Mechanistic Insights and Clinical Implications of ELK1 in Solid Tumors: A Narrative Review
Abstract
1. Introduction
2. ELK1 in Solid Tumors and Metastatic Cells
2.1. Lung Cancer
| Cancer | lncRNA | Regulation by ELK1 | Mechanism of Action | Outcome | Ref. |
|---|---|---|---|---|---|
| NSCLC | lncRNA HOXA10-AS | Upregulation | Positive regulation of Wnt/β-catenin signaling | Promotion of proliferation, migration, and EMT progression | [34] |
| CRC | lncRNA LBX2-AS1 | Upregulation | Blocking of the degradation of S100 calcium binding protein A11 (S100A11 or MLN70) Targets the tumor suppressor miR-491-5p | Promotion of proliferation, migration, and invasion | [37] |
| GC | lncRNA TRPM2-AS | Upregulation | Targets the tumor suppressor miR-195 | Promotion of invasion and increases in metastatic potential | [38] |
| GC | lncRNA MIR100HG | Upregulation | Positive regulation of TGF-β, Wnt/β-catenin, Hippo, and MAPK signaling | Promotion of proliferation, migration, and invasion | [39] |
| PTC | lncRNA LINC01638 | Upregulation | Positive regulation of intracellular signaling cascades leading to c-MYC activation | Promotion of cell cycle progression, proliferation, migration, and invasion | [40] |
| OC | lncRNA LBX2-AS1 | Upregulation | Targets the regulatory micro-RNA miR-4784 | Promotion of cancer progression | [41] |
| Glioma | lncRNA PSMB8-AS1 | Upregulation | lncRNA PSMB8-AS1 downregulates the expression of miR-574-5p | Promotion of proliferation | [42] |
| Osteosarcoma | lncRNA MIR100HG | Upregulation | Positive regulation of TGF-β, Wnt β-catenin, Hippo, and MAPK signaling | Promotion of cancer progression | [43] |
| Osteosarcoma | lncRNA LINC02381 | Upregulation | Targets the regulatory micro-RNA miR-503-5p | Promotion of proliferation | [44] |
2.2. Breast Cancer
| Cancer | Substance | Sample/Model | Effect(s) on ELK1 | Mechanism | Outcome | Ref. |
|---|---|---|---|---|---|---|
| NSCLC | Hexagonal selenium nanoparticles modified by siRNA (HSNM-siRNA) | Human NSCLC cell lines (A549, H1299, H520, and H1975) | Decrease in ELK1 expression | Inhibition of EGFR signaling | Cell cycle arrest, reduced viability, and apoptosis induction | [46] |
| NSCLC | Afatinib | Patient-derived tissues (47 NSCLC patients) and human NSCLC cell lines (H358, H441, A549 H460) | Decrease in ELK1 phosphorylation | Downregulation of CIP2A, promotion of PP2A activity, and decrease in AKT phosphorylation | Apoptosis induction | [49] |
| BC | Withaferin A (WFA) | Human BC cell lines (MCF-7, MDA-MB-231, T-47D, and MDA-MB-468) and mice xenografts | Increase in ELK1 phosphorylation | Upregulation of Death receptor 5 (DR5) expression | Apoptosis induction | [102] |
| BC | Grifolin | Human BC cell lines (MCF-7) | Decrease in ELK1 phosphorylation | Direct inhibition of ERK1/2 activity | Decrease in cell adhesion, migration and invasion | [104] |
| CRC | Curcumin | Human colon cancer cell lines (Moser cells, Caco-2 and HT-29) | Decrease in ELK1 expression | Reduction in the transcriptional activity of EGR1 | Reduced viability | [105] |
| CRC | 6-(Methyl-sulfinyl)-hexyl isothiocyanate (6-MSITC) | Human CRC cell line (HT-116) | Increase in ELK1 phosphorylation | Overexpression of DNA damage-inducible transcript 3 protein (DDIT3) and Death receptor 5 (DR5) | Apoptosis induction | [103] |
| CRC | Monensin | Human CRC cell lines (RKO and HCT-116) | Decrease in ELK1 phosphorylation | Inhibition of IGFR signaling | Decrease in cell cycle progression, proliferation, migration, and invasion | [106] |
| CRC | Gossypol | Human colon cancer cell line (COLO 205-ATCC CCL-222) | Decrease in ELK1 expression | Suppression of CLAUDIN1, ELK1, FAS, GAPDH, IL2, IL8, and ZFAND5 and upregulation of GLUT3 | Reduced the viability | [107] |
| CRC | CYC202 (R-roscovitine) | Human CRC cell lines (HT-29, NIH3T3, and KM-12) | Increase in ELK1 phosphorylation | Inhibition of transcription, possibly via the inhibition of both CDK7 and CDK9 complexes | Cell cycle arrest | [108] |
2.3. Colorectal Cancer
2.4. Prostate Cancer
| Cancer | Substance | Model/Sample | Effect(s) on ELK1 | Mechanism | Outcome | Ref. |
|---|---|---|---|---|---|---|
| PCa | KCI807 | Human PCa cell lines (LNCaP, VCaP, 22Rv1) and mice xenografts | ELK1-AR interaction disruption | Binding to AR, blocks the ELK1 binding and the ELK1-mediated recruitment of AR to chromatin | Growth inhibition | [140] |
| PCa | Quercetin | Human PCa cell lines (PC-3 and LNCaP) | Decrease in ELK1 phosphorylation | Modulation of MAPK- and AKT-associated signaling | Growth inhibition | [163] |
| PCa | Silodosin | Patient-derived tissues (150 PCa patients) and human PCa cell lines (PC-3, DU-145, LNCaP, and C4-2) | Decrease in ELK1 expression and phosphorylation | Selectively blocking of α1A-adrenergic receptors | Reduced migration | [164] |
| PCa | Silibidin | Human PCa cell lines (DU-145 and LNCaP) | Decrease in ELK1 phosphorylation | Inhibition of TGFα/EGFR signaling | Decreases in secreted and cellular TGFα | [166] |
| PCa | Procyanides (as parts of grape seed extract) | Human PCa cell line (DU-145) | Decrease in ELK1 phosphorylation | Inhibition of EGFR signaling and activation of JNK/c-Jun. | Apoptosis induction | [167] |
| PCa | Compound A (CpdA), | Human PCa cell lines (PC-3, DU-145 and LNCaP) | Decrease in ELK1 phosphorylation | Targeting of GR- and AR-mediated signaling via the inhibition of NF-κΒ, AP-1, ETS1, ELK1, SRF, CRE/ATF, and NFATc | Growth inhibition | [168] |
| PCa | Asiatic acid | Human PCa cell lines (PC-3, DU-145 and 22Rv1) | ELK1-MZF1 interaction disruption | Reduced transcription of SNAIL | Reduced migration | [169] |
| PCa | Phyllanthus plant extracts | Human PCa cell line (PC-3) | Decrease in ELK1 phosphorylation | Inhibition of RAS-RAF-MEK-MAPK-ELK1 signaling | Impairment of cell adhesion, apoptosis, metastasis, angiogenesis, and metabolism | [170] |
| PCa | Sulforaphane (SFN), phenethyl isothiocyanate (PEITC) and allyl isothiocyanate (AITC) | Human PCa cell line (PC-3) | Increase in ELK1 phosphorylation | ERK- and JNK-dependent activation of AP-1 | Reduced viability | [171] |
| PCa | Parthenolide (PTL) | Human PCa cell lines (PC-3, DU-145, VCaP and LAPC4), primary prostate TICs, and mice models | Decrease in ELK1 expression | Decrease in the levels of ELK1, FGFR2, PKCs, MEKs, MAPKs, CaMs | Reduced viability | [172] |
2.5. Gastric Cancer
| Cancer | Substance | Model/Sample | Effect(s) on ELK1 | Mechanism | Outcome | Ref. |
|---|---|---|---|---|---|---|
| GC | Doxycycline | Human GC cell lines (AGS, MKN-45 and KATO III) | Decrease in ELK1 phosphorylation | Inhibition of RAS-RAF-MEK-MAPK-ELK1 signaling | Growth inhibition | [193] |
| GC | Grifolin | Human GC cell line (MGC803) | Decrease in ELK1 phosphorylation | Direct inhibition of ERK1/2 activity | Decrease in cell adhesion, migration and invasion | [104] |
| HNSCC | Baicalein | Human OSCC cell lines (SCC-4 and CAL-27) | Decrease in ELK1 phosphorylation | Inhibition of RAS-RAF-MEK-MAPK-ELK1 signaling | Reduced proliferation and migration | [194] |
| HNSCC | Mebendazole (MBZ) | Human HNSCC lines (SCC-15 and CAL27) | Decrease in ELK1 phosphorylation | Modulation of cancer-associated pathways including ELK1/SRF, AP-1, STAT1/2, MYC/MAX | Decrease in cell cycle progression, proliferation, and migration | [178] |
| NPC | Grifolin | Human NPC cell lines (CNE1 and 5–8F) and mice xenografts | Decrease in ELK1 phosphorylation | Direct inhibition of ERK1/2 activity | Decrease in cell adhesion, migration and invasion | [104] |
| HCC | (JS-K) | Human HCC cell lines (HepG2 and Bel-7402)and mice xenografts | Decrease in ELK1 phosphorylation | Activation of JNK and p38 MAPK and inactivation of Raf/MEK/ERK signaling pathways | Apoptosis induction | [100] |
| HCC | TD52 | Human HCC cell lines (Sk-Hep1, PLC5, Hep3B and Huh-7) | Decrease in ELK1 phosphorylation | Inhibition of CIP2A and promotion of PP2A expression | Apoptosis induction | [53] |
| HCC | Withaferin A (WFA) | Mice models, xenografts, and human HCC cell lines (HepG2 and Huh7) | Increase in ELK1 phosphorylation | Crosstalk between ERK/RSK, ELK1, and DR5 | Apoptosis induction | [195] |
| HCC | 2-(2-mercaptoethanol)-3-methyl-1,4-naphthoquinone (Compound 5, Cpd 5) plus EGF | Human HCC cell line (Hep3B) | Increase in ELK1 phosphorylation | Prolonged MAPK phosphorylation | Growth inhibition | [196] |
| CC | Luteolin | Human CC cell line (HeLa) | Decrease in ELK1 expression | Decreases in the expression of several pro-survival genes including ELK1, MAPK14, MAP3K5, MAPK3 and MAPK1 | Proliferation inhibition, apoptosis induction | [197] |
| CC | Kaempferia parviflora plant extract | Human CC cell line (HeLa) | Decrease in ELK1 phosphorylation | Inhibition of MAPK and PI3K-Akt signaling | Apoptosis induction | [198] |
| CC | Tanshinone I | Human CC cell lines (HeLa and C4-1) | Decrease in ELK1 phosphorylation | Downregulation of KRAS expression | Inhibition of metastasis and cisplatin resistance | [199] |
| CC | Grifolin | Human CC cell line (HeLa) | Decrease in ELK1 phosphorylation | Direct inhibition of ERK1/2 activity | Decrease in cell adhesion, migration and invasion | [104] |
2.6. Esophageal, Head, Neck, and Laryngeal Cancers
2.6.1. Esophageal Adenocarcinoma
2.6.2. Esophageal Squamous Cell Carcinoma
2.6.3. Head and Neck Squamous Cell Carcinoma
2.6.4. Laryngeal Squamous Cell Carcinoma
2.6.5. Epidermoid Squamous Cell Carcinoma
2.6.6. Nasopharyngeal Carcinoma
2.7. Liver Cancer
2.8. Thyroid Cancer
2.9. Cervical Cancer
| Cancer | microRNA | Regulation by ELK1 | Mechanism of Action | Outcome | Ref. |
|---|---|---|---|---|---|
| NSCLC | miR-30c | Upregulation | Targets tumor suppressor genes such as NF1, RASA1, BID, RASSF8 | Drug resistance, cell migration and invasion | [35] |
| NSCLC | miR-21 | Upregulation | Targets tumor suppressor genes such as NF1, RASA1, BID, RASSF8 | Drug resistance, cell migration and invasion | [35] |
| BC | miR-200b | Downregulation | miR-200b, upon activation regulates the activity of both ETV4 and ELK1 through regulation of the PIN1-ERK1/2 pathway | Promotion of cancer cell survival during metastasis | [85] |
| CRC | miR-31-5p | Upregulation | Targets the CDIP gene expression | Promotion of metastasis and regulation of autophagy, and apoptosis | [123] |
| CRC | miR-181a-5p | Upregulation | Targets the SOCS3 gene expression | Promotion of TME remodeling | [126] |
| CRC | MIR17HG | Upregulation | Targets the miR-138-5p, a micro-RNA that downregulates HK1 | Promotion of glycolysis | [128] |
| CC | miR-130b-5p | Downregulation | ELK1 suppresses miR-130-5p | Promotion of proliferation | [245] |
| OC | miR-134 | Downregulation | ELK1 downregulates the expression of miR-134 | Drug resistance, cancer progression | [174] |
2.10. Bladder Cancer
| Cancer | Substance | Model/Sample | Effect(s) on ELK1 | Mechanism | Outcome | Ref. |
|---|---|---|---|---|---|---|
| BCa | Silodosin | Patient-derived tissues (from BCa patients) and human urothelial carcinoma cell lines (TCCSUP, UM-UC-3, and 5637) | Decrease in ELK1 expression and phosphorylation | Selectively blocking of α1A-adrenergic receptors and inhibition of RAS-RAF-MEK-MAPK-ELK1 signaling | Reduced viability and migration | [175] |
| BCa | Trametinib | Dog BCa organoids, mice xenografts | Decrease in ELK1 expression and phosphorylation | Inhibition of ERK1/2-mediated signaling and decrease in the levels of ELK1, MYC, SIK1, and PLA2G4A | Reduced viability | [256] |
| PaCa | Everolimus | Human PaCa cell lines (Panc-1 and PaCa) and mice xenografts | Bypass of ELK1’s suppressive activity | Inhibition of the PI3K-Akt signaling pathway and surpass the ELK1-imposed suppression of DEPTOR | Reduced viability and resensitization to gemcitabine | [257] |
| RCC | 6-anilino-5,8-quinolinequinone (LY83583) | Human RCC cell line (786-0) | Decrease in ELK1 phosphorylation | Dephosphorylation of ERK1/2, decline of activated ELK1 levels and subsequent downregulation of PTGS2 and BCL2L1. | Apoptosis induction | [258] |
| EC | Sorafenib | Human EC cell lines (HEC1A, HEC1B, and RL95-2) | Decrease in ELK1 phosphorylation | Dephosphorylation of ERK1/2, decline of activated ELK1 levels and subsequent downregulation of MCL1. | Apoptosis induction | [259] |
| Melanoma | Mebendazole combined with trametinib | Human melanoma cell lines derived from metastatic patients and, established human melanoma cell lines (BAK, BUL, and STU) and mice xenografts | Decrease in ELK1 phosphorylation | Inhibition of RAS-RAF-MEK-MAPK-ELK1 signaling | Decrease in cell cycle progression, proliferation, and migration | [260] |
| Melanoma | Fused naphthofuro [3,2-c] quinoline-6,7,12-triones and pyrano [3,2-c]quinoline-6,7,8,13-tetraones derivatives | In vitro study using the NCI-60 panel of tumor cell lines | Decrease in ELK1 phosphorylation | Inhibition of MAPK activation | Apoptosis induction | [261] |
| Melanoma | Paclitaxel | Human melanoma cell lines (A375 and BLM) | Increase in ELK1 phosphorylation | Persistent RAS-RAF-MEK-MAPK pathway activation | Apoptosis induction | [262] |
| Melanoma | Carvedilol | Human skin cancer (JB6 Cl 41-5a), human melanoma cell line (A375), and mice xenografts | Reversal of EGF-induced activation | ERK1/2 are phosphorylated in the cytoplasm and do not translocate to the nucleus | Melanoma prevention | [263,264] |
2.11. Pancreatic Cancer
2.11.1. Pancreatic Carcinoma
2.11.2. Insulinoma
2.12. Renal Cancer
2.13. Uterine Cancer
2.14. Melanoma
2.15. Ovarian Cancer
| Cancer | Substance | Model/Sample | Effect(s) on ELK1 | Mechanism | Outcome | Ref. |
|---|---|---|---|---|---|---|
| OC | E1A | Human OC cell lines (SKOV3.ip1 and OVCAR-3) | Decrease in ELK1 phosphorylation | Overexpression of PEA15, and inhibition of the translocation of ERK1/2 inside the nucleus | Tumorgenicity suppression | [301] |
| OC | 3,3′-Diindolylmethane (DIM) | Human OC cell lines (SKOV-3, OVCAR-3, and TOV-21G) | Decrease in ELK1 phosphorylation | Inhibition of EGFR-MAPK-ELK1 signaling | Growth inhibition | [302] |
| OC | Monensin | Patient-derived OC tissues and human OC cell lines (SKOV3 and HeyA8) | Decrease in ELK1 phosphorylation | Inhibition of EGFR-MAPK-ELK1 signaling and downregulation SRF, AP-1, NF-κB, and STAT3 activity and EGFR expression | Proliferation inhibition, apoptosis induction | [303] |
| OC | Niclosamide | Human OC cell lines (SKOV3 and HeyA8) | Decrease in ELK1 phosphorylation | Inhibition of IGFR signaling | Reduced cell growth and migration | [304] |
| Glioma | Amitriptyline | Rat glioma cell line (C6) | Increase in ELK1 phosphorylation | Phosphorylation of ERKs and JNKs | Increase in EGR1 transcription | [305] |
| GBM | Anisomycin | Human GBM cell line (U-87 MG) | Increase in ELK1 phosphorylation | Phosphorylation of all three major MAPK classes (ERK1/2, JNK, p38) | Increase in EGR1 transcription | [306] |
| GBM | LY294002 or wortmannin | Human GBM cell line (U-138) | Accumulation of phosphorylated ELK1 in the cytoplasm | AKT inhibition | Growth inhibition | [307] |
| GBM | UO126 | Human GBM cell line (U-138) | Decrease in ELK1 phosphorylation | MEK inhibition | Growth inhibition | [307] |
| GBM | FR180204 | Human GBM cell line (U-138) | Decrease in ELK1 phosphorylation | MAPK inhibition | Growth inhibition | [307] |
| GBM | Curcumin | Human GBM cell line (U-87MG) and rat glioma cell line (C6) | Increase in ELK1 phosphorylation | Phosphorylation of ERKs and JNKs, activation of EGR1, and upregulation of p21Waf1/Cip1 | Growth inhibition | [95] |
2.16. Gliomas
2.16.1. Glioma
2.16.2. Glioblastoma
2.17. Osteosarcoma
3. Conclusions
| Cancer | RNA | Effect(s) on ELK1 | Mechanism | Outcome | Ref. |
|---|---|---|---|---|---|
| BC | miR-135a | Downregulation | Reduction in ELK1/3 levels | Inhibition of proliferation | [86] |
| BC | miR-326 | Downregulation | Reduction in ELK1 levels | Inhibition of proliferation, colony formation, and invasion | [87,89] |
| BC | miR-330-5p | Downregulation | Reduction in ELK1 levels | Inhibition of proliferation and migration, and induction of apoptosis | [89] |
| CRC | miR-206 | Indirect downregulation | Downregulation of the Met/ERK/ELK1/HIF-1α/VEGF-A pathway | Angiogenesis inhibition | [111] |
| CRC | miR-873 | Downregulation | Binding to the 3′UTR of ELK1 and STRN4 mRNAs, inhibiting their translation | Inhibition of proliferation and migration | [124] |
| CRC | miR-382-5p | Downregulation | Reduction in ELK1 levels | Limits cancer progression | [125] |
| PCa | miRNA let-7a | Indirect downregulation | Downregulation of IGF1R’s expression and thus decreased ELK1 activation and c-FOS expression | Limits cancer progression | [161] |
| GC | miR-139-3p | Downregulation | Reduction in ELK1 levels | Limits GC tumorigenesis | [190] |
| ESCA | miR-29a-3p | Downregulation | Reduction in ELK1 levels | Inhibition of proliferation, migration, and invasion | [202] |
| LSCC | miR-340-3p | Downregulation | Reduction in ELK1 levels | Inhibition of proliferation, migration, colony formation, and invasion | [208] |
| HCC | miR-361-3p | Downregulation | Reduction in ELK1 levels | Inhibition of proliferation, migration, and invasion | [21] |
| CC | miR-197-3p | Downregulation | Reduction in ELK1 levels | Inhibition of cell cycle progression, reduction in viability, induction of apoptosis and autophagy | [243] |
| CC | miR-326 | Downregulation | Reduction in ELK1 levels | Inhibition of proliferation, colony formation, and invasion | [88] |
| CC | miR-130b-5p | Downregulation | Overexpression of miR-130b-5p leads to reduction in ELK1 levels | Inhibition of proliferation | [245] |
| CC | miR-330-5p | Downregulation | Reduction in ELK1 levels | Downregulation of ELK1-related gene expression | [90] |
| CC | miR-143-5p | Downregulation | Reduction in ELK1 levels | Limits cancer progression | [246] |
| BCa | miR-2682-5p | Downregulation | Reduction in ELK1 and lncRNA SNHG7 levels | Inhibition of cell proliferation, migration, and invasion | [267] |
| PaCa | miR-217 | Downregulation | Reduction in ELK1 levels | Limits cancer progression and resensitizes cells to gemcitabine | [277] |
| PaCa | miR-597-5p | Downregulation | Reduction in ELK1 levels | Induction of apoptosis, and inhibition of tumor growth | [279] |
| RCC | miR-139-3p | Downregulation | Reduction in ELK1 levels | Limits cancer progression | [286] |
| Osteosarcoma | miR-30b-3p | Downregulation | Binding to the 3′UTR of ELK1 and downregulation of its transcription | Inhibition of proliferation, migration, and invasion | [334] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-Suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Dakal, T.C.; Dhabhai, B.; Pant, A.; Moar, K.; Chaudhary, K.; Yadav, V.; Ranga, V.; Sharma, N.K.; Kumar, A.; Maurya, P.K.; et al. Oncogenes and Tumor Suppressor Genes: Functions and Roles in Cancers. MedComm 2024, 5, e582. [Google Scholar] [CrossRef] [PubMed]
- Stojchevski, R.; Sutanto, E.A.; Sutanto, R.; Hadzi-Petrushev, N.; Mladenov, M.; Singh, S.R.; Sinha, J.K.; Ghosh, S.; Yarlagadda, B.; Singh, K.K.; et al. Translational Advances in Oncogene and Tumor-Suppressor Gene Research. Cancers 2025, 17, 1008. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, K.; Viswakarma, N.; Rana, A.; Rana, B. Transcription Factors in Cancer Development and Therapy. Cancers 2020, 12, 2296. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, L.; Lin, R.Y.-T.; Müschen, M.; Koeffler, H.P. Core Transcriptional Regulatory Circuitries in Cancer. Oncogene 2020, 39, 6633–6646. [Google Scholar] [CrossRef]
- Babal, Y.K.; Sonmez, E.; Aksan Kurnaz, I. Nervous System-Related Gene Regulatory Networks and Functional Evolution of ETS Proteins across Species. Biosystems 2023, 227–228, 104891. [Google Scholar] [CrossRef]
- Gu, M.; Li, X.; Wu, R.; Cheng, X.; Zhou, S.; Gu, X. The Transcription Factor Ets1 Influences Axonal Growth via Regulation of Lcn2. Mol. Neurobiol. 2024, 61, 971–981. [Google Scholar] [CrossRef]
- Yi, L.; Li, J.; He, Y.; Wang, J.; Wang, M.; Guo, S.; Luo, M.; Wu, B.; Xu, M.; Tian, Q.; et al. ELK1 Inhibition Alleviates Amyloid Pathology and Memory Decline by Promoting the SYVN1-Mediated Ubiquitination and Degradation of PS1 in Alzheimer’s Disease. Exp. Mol. Med. 2025, 57, 1032–1046. [Google Scholar] [CrossRef]
- Sharrocks, A.D. The ETS-Domain Transcription Factor Family. Nat. Rev. Mol. Cell Biol. 2001, 2, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Soave, C.; Ducker, C.; Islam, N.; Kim, S.; Yurgelevic, S.; Nicely, N.I.; Pardy, L.; Huang, Y.; Shaw, P.E.; Auner, G.; et al. The Small Molecule Antagonist KCI807 Disrupts Association of the Amino-Terminal Domain of the Androgen Receptor with ELK1 by Modulating the Adjacent DNA Binding Domain. Mol. Pharmacol. 2023, 103, 211–220. [Google Scholar] [CrossRef]
- Lee, C.-J.; Hsu, L.-S.; Yue, C.-H.; Lin, H.; Chiu, Y.-W.; Lin, Y.-Y.; Huang, C.-Y.; Hung, M.-C.; Liu, J.-Y. MZF-1/Elk-1 Interaction Domain as Therapeutic Target for Protein Kinase Cα-Based Triple-Negative Breast Cancer Cells. Oncotarget 2016, 7, 59845–59859. [Google Scholar] [CrossRef]
- Kim, G.-C.; Lee, C.-G.; Verma, R.; Rudra, D.; Kim, T.; Kang, K.; Nam, J.H.; Kim, Y.; Im, S.-H.; Kwon, H.-K. ETS1 Suppresses Tumorigenesis of Human Breast Cancer via Trans-Activation of Canonical Tumor Suppressor Genes. Front. Oncol. 2020, 10, 642. [Google Scholar] [CrossRef]
- Turner, D.P.; Watson, D.K. ETS Transcription Factors: Oncogenes and Tumor Suppressor Genes as Therapeutic Targets for Prostate Cancer. Expert Rev. Anticancer Ther. 2008, 8, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawary, S.I.S.; Pallathadka, H.; Hjazi, A.; Zhumanov, Z.E.; Alazbjee, A.A.A.; Imad, S.; Alsalamy, A.; Hussien, B.M.; Jaafer, N.S.; Mahmoudi, R. ETS Transcription Factor ELK3 in Human Cancers: An Emerging Therapeutic Target. Pathol.-Res. Pract. 2023, 248, 154728. [Google Scholar] [CrossRef] [PubMed]
- Sizemore, G.M.; Pitarresi, J.R.; Balakrishnan, S.; Ostrowski, M.C. The ETS Family of Oncogenic Transcription Factors in Solid Tumours. Nat. Rev. Cancer 2017, 17, 337–351. [Google Scholar] [CrossRef]
- Daws, S.E.; Whittard, J.D.; Jacobs, M.M.; Ren, Y.; Mazloom, A.R.; Caputi, F.F.; Horvath, M.; Keller, E.; Ma’ayan, A.; Pan, Y.-X.; et al. ELK1 Transcription Factor Linked to Dysregulated Striatal Mu Opioid Receptor Signaling Network and OPRM1 Polymorphism in Human Heroin Abusers. Biol. Psychiatry 2013, 74, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.; Galan, B.; Vanhoutte, P.; Caboche, J. Elk-1 a Transcription Factor with Multiple Facets in the Brain. Front. Neurosci. 2011, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Alper, M.; Sav, F.N.; Keleş, Y.; Eroğlu, K.P.; Keskin, S.D.; Köçkar, F. STAT-3, ELK-1, and c- Jun Contributes IL-6 Mediated ADAMTS-8 Upregulation in Colorectal Cancer. Mol. Biol. Rep. 2025, 52, 246. [Google Scholar] [CrossRef]
- Kalampounias, G.; Zafeiropoulou, K.; Androutsopoulou, T.; Alexis, S.; Symeonidis, A.; Katsoris, P. The Transcription Axes ERK-Elk1, JNK-cJun, and JAK-STAT Promote Autophagy Activation and Proteasome Inhibitor Resistance in Prostate Cancer Cells. Curr. Issues Mol. Biol. 2025, 47, 352. [Google Scholar] [CrossRef]
- Hao, X.; Qian, X.; Xie, C.; Wang, Z.; Wang, X.; Ji, Y.; Zhang, X.; Li, Q.; Wan, B.; Cui, H.; et al. CircMFN2/miR-361-3p/ELK1 Feedback Loop Promotes Glutaminolysis and the Progression of Hepatocellular Carcinoma. Cancer Lett. 2025, 614, 217473. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Ding, Y.; Zhang, Z.; Feng, J.; Hu, J.; Chen, J.; Lian, Z.; Chen, Y.; Hu, K.; et al. BCL6 Is Regulated by the MAPK/ELK1 Axis and Promotes KRAS-Driven Lung Cancer. J. Clin. Investig. 2022, 132, e161308. [Google Scholar] [CrossRef]
- Cai, C.; Yao, S.; Zou, Y.; Lu, H.; Chen, X.; Wang, Y.; Zheng, K.; Zhu, F.; Wang, Y.; Xiong, H.; et al. KRASG12C Mutation-Induced TOPK Overexpression Contributes to Tumour Progression in Non-Small Cell Lung Cancer. J. Cell. Mol. Med. 2023, 27, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Herbert, K.J.; Ashton, T.M.; Prevo, R.; Pirovano, G.; Higgins, G.S. T-LAK Cell-Originated Protein Kinase (TOPK): An Emerging Target for Cancer-Specific Therapeutics. Cell Death Dis. 2018, 9, 1089. [Google Scholar] [CrossRef]
- Shen, C.; Liu, W.; Zhang, S.; Pu, L.; Deng, B.; Zeng, Q.; Chen, Z.; Wang, X. Downregulation of miR-541 Induced by Heat Stress Contributes to Malignant Transformation of Human Bronchial Epithelial Cells via HSP27. Environ. Res. 2020, 184, 108954. [Google Scholar] [CrossRef] [PubMed]
- Alam, H.; Li, N.; Dhar, S.S.; Wu, S.J.; Lv, J.; Chen, K.; Flores, E.R.; Baseler, L.; Lee, M.G. HP1γ Promotes Lung Adenocarcinoma by Downregulating the Transcription-Repressive Regulators NCOR2 and ZBTB7A. Cancer Res. 2018, 78, 3834–3848. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, M.; Xia, M.; Liu, Y.; Yan, J.; Ji, H.; Wang, G. Selective Requirement for Mediator MED23 in Ras-Active Lung Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E2813–E2822. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-T.; Zhang, T.; Su, F.; Li, Y.-L.; Shan, L.; Hou, X.-M.; Wang, R.-Z. ELK1 Promotes Epithelial-Mesenchymal Transition and the Progression of Lung Adenocarcinoma by Upregulating B7-H3. Oxidative Med. Cell. Longev. 2021, 2021, 2805576. [Google Scholar] [CrossRef]
- Wu, D.-M.; Deng, S.-H.; Zhou, J.; Han, R.; Liu, T.; Zhang, T.; Li, J.; Chen, J.-P.; Xu, Y. PLEK2 Mediates Metastasis and Vascular Invasion via the Ubiquitin-Dependent Degradation of SHIP2 in Non-Small Cell Lung Cancer. Int. J. Cancer 2020, 146, 2563–2575. [Google Scholar] [CrossRef]
- Khan, P.; Manna, A.; Saha, S.; Mohanty, S.; Mukherjee, S.; Mazumdar, M.; Guha, D.; Das, T. Aspirin Inhibits Epithelial-to-Mesenchymal Transition and Migration of Oncogenic K-Ras-Expressing Non-Small Cell Lung Carcinoma Cells by down-Regulating E-Cadherin Repressor Slug. BMC Cancer 2016, 16, 39. [Google Scholar] [CrossRef]
- Wu, K.; Zeng, J.; Zhou, J.; Fan, J.; Chen, Y.; Wang, Z.; Zhang, T.; Wang, X.; He, D. Slug Contributes to Cadherin Switch and Malignant Progression in Muscle-Invasive Bladder Cancer Development. Urol. Oncol. 2013, 31, 1751–1760. [Google Scholar] [CrossRef]
- Rudisch, A.; Dewhurst, M.R.; Horga, L.G.; Kramer, N.; Harrer, N.; Dong, M.; van der Kuip, H.; Wernitznig, A.; Bernthaler, A.; Dolznig, H.; et al. High EMT Signature Score of Invasive Non-Small Cell Lung Cancer (NSCLC) Cells Correlates with NFκB Driven Colony-Stimulating Factor 2 (CSF2/GM-CSF) Secretion by Neighboring Stromal Fibroblasts. PLoS ONE 2015, 10, e0124283. [Google Scholar] [CrossRef]
- Kossenkov, A.V.; Vachani, A.; Chang, C.; Nichols, C.; Billouin, S.; Horng, W.; Rom, W.N.; Albelda, S.M.; Showe, M.K.; Showe, L.C. Resection of Non-Small Cell Lung Cancers Reverses Tumor-Induced Gene Expression Changes in the Peripheral Immune System. Clin. Cancer Res. 2011, 17, 5867–5877. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Lu, J.; Zhao, H. ELK1-Induced Upregulation of lncRNA HOXA10-AS Promotes Lung Adenocarcinoma Progression by Increasing Wnt/β-Catenin Signaling. Biochem. Biophys. Res. Commun. 2018, 501, 612–618. [Google Scholar] [CrossRef]
- Shi, L.; Middleton, J.; Jeon, Y.-J.; Magee, P.; Veneziano, D.; Laganà, A.; Leong, H.-S.; Sahoo, S.; Fassan, M.; Booton, R.; et al. KRAS Induces Lung Tumorigenesis through microRNAs Modulation. Cell Death Dis. 2018, 9, 219. [Google Scholar] [CrossRef]
- Cho, C.-Y.; Huang, J.-S.; Shiah, S.-G.; Chung, S.-Y.; Lay, J.-D.; Yang, Y.-Y.; Lai, G.-M.; Cheng, A.-L.; Chen, L.-T.; Chuang, S.-E. Negative Feedback Regulation of AXL by miR-34a Modulates Apoptosis in Lung Cancer Cells. RNA 2016, 22, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Dai, W.; Zhang, J.; Li, Q.; Gu, B.; Song, Y.; Yang, X. ELK1-mediated Upregulation of lncRNA LBX2-AS1 Facilitates Cell Proliferation and Invasion via Regulating miR-491-5p/S100A11 Axis in Colorectal Cancer. Int. J. Mol. Med. 2021, 48, 138. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Chang, C.; Wang, B.-L.; Li, H. ELK1-Induced Upregulation of lncRNA TRPM2-AS Promotes Tumor Progression in Gastric Cancer by Regulating miR-195/HMGA1 Axis. J. Cell. Biochem. 2019, 120, 16921–16933. [Google Scholar] [CrossRef]
- Li, P.; Ge, D.; Li, P.; Hu, F.; Chu, J.; Chen, X.; Song, W.; Wang, A.; Tian, G.; Gu, X. CXXC Finger Protein 4 Inhibits the CDK18-ERK1/2 Axis to Suppress the Immune Escape of Gastric Cancer Cells with Involvement of ELK1/MIR100HG Pathway. J. Cell. Mol. Med. 2020, 24, 10151–10165. [Google Scholar] [CrossRef]
- Lv, P.; Xue, Y. ETS Like-1 Protein ELK1-Induced lncRNA LINC01638 Accelerates the Progression of Papillary Thyroid Cancer by Regulating Axin2 through Wnt/β-Catenin Signaling Pathway. Bioengineered 2021, 12, 3873–3885. [Google Scholar] [CrossRef]
- Gu, H.; Lin, R.; Zheng, F.; Zhang, Q. ELK1 Activated-Long Noncoding RNA LBX2-AS1 Aggravates the Progression of Ovarian Cancer through Targeting miR-4784/KDM5C Axis. J. Mol. Histol. 2021, 52, 31–44. [Google Scholar] [CrossRef]
- Shen, G.; Mao, Y.; Su, Z.; Du, J.; Yu, Y.; Xu, F. PSMB8-AS1 Activated by ELK1 Promotes Cell Proliferation in Glioma via Regulating miR-574-5p/RAB10. Biomed. Pharmacother. 2020, 122, 109658. [Google Scholar] [CrossRef]
- Su, X.; Teng, J.; Jin, G.; Li, J.; Zhao, Z.; Cao, X.; Guo, Y.; Guo, M.; Li, X.; Wu, J.; et al. ELK1-Induced Upregulation of Long Non-Coding RNA MIR100HG Predicts Poor Prognosis and Promotes the Progression of Osteosarcoma by Epigenetically Silencing LATS1 and LATS2. Biomed. Pharmacother. 2019, 109, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Sun, Y.-M.; Wang, L.-M.; Shang, Y.-L. ELK1-Induced Upregulation lncRNA LINC02381 Accelerates the Osteosarcoma Tumorigenesis through Targeting CDCA4 via Sponging miR-503-5p. Biochem. Biophys. Res. Commun. 2021, 548, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Chen, M.; Lin, C.; Andresen, B.T.; Parsa, C.; Orlando, R.; Huang, Y. The β-Blocker Carvedilol Prevents Benzo(a)Pyrene-Induced Lung Toxicity, Inflammation and Carcinogenesis. Cancers 2023, 15, 583. [Google Scholar] [CrossRef]
- Kamrani Moghaddam, L.; Ramezani Paschepari, S.; Zaimy, M.A.; Abdalaian, A.; Jebali, A. The Inhibition of Epidermal Growth Factor Receptor Signaling by Hexagonal Selenium Nanoparticles Modified by SiRNA. Cancer Gene Ther. 2016, 23, 321–325. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, D.S.; Lee, O.-H.; Oh, S.-H.; Lippman, S.M.; Lee, H.-Y. Antiangiogenic Antitumor Activities of IGFBP-3 Are Mediated by IGF-Independent Suppression of Erk1/2 Activation and Egr-1–Mediated Transcriptional Events. Blood 2011, 118, 2622–2631. [Google Scholar] [CrossRef]
- Yu, J.; Bulk, E.; Ji, P.; Hascher, A.; Koschmieder, S.; Berdel, W.E.; Müller-Tidow, C. The Kinase Defective EPHB6 Receptor Tyrosine Kinase Activates MAP Kinase Signaling in Lung Adenocarcinoma. Int. J. Oncol. 2009, 35, 175–179. [Google Scholar] [CrossRef]
- Chao, T.-T.; Wang, C.-Y.; Chen, Y.-L.; Lai, C.-C.; Chang, F.-Y.; Tsai, Y.-T.; Chao, C.-H.H.; Shiau, C.-W.; Huang, Y.-C.T.; Yu, C.-J.; et al. Afatinib Induces Apoptosis in NSCLC without EGFR Mutation through Elk-1-Mediated Suppression of CIP2A. Oncotarget 2015, 6, 2164–2179. [Google Scholar] [CrossRef]
- Junttila, M.R.; Puustinen, P.; Niemelä, M.; Ahola, R.; Arnold, H.; Böttzauw, T.; Ala-aho, R.; Nielsen, C.; Ivaska, J.; Taya, Y.; et al. CIP2A Inhibits PP2A in Human Malignancies. Cell 2007, 130, 51–62. [Google Scholar] [CrossRef]
- Khanna, A.; Okkeri, J.; Bilgen, T.; Tiirikka, T.; Vihinen, M.; Visakorpi, T.; Westermarck, J. ETS1 Mediates MEK1/2-Dependent Overexpression of Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A) in Human Cancer Cells. PLoS ONE 2011, 6, e17979. [Google Scholar] [CrossRef]
- Yu, H.-C.; Chen, H.-J.; Chang, Y.-L.; Liu, C.-Y.; Shiau, C.-W.; Cheng, A.-L.; Chen, K.-F. Inhibition of CIP2A Determines Erlotinib-Induced Apoptosis in Hepatocellular Carcinoma. Biochem. Pharmacol. 2013, 85, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-C.; Hung, M.-H.; Chen, Y.-L.; Chu, P.-Y.; Wang, C.-Y.; Chao, T.-T.; Liu, C.-Y.; Shiau, C.-W.; Chen, K.-F. Erlotinib Derivative Inhibits Hepatocellular Carcinoma by Targeting CIP2A to Reactivate Protein Phosphatase 2A. Cell Death Dis. 2014, 5, e1359. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Sun, X.; Zhang, X.; Simone, N.; He, J. ELK1/MTOR/S6K1 Pathway Contributes to Acquired Resistance to Gefitinib in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 2382. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Pang, C.; Chang, N.; Zhang, J.; Liu, W. Overexpression of PAD4 Suppresses Drug Resistance of NSCLC Cell Lines to Gefitinib through Inhibiting Elk1-Mediated Epithelial-Mesenchymal Transition. Oncol. Rep. 2016, 36, 551–558. [Google Scholar] [CrossRef]
- Weaver, Z.; Difilippantonio, S.; Carretero, J.; Martin, P.L.; El Meskini, R.; Iacovelli, A.J.; Gumprecht, M.; Kulaga, A.; Guerin, T.; Schlomer, J.; et al. Temporal Molecular and Biological Assessment of an Erlotinib-Resistant Lung Adenocarcinoma Model Reveals Markers of Tumor Progression and Treatment Response. Cancer Res. 2012, 72, 5921–5933. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wang, Q.; Lv, S.; Mao, G. Elevated Expression of ELK1 Promotes Breast Cancer Cell Growth and Correlates with Poor Prognosis of Breast Cancer Patients. Ann. Med. Surg. 2024, 86, 5767–5775. [Google Scholar] [CrossRef]
- Laliotis, A.; Vrekoussis, T.; Kafousi, M.; Sanidas, E.; Askoxilakis, J.; Melissas, J.; Mavroudis, D.; Castanas, E.; Stathopoulos, E.N. Immunohistochemical Study of pElk-1 Expression in Human Breast Cancer: Association with Breast Cancer Biologic Profile and Clinicopathologic Features. Breast 2013, 22, 89–95. [Google Scholar] [CrossRef]
- Niida, A.; Smith, A.D.; Imoto, S.; Tsutsumi, S.; Aburatani, H.; Zhang, M.Q.; Akiyama, T. Integrative Bioinformatics Analysis of Transcriptional Regulatory Programs in Breast Cancer Cells. BMC Bioinform. 2008, 9, 404. [Google Scholar] [CrossRef]
- Zhang, X.; Gamble, M.J.; Stadler, S.; Cherrington, B.D.; Causey, C.P.; Thompson, P.R.; Roberson, M.S.; Kraus, W.L.; Coonrod, S.A. Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate c-Fos Expression in Breast Cancer Cells. PLoS Genet. 2011, 7, e1002112. [Google Scholar] [CrossRef]
- Yue, C.-H.; Liu, J.-Y.; Chi, C.-S.; Hu, C.-W.; Tan, K.-T.; Huang, F.-M.; Pan, Y.-R.; Lin, K.-I.; Lee, C.-J. Myeloid Zinc Finger 1 (MZF1) Maintains the Mesenchymal Phenotype by Down-Regulating IGF1R/P38 MAPK/ERα Signaling Pathway in High-Level MZF1-Expressing TNBC Cells. Anticancer Res. 2019, 39, 4149–4164. [Google Scholar] [CrossRef]
- Yue, C.-H.; Chiu, Y.-W.; Tung, J.-N.; Tzang, B.-S.; Shiu, J.-J.; Huang, W.-H.; Liu, J.-Y.; Hwang, J.-M. Expression of Protein Kinase C α and the MZF-1 and Elk-1 Transcription Factors in Human Breast Cancer Cells. Chin. J. Physiol. 2012, 55, 31–36. [Google Scholar] [CrossRef]
- Hu, S.; Wang, M.; Ji, A.; Yang, J.; Gao, R.; Li, X.; Sun, L.; Wang, J.; Zhang, Y.; Liu, H. Mutant P53 and ELK1 Co-Drive FRA-1 Expression to Induce Metastasis in Breast Cancer. FEBS Lett. 2023, 597, 3087–3101. [Google Scholar] [CrossRef]
- Castro, A.F.; Campos, T.; Babcock, J.T.; Armijo, M.E.; Martínez-Conde, A.; Pincheira, R.; Quilliam, L.A. M-Ras Induces Ral and JNK Activation to Regulate MEK/ERK-Independent Gene Expression in MCF-7 Breast Cancer Cells. J. Cell. Biochem. 2012, 113, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Tokita, K.; Wada, N.; Ito, K.; Yamauchi, C.; Ito, Y.; Ochiai, A. MEK-ERK Pathway Regulates EZH2 Overexpression in Association with Aggressive Breast Cancer Subtypes. Oncogene 2011, 30, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Fukamachi, K.; Tsuda, H.; Ito, K.; Ito, Y.; Ochiai, A. RAS Oncogenic Signal Upregulates EZH2 in Pancreatic Cancer. Biochem. Biophys. Res. Commun. 2012, 417, 1074–1079. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Xiao, J.; Chen, W.; Chen, W. ELK1/KIFC1 Axis Promotes Breast Cancer Cell Proliferation by Regulating Glutathione Metabolism. J. Obstet. Gynaecol. Res. 2023, 49, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, H.; Liu, H.; Guo, X.; Ma, R.; Zhu, W.; Gao, P. ELK1-Induced up-Regulation of KIF26B Promotes Cell Cycle Progression in Breast Cancer. Med. Oncol. 2021, 39, 15. [Google Scholar] [CrossRef]
- Jo, K.; Santos-Buitrago, B.; Kim, M.; Rhee, S.; Talcott, C.; Kim, S. Logic-Based Analysis of Gene Expression Data Predicts Association between TNF, TGFB1 and EGF Pathways in Basal-like Breast Cancer. Methods 2020, 179, 89–100. [Google Scholar] [CrossRef]
- Lowe, W.L.; Fu, R.; Banko, M. Growth Factor-Induced Transcription via the Serum Response Element Is Inhibited by Cyclic Adenosine 3′,5′-Monophosphate in MCF-7 Breast Cancer Cells. Endocrinology 1997, 138, 2219–2226. [Google Scholar] [CrossRef]
- Booy, E.P.; Henson, E.S.; Gibson, S.B. Epidermal Growth Factor Regulates Mcl-1 Expression through the MAPK-Elk-1 Signalling Pathway Contributing to Cell Survival in Breast Cancer. Oncogene 2011, 30, 2367–2378. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, T.-T.; Huang, C.-T.; Hu, M.-H.; Wang, D.-S.; Wang, W.-L.; Tsai, W.-C.; Lee, C.-H.; Lau, K.-Y.; Yang, H.-P.; et al. EGFR-Independent Elk1/CIP2A Signalling Mediates Apoptotic Effect of an Erlotinib Derivative TD52 in Triple-Negative Breast Cancer Cells. Eur. J. Cancer 2017, 72, 112–123. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Hu, M.-H.; Hsu, C.-J.; Huang, C.-T.; Wang, D.-S.; Tsai, W.-C.; Chen, Y.-T.; Lee, C.-H.; Chu, P.-Y.; Hsu, C.-C.; et al. Lapatinib Inhibits CIP2A/PP2A/p-Akt Signaling and Induces Apoptosis in Triple Negative Breast Cancer Cells. Oncotarget 2016, 7, 9135–9149. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Huang, T.-T.; Chen, Y.-T.; Chen, J.-L.; Chu, P.-Y.; Huang, C.-T.; Wang, W.-L.; Lau, K.-Y.; Dai, M.-S.; Shiau, C.-W.; et al. Targeting SET to Restore PP2A Activity Disrupts an Oncogenic CIP2A-Feedforward Loop and Impairs Triple Negative Breast Cancer Progression. EBioMedicine 2019, 40, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Wyrzykowska, P.; Stalińska, K.; Wawro, M.; Kochan, J.; Kasza, A. Epidermal Growth Factor Regulates PAI-1 Expression via Activation of the Transcription Factor Elk-1. Biochim. Biophys. Acta 2010, 1799, 616–621. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.; Moreno, E.; Hamann, S.; Martín-Villar, E.; Flores, J.M.; et al. Role of Cannabinoid Receptor CB2 in HER2 Pro-Oncogenic Signaling in Breast Cancer. J. Natl. Cancer Inst. 2015, 107, djv077. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Xie, W.; Burghardt, R.C.; Safe, S. Estrogen Receptor-Mediated Activation of the Serum Response Element in MCF-7 Cells through MAPK-Dependent Phosphorylation of Elk-1. J. Biol. Chem. 2001, 276, 11590–11598. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lee, W.-R.; Safe, S. Egr-1 Is Activated by 17beta-Estradiol in MCF-7 Cells by Mitogen-Activated Protein Kinase-Dependent Phosphorylation of ELK-1. J. Cell. Biochem. 2004, 93, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Song, R.X.; Zhang, Z.; Yue, W.; Kumar, R. Adaptive Hypersensitivity to Estrogen: Mechanism for Sequential Responses to Hormonal Therapy in Breast Cancer. Clin. Cancer Res. 2004, 10, 337S–345S. [Google Scholar] [CrossRef]
- Santen, R.J.; Song, R.X.; Zhang, Z.; Kumar, R.; Jeng, M.-H.; Masamura, A.; Lawrence, J.; Berstein, L.; Yue, W. Long-Term Estradiol Deprivation in Breast Cancer Cells up-Regulates Growth Factor Signaling and Enhances Estrogen Sensitivity. Endocr. Relat. Cancer 2005, 12 (Suppl. 1), S61–S73. [Google Scholar] [CrossRef]
- Gutzman, J.H.; Nikolai, S.E.; Rugowski, D.E.; Watters, J.J.; Schuler, L.A. Prolactin and Estrogen Enhance the Activity of Activating Protein 1 in Breast Cancer Cells: Role of Extracellularly Regulated Kinase 1/2-Mediated Signals to c-Fos. Mol. Endocrinol. 2005, 19, 1765–1778. [Google Scholar] [CrossRef]
- Schmitt, J.M.; Abell, E.; Wagner, A.; Davare, M.A. ERK Activation and Cell Growth Require CaM Kinases in MCF-7 Breast Cancer Cells. Mol. Cell. Biochem. 2010, 335, 155–171. [Google Scholar] [CrossRef]
- Vivacqua, A.; De Marco, P.; Santolla, M.F.; Cirillo, F.; Pellegrino, M.; Panno, M.L.; Abonante, S.; Maggiolini, M. Estrogenic Gper Signaling Regulates Mir144 Expression in Cancer Cells and Cancer-Associated Fibroblasts (Cafs). Oncotarget 2015, 6, 16573–16587. [Google Scholar] [CrossRef]
- Chia, K.M.; Liu, J.; Francis, G.D.; Naderi, A. A Feedback Loop between Androgen Receptor and ERK Signaling in Estrogen Receptor-Negative Breast Cancer. Neoplasia 2011, 13, 154–166. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Gao, J.; Wang, X.; Liu, Z. Regulation of the microRNA 200b (miRNA-200b) by Transcriptional Regulators PEA3 and ELK-1 Protein Affects Expression of Pin1 Protein to Control Anoikis. J. Biol. Chem. 2013, 288, 32742–32752. [Google Scholar] [CrossRef]
- Ahmad, A.; Zhang, W.; Wu, M.; Tan, S.; Zhu, T. Tumor-Suppressive miRNA-135a Inhibits Breast Cancer Cell Proliferation by Targeting ELK1 and ELK3 Oncogenes. Genes Genom. 2018, 40, 243–251. [Google Scholar] [CrossRef]
- Zhao, C.; Li, L.; Li, Z.; Xu, J.; Yang, Q.; Shi, P.; Zhang, K.; Jiang, R. A Novel Circular RNA hsa_circRPPH1_015 Exerts an Oncogenic Role in Breast Cancer by Impairing miRNA-326-Mediated ELK1 Inhibition. Front. Oncol. 2020, 10, 906. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Z.; Zhao, L.; Xu, H. Circular RNA Hsa_circ_0000515 Acts as a miR-326 Sponge to Promote Cervical Cancer Progression through up-Regulation of ELK1. Aging 2019, 11, 9982–9999. [Google Scholar] [CrossRef]
- Wang, M.-H.; Liu, Z.-H.; Zhang, H.-X.; Liu, H.-C.; Ma, L.-H. Hsa_circRNA_000166 Accelerates Breast Cancer Progression via the Regulation of the miR-326/ELK1 and miR-330-5p/ELK1 Axes. Ann. Med. 2024, 56, 2424515. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, G.-M.; Wang, W.-L.; Wang, Z.-H.; Fang, Y.; Liu, Y.-L. LncRNA TDRG1 Functions as an Oncogene in Cervical Cancer through Sponging miR-330-5p to Modulate ELK1 Expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7295–7306. [Google Scholar] [CrossRef]
- Maniccia, A.W.; Lewis, C.; Begum, N.; Xu, J.; Cui, J.; Chipitsyna, G.; Aysola, K.; Reddy, V.; Bhat, G.; Fujimura, Y.; et al. Mitochondrial Localization, ELK-1 Transcriptional Regulation and Growth Inhibitory Functions of BRCA1, BRCA1a, and BRCA1b Proteins. J. Cell. Physiol. 2009, 219, 634–641. [Google Scholar] [CrossRef]
- Chai, Y.; Chipitsyna, G.; Cui, J.; Liao, B.; Liu, S.; Aysola, K.; Yezdani, M.; Reddy, E.S.; Rao, V.N. C-Fos Oncogene Regulator Elk-1 Interacts with BRCA1 Splice Variants BRCA1a/1b and Enhances BRCA1a/1b-Mediated Growth Suppression in Breast Cancer Cells. Oncogene 2001, 20, 1357–1367. [Google Scholar] [CrossRef]
- Kim, H.-R.; Lee, H.-N.; Lim, K.; Surh, Y.-J.; Na, H.-K. 15-Deoxy-Δ12,14-Prostaglandin J2 Induces Expression of 15-Hydroxyprostaglandin Dehydrogenase through Elk-1 Activation in Human Breast Cancer MDA-MB-231 Cells. Mutat. Res. 2014, 768, 6–15. [Google Scholar] [CrossRef]
- Kim, C.G.; Choi, B.H.; Son, S.W.; Yi, S.J.; Shin, S.Y.; Lee, Y.H. Tamoxifen-Induced Activation of p21Waf1/Cip1 Gene Transcription Is Mediated by Early Growth Response-1 Protein through the JNK and P38 MAP Kinase/Elk-1 Cascades in MDA-MB-361 Breast Carcinoma Cells. Cell. Signal. 2007, 19, 1290–1300. [Google Scholar] [CrossRef]
- Choi, B.H.; Kim, C.G.; Bae, Y.-S.; Lim, Y.; Lee, Y.H.; Shin, S.Y. P21 Waf1/Cip1 Expression by Curcumin in U-87MG Human Glioma Cells: Role of Early Growth Response-1 Expression. Cancer Res. 2008, 68, 1369–1377. [Google Scholar] [CrossRef]
- Kole, L.; Sarkar, M.; Deb, A.; Giri, B. Pioglitazone, an Anti-Diabetic Drug Requires Sustained MAPK Activation for Its Anti-Tumor Activity in MCF7 Breast Cancer Cells, Independent of PPAR-γ Pathway. Pharmacol. Rep. 2016, 68, 144–154. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Mendonca, P.; Messeha, S.S.; Oriaku, E.T.; Soliman, K.F.A. The Anticancer Effects of Marine Carotenoid Fucoxanthin through Phosphatidylinositol 3-Kinase (PI3K)-AKT Signaling on Triple-Negative Breast Cancer Cells. Molecules 2023, 29, 61. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhu, L.; Zou, J.; Jiang, X.; Chen, M.; Chen, B. Letrozole Improves the Sensitivity of Breast Cancer Cells Overexpressing Aromatase to Cisplatin via Down-Regulation of FEN1. Clin. Transl. Oncol. 2019, 21, 1026–1033. [Google Scholar] [CrossRef]
- Wu, W.; Pew, T.; Zou, M.; Pang, D.; Conzen, S.D. Glucocorticoid Receptor-Induced MAPK Phosphatase-1 (MPK-1) Expression Inhibits Paclitaxel-Associated MAPK Activation and Contributes to Breast Cancer Cell Survival. J. Biol. Chem. 2005, 280, 4117–4124. [Google Scholar] [CrossRef]
- Liu, L.; Xing, Y.; Cao, M.; Xu, J.; Chen, J. Exogenous NO Induces Apoptosis of Hepatocellular Carcinoma Cells via Positive P38/JNK Signaling Pathway and Negative ERK Signaling Pathways. Mol. Cell. Biochem. 2021, 476, 1651–1661. [Google Scholar] [CrossRef]
- Kim, B.-W.; Lee, E.-R.; Min, H.-M.; Jeong, H.-S.; Ahn, J.-Y.; Kim, J.-H.; Choi, H.-Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained ERK Activation Is Involved in the Kaempferol-Induced Apoptosis of Breast Cancer Cells and Is More Evident under 3-D Culture Condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef]
- Nagalingam, A.; Kuppusamy, P.; Singh, S.V.; Sharma, D.; Saxena, N.K. Mechanistic Elucidation of the Antitumor Properties of Withaferin a in Breast Cancer. Cancer Res. 2014, 74, 2617–2629. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.-X. Involvement of ERK1/2-Mediated ELK1/CHOP/DR5 Pathway in 6-(Methylsulfinyl)Hexyl Isothiocyanate-Induced Apoptosis of Colorectal Cancer Cells. Biosci. Biotechnol. Biochem. 2019, 83, 960–969. [Google Scholar] [CrossRef]
- Luo, X.; Yang, L.; Xiao, L.; Xia, X.; Dong, X.; Zhong, J.; Liu, Y.; Li, N.; Chen, L.; Li, H.; et al. Grifolin Directly Targets ERK1/2 to Epigenetically Suppress Cancer Cell Metastasis. Oncotarget 2015, 6, 42704–42716. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xu, J.; Johnson, A.C. Curcumin Inhibits Human Colon Cancer Cell Growth by Suppressing Gene Expression of Epidermal Growth Factor Receptor through Reducing the Activity of the Transcription Factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, Y.; Wang, J.; Yan, Z.; Wei, Q.; Ye, J.; Zhang, J.; He, T.-C.; Qiao, M. Effect of Antibiotic Monensin on Cell Proliferation and IGF1R Signaling Pathway in Human Colorectal Cancer Cells. Ann. Med. 2023, 55, 954–964. [Google Scholar] [CrossRef]
- Cao, H.; Sethumadhavan, K.; Cao, F.; Wang, T.T.Y. Gossypol Decreased Cell Viability and Down-Regulated the Expression of a Number of Genes in Human Colon Cancer Cells. Sci. Rep. 2021, 11, 5922. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.R.; Walton, M.I.; Garrett, M.D.; Workman, P. The Cyclin-Dependent Kinase Inhibitor CYC202 (R-Roscovitine) Inhibits Retinoblastoma Protein Phosphorylation, Causes Loss of Cyclin D1, and Activates the Mitogen-Activated Protein Kinase Pathway. Cancer Res. 2004, 64, 262–272. [Google Scholar] [CrossRef]
- Marks, B.A.; Pipia, I.M.; Mukai, C.; Horibata, S.; Rice, E.J.; Danko, C.G.; Coonrod, S.A. GDNF-RET Signaling and EGR1 Form a Positive Feedback Loop That Promotes Tamoxifen Resistance via Cyclin D1. BMC Cancer 2023, 23, 138. [Google Scholar] [CrossRef]
- Zhao, J.; Ou, B.; Han, D.; Wang, P.; Zong, Y.; Zhu, C.; Liu, D.; Zheng, M.; Sun, J.; Feng, H.; et al. Tumor-Derived CXCL5 Promotes Human Colorectal Cancer Metastasis through Activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-Catenin Pathways. Mol. Cancer 2017, 16, 70. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, C.; Chen, C.; Zong, Y.; Feng, H.; Liu, D.; Feng, W.; Zhao, J.; Lu, A. CCL19 Suppresses Angiogenesis through Promoting miR-206 and Inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A Pathway in Colorectal Cancer. Cell Death Dis. 2018, 9, 974. [Google Scholar] [CrossRef]
- Cao, H. Bacterial Endotoxin Lipopolysaccharides Regulate Gene Expression in Human Colon Cancer Cells. BMC Res. Notes 2023, 16, 216. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Chen, H.; Abbas, M.K.; Yang, L.; Sun, H.; Sun, T.; Wu, B.; Yang, S.; Zhou, D. C-KIT-ERK1/2 Signaling Activated ELK1 and Upregulated Carcinoembryonic Antigen Expression to Promote Colorectal Cancer Progression. Cancer Sci. 2021, 112, 655–667. [Google Scholar] [CrossRef]
- Taniuchi, F.; Higai, K.; Tanaka, T.; Azuma, Y.; Matsumoto, K. Transcriptional Regulation of Fucosyltransferase 1 Gene Expression in Colon Cancer Cells. Sci. World J. 2013, 2013, 105464. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Yuan, X.; Chen, Y.; Cui, Y.; Cao, K. Comprehensive Multi-Omics Analysis Identifies FUT1 as a Prognostic and Therapeutic Biomarker Across Pan-Cancer. Int. J. Med. Sci. 2025, 22, 1313–1328. [Google Scholar] [CrossRef]
- Hennessy, B.A.; Harvey, B.J.; Healy, V. 17beta-Estradiol Rapidly Stimulates c-Fos Expression via the MAPK Pathway in T84 Cells. Mol. Cell. Endocrinol. 2005, 229, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, Y.; Chen, F.; Zhou, L.; Wei, H.; Dong, F.; Wang, X.; Shan, Y.; Chen, T. RPL36A Activates ERK Pathway and Promotes Colorectal Cancer Growth. Transl. Oncol. 2025, 51, 102170. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; An, P.; Xu, J.; Liu, W.; Lin, F.; Yang, Y. A-Kinase Anchor Protein 95 Is Involved in ERK1/2–Elk-1 Signal Transduction in Colon Cancer. Anal. Cell. Pathol. 2023, 2023, 8242646. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, K.M.M.; Malapit, J.R.P.; Yu, R.T.D.; Garrido, J.A.M.G.; Rigor, J.P.T.; Angeles, A.K.J.; Cutiongco-de la Paz, E.M.; Garcia, R.L. Non-Redundant and Overlapping Oncogenic Readouts of Non-Canonical and Novel Colorectal Cancer KRAS and NRAS Mutants. Cells 2019, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Camaj, P.; Heinemann, V.; Schwarz, B.; Jung, A.; Laubender, R.P.; Gamba, S.; Haertl, C.; Stintzing, S.; Primo, S.; et al. KRAS Allel-Specific Activity of Sunitinib in an Isogenic Disease Model of Colorectal Cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 953–961. [Google Scholar] [CrossRef]
- Hollander, D.; Donyo, M.; Atias, N.; Mekahel, K.; Melamed, Z.; Yannai, S.; Lev-Maor, G.; Shilo, A.; Schwartz, S.; Barshack, I.; et al. A Network-Based Analysis of Colon Cancer Splicing Changes Reveals a Tumorigenesis-Favoring Regulatory Pathway Emanating from ELK1. Genome Res. 2016, 26, 541–553. [Google Scholar] [CrossRef]
- Pradhan, M.P.; Prasad, N.K.A.; Palakal, M.J. A Systems Biology Approach to the Global Analysis of Transcription Factors in Colorectal Cancer. BMC Cancer 2012, 12, 331. [Google Scholar] [CrossRef]
- Yan, G.; Lei, W. Role of ELK1 in Regulating Colorectal Cancer Progression: miR-31-5p/CDIP1 Axis in CRC Pathogenesis. PeerJ 2023, 11, e15602. [Google Scholar] [CrossRef]
- Fan, C.; Lin, B.; Huang, Z.; Cui, D.; Zhu, M.; Ma, Z.; Zhang, Y.; Liu, F.; Liu, Y. MicroRNA-873 Inhibits Colorectal Cancer Metastasis by Targeting ELK1 and STRN4. Oncotarget 2019, 10, 4192–4204. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Chen, D.; Zhang, L.; Pan, Y.; Liu, D.; Shen, M.; Chen, M. Circ_0022340 Promotes Colorectal Cancer Progression via HNRNPC/EBF1/SYT7 or miR-382-5p/ELK1 Axis. Cell. Mol. Biol. 2022, 68, 107–116. [Google Scholar] [CrossRef]
- Zhao, S.; Mi, Y.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Xu, Y.; Cai, S.; Li, X.; Li, D. Highly-Metastatic Colorectal Cancer Cell Released miR-181a-5p-Rich Extracellular Vesicles Promote Liver Metastasis by Activating Hepatic Stellate Cells and Remodelling the Tumour Microenvironment. J. Extracell. Vesicles 2022, 11, e12186. [Google Scholar] [CrossRef]
- Chen, C.; Cai, Y.; Hu, W.; Tan, K.; Lu, Z.; Zhu, X.; Liu, Z.; He, C.; Xu, G.; Zhang, R.; et al. Single-Cell eQTL Mapping Reveals Cell Subtype-Specific Genetic Control and Mechanism in Malignant Transformation of Colorectal Cancer. Cancer Discov. 2025, 15, 1649–1675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Guan, B.; Mi, Y.; Shi, D.; Wei, P.; Gu, Y.; Cai, S.; Xu, Y.; Li, X.; Yan, D.; et al. LncRNA MIR17HG Promotes Colorectal Cancer Liver Metastasis by Mediating a Glycolysis-Associated Positive Feedback Circuit. Oncogene 2021, 40, 4709–4724. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Wu, S.; Zheng, Z.; Wang, C.; Li, H.; Zhao, L.; Zhang, X.; Huang, H.; Huang, C.; et al. C4orf19 Inhibits Colorectal Cancer Cell Proliferation by Competitively Binding to Keap1 with TRIM25 via the USP17/Elk-1/CDK6 Axis. Oncogene 2023, 42, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xu, J. Activation of PPAR{gamma} by Curcumin Inhibits Moser Cell Growth and Mediates Suppression of Gene Expression of Cyclin D1 and EGFR. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G447–G456. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, G.; Domeier, P.P.; Ding, W.; Mulder, K.M. Decreased Tumor Progression and Invasion by a Novel Anti-Cell Motility Target for Human Colorectal Carcinoma Cells. PLoS ONE 2013, 8, e66439. [Google Scholar] [CrossRef]
- Morris, J.F.; Sul, J.-Y.; Kim, M.-S.; Klein-Szanto, A.J.; Schochet, T.; Rustgi, A.; Eberwine, J.H. Elk-1 Phosphorylated at Threonine-417 Is Present in Diverse Cancers and Correlates with Differentiation Grade of Colonic Adenocarcinoma. Hum. Pathol. 2013, 44, 766–776. [Google Scholar] [CrossRef]
- Dobre, M.; Trandafir, B.; Milanesi, E.; Salvi, A.; Bucuroiu, I.A.; Vasilescu, C.; Niculae, A.M.; Herlea, V.; Hinescu, M.E.; Constantinescu, G. Molecular Profile of the NF-κB Signalling Pathway in Human Colorectal Cancer. J. Cell. Mol. Med. 2022, 26, 5966–5975. [Google Scholar] [CrossRef]
- Asting, A.G.; Carén, H.; Andersson, M.; Lönnroth, C.; Lagerstedt, K.; Lundholm, K. COX-2 Gene Expression in Colon Cancer Tissue Related to Regulating Factors and Promoter Methylation Status. BMC Cancer 2011, 11, 238. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, J.; Fang, K.; Jiang, H.; Leng, Z.; Wu, H.; Zhang, Z.; Wang, Z.; Li, Z.; Sun, M.; et al. FOXC1-Mediated Serine Metabolism Reprogramming Enhances Colorectal Cancer Growth and 5-FU Resistance under Serine Restriction. Cell Commun. Signal. 2025, 23, 13. [Google Scholar] [CrossRef]
- Pardy, L.; Rosati, R.; Soave, C.; Huang, Y.; Kim, S.; Ratnam, M. The Ternary Complex Factor Protein ELK1 Is an Independent Prognosticator of Disease Recurrence in Prostate Cancer. Prostate 2020, 80, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, L.; Jin, X.; Song, L.; Lv, Y.; Han, Y. Identification of Open Chromosomal Regions and Key Genes in Prostate Cancer via Integrated Analysis of DNase-seq and RNA-seq Data. Mol. Med. Rep. 2018, 18, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Patki, M.; Chari, V.; Sivakumaran, S.; Gonit, M.; Trumbly, R.; Ratnam, M. The ETS Domain Transcription Factor ELK1 Directs a Critical Component of Growth Signaling by the Androgen Receptor in Prostate Cancer Cells. J. Biol. Chem. 2013, 288, 11047–11065. [Google Scholar] [CrossRef]
- Rosa-Ribeiro, R.; Nishan, U.; Vidal, R.O.; Barbosa, G.O.; Reis, L.O.; Cesar, C.L.; Carvalho, H.F. Transcription Factors Involved in Prostate Gland Adaptation to Androgen Deprivation. PLoS ONE 2014, 9, e97080. [Google Scholar] [CrossRef] [PubMed]
- Rosati, R.; Polin, L.; Ducker, C.; Li, J.; Bao, X.; Selvakumar, D.; Kim, S.; Xhabija, B.; Larsen, M.; McFall, T.; et al. Strategy for Tumor-Selective Disruption of Androgen Receptor Function in the Spectrum of Prostate Cancer. Clin. Cancer Res. 2018, 24, 6509–6522. [Google Scholar] [CrossRef]
- Rosati, R.; Patki, M.; Chari, V.; Dakshnamurthy, S.; McFall, T.; Saxton, J.; Kidder, B.L.; Shaw, P.E.; Ratnam, M. The Amino-Terminal Domain of the Androgen Receptor Co-Opts Extracellular Signal-Regulated Kinase (ERK) Docking Sites in ELK1 Protein to Induce Sustained Gene Activation That Supports Prostate Cancer Cell Growth. J. Biol. Chem. 2016, 291, 25983–25998. [Google Scholar] [CrossRef]
- Soave, C.; Ducker, C.; Kim, S.; Strahl, T.; Rosati, R.; Huang, Y.; Shaw, P.E.; Ratnam, M. Identification of ELK1 Interacting Peptide Segments in the Androgen Receptor. Biochem. J. 2022, 479, 1519–1531. [Google Scholar] [CrossRef]
- Xie, W.; Li, S.; Guo, H.; Zhang, J.; Tu, M.; Wang, R.; Lin, B.; Wu, Y.; Wang, X. Androgen Receptor Knockdown Enhances Prostate Cancer Chemosensitivity by Down-Regulating FEN1 through the ERK/ELK1 Signalling Pathway. Cancer Med. 2023, 12, 15317–15336. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Ruoff, R.; Garabedian, M.J. MED19 Alters AR Occupancy and Gene Expression in Prostate Cancer Cells, Driving MAOA Expression and Growth under Low Androgen. PLoS Genet. 2021, 17, e1008540. [Google Scholar] [CrossRef]
- Azami, S.; Vo Nguyen, T.T.; Watanabe, Y.; Kato, M. Cooperative Induction of Transmembrane Prostate Androgen Induced Protein TMEPAI/PMEPA1 by Transforming Growth Factor-β and Epidermal Growth Factor Signaling. Biochem. Biophys. Res. Commun. 2015, 456, 580–585. [Google Scholar] [CrossRef]
- Gregg, J.; Fraizer, G. Transcriptional Regulation of EGR1 by EGF and the ERK Signaling Pathway in Prostate Cancer Cells. Genes Cancer 2011, 2, 900–909. [Google Scholar] [CrossRef]
- Zhang, P.-J.; Hu, X.-Y.; Liu, C.-Y.; Chen, Z.-B.; Ni, N.-N.; Yu, Y.; Yang, L.-N.; Huang, Z.-Q.; Liu, Q.-W.; Jiang, A.-L. The Inhibitory Effects of NKX3.1 on IGF-1R Expression and Its Signalling Pathway in Human Prostatic Carcinoma PC3 Cells. Asian J. Androl. 2012, 14, 493–498. [Google Scholar] [CrossRef]
- Shankar, E.; Song, K.; Corum, S.L.; Bane, K.L.; Wang, H.; Kao, H.-Y.; Danielpour, D. A Signaling Network Controlling Androgenic Repression of C-Fos Protein in Prostate Adenocarcinoma Cells. J. Biol. Chem. 2016, 291, 5512–5526. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; De Castro, I.; Defranco, D.B.; Deng, F.-M.; Melamed, J.; Kapur, P.; Raj, G.V.; Rossi, R.; Hammes, S.R. Paxillin Mediates Extranuclear and Intranuclear Signaling in Prostate Cancer Proliferation. J. Clin. Investig. 2012, 122, 2469–2481. [Google Scholar] [CrossRef]
- Adler, D.; Lindstrot, A.; Langer, B.; Buettner, R.; Wernert, N. Differential Expression of ETS Family Members in Prostate Cancer Tissues and Androgen-Sensitive and Insensitive Prostate Cancer Cell Lines. Int. J. Mol. Med. 2011, 28, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Li, J.; Zhu, Z.; Berk, M.; Hardaway, A.; McManus, J.; Chung, Y.-M.; Alyamani, M.; Valle, S.; Tiwari, R.; et al. Cancer-Associated Fibroblast-Secreted Glucosamine Alters the Androgen Biosynthesis Program in Prostate Cancer via HSD3B1 Upregulation. J. Clin. Investig. 2023, 133, e161913. [Google Scholar] [CrossRef]
- Li, P.; Shi, Y.; Gao, D.; Xu, H.; Zou, Y.; Wang, Z.; Li, W. ELK1-Mediated YTHDF1 Drives Prostate Cancer Progression by Facilitating the Translation of Polo-like Kinase 1 in an m6A Dependent Manner. Int. J. Biol. Sci. 2022, 18, 6145–6162. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Yang, H.; Yang, X.; Zhang, Y.; Yu, X.; Li, Y.; Chen, G.; Yang, Z. The Potential Role of m6A Reader YTHDF1 as Diagnostic Biomarker and the Signaling Pathways in Tumorigenesis and Metastasis in Pan-Cancer. Cell Death Discov. 2023, 9, 34. [Google Scholar] [CrossRef]
- Kalra, R.; Bhagyaraj, E.; Tiwari, D.; Nanduri, R.; Chacko, A.P.; Jain, M.; Mahajan, S.; Khatri, N.; Gupta, P. AIRE Promotes Androgen-Independent Prostate Cancer by Directly Regulating IL-6 and Modulating Tumor Microenvironment. Oncogenesis 2018, 7, 43. [Google Scholar] [CrossRef]
- Jilg, C.A.; Ketscher, A.; Metzger, E.; Hummel, B.; Willmann, D.; Rüsseler, V.; Drendel, V.; Imhof, A.; Jung, M.; Franz, H.; et al. PRK1/PKN1 Controls Migration and Metastasis of Androgen-Independent Prostate Cancer Cells. Oncotarget 2014, 5, 12646–12664. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Qu, X.; Weber, H.C. GRP Receptor-Mediated Immediate Early Gene Expression and Transcription Factor Elk-1 Activation in Prostate Cancer Cells. Regul. Pept. 2002, 109, 141–148. [Google Scholar] [CrossRef]
- Koochekpour, S.; Sartor, O.; Lee, T.-J.; Zieske, A.; Patten, D.Y.; Hiraiwa, M.; Sandhoff, K.; Remmel, N.; Minokadeh, A. Prosaptide TX14A Stimulates Growth, Migration, and Invasion and Activates the Raf-MEK-ERK-RSK-Elk-1 Signaling Pathway in Prostate Cancer Cells. Prostate 2004, 61, 114–123. [Google Scholar] [CrossRef]
- Nishida, M.; Sato, A.; Shimizu, A.; Rahman, N.I.A.; Wada, A.; Kageyama, S.; Ogita, H. EphA-Mediated Regulation of Stomatin Expression in Prostate Cancer Cells. Cancer Med. 2024, 13, e70276. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, C.; Cansino, J.R.; Bethencourt, F.; Pérez-Utrilla, M.; Fraile, B.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; Royuela, M. TNF/IL-1/NIK/NF-Kappa B Transduction Pathway: A Comparative Study in Normal and Pathological Human Prostate (Benign Hyperplasia and Carcinoma). Histopathology 2008, 53, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Berriguete, G.; Fraile, B.; de Bethencourt, F.R.; Prieto-Folgado, A.; Bartolome, N.; Nuñez, C.; Prati, B.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; et al. Role of IAPs in Prostate Cancer Progression: Immunohistochemical Study in Normal and Pathological (Benign Hyperplastic, Prostatic Intraepithelial Neoplasia and Cancer) Human Prostate. BMC Cancer 2010, 10, 18. [Google Scholar] [CrossRef]
- Wang, L.-N.; Chen, W.-W.; Zhang, J.; Li, C.-Y.; Liu, C.-Y.; Xue, J.; Zhang, P.-J.; Jiang, A.-L. The miRNA Let-7a1 Inhibits the Expression of Insulin-like Growth Factor 1 Receptor (IGF1R) in Prostate Cancer PC-3 Cells. Asian J. Androl. 2013, 15, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Lombe, C.P.; Meyer, M.; Pretorius, A. Bioinformatics Prediction and Analysis of MicroRNAs and Their Targets as Biomarkers for Prostate Cancer: A Preliminary Study. Mol. Biotechnol. 2022, 64, 401–412. [Google Scholar] [CrossRef]
- Huynh, H.; Nguyen, T.T.T.; Chan, E.; Tran, E. Inhibition of ErbB-2 and ErbB-3 Expression by Quercetin Prevents Transforming Growth Factor Alpha (TGF-Alpha)- and Epidermal Growth Factor (EGF)-Induced Human PC-3 Prostate Cancer Cell Proliferation. Int. J. Oncol. 2003, 23, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Aljarah, A.K.; Shareef, H.K.; Inoue, S.; Ide, H.; Patterson, J.D.; Kashiwagi, E.; Han, B.; Li, Y.; Zheng, Y.; et al. Silodosin Inhibits Prostate Cancer Cell Growth via ELK1 Inactivation and Enhances the Cytotoxic Activity of Gemcitabine. Prostate 2016, 76, 744–756. [Google Scholar] [CrossRef]
- Hennenberg, M.; Strittmatter, F.; Beckmann, C.; Rutz, B.; Füllhase, C.; Waidelich, R.; Montorsi, F.; Hedlund, P.; Andersson, K.-E.; Stief, C.G.; et al. Silodosin Inhibits Noradrenaline-Activated Transcription Factors Elk1 and SRF in Human Prostate Smooth Muscle. PLoS ONE 2012, 7, e50904. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, Y.; Agarwal, C.; Agarwal, R. Silibinin Impairs Constitutively Active TGFalpha-EGFR Autocrine Loop in Advanced Human Prostate Carcinoma Cells. Pharm. Res. 2008, 25, 2143–2150. [Google Scholar] [CrossRef]
- Tyagi, A.; Agarwal, R.; Agarwal, C. Grape Seed Extract Inhibits EGF-Induced and Constitutively Active Mitogenic Signaling but Activates JNK in Human Prostate Carcinoma DU145 Cells: Possible Role in Antiproliferation and Apoptosis. Oncogene 2003, 22, 1302–1316. [Google Scholar] [CrossRef]
- Yemelyanov, A.; Czwornog, J.; Gera, L.; Joshi, S.; Chatterton, R.T.; Budunova, I. Novel Steroid Receptor Phyto-Modulator Compound a Inhibits Growth and Survival of Prostate Cancer Cells. Cancer Res. 2008, 68, 4763–4773. [Google Scholar] [CrossRef]
- Lai, Y.-W.; Wang, S.-W.; Lin, C.-L.; Chen, S.-S.; Lin, K.-H.; Lee, Y.-T.; Chen, W.-C.; Hsieh, Y.-H. Asiatic Acid Exhibits Antimetastatic Activity in Human Prostate Cancer Cells by Modulating the MZF-1/Elk-1/Snail Signaling Axis. Eur. J. Pharmacol. 2023, 951, 175770. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Q.; Jaganath, I.; Manikam, R.; Sekaran, S.D. Phyllanthus Suppresses Prostate Cancer Cell, PC-3, Proliferation and Induces Apoptosis through Multiple Signalling Pathways (MAPKs, PI3K/Akt, NFκB, and Hypoxia). Evid.-Based Complement. Altern. Med. 2013, 2013, 609581. [Google Scholar] [CrossRef]
- Xu, C.; Shen, G.; Yuan, X.; Kim, J.-H.; Gopalkrishnan, A.; Keum, Y.-S.; Nair, S.; Kong, A.-N.T. ERK and JNK Signaling Pathways Are Involved in the Regulation of Activator Protein 1 and Cell Death Elicited by Three Isothiocyanates in Human Prostate Cancer PC-3 Cells. Carcinogenesis 2006, 27, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, B.T.; Hurt, E.M.; Kalathur, M.; Duhagon, M.A.; Milner, J.A.; Kim, Y.S.; Farrar, W.L. Effects of the Sesquiterpene Lactone Parthenolide on Prostate Tumor-Initiating Cells: An Integrated Molecular Profiling Approach. Prostate 2009, 69, 827–837. [Google Scholar] [CrossRef]
- Zafeiropoulou, K.; Kalampounias, G.; Alexis, S.; Anastasopoulos, D.; Symeonidis, A.; Katsoris, P. Autophagy and Oxidative Stress Modulation Mediate Bortezomib Resistance in Prostate Cancer. PLoS ONE 2024, 19, e0289904. [Google Scholar] [CrossRef]
- Shuang, T.; Wang, M.; Zhou, Y.; Shi, C.; Wang, D. NF-κB1, c-Rel, and ELK1 Inhibit miR-134 Expression Leading to TAB1 Upregulation in Paclitaxel-Resistant Human Ovarian Cancer. Oncotarget 2017, 8, 24853–24868. [Google Scholar] [CrossRef]
- Kawahara, T.; Ide, H.; Kashiwagi, E.; Patterson, J.D.; Inoue, S.; Shareef, H.K.; Aljarah, A.K.; Zheng, Y.; Baras, A.S.; Miyamoto, H. Silodosin Inhibits the Growth of Bladder Cancer Cells and Enhances the Cytotoxic Activity of Cisplatin via ELK1 Inactivation. Am. J. Cancer Res. 2015, 5, 2959–2968. [Google Scholar]
- Rodriguez-Aguayo, C.; Bayraktar, E.; Ivan, C.; Aslan, B.; Mai, J.; He, G.; Mangala, L.S.; Jiang, D.; Nagaraja, A.S.; Ozpolat, B.; et al. PTGER3 Induces Ovary Tumorigenesis and Confers Resistance to Cisplatin Therapy through Up-Regulation Ras-MAPK/Erk-ETS1-ELK1/CFTR1 Axis. EBioMedicine 2019, 40, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gao, Y.; Lin, S.; Li, Y.; Shi, L.; Kan, Q. EGR1-CCL2 Feedback Loop Maintains Epithelial-Mesenchymal Transition of Cisplatin-Resistant Gastric Cancer Cells and Promotes Tumor Angiogenesis. Dig. Dis. Sci. 2022, 67, 3702–3713. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Zhang, H.; Huang, E.; Gao, L.; Luo, W.; Wei, Q.; Fan, J.; Song, D.; Liao, J.; et al. Anthelmintic Mebendazole Enhances Cisplatin’s Effect on Suppressing Cell Proliferation and Promotes Differentiation of Head and Neck Squamous Cell Carcinoma (HNSCC). Oncotarget 2017, 8, 12968–12982. [Google Scholar] [CrossRef] [PubMed]
- Höcker, M.; Henihan, R.J.; Rosewicz, S.; Riecken, E.O.; Zhang, Z.; Koh, T.J.; Wang, T.C. Gastrin and Phorbol 12-Myristate 13-Acetate Regulate the Human Histidine Decarboxylase Promoter through Raf-Dependent Activation of Extracellular Signal-Regulated Kinase-Related Signaling Pathways in Gastric Cancer Cells. J. Biol. Chem. 1997, 272, 27015–27024. [Google Scholar] [CrossRef] [PubMed]
- Meyer-ter-Vehn, T.; Covacci, A.; Kist, M.; Pahl, H.L. Helicobacter Pylori Activates Mitogen-Activated Protein Kinase Cascades and Induces Expression of the Proto-Oncogenes c-Fos and c-Jun. J. Biol. Chem. 2000, 275, 16064–16072. [Google Scholar] [CrossRef]
- Hirata, Y.; Maeda, S.; Mitsuno, Y.; Tateishi, K.; Yanai, A.; Akanuma, M.; Yoshida, H.; Kawabe, T.; Shiratori, Y.; Omata, M. Helicobacter Pylori CagA Protein Activates Serum Response Element-Driven Transcription Independently of Tyrosine Phosphorylation. Gastroenterology 2002, 123, 1962–1971. [Google Scholar] [CrossRef]
- Rieder, G.; Tessier, A.J.; Qiao, X.T.; Madison, B.; Gumucio, D.L.; Merchant, J.L. Helicobacter-Induced Intestinal Metaplasia in the Stomach Correlates with Elk-1 and Serum Response Factor Induction of Villin. J. Biol. Chem. 2005, 280, 4906–4912. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, M.; Aoyagi, K.; Danjoh, I.; Fukaya, M.; Yanagihara, K.; Sakamoto, H.; Yoshida, T.; Sasaki, H. Discovery of Aberrant Expression of R-RAS by Cancer-Linked DNA Hypomethylation in Gastric Cancer Using Microarrays. Cancer Res. 2005, 65, 2115–2124. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Gao, Y.; Pei, L.; Zhou, J.; Gu, L.; Zhang, L.; Zhu, B.; Hattori, N.; Ji, J.; et al. Large-Scale Characterization of DNA Methylation Changes in Human Gastric Carcinomas with and without Metastasis. Clin. Cancer Res. 2014, 20, 4598–4612. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Aoyagi, K.; Fukaya, M.; Danjoh, I.; Ohta, A.; Isohata, N.; Saeki, N.; Taniguchi, H.; Sakamoto, H.; Shimoda, T.; et al. Cross Talk between Hedgehog and Epithelial-Mesenchymal Transition Pathways in Gastric Pit Cells and in Diffuse-Type Gastric Cancers. Br. J. Cancer 2009, 100, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-D.; Jeong, S.-J.; Wang, G.; Park, J.-J.; Lim, D.-S.; Kim, B.-H.; Cho, Y.-I.; Kim, C.-S.; Jeong, M.-J. Secretory Leukocyte Protease Inhibitor Is Associated with MMP-2 and MMP-9 to Promote Migration and Invasion in SNU638 Gastric Cancer Cells. Int. J. Mol. Med. 2011, 28, 527–534. [Google Scholar] [CrossRef]
- Qian, J.; Kong, X.; Deng, N.; Tan, P.; Chen, H.; Wang, J.; Li, Z.; Hu, Y.; Zou, W.; Xu, J.; et al. OCT1 Is a Determinant of Synbindin-Related ERK Signalling with Independent Prognostic Significance in Gastric Cancer. Gut 2015, 64, 37–48. [Google Scholar] [CrossRef]
- Ma, W.; Xu, Z.; Wang, Y.; Li, W.; Wei, Z.; Chen, T.; Mou, T.; Cheng, M.; Luo, J.; Luo, T.; et al. A Positive Feedback Loop of SLP2 Activates MAPK Signaling Pathway to Promote Gastric Cancer Progression. Theranostics 2018, 8, 5744–5757. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dang, Y.; Feng, W.; Qiao, C.; Liu, D.; Zhang, T.; Wang, Y.; Tian, D.; Fan, D.; Nie, Y.; et al. SOX18 Promotes Gastric Cancer Metastasis through Transactivating MCAM and CCL7. Oncogene 2020, 39, 5536–5552. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, Y.; Fu, K.; Lin, Q.; Zhao, T.; Tang, W.; Xi, L.; Sheng, L.; Zhang, H.; Sun, Y. Circ-PTPDC1 Promotes the Progression of Gastric Cancer through Sponging Mir-139-3p by Regulating ELK1 and Functions as a Prognostic Biomarker. Int. J. Biol. Sci. 2021, 17, 4285–4304. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-L.; Shyu, R.-Y.; Yeh, M.-Y.; Jiang, S.-Y. The Retinoid-Inducible Gene I: Effect on Apoptosis and Mitogen-Activated Kinase Signal Pathways. Anticancer Res. 2002, 22, 799–804. [Google Scholar]
- Sang, J.; Chen, Y.; Jiang, L.; Tao, Y.; Wu, Y.; Wang, Y.; Li, Y.; Lan, T.; Shao, G. Type II cGMP-Dependent Protein Kinase Inhibits ERK/JNK-Mediated Activation of Transcription Factors in Gastric Cancer Cells. Mol. Med. Rep. 2012, 6, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Pandian, J.; Panneerpandian, P.; Devanandan, H.J.; Sekar, B.T.; Balakrishnan, K.; Selvarasu, K.; Muthupandi, K.; Ganesan, K. Identification of the Targeted Therapeutic Potential of Doxycycline for a Subset of Gastric Cancer Patients. Ann. N. Y. Acad. Sci. 2020, 1467, 94–111. [Google Scholar] [CrossRef]
- Huang, Y.; Li, F. Baicalein Inhibits Metastasis of Oral Squamous Cell Carcinoma Cells by Regulating ERK/ELK-1/Snail Signaling. Discov. Med. 2024, 36, 1298–1305. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Nagalingam, A.; Muniraj, N.; Saxena, N.K.; Sharma, D. Concomitant Activation of ETS-like Transcription Factor-1 and Death Receptor-5 via Extracellular Signal-Regulated Kinase in Withaferin A-Mediated Inhibition of Hepatocarcinogenesis in Mice. Sci. Rep. 2017, 7, 17943. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kar, S.; Wang, M.; Carr, B.I. Transient and Sustained ERK Phosphorylation and Nuclear Translocation in Growth Control. J. Cell. Physiol. 2002, 192, 151–159. [Google Scholar] [CrossRef]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin Inhibits Proliferation, Triggers Apoptosis and Modulates Akt/mTOR and MAP Kinase Pathways in HeLa Cells. Oncol. Lett. 2021, 21, 192. [Google Scholar] [CrossRef]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia Parviflora Extract Exhibits Anti-Cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef]
- Dun, S.; Gao, L. Tanshinone I Attenuates Proliferation and Chemoresistance of Cervical Cancer in a KRAS-Dependent Manner. J. Biochem. Mol. Toxicol. 2019, 33, e22267. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J.; Ma, S.; Huang, Z.; Zhang, G. Requirement of Osteopontin in the Migration and Protection against Taxol-Induced Apoptosis via the ATX-LPA Axis in SGC7901 Cells. BMC Cell Biol. 2011, 12, 11. [Google Scholar] [CrossRef]
- Wang, M.; An, Q.; Li, Z.; Huang, Z.; Huang, K.; Li, G.; Ma, Q.; Zhao, L. The Alkylglycerone Phosphate Synthase Sustains the Resistance of Gastric Cancer Cells to Ferroptosis Induced by Apatinib. Gastric Cancer 2025, 28, 579–597. [Google Scholar] [CrossRef]
- Tang, M.; Sun, J.; Cai, Z. LncRNA THUMPD3-AS1 Promotes the Proliferation and Migration of Esophageal Cancer Cells through the miR-29a-3p/ELK1/PRDX4 Signaling Pathway. Semin. Oncol. 2025, 52, 152350. [Google Scholar] [CrossRef]
- Chen, A.-G.; Yu, Z.-C.; Yu, X.-F.; Cao, W.-F.; Ding, F.; Liu, Z.-H. Overexpression of Ets-like Protein 1 in Human Esophageal Squamous Cell Carcinoma. World J. Gastroenterol. 2006, 12, 7859–7863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Min, L.; Xu, C.; Shao, L.; Guo, S.; Cheng, R.; Xing, J.; Zhu, S.; Zhang, S. Construction of Disease-Specific Transcriptional Regulatory Networks Identifies Co-Activation of Four Gene in Esophageal Squamous Cell Carcinoma. Oncol. Rep. 2017, 38, 411–417. [Google Scholar] [CrossRef]
- Zheng, Z.-Y.; Chu, M.-Y.; Lin, W.; Zheng, Y.-Q.; Xu, X.-E.; Chen, Y.; Liao, L.-D.; Wu, Z.-Y.; Wang, S.-H.; Li, E.-M.; et al. Blocking STAT3 Signaling Augments MEK/ERK Inhibitor Efficacy in Esophageal Squamous Cell Carcinoma. Cell Death Dis. 2022, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Kanazawa, K.; Nomura, M.; Tanaka, T.; Shigemoto-Kuroda, T.; Fukui, K.; Miura, K.; Kurosawa, K.; Kawai, M.; Kato, H.; et al. Ppp6c Deficiency Accelerates K-rasG12D -Induced Tongue Carcinogenesis. Cancer Med. 2021, 10, 4451–4464. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yuan, W.; An, C.; Tan, Q.; Ma, J. BEST1 Positive Monocytes in Circulation: Visualize Intratumoral Crosstalk between Cancer Cells and Monocytes. Adv. Sci. 2023, 10, e2205915. [Google Scholar] [CrossRef]
- Shuang, Y.; Liu, J.; Niu, J.; Guo, W.; Li, C. A Novel Circular RNA circPPFIA1 Promotes Laryngeal Squamous Cell Carcinoma Progression through Sponging miR-340-3p and Regulating ELK1 Expression. Bioengineered 2021, 12, 5220–5230. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Aubee, J.; DiVito, K.A.; Zhou, H.; Zhang, W.; Chou, F.-P.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S. Id3 Induces an Elk-1–Caspase-8-Dependent Apoptotic Pathway in Squamous Carcinoma Cells. Cancer Med. 2015, 4, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, M.; Wang, J.; Cao, G.; Chen, W.; Xu, J. PCNA-Associated Factor KIAA0101 Transcriptionally Induced by ELK1 Controls Cell Proliferation and Apoptosis in Nasopharyngeal Carcinoma: An Integrated Bioinformatics and Experimental Study. Aging 2020, 12, 5992–6017. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Lu, J.; Zou, Y.S.; Soh-Won, J.; Cohen, D.M.; Buttrick, P.M.; Cooper, D.R.; Steinberg, S.F.; Mackman, N.; Pinsky, D.J.; et al. Hypoxia-Associated Induction of Early Growth Response-1 Gene Expression. J. Biol. Chem. 1999, 274, 15030–15040. [Google Scholar] [CrossRef]
- Shan, J.; Dudenhausen, E.; Kilberg, M.S. Induction of Early Growth Response Gene 1 (EGR1) by Endoplasmic Reticulum Stress Is Mediated by the Extracellular Regulated Kinase (ERK) Arm of the MAPK Pathways. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 371–381. [Google Scholar] [CrossRef]
- Bauer, I.; Hohl, M.; Al-Sarraj, A.; Vinson, C.; Thiel, G. Transcriptional Activation of the Egr-1 Gene Mediated by Tetradecanoylphorbol Acetate and Extracellular Signal-Regulated Protein Kinase. Arch. Biochem. Biophys. 2005, 438, 36–52. [Google Scholar] [CrossRef]
- Shan, J.; Balasubramanian, M.N.; Donelan, W.; Fu, L.; Hayner, J.; Lopez, M.-C.; Baker, H.V.; Kilberg, M.S. A Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Kinase (MEK)-Dependent Transcriptional Program Controls Activation of the Early Growth Response 1 (EGR1) Gene during Amino Acid Limitation. J. Biol. Chem. 2014, 289, 24665–24679. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Donelan, W.; Hayner, J.N.; Zhang, F.; Dudenhausen, E.E.; Kilberg, M.S. MAPK Signaling Triggers Transcriptional Induction of cFOS during Amino Acid Limitation of HepG2 Cells. Biochim. Biophys. Acta 2015, 1853, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.; Rössler, O.G. Resveratrol Stimulates AP-1-Regulated Gene Transcription. Mol. Nutr. Food Res. 2014, 58, 1402–1413. [Google Scholar] [CrossRef]
- Zhong, S.; Fromm, J.; Johnson, D.L. TBP Is Differentially Regulated by C-Jun N-Terminal Kinase 1 (JNK1) and JNK2 through Elk-1, Controlling c-Jun Expression and Cell Proliferation. Mol. Cell. Biol. 2007, 27, 54–64. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, T.; Chen, X.; Zhang, B.; Wang, Y.; Xie, M.; Ji, X.; Sun, M.; Huang, W.; Xia, L. ONECUT2 Facilitates Hepatocellular Carcinoma Metastasis by Transcriptionally Upregulating FGF2 and ACLY. Cell Death Dis. 2021, 12, 1113. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, W.; He, Q.; Li, D.; Wang, Z.; Feng, Y.; Liu, D.; Zhang, T.; Wang, Y.; Xie, M.; et al. FOXC1 Promotes HCC Proliferation and Metastasis by Upregulating DNMT3B to Induce DNA Hypermethylation of CTH Promoter. J. Exp. Clin. Cancer Res. 2021, 40, 50. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, D.; Zhang, Y.; Han, C.-C.; Wei, W. Insulin-Like Growth Factor Binding Protein-2 Promotes Proliferation and Predicts Poor Prognosis in Hepatocellular Carcinoma. OncoTargets Ther. 2020, 13, 5083–5092. [Google Scholar] [CrossRef]
- Huynh, H.; Chow, P.K.H.; Ooi, L.L.P.; Soo, K.-C. A Possible Role for Insulin-like Growth Factor-Binding Protein-3 Autocrine/Paracrine Loops in Controlling Hepatocellular Carcinoma Cell Proliferation. Cell Growth Differ. 2002, 13, 115–122. [Google Scholar]
- Pellegrino, R.; Thavamani, A.; Calvisi, D.F.; Budczies, J.; Neumann, A.; Geffers, R.; Kroemer, J.; Greule, D.; Schirmacher, P.; Nordheim, A.; et al. Serum Response Factor (SRF) Drives the Transcriptional Upregulation of the MDM4 Oncogene in HCC. Cancers 2021, 13, 199. [Google Scholar] [CrossRef]
- Yan, Q.; Lou, G.; Qian, Y.; Qin, B.; Xu, X.; Wang, Y.; Liu, Y.; Dong, X. SPAG9 Is Involved in Hepatocarcinoma Cell Migration and Invasion via Modulation of ELK1 Expression. OncoTargets Ther. 2016, 9, 1067–1075. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Xie, M.; Ji, X.; Luo, X.; Chen, X.; Zhang, B.; Liu, D.; Feng, Y.; Sun, M.; et al. HGF-Mediated Elevation of ETV1 Facilitates Hepatocellular Carcinoma Metastasis through Upregulating PTK2 and c-MET. J. Exp. Clin. Cancer Res. 2022, 41, 275. [Google Scholar] [CrossRef]
- Ma, C.; Huang, S.; Xu, L.; Tian, L.; Yang, Y.; Wang, J. Transcription Co-Activator P300 Activates Elk1-aPKC-ι Signaling Mediated Epithelial-to-Mesenchymal Transition and Malignancy in Hepatocellular Carcinoma. Oncogenesis 2020, 9, 32. [Google Scholar] [CrossRef]
- Wang, J.-M.; Li, Q.; Du, G.-S.; Lu, J.-X.; Zou, S.-Q. Significance and Expression of Atypical Protein Kinase C-Iota in Human Hepatocellular Carcinoma. J. Surg. Res. 2009, 154, 143–149. [Google Scholar] [CrossRef]
- Yue, C.-H.; Huang, C.-Y.; Tsai, J.-H.; Hsu, C.-W.; Hsieh, Y.-H.; Lin, H.; Liu, J.-Y. MZF-1/Elk-1 Complex Binds to Protein Kinase Cα Promoter and Is Involved in Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0127420. [Google Scholar] [CrossRef][Green Version]
- Ye, J.-C.; Hsu, L.-S.; Tsai, J.-H.; Yang, H.-L.; Hsiao, M.-W.; Hwang, J.-M.; Lee, C.-J.; Liu, J.-Y. MZF-1/Elk-1/PKCα Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. J. Cancer 2017, 8, 3028–3036. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Wu, T.-T.; Tsai, J.-H.; Huang, C.-Y.; Hsieh, Y.-S.; Liu, J.-Y. PKCalpha Expression Regulated by Elk-1 and MZF-1 in Human HCC Cells. Biochem. Biophys. Res. Commun. 2006, 339, 217–225. [Google Scholar] [CrossRef]
- An, S.; Zheng, Y.; Bleu, T. Sphingosine 1-Phosphate-Induced Cell Proliferation, Survival, and Related Signaling Events Mediated by G Protein-Coupled Receptors Edg3 and Edg5. J. Biol. Chem. 2000, 275, 288–296. [Google Scholar] [CrossRef]
- Su, T.; Zhang, N.; Wang, T.; Zeng, J.; Li, W.; Han, L.; Yang, M. Super Enhancer-Regulated LncRNA LINC01089 Induces Alternative Splicing of DIAPH3 to Drive Hepatocellular Carcinoma Metastasis. Cancer Res. 2023, 83, 4080–4094. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Iyer, S.V.; Ward, C.; Link, T.; Diaz, F.J.; Dhar, A.; Tawfik, O.W.; Weinman, S.A.; Azuma, Y.; Izumi, T.; et al. MTBP Inhibits the Erk1/2-Elk-1 Signaling in Hepatocellular Carcinoma. Oncotarget 2018, 9, 21429–21443. [Google Scholar] [CrossRef]
- Bi, Q.; Ranjan, A.; Fan, R.; Agarwal, N.; Welch, D.R.; Weinman, S.A.; Ding, J.; Iwakuma, T. MTBP Inhibits Migration and Metastasis of Hepatocellular Carcinoma. Clin. Exp. Metastasis 2015, 32, 301–311. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, H.; Jeong, K.; Pak, Y. Regulation of Cancer Cell Proliferation by Caveolin-2 down-Regulation and Re-Expression. Int. J. Oncol. 2011, 38, 1395–1402. [Google Scholar] [CrossRef]
- Pallai, R.; Bhaskar, A.; Sodi, V.; Rice, L.M. Ets1 and Elk1 Transcription Factors Regulate Cancerous Inhibitor of Protein Phosphatase 2A Expression in Cervical and Endometrial Carcinoma Cells. Transcription 2012, 3, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Ying, T.-H.; Hsieh, Y.-H.; Hsieh, Y.-S.; Liu, J.-Y. Antisense Oligonucleotide Elk-1 Suppresses the Tumorigenicity of Human Hepatocellular Carcinoma Cells. Cell Biol. Int. 2008, 32, 210–216. [Google Scholar] [CrossRef]
- Ma, J.; Zeng, S.; Zhang, Y.; Deng, G.; Qu, Y.; Guo, C.; Yin, L.; Han, Y.; Cai, C.; Li, Y.; et al. BMP4 Promotes Oxaliplatin Resistance by an Induction of Epithelial-Mesenchymal Transition via MEK1/ERK/ELK1 Signaling in Hepatocellular Carcinoma. Cancer Lett. 2017, 411, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Fontaine, J.-F.; Franc, B.; Mirebeau-Prunier, D.; Triau, S.; Savagner, F.; Malthiery, Y. Death-Associated Protein 3 Is Overexpressed in Human Thyroid Oncocytic Tumours. Br. J. Cancer 2009, 101, 132–138. [Google Scholar] [CrossRef]
- Mariani, L.; Beaudry, C.; McDonough, W.S.; Hoelzinger, D.B.; Kaczmarek, E.; Ponce, F.; Coons, S.W.; Giese, A.; Seiler, R.W.; Berens, M.E. Death-Associated Protein 3 (Dap-3) Is Overexpressed in Invasive Glioblastoma Cells in Vivo and in Glioma Cell Lines with Induced Motility Phenotype in Vitro. Clin. Cancer Res. 2001, 7, 2480–2489. [Google Scholar]
- Sasaki, H.; Ide, N.; Yukiue, H.; Kobayashi, Y.; Fukai, I.; Yamakawa, Y.; Fujii, Y. Arg and DAP3 Expression Was Correlated with Human Thymoma Stage. Clin. Exp. Metastasis 2004, 21, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bullock, M.; Lim, G.; Li, C.; Choi, I.H.; Kochhar, S.; Liddle, C.; Zhang, L.; Clifton-Bligh, R.J. Thyroid Transcription Factor FOXE1 Interacts with ETS Factor ELK1 to Co-Regulate TERT. Oncotarget 2016, 7, 85948–85962. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yin, J.; Fu, Y.; Chen, Y.; Zhou, Y.; Geng, X. Suppression of Elk1 Inhibits Thyroid Cancer Progression by Mediating PTEN Expression. Oncol. Rep. 2018, 40, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S. Downregulation of circRNA_0000285 Suppresses Cervical Cancer Development by Regulating miR197-3p–ELK1 Axis. Cancer Manag. Res. 2020, 12, 8663–8674. [Google Scholar] [CrossRef]
- Zhang, P.; Kong, F.; Deng, X.; Yu, Y.; Hou, C.; Liang, T.; Zhu, L. MicroRNA-326 Suppresses the Proliferation, Migration and Invasion of Cervical Cancer Cells by Targeting ELK1. Oncol. Lett. 2017, 13, 2949–2956. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, F. Elevated microRNA-130b-5p or Silenced ELK1 Inhibits Self-Renewal Ability, Proliferation, Migration, and Invasion Abilities, and Promotes Apoptosis of Cervical Cancer Stem Cells. IUBMB Life 2021, 73, 118–129. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Hu, Y.; Ying, F.; Zou, R.; Lin, F.; Shi, Z.; Zhu, X.; Yan, X.; Li, S.; et al. LncRNA-TCONS_00026907 Is Involved in the Progression and Prognosis of Cervical Cancer through Inhibiting miR-143-5p. Cancer Med. 2017, 6, 1409–1423. [Google Scholar] [CrossRef]
- Go, S.-H.; Rho, S.B.; Yang, D.-W.; Kim, B.-R.; Lee, C.H.; Lee, S.-H. HPV-18 E7 Interacts with Elk-1 Leading to Elevation of the Transcriptional Activity of Elk-1 in Cervical Cancer. Biomol. Ther. 2022, 30, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Salmerón-Bárcenas, E.G.; Zacapala-Gómez, A.E.; Lozano-Amado, D.; Castro-Muñoz, L.J.; Leyva-Vázquez, M.A.; Manzo-Merino, J.; Ávila-López, P.A. Comprehensive Bioinformatic Analysis Reveals Oncogenic Role of H2A.Z Isoforms in Cervical Cancer Progression. Iran. J. Basic Med. Sci. 2021, 24, 1470–1481. [Google Scholar] [CrossRef]
- Wang, C.-H.; Shyu, R.-Y.; Wu, C.-C.; Tsai, T.-C.; Wang, L.-K.; Chen, M.-L.; Jiang, S.-Y.; Tsai, F.-M. Phospholipase A/Acyltransferase Enzyme Activity of H-Rev107 Inhibits the H-RAS Signaling Pathway. J. Biomed. Sci. 2014, 21, 36. [Google Scholar] [CrossRef]
- Swiatkowski, S.; Seifert, H.-H.; Steinhoff, C.; Prior, A.; Thievessen, I.; Schliess, F.; Schulz, W.A. Activities of MAP-Kinase Pathways in Normal Uroepithelial Cells and Urothelial Carcinoma Cell Lines. Exp. Cell Res. 2003, 282, 48–57. [Google Scholar] [CrossRef]
- Jou, Y.-C.; Chiu, Y.-W.; Chen, Y.-H.; Hwang, J.-M.; Chao, P.-Y.; Shiu, J.-J.; Hwang, W.-H.; Liu, J.-Y.; Hsu, L.-S. Expression of Protein Kinase Cα and the MZF-1 and Elk-1 Transcription Factors in Human Bladder Transitional Cell Carcinoma Cells. Chin. J. Physiol. 2012, 55, 75–81. [Google Scholar] [CrossRef]
- Kawahara, T.; Shareef, H.K.; Aljarah, A.K.; Ide, H.; Li, Y.; Kashiwagi, E.; Netto, G.J.; Zheng, Y.; Miyamoto, H. ELK1 Is Up-Regulated by Androgen in Bladder Cancer Cells and Promotes Tumor Progression. Oncotarget 2015, 6, 29860–29876. [Google Scholar] [CrossRef]
- Inoue, S.; Ide, H.; Mizushima, T.; Jiang, G.; Kawahara, T.; Miyamoto, H. ELK1 Promotes Urothelial Tumorigenesis in the Presence of an Activated Androgen Receptor. Am. J. Cancer Res. 2018, 8, 2325–2336. [Google Scholar]
- Inoue, S.; Ide, H.; Fujita, K.; Mizushima, T.; Jiang, G.; Kawahara, T.; Yamaguchi, S.; Fushimi, H.; Nonomura, N.; Miyamoto, H. Expression of Phospho-ELK1 and Its Prognostic Significance in Urothelial Carcinoma of the Upper Urinary Tract. Int. J. Mol. Sci. 2018, 19, 777. [Google Scholar] [CrossRef] [PubMed]
- Dawood, E.E.; Awadalla, A.; Hashem, A.; Shokeir, A.A.; Abdel-Aziz, A.F. Evaluation of Molecular Signatures in the Urinary Bladder and Upper Tract Urothelial Carcinomas: A Prospective Controlled Clinical Study. J. Egypt. Natl. Cancer Inst. 2022, 34, 47. [Google Scholar] [CrossRef]
- Elbadawy, M.; Sato, Y.; Mori, T.; Goto, Y.; Hayashi, K.; Yamanaka, M.; Azakami, D.; Uchide, T.; Fukushima, R.; Yoshida, T.; et al. Anti-Tumor Effect of Trametinib in Bladder Cancer Organoid and the Underlying Mechanism. Cancer Biol. Ther. 2021, 22, 357–371. [Google Scholar] [CrossRef]
- Li, T.; Kuang, T.; Yang, Z.; Zhang, Q.; Zhang, W.; Fan, Y. Co-Treatment With Everolimus, an mTOR-Specific Antagonist, or Downregulation of ELK1 Enhances the Sensitivity of Pancreatic Cancer Cells to Genistein. Front. Cell Dev. Biol. 2021, 9, 633035. [Google Scholar] [CrossRef]
- Ambrose, M.; Ryan, A.; O’Sullivan, G.C.; Dunne, C.; Barry, O.P. Induction of Apoptosis in Renal Cell Carcinoma by Reactive Oxygen Species: Involvement of Extracellular Signal-Regulated Kinase 1/2, P38delta/Gamma, Cyclooxygenase-2 down-Regulation, and Translocation of Apoptosis-Inducing Factor. Mol. Pharmacol. 2006, 69, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-K.; Huang, S.-L.; Chang, T.-C.; Chao, C.C.-K. Sorafenib Induces Endometrial Carcinoma Apoptosis by Inhibiting Elk-1-Dependent Mcl-1 Transcription and Inducing Akt/GSK3β-Dependent Protein Degradation. J. Cell. Biochem. 2013, 114, 1819–1831. [Google Scholar] [CrossRef]
- Simbulan-Rosenthal, C.M.; Dakshanamurthy, S.; Gaur, A.; Chen, Y.-S.; Fang, H.-B.; Abdussamad, M.; Zhou, H.; Zapas, J.; Calvert, V.; Petricoin, E.F.; et al. The Repurposed Anthelmintic Mebendazole in Combination with Trametinib Suppresses Refractory NRASQ61K Melanoma. Oncotarget 2017, 8, 12576–12595. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; El-Sheref, E.M.; Bakheet, M.E.M.; Mourad, M.A.E.; Bräse, S.; Ibrahim, M.A.A.; Nieger, M.; Garvalov, B.K.; Dalby, K.N.; Kaoud, T.S. Design, Synthesis and Biological Evaluation of Fused Naphthofuro[3,2-c]Quinoline-6,7,12-Triones and Pyrano[3,2-c]Quinoline-6,7,8,13-Tetraones Derivatives as ERK Inhibitors with Efficacy in BRAF-Mutant Melanoma. Bioorganic Chem. 2019, 82, 290–305. [Google Scholar] [CrossRef]
- Selimovic, D.; Hassan, M.; Haikel, Y.; Hengge, U.R. Taxol-Induced Mitochondrial Stress in Melanoma Cells Is Mediated by Activation of c-Jun N-Terminal Kinase (JNK) and P38 Pathways via Uncoupling Protein 2. Cell. Signal. 2008, 20, 311–322. [Google Scholar] [CrossRef]
- Cleveland, K.H.; Yeung, S.; Huang, K.M.; Liang, S.; Andresen, B.T.; Huang, Y. Phosphoproteome Profiling Provides Insight into the Mechanism of Action for Carvedilol-Mediated Cancer Prevention. Mol. Carcinog. 2018, 57, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Shamim, M.A.; Shahid, A.; Chen, M.; Cleveland, K.H.; Parsa, C.; Orlando, R.; Andresen, B.T.; Huang, Y. Prevention of Skin Carcinogenesis by the Non-β-Blocking R-Carvedilol Enantiomer. Cancer Prev. Res. 2021, 14, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, J.; Wu, X.; Li, Z. ELK1 Suppresses SYTL1 Expression by Recruiting HDAC2 in Bladder Cancer Progression. Hum. Cell 2022, 35, 1961–1975. [Google Scholar] [CrossRef] [PubMed]
- Himura, R.; Kawano, S.; Nagata, Y.; Kawai, M.; Ota, A.; Kudo, Y.; Yoshino, Y.; Fujimoto, N.; Miyamoto, H.; Endo, S.; et al. Inhibition of Aldo-Keto Reductase 1C3 Overcomes Gemcitabine/Cisplatin Resistance in Bladder Cancer. Chem. Biol. Interact. 2024, 388, 110840. [Google Scholar] [CrossRef]
- Wang, W.; Chen, S.; Song, X.; Gui, J.; Li, Y.; Li, M. ELK1/lncRNA-SNHG7/miR-2682-5p Feedback Loop Enhances Bladder Cancer Cell Growth. Life Sci. 2020, 262, 118386. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mizrachi, E.; Wen, W.; Srinivasan, S.; Klenk, A.; Cohen, D.; Permutt, M.A. Activation of Elk-1, an Ets Transcription Factor, by Glucose and EGF Treatment of Insulinoma Cells. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1286–E1299. [Google Scholar] [CrossRef]
- Kent, O.A.; Mendell, J.T.; Rottapel, R. Transcriptional Regulation of miR-31 by Oncogenic KRAS Mediates Metastatic Phenotypes by Repressing RASA1. Mol. Cancer Res. 2016, 14, 267–277. [Google Scholar] [CrossRef]
- Kent, O.A.; Sandí, M.-J.; Burston, H.E.; Brown, K.R.; Rottapel, R. An Oncogenic KRAS Transcription Program Activates the RHOGEF ARHGEF2 to Mediate Transformed Phenotypes in Pancreatic Cancer. Oncotarget 2017, 8, 4484–4500. [Google Scholar] [CrossRef]
- Langfermann, D.S.; Rössler, O.G.; Thiel, G. Stimulation of B-Raf Increases c-Jun and c-Fos Expression and Upregulates AP-1-Regulated Gene Transcription in Insulinoma Cells. Mol. Cell. Endocrinol. 2018, 472, 126–139. [Google Scholar] [CrossRef]
- He, Z.; Zheng, D.; Li, F.; Chen, L.; Wu, C.; Zeng, Z.; Yu, C. TMOD3 Accelerated Resistance to Immunotherapy in KRAS-Mutated Pancreatic Cancer through Promoting Autophagy-Dependent Degradation of ASCL4. Drug Resist. Updates 2025, 78, 101171. [Google Scholar] [CrossRef] [PubMed]
- Köenig, A.; Linhart, T.; Schlengemann, K.; Reutlinger, K.; Wegele, J.; Adler, G.; Singh, G.; Hofmann, L.; Kunsch, S.; Büch, T.; et al. NFAT-Induced Histone Acetylation Relay Switch Promotes c-Myc-Dependent Growth in Pancreatic Cancer Cells. Gastroenterology 2010, 138, 1189–1199.e2. [Google Scholar] [CrossRef]
- Yan, Q.; Ni, C.; Lin, Y.; Sun, X.; Shen, Z.; Zhang, M.; Han, S.; Shi, J.; Mao, J.; Yang, Z.; et al. ELK1 Enhances Pancreatic Cancer Progression Via LGMN and Correlates with Poor Prognosis. Front. Mol. Biosci. 2021, 8, 764900. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, Y.; Liu, X.; Wang, P.; Dai, S.; Zhu, F.; Liu, T.; Xu, W.; Wu, J. Aberrant Expression of CKS2 Induced by ELK1 Contributes to Malignant Progression of Pancreatic Cancer. Mol. Carcinog. 2023, 62, 1947–1959. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhu, Y.; Chen, Z.; Xu, Z.; Miao, Y. Transcriptional Regulation of Human Mucin Gene MUC4 in Pancreatic Cancer Cells. Mol. Biol. Rep. 2010, 37, 2797–2802. [Google Scholar] [CrossRef]
- Panebianco, C.; Trivieri, N.; Villani, A.; Terracciano, F.; Latiano, T.P.; Potenza, A.; Perri, F.; Binda, E.; Pazienza, V. Improving Gemcitabine Sensitivity in Pancreatic Cancer Cells by Restoring miRNA-217 Levels. Biomolecules 2021, 11, 639. [Google Scholar] [CrossRef]
- Zihlif, M.; Hameduh, T.; Bulatova, N.; Hammad, H. Alteration in the Expression of the Chemotherapy Resistance-related Genes in Response to Chronic and Acute Hypoxia in Pancreatic Cancer. Biomed. Rep. 2023, 19, 88. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Xing, X.; Wang, L. miR-597-5p Inhibits Cell Growth and Promotes Cell Apoptosis by Targeting ELK1 in Pancreatic Cancer. Hum. Cell 2020, 33, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, C.; Zhao, B.; Li, Z.; Li, T.; Yang, X.; Zhao, Y.; Wang, W. EGR1 Induces EMT in Pancreatic Cancer via a P300/SNAI2 Pathway. J. Transl. Med. 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.I.; Thiel, G. Calcium Influx into MIN6 Insulinoma Cells Induces Expression of Egr-1 Involving Extracellular Signal-Regulated Protein Kinase and the Transcription Factors Elk-1 and CREB. Eur. J. Cell Biol. 2009, 88, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Karatug Kacar, A. Exploring Dual Effects of Dinutuximab Beta on Cell Death and Proliferation of Insulinoma. Chem. Biol. Drug Des. 2024, 103, e14368. [Google Scholar] [CrossRef]
- Marin-Kuan, M.; Nestler, S.; Verguet, C.; Bezençon, C.; Piguet, D.; Delatour, T.; Mantle, P.; Cavin, C.; Schilter, B. MAPK-ERK Activation in Kidney of Male Rats Chronically Fed Ochratoxin A at a Dose Causing a Significant Incidence of Renal Carcinoma. Toxicol. Appl. Pharmacol. 2007, 224, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, Z.; Wang, K.; Qi, Y.; Yang, Y.; Zhang, Y.; An, X.; Luo, S.; Zheng, J. NDUFA4L2 Is Associated with Clear Cell Renal Cell Carcinoma Malignancy and Is Regulated by ELK1. PeerJ 2017, 5, e4065. [Google Scholar] [CrossRef]
- Oh, S.-I.; Jeong, H.; Park, H.-S.; Choi, K.-A.; Hwang, I.; Lee, J.; Cho, J.; Hong, S. Activation of CXCL12-CXCR4 Signalling Induces Conversion of Immortalised Embryonic Kidney Cells into Cancer Stem-like Cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1303–1313. [Google Scholar] [CrossRef]
- Okada, R.; Goto, Y.; Yamada, Y.; Kato, M.; Asai, S.; Moriya, S.; Ichikawa, T.; Seki, N. Regulation of Oncogenic Targets by the Tumor-Suppressive miR-139 Duplex (miR-139-5p and miR-139-3p) in Renal Cell Carcinoma. Biomedicines 2020, 8, 599. [Google Scholar] [CrossRef]
- Taniguchi, F.; Harada, T.; Sakamoto, Y.; Yamauchi, N.; Yoshida, S.; Iwabe, T.; Terakawa, N. Activation of Mitogen-Activated Protein Kinase Pathway by Keratinocyte Growth Factor or Fibroblast Growth Factor-10 Promotes Cell Proliferation in Human Endometrial Carcinoma Cells. J. Clin. Endocrinol. Metab. 2003, 88, 773–780. [Google Scholar] [CrossRef]
- Wei, S.; Yu, Z.; Shi, R.; An, L.; Zhang, Q.; Zhang, Q.; Zhang, T.; Zhang, J.; Wang, H. GPX4 Suppresses Ferroptosis to Promote Malignant Progression of Endometrial Carcinoma via Transcriptional Activation by ELK1. BMC Cancer 2022, 22, 881. [Google Scholar] [CrossRef]
- Singh, K.; Baird, M.; Fischer, R.; Chaitankar, V.; Seifuddin, F.; Chen, Y.-C.; Tunc, I.; Waterman, C.M.; Pirooznia, M. Misregulation of ELK1, AP1, and E12 Transcription Factor Networks Is Associated with Melanoma Progression. Cancers 2020, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, V.; Maresca, L.; Apollo, A.; Pepe, S.; Carr, R.M.; Fernandez-Zapico, M.E.; Stecca, B. E3 Ubiquitin Ligase PARK2, an Inhibitor of Melanoma Cell Growth, Is Repressed by the Oncogenic ERK1/2-ELK1 Transcriptional Axis. J. Biol. Chem. 2020, 295, 16058–16071. [Google Scholar] [CrossRef] [PubMed]
- Chava, S.; Bugide, S.; Malvi, P.; Gupta, R. Co-Targeting of Specific Epigenetic Regulators in Combination with CDC7 Potently Inhibit Melanoma Growth. iScience 2022, 25, 104752. [Google Scholar] [CrossRef]
- Makino, E.; Fröhlich, L.M.; Sinnberg, T.; Kosnopfel, C.; Sauer, B.; Garbe, C.; Schittek, B. Targeting Rad51 as a Strategy for the Treatment of Melanoma Cells Resistant to MAPK Pathway Inhibition. Cell Death Dis. 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Q.; Jaganath, I.B.; Manikam, R.; Sekaran, S.D. Inhibition of MAPKs, Myc/Max, NFκB, and Hypoxia Pathways by Phyllanthus Prevents Proliferation, Metastasis and Angiogenesis in Human Melanoma (MeWo) Cancer Cell Line. Int. J. Med. Sci. 2014, 11, 564–577. [Google Scholar] [CrossRef]
- Chang, A.; Yeung, S.; Thakkar, A.; Huang, K.M.; Liu, M.M.; Kanassatega, R.-S.; Parsa, C.; Orlando, R.; Jackson, E.K.; Andresen, B.T.; et al. Prevention of Skin Carcinogenesis by the β-Blocker Carvedilol. Cancer Prev. Res. 2015, 8, 27–36. [Google Scholar] [CrossRef]
- Huang, K.M.; Liang, S.; Yeung, S.; Oiyemhonlan, E.; Cleveland, K.H.; Parsa, C.; Orlando, R.; Meyskens, F.L.; Andresen, B.T.; Huang, Y. Topically Applied Carvedilol Attenuates Solar Ultraviolet Radiation Induced Skin Carcinogenesis. Cancer Prev. Res. 2017, 10, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-C.; Kang, S.K.; Tai, C.-J.; Auersperg, N.; Leung, P.C.K. Follicle-Stimulating Hormone Activates Mitogen-Activated Protein Kinase in Preneoplastic and Neoplastic Ovarian Surface Epithelial Cells. J. Clin. Endocrinol. Metab. 2002, 87, 2245–2253. [Google Scholar] [CrossRef]
- Choi, K.-C.; Tai, C.-J.; Tzeng, C.-R.; Auersperg, N.; Leung, P.C.K. Adenosine Triphosphate Activates Mitogen-Activated Protein Kinase in Pre-Neoplastic and Neoplastic Ovarian Surface Epithelial Cells. Biol. Reprod. 2003, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.Y.W.; Gilad, E.; Rothman, K.; Peyrollier, K. Hyaluronan-CD44 Interaction with IQGAP1 Promotes Cdc42 and ERK Signaling, Leading to Actin Binding, Elk-1/Estrogen Receptor Transcriptional Activation, and Ovarian Cancer Progression. J. Biol. Chem. 2005, 280, 11961–11972. [Google Scholar] [CrossRef]
- Al-Ayoubi, A.; Tarcsafalvi, A.; Zheng, H.; Sakati, W.; Eblen, S.T. ERK Activation and Nuclear Signaling Induced by the Loss of Cell/Matrix Adhesion Stimulates Anchorage-Independent Growth of Ovarian Cancer Cells. J. Cell. Biochem. 2008, 105, 875–884. [Google Scholar] [CrossRef]
- Goncharenko-Khaider, N.; Matte, I.; Lane, D.; Rancourt, C.; Piché, A. Ovarian Cancer Ascites Increase Mcl-1 Expression in Tumor Cells through ERK1/2-Elk-1 Signaling to Attenuate TRAIL-Induced Apoptosis. Mol. Cancer 2012, 11, 84. [Google Scholar] [CrossRef]
- Bartholomeusz, C.; Itamochi, H.; Nitta, M.; Saya, H.; Ginsberg, M.H.; Ueno, N.T. Antitumor Effect of E1A in Ovarian Cancer by Cytoplasmic Sequestration of Activated ERK by PEA15. Oncogene 2006, 25, 79–90. [Google Scholar] [CrossRef]
- Kandala, P.K.; Wright, S.E.; Srivastava, S.K. Blocking Epidermal Growth Factor Receptor Activation by 3,3′-Diindolylmethane Suppresses Ovarian Tumor Growth in Vitro and in Vivo. J. Pharmacol. Exp. Ther. 2012, 341, 24–32. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.; Wang, Z.; Yan, Z.; Qiao, M.; Ye, J.; Wei, Q.; Wang, J.; Wang, X.; Zhao, L.; et al. Antibiotic Monensin Synergizes with EGFR Inhibitors and Oxaliplatin to Suppress the Proliferation of Human Ovarian Cancer Cells. Sci. Rep. 2015, 5, 17523. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Zhang, F.; Qiao, M.; Yan, Z.; Wei, Q.; Wang, J.; Liu, H.; Fan, J.; Zou, Y.; et al. A Blockade of IGF Signaling Sensitizes Human Ovarian Cancer Cells to the Anthelmintic Niclosamide-Induced Anti-Proliferative and Anticancer Activities. Cell Physiol. Biochem. 2016, 39, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Shin, S.Y.; Lee, Y.H. Amitriptyline Induces Early Growth Response-1 Gene Expression via ERK and JNK Mitogen-Activated Protein Kinase Pathways in Rat C6 Glial Cells. Neurosci. Lett. 2007, 422, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Lee, J.H.; Min, B.; Lee, Y.H. The Translation Inhibitor Anisomycin Induces Elk-1-Mediated Transcriptional Activation of Egr-1 through Multiple Mitogen-Activated Protein Kinase Pathways. Exp. Mol. Med. 2006, 38, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Mut, M.; Lule, S.; Demir, O.; Kurnaz, I.A.; Vural, I. Both Mitogen-Activated Protein Kinase (MAPK)/Extracellular-Signal-Regulated Kinases (ERK) 1/2 and Phosphatidylinositide-3-OH Kinase (PI3K)/Akt Pathways Regulate Activation of E-Twenty-Six (ETS)-like Transcription Factor 1 (Elk-1) in U138 Glioblastoma Cells. Int. J. Biochem. Cell Biol. 2012, 44, 302–310. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Choi, K.-C.; Park, S.-H.; Auersperg, N.; Leung, P.C.K. Extracellular Signal-Regulated Protein Kinase, but Not c-Jun N-Terminal Kinase, Is Activated by Type II Gonadotropin-Releasing Hormone Involved in the Inhibition of Ovarian Cancer Cell Proliferation. J. Clin. Endocrinol. Metab. 2005, 90, 1670–1677. [Google Scholar] [CrossRef]
- Sahin, A.; Vercamer, C.; Kaminski, A.; Fuchs, T.; Florin, A.; Hahne, J.C.; Mattot, V.; Pourtier-Manzanedo, A.; Pietsch, T.; Fafeur, V.; et al. Dominant-Negative Inhibition of Ets 1 Suppresses Tumor Growth, Invasion and Migration in Rat C6 Glioma Cells and Reveals Differentially Expressed Ets 1 Target Genes. Int. J. Oncol. 2009, 34, 377–389. [Google Scholar] [PubMed]
- Wang, G.; Ren, X.; Li, J.; Cui, R.; Zhao, X.; Sui, F.; Liu, J.; Chen, P.; Yang, Q.; Ji, M.; et al. High Expression of RTEL1 Predicates Worse Progression in Gliomas and Promotes Tumorigenesis through JNK/ELK1 Cascade. BMC Cancer 2024, 24, 385. [Google Scholar] [CrossRef]
- Asensi-Cantó, A.; López-Abellán, M.D.; Castillo-Guardiola, V.; Hurtado, A.M.; Martínez-Penella, M.; Luengo-Gil, G.; Conesa-Zamora, P. Antitumoral Effects of Tricyclic Antidepressants: Beyond Neuropathic Pain Treatment. Cancers 2022, 14, 3248. [Google Scholar] [CrossRef]
- Evason, K.J.; Francisco, M.T.; Juric, V.; Balakrishnan, S.; Lopez Pazmino, M.D.P.; Gordan, J.D.; Kakar, S.; Spitsbergen, J.; Goga, A.; Stainier, D.Y.R. Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish. PLoS Genet. 2015, 11, e1005305. [Google Scholar] [CrossRef]
- Bielecka-Wajdman, A.M.; Ludyga, T.; Machnik, G.; Gołyszny, M.; Obuchowicz, E. Tricyclic Antidepressants Modulate Stressed Mitochondria in Glioblastoma Multiforme Cells. Cancer Control 2018, 25, 1073274818798594. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Xu, L.; Zhang, X.; Peng, W.; Tang, Q.; Feng, C. The Proliferation Effects of Fluoxetine and Amitriptyline on Human Breast Cancer Cells and the Underlying Molecular Mechanisms. Environ. Toxicol. Pharmacol. 2021, 83, 103586. [Google Scholar] [CrossRef] [PubMed]
- Zinnah, K.M.A.; Park, S.-Y. Sensitizing TRAIL-resistant A549 Lung Cancer Cells and Enhancing TRAIL-induced Apoptosis with the Antidepressant Amitriptyline. Oncol. Rep. 2021, 46, 144. [Google Scholar] [CrossRef]
- Parker, K.A.; Glaysher, S.; Hurren, J.; Knight, L.A.; McCormick, D.; Suovouri, A.; Amberger-Murphy, V.; Pilkington, G.J.; Cree, I.A. The Effect of Tricyclic Antidepressants on Cutaneous Melanoma Cell Lines and Primary Cell Cultures. Anticancer Drugs 2012, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Hou, T.; Cao, B.; Wang, W.; Li, Z.; Chen, S.; Fei, M.; Hurren, R.; Gronda, M.; Wu, D.; et al. The Tricyclic Antidepressant Amitriptyline Inhibits D-Cyclin Transactivation and Induces Myeloma Cell Apoptosis by Inhibiting Histone Deacetylases: In Vitro and in Silico Evidence. Mol. Pharmacol. 2011, 79, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, X.; Zhao, C.; Cao, B.; Zhao, Y.; Mao, X. The Antidepressant Amitriptyline Shows Potent Therapeutic Activity against Multiple Myeloma. Anticancer Drugs 2013, 24, 792–798. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeong, I.-Y.; Lim, Y.; Lee, Y.H.; Shin, S.Y. Estrogen Receptor Beta Stimulates Egr-1 Transcription via MEK1/Erk/Elk-1 Cascade in C6 Glioma Cells. BMB Rep. 2011, 44, 452–457. [Google Scholar] [CrossRef]
- Huang, G.-D.; Cui, P.; Ma, G.-X.; Chen, F.-F.; Chen, Z.-B.; Li, X.-J.; Liao, Z.-J.; Li, W.-P.; Li, Z.-Y.; Chen, L. Phragmunis a Suppresses Glioblastoma through the Regulation of MCL1-FBXW7 by Blocking ELK1-SRF Complex-Dependent Transcription. Neurochem. Int. 2021, 147, 105051. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Meng, X.; Liang, H. Modulation of Transcriptional Activity in Brain Lower Grade Glioma by Alternative Splicing. PeerJ 2018, 6, e4686. [Google Scholar] [CrossRef]
- Mahdi, A.; Aittaleb, M.; Tissir, F. Targeting Glioma Stem Cells: Therapeutic Opportunities and Challenges. Cells 2025, 14, 675. [Google Scholar] [CrossRef]
- Tang, J.; Amin, M.A.; Campian, J.L. Glioblastoma Stem Cells at the Nexus of Tumor Heterogeneity, Immune Evasion, and Therapeutic Resistance. Cells 2025, 14, 562. [Google Scholar] [CrossRef]
- Tang, J.; Karbhari, N.; Campian, J.L. Therapeutic Targets in Glioblastoma: Molecular Pathways, Emerging Strategies, and Future Directions. Cells 2025, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Golovin, A.; Dzarieva, F.; Rubetskaya, K.; Shamadykova, D.; Usachev, D.; Pavlova, G.; Kopylov, A. In Silico Born Designed Anti-EGFR Aptamer Gol1 Has Anti-Proliferative Potential for Patient Glioblastoma Cells. Int. J. Mol. Sci. 2025, 26, 1072. [Google Scholar] [CrossRef] [PubMed]
- Courot, H.; Rigal, E.; Adib, N.; Criton, M.; Cookson, A.; Fauvel, B.; Presumey, J. In Vitro Evaluation of Genetically Unmodified Ligand-Armed Allogeneic Natural Killer Cells to Treat EGFR-Positive Glioblastoma. Cells 2025, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Adiseshaiah, P.; Kalvakolanu, D.V.; Reddy, S.P. A Phosphatidylinositol 3-Kinase-Regulated Akt-Independent Signaling Promotes Cigarette Smoke-Induced FRA-1 Expression. J. Biol. Chem. 2006, 281, 10174–10181. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, H.; Sun, C.; Xu, L.; Chen, Y.; Zhu, Q.; Zhao, H.; Huang, Q.; Dong, J.; Lan, Q. GATA2 Promotes Glioma Progression through EGFR/ERK/Elk-1 Pathway. Med. Oncol. 2015, 32, 87. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, X.; Wu, Y.; Zhang, G.; Liu, X.; Li, Y.; Bao, Y.; Yang, W.; Cui, H. EGFR Activates GDH1 Transcription to Promote Glutamine Metabolism through MEK/ERK/ELK1 Pathway in Glioblastoma. Oncogene 2020, 39, 2975–2986. [Google Scholar] [CrossRef]
- Uht, R.M.; Amos, S.; Martin, P.M.; Riggan, A.E.; Hussaini, I.M. The Protein Kinase C-Eta Isoform Induces Proliferation in Glioblastoma Cell Lines through an ERK/Elk-1 Pathway. Oncogene 2007, 26, 2885–2893. [Google Scholar] [CrossRef]
- Bébien, M.; Salinas, S.; Becamel, C.; Richard, V.; Linares, L.; Hipskind, R.A. Immediate-Early Gene Induction by the Stresses Anisomycin and Arsenite in Human Osteosarcoma Cells Involves MAPK Cascade Signaling to Elk-1, CREB and SRF. Oncogene 2003, 22, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-H.; Lin, F.-L.; Hou, S.-M.; Liu, J.-F. Cyr61 Promotes Epithelial-Mesenchymal Transition and Tumor Metastasis of Osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 Signaling Pathway. Mol. Cancer 2014, 13, 236. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, H.C.; Choe, Y.-J.; Shin, S.J.; Lee, S.H.; Kim, H.-S. Nutlin-3 Induces BCL2A1 Expression by Activating ELK1 through the Mitochondrial P53-ROS-ERK1/2 Pathway. Int. J. Oncol. 2014, 45, 675–682. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Z.; Wang, X.; Xia, S.; Cai, P.; Wang, M.; Gao, Z. Knockdown of lncRNA LINC00662 Suppresses Malignant Behaviour of Osteosarcoma Cells via Competition with miR-30b-3p to Regulate ELK1 Expression. J. Orthop. Surg. Res. 2022, 17, 74. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Zhang, X.; Cao, L.; Wu, Y.; Miao, X. Transcription Factor ELK1 Accelerates Aerobic Glycolysis to Enhance Osteosarcoma Chemoresistance through miR-134/PTBP1 Signaling Cascade. Aging 2021, 13, 6804–6819. [Google Scholar] [CrossRef]
- Cesari, F.; Brecht, S.; Vintersten, K.; Vuong, L.G.; Hofmann, M.; Klingel, K.; Schnorr, J.-J.; Arsenian, S.; Schild, H.; Herdegen, T.; et al. Mice Deficient for the Ets Transcription Factor Elk-1 Show Normal Immune Responses and Mildly Impaired Neuronal Gene Activation. Mol. Cell. Biol. 2004, 24, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lesch, A.; Backes, T.M.; Langfermann, D.S.; Rössler, O.G.; Laschke, M.W.; Thiel, G. Ternary Complex Factor Regulates Pancreatic Islet Size and Blood Glucose Homeostasis in Transgenic Mice. Pharmacol. Res. 2020, 159, 104983. [Google Scholar] [CrossRef] [PubMed]


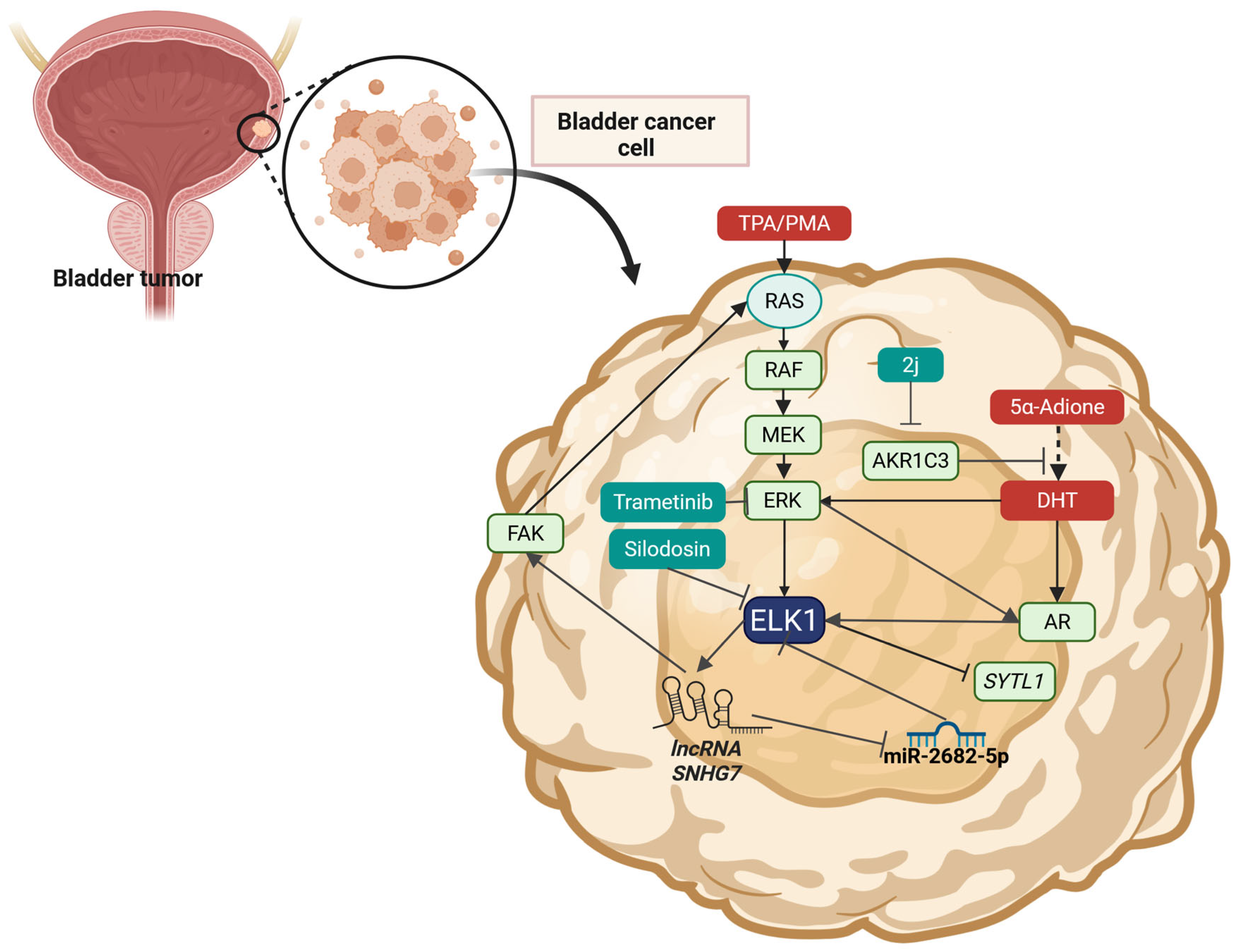
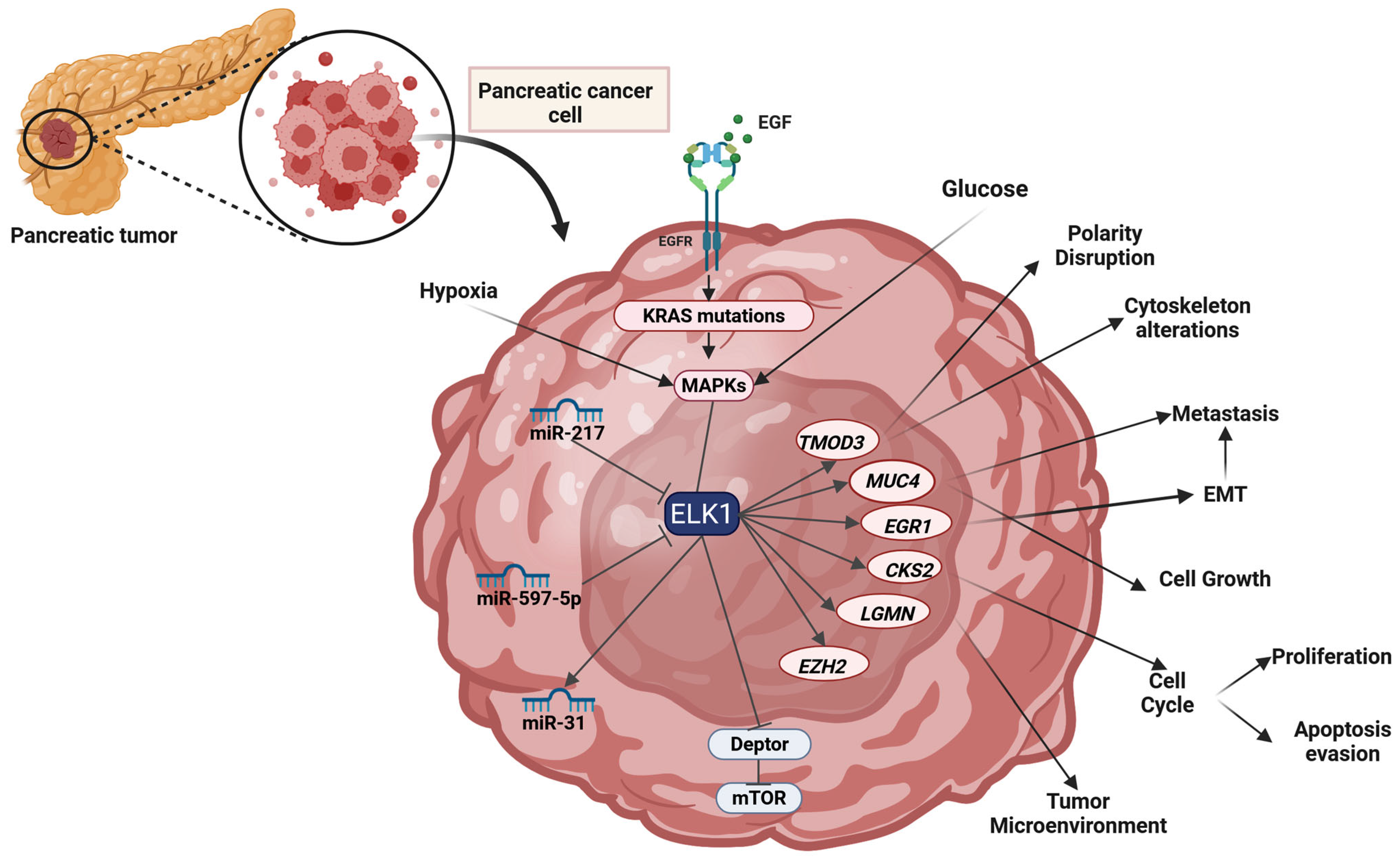
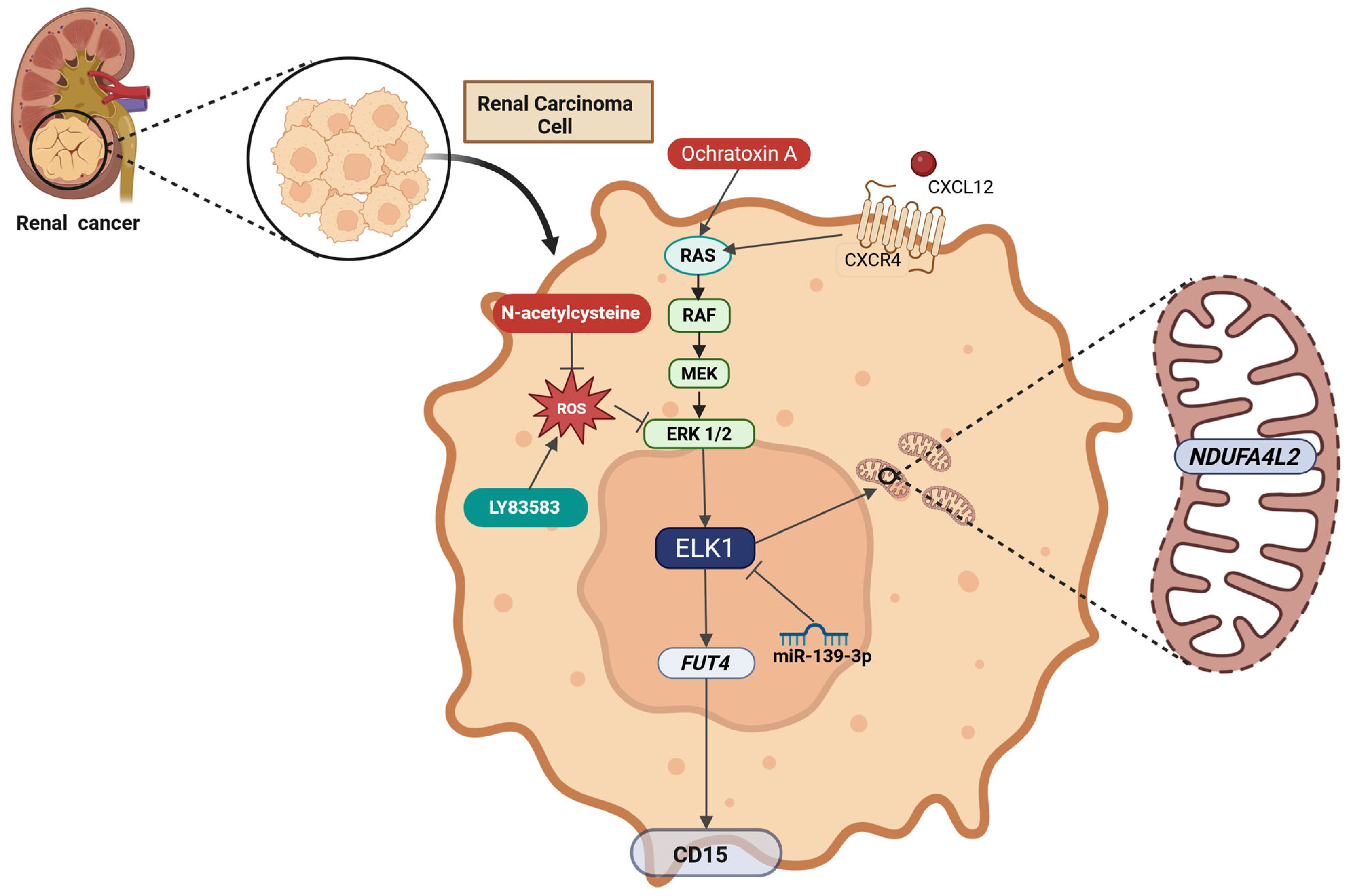

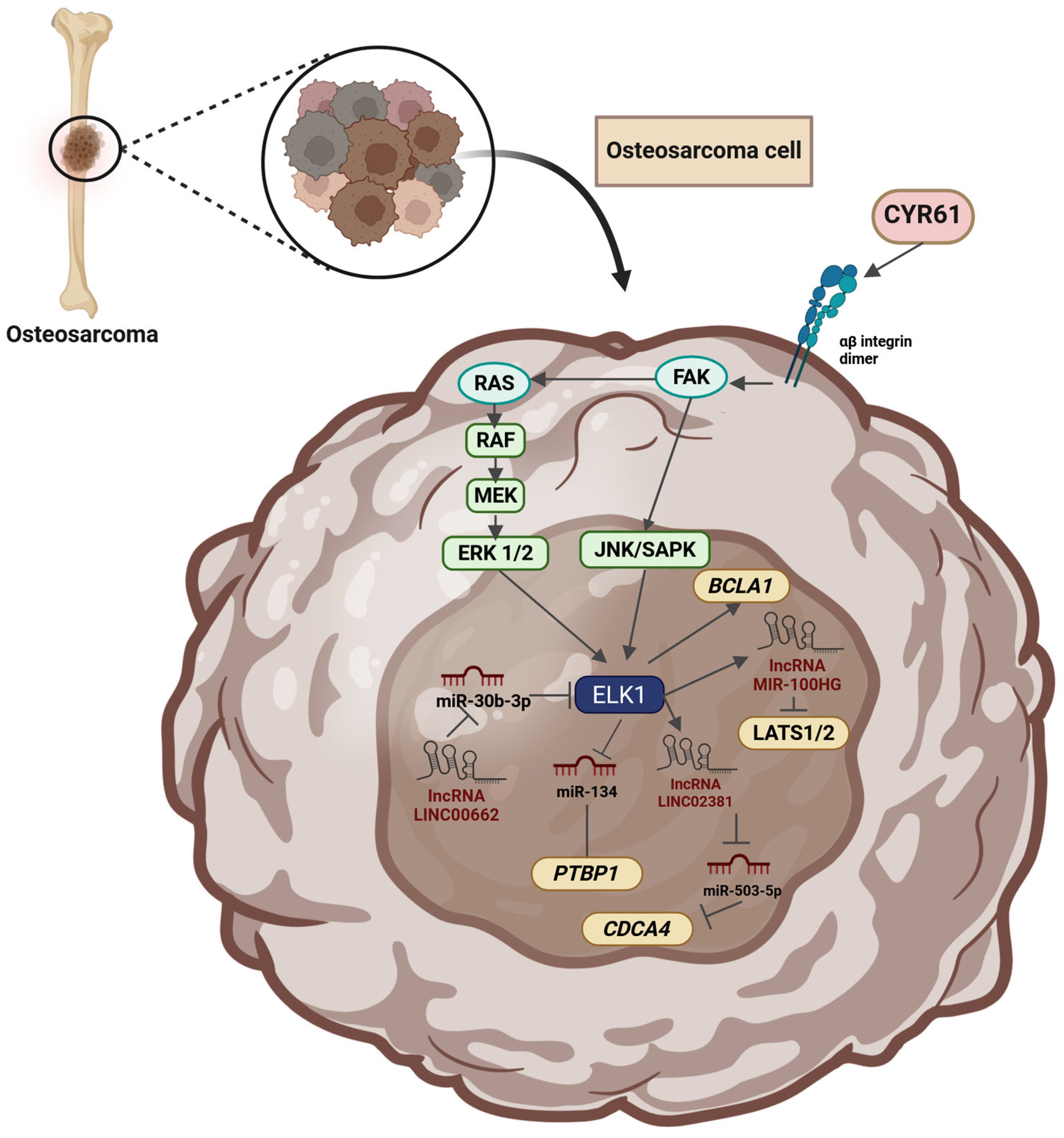
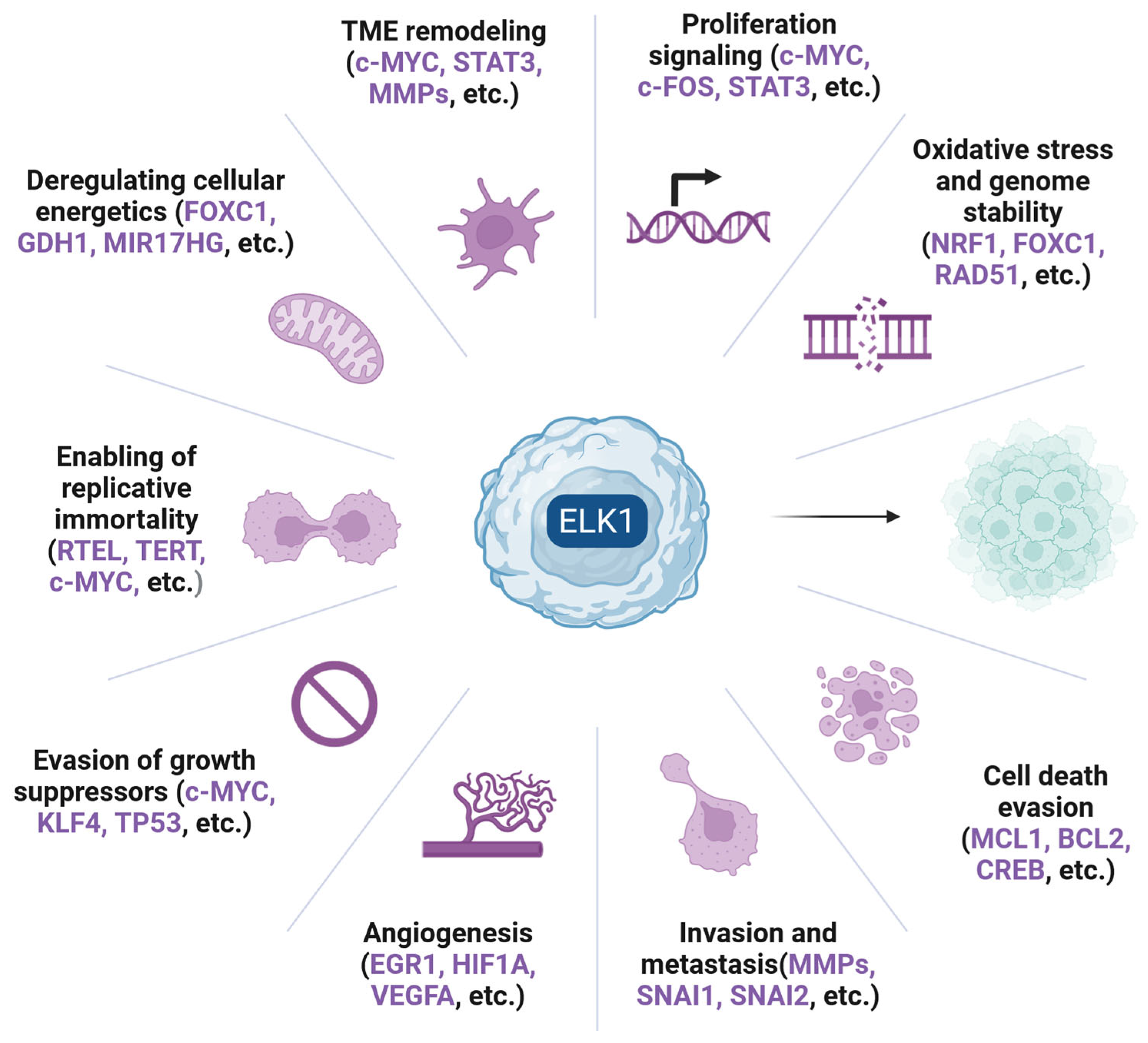
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalampounias, G.; Androutsopoulou, T.; Katsoris, P. Mechanistic Insights and Clinical Implications of ELK1 in Solid Tumors: A Narrative Review. Cells 2025, 14, 1257. https://doi.org/10.3390/cells14161257
Kalampounias G, Androutsopoulou T, Katsoris P. Mechanistic Insights and Clinical Implications of ELK1 in Solid Tumors: A Narrative Review. Cells. 2025; 14(16):1257. https://doi.org/10.3390/cells14161257
Chicago/Turabian StyleKalampounias, Georgios, Theodosia Androutsopoulou, and Panagiotis Katsoris. 2025. "Mechanistic Insights and Clinical Implications of ELK1 in Solid Tumors: A Narrative Review" Cells 14, no. 16: 1257. https://doi.org/10.3390/cells14161257
APA StyleKalampounias, G., Androutsopoulou, T., & Katsoris, P. (2025). Mechanistic Insights and Clinical Implications of ELK1 in Solid Tumors: A Narrative Review. Cells, 14(16), 1257. https://doi.org/10.3390/cells14161257









