Adenosine A2a Receptor Stimulation Mitigates Periodontitis and Is Mitoprotective in Gingival Fibroblasts Promoting Cellular Resilience
Abstract
1. Introduction
2. Methods
2.1. Animal Ligature-Induced Periodontitis (LIP) and Adenosine A2aR Treatment in Vivo
2.2. Primary Murine Fibroblast Cell Culture and Treatments
2.3. RNA Isolation, cDNA Synthesis, and Quantitative PCR (RT-qPCR)
2.4. ELISA
2.5. Western Blot
2.6. Antibodies
2.7. Oxygen Consumption Rate (OCR) Measurements via Seahorse Assay
2.8. Mitotracker Red and CellROX Green Staining
2.9. Assessment of Mitochondrial Membrane Potential
2.10. Mitochondrial DNA (mtDNA) Copy Number Quantification
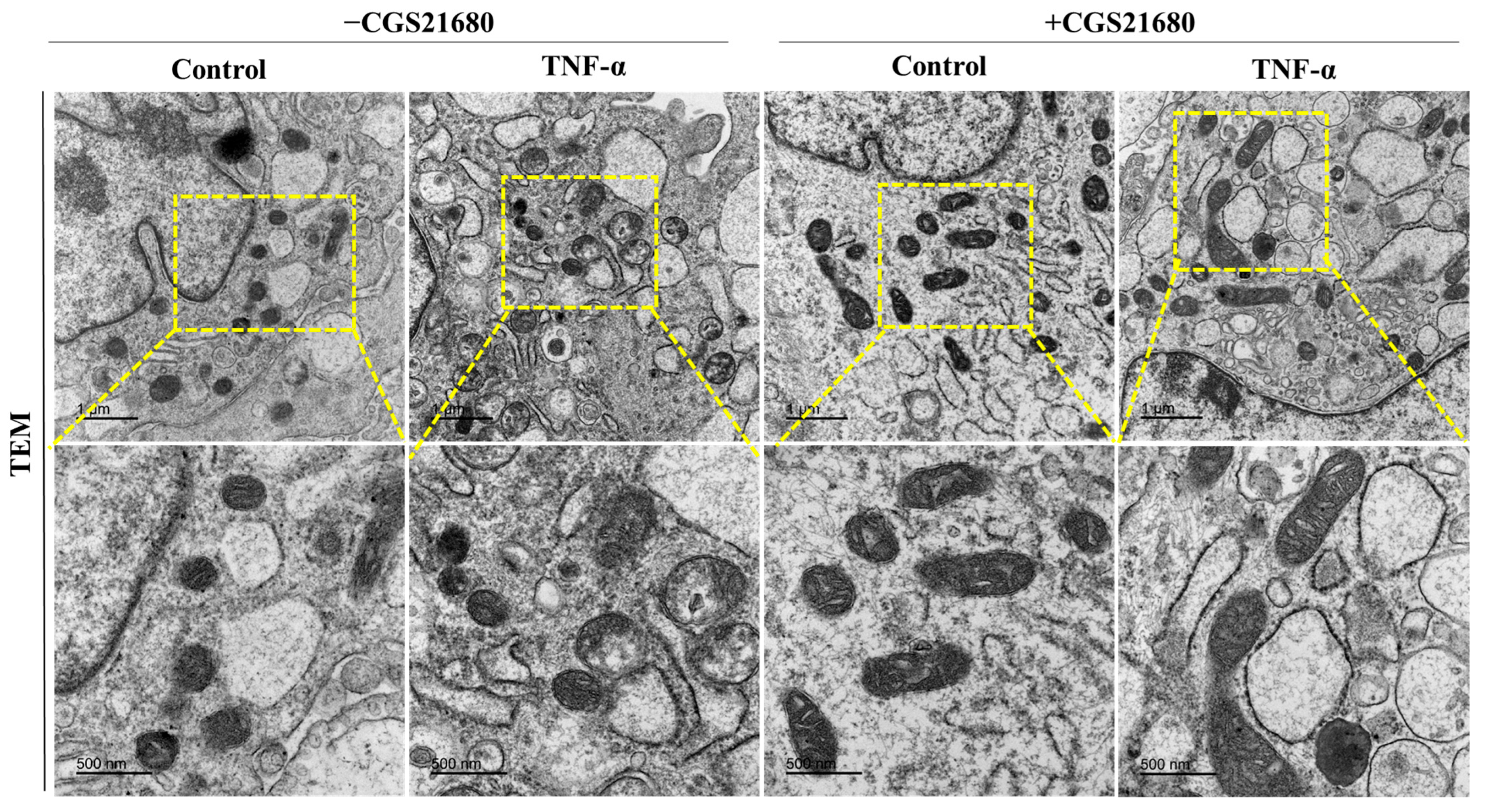
2.11. Transmission Electron Microscopy Analysis
2.12. Statistical Analysis
3. Results
3.1. Adenosine A2a Receptor Stimulation Is Protective to Alveolar Bone Loss and Mitigates Gingival Inflammation
3.2. A2aR Stimulation Promotes Healthy Mitochondrial Function
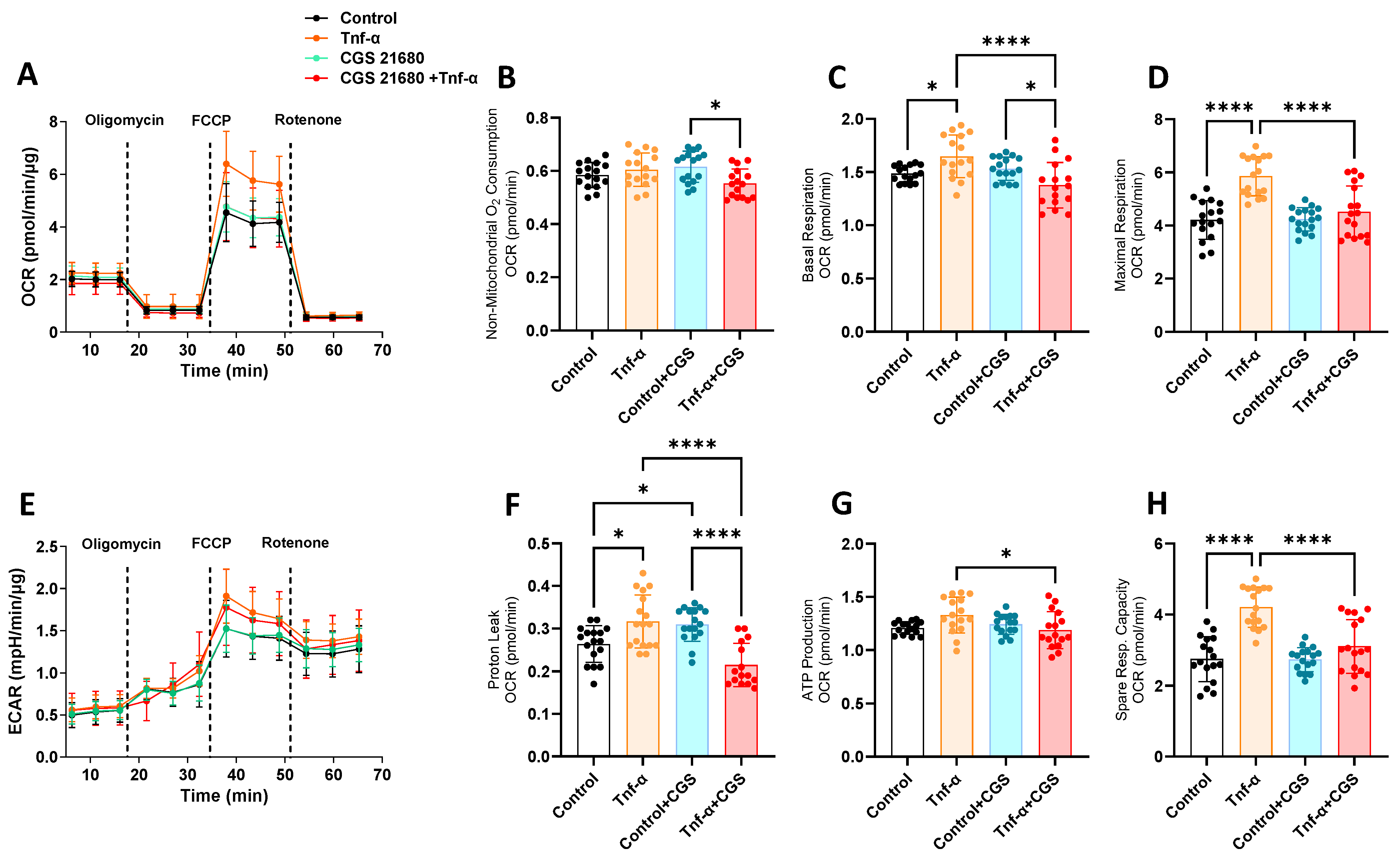
3.3. A2aR Stimulation Leads to Decreased Tnf-α-Induced Mitochondrial Stress and Elongated Mitochondria
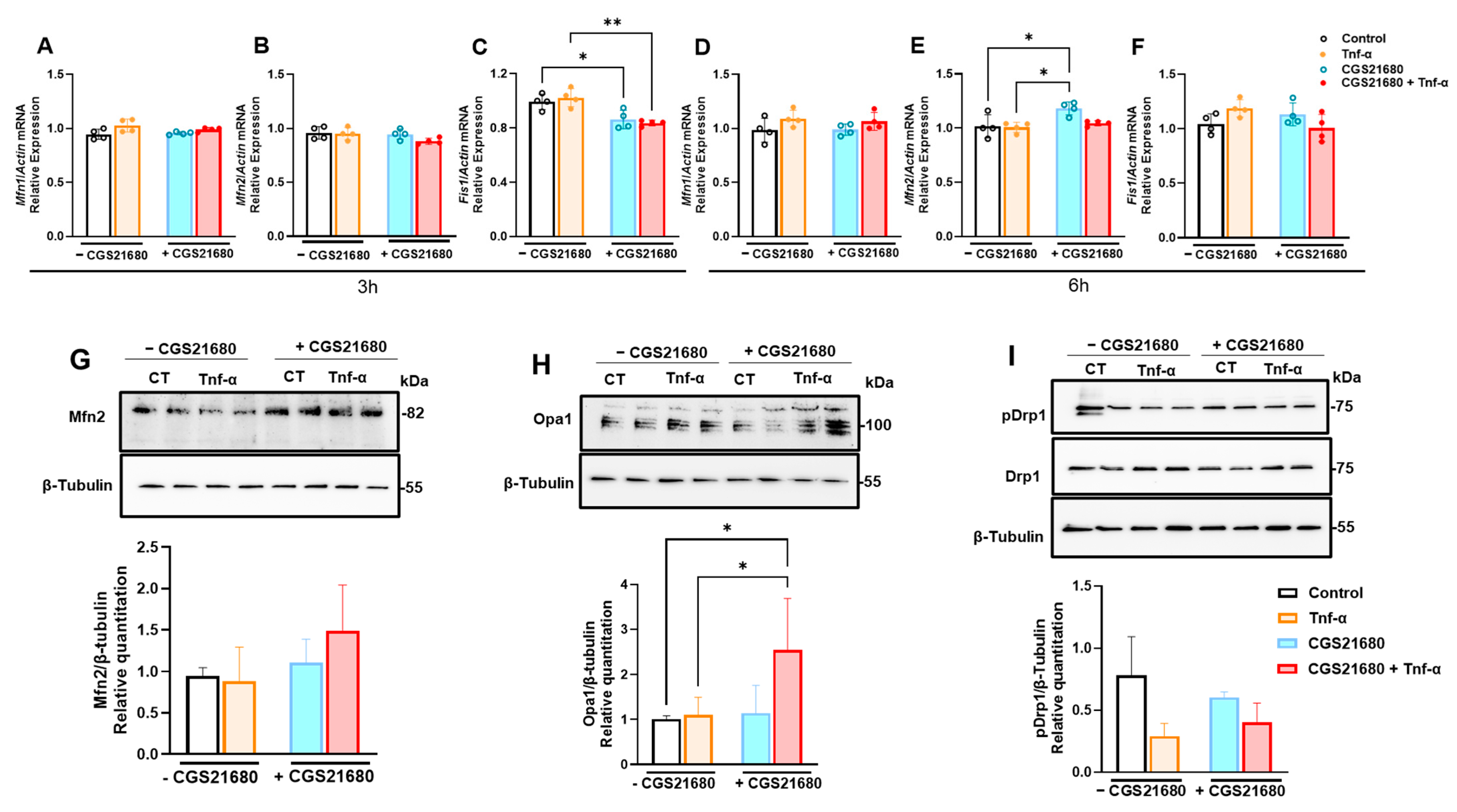
3.4. Mitoprotective Effect of A2aR Stimulation Is Due to Improved Mitochondrial Dynamics Through Increased Mitochondrial Fusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darveau, R.P.; Tanner, A.; Page, R.C. The microbial challenge in periodontitis. Periodontol. 2000 1997, 14, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pinedo, A.; Solbiati, J.O.; Teles, F.; Yanping, Z.; Frias-Lopez, J. Longitudinal host-microbiome dynamics of metatranscription identify hallmarks of progression in periodontitis. Microbiome 2025, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. Host modulation: Controlling the inflammation to control the infection. Periodontol. 2000 2017, 75, 317–329. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Sabbah, W.; Netuveli, G.; Donos, N.; Hingorani, A.D.; Deanfield, J.; Tsakos, G. Association of the metabolic syndrome with severe periodontitis in a large U.S. population-based survey. J. Clin. Endocrinol. Metab. 2008, 93, 3989–3994. [Google Scholar] [CrossRef]
- Marruganti, C.; Suvan, J.E.; D’Aiuto, F. Periodontitis and metabolic diseases (diabetes and obesity): Tackling multimorbidity. Periodontol. 2000 2023. [Google Scholar] [CrossRef]
- Villoria, G.E.M.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal disease: A systemic condition. Periodontol. 2000 2024, 96, 7–19. [Google Scholar] [CrossRef]
- Paladines, N.; Dawson, S.; Ryan, W.; Serrano-Lopez, R.; Messer, R.; Huo, Y.; Cutler, C.W.; Ramos-Junior, E.S.; Morandini, A.C. Metabolic reprogramming through mitochondrial biogenesis drives adenosine anti-inflammatory effects: New mechanism controlling gingival fibroblast hyper-inflammatory state. Front. Immunol. 2023, 14, 1148216. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Junior, E.S.; Pedram, M.; Lee, R.E.; Exstrom, D.; Yilmaz, O.; Coutinho-Silva, R.; Ojcius, D.M.; Morandini, A.C. CD73-dependent adenosine dampens interleukin-1beta-induced CXCL8 production in gingival fibroblasts: Association with heme oxygenase-1 and adenosine monophosphate-activated protein kinase. J. Periodontol. 2020, 91, 253–262. [Google Scholar] [CrossRef]
- Ramos-Junior, E.S.; Dawson, S.; Ryan, W.; Clinebell, B.; Serrano-Lopez, R.; Russell, M.; Brumbaugh, R.; Zhong, R.; Goncalves Fernandes, J.; Shaddox, L.M.; et al. The protective role of CD73 in periodontitis: Preventing hyper-inflammatory fibroblasts and driving osteoclast energy metabolism. Front. Oral Health 2023, 4, 1308657. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Hasko, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Hasko, G.; Cronstein, B. Regulation of inflammation by adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef]
- Mediero, A.; Cronstein, B.N. Adenosine and bone metabolism. Trends Endocrinol. Metab. 2013, 24, 290–300. [Google Scholar] [CrossRef]
- Sitkovsky, M.V. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem. Pharmacol. 2003, 65, 493–501. [Google Scholar] [CrossRef]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, K.A.; Boodoo, S.; Collins, S.; Black, K.E.; Chan-Li, Y.; Zarek, P.; Powell, J.D.; Horton, M.R. The adenosine a2a receptor inhibits matrix-induced inflammation in a novel fashion. Am. J. Respir. Cell Mol. Biol. 2009, 40, 251–259. [Google Scholar] [CrossRef]
- Castro, C.M.; Corciulo, C.; Solesio, M.E.; Liang, F.; Pavlov, E.V.; Cronstein, B.N. Adenosine A2A receptor (A2AR) stimulation enhances mitochondrial metabolism and mitigates reactive oxygen species-mediated mitochondrial injury. FASEB J. 2020, 34, 5027–5045. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Harris, G.; Leist, M.; Hartung, T. Cellular resilience. Altex 2015, 32, 247–260. [Google Scholar] [CrossRef]
- Morandini, A.C.; Ramos-Junior, E.S. Mitochondrial function in oral health and disease. J. Immunol. Methods 2024, 532, 113729. [Google Scholar] [CrossRef] [PubMed]
- Cherezova, A.; Sudarikova, A.; Vasileva, V.; Iurchenko, R.; Nikiforova, A.; Spires, D.R.; Zamaro, A.S.; Jones, A.C.; Schibalski, R.S.; Dong, Z.; et al. The effects of the atrial natriuretic peptide deficiency on renal cortical mitochondrial bioenergetics in the Dahl SS rat. FASEB J. 2024, 38, e23891. [Google Scholar] [CrossRef]
- Lew, S.Y.; Mohd Hisam, N.S.; Phang, M.W.L.; Syed Abdul Rahman, S.N.; Poh, R.Y.Y.; Lim, S.H.; Kamaruzzaman, M.A.; Chau, S.C.; Tsui, K.C.; Lim, L.W.; et al. Adenosine Improves Mitochondrial Function and Biogenesis in Friedreich’s Ataxia Fibroblasts Following L-Buthionine Sulfoximine-Induced Oxidative Stress. Biology 2023, 12, 559. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Scorrano, L. Determinants and outcomes of mitochondrial dynamics. Mol. Cell 2023, 83, 857–876. [Google Scholar] [CrossRef]
- Mediero, A.; Kara, F.M.; Wilder, T.; Cronstein, B.N. Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am. J. Pathol. 2012, 180, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Mediero, A.; Wilder, T.; Shah, L.; Cronstein, B.N. Adenosine A(2A) receptor (A2AR) stimulation modulates expression of semaphorins 4D and 3A, regulators of bone homeostasis. FASEB J. 2018, 32, 3487–3501. [Google Scholar] [CrossRef]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, M.J.; Kweon, D.K.; Lim, S.T.; Lee, S.J. Polydeoxyribonucleotide Activates Mitochondrial Biogenesis but Reduces MMP-1 Activity and Melanin Biosynthesis in Cultured Skin Cells. Appl. Biochem. Biotechnol. 2020, 191, 540–554. [Google Scholar] [CrossRef]
- Toller-Kawahisa, J.E.; Viacava, P.R.; Palsson-McDermott, E.M.; Nascimento, D.C.; Cervantes-Silva, M.P.; O’Carroll, S.M.; Zotta, A.; Damasceno, L.E.A.; Publio, G.A.; Forti, P.; et al. Metabolic reprogramming of macrophages by PKM2 promotes IL-10 production via adenosine. Cell Rep. 2025, 44, 115172. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, P.; Amati, A.; Perrone, M.; Petruzzella, V. The Balance of MFN2 and OPA1 in Mitochondrial Dynamics, Cellular Homeostasis, and Disease. Biomolecules 2025, 15, 433. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [Google Scholar] [CrossRef]
- Ihenacho, U.K.; Meacham, K.A.; Harwig, M.C.; Widlansky, M.E.; Hill, R.B. Mitochondrial Fission Protein 1: Emerging Roles in Organellar Form and Function in Health and Disease. Front. Endocrinol. 2021, 12, 660095. [Google Scholar] [CrossRef]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial Dynamics, ROS, and Cell Signaling: A Blended Overview. Life 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morandini, A.C.; Dawson, S.; Paladines, N.; Adams, N.; Ramos-Junior, E.S. Adenosine A2a Receptor Stimulation Mitigates Periodontitis and Is Mitoprotective in Gingival Fibroblasts Promoting Cellular Resilience. Cells 2025, 14, 1266. https://doi.org/10.3390/cells14161266
Morandini AC, Dawson S, Paladines N, Adams N, Ramos-Junior ES. Adenosine A2a Receptor Stimulation Mitigates Periodontitis and Is Mitoprotective in Gingival Fibroblasts Promoting Cellular Resilience. Cells. 2025; 14(16):1266. https://doi.org/10.3390/cells14161266
Chicago/Turabian StyleMorandini, A. C., S. Dawson, N. Paladines, N. Adams, and E. S. Ramos-Junior. 2025. "Adenosine A2a Receptor Stimulation Mitigates Periodontitis and Is Mitoprotective in Gingival Fibroblasts Promoting Cellular Resilience" Cells 14, no. 16: 1266. https://doi.org/10.3390/cells14161266
APA StyleMorandini, A. C., Dawson, S., Paladines, N., Adams, N., & Ramos-Junior, E. S. (2025). Adenosine A2a Receptor Stimulation Mitigates Periodontitis and Is Mitoprotective in Gingival Fibroblasts Promoting Cellular Resilience. Cells, 14(16), 1266. https://doi.org/10.3390/cells14161266







