The Role of Extracellular Vesicles in Mediating Signaling in Biliary Epithelial Cell Activation and Cholangiopathies
Abstract
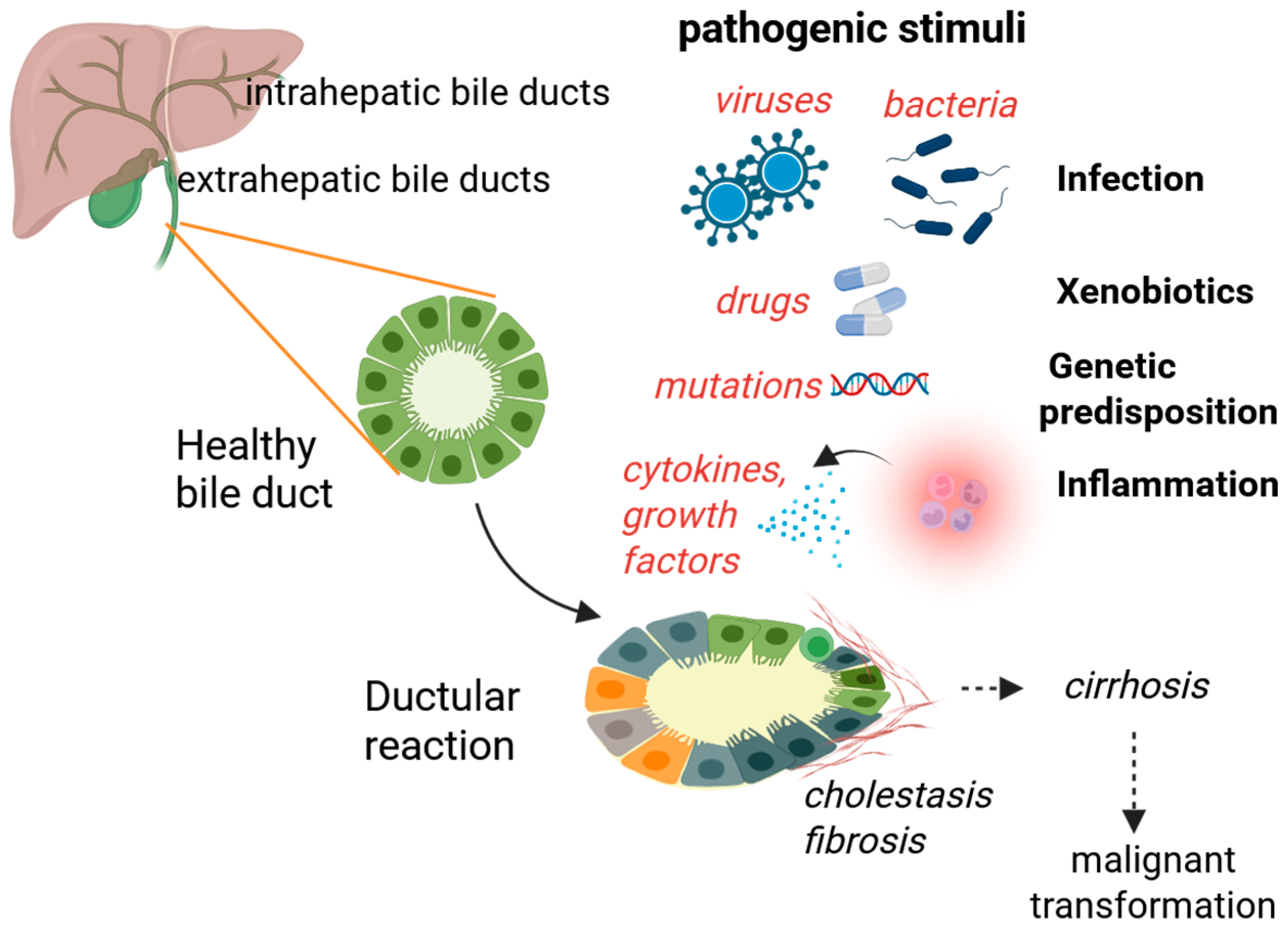
1. Introduction
2. Cholangiocyte Function and Activation
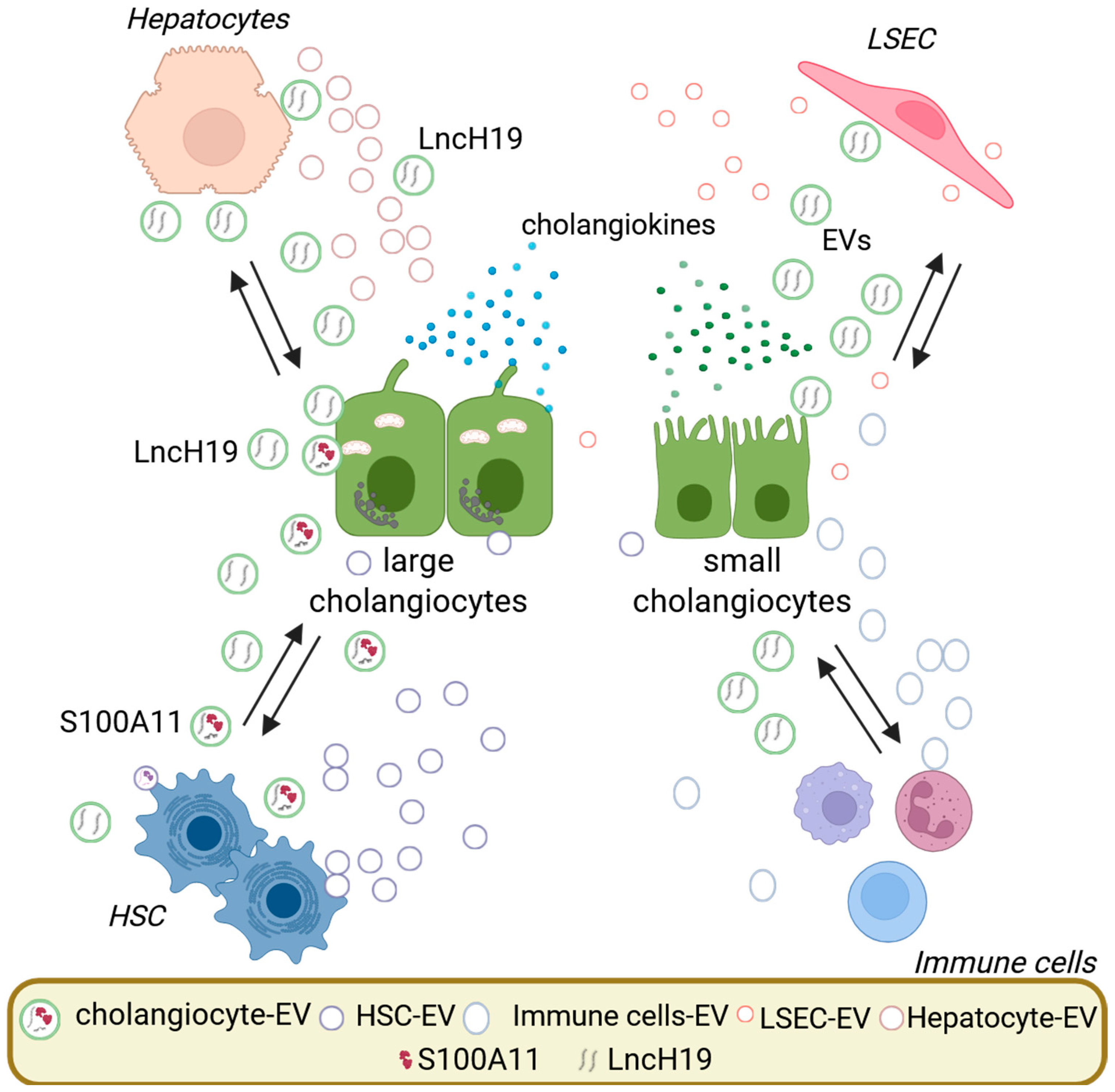
3. Extracellular Vesicles in Cholangiocyte Biology and Pathology
3.1. Cholangiocyte-Derived Extracellular Vesicles
3.2. Extracellular Vesicles Cargo Relevant to Cholangiocyte and Liver Pathology
3.3. Senescent Cholangiocyte-Associated Extracellular Vesicles
3.4. Action on Immune System
3.5. Fibrogenesis
3.6. Extracellular Vesicle Uptake Mechanisms
4. EV-Mediated Signaling in Cholangiocyte Activation
5. EVs in Specific Hepatobiliary Diseases and Clinical Implications
5.1. Primary Sclerosing Cholangitis
5.2. Cholangiocarcinoma
5.3. Biliary Atresia and Pediatric Disorders
6. Future Directions
6.1. Diagnostic Potential: Extracellular Vesicles as Non-Invasive Biomarkers in Cholangiopathies
6.2. Therapeutic Strategies: Modulating Extracellular Vesicles and Harnessing Them for Drug Delivery
6.3. Technical and Biological Challenges
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAF(s) | Cancer-associated fibroblast(s) |
| CCA | Cholangiocarcinoma |
| DAMP | Damage-associated molecular patterns |
| EMT | Epithelial-mesenchymal transition |
| EV(s) | Extracellular vesicle(s) |
| HPGD | Hydroxyprostaglandin dehydrogenase |
| HSCs | Hepatic stellate cells |
| LSEC | Liver sinusoidal endothelial cell |
| MAFLD | Metabolic associated fatty liver disease |
| MASH | Metabolic associated steatohepatitis |
| MVBs | Multivesicular bodies |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| PBC | Primary biliary cholangitis |
| PSC | Primary sclerosing cholangitis |
| sEVs | Small extracellular vesicles |
| TLR(s) | Toll-like receptor(s) |
References
- Lazaridis, K.N.; LaRusso, N.F. The Cholangiopathies. Mayo Clin. Proc. 2015, 90, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Trussoni, C.E.; LaRusso, N.F.; Gores, G.J. The Spectrum of Reactive Cholangiocytes in Primary Sclerosing Cholangitis. Hepatology 2020, 71, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Spirli, C.; Cadamuro, M.; Fiorotto, R.; Strazzabosco, M. Emerging concepts in biliary repair and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G102–G116. [Google Scholar] [CrossRef]
- Rejas, C.; Junger, H. Cholangiocyte Organoids in Liver Transplantation; a Comprehensive Review. Transpl. Int. 2024, 37, 12708. [Google Scholar] [CrossRef] [PubMed]
- Gadd, V.L.; Skoien, R.; Powell, E.E.; Fagan, K.J.; Winterford, C.; Horsfall, L.; Irvine, K.; Clouston, A.D. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 2014, 59, 1393–1405. [Google Scholar] [CrossRef]
- Maillette de Buy Wenniger, L.J.; Oude Elferink, R.P.; Beuers, U. Molecular targets for the treatment of fibrosing cholangiopathies. Clin. Pharmacol. Ther. 2012, 92, 381–387. [Google Scholar] [CrossRef]
- Gerussi, A.; Lucà, M.; Cristoferi, L.; Ronca, V.; Mancuso, C.; Milani, C.; D’Amato, D.; O’Donnell, S.E.; Carbone, M.; Invernizzi, P. New Therapeutic Targets in Autoimmune Cholangiopathies. Front. Med. 2020, 7, 117. [Google Scholar] [CrossRef]
- Re, O.L.; López-López, V.; Balaguer-Román, A.; Martínez-Sánchez, M.A.; Eshmuminov, D.; Llamoza-Torres, C.J.; Miura, K.; Baroja-Mazo, A.; Ramírez, P.; Robles-Campos, R.; et al. New challenges in cholangiocarcinoma candidates for elective surgery: Harnessing the microbiome dysbiosis. Langenbeck’s Arch. Surg. 2023, 408, 134. [Google Scholar] [CrossRef]
- Sun, D.; Xie, C.; Zhao, Y.; Liao, J.; Li, S.; Zhang, Y.; Wang, D.; Hua, K.; Gu, Y.; Du, J.; et al. The gut microbiota-bile acid axis in cholestatic liver disease. Mol. Med. 2024, 30, 104. [Google Scholar] [CrossRef]
- Maroni, L.; Haibo, B.; Ray, D.; Zhou, T.; Wan, Y.; Meng, F.; Marzioni, M.; Alpini, G. Functional and structural features of cholangiocytes in health and disease. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 368–380. [Google Scholar] [CrossRef]
- Mancinelli, R.; Franchitto, A.; Gaudio, E.; Onori, P.; Glaser, S.; Francis, H.; Venter, J.; Demorrow, S.; Carpino, G.; Kopriva, S.; et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am. J. Pathol. 2010, 176, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Mansini, A.P.; Peixoto, E.; Thelen, K.M.; Gaspari, C.; Jin, S.; Gradilone, S.A. The cholangiocyte primary cilium in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1245–1253. [Google Scholar] [CrossRef]
- O’Hara, S.P.; Tabibian, J.H.; Splinter, P.L.; LaRusso, N.F. The dynamic biliary epithelia: Molecules, pathways, and disease. J. Hepatol. 2013, 58, 575–582. [Google Scholar] [CrossRef]
- Cai, X.; Tacke, F.; Guillot, A.; Liu, H. Cholangiokines: Undervalued modulators in the hepatic microenvironment. Front. Immunol. 2023, 14, 1192840. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Ceci, L.; Gaudio, E.; Kennedy, L. Cellular Interactions and Crosstalk Facilitating Biliary Fibrosis in Cholestasis. Cell Mol. Gastroenterol. Hepatol. 2024, 17, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Fagoonee, S. Stem Cell-Derived Extracellular Vesicles for Therapy of Human Diseases; Edizioni Minerva Medica: Rome, Italy, 2021. [Google Scholar]
- Fagoonee, S.; Weiskirchen, R. MicroRNAs and RNA-Binding Protein-Based Regulation of Bone Metastasis from Hepatobiliary Cancers and Potential Therapeutic Strategies. Cells 2024, 13, 1935. [Google Scholar] [CrossRef]
- Ferro, A.; Saccu, G.; Mattivi, S.; Gaido, A.; Herrera Sanchez, M.B.; Haque, S.; Silengo, L.; Altruda, F.; Durazzo, M.; Fagoonee, S. Extracellular Vesicles as Delivery Vehicles for Non-Coding RNAs: Potential Biomarkers for Chronic Liver Diseases. Biomolecules 2024, 14, 277. [Google Scholar] [CrossRef]
- Miceli, R.T.; Chen, T.Y.; Nose, Y.; Tichkule, S.; Brown, B.; Fullard, J.F.; Saulsbury, M.D.; Heyliger, S.O.; Gnjatic, S.; Kyprianou, N.; et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations. J. Extracell. Vesicles 2024, 13, e70005. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic potential of RNA-enriched extracellular vesicles: The next generation in RNA delivery via biogenic nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Li, S.; Cheng, F.; Zhang, Z.; Xu, R.; Shi, H.; Yan, Y. The role of hepatocyte-derived extracellular vesicles in liver and extrahepatic diseases. Biomed. Pharmacother. 2024, 180, 117502. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, J.; Wang, M.; Wang, Y.; Dong, M.; Ma, X.; Sun, B.; Liu, S.; Zhao, Z.; Chen, L.; et al. Lipid-induced DRAM recruits STOM to lysosomes and induces LMP to promote exosome release from hepatocytes in NAFLD. Sci. Adv. 2021, 7, eabh1541. [Google Scholar] [CrossRef]
- Davies, B.A.; Morton, L.O.; Jefferson, J.R.; Rozeveld, C.N.; Doskey, L.C.; LaRusso, N.F.; Katzmann, D.J. Polarized human cholangiocytes release distinct populations of apical and basolateral small extracellular vesicles. Mol. Biol. Cell 2020, 31, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Botts, S.R.; Blaser, M.C.; Abdul-Samad, M.; Prajapati, K.; Khosraviani, N.; Ho, T.W.W.; Breda, L.C.D.; Ching, C.; Galant, N.J.; et al. Directional Endothelial Communication by Polarized Extracellular Vesicle Release. Circ. Res. 2024, 134, 269–289. [Google Scholar] [CrossRef]
- Ge, T.; Shao, Y.; Bao, X.; Xu, W.; Lu, C. Cellular senescence in liver diseases: From mechanisms to therapies. Int. Immunopharmacol. 2023, 121, 110522. [Google Scholar] [CrossRef]
- Tabibian, J.H.; O’Hara, S.P.; Splinter, P.L.; Trussoni, C.E.; LaRusso, N.F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014, 59, 2263–2275. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Fujita, Y.; Yoshioka, Y.; Araya, J.; Kuwano, K.; Ochiya, T. Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases. Mol. Aspects Med. 2018, 60, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Al Suraih, M.S.; Trussoni, C.E.; Splinter, P.L.; LaRusso, N.F.; O’Hara, S.P. Senescent cholangiocytes release extracellular vesicles that alter target cell phenotype via the epidermal growth factor receptor. Liver Int. 2020, 40, 2455–2468. [Google Scholar] [CrossRef]
- Morland, C.M.; Fear, J.; Joplin, R.; Adams, D.H. Inflammatory cytokines stimulate human biliary epithelial cells to express interleukin-8 and monocyte chemotactic protein-1. Biochem. Soc. Trans. 1997, 25, 232S. [Google Scholar] [CrossRef]
- Katsumi, T.; Guicciardi, M.E.; Azad, A.; Bronk, S.F.; Krishnan, A.; Gores, G.J. Activated cholangiocytes release macrophage-polarizing extracellular vesicles bearing the DAMP S100A11. Am. J. Physiol. Cell Physiol. 2019, 317, C788–C799. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Wang, Y.; Zhu, W.; Zhao, D.; Wang, X.; Yang, H.; Gurley, E.C.; Chen, W.; Hylemon, P.B.; et al. Cholangiocyte-Derived Exosomal lncRNA H19 Promotes Macrophage Activation and Hepatic Inflammation under Cholestatic Conditions. Cells 2020, 9, 190. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Huang, Z.; Gurley, E.C.; Wang, X.; Wang, J.; He, H.; Yang, H.; Lai, G.; Zhang, L.; et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology 2018, 68, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Zhu, W.; Wang, Y.; Zhao, D.; Wang, X.; Gurley, E.C.; Liang, G.; Chen, W.; Lai, G.; et al. Cholangiocyte-Derived Exosomal Long Noncoding RNA H19 Promotes Hepatic Stellate Cell Activation and Cholestatic Liver Fibrosis. Hepatology 2019, 70, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, S.; Manco, M.; Altruda, F.; Fagoonee, S.; Tolosano, E. Liver Sinusoidal Endothelial Cells at the Crossroad of Iron Overload and Liver Fibrosis. Antioxid. Redox Signal. 2021, 35, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Savage, T.M.; Fortson, K.T.; de Los Santos-Alexis, K.; Oliveras-Alsina, A.; Rouanne, M.; Rae, S.S.; Gamarra, J.R.; Shayya, H.; Kornberg, A.; Cavero, R.; et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in non-alcoholic steatohepatitis. Immunity 2024, 57, 303–318.e306. [Google Scholar] [CrossRef]
- McKee, C.; Sigala, B.; Soeda, J.; Mouralidarane, A.; Morgan, M.; Mazzoccoli, G.; Rappa, F.; Cappello, F.; Cabibi, D.; Pazienza, V.; et al. Amphiregulin activates human hepatic stellate cells and is upregulated in non alcoholic steatohepatitis. Sci. Rep. 2015, 5, 8812. [Google Scholar] [CrossRef]
- Santamaría, E.; Rodríguez-Ortigosa, C.M.; Uriarte, I.; Latasa, M.U.; Urtasun, R.; Alvarez-Sola, G.; Bárcena-Varela, M.; Colyn, L.; Arcelus, S.; Jiménez, M.; et al. The Epidermal Growth Factor Receptor Ligand Amphiregulin Protects From Cholestatic Liver Injury and Regulates Bile Acids Synthesis. Hepatology 2019, 69, 1632–1647. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Cong, M.; Liu, T.; Sun, S.; Ma, H.; You, H.; Jia, J.; Wang, P. EGF neutralization antibodies attenuate liver fibrosis by inhibiting myofibroblast proliferation in bile duct ligation mice. Histochem. Cell Biol. 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Fuchs, B.C.; Hoshida, Y.; Fujii, T.; Wei, L.; Yamada, S.; Lauwers, G.Y.; McGinn, C.M.; DePeralta, D.K.; Chen, X.; Kuroda, T.; et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 2014, 59, 1577–1590. [Google Scholar] [CrossRef]
- Calder, A.N.; Sakaguchi, T.; Peter, M.; Tobias, J.; Frankel, T.; Razumilava, N. EGFR activation in cholangiocytes promotes extrahepatic bile duct regeneration after injury. bioRxiv 2025. [Google Scholar] [CrossRef]
- Geervliet, E.; Terstappen, L.W.M.M.; Bansal, R. Hepatocyte survival and proliferation by fibroblast growth factor 7 attenuates liver inflammation, and fibrogenesis during acute liver injury via paracrine mechanisms. Biomed. Pharmacother. 2023, 167, 115612. [Google Scholar] [CrossRef]
- Sato, K.; Meng, F.; Venter, J.; Giang, T.; Glaser, S.; Alpini, G. The role of the secretin/secretin receptor axis in inflammatory cholangiocyte communication via extracellular vesicles. Sci. Rep. 2017, 7, 11183. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, D.M.W. Amphiregulin as a driver of tissue fibrosis. Am. J. Transplant. 2020, 20, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Oszvald, Á.; Szvicsek, Z.; Pápai, M.; Kelemen, A.; Varga, Z.; Tölgyes, T.; Dede, K.; Bursics, A.; Buzás, E.I.; Wiener, Z. Fibroblast-Derived Extracellular Vesicles Induce Colorectal Cancer Progression by Transmitting Amphiregulin. Front. Cell Dev. Biol. 2020, 8, 558. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150.e136. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.; Sadowska, A.; Piotrowska-Tomala, K.; Szóstek-Mioduchowska, A. The effect of amphiregulin on equine endometrial fibroblasts: In vitro responses of fibroblast derived from non-fibrotic and fibrotic endometrium. Reprod. Biol. 2025, 25, 101018. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Tameda, M.; Eguchi, A.; Ren, W.; Kim, J.; Myers, R.; Goodman, Z.D.; Harrison, S.A.; Sanyal, A.J.; Bosch, J.; et al. Protein and miRNA profile of circulating extracellular vesicles in patients with primary sclerosing cholangitis. Sci. Rep. 2022, 12, 3027. [Google Scholar] [CrossRef]
- Fagoonee, S.; Arigoni, M.; Manco, M.; Olivero, M.; Bizzaro, F.; Magagnotti, C.; Andolfo, A.; Miniscalco, B.; Forni, M.; Todeschi, S.; et al. Circulating Extracellular Vesicles Contain Liver-Derived RNA Species as Indicators of Severe Cholestasis-Induced Early Liver Fibrosis in Mice. Antioxid. Redox Signal. 2022, 36, 480–504. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Sotillo, J.; Krueajampa, W.; Thongsen, S.; Smout, M.; Brindley, P.J.; Laha, T.; Loukas, A. Silencing of Opisthorchis viverrini Tetraspanin Gene Expression Results in Reduced Secretion of Extracellular Vesicles. Front. Cell. Infect. Microbiol. 2022, 12, 827521. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Casella, G.; Podini, P.; Finardi, A.; Racchetti, G.; Norton, E.G.; Cocucci, E.; Furlan, R. Polarized cells display asymmetric release of extracellular vesicles. Traffic 2021, 22, 98–110. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Huang, B.Q.; Ward, C.J.; Gradilone, S.A.; Banales, J.M.; Masyuk, T.V.; Radtke, B.; Splinter, P.L.; LaRusso, N.F. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G990–G999. [Google Scholar] [CrossRef]
- McDaniel, K.; Wu, N.; Zhou, T.; Huang, L.; Sato, K.; Venter, J.; Ceci, L.; Chen, D.; Ramos-Lorenzo, S.; Invernizzi, P.; et al. Amelioration of Ductular Reaction by Stem Cell Derived Extracellular Vesicles in MDR2 Knockout Mice via Lethal-7 microRNA. Hepatology 2019, 69, 2562–2578. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrabim, S.H.; Gores, G.J.; Malhi, H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to NASH pathogenesis. J. Lipid. Res. 2016, 57, 1758–1770. [Google Scholar] [CrossRef] [PubMed]
- He, Y.H.; Pan, J.X.; Xu, L.M.; Gu, T.; Chen, Y.W. Ductular reaction in non-alcoholic fatty liver disease: When Macbeth is perverted. World J. Hepatol. 2023, 15, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef]
- Chen, X.M.; O’Hara, S.P.; Nelson, J.B.; Splinter, P.L.; Small, A.J.; Tietz, P.S.; Limper, A.H.; LaRusso, N.F. Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol. 2005, 175, 7447–7456. [Google Scholar] [CrossRef]
- Bordin, A.; Chirivì, M.; Pagano, F.; Milan, M.; Iuliano, M.; Scaccia, E.; Fortunato, O.; Mangino, G.; Dhori, X.; De Marinis, E.; et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell Prolif. 2022, 55, e13312. [Google Scholar] [CrossRef]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef]
- Eustes, A.S.; Dayal, S. The Role of Platelet-Derived Extracellular Vesicles in Immune-Mediated Thrombosis. Int. J. Mol. Sci. 2022, 23, 7837. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Trussoni, C.E.; Krishnan, A.; Bronk, S.F.; Lorenzo Pisarello, M.J.; O’Hara, S.P.; Splinter, P.L.; Gao, Y.; Vig, P.; Revzin, A.; et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol. 2018, 69, 676–686. [Google Scholar] [CrossRef]
- Chi, Y.; Jiang, H.; Yin, Y.; Zhou, X.; Shao, Y.; Li, Y.; Rao, J. Macrophage Signaling Pathways in Health and Disease: From Bench to Bedside Applications. MedComm 2025, 6, e70256. [Google Scholar] [CrossRef]
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pépin, G.; Germain, M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, C.; Wang, X.; Peng, R.; Lu, G.; Zhang, J. Phosphatidylserine induce thrombotic tendency and liver damage in obstructive jaundice. BMC Gastroenterol. 2025, 25, 146. [Google Scholar] [CrossRef] [PubMed]
- Dorner, H.; Stolzer, I.; Mattner, J.; Kaminski, S.; Leistl, S.; Edrich, L.M.; Schwendner, R.; Hobauer, J.; Sebald, A.; Leikam, S.; et al. Gut Pathobiont-Derived Outer Membrane Vesicles Drive Liver Inflammation and Fibrosis in Primary Sclerosing Cholangitis-Associated Inflammatory Bowel Disease. Gastroenterology 2024, 167, 1183–1197.e1116. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Wu, L.; Li, Y. Extracellular vesicles in tumor immunity: Mechanisms and novel insights. Mol. Cancer 2025, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Fu, W.; Su, G.; Cao, J.; Gao, L.; Huang, C.; Ma, H.; Zhang, J.; Yue, P.; Bai, B.; et al. The role of extracellular vesicles in cholangiocarcinoma. Cancer Cell Int. 2020, 20, 435. [Google Scholar] [CrossRef]
- Oliviero, B.; Dei Cas, M.; Zulueta, A.; Maiello, R.; Villa, A.; Martinelli, C.; Del Favero, E.; Falleni, M.; Montavoci, L.; Varchetta, S.; et al. Ceramide present in cholangiocarcinoma-derived extracellular vesicle induces a pro-inflammatory state in monocytes. Sci. Rep. 2023, 13, 7766. [Google Scholar] [CrossRef]
- Zhang, N.; Shu, L.; Liu, Z.; Shi, A.; Zhao, L.; Huang, S.; Sheng, G.; Yan, Z.; Song, Y.; Huang, F.; et al. The role of extracellular vesicles in cholangiocarcinoma tumor microenvironment. Front. Pharmacol. 2023, 14, 1336685. [Google Scholar] [CrossRef]
- Guo, R.; Wang, P. Tumor-derived extracellular vesicles: Hijacking T cell function through exhaustion. Pathol. Res. Pract. 2025, 269, 155948. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Dominiak, A.; Żołnierzak, A.; Kubiak-Tomaszewska, G.; Lorenc, T. Tumor-Derived Exosomes in Immunosuppression and Immunotherapy. J. Immunol. Res. 2020, 2020, 6272498. [Google Scholar] [CrossRef]
- Shu, L.; Li, X.; Liu, Z.; Li, K.; Shi, A.; Tang, Y.; Zhao, L.; Huang, L.; Zhang, Z.; Zhang, D.; et al. Bile exosomal miR-182/183-5p increases cholangiocarcinoma stemness and progression by targeting HPGD and increasing PGE2 generation. Hepatology 2024, 79, 307–322. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Agüero, R.; Lacasta, A.; et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef]
- Amarachintha, S.P.; Mourya, R.; Ayabe, H.; Yang, L.; Luo, Z.; Li, X.; Thanekar, U.; Shivakumar, P.; Bezerra, J.A. Biliary organoids uncover delayed epithelial development and barrier function in biliary atresia. Hepatology 2022, 75, 89–103. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhan, Y.; Chen, G.; Shen, Z.; Zheng, S.; Dong, R. The synthetic toxin biliatresone causes biliary atresia in mice. Lab. Investig. 2020, 100, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Lieshout, R.; van Tienderen, G.S.; de Ruiter, V.; van Royen, M.E.; Boor, P.P.C.; Magré, L.; Desai, J.; Köten, K.; Kan, Y.Y.; et al. Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells. Br. J. Cancer 2022, 127, 649–660. [Google Scholar] [CrossRef]
- Schuster, M.; Braun, F.K.; Chiang, D.M.; Ludwig, C.; Meng, C.; Grätz, C.; Kirchner, B.; Proescholdt, M.; Hau, P.; Steinlein, O.K.; et al. Extracellular vesicles secreted by 3D tumor organoids are enriched for immune regulatory signaling biomolecules compared to conventional 2D glioblastoma cell systems. Front. Immunol. 2024, 15, 1388769. [Google Scholar] [CrossRef] [PubMed]
- Letelier, P.; Riquelme, I.; Hernández, A.H.; Guzmán, N.; Farías, J.G.; Roa, J.C. Circulating MicroRNAs as Biomarkers in Biliary Tract Cancers. Int. J. Mol. Sci. 2016, 17, 791. [Google Scholar] [CrossRef]
- Olaizola, P.; Lee-Law, P.Y.; Arbelaiz, A.; Lapitz, A.; Perugorria, M.J.; Bujanda, L.; Banales, J.M. MicroRNAs and extracellular vesicles in cholangiopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Atai, N.A.; Balaj, L.; van Veen, H.; Breakefield, X.O.; Jarzyna, P.A.; Van Noorden, C.J.; Skog, J.; Maguire, C.A. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neurooncol. 2013, 115, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Menjivar, N.G.; Oropallo, J.; Gebremedhn, S.; Souza, L.A.; Gad, A.; Puttlitz, C.M.; Tesfaye, D. MicroRNA Nano-Shuttles: Engineering Extracellular Vesicles as a Cutting-Edge Biotechnology Platform for Clinical Use in Therapeutics. Biol. Proced. Online 2024, 26, 14. [Google Scholar] [CrossRef]
- Teixeira, A.F.; Wang, Y.; Iaria, J.; Ten Dijke, P.; Zhu, H.J. Simultaneously targeting extracellular vesicle trafficking and TGF-β receptor kinase activity blocks signaling hyperactivation and metastasis. Signal Transduct. Target. Ther. 2023, 8, 456. [Google Scholar] [CrossRef]
- Vuckovic, S.; Vandyke, K.; Rickards, D.A.; McCauley Winter, P.; Brown, S.H.J.; Mitchell, T.W.; Liu, J.; Lu, J.; Askenase, P.W.; Yuriev, E.; et al. The cationic small molecule GW4869 is cytotoxic to high phosphatidylserine-expressing myeloma cells. Br. J. Haematol. 2017, 177, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Coffey, R.J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat. Protoc. 2023, 18, 1462–1487. [Google Scholar] [CrossRef]
- Sódar, B.W.; Kittel, Á.; Pálóczi, K.; Vukman, K.V.; Osteikoetxea, X.; Szabó-Taylor, K.; Németh, A.; Sperlágh, B.; Baranyai, T.; Giricz, Z.; et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 2016, 6, 24316. [Google Scholar] [CrossRef]
| Growth Factor | Role in Fibrosis | Mechanism | References |
|---|---|---|---|
| Amphiregulin (EGFR ligand) | Pro-fibrotic | Upregulated in fibrotic livers in NASH. Activates EGFR on HSCs, leading to collagen production; involving AKT, ERK1/2, and p38 MAP kinases. | [39,40] |
| Anti-fibrotic | Upregulated in fibrotic livers in PSC and PBC. Regulates CYP7A1 expression, serum cholesterol, and biliary acid levels through FXR. | [41] | |
| EGF | Pro-fibrotic | EGFR activation promotes cell proliferation (myofibroblasts, HSCs), α-SMA expression, and fibrosis. Inhibiting EGFR (e.g., with EGF neutralization antibodies) reduces biliary fibrosis in rodent models. | [42,43] |
| Anti-fibrotic | EGF signaling enhances intrahepatic cholangiocyte proliferation. Inhibiting EGFR (e.g., with erlotinib) dampens biliary proliferation in rodent models. | [44] | |
| FGF7 (also known as KGF) | Generally antifibrotic/regenerative | Promotes epithelial repair and hepatocyte regeneration. Can reduce liver injury and fibrosis in some models by restoring epithelial integrity. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagoonee, S.; Bolontrade, M.F.; Defilippi, P.; Weiskirchen, R. The Role of Extracellular Vesicles in Mediating Signaling in Biliary Epithelial Cell Activation and Cholangiopathies. Cells 2025, 14, 1274. https://doi.org/10.3390/cells14161274
Fagoonee S, Bolontrade MF, Defilippi P, Weiskirchen R. The Role of Extracellular Vesicles in Mediating Signaling in Biliary Epithelial Cell Activation and Cholangiopathies. Cells. 2025; 14(16):1274. https://doi.org/10.3390/cells14161274
Chicago/Turabian StyleFagoonee, Sharmila, Marcela Fabiana Bolontrade, Paola Defilippi, and Ralf Weiskirchen. 2025. "The Role of Extracellular Vesicles in Mediating Signaling in Biliary Epithelial Cell Activation and Cholangiopathies" Cells 14, no. 16: 1274. https://doi.org/10.3390/cells14161274
APA StyleFagoonee, S., Bolontrade, M. F., Defilippi, P., & Weiskirchen, R. (2025). The Role of Extracellular Vesicles in Mediating Signaling in Biliary Epithelial Cell Activation and Cholangiopathies. Cells, 14(16), 1274. https://doi.org/10.3390/cells14161274









