The Dark Side of Vascular Aging: Noncoding Ribonucleic Acids in Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. The Landscape of Vascular Aging in Cardiovascular Disease
2.1. Classification of Vascular Aging: Macrovascular vs. Microvascular and Physiological vs. Pathological
2.2. Cellular Hallmarks: Endothelial Senescence, VSMC Phenotype Switching, and ECM Remodeling
2.3. Systemic Implications: Inflammaging, Oxidative Stress, Arterial Stiffness, and Reduced NO Bioavailability
3. Vascular Aging in the Pathogenesis of HFpEF
3.1. Arterial Stiffness and Increased Ventricular Afterload
3.2. Coronary Microvascular Dysfunction and Impaired Myocardial Perfusion
3.3. The Vascular–Cardiac Axis: Inflammatory, Hemodynamic, and Metabolic Crosstalk
4. Noncoding RNAs as Regulators of Vascular Aging
4.1. MiRNA
4.2. LncRNA
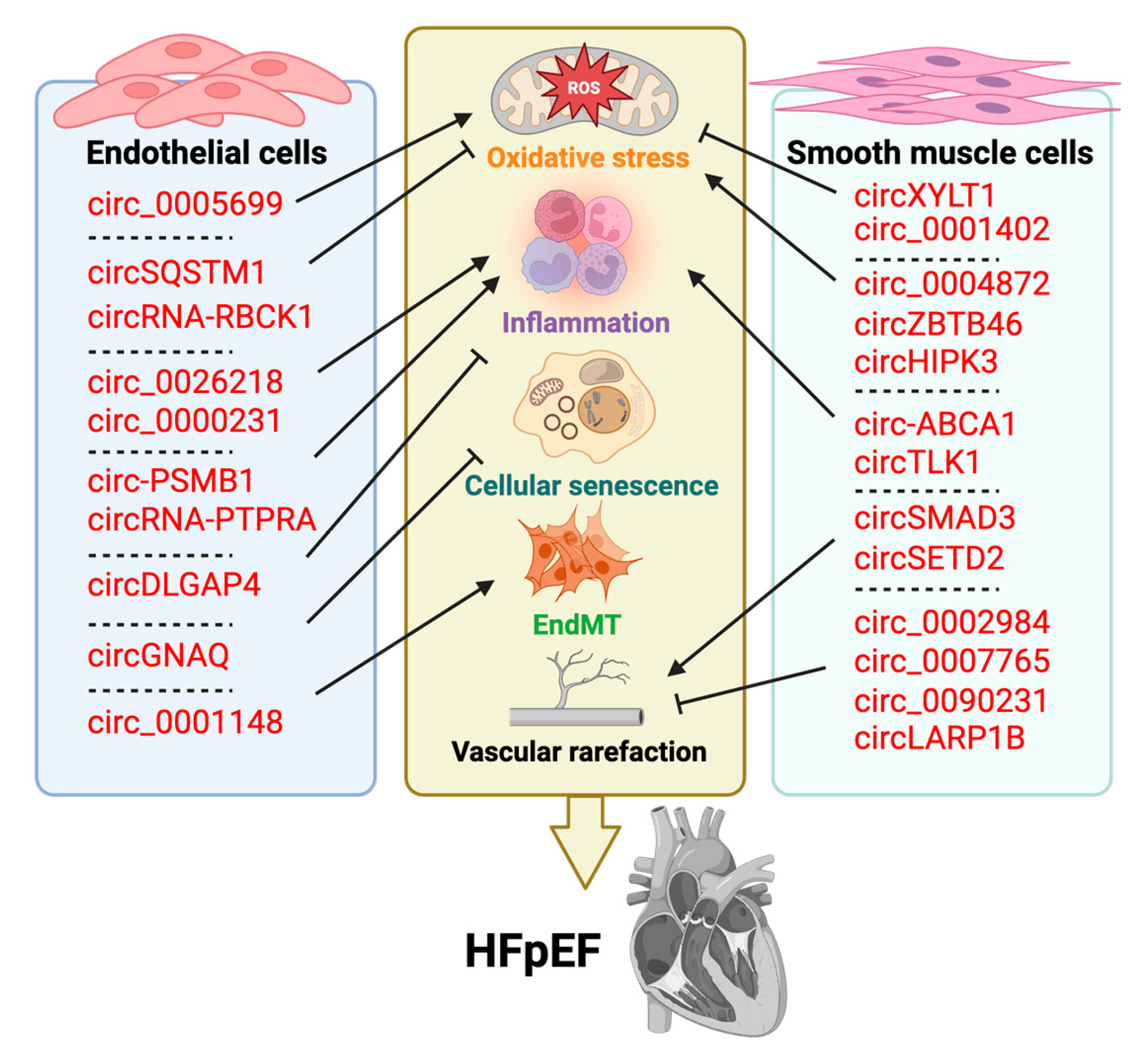
4.3. CircRNA
4.4. PiRNA
4.5. TsRNA
4.6. SnRNA
4.7. SnoRNA
5. Clinical Translation and Therapeutic Perspectives
5.1. Diagnostic and Prognostic Value of Circulating ncRNAs in Heart Failure
5.2. Therapeutic Targeting of ncRNAs: ASOs, Mimics, and Inhibitors
- (1)
- Ligand-targeted nanoparticles functionalized with antibodies or peptides that recognize vascular cell markers (e.g., ICAM-1, E-selectin) enrich payloads in diseased vasculature.
- (2)
- Exosome-based vehicles, leveraging the natural tropism of engineered exosomes carrying therapeutic ncRNAs, can home to injured myocardium and endothelium while evading immune surveillance.
- (3)
- Virus-derived vectors (AAV, lentivirus) offer prolonged expression of miRNA sponges or decoy RNAs but require rigorous screening to mitigate insertional mutagenesis and immunogenicity.
- (4)
- Cell-type–specific promoters incorporated into plasmid or viral constructs restrict ncRNA modulation to endothelial cells (e.g., using VE-cadherin promoter) or cardiomyocytes (e.g., α-MHC promoter), reducing off-target effects.
5.3. Personalized Therapies Informed by Multi-Omics and ncRNA Profiling
6. Conclusions and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Vitale, C.; Spoletini, I. Precision Cardiology: Phenotype-Targeted Therapies for HFmrEF and HFpEF. Int. J. Heart Fail. 2024, 6, 47–55. [Google Scholar] [CrossRef]
- Banthiya, S.; Check, L.; Atkins, J. Hypertrophic Cardiomyopathy as a Form of Heart Failure with Preserved Ejection Fraction: Diagnosis, Drugs, and Procedures. US Cardiol. 2024, 18, e17. [Google Scholar] [CrossRef]
- Ovchinnikov, A.; Potekhina, A.; Arefieva, T.; Filatova, A.; Ageev, F.; Belyavskiy, E. Use of Statins in Heart Failure with Preserved Ejection Fraction: Current Evidence and Perspectives. Int. J. Mol. Sci. 2024, 25, 4958. [Google Scholar] [CrossRef]
- Rusu, M.E.; Bigman, G.; Ryan, A.S.; Popa, D.-S. Investigating the Effects and Mechanisms of Combined Vitamin D and K Supplementation in Postmenopausal Women: An Up-to-Date Comprehensive Review of Clinical Studies. Nutrients 2024, 16, 2356. [Google Scholar] [CrossRef]
- Jheng, J.-R.; DesJardin, J.T.; Chen, Y.-Y.; Huot, J.R.; Bai, Y.; Cook, T.; Hibbard, L.M.; Rupp, J.M.; Fisher, A.; Zhang, Y.; et al. Plasma Proteomics Identifies B2M as a Regulator of Pulmonary Hypertension in Heart Failure with Preserved Ejection Fraction. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Y.; Chen, Q.; Zheng, J. Prognostic Impact of Coronary Microvascular Dysfunction Assessed by AMR in Acute Coronary Syndrome Patients with Chronic Kidney Disease. Front. Cardiovasc. Med. 2024, 11, 1489403. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.-H.; Ryu, J.; Kim, Y.-K.; Kook, H. Roles of Histone Acetylation Modifiers and Other Epigenetic Regulators in Vascular Calcification. Int. J. Mol. Sci. 2020, 21, 3246. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Bennett, J.; Van Dinther, M.; Voorter, P.; Backes, W.; Barnes, J.; Barkhof, F.; Captur, G.; Hughes, A.D.; Sudre, C.; Treibel, T.A. Assessment of Microvascular Disease in Heart and Brain by MRI: Application in Heart Failure with Preserved Ejection Fraction and Cerebral Small Vessel Disease. Medicina 2023, 59, 1596. [Google Scholar] [CrossRef]
- Al-Gully, J.; Oliveri, F.; Forouzanfar, J.P.; Montero-Cabezas, J.M.; Jukema, J.W.; den Haan, M.C.; Al Amri, I.; Bingen, B.O. Prognostic Role of Con-/Discordant Coronary Flow Reserve and Microvascular Resistance in Coronary Microvascular Disease: A Systematic Review and Network Meta-Analysis. Open Heart 2025, 12, e003055. [Google Scholar] [CrossRef]
- Rossi, C.; Venturin, M.; Gubala, J.; Frasca, A.; Corsini, A.; Battaglia, C.; Bellosta, S. PURPL and NEAT1 Long Non-Coding RNAs Are Modulated in Vascular Smooth Muscle Cell Replicative Senescence. Biomedicines 2023, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Habibi, J.; DeMarco, V.G.; Hulse, J.L.; Hayden, M.R.; Whaley-Connell, A.; Hill, M.A.; Sowers, J.R.; Jia, G. Inhibition of Sphingomyelinase Attenuates Diet—Induced Increases in Aortic Stiffness. J. Mol. Cell. Cardiol. 2022, 167, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Wu, T.; Gu, X.; Wu, L. Endothelial Activation and Stress Index Predicts All-Cause and Cardiovascular Mortality in Hypertensive Individuals: A Nationwide Study. J. Clin. Hypertens. 2025, 27, e70057. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Lekakis, J.P.; Nikolaou, M.; Paraskevaidis, I.; Andreadou, I.; Kaplanoglou, T.; Katsimbri, P.; Skarantavos, G.; Soucacos, P.N.; Kremastinos, D.T. Inhibition of Interleukin-1 by Anakinra Improves Vascular and Left Ventricular Function in Patients with Rheumatoid Arthritis. Circulation 2008, 117, 2662–2669. [Google Scholar] [CrossRef]
- Fan, J.; Wang, S.; Chen, K.; Sun, Z. Aging Impairs Arterial Compliance via Klotho-Mediated Downregulation of B-Cell Population and IgG Levels. Cell. Mol. Life Sci. 2022, 79, 494. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-Related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P.; Asmar, R.; Gautier, I.; Laloux, B.; Guize, L.; Ducimetiere, P.; Benetos, A. Aortic Stiffness Is an Independent Predictor of All-Cause and Cardiovascular Mortality in Hypertensive Patients. Hypertension 2001, 37, 1236–1241. [Google Scholar] [CrossRef]
- Shah, S.J.; Bonderman, D.; Borlaug, B.A.; Cleland, J.G.F.; Lack, G.; Lu, W.; Voors, A.A.; Zannad, F.; Gladwin, M.T. Macitentan for Heart Failure with Preserved or Mildly Reduced Ejection Fraction and Pulmonary Vascular Disease: Results of the SERENADE Randomized Clinical Trial and Open-Label Extension Study. Circ. Heart Fail. 2025, 18, e011381. [Google Scholar] [CrossRef]
- Roh, K.; Li, H.; Freeman, R.N.; Zazzeron, L.; Lee, A.; Zhou, C.; Shen, S.; Xia, P.; Guerra, J.R.B.; Sheffield, C.; et al. Exercise-Induced Cardiac Lymphatic Remodeling Mitigates Inflammation in the Aging Heart. Aging Cell 2025, 24, e70043. [Google Scholar] [CrossRef]
- Guo, X.; Huang, C.; Zhang, L.; Hu, G.; Du, Y.; Chen, X.; Sun, F.; Li, T.; Cui, Z.; Li, C.; et al. Lymphatic Endothelial Branched-Chain Amino Acid Catabolic Defects Undermine Cardiac Lymphatic Integrity and Drive HFpEF. Circulation 2025, 151, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Andersen, M.J.; Obokata, M.; Koepp, K.E.; Kane, G.C.; Melenovsky, V.; Olson, T.P.; Borlaug, B.A. Arterial Stiffening with Exercise in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Nicklas, B.; Kraus, W.E.; Lyles, M.F.; Eggebeen, J.; Morgan, T.M.; Haykowsky, M. Skeletal Muscle Abnormalities and Exercise Intolerance in Older Patients with Heart Failure and Preserved Ejection Fraction. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1364–H1370. [Google Scholar] [CrossRef]
- Ajmal, M.; Ajmal, A.; Huang, L.; Zeng, L. The Potential Therapeutic Role of Celastrol in Patients with Heart Failure with Preserved Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 725602. [Google Scholar] [CrossRef]
- Raucci, A.; Macrì, F.; Castiglione, S.; Badi, I.; Vinci, M.C.; Zuccolo, E. MicroRNA-34a: The Bad Guy in Age-Related Vascular Diseases. Cell. Mol. Life Sci. 2021, 78, 7355–7378. [Google Scholar] [CrossRef]
- Mensà, E.; Guescini, M.; Giuliani, A.; Bacalini, M.G.; Ramini, D.; Corleone, G.; Ferracin, M.; Fulgenzi, G.; Graciotti, L.; Prattichizzo, F.; et al. Small Extracellular Vesicles Deliver miR-21 and miR-217 as pro-Senescence Effectors to Endothelial Cells. J. Extracell. Vesicles 2020, 9, 1725285. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.-F.; Li, R.; Zhao, L.; Qin, S.-G.; Pan, L.-F.; Gao, Y.-X. MiR-217 Inhibits Apoptosis of Atherosclerotic Endothelial Cells via the TLR4/PI3K/Akt/NF-κB Pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12867–12877. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, J.; Xing, W.; Mu, F.; Wu, Y.; Zhao, J.; Liu, X.; Wang, D.; Wang, J.; Li, X.; et al. Hypoxic Glioma-Derived Exosomal miR-25-3p Promotes Macrophage M2 Polarization by Activating the PI3K-AKT-mTOR Signaling Pathway. J. Nanobiotechnol. 2024, 22, 628. [Google Scholar] [CrossRef]
- Ruan, J.; Xia, Y.; Ma, Y.; Xu, X.; Luo, S.; Yi, J.; Wu, B.; Chen, R.; Wang, H.; Yu, H.; et al. Milk-Derived Exosomes as Functional Nanocarriers in Wound Healing: Mechanisms, Applications, and Future Directions. Mater. Today Bio 2025, 32, 101715. [Google Scholar] [CrossRef]
- Ding, Q.; Shao, C.; Rose, P.; Zhu, Y.Z. Epigenetics and Vascular Senescence-Potential New Therapeutic Targets? Front. Pharmacol. 2020, 11, 535395. [Google Scholar] [CrossRef]
- Efovi, D.; Xiao, Q. Noncoding RNAs in Vascular Cell Biology and Restenosis. Biology 2022, 12, 24. [Google Scholar] [CrossRef]
- Tao, W.; Hong, Y.; He, H.; Han, Q.; Mao, M.; Hu, B.; Zhang, H.; Huang, X.; You, W.; Liang, X.; et al. MicroRNA-199a-5p Aggravates Angiotensin II-Induced Vascular Smooth Muscle Cell Senescence by Targeting Sirtuin-1 in Abdominal Aortic Aneurysm. J. Cell. Mol. Med. 2021, 25, 6056–6069. [Google Scholar] [CrossRef]
- Tong, P.; Zhang, J.; Liu, S.; An, J.; Jing, G.; Ma, L.; Wang, R.; Wang, Z. miRNA-142-3p Aggravates Hydrogen Peroxide-Induced Human Umbilical Vein Endothelial Cell Premature Senescence by Targeting SIRT1. Biosci. Rep. 2024, 44, BSR20231511. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, Y.; Zhang, C.; Wang, C. Palmitic Acid Inhibits Vascular Smooth Muscle Cell Switch to Synthetic Phenotype via Upregulation of miR-22 Expression. Aging 2022, 14, 8046–8060. [Google Scholar] [CrossRef]
- Irie, K.; Tsujimura, K.; Nakashima, H.; Nakashima, K. MicroRNA-214 Promotes Dendritic Development by Targeting the Schizophrenia-Associated Gene Quaking (Qki). J. Biol. Chem. 2016, 291, 13891–13904. [Google Scholar] [CrossRef]
- Shiau, J.-P.; Chuang, Y.-T.; Yen, C.-Y.; Chang, F.-R.; Yang, K.-H.; Hou, M.-F.; Tang, J.-Y.; Chang, H.-W. Modulation of AKT Pathway-Targeting miRNAs for Cancer Cell Treatment with Natural Products. Int. J. Mol. Sci. 2023, 24, 3688. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, L.; Ji, S.; Shen, W. LncRNA ANRIL-Mediated miR-181b-5p/S1PR1 Axis Is Involved in the Progression of Uremic Cardiomyopathy through Activating T Cells. Sci. Rep. 2022, 12, 18027. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, X.; Ma, C.; Chang, H.; Li, H.; Liu, F.; Lu, J. Low Expression of miR-448 Induces EMT and Promotes Invasion by Regulating ROCK2 in Hepatocellular Carcinoma. Cell Physiol. Biochem. 2015, 36, 487–498. [Google Scholar] [CrossRef]
- Ma, W.-Y.; Song, R.-J.; Xu, B.-B.; Xu, Y.; Wang, X.-X.; Sun, H.-Y.; Li, S.-N.; Liu, S.-Z.; Yu, M.-X.; Yang, F.; et al. Melatonin Promotes Cardiomyocyte Proliferation and Heart Repair in Mice with Myocardial Infarction via miR-143-3p/Yap/Ctnnd1 Signaling Pathway. Acta Pharmacol. Sin. 2021, 42, 921–931. [Google Scholar] [CrossRef]
- Zha, Y.; Zhuang, W.; Yang, Y.; Zhou, Y.; Li, H.; Liang, J. Senescence in Vascular Smooth Muscle Cells and Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 910580. [Google Scholar] [CrossRef]
- Moskalik, A.; Ratajska, A.; Majchrzak, B.; Jankowska-Steifer, E.; Bartkowiak, K.; Bartkowiak, M.; Niderla-Bielińska, J. miR-31-5p-Modified RAW 264.7 Macrophages Affect Profibrotic Phenotype of Lymphatic Endothelial Cells In Vitro. Int. J. Mol. Sci. 2022, 23, 13193. [Google Scholar] [CrossRef] [PubMed]
- de Yébenes, V.G.; Briones, A.M.; Martos-Folgado, I.; Mur, S.M.; Oller, J.; Bilal, F.; González-Amor, M.; Méndez-Barbero, N.; Silla-Castro, J.C.; Were, F.; et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2408–2424. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. MicroRNA-126 Regulates Hypoxia-Inducible Factor-1α Which Inhibited Migration, Proliferation, and Angiogenesis in Replicative Endothelial Senescence. Sci. Rep. 2019, 9, 7381. [Google Scholar] [CrossRef]
- Tian, Q.; Leung, F.P.; Chen, F.M.; Tian, X.Y.; Chen, Z.; Tse, G.; Ma, S.; Wong, W.T. Butyrate Protects Endothelial Function through PPARδ/miR-181b Signaling. Pharmacol. Res. 2021, 169, 105681. [Google Scholar] [CrossRef]
- Cheng, M.; Yang, Z.; Qiao, L.; Yang, Y.; Deng, Y.; Zhang, C.; Mi, T. AGEs Induce Endothelial Cells Senescence and Endothelial Barrier Dysfunction via miR-1-3p/MLCK Signaling Pathways. Gene 2023, 851, 147030. [Google Scholar] [CrossRef]
- Lan, Y.; Li, Y.-J.; Li, D.-J.; Li, P.; Wang, J.-Y.; Diao, Y.-P.; Ye, G.-D.; Li, Y.-F. Long Noncoding RNA MEG3 Prevents Vascular Endothelial Cell Senescence by Impairing miR-128-Dependent Girdin Downregulation. Am. J. Physiol. Cell Physiol. 2019, 316, C830–C843. [Google Scholar] [CrossRef]
- Lee, I.; Piao, S.; Kim, S.; Nagar, H.; Choi, S.-J.; Kim, M.; Vu, G.-H.; Jeon, B.-H.; Kim, C.-S. IDH2 Deficiency Promotes Endothelial Senescence by Eliciting miR-34b/c-Mediated Suppression of Mitophagy and Increased ROS Production. Antioxidants 2023, 12, 585. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c Is Upregulated by Oxidative Stress and Induces Endothelial Cell Apoptosis and Senescence via ZEB1 Inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Li, Y.; Cui, Y.; Zhang, Y.; Shi, W.; Wang, J.; Wu, X.; Liang, R.; Wang, X.; et al. The lncRNA OIP5-AS1/miR-4500 Axis Targeting ARG2 Modulates Oxidative Stress-Induced Premature Senescence in Endothelial Cells: Implications for Vascular Aging. Expert. Opin. Ther. Targets 2023, 27, 393–407. [Google Scholar] [CrossRef]
- Lyu, G.; Guan, Y.; Zhang, C.; Zong, L.; Sun, L.; Huang, X.; Huang, L.; Zhang, L.; Tian, X.-L.; Zhou, Z.; et al. TGF-β Signaling Alters H4K20me3 Status via miR-29 and Contributes to Cellular Senescence and Cardiac Aging. Nat. Commun. 2018, 9, 2560. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-Derived Extracellular Vesicles Promote Endothelial Inflammation and Atherogenesis via microRNA-1. J. Hepatol. 2020, 72, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mi, X.; Chen, Y.; Feng, C.; Hou, Z.; Hui, R.; Zhang, W. MicroRNA-216a Induces Endothelial Senescence and Inflammation via Smad3/IκBα Pathway. J. Cell. Mol. Med. 2018, 22, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Manakanatas, C.; Ghadge, S.K.; Agic, A.; Sarigol, F.; Fichtinger, P.; Fischer, I.; Foisner, R.; Osmanagic-Myers, S. Endothelial and Systemic Upregulation of miR-34a-5p Fine-Tunes Senescence in Progeria. Aging 2022, 14, 195–224. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, Y.; Wang, G.; Yin, Y.; Han, S.; Zheng, D.; Zhou, S.; Zhao, Y.; Chen, Y.; Jin, Y. Low Shear Stress Regulates Vascular Endothelial Cell Pyroptosis through miR-181b-5p/STAT-3 Axis. J. Cell. Physiol. 2021, 236, 318–327. [Google Scholar] [CrossRef]
- Guo, Y.; Chao, L.; Chao, J. Kallistatin Attenuates Endothelial Senescence by Modulating Let-7g-Mediated miR-34a-SIRT1-eNOS Pathway. J. Cell. Mol. Med. 2018, 22, 4387–4398. [Google Scholar] [CrossRef]
- Song, C.-Y.; Huang, H.-Z.; Yan, T.-T.; Cui, C.-X.; Wu, H.-Y.; Chen, J.; Peng, J.-H.; Chen, N.-Y.; Tang, J.; Pan, S.-L. Downregulation of miR-27a-3p Induces Endothelial Injury and Senescence and Its Significance in the Development of Coronary Heart Disease. Cell. Signal. 2025, 131, 111759. [Google Scholar] [CrossRef]
- Lian, C.; Zhao, L.; Qiu, J.; Wang, Y.; Chen, R.; Liu, Z.; Cui, J.; Zhu, X.; Wen, X.; Wang, S.; et al. miR-25-3p Promotes Endothelial Cell Angiogenesis in Aging Mice via TULA-2/SYK/VEGFR-2 Downregulation. Aging 2020, 12, 22599–22613. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, D.; Cheng, H.S.; McCoy, M.G.; Pérez-Cremades, D.; Haemmig, S.; Wong, D.; Chen, L.; Feinberg, M.W. miR-181b Regulates Vascular Endothelial Aging by Modulating an MAP3K3 Signaling Pathway. FASEB J. 2022, 36, e22353. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, D.; Zhang, N.; Yuan, T.; Tao, H. MiR-217 Promotes Endothelial Cell Senescence through the SIRT1/P53 Signaling Pathway. J. Mol. Histol. 2021, 52, 257–267. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, J.-Y.; Bae, J.; Kim, Y.-M.; Won, M.-H.; Ha, K.-S.; Kwon, Y.-G.; Kim, Y.-M. Korean Red Ginseng Prevents Endothelial Senescence by Downregulating the HO-1/NF-κB/miRNA-155-5p/eNOS Pathway. J. Ginseng Res. 2021, 45, 344–353. [Google Scholar] [CrossRef]
- Zhu, S.; Deng, S.; Ma, Q.; Zhang, T.; Jia, C.; Zhuo, D.; Yang, F.; Wei, J.; Wang, L.; Dykxhoorn, D.M.; et al. MicroRNA-10A* and MicroRNA-21 Modulate Endothelial Progenitor Cell Senescence via Suppressing High-Mobility Group A2. Circ. Res. 2013, 112, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-H.; Lee, Y.-N.; Su, C.-H.; Lee, H.-I.; Hsieh, C.-L.; Tien, T.-Y.; Lin, C.-F.; Yeh, H.-I.; Wu, Y.-J. Senescence Induces miR-409 to down-Regulate CCL5 and Impairs Angiogenesis in Endothelial Progenitor Cells. J. Cell. Mol. Med. 2024, 28, e18489. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-N.; Wu, Y.-J.; Lee, H.-I.; Wang, H.-H.; Hung, C.-L.; Chang, C.-Y.; Chou, Y.-H.; Tien, T.-Y.; Lee, C.-W.; Lin, C.-F.; et al. Hsa-miR-409-3p Regulates Endothelial Progenitor Senescence via PP2A-P38 and Is a Potential Ageing Marker in Humans. J. Cell. Mol. Med. 2023, 27, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, T.; Duan, J.; Mu, N.; Zhang, T. MALAT1/miR-15b-5p/MAPK1 Mediates Endothelial Progenitor Cells Autophagy and Affects Coronary Atherosclerotic Heart Disease via mTOR Signaling Pathway. Aging 2019, 11, 1089–1109. [Google Scholar] [CrossRef]
- Manni, E.; Jeffery, N.; Chambers, D.; Slade, L.; Etheridge, T.; Harries, L.W. An Evaluation of the Role of miR-361-5p in Senescence and Systemic Ageing. Exp. Gerontol. 2023, 174, 112127. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, M.; Yu, H.; Wang, L.; Li, X.; Rak, J.; Wang, S.; Zhao, R.C. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Mitigate Oxidative Stress-Induced Senescence in Endothelial Cells via Regulation of miR-146a/Src. Signal Transduct. Target. Ther. 2021, 6, 354. [Google Scholar] [CrossRef]
- Wagner, J.U.G.; Tombor, L.S.; Malacarne, P.F.; Kettenhausen, L.-M.; Panthel, J.; Kujundzic, H.; Manickam, N.; Schmitz, K.; Cipca, M.; Stilz, K.A.; et al. Aging Impairs the Neurovascular Interface in the Heart. Science 2023, 381, 897–906. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Li, Y.; Lin, Y.-J.; Shi, M.-M.; Fu, M.-X.; Li, Z.-Q.; Ning, D.-S.; Zeng, X.-M.; Liu, X.; Cui, Q.-H.; et al. Aging Aggravates Aortic Aneurysm and Dissection via miR-1204-MYLK Signaling Axis in Mice. Nat. Commun. 2024, 15, 5985. [Google Scholar] [CrossRef]
- Qian, Z.; Huang, Y.; Yang, N.; Fang, Z.; Zhang, Y.; Huang, Y.; Luo, M.; Ji, T.; Chen, Z.; Gao, S.; et al. miR-34a-5p/MARCHF8/ADAM10 Axis in the Regulation of Vascular Endothelial Cell Dysfunction and Senescence. Mech. Ageing Dev. 2025, 225, 112060. [Google Scholar] [CrossRef]
- Yan, L.; Xie, X.; Niu, B.-X.; Wu, M.-T.; Tong, W.-Q.; He, S.-Y.; Huang, C.-Y.; Zhao, W.-C.; Li, G.; Li, N.-S.; et al. Involvement of miR-199a-3p/DDR1 in Vascular Endothelial Cell Senescence in Diabetes. Eur. J. Pharmacol. 2021, 908, 174317. [Google Scholar] [CrossRef]
- van Balkom, B.W.M.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.J.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial Cells Require miR-214 to Secrete Exosomes That Suppress Senescence and Induce Angiogenesis in Human and Mouse Endothelial Cells. Blood 2013, 121, 3997–4006, S1-15. [Google Scholar] [CrossRef]
- He, X.; Zhao, X.; Wang, H. LPI-GPR55 Promotes Endothelial Cell Activation and Inhibits Autophagy through Inducing LINC01235 Expression. Ann. Med. 2024, 56, 2407525. [Google Scholar] [CrossRef] [PubMed]
- Yilihamu, Y.; Xu, R.; Jia, W.; Kukun, H.; Aihemaiti, D.; Chang, Y.; Ding, S.; Wang, Y. Role of Long Non-Coding RNA TCONS_02443383 in Regulating Cell Adhesion and Peroxisome Proliferator-Activated Receptor (PPAR) Signaling Genes in Atherosclerosis: A New Zealand White Rabbit Model Study. Gene 2024, 927, 148694. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, F.; Yue, A.; Xuan, Y.; Huang, Y.; Xu, J.; Weng, J.; Li, Y.; Sun, K. LncRNA Uc003pxg.1 Interacts With miR-339-5p Promote Vascular Endothelial Cell Proliferation, Migration and Angiogenesis. Korean Circ. J. 2025, 55, 440–455. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Pu, J.; Ge, H.; Liu, T.; Lv, X.; Zhang, Y.; Cao, J.; Yu, H.; Lu, Z.; Du, F. Elevated Circulating LncRNA NORAD Fosters Endothelial Cell Growth and Averts Ferroptosis by Modulating the miR-106a/CCND1 Axis in CAD Patients. Sci. Rep. 2024, 14, 24223. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chu, H.; Ran, Y.; Lin, R.; Cai, Y.; Guan, X.; Cui, X.; Zhang, X.; Li, H.; Cheng, M. Linc-ROR Modulates the Endothelial-Mesenchymal Transition of Endothelial Progenitor Cells through the miR-145/Smad3 Signaling Pathway. Physiol. Res. 2024, 73, 565–576. [Google Scholar] [CrossRef]
- Wang, K.-J.; Zhang, Y.-X.; Mo, Z.-W.; Li, Z.-L.; Wang, M.; Wang, R.; Wang, Z.-C.; Chang, G.-Q.; Wu, W.-B. Upregulation of Long Noncoding RNA MAGOH-DT Mediates TNF-α and High Glucose-Induced Endothelial-Mesenchymal Transition in Arteriosclerosis Obliterans. Tohoku J. Exp. Med. 2024, 263, 227–238. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.-F.; Dong, N.; Wang, G.-N.; Qi, M.-X.; Tan, J.-K. LncRNA PVT1 Inhibits Endothelial Cells Apoptosis in Coronary Heart Disease through Regulating MAPK1 Expression via miR-532-3p. Acta Cardiol. 2024, 79, 295–303. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ren, M.; He, S.; Bie, H.; Duan, M.; Chen, Z.; Jia, Q.; Chi, B.; Gan, X.; et al. LncRNA LUCAT1 Offers Protection against Human Coronary Artery Endothelial Cellular Oxidative Stress Injury through Modulating Hsa-miR-6776-5p/LRRC25 Axis and Activating Autophagy Flux. J. Transl. Med. 2024, 22, 1171. [Google Scholar] [CrossRef]
- Cheng, X.; Shihabudeen Haider Ali, M.S.; Baki, V.B.; Moran, M.; Su, H.; Sun, X. Multifaceted Roles of Meg3 in Cellular Senescence and Atherosclerosis. Atherosclerosis 2024, 392, 117506. [Google Scholar] [CrossRef]
- Bian, W.; Jing, X.; Yang, Z.; Shi, Z.; Chen, R.; Xu, A.; Wang, N.; Jiang, J.; Yang, C.; Zhang, D.; et al. Downregulation of LncRNA NORAD Promotes Ox-LDL-Induced Vascular Endothelial Cell Injury and Atherosclerosis. Aging 2020, 12, 6385–6400. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Ge, Y.; Zhuge, Y.; Liu, X.; Chen, H.; Liu, J.; Li, W.; Wang, X.; Shen, G.; Wang, Q.; et al. LncRNA MIR181A1HG Deficiency Attenuates Vascular Inflammation and Atherosclerosis. Circ. Res. 2025, 136, 862–883. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Tian, L.-H.; Zhu, X.-H.; Yang, S.; Zeng, H.; Yang, Y.-Y. METTL3-Mediated m6A Modification Enhances lncRNA H19 Stability to Promote Endothelial Cell Inflammation and Pyroptosis to Aggravate Atherosclerosis. FASEB J. 2024, 38, e70090. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Warwick, T.; Zehr, S.; Günther, S.; Wolf, S.; Schmachtel, T.; Izquierdo Ponce, J.; Pálfi, K.; Teichmann, T.; Schneider, A.; et al. RUNX1 Interacts with lncRNA SMANTIS to Regulate Monocytic Cell Functions. Commun. Biol. 2024, 7, 1131. [Google Scholar] [CrossRef]
- Gu, X.; Hou, J.; Rao, J.; Weng, R.; Liu, S. LncRNA MALAT1 Suppresses Monocyte-Endothelial Cell Interactions by Targeting miR-30b-5p and Enhancing ATG5-Mediated Autophagy. Heliyon 2024, 10, e28882. [Google Scholar] [CrossRef]
- Liu, X.; Huang, R.; Wan, J.; Niu, T. LncRNA MIR4697HG Alleviates Endothelial Cell Injury and Atherosclerosis Progression in Mice via the FUS/ANXA5 Axis. Biochem. Genet. 2024, 62, 3155–3173. [Google Scholar] [CrossRef]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel Growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef]
- Ghiasvand, T.; Karimi, J.; Khodadadi, I.; Yazdi, A.; Khazaei, S.; Kichi, Z.A.; Hosseini, S.K. The Interplay of LDLR, PCSK9, and lncRNA- LASER Genes Expression in Coronary Artery Disease: Implications for Therapeutic Interventions. Prostaglandins Other Lipid Mediat. 2025, 177, 106969. [Google Scholar] [CrossRef]
- Hofmann, P.; Sommer, J.; Theodorou, K.; Kirchhof, L.; Fischer, A.; Li, Y.; Perisic, L.; Hedin, U.; Maegdefessel, L.; Dimmeler, S.; et al. Long Non-Coding RNA H19 Regulates Endothelial Cell Aging via Inhibition of STAT3 Signalling. Cardiovasc. Res. 2019, 115, 230–242. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chen, A.T.; Burns, A.B.; Sriram, K.; Luo, Y.; Tang, X.; Branciamore, S.; O’Meally, D.; Chang, S.-L.; Huang, P.-H.; et al. RAMP2-AS1 Regulates Endothelial Homeostasis and Aging. Front. Cell Dev. Biol. 2021, 9, 635307. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Liang, Y.; Wang, K.-J.; Jin, S.-N.; Yu, X.-M.; Zhang, Q.; Wei, J.-Y.; Liu, H.; Fang, W.-G.; Zhao, W.-D.; et al. Endothelial lincRNA-P21 Alleviates Cerebral Ischemia/Reperfusion Injury by Maintaining Blood-Brain Barrier Integrity. J. Cereb. Blood Flow. Metab. 2024, 44, 1532–1550. [Google Scholar] [CrossRef]
- Song, X.-W.; He, W.-X.; Su, T.; Li, C.-J.; Jiang, L.-L.; Huang, S.-Q.; Li, S.-H.; Guo, Z.-F.; Zhang, B.-L. Abnormal Expression of PRKAG2-AS1 in Endothelial Cells Induced Inflammation and Apoptosis by Reducing PRKAG2 Expression. Noncoding RNA Res. 2024, 9, 536–546. [Google Scholar] [CrossRef]
- Huang, L.; Ye, Y.; Sun, Y.; Zhou, Z.; Deng, T.; Liu, Y.; Wu, R.; Wang, K.; Yao, C. LncRNA H19/miR-107 Regulates Endothelial Progenitor Cell Pyroptosis and Promotes Flow Recovery of Lower Extremity Ischemia through Targeting FADD. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167323. [Google Scholar] [CrossRef]
- Drekolia, M.-K.; Talyan, S.; Cordellini Emídio, R.; Boon, R.A.; Guenther, S.; Looso, M.; Dumbović, G.; Bibli, S.-I. Unravelling the Impact of Aging on the Human Endothelial lncRNA Transcriptome. Front. Genet. 2022, 13, 1035380. [Google Scholar] [CrossRef]
- Scacalossi, K.R.; van Solingen, C.; Moore, K.J. Long Non-Coding RNAs Regulating Macrophage Functions in Homeostasis and Disease. Vasc. Pharmacol. 2019, 114, 122–130. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, Y.; Wang, R.; Wang, L. Prospective Study of lncRNA NORAD for Predicting Cerebrovascular Events in Asymptomatic Patients with Carotid Artery Stenosis. Clin. Appl. Thromb. Hemost. 2025, 31, 10760296241299792. [Google Scholar] [CrossRef] [PubMed]
- Tavares e Silva, J.; Pessoa, J.; Nóbrega-Pereira, S.; Bernardes de Jesus, B. The Impact of Long Noncoding RNAs in Tissue Regeneration and Senescence. Cells 2024, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, X.; Han, Y.; Tian, E.; Cheng, J. LncRNA MALAT1 Protects Cardiomyocytes from Isoproterenol-Induced Apoptosis through Sponging miR-558 to Enhance ULK1-Mediated Protective Autophagy. J. Cell. Physiol. 2019, 234, 10842–10854. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wu, Z.; Zhu, S. The Roles and Mechanisms of the lncRNA-miRNA Axis in the Progression of Esophageal Cancer: A Narrative Review. J. Thorac. Dis. 2022, 14, 4545–4559. [Google Scholar] [CrossRef]
- Huang, S.; Qi, P.; Zhang, T.; Li, F.; He, X. The HIF-1α/miR-224-3p/ATG5 Axis Affects Cell Mobility and Chemosensitivity by Regulating Hypoxia-induced Protective Autophagy in Glioblastoma and Astrocytoma. Oncol. Rep. 2019, 41, 1759–1768. [Google Scholar] [CrossRef]
- Xia, W.; Zhuang, L.; Deng, X.; Hou, M. Long Noncoding RNA-p21 Modulates Cellular Senescence via the Wnt/Β-catenin Signaling Pathway in Mesenchymal Stem Cells. Mol. Med. Rep. 2017, 16, 7039–7047. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.A.; Biswas, S.; Feng, B.; Chen, S.; Gonder, J.; Chakrabarti, S. lncRNA H19 Prevents Endothelial-Mesenchymal Transition in Diabetic Retinopathy. Diabetologia 2019, 62, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Yao, S. MicroRNA Biogenesis and Their Functions in Regulating Stem Cell Potency and Differentiation. Biol. Proced. Online 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, X.; Li, J.; Yang, W.; Ma, H.; Zhang, Z. P53-Targeted lincRNA-P21 Acts as a Tumor Suppressor by Inhibiting JAK2/STAT3 Signaling Pathways in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2019, 18, 38. [Google Scholar] [CrossRef]
- Fan, X.; Qi, Z.; Deng, Y.; Yang, Z.; Sun, L.; Li, G.; Liang, J.; Wu, F.; Yuan, L. [LncRNA MAGI2-AS3 enhances cisplatin sensitivity of non-small cell lung cancer cells by regulating the miR-1269a/PTEN/AKT pathway]. Nan Fang Yi Ke Da Xue Xue Bao 2024, 44, 2033–2043. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, L.; Zhao, Y.; Fei, J.; Zhang, W. Circular RNA Circ_0029589 Regulates Proliferation, Migration, Invasion, and Apoptosis in Ox-LDL-Stimulated VSMCs by Regulating miR-424-5p/IGF2 Axis. Vasc. Pharmacol. 2020, 135, 106782. [Google Scholar] [CrossRef]
- Qu, X.; Du, Y.; Shu, Y.; Gao, M.; Sun, F.; Luo, S.; Yang, T.; Zhan, L.; Yuan, Y.; Chu, W.; et al. MIAT Is a Pro-Fibrotic Long Non-Coding RNA Governing Cardiac Fibrosis in Post-Infarct Myocardium. Sci. Rep. 2017, 7, 42657. [Google Scholar] [CrossRef]
- Thomas, J.T.; Grant, M.E. Cartilage Proteoglycan Aggregate and Fibronectin Can Modulate the Expression of Type X Collagen by Embryonic Chick Chondrocytes Cultured in Collagen Gels. Biosci. Rep. 1988, 8, 163–171. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Z. Effects of LncRNA MYOSLID and MiR-29c-3p on the Proliferation and Migration of Angiotensin II-Induced Vascular Smooth Muscle Cells. Int. Heart J. 2025, 66, 164–174. [Google Scholar] [CrossRef]
- Gu, J.; Chen, J.; Yin, Q.; Dong, M.; Zhang, Y.; Chen, M.; Chen, X.; Min, J.; He, X.; Tan, Y.; et al. lncRNA JPX-Enriched Chromatin Microenvironment Mediates Vascular Smooth Muscle Cell Senescence and Promotes Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 156–176. [Google Scholar] [CrossRef]
- Yin, C.; Ge, Z.; Yuan, J.; Chen, Y.; Tang, Y.; Xiang, Y.; Zhang, Y. NEAT1 Regulates VSMC Differentiation and Calcification in as Long Noncoding RNA NEAT1 Enhances Phenotypic and Osteogenic Switching of Vascular Smooth Muscle Cells in Atherosclerosis via Scaffolding EZH2. Am. J. Physiol. Cell Physiol. 2024, 326, C1721–C1734. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Wang, S.; Li, Q. Tanshinone IIA Attenuates Ox-LDL-Induced Endothelial Cell Injury by Inhibiting NF-kapaB Pathway via Circ_0000231/miR-590-5p/TXNIP Axis. Chem. Biol. Drug Des. 2024, 103, e14394. [Google Scholar] [CrossRef]
- Yu, S.-M.; Liu, J.-Q.; Zhang, L.-L.; Ma, Y.-T.; Yin, F.-Y.; Liu, S. Mmu_circ_0001148 Promotes Endothlial-Mesenchymal Transition via Regulating miR-218-5p/JMY Axis and Drives Progression of Atherosclerosis. Int. J. Biol. Macromol. 2025, 293, 139305. [Google Scholar] [CrossRef]
- Wan, H.; You, T.; Luo, W. Circ_0003204 Regulates Cell Growth, Oxidative Stress, and Inflammation in Ox-LDL-Induced Vascular Endothelial Cells via Regulating miR-942-5p/HDAC9 Axis. Front. Cardiovasc. Med. 2021, 8, 646832. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, L.; Ye, B.; Chen, W.; Zheng, G.; Xie, H.; Guo, Y. Knockdown of Hsa_circ_0005699 Attenuates Inflammation and Apoptosis Induced by Ox-LDL in Human Umbilical Vein Endothelial Cells through Regulation of the miR-450b-5p/NFKB1 Axis. Mol. Med. Rep. 2022, 26, 290. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, J.; He, L.; Liu, C. Circ_0005699 Expedites Ox-LDL-Triggered Endothelial Cell Injury via Targeting miR-384/ASPH Axis. Cardiovasc. Toxicol. 2024, 24, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Yu, Z.; Zhang, T. Circ_0026218 Ameliorates Oxidized Low-Density Lipoprotein-Induced Vascular Endothelial Cell Dysfunction by Regulating miR-188-3p/TLR4/NF-κB Pathway. Cardiovasc. Drugs Ther. 2024, 38, 263–277. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Chen, X. Exosomal Circular RNA Circ_0074673 Regulates the Proliferation, Migration, and Angiogenesis of Human Umbilical Vein Endothelial Cells via the microRNA-1200/MEOX2 Axis. Bioengineered 2021, 12, 6782–6792. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Y.; Zhu, J.; Xie, Y.; Wu, R.; Zhong, J.; Qiu, Z.; Jiang, L. Circ_0086296 Induced Atherosclerotic Lesions via the IFIT1/STAT1 Feedback Loop by Sponging miR-576-3p. Cell. Mol. Biol. Lett. 2022, 27, 80. [Google Scholar] [CrossRef]
- Wang, H.-G.; Yan, H.; Wang, C.; Li, M.-M.; Lv, X.-Z.; Wu, H.-D.; Fang, Z.-H.; Mo, D.-L.; Zhang, Z.-Y.; Liang, B.; et al. circAFF1 Aggravates Vascular Endothelial Cell Dysfunction Mediated by miR-516b/SAV1/YAP1 Axis. Front. Physiol. 2020, 11, 899. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.; Di, Y.; Sun, S.; Yang, L.; Wang, B.; Ji, Z. CircCHMP5 Contributes to Ox-LDL-Induced Endothelial Cell Injury Through the Regulation of MiR-532-5p/ROCK2 Axis. Cardiovasc. Drugs Ther. 2023, 37, 1–12. [Google Scholar] [CrossRef]
- Zou, J.; Liu, K.-C.; Wang, W.-P.; Xu, Y. Circular RNA COL1A2 Promotes Angiogenesis via Regulating miR-29b/VEGF Axis in Diabetic Retinopathy. Life Sci. 2020, 256, 117888. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, H.; Chen, F.; Tang, Y. CircDLGAP4 Induces Autophagy and Improves Endothelial Cell Dysfunction in Atherosclerosis by Targeting PTPN4 with miR-134-5p. Environ. Toxicol. 2023, 38, 2952–2966. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-P.; Zhou, M.-Y.; Liu, D.-L.; Min, X.; Shao, T.; Xu, Z.-Y.; Jing, X.; Cai, M.-Y.; Xu, S.; Liang, X.; et al. circGNAQ, a Circular RNA Enriched in Vascular Endothelium, Inhibits Endothelial Cell Senescence and Atherosclerosis Progression. Mol. Ther. Nucleic Acids 2021, 26, 374–387. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, R.; Zhao, L.; Lian, C.; Liu, Z.; Zhu, X.; Cui, J.; Wang, S.; Wang, M.; Huang, Y.; et al. Circular RNA circGSE1 Promotes Angiogenesis in Ageing Mice by Targeting the miR-323-5p/NRP1 Axis. Aging 2022, 14, 3049–3069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, Y. Circ-PSMB1 Knockdown Inhibits the Pyroptosis of Ox-LDL Treated Human Aortic Cells via the miR-624-3p/ASC Axis. J. Cardiothorac. Surg. 2025, 20, 226. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Shi, W.; Xie, H.; Chen, R.; Shao, Y.; Liang, W. CircRNA PVT1 Promotes Proliferation and Chemoresistance of Osteosarcoma Cells via the miR-24-3p/KLF8 Axis. Int. J. Clin. Oncol. 2022, 27, 811–822. [Google Scholar] [CrossRef]
- Li, B.; Bai, W.-W.; Guo, T.; Tang, Z.-Y.; Jing, X.-J.; Shan, T.-C.; Yin, S.; Li, Y.; Wang, F.; Zhu, M.-L.; et al. Statins Improve Cardiac Endothelial Function to Prevent Heart Failure with Preserved Ejection Fraction through Upregulating circRNA-RBCK1. Nat. Commun. 2024, 15, 2953. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, Y.; Chen, Z.; Ma, D.; Zhang, X.; Wu, G.; Fan, W. Shear-Sensitive circRNA-LONP2 Promotes Endothelial Inflammation and Atherosclerosis by Targeting NRF2/HO1 Signaling. JACC Basic. Transl. Sci. 2024, 9, 652–670. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, X. CircRNA-PTPRA Knockdown Inhibits Atherosclerosis Progression by Repressing Ox-LDL-Induced Endothelial Cell Injury via Sponging of miR-671-5p. Biochem. Genet. 2023, 61, 187–201. [Google Scholar] [CrossRef]
- Wei, Z.; Ran, H.; Yang, C. CircRSF1 Contributes to Endothelial Cell Growth, Migration and Tube Formation under Ox-LDL Stress through Regulating miR-758/CCND2 Axis. Life Sci. 2020, 259, 118241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, R.; Zhu, Y.; Huang, Z.; Yang, X.; Li, Q.; Zhong, M.; Zhang, W.; Chen, L.; Wu, W.; et al. A Novel Circular RNA, circSQSTM1, Protects the Endothelial Function in Atherosclerosis. Free Radic. Biol. Med. 2023, 209, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Jiang, P.; Du, Y.; Zeng, D.; Zhao, J.; Li, M.; Xia, C.; Xie, Z.; Wu, J. Oxidized Low-Density Lipoprotein Accelerates the Injury of Endothelial Cells via Circ-USP36/miR-98-5p/VCAM1 Axis. IUBMB Life 2021, 73, 177–187. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, Q.; Ren, M.; Bie, H.; Zhang, X.; Zhang, Q.; He, S.; Li, C.; Zhou, H.; Wang, Y.; et al. Circular RNA ZBTB46 Depletion Alleviates the Progression of Atherosclerosis by Regulating the Ubiquitination and Degradation of hnRNPA2B1 via the AKT/mTOR Pathway. Immun. Ageing 2023, 20, 66. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Li, J.; Zhang, Y.; Wang, W.; Xu, P.; Li, Y. Endothelial Cell-Derived Exosomal circHIPK3 Promotes the Proliferation of Vascular Smooth Muscle Cells Induced by High Glucose via the miR-106a-5p/Foxo1/Vcam1 Pathway. Aging 2021, 13, 25241–25255. [Google Scholar] [CrossRef]
- Zheng, B.; Bernier, M.; Zhang, X.; Suzuki, T.; Nie, C.; Li, Y.H.; Zhang, Y.; Song, L.-L.; Shi, H.; Liu, Y.; et al. miR-200c-SUMOylated KLF4 Feedback Loop Acts as a Switch in Transcriptional Programs That Control VSMC Proliferation. J. Mol. Cell. Cardiol. 2015, 82, 201–212. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Sheu, J.-J.; Sun, C.-K.; Huang, T.-H.; Lin, Y.-P.; Yip, H.-K. MicroRNA-214 Modulates the Senescence of Vascular Smooth Muscle Cells in Carotid Artery Stenosis. Mol. Med. 2020, 26, 46. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Niu, F.; Luo, X.; Li, J.; Hu, W. MicroRNA-30a-3p: A Potential Noncoding RNA Target for the Treatment of Arteriosclerosis Obliterans. Aging 2023, 15, 11875–11890. [Google Scholar] [CrossRef]
- Li, F.-J.; Zhang, C.-L.; Luo, X.-J.; Peng, J.; Yang, T.-L. Involvement of the MiR-181b-5p/HMGB1 Pathway in Ang II-Induced Phenotypic Transformation of Smooth Muscle Cells in Hypertension. Aging Dis. 2019, 10, 231–248. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, X.-L.; Peng, D.-Q.; Zhao, S.-P. Myocyte Enhancer Factor 2A Regulates Hydrogen Peroxide-Induced Senescence of Vascular Smooth Muscle Cells Via microRNA-143. J. Cell. Physiol. 2015, 230, 2202–2211. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Yang, S.; Qian, D. Downregulation of miR-542-3p Promotes Osteogenic Transition of Vascular Smooth Muscle Cells in the Aging Rat by Targeting BMP7. Hum. Genom. 2019, 13, 67. [Google Scholar] [CrossRef]
- Tan, P.; Wang, H.; Zhan, J.; Ma, X.; Cui, X.; Wang, Y.; Wang, Y.; Zhong, J.; Liu, Y. Rapamycin-induced miR-30a Downregulation Inhibits Senescence of VSMCs by Targeting Beclin1. Int. J. Mol. Med. 2019, 43, 1311–1320. [Google Scholar] [CrossRef]
- Chen, T.; Liang, Q.; Xu, J.; Zhang, Y.; Zhang, Y.; Mo, L.; Zhang, L. MiR-665 Regulates Vascular Smooth Muscle Cell Senescence by Interacting with LncRNA GAS5/SDC1. Front. Cell Dev. Biol. 2021, 9, 700006. [Google Scholar] [CrossRef] [PubMed]
- Peymani, P.; Dehesh, T.; Aligolighasemabadi, F.; Sadeghdoust, M.; Kotfis, K.; Ahmadi, M.; Mehrbod, P.; Iranpour, P.; Dastghaib, S.; Nasimian, A.; et al. Statins in Patients with COVID-19: A Retrospective Cohort Study in Iranian COVID-19 Patients. Transl. Med. Commun. 2021, 6, 3. [Google Scholar] [CrossRef]
- Wang, S.-F.; Yang, L.-Y.; Zhao, A.-Q.; Wang, Z.-Y.; Wang, S.; Gong, M.; Zheng, M.-Q.; Liu, G.; Yang, S.-Y.; Lin, J.-J.; et al. A Novel Hidden Protein P-414aa Encoded by circSETD2(14,15) Inhibits Vascular Remodeling. Circulation 2025, 151, 1568–1582. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Ma, X.; Zhou, L.; Wuyun, Q.; Cai, Z.; Yan, J.; Ding, H. CircSMAD3 Represses VSMC Phenotype Switching and Neointima Formation via Promoting hnRNPA1 Ubiquitination Degradation. Cell Prolif. 2025, 58, e13742. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, R.; Yu, X. Circular RNA Circ_0002984 Facilitates the Proliferation and Migration of Ox-LDL-Induced Vascular Smooth Muscle Cells via the Let-7a-5p/KLF5 Pathway. Cardiovasc. Toxicol. 2024, 24, 1253–1267. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhang, Y.; Che, P.; Qin, W.; Wu, X.; Liu, Y.; Hu, B. Circ_0090231 Knockdown Protects Vascular Smooth Muscle Cells from Ox-LDL-Induced Proliferation, Migration and Invasion via miR-942-5p/PPM1B Axis during Atherosclerosis. Mol. Cell. Biochem. 2024, 479, 2035–2045. [Google Scholar] [CrossRef]
- Lu, P.; Fan, J.; Li, B.; Wang, X.; Song, M. A Novel Protein Encoded by circLARP1B Promotes the Proliferation and Migration of Vascular Smooth Muscle Cells by Suppressing cAMP Signaling. Atherosclerosis 2024, 395, 117575. [Google Scholar] [CrossRef]
- Li, G.; Li, D.; Li, Y.; Liu, B. CircXYLT1 Suppresses Oxidative Stress and Promotes Vascular Remodeling in Aging Mice Carotid Artery Injury Model of Atherosclerosis via PTBP1. Exp. Gerontol. 2025, 201, 112690. [Google Scholar] [CrossRef]
- Qian, P.; Cao, X.; Zhang, Q.; Gao, M.; Liu, X.; Yan, L. Circ_0004872 Deficiency Attenuates Ox-LDL-Induced Vascular Smooth Muscle Cell Dysfunction by miR-424-5p-Dependent Regulation of FRS2. Mol. Cell. Biochem. 2024, 479, 3425–3435. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Liu, W.; Wang, Z.; Rong, J.; Long, X.; Liu, Z.; Ge, J.; Shi, B. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7954657. [Google Scholar] [CrossRef]
- Yu, F.; Liu, J.; Wei, X. Circ-ABCA1 Promotes Oxidized Low-Density Lipoprotein-Induced Inflammation and Phenotypic Switch in Vascular Smooth Muscle Cells. Clinics 2024, 79, 100343. [Google Scholar] [CrossRef]
- Zhang, F.; Xiang, X.; Wen, C.; Guo, X.; Nie, L.; Chen, J.; Xia, Y.; Hu, B.; Mao, L. CircTLK1: A Novel Regulator Involved in VSMC Phenotypic Switching and the Developmental Process of Atherosclerosis. FASEB J. 2024, 38, e23557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yan, C. LncRNA MAGI2-AS3 Promotes the Progression of Atherosclerosis by Sponging miR-525-5p. J. Cardiothorac. Surg. 2025, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, X.; Cai, X.; Yu, Y.; Hu, D. LncRNA MBNL1-AS1 Functions as an Alternative Atherosclerosis Biomarker in Elderly Hypertensive Patients and Regulates Vascular Smooth Muscle Cell Function. Minerva Cardiol. Angiol. 2025, 73, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Luo, Y.; Zhai, H.; Lu, J.; Zhang, M.; Wang, N. Long Noncoding RNA MIAT Regulates VSMC Migration by Sponging miR-326. Sci. Prog. 2025, 108, 368504251335854. [Google Scholar] [CrossRef]
- Ballantyne, M.D.; Pinel, K.; Dakin, R.; Vesey, A.T.; Diver, L.; Mackenzie, R.; Garcia, R.; Welsh, P.; Sattar, N.; Hamilton, G.; et al. Smooth Muscle Enriched Long Noncoding RNA (SMILR) Regulates Cell Proliferation. Circulation 2016, 133, 2050–2065. [Google Scholar] [CrossRef]
- Fasolo, F.; Jin, H.; Winski, G.; Chernogubova, E.; Pauli, J.; Winter, H.; Li, D.Y.; Glukha, N.; Bauer, S.; Metschl, S.; et al. Long Noncoding RNA MIAT Controls Advanced Atherosclerotic Lesion Formation and Plaque Destabilization. Circulation 2021, 144, 1567–1583. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Yue, M.; Li, Y.; Bi, J.; Liu, H. Angiotensin II Inhibits Apoptosis of Mouse Aortic Smooth Muscle Cells through Regulating the circNRG-1/miR-193b-5p/NRG-1 Axis. Cell Death Dis. 2019, 10, 362. [Google Scholar] [CrossRef]
- Zeng, Z.; Xia, L.; Fan, S.; Zheng, J.; Qin, J.; Fan, X.; Liu, Y.; Tao, J.; Liu, Y.; Li, K.; et al. Circular RNA CircMAP3K5 Acts as a MicroRNA-22-3p Sponge to Promote Resolution of Intimal Hyperplasia Via TET2-Mediated Smooth Muscle Cell Differentiation. Circulation 2021, 143, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, L.; Zheng, B.; Zhang, X.; Zhang, H.; Zhao, A.; Yu, J.; Yang, Z.; Wen, J. circACTA2 Inhibits NLRP3 Inflammasome-Mediated Inflammation via Interacting with NF-κB in Vascular Smooth Muscle Cells. Cell. Mol. Life Sci. 2023, 80, 229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Wang, Z.; Lin, X.; Fu, X.; He, X.; Liu, M.; Wang, J.-X.; Yu, T.; Sun, P. CircHIPK3 Targets DRP1 to Mediate Hydrogen Peroxide-Induced Necroptosis of Vascular Smooth Muscle Cells and Atherosclerotic Vulnerable Plaque Formation. J. Adv. Res. 2025, 69, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Tian, M.; Cao, N.; Yang, P.; Xu, Z.; Zheng, S.; Liao, Q.; Chen, C.; Zeng, C.; Jose, P.A.; et al. Circular RNA circEsyt2 Regulates Vascular Smooth Muscle Cell Remodeling via Splicing Regulation. J. Clin. Investig. 2021, 131, e147031. [Google Scholar] [CrossRef]
- Hall, I.F.; Climent, M.; Quintavalle, M.; Farina, F.M.; Schorn, T.; Zani, S.; Carullo, P.; Kunderfranco, P.; Civilini, E.; Condorelli, G.; et al. Circ_Lrp6, a Circular RNA Enriched in Vascular Smooth Muscle Cells, Acts as a Sponge Regulating miRNA-145 Function. Circ. Res. 2019, 124, 498–510. [Google Scholar] [CrossRef]
- Kou, L.; Yang, N.; Dong, B.; Yang, J.; Song, Y.; Li, Y.; Qin, Q. Circular RNA Testis-Expressed 14 Overexpression Induces Apoptosis and Suppresses Migration of Ox-LDL-Stimulated Vascular Smooth Muscle Cells via Regulating the microRNA 6509-3p/Thanatos-Associated Domain-Containing Apoptosis-Associated Protein 1 Axis. Bioengineered 2022, 13, 13150–13161. [Google Scholar] [CrossRef]
- Pu, Z.; Lu, J.; Yang, X. Emerging Roles of Circular RNAs in Vascular Smooth Muscle Cell Dysfunction. Front. Genet. 2021, 12, 749296. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Zhang, X.; Ma, X.; He, X.; Gan, C.; Zou, X.; Wang, S.; Shu, K.; Lei, T.; et al. CircZXDC Promotes Vascular Smooth Muscle Cell Transdifferentiation via Regulating miRNA-125a-3p/ABCC6 in Moyamoya Disease. Cells 2022, 11, 3792. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Zhao, H.; Wang, M.; Zhang, Y. Circular RNA circCHFR Facilitates the Proliferation and Migration of Vascular Smooth Muscle via miR-370/FOXO1/Cyclin D1 Pathway. Mol. Ther. Nucleic Acids 2019, 16, 434–441. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Yuan, J.; Zhang, Y.; Sang, H. Circular RNA WDR77 Target FGF-2 to Regulate Vascular Smooth Muscle Cells Proliferation and Migration by Sponging miR-124. Biochem. Biophys. Res. Commun. 2017, 494, 126–132. [Google Scholar] [CrossRef]
- Teefy, B.B.; Adler, A.; Xu, A.; Hsu, K.; Singh, P.P.; Benayoun, B.A. Dynamic Regulation of Gonadal Transposon Control across the Lifespan of the Naturally Short-Lived African Turquoise Killifish. Genome Res. 2023, 33, 141–153. [Google Scholar] [CrossRef]
- Kasper, D.M.; Wang, G.; Gardner, K.E.; Johnstone, T.G.; Reinke, V. The C. Elegans SNAPc Component SNPC-4 Coats piRNA Domains and Is Globally Required for piRNA Abundance. Dev. Cell 2014, 31, 145–158. [Google Scholar] [CrossRef]
- Teefy, B.B.; Siebert, S.; Cazet, J.F.; Lin, H.; Juliano, C.E. PIWI-piRNA Pathway-Mediated Transposable Element Repression in Hydra Somatic Stem Cells. RNA 2020, 26, 550–563. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, C.; Ding, Y.-H.; Mello, C. Casein Kinase II Promotes piRNA Production through Direct Phosphorylation of USTC Component TOFU-4. Nat. Commun. 2024, 15, 2727. [Google Scholar] [CrossRef]
- Yu, Y.-F.; Yao, P.-Q.; Wang, Z.-K.; Xie, W.-W. MiR-137 Promotes TLR4/NF-κB Pathway Activity through Targeting KDM4A, Inhibits Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells and Aggravates Osteoporosis. J. Orthop. Surg. Res. 2023, 18, 444. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Y.; Kong, L.-J.; Li, D.; Tong, M.; Li, X.-M.; Zhao, C.; Jiang, Q.; Yan, B. Targeting Endothelial Glycolytic Reprogramming by tsRNA-1599 for Ocular Anti-Angiogenesis Therapy. Theranostics 2024, 14, 3509–3525. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Y.; Zhang, H.; Ma, G.; Wu, G.; Xiang, A.; Shi, X.; Yang, G.S.; Sun, S. MicroRNA-106a-5p Inhibited C2C12 Myogenesis via Targeting PIK3R1 and Modulating the PI3K/AKT Signaling. Genes 2018, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qian, B.; Wang, F.; Huang, Y.; Yan, X.; Li, P.; Zhang, Q.; Li, Y.; Sun, K. Global Profile of tRNA-Derived Small RNAs in Pathological Cardiac Hypertrophy Plasma and Identification of tRF-21-NB8PLML3E as a New Hypertrophy Marker. Diagnostics 2023, 13, 2065. [Google Scholar] [CrossRef]
- Bao, P.; Höbartner, C.; Hartmuth, K.; Lührmann, R. Yeast Prp2 Liberates the 5’ Splice Site and the Branch Site Adenosine for Catalysis of Pre-mRNA Splicing. RNA 2017, 23, 1770–1779. [Google Scholar] [CrossRef]
- Shen, A.; Hencel, K.; Parker, M.T.; Scott, R.; Skukan, R.; Adesina, A.S.; Metheringham, C.L.; Miska, E.A.; Nam, Y.; Haerty, W.; et al. U6 snRNA m6A Modification Is Required for Accurate and Efficient Cis- and Trans-Splicing of C. Elegans mRNAs. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rhine, K.; Li, R.; Kopalle, H.M.; Rothamel, K.; Ge, X.; Epstein, E.; Mizrahi, O.; Madrigal, A.A.; Her, H.-L.; Gomberg, T.A.; et al. Neuronal Aging Causes Mislocalization of Splicing Proteins and Unchecked Cellular Stress. Nat. Neurosci. 2025, 28, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.; Hencel, K.; Parker, M.T.; Scott, R.; Skukan, R.; Adesina, A.S.; Metheringham, C.L.; Miska, E.A.; Nam, Y.; Haerty, W.; et al. U6 snRNA m6A Modification Is Required for Accurate and Efficient Splicing of C. Elegans and Human Pre-mRNAs. Nucleic Acids Res. 2024, 52, 9139–9160. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Bernabéu, C. The Splicing Factor SRSF1 as a Marker for Endothelial Senescence. Front. Physiol. 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gan, X.; Qiu, C.; Yang, D.; Sun, X.; Zeng, Z. Role of Polypyrimidine Tract-Binding Protein 1/Yin Yang 2 Signaling in Regulating Vascular Smooth Muscle Cell Proliferation and Neointima Hyperplasia. Toxicol. Appl. Pharmacol. 2019, 383, 114747. [Google Scholar] [CrossRef]
- Watson, C.N.; Belli, A.; Di Pietro, V. Small Non-Coding RNAs: New Class of Biomarkers and Potential Therapeutic Targets in Neurodegenerative Disease. Front. Genet. 2019, 10, 364. [Google Scholar] [CrossRef]
- Xu, B.; Gogol, M.; Gaudenz, K.; Gerton, J.L. Improved Transcription and Translation with L-Leucine Stimulation of mTORC1 in Roberts Syndrome. BMC Genom. 2016, 17, 25. [Google Scholar] [CrossRef]
- Suthahar, N.; Lau, E.S.; Blaha, M.J.; Paniagua, S.M.; Larson, M.G.; Psaty, B.M.; Benjamin, E.J.; Allison, M.A.; Bartz, T.M.; Januzzi, J.L.; et al. Sex-Specific Associations of Cardiovascular Risk Factors and Biomarkers with Incident Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 1455–1465. [Google Scholar] [CrossRef]
- Jaé, N.; Dimmeler, S. Noncoding RNAs in Vascular Diseases. Circ. Res. 2020, 126, 1127–1145. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Wen, J.; Chen, X.; Xie, B.; Zhao, Y. Enhancer RNAs: Mechanisms in Transcriptional Regulation and Functions in Diseases. Cell Commun. Signal. 2023, 21, 191. [Google Scholar] [CrossRef]
- Nagasawa, C.; Ogren, A.; Kibiryeva, N.; Marshall, J.; O’Brien, J.E.; Kenmochi, N.; Bittel, D.C. The Role of scaRNAs in Adjusting Alternative mRNA Splicing in Heart Development. J. Cardiovasc. Dev. Dis. 2018, 5, 26. [Google Scholar] [CrossRef]
- Büscher, M.; Horos, R.; Hentze, M.W. ‘High Vault-Age’: Non-Coding RNA Control of Autophagy. Open Biol. 2020, 10, 190307. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.; Røsjø, H.; Aspenes, S.T.; Condorelli, G.; Omland, T.; Wisløff, U. Circulating microRNAs and Aerobic Fitness--the HUNT-Study. PLoS ONE 2013, 8, e57496. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Silvello, D.; Martinelli, N.; Garbin, A.; Biolo, A.; Clausell, N.; Andrades, M.; Dos Santos, K.; Rohde, L. Plasma Levels of microRNA-21, -126 and -423-5p Alter during Clinical Improvement and Are Associated with the Prognosis of Acute Heart Failure. Mol. Med. Rep. 2018, 17, 4736–4746. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Bauters, C.; Volkmann, I.; Maury, F.; Fetisch, J.; Holzmann, A.; Lemesle, G.; de Groote, P.; Pinet, F.; Thum, T. Circulating Long Noncoding RNA, LIPCAR, Predicts Survival in Patients with Heart Failure. Circ. Res. 2014, 114, 1569–1575. [Google Scholar] [CrossRef]
- Holdt, L.M.; Beutner, F.; Scholz, M.; Gielen, S.; Gäbel, G.; Bergert, H.; Schuler, G.; Thiery, J.; Teupser, D. ANRIL Expression Is Associated with Atherosclerosis Risk at Chromosome 9p21. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 620–627. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Shao, C.; Xu, J. Expression and Diagnostic Value of lncRNA MALAT1 and NLRP3 in Lower Limb Atherosclerosis in Diabetes. BMC Endocr. Disord. 2024, 24, 28. [Google Scholar] [CrossRef]
- Jiang, R.; He, X.; Chen, W.; Cai, H.; Su, Z.; Xie, Z.; Zhang, B.; Yang, J.; Wang, Y.; Huang, L.; et al. lncRNA H19 Facilitates Vascular Neointima Formation by Targeting miR-125a-3p/FLT1 Axis. Acta Biochim. Biophys. Sin. 2024, 56, 1437–1445. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Endothelial Progenitor Cells Protect the Kidney from Ischemia-Reperfusion Injury by microRNA-Dependent Reprogramming of Resident Renal Cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef]
- Smits, P.C.; van Langenhove, G.; Schaar, M.; Reijs, A.; Bakker, W.H.; van der Giessen, W.J.; Verdouw, P.D.; Krenning, E.P.; Serruys, P.W. Efficacy of Percutaneous Intramyocardial Injections Using a Nonfluoroscopic 3-D Mapping Based Catheter System. Cardiovasc. Drugs Ther. 2002, 16, 527–533. [Google Scholar] [CrossRef]
- Mikutis, S.; Bernardes, G.J.L. Technologies for Targeted RNA Degradation and Induced RNA Decay. Chem. Rev. 2024, 124, 13301–13330. [Google Scholar] [CrossRef]
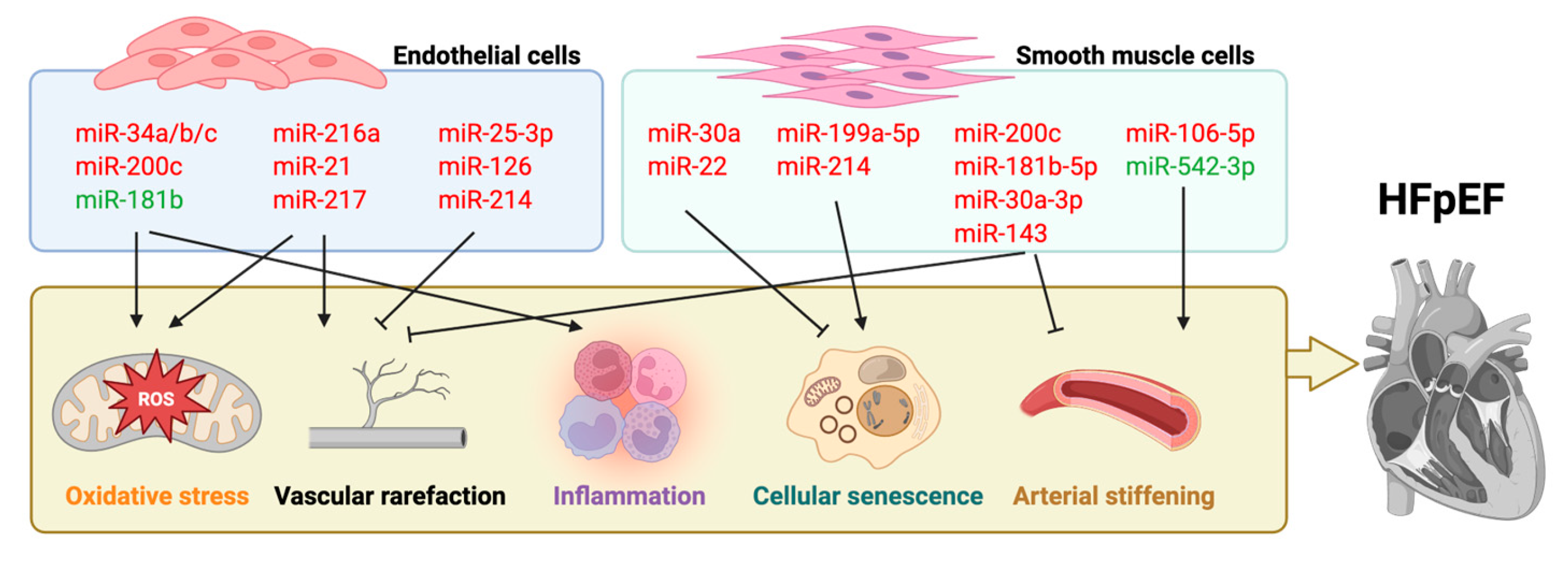

| miRNA | Regulatory Mechanisms | Target(s) | |
|---|---|---|---|
| miR-217 | miR-217 inhibits eNOS and upstream VEGF/Apelin signaling, contributing to endothelial dysfunction and atherosclerosis. | eNOS | [42] |
| miR-126 | miR-126 maintains HIF-1α levels, supporting endothelial angiogenesis and repair; its downregulation impairs function. | HIF-1α | [43] |
| miR-181b | Butyrate activates PPARδ to upregulate miR-181b, suppressing NOX2-mediated ROS and improving endothelial function. | NOX2 | [44] |
| miR-1-3p | AGEs downregulate miR-1-3p, activating the MLCK/p-MLC pathway to induce endothelial senescence and barrier dysfunction. | MLCK; p-MLC | [45] |
| miR-128 | MEG3 inhibits miR-128 to upregulate Girdin, promoting antioxidation and delaying endothelial senescence. | Girdin | [46] |
| miR-34b | IDH2 deficiency upregulates miR-34b/c, inhibiting mitophagy and reducing Sirt3, inducing ROS accumulation and senescence. | Sirt3 | [47] |
| miR-200c | ROS induces miR-200c, which inhibits ZEB1 and promotes endothelial growth arrest, apoptosis, and senescence. | ZEB1 | [48] |
| miR-4500 | Oxidative stress induces OIP5-AS1, which inhibits miR-4500 and upregulates ARG2, promoting endothelial dysfunction. | ARG2 | [49] |
| miR-29 | TGF-β/Smad signaling upregulates miR-29a/c, suppressing Suv4-20h and impairing DNA repair to drive senescence. | Suv4-20h | [50] |
| miR-1 | Hepatocyte-derived EVs carrying miR-1 suppress KLF4 and activate NF-κB, inducing endothelial inflammation and atherosclerosis. | KLF4 | [51] |
| miR-216a | miR-216a promotes endothelial senescence and inflammation by targeting Smad3 and destabilizing IκBα. | Smad3 | [52] |
| miR-34a-5p | miR-34a-5p activates p53 and p16 pathways to drive endothelial inflammation and senescence. | p53; p16 | [53] |
| miR-181b-5p | Low shear stress downregulates miR-181b-5p, relieving suppression of STAT3 and activating NLRP3 inflammasome. | STAT3 | [54] |
| miR-34a | Kallistatin upregulates Let-7g and suppresses miR-34a to enhance SIRT1/eNOS and antioxidant enzymes. | SIRT1; eNOS | [55] |
| miR-27a-3p | miR-27a-3p suppresses GRIN2D and the PKC/MEK/ERK pathway to alleviate endothelial oxidative stress and senescence. | GRIN2D | [56] |
| miR-25-3p | miR-25-3p targets TULA-2 to activate SYK/VEGFR-2 phosphorylation and improve angiogenesis in aging. | SYK; VEGFR-2 | [57] |
| miR-217 | miR-21 and miR-217 in EVs suppress DNMT1 and SIRT1 in recipient cells to induce senescence via paracrine signaling. | DNMT1; SIRT1 | [26] |
| miR-181b | miR-181b targets MAP3K3 to inhibit MAPK signaling and reduce oxidative stress-induced endothelial apoptosis. | MAP3K3 | [58] |
| miR-217 | miR-217 suppresses SIRT1 and activates p53, impairing endothelial proliferation and angiogenesis. | SIRT1 | [59] |
| miR-217 | miR-217 inhibits VEGF/apelin/eNOS signaling, impairing NO generation and promoting dysfunction. | eNOS; VEGF | [42] |
| miR-155-5p | KRGE induces HO-1 and suppresses NF-κB/miR-155-5p to restore eNOS and delay senescence. | eNOS | [60] |
| miR-10A | miR-10A and miR-21 suppress HMGA2, activating p16/p19 to induce EPC senescence and impair repair. | HMGA2 | [61] |
| miR-409 | miR-409 suppresses CCL5/VEGF signaling in EPCs, reducing angiogenesis and promoting senescence. | VEGF | [62] |
| Hsa-miR-409-3p | miR-409-3p targets PPP2CA to activate p38/JNK and impair EPC angiogenesis, promoting senescence. | PPP2CA | [63] |
| miR-15b-5p | MALAT1 sponges miR-15b-5p to upregulate MAPK1 and activate mTOR, suppressing EPC autophagy and increasing inflammation. | MAPK1 | [64] |
| miR-361-5p | miR-361-5p targets cytoskeletal/ECM genes; restoration reduces senescence but requires precise control. | [65] | |
| miR-146a | MSC-sEV-delivered miR-146a suppresses Src kinase and stabilizes VE-cadherin, reducing inflammation and restoring angiogenesis. | Src | [66] |
| miR-145 | miR-145 suppresses Sema3a to maintain the neurovascular interface; its downregulation in aging impairs innervation and function. | Sema3a | [67] |
| miR1204 | miR-1204 downregulates MYLK, promoting SASP and inflammatory phenotype in VSMCs, exacerbating vascular aging. | MYLK | [68] |
| miR-34a-5p | miR-34a-5p suppresses MARCHF8, increasing ADAM10, leading to endothelial dysfunction and senescence. | MARCHF8 | [69] |
| miR-199a-3p | High glucose downregulates miR-199a-3p, relieving DDR1 inhibition and activating p53/p21 to induce senescence. | DDR1 | [70] |
| miR-214 | miR-214 in EC-derived exosomes suppresses ATM in recipient cells to reduce senescence and promote angiogenesis. | ATM | [71] |
| LncRNA | Regulatory Mechanisms | Target(s) | |
|---|---|---|---|
| LINC01235 | Inhibits autophagy via miR-224-3p/RABEP1 | miR-224-3p; RABEP1 | [72] |
| TCONS_02443383 | Activates PPAR and adhesion genes | PPAR | [73] |
| uc003pxg.1 | Enhances TGF-β1/α-SMA via miR-339-5p | miR-339-5; TGF-β1; α-SMA | [74] |
| NORAD | Promotes EC proliferation, suppresses ferroptosis via miR-106a/CCND1 | miR-106a; CCND1 | [75] |
| linc-ROR | Promotes EndMT via miR-145/Smad3 axis | miR-145; Smad3 | [76] |
| MAGOH-DT | Enhances TGF-β2 translation via HNRPC binding | HNRPC; TGF-β2 | [77] |
| PVT1 | Promotes EC repair via miR-532-3p/MAPK1 | miR-532-3p; MAPK1 | [78] |
| LUCAT1 | Resists oxidative stress via miR-6776-5p/LRRC25 | miR-6776-5p; LRRC25 | [79] |
| Meg3 | Induces EC senescence via p21/p16 and mitochondrial dysfunction | p21; p16 | [80] |
| NORAD | Blocks IL-8/NF-κB/p53-p21 to inhibit apoptosis and senescence | IL-8; NF-κB; p53; p21 | [81] |
| MIR181A1HG | Activates NLRP3 inflammasome via Foxp1/NF-κB | Foxp1; NF-κB/p65; NLRP3 | [82] |
| H19 | Stabilizes H19 via METTL3-mediated m6A, promotes pyroptosis | IGF2BP2 | [83] |
| SMANTIS | Modulates RUNX1 binding via Alu elements | RUNX1; EP300/CBFB | [84] |
| MALAT1 | Promotes autophagy, suppresses adhesion via miR-30b-5p/ATG5 | miR-30b-5p; ATG5 | [85] |
| MIR4697HG | Inhibits EC damage via FUS/ANXA5 axis | FUS; ANXA5 | [86] |
| MALAT1 | Regulates VSMC and macrophage behavior in AS | EGR1-ELK1-ERK; KLF4 | [87] |
| LASER | Inhibits LDL clearance via PCSK9/LDLR suppression | PCSK9; PCSK9 | [88] |
| H19 | Blocks STAT3 signaling to prevent EC senescence | STAT3 | [89] |
| RAMP2-AS1 | Cis-activation of RAMP2 and IL-8 regulation via SFPQ | ADM-RAMP2 | [90] |
| lincRNA-p21 | Inhibits autophagy, stabilizes junctions via PI3K/AKT/mTOR | PI3K/AKT/mTOR; miR-101-3p | [91] |
| PRKAG2-AS1 | Activates PRKAG2, suppresses inflammation and apoptosis | PRKAG2 | [92] |
| H19 | Promotes EPC regeneration via miR-107/FADD, inhibits pyroptosis | miR-107; FADD | [93] |
| CircRNA | Regulatory Mechanisms | Target(s) | |
|---|---|---|---|
| Circ_0000231 | Sponges miR-590-5p to upregulate TXNIP and activate NF-κB signaling | TXNIP; NF-κB | [112] |
| Circ_0001148 | Sponges miR-218-5p to upregulate JMY and promote EndMT | miR-218-5p; JMY | [113] |
| Circ_0003204 | Increases E-cadherin and HDAC9 by sponging miR-942-5p | miR-942-5p; HDAC9 | [114] |
| Circ_0005699 | Regulates the miR-450b-5P/NFKB1 axis | miR-450b-5P; NFKB1 | [115] |
| Circ_0005699 | Sponges miR-384 to upregulate ASPH and promote ox-LDL-induced EC injury | miR-384; ASPH | [116] |
| Circ_0026218 | Sponges miR-188-3p to upregulate TLR4, activates NF-κB and propagates via exosomes | miR-188-3p; TLR4 | [117] |
| Circ_0074673 | Regulates the miR-1200/MEOX2 axis | miR-1200; MEOX2 | [118] |
| Circ_0086296 | Forms circ_0086296/miR-576-3p/IFIT1/STAT1 feedback loop | miR-576-3p; IFIT1; STAT1 | [119] |
| Circ-AFF1 | Regulates the miR-516b/SAV1/YAP1 axis | miR-516b; SAV1; YAP1 | [120] |
| Circ-CHMP5 | Sponges miR-532-5p to upregulate ROCK2 and promote EC injury | miR-532-5p; ROCK2 | [121] |
| Circ-COL1A2 | Regulates the miR-29b/VEGF axis | miR-29b; VEGF | [122] |
| Circ-DLGAP4 | Sponges miR-134-5p to upregulate PTPN4 and induce autophagy, reduce apoptosis/inflammation | miR-134-5p; PTPN4 | [123] |
| Circ-GNAQ | Sponges miR-146a-5p to upregulate PLK2 and delay EC senescence | miR-146a-5p; PLK2 | [124] |
| Circ-GSE1 | Regulates the miR-323-5p/NRP1 axis | miR-323-5p; NRP1 | [125] |
| Circ-PSMB1 | Sponges miR-624-3p to upregulate ASC and activate NLRP3 inflammasome | miR-624-3p; NLRP3 | [126] |
| Circ-PVT1 | Regulates the miR-24-3p/CDK4/pRb pathway | miR-24-3p; CDK4; pRb | [127] |
| Circ-RBCK1 | Sponges miR-133a to block pathogenic targeting under statin stimulation | miR-133a | [128] |
| CircRNA-LONP2 | Sponges miR-200a-3p to upregulate Keap1/YAP1/EZH2, suppress Nrf2/HO-1, promote stress/inflammation | miR-200a-3p; Keap1; YAP1 | [129] |
| CircRNA-PTPRA | Sponges miR-671-5p to modulate inflammation and apoptosis | miR-671-5p | [130] |
| Circ-RSF1 | Regulates the miR-758/CCND2 and miR-135b-5p/HDAC1 axes | miR-758; CCND2 | [131] |
| Circ-SQSTM1 | Sponges miR-23b-3p to upregulate Sirt1; promotes FOXO1 mRNA export and Sirt1 transcription | miR-23b-3p; Sirt1 | [132] |
| Circ-USP36 | Sponges miR-98-5p to elevate VCAM1; sponges miR-637 to enhance WNT4 | miR-98-5p; VCAM1 | [133] |
| Circ-ZBTB46 | Stabilizes hnRNPA2B1, activates PTEN/AKT/mTOR, enhances proliferation/migration, inhibits apoptosis | hnRNPA2B1 | [134] |
| miRNA | Regulatory Mechanisms | Target(s) | |
|---|---|---|---|
| miR-106a-5p | circHIPK3-enriched exosomes from ECs promote VSMC proliferation and inhibit apoptosis via miR-106a-5p/Foxo1/Vcam1 axis. | Foxo1; Vcam1 | [135] |
| miR-199a-5p | miR-199a-5p upregulation promotes ROS by repressing Sirt1, exacerbating Ang II-induced VSMC senescence in AAA. | Sirt1 | [32] |
| miR-200c | miR-200c disrupts SUMOylated KLF4 feedback loop by inhibiting Ubc9 and KLF4, suppressing VSMC proliferation and remodeling. | Ubc9; KLF4 | [136] |
| miR-214 | miR-214 suppresses Quaking to impair angiogenesis and promote VSMC senescence, serving as a vascular aging biomarker. | Quaking | [137] |
| miR-30a-3p | miR-30a-3p targets ROCK2 to suppress VSMC proliferation, migration, and phenotypic switching in ASO. | ROCK2 | [138] |
| miR-22 | Palmitic acid upregulates miR-22 to inhibit EVI1, preventing VSMC synthetic phenotype switching. | EVI1 | [34] |
| miR-181b-5p | miR-181b-5p downregulation derepresses HMGB1, driving VSMC phenotype switching and vascular remodeling in hypertension. | HMGB1 | [139] |
| miR-143 | MEF2A induces miR-143 to inhibit AKT pathway, promoting H2O2-induced VSMC senescence. | AKT | [140] |
| miR-542-3p | miR-542-3p targets BMP7 to suppress osteogenic differentiation; its downregulation promotes VSMC calcification in aging. | BMP7 | [141] |
| miR-30a | Rapamycin downregulates miR-30a to activate Beclin1-mediated autophagy and inhibit VSMC senescence. | Beclin1 | [142] |
| miR-665 | GAS5 sponges miR-665 to derepress SDC1, delaying VSMC senescence and protecting against vascular aging. | SDC1 | [143] |
| LncRNA | Regulatory Mechanisms | Target(s) | |
|---|---|---|---|
| MAGI2-AS3 | Sponges miR-525-5p to promote pathological VSMC proliferation and migration | miR-525-5p | [155] |
| MBNL1-AS1 | Sponges miR-424-5p to modulate MAPK/PI3K-Akt signaling | miR-424-5p; MAPK; PI3K-Akt | [156] |
| MYOSLID | Sponges miR-29c-3p to suppress Ang II-induced VSMC migration, proliferation, and apoptosis | miR-29c-3p | [109] |
| MIAT | Sponges miR-326 to enhance MCP-1 expression and VSMC migration | miR-326; MCP-1 | [157] |
| JPX | Enhancer-like RNA that activates SASP via chromatin remodeling and the STING pathway | SASP; VDAC1 | [110] |
| NEAT1 | Guides EZH2 to repress P16/P21/TIMP3 via histone methylation | EZH2; P16, P21 and TIMP3 | [111] |
| SMILR | Acts in cis to scaffold chromatin and promote HAS2 transcription | CENPF | [158] |
| MIAT | Controls proliferation, apoptosis, and phenotypic switching in advanced AS | EGR1-ELK1-ERK; KLF4 | [159] |
| CircRNA | Regulatory Mechanisms | Target(s) | |
|---|---|---|---|
| Circ-NRG-1 | miR-193b-5p/NRG-1 signaling modulation | miR-193b-5p; NRG-1 | [160] |
| Circ-MAP3K5 | miR-22-3p/TET2 axis regulation | miR-22-3p; TET2 | [161] |
| Circ-ACTA2 | NF-κB/NLRP3 pathway activation | NF-κB; NLRP3 | [162] |
| Circ-SETD2(14,15) | HuR/C-FOS axis regulation by circSETD2(14,15)-encoded protein | p-414aa; HuR; C-FOS | [145] |
| Circ-HIPK3 | DRP1/ROS-mediated mitochondrial fragmentation | DRP1 | [163] |
| Circ-XYLT1 | PTBP1-dependent chemokine signaling modulation | PTBP1 | [150] |
| Circ-Esyt2 | PCBP1-mediated p53β splicing regulation | p53β; PCBP1 | [164] |
| Circ-Lrp6 | miR-145 repression | miR-145 | [165] |
| Circ-TEX14 | miR-6509-3p/THAP1 pathway regulation | miR-6509-3p; THAP1 | [166] |
| Circ-Diaph3 | IGF-1 pathway activation via miR-148a-5p inhibition | miR-148a-5p; IGF1 | [167] |
| Circ-ZXDC | miR-125a-3p/ABCC6 signaling regulation | miR-125a-3p; ABCC6 | [168] |
| Circ-CHFR | miR-370/FOXO1/Cyclin D1 modulation | miR-370; FOXO1; Cyclin D1 | [169] |
| Circ-WDR77 | miR-124/FGF2 signaling regulation | miR-124; FGF2 | [170] |
| Circ-ABCA1 | miR-885-5p/ROCK2 axis control in VSMCs | miR-885-5p; ROCK2 | [153] |
| Circ-LARP1B | cAMP pathway suppression via PDE4C induction | PDE4C; cAMP | [149] |
| Circ-SMAD3 | hnRNPA1 degradation and p53γ splicing regulation | hnRNPA1; p53γ | [146] |
| Circ-TLK1 | miR-513a-3p/KLF4 axis modulation | miR-513a-3p; KLF4 | [154] |
| Circ-ZBTB46 | hnRNPA2B1 stabilization and PTEN/AKT/mTOR pathway regulation | hnRNPA2B1; PTEN/AKT/mTOR | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Xiao, X.; Zhou, C.; Zhang, Y.; Rhee, J.; Li, H. The Dark Side of Vascular Aging: Noncoding Ribonucleic Acids in Heart Failure with Preserved Ejection Fraction. Cells 2025, 14, 1269. https://doi.org/10.3390/cells14161269
Chen J, Xiao X, Zhou C, Zhang Y, Rhee J, Li H. The Dark Side of Vascular Aging: Noncoding Ribonucleic Acids in Heart Failure with Preserved Ejection Fraction. Cells. 2025; 14(16):1269. https://doi.org/10.3390/cells14161269
Chicago/Turabian StyleChen, Jianning, Xiao Xiao, Charles Zhou, Yajing Zhang, James Rhee, and Haobo Li. 2025. "The Dark Side of Vascular Aging: Noncoding Ribonucleic Acids in Heart Failure with Preserved Ejection Fraction" Cells 14, no. 16: 1269. https://doi.org/10.3390/cells14161269
APA StyleChen, J., Xiao, X., Zhou, C., Zhang, Y., Rhee, J., & Li, H. (2025). The Dark Side of Vascular Aging: Noncoding Ribonucleic Acids in Heart Failure with Preserved Ejection Fraction. Cells, 14(16), 1269. https://doi.org/10.3390/cells14161269







