Impact of Novel Agents on Allogeneic Hematopoietic Cell Transplantation in Patients with T-Cell Lymphomas
Abstract
1. Introduction
2. Antibody Drugs
2.1. Mogamulizumab (MOG)
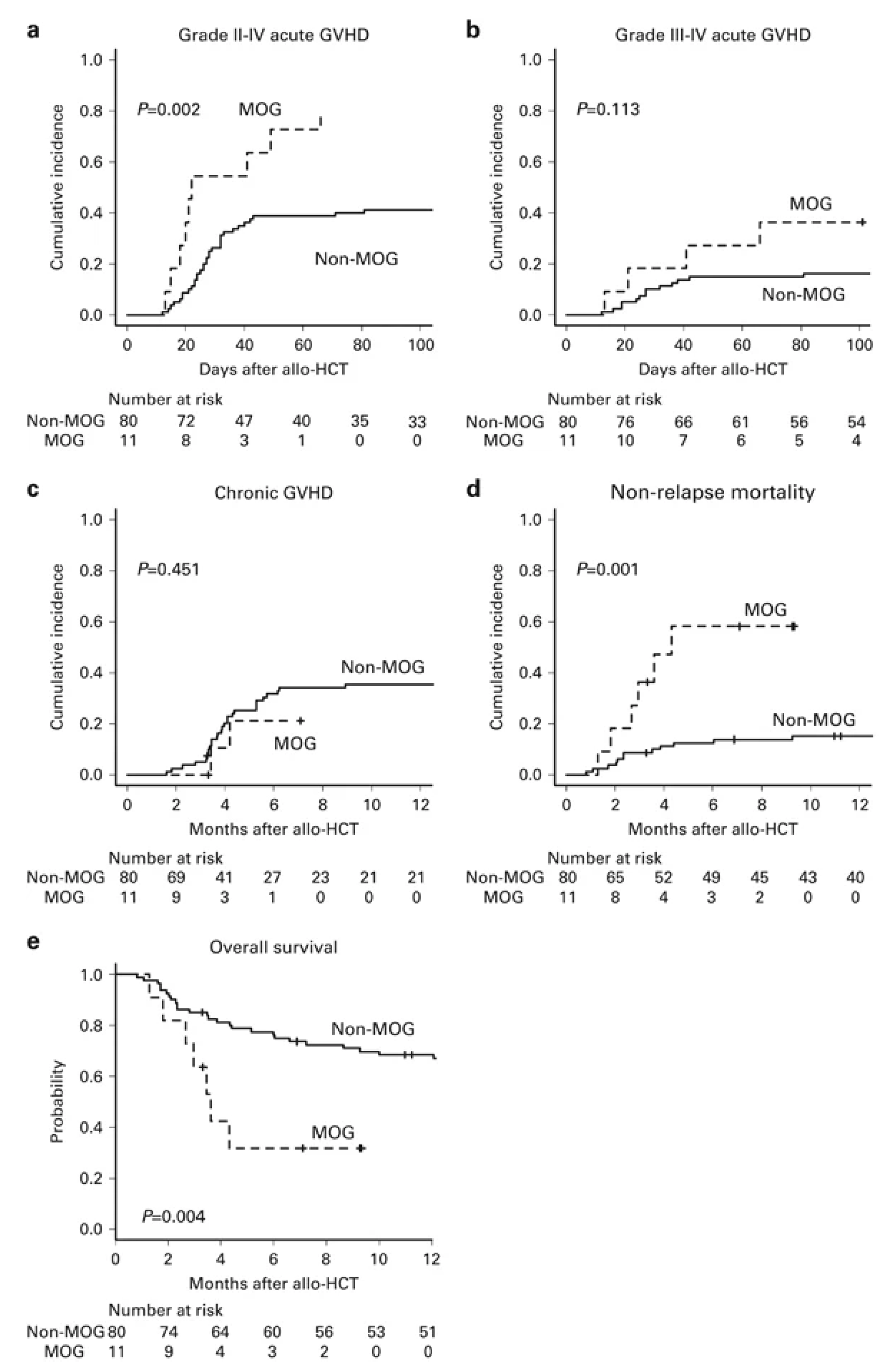
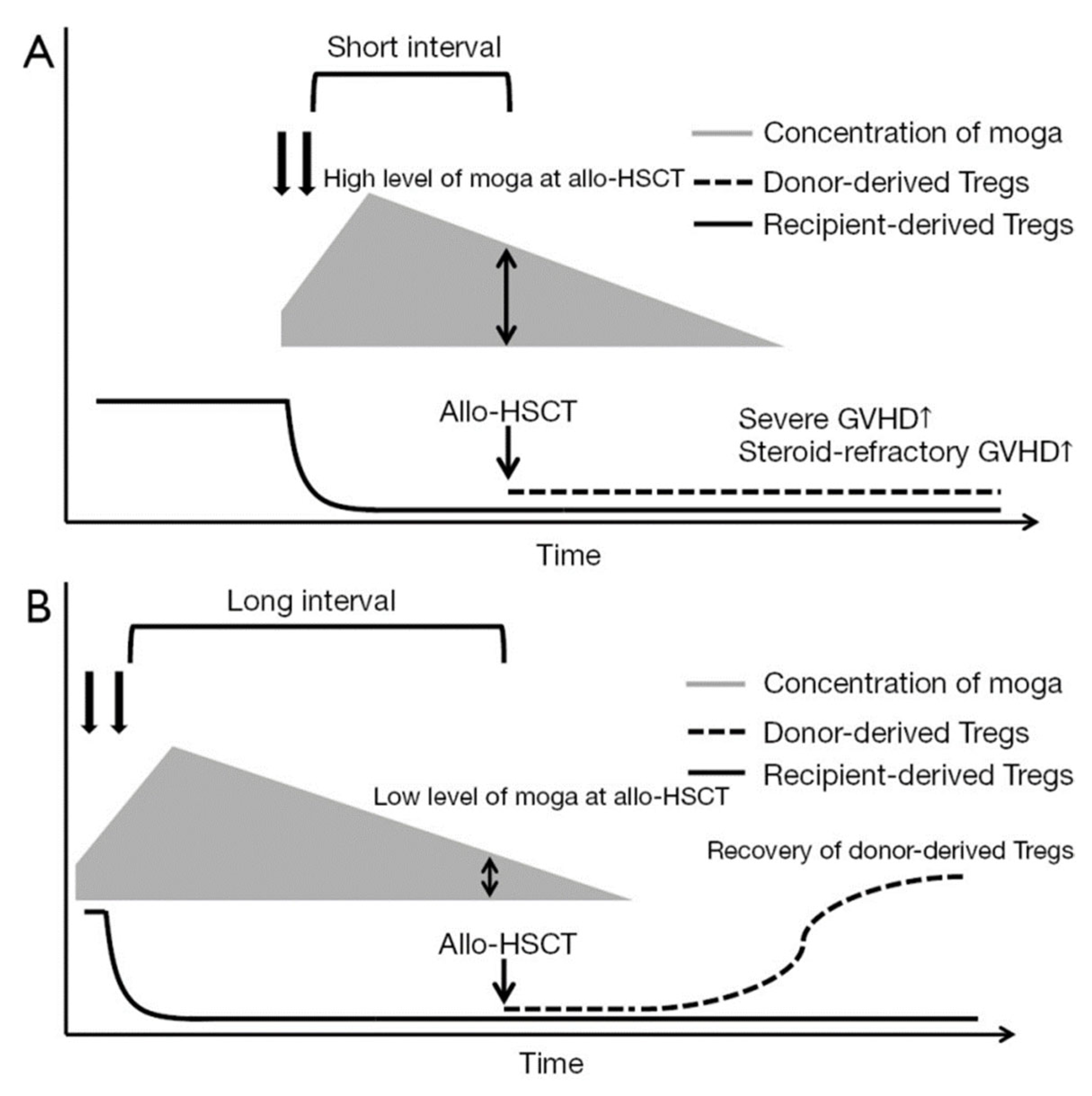
2.1.1. Impact of Pre-HCT Use
2.1.2. Impact of Post-HCT Use
2.2. Brentuximab Vedotin (BV)
2.2.1. Impact of Pre-HCT Use
2.2.2. Impact of Post-HCT Use
3. Lenalidomide (LEN)
Impact of Post-HCT Use
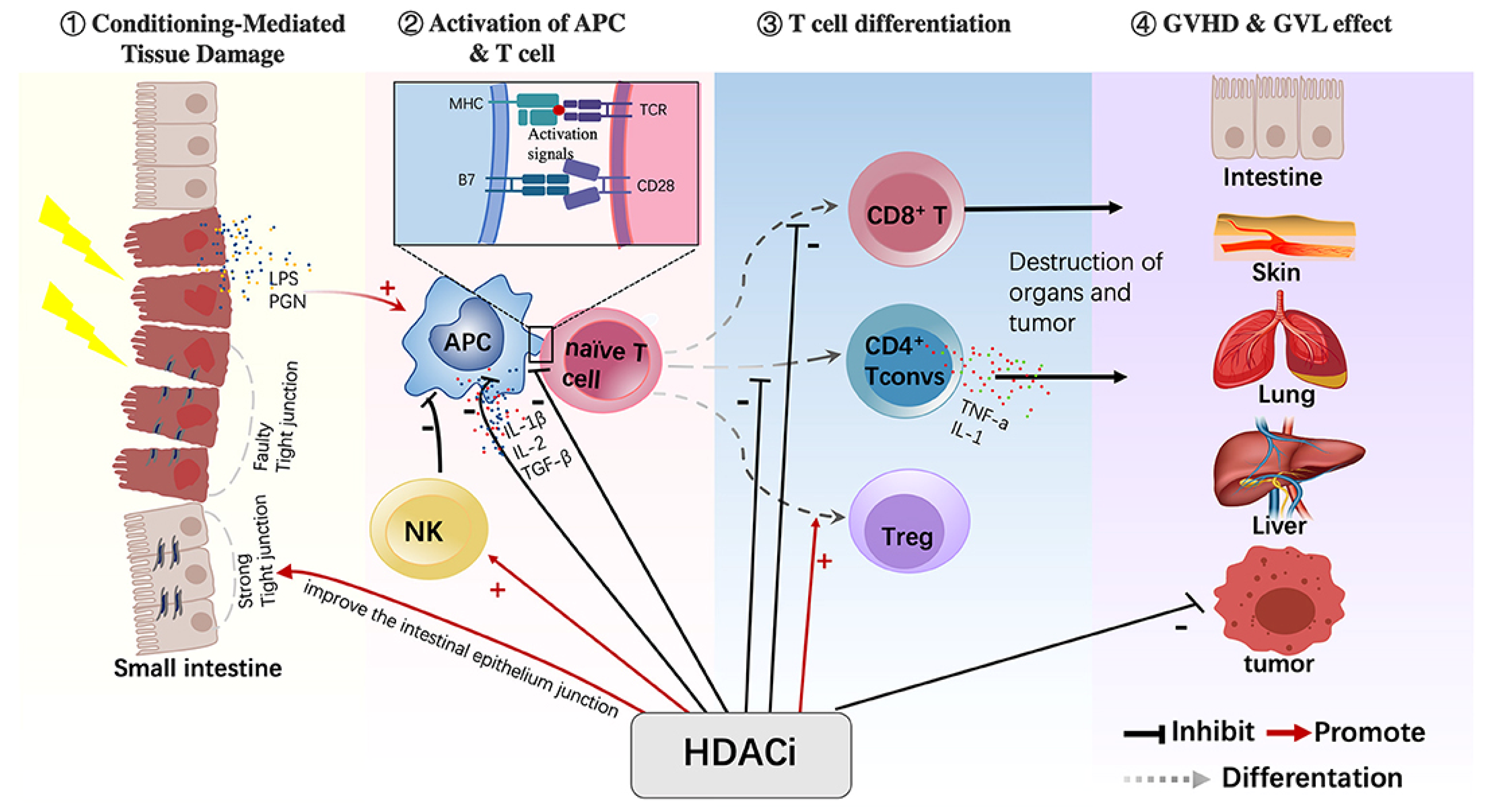
4. HDAC Inhibitors (Vorinostat, Romidepsin, Belinostat, Chidamide (Tucidinostat))
Control of GVHD by HDACis
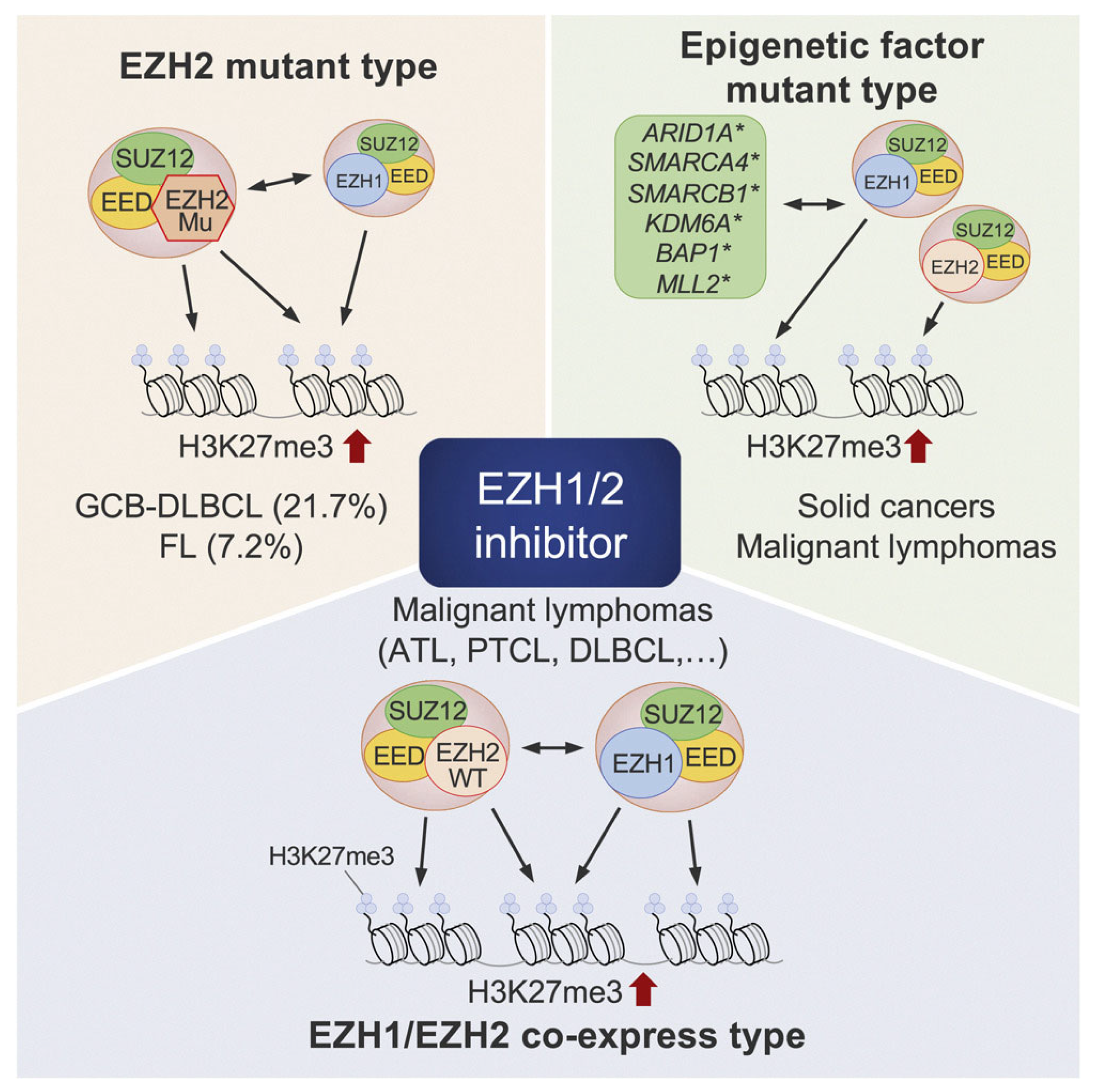
5. EZH1/2 Inhibitors
Impact of EZH1/2 Inhibition on GVHD
6. Immune Checkpoint Inhibitors (ICIs)
Impact of Pre- and Post-HCT Use
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | antigen-presenting cell(s) |
| ATL | adult T-cell leukemia/lymphoma |
| AZA | azacitidine |
| BV | brentuximab vedotin |
| EZH1/2 | enhancer of zeste homolog 1/2 |
| GVHD | graft-versus-host disease |
| GVL | graft-versus-lymphoma |
| HCT | allogenic hematopoietic cell transplantation |
| HDACis | histone deacetylase inhibitors |
| irAEs | immune-related adverse events |
| LEN | lenalidomide |
| MOG | mogamulizumab |
| NK | natural killer |
| NRM | non-relapse mortality |
| PD-1 | programmed cell death protein 1 |
| PTCy | post-transplant cyclophosphamide |
| TCLs | T-cell Lymphomas |
| Treg | regulatory T cell(s) |
References
- Vose, J.; Armitage, J.; Weisenburger, D.; International, T.C.L.P. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar] [CrossRef] [PubMed]
- Chihara, D.; Ito, H.; Matsuda, T.; Shibata, A.; Katsumi, A.; Nakamura, S.; Tomotaka, S.; Morton, L.M.; Weisenburger, D.D.; Matsuo, K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br. J. Haematol. 2014, 164, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Newcomb, P.A.; Shustov, A.R. Racial Patterns of Peripheral T-Cell Lymphoma Incidence and Survival in the United States. J. Clin. Oncol. 2016, 34, 963–971. [Google Scholar] [CrossRef]
- Han, J.X.; Koh, M.J.; Boussi, L.; Sorial, M.; McCabe, S.M.; Peng, L.; Singh, S.; Eche-Ugwu, I.J.; Gabler, J.; Fernandez Turizo, M.J.; et al. Global outcomes and prognosis for relapsed/refractory mature T-cell and NK-cell lymphomas: Results from the PETAL consortium. Blood Adv. 2025, 9, 583–602. [Google Scholar] [CrossRef]
- Dobos, G.; Pohrt, A.; Ram-Wolff, C.; Lebbe, C.; Bouaziz, J.D.; Battistella, M.; Bagot, M.; de Masson, A. Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 Patients. Cancers 2020, 12, 2921. [Google Scholar] [CrossRef]
- Lone, W.; Alkhiniji, A.; Manikkam Umakanthan, J.; Iqbal, J. Molecular Insights Into Pathogenesis of Peripheral T Cell Lymphoma: A Review. Curr. Hematol. Malig. Rep. 2018, 13, 318–328. [Google Scholar] [CrossRef]
- Mak, V.; Hamm, J.; Chhanabhai, M.; Shenkier, T.; Klasa, R.; Sehn, L.H.; Villa, D.; Gascoyne, R.D.; Connors, J.M.; Savage, K.J. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: Spectrum of disease and rare long-term survivors. J. Clin. Oncol. 2013, 31, 1970–1976. [Google Scholar] [CrossRef]
- Chihara, D.; Fanale, M.A.; Miranda, R.N.; Noorani, M.; Westin, J.R.; Nastoupil, L.J.; Hagemeister, F.B.; Fayad, L.E.; Romaguera, J.E.; Samaniego, F.; et al. The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma. Br. J. Haematol. 2017, 176, 750–758. [Google Scholar] [CrossRef]
- Butcher, B.W.; Collins, R.H., Jr. The graft-versus-lymphoma effect: Clinical review and future opportunities. Bone Marrow Transplant. 2005, 36, 1–17. [Google Scholar] [CrossRef]
- Boo, Y.L.; Koh, L.P. Hematopoietic Stem Cell Transplantation in T Cell and Natural Killer Cell Lymphomas: Update on Recent Advances. Transplant. Cell Ther. 2021, 27, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Dreger, P.; Schmitz, N. The role of stem cell transplant (auto and allo) in PTCL and CTCL. Hematology 2024, 2024, 69–77. [Google Scholar] [CrossRef]
- Weng, W.K.; Arai, S.; Rezvani, A.; Johnston, L.; Lowsky, R.; Miklos, D.; Shizuru, J.; Muffly, L.; Meyer, E.; Negrin, R.S.; et al. Nonmyeloablative allogeneic transplantation achieves clinical and molecular remission in cutaneous T-cell lymphoma. Blood Adv. 2020, 4, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Domenech, E.; Duarte, R.F.; Boumedil, A.; Onida, F.; Gabriel, I.; Finel, H.; Arcese, W.; Browne, P.; Beelen, D.; Kobbe, G.; et al. Allogeneic hematopoietic stem cell transplantation for advanced mycosis fungoides and Sézary syndrome. An updated experience of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2021, 56, 1391–1401. [Google Scholar] [CrossRef]
- Goyal, A.; O’Leary, D.; Foss, F. Allogeneic stem cell transplant for treatment of mycosis fungoides and Sezary syndrome: A systematic review and meta-analysis. Bone Marrow Transplant. 2024, 59, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Ahn, K.W.; Litovich, C.; Ahmed, S.; Battiwalla, M.; Cohen, J.B.; Dahi, P.; Farhadfar, N.; Farooq, U.; Freytes, C.O.; et al. Allogeneic hematopoietic cell transplantation provides effective salvage despite refractory disease or failed prior autologous transplant in angioimmunoblastic T-cell lymphoma: A CIBMTR analysis. J. Hematol. Oncol. 2019, 12, 6. [Google Scholar] [CrossRef]
- Cook, L.B.; Fuji, S.; Hermine, O.; Bazarbachi, A.; Ramos, J.C.; Ratner, L.; Horwitz, S.; Fields, P.; Tanase, A.; Bumbea, H.; et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J. Clin. Oncol. 2019, 37, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Nakano, N.; Tanaka, T.; Fuji, S.; Makiyama, J.; Inoue, Y.; Choi, I.; Nakamae, H.; Nagafuji, K.; Takase, K.; et al. Improved survival of patients with aggressive ATL by increased use of allo-HCT: A prospective observational study. Blood Adv. 2021, 5, 4156–4166. [Google Scholar] [CrossRef]
- Foley, N.C.; Mehta-Shah, N. Management of Peripheral T-cell Lymphomas and the Role of Transplant. Curr. Oncol. Rep. 2022, 24, 1489–1499. [Google Scholar] [CrossRef]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Advani, R.H.; Bartlett, N.L.; Jacobsen, E.D.; Sharman, J.P.; O’Connor, O.A.; Siddiqi, T.; Kennedy, D.A.; Oki, Y. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 2014, 123, 3095–3100. [Google Scholar] [CrossRef]
- Ishida, T.; Fujiwara, H.; Nosaka, K.; Taira, N.; Abe, Y.; Imaizumi, Y.; Moriuchi, Y.; Jo, T.; Ishizawa, K.; Tobinai, K.; et al. Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J. Clin. Oncol. 2016, 34, 4086–4093. [Google Scholar] [CrossRef]
- Duvic, M.; Talpur, R.; Ni, X.; Zhang, C.; Hazarika, P.; Kelly, C.; Chiao, J.H.; Reilly, J.F.; Ricker, J.L.; Richon, V.M.; et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 2007, 109, 31–39. [Google Scholar] [CrossRef]
- Coiffier, B.; Pro, B.; Prince, H.M.; Foss, F.; Sokol, L.; Greenwood, M.; Caballero, D.; Borchmann, P.; Morschhauser, F.; Wilhelm, M.; et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J. Clin. Oncol. 2012, 30, 631–636. [Google Scholar] [CrossRef]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; Van Hoof, A.; Brown, P.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Kim, W.S.; Ando, K.; Choi, I.; Izutsu, K.; Tsukamoto, N.; Yokoyama, M.; Tsukasaki, K.; Kuroda, J.; Ando, J.; et al. Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Phase IIb results. Haematologica 2023, 108, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Izutsu, K.; Mehta-Shah, N.; Barta, S.K.; Ishitsuka, K.; Córdoba, R.; Kusumoto, S.; Bachy, E.; Cwynarski, K.; Gritti, G.; et al. Valemetostat for patients with relapsed or refractory peripheral T-cell lymphoma (VALENTINE-PTCL01): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2024, 25, 1602–1613. [Google Scholar] [CrossRef]
- Kwong, Y.L.; Chan, T.S.Y.; Tan, D.; Kim, S.J.; Poon, L.M.; Mow, B.; Khong, P.L.; Loong, F.; Au-Yeung, R.; Iqbal, J.; et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood 2017, 129, 2437–2442. [Google Scholar] [CrossRef]
- Mamez, A.C.; Dupont, A.; Blaise, D.; Chevallier, P.; Forcade, E.; Ceballos, P.; Mohty, M.; Suarez, F.; Beguin, Y.; Peffault De Latour, R.; et al. Allogeneic stem cell transplantation for peripheral T cell lymphomas: A retrospective study in 285 patients from the Societe Francophone de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). J. Hematol. Oncol. 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Utsunomiya, A.; Tobinai, K.; Tsukasaki, K.; Uike, N.; Uozumi, K.; Yamaguchi, K.; Yamada, Y.; Hanada, S.; Tamura, K.; et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J. Clin. Oncol. 2010, 28, 1591–1598. [Google Scholar] [CrossRef]
- Imai, T.; Nagira, M.; Takagi, S.; Kakizaki, M.; Nishimura, M.; Wang, J.; Gray, P.W.; Matsushima, K.; Yoshie, O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 1999, 11, 81–88. [Google Scholar] [CrossRef]
- Baatar, D.; Olkhanud, P.; Sumitomo, K.; Taub, D.; Gress, R.; Biragyn, A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4- Tregs, regulate effector T cells using FasL. J. Immunol. 2007, 178, 4891–4900. [Google Scholar] [CrossRef]
- Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D’Ambrosio, D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001, 194, 847–853. [Google Scholar] [CrossRef]
- Ito, A.; Ishida, T.; Yano, H.; Inagaki, A.; Suzuki, S.; Sato, F.; Takino, H.; Mori, F.; Ri, M.; Kusumoto, S.; et al. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol. Immunother. 2009, 58, 1195–1206. [Google Scholar] [CrossRef]
- Kurose, K.; Ohue, Y.; Wada, H.; Iida, S.; Ishida, T.; Kojima, T.; Doi, T.; Suzuki, S.; Isobe, M.; Funakoshi, T.; et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin. Cancer Res. 2015, 21, 4327–4336. [Google Scholar] [CrossRef]
- Kim, Y.H.; Bagot, M.; Pinter-Brown, L.; Rook, A.H.; Porcu, P.; Horwitz, S.M.; Whittaker, S.; Tokura, Y.; Vermeer, M.; Zinzani, P.L.; et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Fuji, S.; Tanosaki, R.; Inamoto, Y.; Tanaka, T.; Ito, A.; Okinaka, K.; Kurosawa, S.; Kim, S.W.; Nakagama, H.; et al. Prognostic importance of pretransplant disease status for posttransplant outcomes in patients with adult T cell leukemia/lymphoma. Bone Marrow Transplant. 2018, 53, 1105–1115. [Google Scholar] [CrossRef]
- Ishida, T.; Jo, T.; Takemoto, S.; Suzushima, H.; Uozumi, K.; Yamamoto, K.; Uike, N.; Saburi, Y.; Nosaka, K.; Utsunomiya, A.; et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: A randomized phase II study. Br. J. Haematol. 2015, 169, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Fuji, S.; Tanosaki, R.; Fukuda, T. Pretransplant mogamulizumab against ATLL might increase the risk of acute GVHD and non-relapse mortality. Bone Marrow Transplant. 2016, 51, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Fuji, S.; Inoue, Y.; Utsunomiya, A.; Moriuchi, Y.; Uchimaru, K.; Choi, I.; Otsuka, E.; Henzan, H.; Kato, K.; Tomoyose, T.; et al. Pretransplantation Anti-CCR4 Antibody Mogamulizumab Against Adult T-Cell Leukemia/Lymphoma Is Associated With Significantly Increased Risks of Severe and Corticosteroid-Refractory Graft-Versus-Host Disease, Nonrelapse Mortality, and Overall Mortality. J. Clin. Oncol. 2016, 34, 3426–3433. [Google Scholar] [CrossRef]
- Shichijo, T.; Nosaka, K.; Tatetsu, H.; Higuchi, Y.; Endo, S.; Inoue, Y.; Toyoda, K.; Kikukawa, Y.; Kawakita, T.; Yasunaga, J.I.; et al. Beneficial impact of first-line mogamulizumab-containing chemotherapy in adult T-cell leukaemia-lymphoma. Br. J. Haematol. 2022, 198, 983–987. [Google Scholar] [CrossRef]
- Fuji, S.; Tokunaga, M.; Kato, K.; Nakano, N.; Shindo, T.; Makiyama, J.; Itonaga, H.; Ito, A.; Eto, T.; Ishikawa, J.; et al. Significant detrimental impact of pre-transplant mogamulizumab on the post-transplant outcome with a short interval between the last mogamulizumab and transplantation. Bone Marrow Transplant. 2025, 60, 552–554. [Google Scholar] [CrossRef]
- Inoue, Y.; Nishimura, N.; Murai, M.; Matsumoto, M.; Watanabe, M.; Yamada, A.; Izaki, M.; Nosaka, K.; Matsuoka, M. Prevention of acute graft-versus-host disease in adult T-cell leukemia-lymphoma patients who received mogamulizumab before allogeneic hematopoietic cell transplantation. Int. J. Hematol. 2022, 115, 435–439. [Google Scholar] [CrossRef]
- Fuji, S.; Shindo, T. Friend or foe? Mogamulizumab in allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia/lymphoma. Stem Cell Investig. 2016, 3, 70. [Google Scholar] [CrossRef] [PubMed]
- Tsubokura, Y.; Satake, A.; Hotta, M.; Yoshimura, H.; Fujita, S.; Azuma, Y.; Nakanishi, T.; Nakaya, A.; Ito, T.; Ishii, K.; et al. Successful treatment with mogamulizumab followed by allogeneic hematopoietic stem-cell transplantation in adult T-cell leukemia/lymphoma: A report of two cases. Int. J. Hematol. 2016, 104, 744–748. [Google Scholar] [CrossRef]
- Hirosawa, M.; Higashi, T.; Iwashige, A.; Yamaguchi, T.; Tsukada, J. HLA-haploidentical hematopoietic stem cell transplantation with low-dose thymoglobulin GVHD prophylaxis for an adult T cell leukemia/lymphoma patient treated with pretransplant mogamulizumab. Ann. Hematol. 2017, 96, 327–328. [Google Scholar] [CrossRef]
- Motohashi, K.; Suzuki, T.; Kishimoto, K.; Numata, A.; Nakajima, Y.; Tachibana, T.; Ohshima, R.; Kuwabara, H.; Tanaka, M.; Tomita, N.; et al. Successful treatment of a patient with adult T cell leukemia/lymphoma using anti-CC chemokine receptor 4 monoclonal antibody mogamulizumab followed by allogeneic hematopoietic stem cell transplantation. Int. J. Hematol. 2013, 98, 258–260. [Google Scholar] [CrossRef]
- Nakata, K.; Fuji, S.; Koike, M.; Tada, Y.; Masaie, H.; Yoshida, H.; Watanabe, E.; Kobayashi, S.; Tojo, A.; Uchimaru, K.; et al. Successful treatment strategy in corporating mogamulizumab and cord blood transplantation in aggressive adult T-cell leukemia-lymphoma: A case report. Blood Cell Ther. 2020, 3, 6–10. [Google Scholar] [CrossRef]
- Ganguly, S.; Ross, D.B.; Panoskaltsis-Mortari, A.; Kanakry, C.G.; Blazar, B.R.; Levy, R.B.; Luznik, L. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014, 124, 2131–2141. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakamae, H.; Ito, A.; Fuji, S.; Hirose, A.; Eto, T.; Henzan, H.; Takase, K.; Yamasaki, S.; Makiyama, J.; et al. A Phase I/II Multicenter Trial of HLA-Haploidentical PBSCT with PTCy for Aggressive Adult T Cell Leukemia/Lymphoma. Transplant. Cell Ther. 2021, 27, 928.e921–928.e927. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Uike, N.; Wake, A.; Yoshimitsu, M.; Tobai, T.; Sawayama, Y.; Takatsuka, Y.; Fukuda, T.; Uchida, N.; Eto, T.; et al. The outcome and characteristics of patients with relapsed adult T cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation. Hematol. Oncol. 2019, 37, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, H.; Tsushima, H.; Taguchi, J.; Fukushima, T.; Taniguchi, H.; Sato, S.; Ando, K.; Sawayama, Y.; Matsuo, E.; Yamasaki, R.; et al. Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: The Nagasaki Transplant Group experience. Blood 2013, 121, 219–225. [Google Scholar] [CrossRef]
- Inoue, Y.; Endo, S.; Matsuno, N.; Kikukawa, Y.; Shichijo, T.; Koga, K.; Takaki, A.; Iwanaga, K.; Nishimura, N.; Fuji, S.; et al. Safety of mogamulizumab for relapsed ATL after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019, 54, 338–342. [Google Scholar] [CrossRef]
- Sakamoto, H.; Itonaga, H.; Sawayama, Y.; Furumoto, T.; Fujioka, M.; Chiwata, M.; Toriyama, E.; Kasai, S.; Nakashima, J.; Horai, M.; et al. Treatment with mogamulizumab or lenalidomide for relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: The Nagasaki transplant group experience. Hematol. Oncol. 2020, 38, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Tajika, K.; Nakayama, K.; Arai, A. Treatment of relapsed aggressive adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation with mogamulizumab followed by lenalidomide. Bone Marrow Transplant. 2021, 56, 2862–2863. [Google Scholar] [CrossRef]
- Hirosawa, M.; Goto, M.; Oku, M.; Akao, K.; Kitamura, N.; Nakanishi, T.; Tanaka, A.; Niino, D.; Higashi, T.; Morimoto, H.; et al. Mogamulizumab for post-transplant relapse of adult T-cell leukemia/lymphoma: A case study. Int. J. Hematol. 2023, 117, 143–148. [Google Scholar] [CrossRef]
- Sugiyama, D.; Nishikawa, H.; Maeda, Y.; Nishioka, M.; Tanemura, A.; Katayama, I.; Ezoe, S.; Kanakura, Y.; Sato, E.; Fukumori, Y.; et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 17945–17950. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef]
- Matsumoto, K.; Terakawa, M.; Miura, K.; Fukuda, S.; Nakajima, T.; Saito, H. Extremely Rapid and Intense Induction of Apoptosis in Human Eosinophils by Anti-CD30 Antibody Treatment In Vitro. J. Immunol. 2004, 172, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Oak, E.; Bartlett, N.L. A safety evaluation of brentuximab vedotin for the treatment of Hodgkin lymphoma. Expert Opin. Drug Saf. 2016, 15, 875–882. [Google Scholar] [CrossRef]
- Chen, R.; Palmer, J.M.; Thomas, S.H.; Tsai, N.C.; Farol, L.; Nademanee, A.; Forman, S.J.; Gopal, A.K. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood 2012, 119, 6379–6381. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Boumendil, A.; Finel, H.; Mohty, M.; Castagna, L.; Peggs, K.S.; Blaise, D.; Afanasyev, B.; Diez-Martin, J.L.; Sierra, J.; et al. Brentuximab vedotin prior to allogeneic stem cell transplantation in Hodgkin lymphoma: A report from the EBMT Lymphoma Working Party. Br. J. Haematol. 2018, 181, 86–96. [Google Scholar] [CrossRef]
- Garciaz, S.; Loschi, M.; De Masson, A.; Biard, L.; Mercier, M.; Tomowiak, C.; Delage, J.; Labussiere-Wallet, H.; Sibon, D.; Cassuto, O.; et al. Brentuximab vedotin as a bridge to allogeneic stem-cell transplantation for refractory or relapsing patients with CD30 positive anaplastic or T-cell non-Hodgkin lymphomas: A study on behalf of the SFGM-TC. Leuk. Lymphoma 2019, 60, 2802–2805. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.; Jones, C.; Bloor, A.; Kulkarni, S.; Illidge, T.; Linton, K.; Radford, J. Brentuximab vedotin in refractory CD30+ lymphomas: A bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica 2013, 98, 611–614. [Google Scholar] [CrossRef]
- Ordemann, R.; Stohlmacher, J.; Beuthien-Baumann, B.; Platzek, I.; van den Hoff, J.; Kroschinsky, F.; Middeke, J.M.; Platzbecker, U.; Zietz, C.; Bornhauser, M.; et al. Use of targeted therapy for refractory ALK-positive anaplastic large cell lymphoma as a bridging strategy prior to allogeneic transplantation. Ann. Hematol. 2013, 92, 125–127. [Google Scholar] [CrossRef]
- Illidge, T.; Bouabdallah, R.; Chen, R.; Gopal, A.K.; Moskowitz, C.H.; Ramchandren, R.; Shustov, A.R.; Tilly, H.; Trippett, T.M.; Gibb, A.; et al. Allogeneic transplant following brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk. Lymphoma 2015, 56, 703–710. [Google Scholar] [CrossRef]
- Mediwake, H.; Morris, K.; Curley, C.; Butler, J.; Kennedy, G. Use of brentuximab vedotin as salvage therapy pre-allogeneic stem cell transplantation in relapsed/refractory CD30 positive lympho-proliferative disorders: A single centre experience. Intern. Med. J. 2017, 47, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.K.; Ramchandren, R.; O’Connor, O.A.; Berryman, R.B.; Advani, R.H.; Chen, R.; Smith, S.E.; Cooper, M.; Rothe, A.; Matous, J.V.; et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood 2012, 120, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Carlo-Stella, C.; Ricci, F.; Dalto, S.; Mazza, R.; Malagola, M.; Patriarca, F.; Viviani, S.; Russo, D.; Giordano, L.; Castagna, L.; et al. Brentuximab vedotin in patients with Hodgkin lymphoma and a failed allogeneic stem cell transplantation: Results from a named patient program at four Italian centers. Oncologist 2015, 20, 323–328. [Google Scholar] [CrossRef]
- Tsirigotis, P.; Danylesko, I.; Gkirkas, K.; Shem-Tov, N.; Yerushalmi, R.; Stamouli, M.; Avigdor, A.; Spyridonidis, A.; Gauthier, J.; Goldstein, G.; et al. Brentuximab vedotin in combination with or without donor lymphocyte infusion for patients with Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016, 51, 1313–1317. [Google Scholar] [CrossRef]
- Chen, Y.B.; McDonough, S.; Hasserjian, R.; Chen, H.; Coughlin, E.; Illiano, C.; Park, I.S.; Jagasia, M.; Spitzer, T.R.; Cutler, C.S.; et al. Expression of CD30 in patients with acute graft-versus-host disease. Blood 2012, 120, 691–696. [Google Scholar] [CrossRef]
- Verhelle, D.; Corral, L.G.; Wong, K.; Mueller, J.H.; Moutouh-de Parseval, L.; Jensen-Pergakes, K.; Schafer, P.H.; Chen, R.; Glezer, E.; Ferguson, G.D.; et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007, 67, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Liu, N.; Klimek, V.; Hassoun, H.; Mazumder, A.; Nimer, S.D.; Jagannath, S.; Dhodapkar, M.V. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: Therapeutic implications. Blood 2006, 108, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Dredge, K.; Marriott, J.B.; Todryk, S.M.; Muller, G.W.; Chen, R.; Stirling, D.I.; Dalgleish, A.G. Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Th1-type immunity. J. Immunol. 2002, 168, 4914–4919. [Google Scholar] [CrossRef]
- Govindaraj, C.; Madondo, M.; Kong, Y.Y.; Tan, P.; Wei, A.; Plebanski, M. Lenalidomide-based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am. J. Hematol. 2014, 89, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Dredge, K.; Horsfall, R.; Robinson, S.P.; Zhang, L.H.; Lu, L.; Tang, Y.; Shirley, M.A.; Muller, G.; Schafer, P.; Stirling, D.; et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc. Res. 2005, 69, 56–63. [Google Scholar] [CrossRef]
- Lu, G.; Middleton, R.E.; Sun, H.; Naniong, M.; Ott, C.J.; Mitsiades, C.S.; Wong, K.K.; Bradner, J.E.; Kaelin, W.G., Jr. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014, 343, 305–309. [Google Scholar] [CrossRef]
- Krönke, J.; Udeshi, N.D.; Narla, A.; Grauman, P.; Hurst, S.N.; McConkey, M.; Svinkina, T.; Heckl, D.; Comer, E.; Li, X.; et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–305. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Pellegrini, C.; Broccoli, A.; Stefoni, V.; Gandolfi, L.; Quirini, F.; Argnani, L.; Berti, E.; Derenzini, E.; Pileri, S.; et al. Lenalidomide monotherapy for relapsed/refractory peripheral T-cell lymphoma not otherwise specified. Leuk. Lymphoma 2011, 52, 1585–1588. [Google Scholar] [CrossRef]
- Morschhauser, F.; Fitoussi, O.; Haioun, C.; Thieblemont, C.; Quach, H.; Delarue, R.; Glaisner, S.; Gabarre, J.; Bosly, A.; Lister, J.; et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: The EXPECT trial. Eur. J. Cancer 2013, 49, 2869–2876. [Google Scholar] [CrossRef]
- Toumishey, E.; Prasad, A.; Dueck, G.; Chua, N.; Finch, D.; Johnston, J.; van der Jagt, R.; Stewart, D.; White, D.; Belch, A.; et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer 2015, 121, 716–723. [Google Scholar] [CrossRef]
- Kneppers, E.; van der Holt, B.; Kersten, M.J.; Zweegman, S.; Meijer, E.; Huls, G.; Cornelissen, J.J.; Janssen, J.J.; Huisman, C.; Cornelisse, P.B.; et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: Results of the HOVON 76 Trial. Blood 2011, 118, 2413–2419. [Google Scholar] [CrossRef]
- Sockel, K.; Bornhaeuser, M.; Mischak-Weissinger, E.; Trenschel, R.; Wermke, M.; Unzicker, C.; Kobbe, G.; Finke, J.; Germing, U.; Mohr, B.; et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): Results of the LENAMAINT trial. Haematologica 2012, 97, e34–e35. [Google Scholar] [CrossRef] [PubMed]
- Wolschke, C.; Stubig, T.; Hegenbart, U.; Schonland, S.; Heinzelmann, M.; Hildebrandt, Y.; Ayuk, F.; Atanackovic, D.; Dreger, P.; Zander, A.; et al. Postallograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for T cell-mediated GvHD: Results from a phase I/II dose-finding study. Exp. Hematol. 2013, 41, 134–142.e133. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Becker, P.S.; Zhong, X.; Adams, A.; Hari, P.; Rowley, S.; Stadtmauer, E.A.; Vesole, D.H.; Logan, B.; Weisdorf, D.; et al. Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, W.I.; Green, D.J.; Burwick, N.; Becker, P.S. A prospective study of lenalidomide monotherapy for relapse after Allo-SCT for multiple myeloma. Bone Marrow Transplant. 2014, 49, 492–495. [Google Scholar] [CrossRef]
- Khouri, M.R.; Jabbour, E.J.; Gulbis, A.M.; Turturro, F.; Ledesma, C.; Korbling, M.; Samuels, B.I.; Ahmed, S.; Alousi, A.M.; Ciurea, S.O.; et al. Feasibility of Lenalidomide Therapy for Persistent Chronic Lymphocytic Leukemia after Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 1405–1410. [Google Scholar] [CrossRef]
- Pham, B.; Hoeg, R.; Krishnan, R.; Richman, C.; Tuscano, J.; Abedi, M. Safety and tolerability of lenalidomide maintenance in post-transplant acute myeloid leukemia and high-risk myelodysplastic syndrome. Bone Marrow Transplant. 2021, 56, 2975–2980. [Google Scholar] [CrossRef]
- Craddock, C.; Slade, D.; De Santo, C.; Wheat, R.; Ferguson, P.; Hodgkinson, A.; Brock, K.; Cavenagh, J.; Ingram, W.; Dennis, M.; et al. Combination Lenalidomide and Azacitidine: A Novel Salvage Therapy in Patients Who Relapse After Allogeneic Stem-Cell Transplantation for Acute Myeloid Leukemia. J. Clin. Oncol. 2019, 37, 580–588. [Google Scholar] [CrossRef]
- Schroeder, T.; Stelljes, M.; Christopeit, M.; Esseling, E.; Scheid, C.; Mikesch, J.H.; Rautenberg, C.; Jager, P.; Cadeddu, R.P.; Drusenheimer, N.; et al. Azacitidine, lenalidomide and donor lymphocyte infusions for relapse of myelodysplastic syndrome, acute myeloid leukemia and chronic myelomonocytic leukemia after allogeneic transplant: The Azalena-Trial. Haematologica 2023, 108, 3001–3010. [Google Scholar] [CrossRef]
- Morishige, S.; Nishi, M.; Saruta, H.; Arakawa, F.; Yamasaki, Y.; Oya, S.; Nakamura, T.; Seki, R.; Yamaguchi, M.; Aoyama, K.; et al. Complete response following toxic epidermal necrolysis in relapsed adult T cell leukemia/lymphoma after haploidentical stem cell transplantation. Int. J. Hematol. 2019, 110, 506–511. [Google Scholar] [CrossRef]
- Tanaka, T.; Inamoto, Y.; Ito, A.; Watanabe, M.; Takeda, W.; Aoki, J.; Kim, S.W.; Fukuda, T. Lenalidomide treatment for recurrent adult T-cell leukemia/lymphoma after allogeneic hematopoietic cell transplantation. Hematol. Oncol. 2023, 41, 389–395. [Google Scholar] [CrossRef]
- Pizzi, M.; Margolskee, E.; Inghirami, G. Pathogenesis of Peripheral T Cell Lymphoma. Annu. Rev. Pathol. 2018, 13, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Irimia, R.; Piccaluga, P.P. Histone Deacetylase Inhibitors for Peripheral T-Cell Lymphomas. Cancers 2024, 16, 3359. [Google Scholar] [CrossRef]
- Meng-Meng, J.; Yao-Hui, H.; Jin-Yan, H.; Zhao-Fu, W.; Di, F.; Han, L.; Feng, L.; Christophe, L.; Li, W.; Jing, Y.; et al. Histone modifier gene mutations in peripheral T-cell lymphoma not otherwise specified. Haematologica 2018, 103, 679–687. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Zhu, M.; Hu, P.; Liu, X.; Qing, Y.; Wang, X.; Wang, H.; Wang, Z.; Xu, J.; et al. Involvement of p53 Acetylation in Growth Suppression of Cutaneous T-Cell Lymphomas Induced by HDAC Inhibition. J. Investig. Dermatol. 2020, 140, 2009–2022.e2004. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Liu, J. Role of HDACs in normal and malignant hematopoiesis. Mol. Cancer 2020, 19, 5. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Olsen, E.A.; Kim, Y.H.; Kuzel, T.M.; Pacheco, T.R.; Foss, F.M.; Parker, S.; Frankel, S.R.; Chen, C.; Ricker, J.L.; Arduino, J.M.; et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 2007, 25, 3109–3115. [Google Scholar] [CrossRef] [PubMed]
- Piekarz, R.L.; Frye, R.; Turner, M.; Wright, J.J.; Allen, S.L.; Kirschbaum, M.H.; Zain, J.; Prince, H.M.; Leonard, J.P.; Geskin, L.J.; et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 2009, 27, 5410–5417. [Google Scholar] [CrossRef]
- Shi, Y.; Dong, M.; Hong, X.; Zhang, W.; Feng, J.; Zhu, J.; Yu, L.; Ke, X.; Huang, H.; Shen, Z.; et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol. 2015, 26, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Chen, J.; Yu, Z. Histone Deacetylases (HDACs) Guided Novel Therapies for T-cell lymphomas. Int. J. Med. Sci. 2019, 16, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.; Jacob, A.T.; et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: A multicenter phase 2 study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef]
- Xu, X.; Li, X.; Zhao, Y.; Huang, H. Immunomodulatory Effects of Histone Deacetylation Inhibitors in Graft-vs.-Host Disease After Allogeneic Stem Cell Transplantation. Front. Immunol. 2021, 12, 641910. [Google Scholar] [CrossRef]
- Reddy, P.; Maeda, Y.; Hotary, K.; Liu, C.; Reznikov, L.L.; Dinarello, C.A.; Ferrara, J.L. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl. Acad. Sci. USA 2004, 101, 3921–3926. [Google Scholar] [CrossRef]
- Kim, E.S.; Lee, J.K. Histone deacetylase inhibitors decrease the antigen presenting activity of murine bone marrow derived dendritic cells. Cell Immunol. 2010, 262, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; de Zoeten, E.F.; Ozkaynak, E.; Chen, C.; Wang, L.; Porrett, P.M.; Li, B.; Turka, L.A.; Olson, E.N.; Greene, M.I.; et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007, 13, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Braun, T.; Henig, I.; Gatza, E.; Magenau, J.; Parkin, B.; Pawarode, A.; Riwes, M.; Yanik, G.; Dinarello, C.A.; et al. Vorinostat plus tacrolimus/methotrexate to prevent GVHD after myeloablative conditioning, unrelated donor HCT. Blood 2017, 130, 1760–1767. [Google Scholar] [CrossRef]
- Idso, J.M.; Lao, S.; Schloemer, N.J.; Knipstein, J.; Burns, R.; Thakar, M.S.; Malarkannan, S. Entinostat augments NK cell functions via epigenetic upregulation of IFIT1-STING-STAT4 pathway. Oncotarget 2020, 11, 1799–1815. [Google Scholar] [CrossRef]
- Zhou, X.; Hua, X.; Ding, X.; Bian, Y.; Wang, X. Trichostatin differentially regulates Th1 and Th2 responses and alleviates rheumatoid arthritis in mice. J. Clin. Immunol. 2011, 31, 395–405. [Google Scholar] [CrossRef]
- Choi, S.W.; Braun, T.; Chang, L.; Ferrara, J.L.; Pawarode, A.; Magenau, J.M.; Hou, G.; Beumer, J.H.; Levine, J.E.; Goldstein, S.; et al. Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: A phase 1/2 trial. Lancet. Oncol. 2014, 15, 87–95. [Google Scholar] [CrossRef]
- Bug, G.; Burchert, A.; Wagner, E.M.; Kroger, N.; Berg, T.; Guller, S.; Metzelder, S.K.; Wolf, A.; Hunecke, S.; Bader, P.; et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia 2017, 31, 2523–2525. [Google Scholar] [CrossRef]
- Kalin, B.; van Norden, Y.; van Gelder, M.; Breems, D.; Maertens, J.; Jongen-Lavrencic, M.; Broers, A.E.C.; Braakman, E.; Grob, T.; Zeijlemaker, W.; et al. Panobinostat and decitabine prior to donor lymphocyte infusion in allogeneic stem cell transplantation. Blood Adv. 2020, 4, 4430–4437. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kitabayashi, I. Oncogenic roles of enhancer of zeste homolog 1/2 in hematological malignancies. Cancer Sci. 2018, 109, 2342–2348. [Google Scholar] [CrossRef]
- Proudman, D.G.; Gupta, D.; Nellesen, D.; Yang, J.; Kamp, B.A.; Mamlouk, K.; Cheson, B.D. Tazemetostat in relapsed/refractory follicular lymphoma: A propensity score-matched analysis of E7438-G000-101 trial outcomes. Oncotarget 2022, 13, 677–683. [Google Scholar] [CrossRef]
- Yamagishi, M.; Hori, M.; Fujikawa, D.; Ohsugi, T.; Honma, D.; Adachi, N.; Katano, H.; Hishima, T.; Kobayashi, S.; Nakano, K.; et al. Targeting Excessive EZH1 and EZH2 Activities for Abnormal Histone Methylation and Transcription Network in Malignant Lymphomas. Cell Rep. 2019, 29, 2321–2337.e2327. [Google Scholar] [CrossRef]
- Honma, D.; Kanno, O.; Watanabe, J.; Kinoshita, J.; Hirasawa, M.; Nosaka, E.; Shiroishi, M.; Takizawa, T.; Yasumatsu, I.; Horiuchi, T.; et al. Novel orally bioavailable EZH1/2 dual inhibitors with greater antitumor efficacy than an EZH2 selective inhibitor. Cancer Sci. 2017, 108, 2069–2078. [Google Scholar] [CrossRef]
- Izutsu, K.; Makita, S.; Nosaka, K.; Yoshimitsu, M.; Utsunomiya, A.; Kusumoto, S.; Morishima, S.; Tsukasaki, K.; Kawamata, T.; Ono, T.; et al. An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma. Blood 2023, 141, 1159–1168. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Izutsu, K.; Maruyama, D.; Makita, S.; Jacobsen, E.D.; Horwitz, S.; Kusumoto, S.; Allen, P.; Porcu, P.; Imaizumi, Y.; et al. First-in-Human Study of the Ezh1 and Ezh2 Dual Inhibitor Valemetostat Tosylate (Ds-3201b) in Patients with Relapsed or Refractory Non-Hodgkin Lymphomas. Hematol. Oncol. 2021, 39, 38. [Google Scholar] [CrossRef]
- Alahmari, B.; Cooper, M.; Ziga, E.; Ritchey, J.; DiPersio, J.F.; Choi, J. Selective targeting of histone modification fails to prevent graft versus host disease after hematopoietic cell transplantation. PLoS ONE 2018, 13, e0207609. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xie, F.; Liu, Y.; Tong, Q.; Mochizuki, K.; Lapinski, P.E.; Mani, R.S.; Reddy, P.; Mochizuki, I.; Chinnaiyan, A.M.; et al. The histone methyltransferase Ezh2 is a crucial epigenetic regulator of allogeneic T-cell responses mediating graft-versus-host disease. Blood 2013, 122, 4119–4128. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, J.; Kato, K.; Xie, F.; Varambally, S.; Mineishi, S.; Kuick, R.; Mochizuki, K.; Liu, Y.; Nieves, E.; et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood 2012, 119, 1274–1282. [Google Scholar] [CrossRef]
- Huang, Q.; He, S.; Tian, Y.; Gu, Y.; Chen, P.; Li, C.; Huang, J.; Liu, Y.; Yu, H.; Jin, M.; et al. Hsp90 inhibition destabilizes Ezh2 protein in alloreactive T cells and reduces graft-versus-host disease in mice. Blood 2017, 129, 2737–2748. [Google Scholar] [CrossRef]
- Bagnato, G.; Stefoni, V.; Broccoli, A.; Argnani, L.; Pellegrini, C.; Casadei, B.; Bonifazi, F.; Zinzani, P.L. Successful Bridging to Allogeneic Transplantation With Valemetostat in Two Refractory/relapsed Peripheral T-cell lymphoma patients. Mediterr. J. Hematol. Infect. Dis. 2024, 16, e2024004. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Mc Neil, V.; Lee, S.W. Advancing Cancer Treatment: A Review of Immune Checkpoint Inhibitors and Combination Strategies. Cancers 2025, 17, 1408. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Wei, W.; Zou, L. Immune Checkpoint Inhibitors in Peripheral T-Cell Lymphoma. Front. Pharmacol. 2022, 13, 869488. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, M.; Yan, J.; Li, L.; Fu, X.; Zhang, X.; Chang, Y.; Sun, Z.; Yu, H.; et al. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J. Hematol. Oncol. 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018, 378, 1947–1948. [Google Scholar] [CrossRef] [PubMed]
- Casadei, B.; Broccoli, A.; Stefoni, V.; Pellegrini, C.; Marangon, M.; Morigi, A.; Nanni, L.; Lolli, G.; Carella, M.; Argnani, L.; et al. PD-1 blockade as bridge to allogeneic stem cell transplantation in relapsed/refractory Hodgkin lymphoma patients: A retrospective single center case series. Haematologica 2019, 104, e521–e522. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Khan, A.Y.; Malik, S.U.; Faridi, W.; Fraz, M.A.; Usman, M.; Tariq, M.J.; Durer, S.; Durer, C.; Russ, A.; et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors before and after Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 94–99. [Google Scholar] [CrossRef]
- Soiffer, R.J. Checkpoint inhibition to prevent or treat relapse in allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2019, 54, 798–802. [Google Scholar] [CrossRef]
- Bobillo, S.; Nieto, J.C.; Barba, P. Use of checkpoint inhibitors in patients with lymphoid malignancies receiving allogeneic cell transplantation: A review. Bone Marrow Transplant. 2021, 56, 1784–1793. [Google Scholar] [CrossRef]
- Merryman, R.W.; Kim, H.T.; Zinzani, P.L.; Carlo-Stella, C.; Ansell, S.M.; Perales, M.A.; Avigdor, A.; Halwani, A.S.; Houot, R.; Marchand, T.; et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood 2017, 129, 1380–1388. [Google Scholar] [CrossRef]
- Merryman, R.W.; Castagna, L.; Giordano, L.; Ho, V.T.; Corradini, P.; Guidetti, A.; Casadei, B.; Bond, D.A.; Jaglowski, S.; Spinner, M.A.; et al. Allogeneic transplantation after PD-1 blockade for classic Hodgkin lymphoma. Leukemia 2021, 35, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Min, K.; Zhou, H.; Gao, X. The influence of immune checkpoint blockade on the outcomes of allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2024, 15, 1491330. [Google Scholar] [CrossRef]
- Norde, W.J.; Maas, F.; Hobo, W.; Korman, A.; Quigley, M.; Kester, M.G.; Hebeda, K.; Falkenburg, J.H.; Schaap, N.; de Witte, T.M.; et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011, 71, 5111–5122. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Yang, T.; Mo, Z.; Wei, G.; Jing, R.; Zhao, H.; Chen, R.; Zu, C.; Gu, T.; et al. Sequential CD7 CAR T-Cell Therapy and Allogeneic HSCT without GVHD Prophylaxis. N. Engl. J. Med. 2024, 390, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.; Harstrick, A.; Emig, M.; Overesch, A.; Pinto, S.; Ravenstijn, P.; Schlüter, T.; Rubel, J.; Rebscher, H.; Graefe, T.; et al. AFM13 in Combination with Allogeneic Natural Killer Cells (AB-101) in Relapsed or Refractory Hodgkin Lymphoma and CD30 + Peripheral T-Cell Lymphoma: A Phase 2 Study (LuminICE). Blood 2023, 142, 4855. [Google Scholar] [CrossRef]
- Grover, N.S.; Hucks, G.; Riches, M.L.; Ivanova, A.; Moore, D.T.; Shea, T.C.; Seegars, M.B.; Armistead, P.M.; Kasow, K.A.; Beaven, A.W.; et al. Anti-CD30 CAR T cells as consolidation after autologous haematopoietic stem-cell transplantation in patients with high-risk CD30+ lymphoma: A phase 1 study. Lancet Haematol. 2024, 11, e358–e367. [Google Scholar] [CrossRef] [PubMed]
- Nieto, Y.; Banerjee, P.; Kaur, I.; Basar, R.; Li, Y.; Daher, M.; Rafei, H.; Kerbauy, L.N.; Kaplan, M.; Marin, D.; et al. Allogeneic NK cells with a bispecific innate cell engager in refractory relapsed lymphoma: A phase 1 trial. Nat. Med. 2025, 31, 1987–1993. [Google Scholar] [CrossRef]
- Iyer, S.P.; Sica, R.A.; Ho, P.J.; Prica, A.; Zain, J.; Foss, F.M.; Hu, B.; Beitinjaneh, A.; Weng, W.-K.; Kim, Y.H.; et al. Safety and activity of CTX130, a CD70-targeted allogeneic CRISPR-Cas9-engineered CAR T-cell therapy, in patients with relapsed or refractory T-cell malignancies (COBALT-LYM): A single-arm, open-label, phase 1, dose-escalation study. Lancet Oncol. 2025, 26, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Nichakawade, T.D.; Ge, J.; Mog, B.J.; Lee, B.S.; Pearlman, A.H.; Hwang, M.S.; DiNapoli, S.R.; Wyhs, N.; Marcou, N.; Glavaris, S.; et al. TRBC1-targeting antibody-drug conjugates for the treatment of T cell cancers. Nature 2024, 628, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Cwynarski, K.; Iacoboni, G.; Tholouli, E.; Menne, T.; Irvine, D.A.; Balasubramaniam, N.; Wood, L.; Shang, J.; Xue, E.; Zhang, Y.; et al. TRBC1-CAR T cell therapy in peripheral T cell lymphoma: A phase 1/2 trial. Nat. Med. 2025, 31, 137–143. [Google Scholar] [CrossRef] [PubMed]
| Novel Agents for TCLs | Effects on HCT Immunity | Pre-HCT Use | Post-HCT Use | |
|---|---|---|---|---|
| Conventional salvage chemotherapy | Non-specific cytotoxic effects on both malignant and normal hematopoietic/immune cells; potential impairment of immune reconstitution. | Commonly used as salvage therapy to achieve disease control; responses are often short-lived in chemotherapy-refractory TCLs. | Limited role due to myelosuppression and cumulative toxicity; generally avoided except in selected relapse cases. | |
| Antibody drugs | MOG | Depletes Tregs. | Increase the risk of steroid-refractory GVHD and NRM (especially with a short MOG-to-HCT interval). | ∙Relatively safe ≥ 3 months post-HCT. ∙Effective against ATL peripheral blood lesions. |
| BV | Depletes activated CD30+ T cells. | Safe as bridging therapy without affecting engraftment or GVHD. | May help reduce GVHD. | |
| LEN | ∙Activates T and NK cells. ∙Increases cytokine production. ∙Suppresses Treg function. | Data limited. | ∙Early use after HCT increases the risk of GVHD. ∙Delayed use or combination with AZA may reduce GVHD risk. ∙Myelosuppression is a concern. | |
| HDACis | ∙Suppress cytokine production. ∙Stabilize Tregs ∙Modulate APC and NK cell function. | Data limited. | Potential for GVHD prevention; well tolerated. | |
| EZH1/2 inhibitors | May modulate GVHD via epigenetics. | Limited reports; no clear harm observed. | No established role; further studies needed. | |
| Immune checkpoint inhibitors | Reduced PD-1+ T cells and Tregs. | Increased risk of GVHD; however, this may be mitigated by the use of PTCy as GVHD prophylaxis. | ∙May enhance the GVL effect. ∙Requires monitoring for both GVHD and irAEs. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Yasunaga, J.-i. Impact of Novel Agents on Allogeneic Hematopoietic Cell Transplantation in Patients with T-Cell Lymphomas. Cells 2025, 14, 1306. https://doi.org/10.3390/cells14171306
Inoue Y, Yasunaga J-i. Impact of Novel Agents on Allogeneic Hematopoietic Cell Transplantation in Patients with T-Cell Lymphomas. Cells. 2025; 14(17):1306. https://doi.org/10.3390/cells14171306
Chicago/Turabian StyleInoue, Yoshitaka, and Jun-ichirou Yasunaga. 2025. "Impact of Novel Agents on Allogeneic Hematopoietic Cell Transplantation in Patients with T-Cell Lymphomas" Cells 14, no. 17: 1306. https://doi.org/10.3390/cells14171306
APA StyleInoue, Y., & Yasunaga, J.-i. (2025). Impact of Novel Agents on Allogeneic Hematopoietic Cell Transplantation in Patients with T-Cell Lymphomas. Cells, 14(17), 1306. https://doi.org/10.3390/cells14171306






