A Biophysics of Epigenetic Rejuvenation
Abstract
1. Introduction
1.1. Epigenetics: H3K9me3-Marked Heterochromatin-Like Domains/Complexes (HLD/Cs) and H3K27me3-Marked Polycomb-Group (PcG) Domains
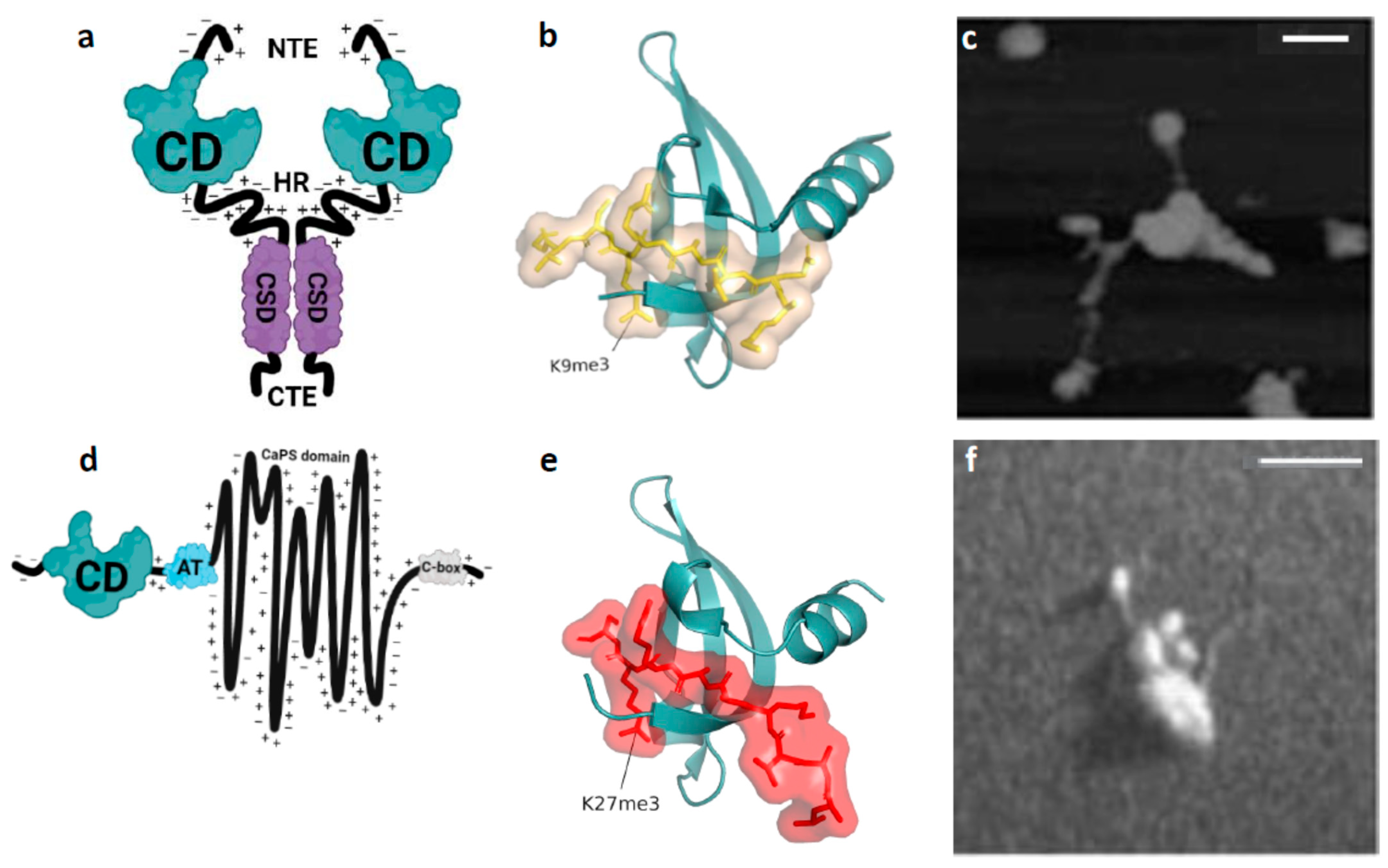
1.2. Machine Learning: Linear vs. Non-Linear Epigenetic “Clocks”
1.3. Polymer Physics: The Flory–Huggins Parameter χ
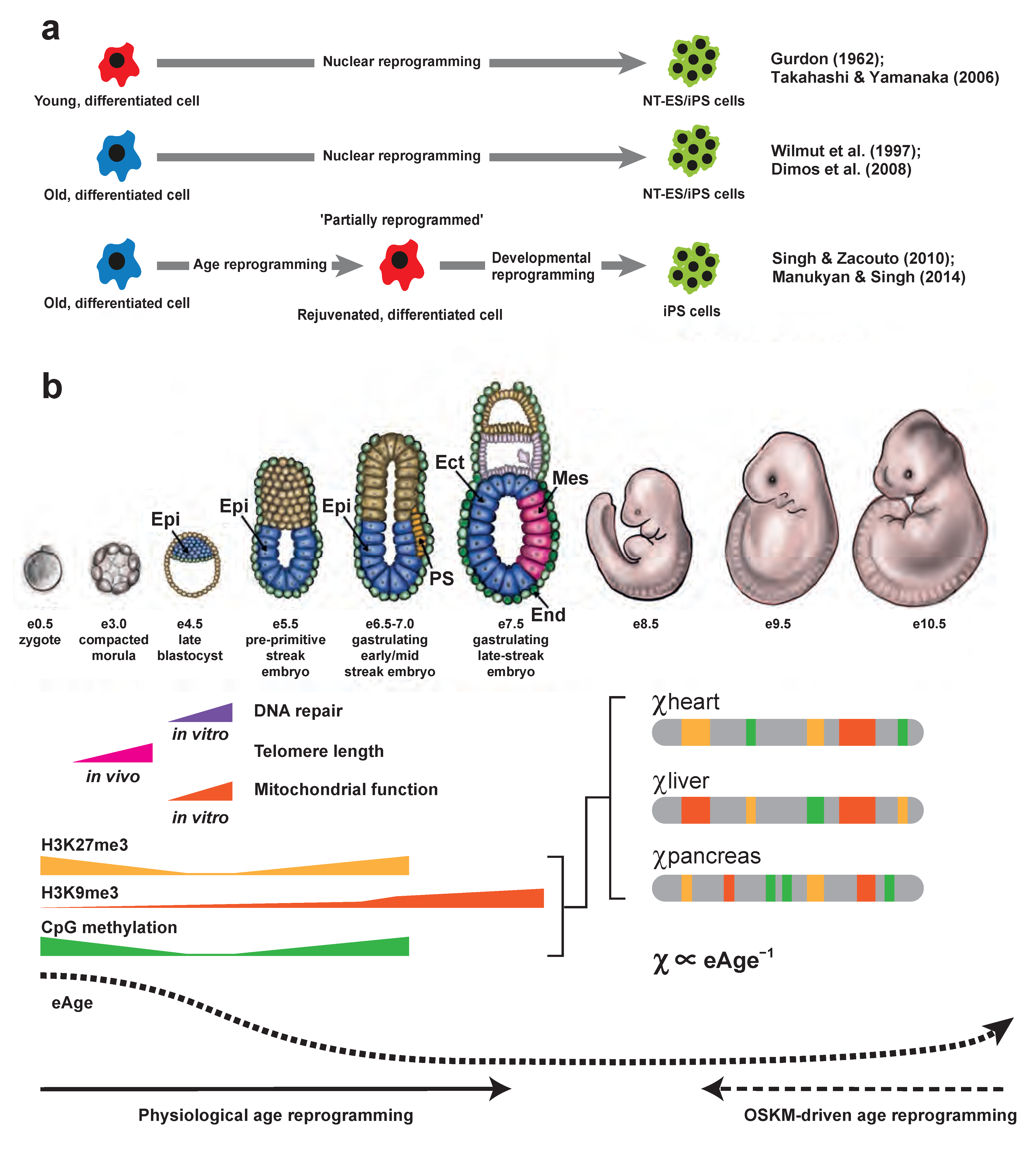
2. A Biophysical Approach for Estimation of χ Consistent with the Nuclear Environment: Changes in χ During Ageing and OSKM-Driven Age Reprogramming
3. OSKM-Driven Age Reprogramming vs. Physiological Age Reprograming: Relationship Between χ, eAge and Shannon Entropy
4. Perspectives
5. Conclusions
- . The sign and magnitude of χHC and χPC ultimately drives micro-phase separation and segregation of HLD/Cs and PcG domains away from euchromatin. The clutch equation (Equation (1)) predicts that as H3K9me3 and H3K27me3 levels decline (while eAge increases), χHC and χPC for HLD/Cs and PcG domains, respectively, decrease leading to a reduction in A/B compartmentalization along with increased A/B compartment switching. This has been observed as cells age. When H3K9me3 and H3K27me3 levels are restored during OSKM-driven epigenetic rejuvenation, χHC and χPC increase resulting in de-mixing of HLD/Cs and PcG domains away from euchromatin. Experimentally this would be observed as enhanced (sharper, more discrete) A/B compartmentalization. Use of specific inhibitors of HMTases that generate H3K9me3 and H3K27me3 would confirm that compartmentalization is driven by restoration of the epigenetic histone modifications.
- . This can be tested indirectly using, as a measure, Shannon entropy associated with the methylome. The clutch equation (Equation (1)) predicts that as H3K9me3 and H3K27me3 levels decline (while eAge increases) χHC and χPC for HLD/Cs and PcG domains, respectively, will decrease. This leads to increased disorder, i.e., increased combinatorial entropy. Given the known cross-talk between chromatin-template-dependent pathways (HLD/Cs and PcG domains) and the DNA de/methylation machinery the increase in disorder of chromatin-template dependent pathways can be measured by an increase in Shannon entropy associated with the methylome. Restoration of H3K9me3 and H3K27me3 levels by OSKM-driven epigenetic rejuvenation will increase χHC and χPC for HLD/Cs and PcG domains that can be measured by a reduction in Shannon entropy associated with the methylome. Use of specific inhibitors of HMTases that generate H3K9me3 and H3K27me3 would confirm that reduced Shannon entropy of the methylome is driven by restoration of the epigenetic histone modifications.
Funding
Acknowledgments
Conflicts of Interest
References
- Allis, D.; Caparros, M.L.; Jenuwein, T.; Reinberg, D. Epigenetics, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015. [Google Scholar]
- Reinberg, D.; Vales, L.D. Chromatin Domains Rich in Inheritance. Science 2018, 361, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Cowell, I.; Aucott, R.; Mahadevaiah, S.; Huskisson, N.; Bongiorni, S.; Prantera, G.; Fanti, L.; Pimpinelli, S.; Wu, R.; Gilbert, D.; et al. Heterochromatin, HP1 and Methylation at Lysine 9 of Histone H3 in Animals. Chromosoma 2002, 111, 22–36. [Google Scholar] [CrossRef]
- Grewal, S.I.S.; Elgin, S.C.R. Heterochromatin: New Possibilities for the Inheritance of Structure. Curr. Opin. Genet. Dev. 2002, 12, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Schuettengruber, B.; Bourbon, H.-M.; Di Croce, L.; Cavalli, G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 2017, 171, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Lachner, M.; O’Carroll, D.; Rea, S.; Mechtler, K.; Jenuwein, T. Methylation of Histone H3 Lysine 9 Creates a Binding Site for HP1 Proteins. Nature 2001, 410, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Zegerman, P.; Partridge, J.F.; Miska, E.A.; Thomas, J.O.; Allshire, R.C.; Kouzarides, T. Selective Recognition of Methylated Lysine 9 on Histone H3 by the HP1 Chromo Domain. Nature 2001, 410, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.R.; Nietlispach, D.; Mott, H.R.; Callaghan, J.; Bannister, A.; Kouzarides, T.; Murzin, A.G.; Murzina, N.V.; Laue, E.D. Structure of the HP1 Chromodomain Bound to Histone H3 Methylated at Lysine 9. Nature 2002, 416, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Takizawa, Y.; Ishimaru, M.; Sugita, Y.; Sekine, S.; Nakayama, J.; Wolf, M.; Kurumizaka, H. Structural Basis of Heterochromatin Formation by Human HP1. Mol. Cell 2018, 69, 385–397.e8. [Google Scholar] [CrossRef] [PubMed]
- Azzaz, A.M.; Vitalini, M.W.; Thomas, A.S.; Price, J.P.; Blacketer, M.J.; Cryderman, D.E.; Zirbel, L.N.; Woodcock, C.L.; Elcock, A.H.; Wallrath, L.L.; et al. Human Heterochromatin Protein 1α Promotes Nucleosome Associations That Drive Chromatin Condensation. J. Biol. Chem. 2014, 289, 6850–6861. [Google Scholar] [CrossRef]
- Hiragami-Hamada, K.; Soeroes, S.; Nikolov, M.; Wilkins, B.; Kreuz, S.; Chen, C.; De La Rosa-Velázquez, I.A.; Zenn, H.M.; Kost, N.; Pohl, W.; et al. Dynamic and Flexible H3K9me3 Bridging via HP1β Dimerization Establishes a Plastic State of Condensed Chromatin. Nat. Commun. 2016, 7, 11310. [Google Scholar] [CrossRef]
- Singh, P.B.; Miller, J.R.; Pearce, J.; Kothary, R.; Burton, R.D.; Paro, R.; James, T.C.; Gaunt, S.J. A Sequence Motif Found in a Drosophila Heterochromatin Protein Is Conserved in Animals and Plants. Nucleic Acids Res. 1991, 19, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.B. Molecular Mechanisms of Cellular Determination: Their Relation to Chromatin Structure and Parental Imprinting. J. Cell Sci. 1994, 107, 2653–2668. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.B. Heterochromatin and the Molecular Mechanisms of ‘Parent-of-Origin’ Effects in Animals. J. Biosci. 2016, 41, 759–786. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.B.; Newman, A.G. On the relations of phase separation and Hi-C maps to epigenetics. R. Soc. Open Sci. 2020, 7, 191976. [Google Scholar] [CrossRef]
- Singh, P.B.; Newman, A.G. HP1-Driven Micro-Phase Separation of Heterochromatin-Like Domains/Complexes. Epigenet. Insights 2022, 15, 25168657221109766. [Google Scholar] [CrossRef]
- Singh, P.B.; Belyakin, S.N.; Laktionov, P.P. Biology and Physics of Heterochromatin-Like Domains/Complexes. Cells 2020, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Merino-Urteaga, R.; Karwacki-Neisius, V.; Carrizo, G.E.; Athreya, A.; Marin-Gonzalez, A.; Benning, N.A.; Park, J.; Mitchener, M.M.; Bhanu, N.V.; et al. Native Nucleosomes Intrinsically Encode Genome Organization Principles. Nature 2025, 643, 572–581. [Google Scholar] [CrossRef]
- Spracklin, G.; Abdennur, N.; Imakaev, M.; Chowdhury, N.; Pradhan, S.; Mirny, L.A.; Dekker, J. Diverse Silent Chromatin States Modulate Genome Compartmentalization and Loop Extrusion Barriers. Nat. Struct. Mol. Biol. 2023, 30, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Donahue, G.; Gilbert, M.B.; Lapidot, T.; Nicetto, D.; Zaret, K.S. Distinct H3K9me3 Heterochromatin Maintenance Dynamics Govern Different Gene Programmes and Repeats in Pluripotent Cells. Nat. Cell Biol. 2024, 26, 2115–2128. [Google Scholar] [CrossRef]
- Miller, E.L.; Hargreaves, D.C.; Kadoch, C.; Chang, C.-Y.; Calarco, J.P.; Hodges, C.; Buenrostro, J.D.; Cui, K.; Greenleaf, W.J.; Zhao, K.; et al. TOP2 Synergizes with BAF Chromatin Remodeling for Both Resolution and Formation of Facultative Heterochromatin. Nat. Struct. Mol. Biol. 2017, 24, 344–352. [Google Scholar] [CrossRef]
- Blackledge, N.P.; Klose, R.J. The Molecular Principles of Gene Regulation by Polycomb Repressive Complexes. Nat. Rev. Mol. Cell Biol. 2021, 22, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kingston, R.E. Context-Specific Polycomb Mechanisms in Development. Nat. Rev. Genet. 2022, 23, 680–695. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Y.; Xu, R.-M. Structural Basis for Specific Binding of Polycomb Chromodomain to Histone H3 Methylated at Lys 27. Genes Dev. 2003, 17, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Grau, D.J.; Chapman, B.A.; Garlick, J.D.; Borowsky, M.; Francis, N.J.; Kingston, R.E. Compaction of Chromatin by Diverse Polycomb Group Proteins Requires Localized Regions of High Charge. Genes Dev. 2011, 25, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kingston, R.E. The CBX Family of Proteins in Transcriptional Repression and Memory. J. Biosci. 2020, 45, 16. [Google Scholar] [CrossRef]
- Vogel, M.J.; Guelen, L.; de Wit, E.; Hupkes, D.P.; Lodén, M.; Talhout, W.; Feenstra, M.; Abbas, B.; Classen, A.-K.; van Steensel, B. Human Heterochromatin Proteins Form Large Domains Containing KRAB-ZNF Genes. Genome Res. 2006, 16, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Groner, A.C.; Meylan, S.; Ciuffi, A.; Zangger, N.; Ambrosini, G.; Dénervaud, N.; Bucher, P.; Trono, D. KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading. PLoS Genet. 2010, 6, e1000869. [Google Scholar] [CrossRef]
- Rao, S.S.P.; Huntley, M.H.; Durand, N.C.; Stamenova, E.K.; Bochkov, I.D.; Robinson, J.T.; Sanborn, A.L.; Machol, I.; Omer, A.D.; Lander, E.S.; et al. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell 2014, 159, 1665–1680. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Jiang, Q.; Gong, T.; Fan, D.; Zhang, J.; Chen, F.; Zhu, X.; Wang, X.; Qiao, Y.; Chen, H.; et al. Loss of Grand Histone H3 Lysine 27 Trimethylation Domains Mediated Transcriptional Activation in Esophageal Squamous Cell Carcinoma. NPJ Genom. Med. 2021, 6, 65. [Google Scholar] [CrossRef]
- Hildebrand, E.M.; Dekker, J. Mechanisms and Functions of Chromosome Compartmentalization. Trends Biochem. Sci. 2020, 45, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Davletgildeeva, A.T.; Kuznetsov, N.A. The Role of DNMT Methyltransferases and TET Dioxygenases in the Maintenance of the DNA Methylation Level. Biomolecules 2024, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Johnson, L.M.; Jacobsen, S.E.; Patel, D.J. DNA Methylation Pathways and Their Crosstalk with Histone Methylation. Nat. Rev. Mol. Cell Biol. 2015, 16, 519–532. [Google Scholar] [CrossRef]
- Allshire, R.C.; Madhani, H.D. Ten Principles of Heterochromatin Formation and Function. Nat. Rev. Mol. Cell Biol. 2018, 19, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, H.; Wang, Q.; Zhou, C.; Wei, L.; Liu, X.; Zhang, W.; Zhang, Y.; Du, Z.; Wang, X.; et al. Genome-Wide Analyses Reveal a Role of Polycomb in Promoting Hypomethylation of DNA Methylation Valleys. Genome Biol. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R.; Klose, R.J. Understanding the Relationship between DNA Methylation and Histone Lysine Methylation. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2014, 1839, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Seman, M.; Fyodorova, Y.; Farhat, A.; Ames, A.; Levashkevich, A.; Biswas, S.; Huang, F.; Freddolino, L.; Biteen, J.S.; et al. Tracking Live-Cell Single-Molecule Dynamics Enables Measurements of Heterochromatin-Associated Protein–Protein Interactions. Nucleic Acids Res 2024, 52, 10731–10746. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Sun, L.; Kokura, K.; Horton, J.R.; Fukuda, M.; Espejo, A.; Izumi, V.; Koomen, J.M.; Bedford, M.T.; Zhang, X.; et al. MPP8 Mediates the Interactions between DNA Methyltransferase Dnmt3a and H3K9 Methyltransferase GLP/G9a. Nat Commun 2011, 2, 533. [Google Scholar] [CrossRef] [PubMed]
- Fuks, F. The DNA Methyltransferases Associate with HP1 and the SUV39H1 Histone Methyltransferase. Nucleic Acids Res. 2003, 31, 2305–2312. [Google Scholar] [CrossRef]
- Smallwood, A.; Estève, P.-O.; Pradhan, S.; Carey, M. Functional Cooperation between HP1 and DNMT1 Mediates Gene Silencing. Genes Dev. 2007, 21, 1169–1178. [Google Scholar] [CrossRef]
- Taglini, F.; Kafetzopoulos, I.; Rolls, W.; Musialik, K.I.; Lee, H.Y.; Zhang, Y.; Marenda, M.; Kerr, L.; Finan, H.; Rubio-Ramon, C.; et al. DNMT3B PWWP Mutations Cause Hypermethylation of Heterochromatin. EMBO Rep. 2024, 25, 1130–1155. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.; Koche, R.P.; Rheinbay, E.; Mendenhall, E.M.; Endoh, M.; Mikkelsen, T.S.; Presser, A.; Nusbaum, C.; Xie, X.; Chi, A.S.; et al. Genomewide Analysis of PRC1 and PRC2 Occupancy Identifies Two Classes of Bivalent Domains. PLoS Genet. 2008, 4, e1000242. [Google Scholar] [CrossRef] [PubMed]
- Long, H.K.; Sims, D.; Heger, A.; Blackledge, N.P.; Kutter, C.; Wright, M.L.; Grützner, F.; Odom, D.T.; Patient, R.; Ponting, C.P.; et al. Epigenetic Conservation at Gene Regulatory Elements Revealed by Non-Methylated DNA Profiling in Seven Vertebrates. eLife 2013, 2, e00348. [Google Scholar] [CrossRef]
- Hon, G.C.; Rajagopal, N.; Shen, Y.; McCleary, D.F.; Yue, F.; Dang, M.D.; Ren, B. Epigenetic Memory at Embryonic Enhancers Identified in DNA Methylation Maps from Adult Mouse Tissues. Nat. Genet. 2013, 45, 1198–1206. [Google Scholar] [CrossRef]
- Neri, F.; Incarnato, D.; Krepelova, A.; Rapelli, S.; Pagnani, A.; Zecchina, R.; Parlato, C.; Oliviero, S. Genome-Wide Analysis Identifies a Functional Association of Tet1 and Polycomb Repressive Complex 2 in Mouse Embryonic Stem Cells. Genome Biol. 2013, 14, R91. [Google Scholar] [CrossRef]
- Williams, K.; Christensen, J.; Helin, K. DNA Methylation: TET Proteins—Guardians of CpG Islands? EMBO Rep. 2012, 13, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, J.; Oh, H.; Wyss-Coray, T. Measuring Biological Age Using Omics Data. Nat. Rev. Genet. 2022, 23, 715–727. [Google Scholar] [CrossRef]
- Simpson, D.J.; Chandra, T. Epigenetic Age Prediction. Aging Cell 2021, 20, e13452. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.B.; Zhakupova, A. Age Reprogramming: Cell Rejuvenation by Partial Reprogramming. Development 2022, 149, dev200755. [Google Scholar] [CrossRef] [PubMed]
- Paine, P.T.; Nguyen, A.; Ocampo, A. Partial Cellular Reprogramming: A Deep Dive into an Emerging Rejuvenation Technology. Aging Cell 2024, 23, e14039. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, M.; Singh, P.B. Epigenome Rejuvenation: HP1β Mobility as a Measure of Pluripotent and Senescent Chromatin Ground States. Sci. Rep. 2014, 4, 4789. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, C.; Ji, Y.; Yang, B.; Shu, J.; Shi, K.; Wang, L.; Wang, S.; Zhang, Y.; Huang, X.; et al. Partial Reprogramming Strategy for Intervertebral Disc Rejuvenation by Activating Energy Switch. Aging Cell 2022, 21, e13577. [Google Scholar] [CrossRef]
- Gill, D.; Parry, A.; Santos, F.; Okkenhaug, H.; Todd, C.D.; Hernando-Herraez, I.; Stubbs, T.M.; Milagre, I.; Reik, W. Multi-Omic Rejuvenation of Human Cells by Maturation Phase Transient Reprogramming. eLife 2022, 11, e71624. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E.; et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 2016, 167, 1719–1733.e12. [Google Scholar] [CrossRef]
- Rodríguez-Matellán, A.; Alcazar, N.; Hernández, F.; Serrano, M.; Ávila, J. In Vivo Reprogramming Ameliorates Aging Features in Dentate Gyrus Cells and Improves Memory in Mice. Stem. Cell Rep. 2020, 15, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S.; et al. Transient Non-Integrative Expression of Nuclear Reprogramming Factors Promotes Multifaceted Amelioration of Aging in Human Cells. Nat. Commun. 2020, 11, 1545. [Google Scholar] [CrossRef] [PubMed]
- Olova, N.; Simpson, D.J.; Marioni, R.E.; Chandra, T. Partial Reprogramming Induces a Steady Decline in Epigenetic Age before Loss of Somatic Identity. Aging Cell 2019, 18, e12877. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.; Moqri, M.; Maybury-Lewis, S.Y.; Rogers-Hammond, R.; de Jong, T.A.; Parker, A.; Rasouli, S.; Schöler, H.R.; Sinclair, D.A.; Sebastiano, V. Mechanisms, Pathways and Strategies for Rejuvenation through Epigenetic Reprogramming. Nat. Aging 2023, 4, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef]
- Raj, K.; Horvath, S. Current Perspectives on the Cellular and Molecular Features of Epigenetic Ageing. Exp. Biol. Med. 2020, 245, 1532–1542. [Google Scholar] [CrossRef]
- Olova, N.N. Epigenetic rejuvenation: A journey backwards towards an epigenomic ground state. Epigenomics 2025, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.T.; Fei, Z.; Haghani, A.; Robeck, T.R.; Zoller, J.A.; Li, C.Z.; Lowe, R.; Yan, Q.; Zhang, J.; Vu, H.; et al. Universal DNA Methylation Age across Mammalian Tissues. Nat. Aging 2023, 3, 1144–1166. [Google Scholar] [CrossRef] [PubMed]
- Moqri, M.; Cipriano, A.; Simpson, D.J.; Rasouli, S.; Murty, T.; de Jong, T.A.; Nachun, D.; de Sena Brandine, G.; Ying, K.; Tarkhov, A.; et al. PRC2-AgeIndex as a Universal Biomarker of Aging and Rejuvenation. Nat. Commun. 2024, 15, 5956. [Google Scholar] [CrossRef]
- de Lima Camillo, L.P.; Lapierre, L.R.; Singh, R. A Pan-Tissue DNA-Methylation Epigenetic Clock Based on Deep Learning. NPJ Aging 2022, 8, 4. [Google Scholar] [CrossRef]
- Bates, F.S. Polymer-Polymer Phase Behavior. Science 1991, 251, 898–905. [Google Scholar] [CrossRef]
- Jost, D.; Carrivain, P.; Cavalli, G.; Vaillant, C. Modeling Epigenome Folding: Formation and Dynamics of Topologically Associated Chromatin Domains. Nucleic Acids Res. 2014, 42, 9553–9561. [Google Scholar] [CrossRef]
- Chiariello, A.M.; Annunziatella, C.; Bianco, S.; Esposito, A.; Nicodemi, M. Polymer Physics of Chromosome Large-Scale 3D Organisation. Sci. Rep. 2016, 6, 29775. [Google Scholar] [CrossRef]
- Nuebler, J.; Fudenberg, G.; Imakaev, M.; Abdennur, N.; Mirny, L.A. Chromatin Organization by an Interplay of Loop Extrusion and Compartmental Segregation. Proc. Natl. Acad. Sci. USA 2018, 115, E6697–E6706. [Google Scholar] [CrossRef]
- Zenk, F.; Zhan, Y.; Kos, P.; Löser, E.; Atinbayeva, N.; Schächtle, M.; Tiana, G.; Giorgetti, L.; Iovino, N. HP1 Drives de Novo 3D Genome Reorganization in Early Drosophila Embryos. Nature 2021, 593, 289–293. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1941, 9, 660. [Google Scholar] [CrossRef]
- Huggins, M.L. Solutions of Long Chain Compounds. J. Chem. Phys. 1941, 9, 440. [Google Scholar] [CrossRef]
- Belaghzal, H.; Borrman, T.; Stephens, A.D.; Lafontaine, D.L.; Venev, S.V.; Weng, Z.; Marko, J.F.; Dekker, J. Liquid Chromatin Hi-C Characterizes Compartment-Dependent Chromatin Interaction Dynamics. Nat. Genet. 2021, 53, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Thorn, G.J.; Clarkson, C.T.; Rademacher, A.; Mamayusupova, H.; Schotta, G.; Rippe, K.; Teif, V.B. DNA Sequence-Dependent Formation of Heterochromatin Nanodomains. Nat. Commun. 2022, 13, 1861. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.A.; Manzo, C.; García-Parajo, M.F.; Lakadamyali, M.; Cosma, M.P. Chromatin Fibers Are Formed by Heterogeneous Groups of Nucleosomes In Vivo. Cell 2015, 160, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Ruan, J.; Xu, C.; Tong, Y.; Pan, P.W.; Tempel, W.; Crombet, L.; Min, J.; Zang, J. Structural Basis for Specific Binding of Human MPP8 Chromodomain to Histone H3 Methylated at Lysine 9. PLoS ONE 2011, 6, e25104. [Google Scholar] [CrossRef]
- Kaustov, L.; Ouyang, H.; Amaya, M.; Lemak, A.; Nady, N.; Duan, S.; Wasney, G.A.; Li, Z.; Vedadi, M.; Schapira, M.; et al. Recognition and Specificity Determinants of the Human Cbx Chromodomains. J. Biol. Chem. 2011, 286, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.H.; Corces, V.G. Principles of 3D Compartmentalization of the Human Genome. Cell Rep. 2021, 35, 109330. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, C.; Yang, N.; Desdín-Micó, G.; Alonso-Calleja, A.; Vílchez-Acosta, A.; Pico, S.; Parras, A.; Piao, Y.; Schoenfeldt, L.; Luo, S.; et al. Loss of H3K9 Trimethylation Leads to Premature Aging. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, E.W.; Croteau, D.L.; Bohr, V.A. Heterochromatin: An Epigenetic Point of View in Aging. Exp. Mol. Med. 2020, 52, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Cheutin, T.; McNairn, A.J.; Jenuwein, T.; Gilbert, D.M.; Singh, P.B.; Misteli, T. Maintenance of Stable Heterochromatin Domains by Dynamic HP1 Binding. Science 2003, 299, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Fischle, W.; Tseng, B.S.; Dormann, H.L.; Ueberheide, B.M.; Garcia, B.A.; Shabanowitz, J.; Hunt, D.F.; Funabiki, H.; Allis, C.D. Regulation of HP1–Chromatin Binding by Histone H3 Methylation and Phosphorylation. Nature 2005, 438, 1116–1122. [Google Scholar] [CrossRef]
- Wenger, A.; Biran, A.; Alcaraz, N.; Redó-Riveiro, A.; Sell, A.C.; Krautz, R.; Flury, V.; Reverón-Gómez, N.; Solis-Mezarino, V.; Völker-Albert, M.; et al. Symmetric Inheritance of Parental Histones Governs Epigenome Maintenance and Embryonic Stem Cell Identity. Nat. Genet. 2023, 55, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Occean, J.R.; Melters, D.P.; Shi, C.; Wang, L.; Stransky, S.; Doyle, M.E.; Cui, C.-Y.; Delannoy, M.; Fan, J.; et al. A Hyper-Quiescent Chromatin State Formed during Aging Is Reversed by Regeneration. Mol. Cell 2023, 83, 1659–1676.e11. [Google Scholar] [CrossRef]
- Zhen, C.Y.; Duc, H.N.; Kokotovic, M.; Phiel, C.J.; Ren, X. Cbx2 Stably Associates with Mitotic Chromosomes via a PRC2- or PRC1-Independent Mechanism and Is Needed for Recruiting PRC1 Complex to Mitotic Chromosomes. Mol. Biol. Cell 2014, 25, 3726–3739. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Horsley, D.; Ma, A.; Otte, A.P.; Hutchings, A.; Butcher, G.W.; Singh, P.B. M33, a Mammalian Homologue of Drosophila Polycomb Localises to Euchromatin within Interphase Nuclei but Is Enriched within the Centromeric Heterochromatin of Metaphase Chromosomes. Cytogenet. Genome Res. 1997, 78, 50–55. [Google Scholar] [CrossRef]
- Liu, Z.; Belmonte, J.C.I.; Zhang, W.; Qu, J.; Liu, G.-H. Deciphering Aging at Three-Dimensional Genomic Resolution. Cell Insight 2022, 1, 100034. [Google Scholar] [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to Recover Youthful Epigenetic Information and Restore Vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef]
- Browder, K.C.; Reddy, P.; Yamamoto, M.; Haghani, A.; Guillen, I.G.; Sahu, S.; Wang, C.; Luque, Y.; Prieto, J.; Shi, L.; et al. In Vivo Partial Reprogramming Alters Age-Associated Molecular Changes during Physiological Aging in Mice. Nat. Aging 2022, 2, 243–253. [Google Scholar] [CrossRef]
- Gladyshev, V.N. The Ground Zero of Organismal Life and Aging. Trends Mol. Med. 2021, 27, 11–19. [Google Scholar] [CrossRef]
- Gurdon, J.B. The Developmental Capacity of Nuclei Taken from Intestinal Epithelium Cells of Feeding Tadpoles. J. Embryol. Exp. Morphol. 1962, 10, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H.S. Viable Offspring Derived from Fetal and Adult Mammalian Cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef]
- Singh, P.B.; Zacouto, F. Nuclear Reprogramming and Epigenetic Rejuvenation. J. Biosci. 2010, 35, 315–319. [Google Scholar] [CrossRef]
- Nicetto, D.; Donahue, G.; Jain, T.; Peng, T.; Sidoli, S.; Sheng, L.; Montavon, T.; Becker, J.S.; Grindheim, J.M.; Blahnik, K.; et al. H3K9me3-Heterochromatin Loss at Protein-Coding Genes Enables Developmental Lineage Specification. Science 2019, 363, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Gao, Y.; Yang, L.; Li, C.; Liu, W.; Chen, C.; Kou, X.; Zhao, Y.; Chen, J.; et al. Reprogramming of H3K9me3-Dependent Heterochromatin during Mammalian Embryo Development. Nat. Cell Biol. 2018, 20, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, B.; Zhang, B.; Xiang, Y.; Du, Z.; Xu, Q.; Li, Y.; Wang, Q.; Ma, J.; Peng, X.; et al. Resetting Epigenetic Memory by Reprogramming of Histone Modifications in Mammals. Mol. Cell 2016, 63, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Auclair, G.; Guibert, S.; Bender, A.; Weber, M. Ontogeny of CpG Island Methylation and Specificity of DNMT3 Methyltransferases during Embryonic Development in the Mouse. Genome Biol. 2014, 15, 545. [Google Scholar] [CrossRef]
- Kerepesi, C.; Zhang, B.; Lee, S.-G.; Trapp, A.; Gladyshev, V.N. Epigenetic Clocks Reveal a Rejuvenation Event during Embryogenesis Followed by Aging. Sci. Adv. 2021, 7, eabg6082. [Google Scholar] [CrossRef]
- Argelaguet, R.; Clark, S.J.; Mohammed, H.; Stapel, L.C.; Krueger, C.; Kapourani, C.-A.; Imaz-Rosshandler, I.; Lohoff, T.; Xiang, Y.; Hanna, C.W.; et al. Multi-Omics Profiling of Mouse Gastrulation at Single-Cell Resolution. Nature 2019, 576, 487–491. [Google Scholar] [CrossRef]
- Duboule, D. Temporal Colinearity and the Phylotypic Progression: A Basis for the Stability of a Vertebrate Bauplan and the Evolution of Morphologies through Heterochrony. Dev. Suppl. 1994, 1994, 135–142. [Google Scholar] [CrossRef]
- Nicetto, D.; Zaret, K.S. Role of H3K9me3 Heterochromatin in Cell Identity Establishment and Maintenance. Curr. Opin. Genet. Dev. 2019, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Noordermeer, D.; Leleu, M.; Splinter, E.; Rougemont, J.; De Laat, W.; Duboule, D. The Dynamic Architecture of Hox Gene Clusters. Science 2011, 334, 222–225. [Google Scholar] [CrossRef]
- Deschamps, J.; Duboule, D. Embryonic Timing, Axial Stem Cells, Chromatin Dynamics, and the Hox Clock. Genes Dev. 2017, 31, 1406–1416. [Google Scholar] [CrossRef]
- Bogdanović, O.; Smits, A.H.; de la Calle Mustienes, E.; Tena, J.J.; Ford, E.; Williams, R.; Senanayake, U.; Schultz, M.D.; Hontelez, S.; van Kruijsbergen, I.; et al. Active DNA Demethylation at Enhancers during the Vertebrate Phylotypic Period. Nat. Genet. 2016, 48, 417–426. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Sys. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Krone, M.W.; Albanese, K.I.; Leighton, G.O.; He, C.Q.; Lee, G.Y.; Garcia-Borràs, M.; Guseman, A.J.; Williams, D.C.; Houk, K.N.; Brustad, E.M.; et al. Thermodynamic Consequences of Tyr to Trp Mutations in the Cation–π-Mediated Binding of Trimethyllysine by the HP1 Chromodomain. Chem. Sci. 2020, 11, 3495–3500. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.L.; Kaeding, K.E.; Keller, S.H.; Zhong, Y.; Xu, L.; Hsieh, A.; Hou, Y.; Donahue, G.; Becker, J.S.; Alberto, O.; et al. Diverse Heterochromatin-Associated Proteins Repress Distinct Classes of Genes and Repetitive Elements. Nat. Cell Biol. 2021, 23, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Eskeland, R.; Eberharter, A.; Imhof, A. HP1 Binding to Chromatin Methylated at H3K9 Is Enhanced by Auxiliary Factors. Mol. Cell Biol. 2007, 27, 453–465. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, Y.; Lin, E.; Liu, F.; Shu, L.; Jing, D.; Wang, B.; Wang, M.; Shan, F.; Zhang, L.; et al. Genome Folding Principles Uncovered in Condensin-Depleted Mitotic Chromosomes. Nat. Genet. 2024, 56, 1213–1224. [Google Scholar] [CrossRef]
- Lu, Y.R.; Tian, X.; Sinclair, D.A. The Information Theory of Aging. Nat. Aging 2023, 3, 1486–1499. [Google Scholar] [CrossRef] [PubMed]
- Hishida, T.; Yamamoto, M.; Hishida-Nozaki, Y.; Shao, C.; Huang, L.; Wang, C.; Shojima, K.; Xue, Y.; Hang, Y.; Shokhirev, M.; et al. In Vivo Partial Cellular Reprogramming Enhances Liver Plasticity and Regeneration. Cell Rep. 2022, 39, 110730. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.J.; Zhao, Q.; Olova, N.N.; Dabrowski, J.; Xie, X.; Latorre-Crespo, E.; Chandra, T. Region-Based Epigenetic Clock Design Improves RRBS-Based Age Prediction. Aging Cell 2023, 22, e13866. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Rubbi, L.; Pellegrini, M. DNA Methylation Entropy Is a Biomarker for Aging. Aging 2025, 17, 685–698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.B. A Biophysics of Epigenetic Rejuvenation. Cells 2025, 14, 1249. https://doi.org/10.3390/cells14161249
Singh PB. A Biophysics of Epigenetic Rejuvenation. Cells. 2025; 14(16):1249. https://doi.org/10.3390/cells14161249
Chicago/Turabian StyleSingh, Prim B. 2025. "A Biophysics of Epigenetic Rejuvenation" Cells 14, no. 16: 1249. https://doi.org/10.3390/cells14161249
APA StyleSingh, P. B. (2025). A Biophysics of Epigenetic Rejuvenation. Cells, 14(16), 1249. https://doi.org/10.3390/cells14161249




