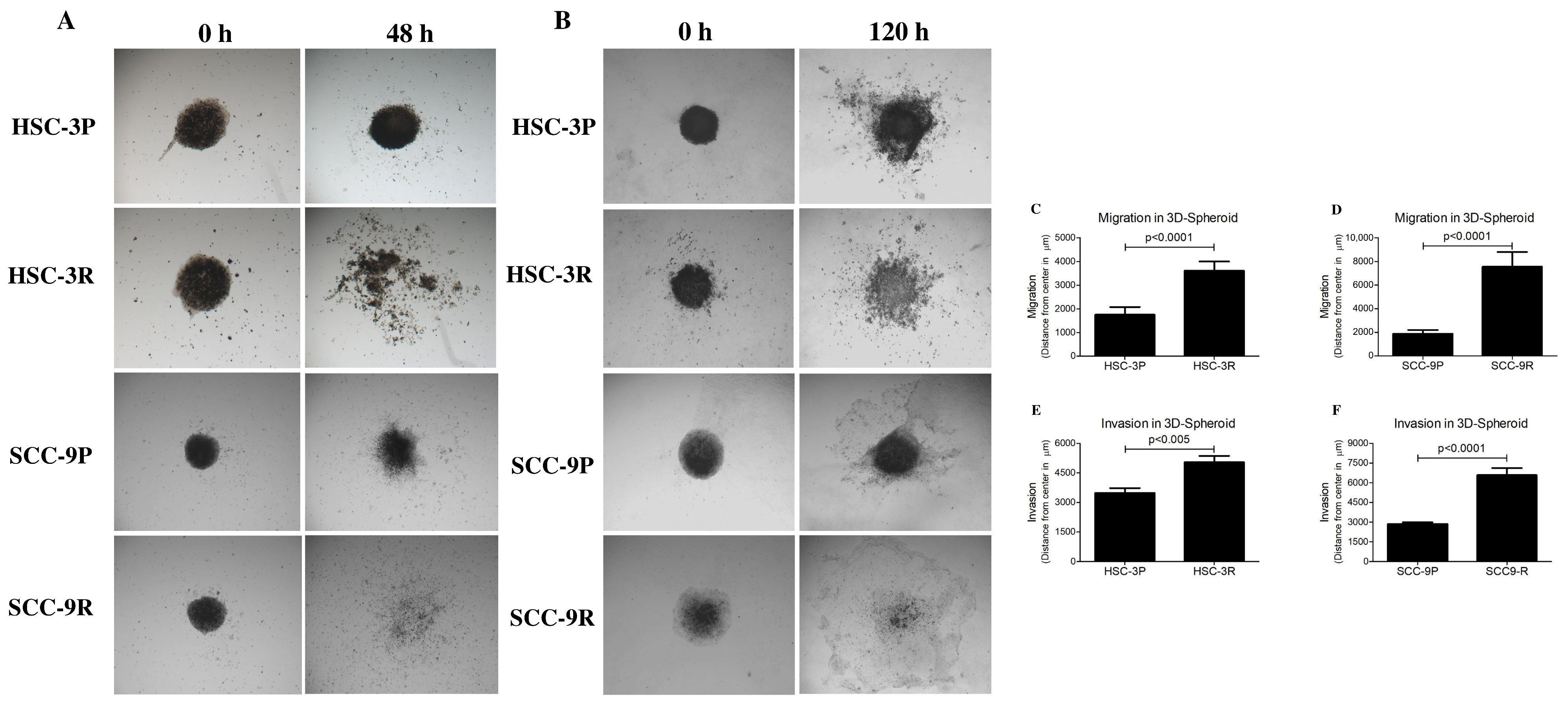
3.4. Chemoresistant Cells Exhibit Enhanced Migratory and Invasive Capacities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine Serum Albumin |
| CAF | Cancer-Associated Fibroblast |
| cDNA | Complementary DNA |
| DEG | Differentially Expressed Gene |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMEM/F12 | Dulbecco’s Modified Eagle’s Medium / Ham’s F12 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| EMT | Epithelial–Mesenchymal Transition |
| FBS | Fetal Bovine Serum |
| FDR | False Discovery Rate |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| GSVA | Gene Set Variation Analysis |
| IC50, IC5, etc. | Inhibitory Concentration 50%/5%, etc. |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MMP | Matrix Metalloproteinase |
| NaCl | Sodium Chloride |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| NSCLC | Non-Small Cell Lung Cancer |
| OSCC | Oral Squamous Cell Carcinoma |
| PBS | Phosphate-Buffered Saline |
| PE | Paired-End (sequencing) |
| qPCR | Quantitative Polymerase Chain Reaction |
| RNA-seq | RNA Sequencing |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| SD | Standard Deviation |
| UV | Ultraviolet |
| VST | Variance-Stabilizing Transformation |
References
- Coletta, R.D.; Yeudall, W.A.; Salo, T. Grand challenges in oral cancers. Front. Oral Health 2020, 1, 3. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- de Morais, E.F.; Almangush, A.; Salo, T.; da Silva, S.D.; Kujan, O.; Coletta, R.D. Emerging histopathological parameters in the prognosis of oral squamous cell carcinomas. Histol. Histopathol. 2024, 39, 1–12. [Google Scholar] [PubMed]
- Mohamad, I.; Glaun, M.D.E.; Prabhash, K.; Busheri, A.; Lai, S.Y.; Noronha, V.; Hosni, A. Current treatment strategies and risk stratification for oral carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389810. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, Y.K.; Yun, P.Y. Cisplatin plus cetuximab inhibits cisplatin-resistant human oral squamous cell carcinoma cell migration and proliferation but does not enhance apoptosis. Int. J. Mol. Sci. 2021, 22, 8167. [Google Scholar] [CrossRef]
- Atashi, F.; Vahed, N.; Emamverdizadeh, P.; Fattahi, S.; Paya, L. Drug resistance against 5-fluorouracil and cisplatin in the treatment of head and neck squamous cell carcinoma: A systematic review. J. Dent. Res. Dent. Clin. Dent. Prospects 2021, 15, 219–225. [Google Scholar] [CrossRef]
- Shriwas, O.; Arya, R.; Mohanty, S.; Mohapatra, P.; Kumar, S.; Rath, R.; Kaushik, S.R.; Pahwa, F.; Murmu, K.C.; Majumdar, S.K.D.; et al. RRBP1 rewires cisplatin resistance in oral squamous cell carcinoma by regulating Hippo pathway. Br. J. Cancer 2021, 124, 2004–2016. [Google Scholar] [CrossRef]
- Hu, H.; Li, B.; Wang, J.; Tan, Y.; Xu, M.; Xu, W.; Lu, H. New advances into cisplatin resistance in head and neck squamous carcinoma: Mechanisms and therapeutic aspects. Biomed. Pharmacother. 2023, 163, 114778. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Polpaya, K.; Kunale, M.; Kodiveri Muthukaliannan, G.; Shetty, S.; Baxi, S.; Mani, R.R.; Paranjothy, C.; Purushothaman, V.; Kayarohanam, S.; et al. Clinical investigation of chemotherapeutic resistance and miRNA expressions in head and neck cancers: A thorough PRISMA compliant systematic review and comprehensive meta-analysis. Genes 2022, 13, 2325. [Google Scholar] [CrossRef] [PubMed]
- Lima de Oliveira, J.; Moré Milan, T.; Longo Bighetti-Trevisan, R.; Fernandes, R.R.; Machado Leopoldino, A.; Oliveira de Almeida, L. Epithelial-mesenchymal transition and cancer stem cells: A route to acquired cisplatin resistance through epigenetics in HNSCC. Oral Dis. 2023, 29, 1991–2005. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Manavi, M.S.; Faghihkhorasani, F.; Fakhr, S.S.; Baei, F.J.; Khorasani, F.F.; Zare, M.M.; Far, N.P.; Rezaei-Tazangi, F.; Ren, J.; et al. Harnessing function of EMT in cancer drug resistance: A metastasis regulator determines chemotherapy response. Cancer Metastasis Rev. 2024, 43, 457–479. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- de Morais, E.F.; de Farias Morais, H.G.; de Moura Santos, E.; Barboza, C.A.G.; Téo, F.H.; Salo, T.; Coletta, R.D.; de Almeida Freitas, R. TWIST1 regulates proliferation, migration, and invasion and is a prognostic marker for oral tongue squamous cell carcinoma. J. Oral Pathol. Med. 2023, 52, 127–135. [Google Scholar] [CrossRef] [PubMed]
- de Morais, E.F.; Morais, H.G.F.; de França, G.M.; Téo, F.H.; Galvão, H.C.; Salo, T.; Coletta, R.D.; Freitas, R.A. SNAIL1 is involved in the control of the epithelial-mesenchymal transition in oral tongue squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 2018, 8, 2918. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Seyedi Asl, F.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The impact of epithelial-mesenchymal transition (EMT) induced by metabolic processes and intracellular signaling pathways on chemo-resistance, metastasis, and recurrence in solid tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef]
- Tangsiri, M.; Hheidari, A.; Liaghat, M.; Razlansari, M.; Ebrahimi, N.; Akbari, A.; Varnosfaderani, S.M.N.; Maleki-Sheikhabadi, F.; Norouzi, A.; Bakhtiyari, M.; et al. Promising applications of nanotechnology in inhibiting chemo-resistance in solid tumors by targeting epithelial-mesenchymal transition (EMT). Biomed. Pharmacother. 2024, 170, 115973. [Google Scholar] [CrossRef]
- Fabregat, I.; Malfettone, A.; Soukupova, J. New insights into the crossroads between EMT and stemness in the context of cancer. J. Clin. Med. 2016, 5, 37. [Google Scholar] [CrossRef]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by EMT: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef]
- Williams, E.D.; Gao, D.; Redfern, A.; Thompson, E.W. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 2019, 19, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Sobral, L.M.; Bufalino, A.; Lopes, M.A.; Graner, E.; Salo, T.; Coletta, R.D. Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol. 2011, 47, 840–846. [Google Scholar] [CrossRef]
- Dourado, M.R.; Elseragy, A.; da Costa, B.C.; Téo, F.H.; Guimarães, G.N.; Machado, R.A.; Risteli, M.; Wahbi, W.; Gurgel Rocha, C.A.; Paranaíba, L.M.R.; et al. Stress induced phosphoprotein 1 overexpression controls proliferation, migration and invasion and is associated with poor survival in oral squamous cell carcinoma. Front. Oncol. 2023, 12, 1085917. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yorioka, C.W.; Coletta, R.D.; Alves, F.; Nishimoto, I.N.; Kowalski, L.P.; Graner, E. Matrix metalloproteinase-2 and -9 activities correlate with the disease-free survival of oral squamous cell carcinoma patients. Int. J. Oncol. 2002, 20, 189–194. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, S.; Gao, L.; Zhi, K.; Ren, W. The molecular basis and therapeutic aspects of cisplatin resistance in oral squamous cell carcinoma. Front. Oncol. 2021, 11, 761379. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, Y.K.; Yun, P.Y. Upregulation of MDR- and EMT-related molecules in cisplatin-resistant human oral squamous cell carcinoma cell lines. Int. J. Mol. Sci. 2019, 20, 3034. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.A.; Gondaliya, P.; Mali, M.; Pawar, A.; Bhat, P.; Khairnar, A.; Arya, N.; Kalia, K. MiR-155 inhibitor-laden exosomes reverse resistance to cisplatin in a 3D tumor spheroid and xenograft model of oral cancer. Mol. Pharm. 2021, 18, 3010–3025. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, J.Y.; Kim, H.S.; Yoon, H. Establishment of acquired cisplatin resistance in ovarian cancer cell lines characterized by enriched metastatic properties with increased Twist expression. Int. J. Mol. Sci. 2020, 21, 7613. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic plasticity: Driver of cancer initiation, progression, and therapy resistance. Cell Stem Cell 2019, 24, 65–78. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ling, M.T.; Guan, X.Y.; Tsao, S.W.; Cheung, H.W.; Lee, D.T.; Wong, Y.C. Identification of a novel function of TWIST, a bHLH protein, in the development of acquired taxol resistance in human cancer cells. Oncogene 2004, 23, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Lu, H.W.; Li, Y.M.; Lu, L.; Wang, J.L.; Zhang, Y.F.; Shang, H. Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15. Mol. Med. Rep. 2015, 12, 3841–3848. [Google Scholar] [CrossRef]
- Roberts, C.M.; Tran, M.A.; Pitruzzello, M.C.; Wen, W.; Loeza, J.; Dellinger, T.H.; Mor, G.; Glackin, C.A. TWIST1 drives cisplatin resistance and cell survival in an ovarian cancer model, via upregulation of GAS6, L1CAM, and Akt signalling. Sci. Rep. 2016, 6, 37652. [Google Scholar] [CrossRef]
- Youssef, K.K.; Narwade, N.; Arcas, A.; Marquez-Galera, A.; Jiménez-Castaño, R.; Lopez-Blau, C.; Fazilaty, H.; García-Gutierrez, D.; Cano, A.; Galcerán, J.; et al. Two distinct epithelial-to-mesenchymal transition programs control invasion and inflammation in segregated tumor cell populations. Nat. Cancer 2024, 5, 1660–1680. [Google Scholar] [CrossRef] [PubMed]
- Işeri, O.D.; Kars, M.D.; Arpaci, F.; Atalay, C.; Pak, I.; Gündüz, U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed. Pharmacother. 2011, 65, 40–45. [Google Scholar] [CrossRef]
- Bitting, R.L.; Schaeffer, D.; Somarelli, J.A.; Garcia-Blanco, M.A.; Armstrong, A.J. The role of epithelial plasticity in prostate cancer dissemination and treatment resistance. Cancer Metastasis Rev. 2014, 33, 441–468. [Google Scholar] [CrossRef]
- Ruscetti, M.; Dadashian, E.L.; Guo, W.; Quach, B.; Mulholland, D.J.; Park, J.W.; Tran, L.M.; Kobayashi, N.; Bianchi-Frias, D.; Xing, Y.; et al. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene 2016, 35, 3781–3795. [Google Scholar] [CrossRef]
- Jia, D.; Jolly, M.K.; Kulkarni, P.; Levine, H. Phenotypic plasticity and cell fate decisions in cancer: Insights from dynamical systems theory. Cancers 2017, 9, 70. [Google Scholar] [CrossRef]
- Jolly, M.K.; Tripathi, S.C.; Somarelli, J.A.; Hanash, S.M.; Levine, H. Epithelial/mesenchymal plasticity: How have quantitative mathematical models helped improve our understanding? Mol. Oncol. 2017, 11, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Sinha, S.K.; Levine, H.; Jolly, M.K.; Dutta, P.S. Anticipating critical transitions in epithelial-hybrid-mesenchymal cell-fate determination. Proc. Natl. Acad. Sci. USA 2019, 116, 26343–26352. [Google Scholar] [CrossRef] [PubMed]
- Sarrió, D.; Rodriguez-Pinilla, S.M.; Hardisson, D.; Cano, A.; Moreno-Bueno, G.; Palacios, J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008, 68, 989–997. [Google Scholar] [CrossRef]
- Norouzi, S.; Gorgi Valokala, M.; Mosaffa, F.; Zirak, M.R.; Zamani, P.; Behravan, J. Crosstalk in cancer resistance and metastasis. Crit. Rev. Oncol. Hematol. 2018, 132, 145–153. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Horta, C.A.; Yang, J. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics. Semin. Cancer Biol. 2023, 88, 46–66. [Google Scholar] [CrossRef]
- Lotfi, A.; Mohammadi, G.; Tavassoli, A.; Mousaviagdas, M.; Chavoshi, H.; Saniee, L. Serum levels of MMP9 and MMP2 in patients with oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 1327–1330. [Google Scholar] [CrossRef]
- Renu, K.; Vinayagam, S.; Veeraraghavan, V.P.; Mukherjee, A.G.; Wanjari, U.R.; Prabakaran, D.S.; Ganesan, R.; Dey, A.; Vellingiri, B.; Kandasamy, S.; et al. Molecular crosstalk between the immunological mechanism of the tumor microenvironment and epithelial-mesenchymal transition in oral cancer. Vaccines 2022, 10, 1490. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, E.F.; de Oliveira, L.Q.R.; Marques, C.E.; Téo, F.H.; Rocha, G.V.; Rodini, C.O.; Gurgel, C.A.; Salo, T.; Graner, E.; Coletta, R.D. Generation and Characterization of Cisplatin-Resistant Oral Squamous Cell Carcinoma Cells Displaying an Epithelial–Mesenchymal Transition Signature. Cells 2025, 14, 1311. https://doi.org/10.3390/cells14171311
de Morais EF, de Oliveira LQR, Marques CE, Téo FH, Rocha GV, Rodini CO, Gurgel CA, Salo T, Graner E, Coletta RD. Generation and Characterization of Cisplatin-Resistant Oral Squamous Cell Carcinoma Cells Displaying an Epithelial–Mesenchymal Transition Signature. Cells. 2025; 14(17):1311. https://doi.org/10.3390/cells14171311
Chicago/Turabian Stylede Morais, Everton Freitas, Lilianny Querino Rocha de Oliveira, Cintia Eliza Marques, Fábio Haach Téo, Gisele Vieira Rocha, Camila Oliveira Rodini, Clarissa A. Gurgel, Tuula Salo, Edgard Graner, and Ricardo D. Coletta. 2025. "Generation and Characterization of Cisplatin-Resistant Oral Squamous Cell Carcinoma Cells Displaying an Epithelial–Mesenchymal Transition Signature" Cells 14, no. 17: 1311. https://doi.org/10.3390/cells14171311
APA Stylede Morais, E. F., de Oliveira, L. Q. R., Marques, C. E., Téo, F. H., Rocha, G. V., Rodini, C. O., Gurgel, C. A., Salo, T., Graner, E., & Coletta, R. D. (2025). Generation and Characterization of Cisplatin-Resistant Oral Squamous Cell Carcinoma Cells Displaying an Epithelial–Mesenchymal Transition Signature. Cells, 14(17), 1311. https://doi.org/10.3390/cells14171311









