Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now?
Abstract
1. Introduction
2. Burden of Autoimmune Rheumatic Disorders
3. Clinical Studies on CAR-T Cell Immunotherapy for Autoimmune Rheumatic Disorders
3.1. Systemic Lupus Erythematosus (SLE)
3.2. Systemic Sclerosis (SSc)
3.3. Rheumatoid Arthritis (RA)
3.4. IIMs
3.5. Antiphospholipid Syndrome (APS)
3.6. Primary Sjögren’s Syndrome (pSS)
3.7. Juvenile Idiopathic Arthritis (JIA) and Juvenile Dermatomyositis (JDM)
4. CAR-T Cell Products in Autoimmune Rheumatic Disorders: Developments and Ongoing Clinical Trials
4.1. Anti-CD19 CAR-T Cell Products
4.2. Anti-BCMA CAR-T Cell Products
4.3. Anti-CD20, CD22, and CD70 CAR-T Cell Products
4.4. Bispecific (Dual Target) and Trispecific (Triple Target) CAR-T Cell Products
5. Conclusions and Future Perspectives
- The role of different LDC regimens in CAR-T cell therapy outcomes and disease control.
- The potential role of tocilizumab prophylaxis in patients who receive CAR-T cell therapy for autoimmune rheumatic disorders.
- Direct comparison of CAR-T cell products with auto-HCT in patients with SSc, and potentially with other autoimmune diseases.
- The endothelial activation and stress index (EASIX) and its modified version (mEASIX) in the prediction of clinical outcomes of these patients, as has been successfully used in the setting of CAR-T cell therapy for hematological malignancies [117].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vukovic, J.; Abazovic, D.; Vucetic, D.; Medenica, S. CAR-Engineered T Cell Therapy as an Emerging Strategy for Treating Autoimmune Diseases. Front. Med. 2024, 11, 1447147. [Google Scholar] [CrossRef]
- Davila, M.L.; Brentjens, R.J. CD19-Targeted CAR T Cells as Novel Cancer Immunotherapy for Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. Clin. Adv. Hematol. Oncol. 2016, 14, 802–808. [Google Scholar]
- Goyco Vera, D.; Waghela, H.; Nuh, M.; Pan, J.; Lulla, P. Approved CAR-T Therapies Have Reproducible Efficacy and Safety in Clinical Practice. Hum. Vaccin. Immunother. 2024, 20, 2378543. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, T.; Liu, D. BCMA-Targeted Immunotherapy for Multiple Myeloma. J. Hematol. Oncol. 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Sheykhhasan, M.; Ahmadieh-Yazdi, A.; Vicidomini, R.; Poondla, N.; Tanzadehpanah, H.; Dirbaziyan, A.; Mahaki, H.; Manoochehri, H.; Kalhor, N.; Dama, P. CAR T Therapies in Multiple Myeloma: Unleashing the Future. Cancer Gene Ther. 2024, 31, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Evangelidis, P.; Tragiannidis, K.; Vyzantiadis, A.; Evangelidis, N.; Kalmoukos, P.; Vyzantiadis, T.-A.; Tragiannidis, A.; Kourti, M.; Gavriilaki, E. Invasive Fungal Disease After Chimeric Antigen Receptor-T Immunotherapy in Adult and Pediatric Patients. Pathogens 2025, 14, 170. [Google Scholar] [CrossRef] [PubMed]
- Strongyli, E.; Evangelidis, P.; Sakellari, I.; Gavriilaki, M.; Gavriilaki, E. Change in Neurocognitive Function in Patients Who Receive CAR-T Cell Therapies: A Steep Hill to Climb. Pharmaceuticals 2024, 17, 591. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Gavriilaki, M.; Anagnostopoulos, A. A New Era in Endothelial Injury Syndromes: Toxicity of CAR-T Cells and the Role of Immunity. Int. J. Mol. Sci. 2020, 21, 3886. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chen, D.H.; Guha, A.; Mackenzie, S.; Walker, J.M.; Roddie, C. CAR T Cell Therapy–Related Cardiovascular Outcomes and Management. JACC CardioOncol. 2020, 2, 97–109. [Google Scholar] [CrossRef]
- Evangelidis, P.; Gavriilaki, E.; Tsakiris, D.A. Thrombotic Complications after Hematopoietic Stem Cell Transplantation and Other Cellular Therapies. Thromb. Update 2024, 16, 100186. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Sheth, V.S.; Gauthier, J. Taming the Beast: CRS and ICANS after CAR T-Cell Therapy for ALL. Bone Marrow Transplant. 2021, 56, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Evangelidis, P.; Evangelidis, N.; Kalmoukos, P.; Kourti, M.; Tragiannidis, A.; Gavriilaki, E. Genetic Susceptibility in Endothelial Injury Syndromes after Hematopoietic Cell Transplantation and Other Cellular Therapies: Climbing a Steep Hill. Curr. Issues Mol. Biol. 2024, 46, 4787–4802. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.D.; Smith, M.; Shah, N.N. How I Treat Refractory CRS and ICANS Following CAR T-Cell Therapy. Blood 2023, 141, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, Prevalence, and Co-Occurrence of Autoimmune Disorders over Time and by Age, Sex, and Socioeconomic Status: A Population-Based Cohort Study of 22 Million Individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef]
- Anyfanti, P.; Dara, A.; Angeloudi, E.; Bekiari, E.; Dimitroulas, T.; Kitas, G.D. Monitoring and Managing Cardiovascular Risk in Immune Mediated Inflammatory Diseases. J. Inflamm. Res. 2021, 14, 6893–6906. [Google Scholar] [CrossRef]
- Moutsopoulos, H.M. Autoimmune Rheumatic Diseases: One or Many Diseases? J. Transl. Autoimmun. 2021, 4, 100129. [Google Scholar] [CrossRef]
- Sakkas, L.I. Regulatory B Cells in Autoimmune Rheumatic Diseases. Mediterr. J. Rheumatol. 2017, 28, 75–79. [Google Scholar] [CrossRef]
- Anyfanti, P.; Ainatzoglou, A.; Angeloudi, E.; Michailou, O.; Defteraiou, K.; Bekiari, E.; Kitas, G.D.; Dimitroulas, T. Cardiovascular Risk in Rheumatoid Arthritis: Considerations on Assessment and Management. Mediterr. J. Rheumatol. 2024, 35, 402–410. [Google Scholar] [CrossRef]
- Sureda, A.; Corbacioglu, S.; Greco, R.; Kröger, N.; Carreras, E. (Eds.) The EBMT Handbook; Springer International Publishing: Cham, Switzerland, 2024; ISBN 978-3-031-44079-3. [Google Scholar]
- Dai, X.; Fan, Y.; Zhao, X. Systemic Lupus Erythematosus: Updated Insights on the Pathogenesis, Diagnosis, Prevention and Therapeutics. Signal Transduct. Target. Ther. 2025, 10, 102. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Olferiev, M.; Crow, M.K. Systemic Lupus Erythematosus Genetics: Insights into Pathogenesis and Implications for Therapy. Nat. Rev. Rheumatol. 2024, 20, 635–648. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Krönke, G.; Völkl, S.; Kretschmann, S.; Aigner, M.; Kharboutli, S.; Böltz, S.; Manger, B.; Mackensen, A.; Schett, G. CD19-Targeted CAR T Cells in Refractory Systemic Lupus Erythematosus. N. Engl. J. Med. 2021, 385, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T Cell Therapy for Refractory Systemic Lupus Erythematosus. Nat. Med. 2022, 28, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Taubmann, J.; Bucci, L.; Wilhelm, A.; Bergmann, C.; Völkl, S.; Aigner, M.; Rothe, T.; Minopoulou, I.; Tur, C.; et al. CD19 CAR T-Cell Therapy in Autoimmune Disease-A Case Series with Follow-Up. N. Engl. J. Med. 2024, 390, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Taubmann, J.; Müller, F.; Yalcin Mutlu, M.; Völkl, S.; Aigner, M.; Bozec, A.; Mackensen, A.; Grieshaber-Bouyer, R.; Schett, G. CD19 Chimeric Antigen Receptor T Cell Treatment: Unraveling the Role of B Cells in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2024, 76, 497–504. [Google Scholar] [CrossRef]
- Hagen, M.; Müller, F.; Wirsching, A.; Kharboutli, S.; Spörl, S.; Aigner, M.; Völkl, S.; Köhler, B.; Dörfler, A.; Grieshaber-Bouyer, R.; et al. Treatment of CNS Systemic Lupus Erythematosus with CD19 CAR T Cells. Lancet 2024, 404, 2158–2160. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, J.; Cinquina, A.; Wang, Q.; Xu, H.; Zhang, Q.; Sun, L.; Chen, Q.; Xu, L.; Pinz, K.; et al. Treatment of Systemic Lupus Erythematosus Using BCMA-CD19 Compound CAR. Stem Cell Rev. Rep. 2021, 17, 2120–2123. [Google Scholar] [CrossRef]
- He, X.; Hu, B.; Zhang, Y.; Liu, F.; Li, Q.; Zheng, C.; Shen, J.; Yang, Z.; Wang, J.; Ma, D.; et al. Treatment of Two Pediatric Patients with Refractory Systemic Lupus Erythematosus Using CD19-Targeted CAR T-Cells. Autoimmun. Rev. 2025, 24, 103692. [Google Scholar] [CrossRef]
- Denton, C.P.; Khanna, D. Systemic Sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Giannakoulas, G.; Karvounis, H.; Garyfallos, A.; Settas, L.; Kitas, G.D. Micro- and Macrovascular Treatment Targets in Scleroderma Heart Disease. Curr. Pharm. Des. 2014, 20, 536–544. [Google Scholar] [CrossRef]
- Bergmann, C.; Müller, F.; Distler, J.H.W.; Györfi, A.-H.; Völkl, S.; Aigner, M.; Kretschmann, S.; Reimann, H.; Harrer, T.; Bayerl, N.; et al. Treatment of a Patient with Severe Systemic Sclerosis (SSc) Using CD19-Targeted CAR T Cells. Ann. Rheum. Dis. 2023, 82, 1117–1120. [Google Scholar] [CrossRef]
- Auth, J.; Müller, F.; Völkl, S.; Bayerl, N.; Distler, J.H.W.; Tur, C.; Raimondo, M.G.; Chenguiti Fakhouri, S.; Atzinger, A.; Coppers, B.; et al. CD19-Targeting CAR T-Cell Therapy in Patients with Diffuse Systemic Sclerosis: A Case Series. Lancet Rheumatol. 2025, 7, e83–e93. [Google Scholar] [CrossRef]
- Merkt, W.; Freitag, M.; Claus, M.; Kolb, P.; Falcone, V.; Röhrich, M.; Rodon, L.; Deicher, F.; Andreeva, I.; Tretter, T.; et al. Third-Generation CD19.CAR-T Cell-Containing Combination Therapy in Scl70+ Systemic Sclerosis. Ann. Rheum. Dis. 2024, 83, 543–546. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Dimitroulas, T.; Bucciarelli-Ducci, C.; Ardoin, S.; Sfikakis, P.P.; Kolovou, G.; Kitas, G.D. Rheumatoid Arthritis: An Autoimmune Disease with Female Preponderance and Cardiovascular Risk Equivalent to Diabetes Mellitus: Role of Cardiovascular Magnetic Resonance. Inflamm. Allergy Drug Targets 2014, 13, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Szabo, D.; Balogh, A.; Gopcsa, L.; Giba-Kiss, L.; Lakatos, G.; Paksi, M.; Reti, M.; Takacs, P.; van Heteren, P.; Zadoyan, G.; et al. Sustained Drug-Free Remission in Rheumatoid Arthritis Associated with Diffuse Large B-Cell Lymphoma Following Tandem CD20-CD19-Directed Non-Cryopreserved CAR-T Cell Therapy Using Zamtocabtagene Autoleucel. RMD Open 2024, 10, e004727. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Sfikakis, P.P.; Dimitroulas, T.; Kolovou, G.; Kitas, G.D. Cardiac and Muscular Involvement in Idiopathic Inflammatory Myopathies: Noninvasive Diagnostic Assessment and the Role of Cardiovascular and Skeletal Magnetic Resonance Imaging. Inflamm. Allergy Drug Targets 2014, 13, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Boeltz, S.; Knitza, J.; Aigner, M.; Völkl, S.; Kharboutli, S.; Reimann, H.; Taubmann, J.; Kretschmann, S.; Rösler, W.; et al. CD19-Targeted CAR T Cells in Refractory Antisynthetase Syndrome. Lancet 2023, 401, 815–818. [Google Scholar] [CrossRef]
- Pecher, A.-C.; Hensen, L.; Klein, R.; Schairer, R.; Lutz, K.; Atar, D.; Seitz, C.; Stanger, A.; Schneider, J.; Braun, C.; et al. CD19-Targeting CAR T Cells for Myositis and Interstitial Lung Disease Associated With Antisynthetase Syndrome. JAMA 2023, 329, 2154–2162. [Google Scholar] [CrossRef]
- Volkov, J.; Nunez, D.; Mozaffar, T.; Stadanlick, J.; Werner, M.; Vorndran, Z.; Ellis, A.; Williams, J.; Cicarelli, J.; Lam, Q.; et al. Case Study of CD19 CAR T Therapy in a Subject with Immune-Mediate Necrotizing Myopathy Treated in the RESET-Myositis Phase I/II Trial. Mol. Ther. 2024, 32, 3821–3828. [Google Scholar] [CrossRef]
- Friedberg, E.; Wohlfarth, P.; Schiefer, A.I.; Skrabs, C.; Pickl, W.F.; Worel, N.; Staber, P.; Jäger, U.; Ay, C. Disappearance of Antiphospholipid Antibodies after Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy of B-Cell Lymphoma in a Patient with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. J. Thromb. Haemost. 2025, 23, 262–266. [Google Scholar] [CrossRef]
- Schmelz, J.L.; Navsaria, L.; Goswamy, R.; Chuang, H.H.; Miranda, R.N.; Lee, H.J. Chimeric Antigen Receptor T-cell Therapy’s Role in Antiphospholipid Syndrome: A Case Report. Br. J. Haematol. 2020, 188, e5. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the Pathophysiology and Treatment of Primary Sjögren Syndrome. Nat. Rev. Rheumatol. 2024, 20, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhang, Y.; Song, Q.; Jiang, X.; Cao, W.; Li, L.; Yi, H.; Weng, X.; Chen, S.; Wang, Z.; et al. Concurrent Remission of Lymphoma and Sjögren’s Disease Following Anti-CD19 Chimeric Antigen Receptor-T Cell Therapy for Diffuse Large B-Cell Lymphoma: A Case Report. Front. Immunol. 2023, 14, 1298815. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Bhandari, S.; Bhandari, S. Chimeric Antigen Receptor T Cell Therapy for the Treatment of Systemic Rheumatic Diseases: A Comprehensive Review of Recent Literature. Ann. Med. Surg. 2023, 85, 3512–3518. [Google Scholar] [CrossRef]
- Dimopoulou, D.; Trachana, M.; Pratsidou-Gertsi, P.; Sidiropoulos, P.; Kanakoudi-Tsakalidou, F.; Dimitroulas, T.; Garyfallos, A. Predictors and Long-Term Outcome in Greek Adults with Juvenile Idiopathic Arthritis: A 17-Year Continuous Follow-up Study. Rheumatology 2017, 56, 1928–1938. [Google Scholar] [CrossRef]
- París-Muñoz, A.; Alcobendas-Rueda, R.M.; Verdú-Sánchez, C.; Udaondo, C.; Galán-Gómez, V.; González-Martínez, B.; Menéndez, J.J.; Martínez-Romera, I.; Minguillón, J.; Pertíñez, L.; et al. CD19 CAR-T Cell Therapy in a Pediatric Patient with MDA5+ Dermatomyositis and Rapidly Progressive Interstitial Lung Disease. Med 2025, 100676. [Google Scholar] [CrossRef]
- Tedder, T.F.; Zhou, L.-J.; Engel, P. The CD19/CD21 Signal Transduction Complex of B Lymphocytes. Immunol. Today 1994, 15, 437–442. [Google Scholar] [CrossRef]
- Fearon, D.T.; Carroll, M.C.; Carroll, M.C. Regulation of B Lymphocyte Responses to Foreign and Self-Antigens by the CD19/CD21 Complex. Annu. Rev. Immunol. 2000, 18, 393–422. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The Generation of Antibody-Secreting Plasma Cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Jellusova, J.; Rickert, R.C. The PI3K Pathway in B Cell Metabolism. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 359–378. [Google Scholar] [CrossRef]
- Komura, K. CD19: A Promising Target for Systemic Sclerosis. Front. Immunol. 2024, 15, 1454913. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Kuroki, K.; Fujimoto, M.; Murakami, Y.; Tedder, T.F.; Tokunaga, K.; Takehara, K.; Sato, S. Association of a Functional CD19 Polymorphism with Susceptibility to Systemic Sclerosis. Arthritis Rheum. 2004, 50, 4002–4007. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Fujimoto, M.; Hasegawa, M.; Komura, K.; Hamaguchi, Y.; Kaburagi, Y.; Nagaoka, T.; Takehara, K.; Tedder, T.F.; Sato, S. CD19-Dependent B Lymphocyte Signaling Thresholds Influence Skin Fibrosis and Autoimmunity in the Tight-Skin Mouse. J. Clin. Investig. 2002, 109, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Reijm, S.; Kwekkeboom, J.C.; Blomberg, N.J.; Suurmond, J.; van der Woude, D.; Toes, R.E.M.; Scherer, H.U. Autoreactive B Cells in Rheumatoid Arthritis Include Mainly Activated CXCR3+ Memory B Cells and Plasmablasts. JCI Insight 2023, 8, e172006. [Google Scholar] [CrossRef]
- Herbst, R.; Wang, Y.; Gallagher, S.; Mittereder, N.; Kuta, E.; Damschroder, M.; Woods, R.; Rowe, D.C.; Cheng, L.; Cook, K.; et al. B-Cell Depletion In Vitro and In Vivo with an Afucosylated Anti-CD19 Antibody. J. Pharmacol. Exp. Ther. 2010, 335, 213–222. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Bennett, J.L.; Kim, H.J.; Weinshenker, B.G.; Pittock, S.J.; Wingerchuk, D.M.; Fujihara, K.; Paul, F.; Cutter, G.R.; Marignier, R.; et al. Inebilizumab for the Treatment of Neuromyelitis Optica Spectrum Disorder (N-MOmentum): A Double-Blind, Randomised Placebo-Controlled Phase 2/3 Trial. Lancet 2019, 394, 1352–1363. [Google Scholar] [CrossRef]
- Duddy, M.E.; Alter, A.; Bar-Or, A. Distinct Profiles of Human B Cell Effector Cytokines: A Role in Immune Regulation? J. Immunol. 2004, 172, 3422–3427. [Google Scholar] [CrossRef]
- Chu, S.Y.; Pong, E.; Bonzon, C.; Yu, N.; Jacob, C.O.; Chalmers, S.A.; Putterman, C.; Szymkowski, D.E.; Stohl, W. Inhibition of B Cell Activation Following in Vivo Co-Engagement of B Cell Antigen Receptor and Fcγ Receptor IIb in Non-Autoimmune-Prone and SLE-Prone Mice. J. Transl. Autoimmun. 2021, 4, 100075. [Google Scholar] [CrossRef]
- Merrill, J.; June, J.; Koumpouras, F.; Machua, W.; Khan, M.F.; Askanase, A.; Khosroshahi, A.; Sheikh, S.; James, J.A.; Guthridge, J.; et al. FRI0176 phase 2, double-blind, randomized, placebo-controlled study of a reversible b cell inhibitor, XMAB®5871, in systemic lupus erythematosus (SLE). Ann. Rheum. Dis. 2019, 78, 761–762. [Google Scholar] [CrossRef]
- Kamburova, E.G.; Koenen, H.J.P.M.; Borgman, K.J.E.; ten Berge, I.J.; Joosten, I.; Hilbrands, L.B. A Single Dose of Rituximab Does Not Deplete B Cells in Secondary Lymphoid Organs but Alters Phenotype and Function. Am. J. Transplant. 2013, 13, 1503–1511. [Google Scholar] [CrossRef]
- Bhoj, V.G.; Arhontoulis, D.; Wertheim, G.; Capobianchi, J.; Callahan, C.A.; Ellebrecht, C.T.; Obstfeld, A.E.; Lacey, S.F.; Melenhorst, J.J.; Nazimuddin, F.; et al. Persistence of Long-Lived Plasma Cells and Humoral Immunity in Individuals Responding to CD19-Directed CAR T-Cell Therapy. Blood 2016, 128, 360–370. [Google Scholar] [CrossRef]
- CD19-Directed Chimeric Antigen Receptor Autologous T Cells (CART19) for Lupus. Available online: https://clinicaltrials.gov/study/NCT06839976 (accessed on 6 August 2025).
- REACT-01: Reversing Autoimmunity Through Cell Therapy. Available online: https://clinicaltrials.gov/study/NCT06465147 (accessed on 6 August 2025).
- A Study to Assess CLBR001+SWI019 in Subjects With Autoimmune Diseases. Available online: https://www.clinicaltrials.gov/study/NCT06913608?cond=Myopathy%20in%20systemic%20lupus%20erythematosus&rank=9 (accessed on 6 August 2025).
- A Phase 1 Study of SYNCAR-001 + STK-009 Without Conditioning Chemotherapy (Lymphodepletion) in Subjects With Severe, Refractory Systemic Autoimmune Rheumatic Disease. Available online: https://www.clinicaltrials.gov/study/NCT06544330 (accessed on 6 August 2025).
- A Study of CC-97540, CD-19-Targeted Nex-T CAR T Cells, in Participants With Severe, Refractory Autoimmune Diseases (Breakfree-1). Available online: https://clinicaltrials.gov/study/NCT05869955 (accessed on 6 August 2025).
- Comparison of B-Cell Depletion by Rituximab and Anti-CD 19 CAR-T Therapy in Patients With Rheumatoid Arthritis (COMPARE). Available online: https://clinicaltrials.gov/study/NCT06475495 (accessed on 6 August 2025).
- Schneider, P.; MacKay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a Novel Ligand of the Tumor Necrosis Factor Family, Stimulates B Cell Growth. J. Exp. Med. 1999, 189, 1747–1756. [Google Scholar] [CrossRef]
- Kim, J.; Gross, J.A.; Dillon, S.R.; Min, J.-K.; Elkon, K.B. Increased BCMA Expression in Lupus Marks Activated B Cells, and BCMA Receptor Engagement Enhances the Response to TLR9 Stimulation. Autoimmunity 2011, 44, 69–81. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.-L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA Is Essential for the Survival of Long-Lived Bone Marrow Plasma Cells. J. Exp. Med. 2004, 199, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Guldenpfennig, C.; Teixeiro, E.; Daniels, M. NF-KB’s Contribution to B Cell Fate Decisions. Front. Immunol. 2023, 14, 1214095. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.-H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.M.; Schuh, E.; Krumbholz, M.; Rübsamen, H.; et al. γ-Secretase Directly Sheds the Survival Receptor BCMA from Plasma Cells. Nat. Commun. 2015, 6, 7333. [Google Scholar] [CrossRef]
- Salazar-Camarena, D.C.; Palafox-Sánchez, C.A.; Cruz, A.; Marín-Rosales, M.; Muñoz-Valle, J.F. Analysis of the Receptor BCMA as a Biomarker in Systemic Lupus Erythematosus Patients. Sci. Rep. 2020, 10, 6236. [Google Scholar] [CrossRef]
- Salazar-Camarena, D.C.; Ortiz-Lazareno, P.C.; Cruz, A.; Oregon-Romero, E.; Machado-Contreras, J.R.; Muñoz-Valle, J.F.; Orozco-López, M.; Marín-Rosales, M.; Palafox-Sánchez, C.A. Association of BAFF, APRIL Serum Levels, BAFF-R, TACI and BCMA Expression on Peripheral B-Cell Subsets with Clinical Manifestations in Systemic Lupus Erythematosus. Lupus 2016, 25, 582–592. [Google Scholar] [CrossRef]
- Sanges, S.; Guerrier, T.; Duhamel, A.; Guilbert, L.; Hauspie, C.; Largy, A.; Balden, M.; Podevin, C.; Lefèvre, G.; Jendoubi, M.; et al. Soluble Markers of B Cell Activation Suggest a Role of B Cells in the Pathogenesis of Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. Front. Immunol. 2022, 13, 954007. [Google Scholar] [CrossRef]
- Pecher, A.-C.; Hensen, L.; Lengerke, C.; Henes, J. The Future of CAR T Therapeutics to Treat Autoimmune Disorders. Mol. Diagn. Ther. 2024, 28, 593–600. [Google Scholar] [CrossRef]
- Dabkowska, A.; Domka, K.; Firczuk, M. Advancements in Cancer Immunotherapies Targeting CD20: From Pioneering Monoclonal Antibodies to Chimeric Antigen Receptor-Modified T Cells. Front. Immunol. 2024, 15, 1363102. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Gómez-Puerta, J.A.; Ramos-Casals, M.; Lozano, F.; Bosch, X. Therapeutic Targeting of B Cells for Rheumatic Autoimmune Diseases. Pharmacol. Rev. 2011, 63, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, G.E.; Soulaidopoulos, S.; Sfikakis, P.P.; Dimitroulas, T.; D Kitas, G. Effect of Biologics on Cardiovascular Inflammation: Mechanistic Insights and Risk Reduction. J. Inflamm. Res. 2021, 14, 1915–1931. [Google Scholar] [CrossRef]
- Melissaropoulos, K.; Kraniotis, P.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.; Daoussis, D. Targeting Very Early Systemic Sclerosis: A Case-Based Review. Rheumatol. Int. 2019, 39, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Du, F.H.; Mills, E.A.; Mao-Draayer, Y. Next-Generation Anti-CD20 Monoclonal Antibodies in Autoimmune Disease Treatment. Autoimmun. Highlights 2017, 8, 12. [Google Scholar] [CrossRef]
- Tan Su Yin, E.; Hu, Y.X.; Huang, H. The Breakthrough and the Future: CD20 Chimeric Antigen Receptor T-cell Therapy for Hematologic Malignancies. ImmunoMedicine 2022, 2, e1039. [Google Scholar] [CrossRef]
- A Phase 1 Study of ADI-001 in Autoimmune Disease. Available online: https://clinicaltrials.gov/study/NCT06375993 (accessed on 6 August 2025).
- Poe, J.C.; Hasegawa, M.; Tedder, T.F. CD19, CD21, and CD22: Multifaceted Response Regulators of B Lymphocyte Signal Transduction. Int. Rev. Immunol. 2001, 20, 739–762. [Google Scholar] [CrossRef]
- Nitschke, L. The Role of CD22 and Other Inhibitory Co-Receptors in B-Cell Activation. Curr. Opin. Immunol. 2005, 17, 290–297. [Google Scholar] [CrossRef]
- Cyster, J.G.; Goodnow, C.C. Tuning Antigen Receptor Signaling by CD22: Integrating Cues from Antigens and the Microenvironment. Immunity 1997, 6, 509–517. [Google Scholar] [CrossRef]
- A Study of LCAR-AIO in Subjects with Relapsed/Refractory Systemic Lupus Erythematosus. Available online: https://clinicaltrials.gov/study/NCT06653556 (accessed on 6 August 2025).
- A Study of LCAR-AIO in Subjects with Relapsed/Refractory Autoimmune Diseases. Available online: https://asgct.careboxhealth.com/en-US/trial/listing/583155 (accessed on 6 August 2025).
- Study of BCMA/CD70 CAR-T Therapy for Refractory CSLE. Available online: https://www.clinicaltrials.gov/study/NCT06934447?term=AREA%5BConditionSearch%5D(car%20t%20therapy)%20AND%20AREA%5BBasicSearch%5D(single%20arm)&rank=9 (accessed on 6 August 2025).
- Universal CAR-T (CHT101) Cell Therapy for Relapsed Refractory Systemic Lupus Erythematosus. Available online: https://clinicaltrials.gov/study/NCT06946485 (accessed on 6 August 2025).
- van de Donk, N.W.C.J.; O’Neill, C.; de Ruijter, M.E.M.; Verkleij, C.P.M.; Zweegman, S. T-Cell Redirecting Bispecific and Trispecific Antibodies in Multiple Myeloma beyond BCMA. Curr. Opin. Oncol. 2023, 35, 601–611. [Google Scholar] [CrossRef]
- Trabolsi, A.; Arumov, A.; Schatz, J.H. Bispecific Antibodies and CAR-T Cells: Dueling Immunotherapies for Large B-Cell Lymphomas. Blood Cancer J. 2024, 14, 27. [Google Scholar] [CrossRef]
- Gómez-Melero, S.; Hassouneh, F.; Vallejo-Bermúdez, I.M.; Agüera-Morales, E.; Solana, R.; Caballero-Villarraso, J. Tandem CAR-T Cell Therapy: Recent Advances and Current Challenges. Front. Immunol. 2025, 16, 1546172. [Google Scholar] [CrossRef]
- FKC288 in Participants With Autoimmune Kidney Diseases. Available online: https://clinicaltrials.gov/study/NCT06285279 (accessed on 6 August 2025).
- CD19-BCMA CART Cell Therapy for Refractory SLE-LN, SSc, and PSS-PAH: A Single-Center, Open, Non-Randomized, Single-Arm Clinical Study. Available online: https://clinicaltrials.gov/study/NCT06947460 (accessed on 6 August 2025).
- US Zamto-Cel Autoimmune Diseases. Available online: https://clinicaltrials.gov/study/NCT06708845 (accessed on 6 August 2025).
- CD19/BCMA CAR-T Cell Therapy for Refractory/Relapsed Lupus Nephritis. Available online: https://clinicaltrials.gov/study/NCT06785519?cond=lupus&term=CAR-T&rank=10 (accessed on 6 August 2025).
- BCMA-CD19 CAR-T Therapy for Refractory Autoimmune Diseases. Available online: https://clinicaltrials.gov/study/NCT06794008?cond=IgG4-related%20ophthalmic%20disease&viewType=Table&rank=7 (accessed on 6 August 2025).
- Efficacy of AZD0120 in Adults with Refractory SLE. Available online: https://www.clinicaltrials.gov/study/NCT06897930?term=AREA%5BBasicSearch%5D(antigen%20specific%20systemic%20lupus)&rank=1 (accessed on 6 August 2025).
- UCAR T-Cell Therapy Targeting CD19/BCMA in Patients With r/r Systemic Lupus Erythematosus. Available online: https://clinicaltrials.gov/study/NCT06920433?cond=%22Systemic%20lupus%20erythematosus%22&rank=10 (accessed on 6 August 2025).
- T Cell Therapy for Refractory Autoimmune Disease. Available online: https://www.clinicaltrials.gov/study/NCT07052565?cond=T%20Cell%20Therapy%20for%20Refractory%20Autoimmune%20Disease&rank=2 (accessed on 6 August 2025).
- JWCAR201 for the Treatment of Hematology Malignancy and Autoimmune Diseases. Available online: https://www.clinicaltrials.gov/study/NCT06567080?cond=JWCAR201%20for%20the%20Treatment%20of%20Hematology%20Malignancy%20and%20Autoimmune%20Diseases&rank=1 (accessed on 6 August 2025).
- A Study of GC012F Injection in Subjects with Refractory Systemic Lupus Erythematosus. Available online: https://www.clinicaltrials.gov/study/NCT06530849?term=AREA%5BBasicSearch%5D(GC012F)&rank=7 (accessed on 6 August 2025).
- An Exploratory Clinical Study of SCAR02 Targeting BCMA and CD19 for the Treatment of Refractory Autoimmune Diseases (SCAR02). Available online: https://clinicaltrials.gov/study/NCT06503224?cond=An%20Exploratory%20Clinical%20Study%20of%20SCAR02%20Targeting%20BCMA%20and%20CD19%20for%20the%20Treatment%20of%20Refractory%20Autoimmune%20Diseases%20(SCAR02)&rank=1 (accessed on 6 August 2025).
- Safety and Efficacy of PRG-2311 for Refractory Lupus Nephritis and IgG4-Related Disease. Available online: https://www.clinicaltrials.gov/study/NCT06497361?term=AREA%5BBasicSearch%5D(AREA%5BConditionSearch%5D(IgG4-related%20disease%20OR%20%22IgG4-RD%22%20OR%20%22%20IgG4-related%20sclerosing%20disease%22%20OR%20%22%20IgG4-related%20systemic%20disease%22%20OR%20%22%20Immunoglobulin%20G4-related%20sclerosing%20disease%22)%20AND%20AREA%5BOverallStatus%5D(NOT_YET_RECRUITING%20OR%20RECRUITING))&rank=10 (accessed on 6 August 2025).
- IMPT-514 in Systemic Lupus Erythematosus, Anca-Associated Vasculitis, and Idiopathic Inflammatory Myopathy. Available online: https://clinicaltrials.gov/study/NCT06462144 (accessed on 6 August 2025).
- Sequential CAR-T Cells Targeting BCMA/CD19 in Patients with Relapsed/Refractory Autoimmune Diseases (BAH247). Available online: https://clinicaltrials.gov/study/NCT06428188 (accessed on 6 August 2025).
- Anti-CD19-CD3E-CAR-T Cells in Relapsed/Refractory Autoimmune Disease. Available online: https://www.clinicaltrials.gov/study/NCT06373081 (accessed on 6 August 2025).
- Fourth-Gen CAR T Cells Targeting BCMA/CD19 for Refractory Systemic Lupus Erythematosus (SLE) (BAH242). Available online: https://clinicaltrials.gov/study/NCT06350110 (accessed on 6 August 2025).
- CD19/BCMA CAR-T Cell Therapy for Refractory/Moderate-to-Severe Systemic Lupus Erythematosus. Available online: https://clinicaltrials.gov/study/NCT06349343 (accessed on 6 August 2025).
- Dual Target CAR-T Cell Treatment for Refractory Systemic Lupus Erythematosus (SLE) Patients. Available online: https://www.clinicaltrials.gov/study/NCT05858684?cond=car-t&viewType=Table&rank=5 (accessed on 6 August 2025).
- Umbilical Cord Blood CD19-BCMA CART Cell Therapy for SLE-LN, SSc, andpSS PAH. Available online: https://www.clinicaltrials.gov/study/NCT06947473?cond=Peeling%20skin%20syndrome%20OR%20%22deciduous%20skin%22%20OR%20%22%20familial%20continuous%20skin%20peeling%20syndrome%22%20OR%20%22%20idiopathic%20deciduous%20skin%22%20OR%20%22%20keratosis%20exfoliativa%20congenita%22%20OR%20%22%20peeling%20skin%20disease%22%20OR%20%22%20PSS%22&aggFilters=status:not%20rec&viewType=Table&rank=8 (accessed on 6 August 2025).
- Clinical Study on Targeted CD19 or CD19-BCMA CAR-T Therapy for Autoimmune Diseases. Available online: https://clinicaltrials.gov/study/NCT06548607?term=AAV&cond=%22Xerostomia%22&viewType=Table&rank=4 (accessed on 6 August 2025).
- A Study of CD19/BCMA Chimeric Antigen Receptor T Cells Therapy for Patients with Refractory Sjogren’s Syndrome. Available online: https://clinicaltrials.gov/study/NCT05085431 (accessed on 6 August 2025).
- CAR T-Cell Therapy Targeting CD19 and BCMA in Patients with Relapse/Refractory Autoimmune Diseases. Available online: https://clinicaltrials.gov/study/NCT06941129?term=AREA%5BConditionSearch%5D(%22Inclusion%20Body%20Myositis%22)%20AND%20AREA%5BInterventionSearch%5D(%22vaccines%22)&rank=1 (accessed on 6 August 2025).
- Gavriilaki, E.; Tzannou, I.; Batsis, I.; Tsonis, I.; Liga, M.; Gkirkas, K.; Ximeri, M.; Dolgyras, P.; Bampali, V.; Evangelidis, P.; et al. EASIX and M-EASIX Predict Severe Cytokine Release Syndrome and Overall Survival after CAR T-Cell Therapy. Blood Vessel. Thromb. Hemost. 2024, 1, 100025. [Google Scholar] [CrossRef]
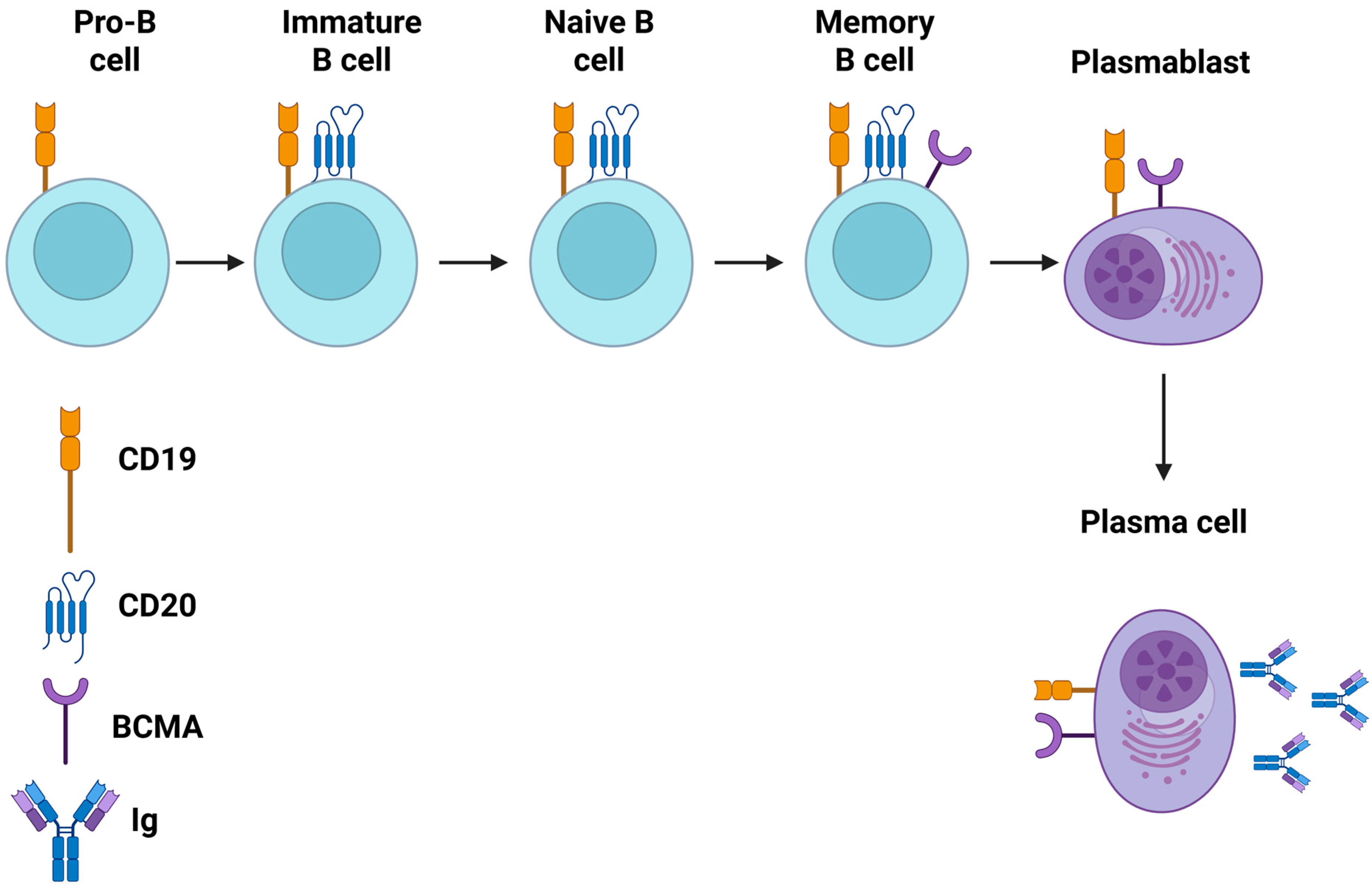
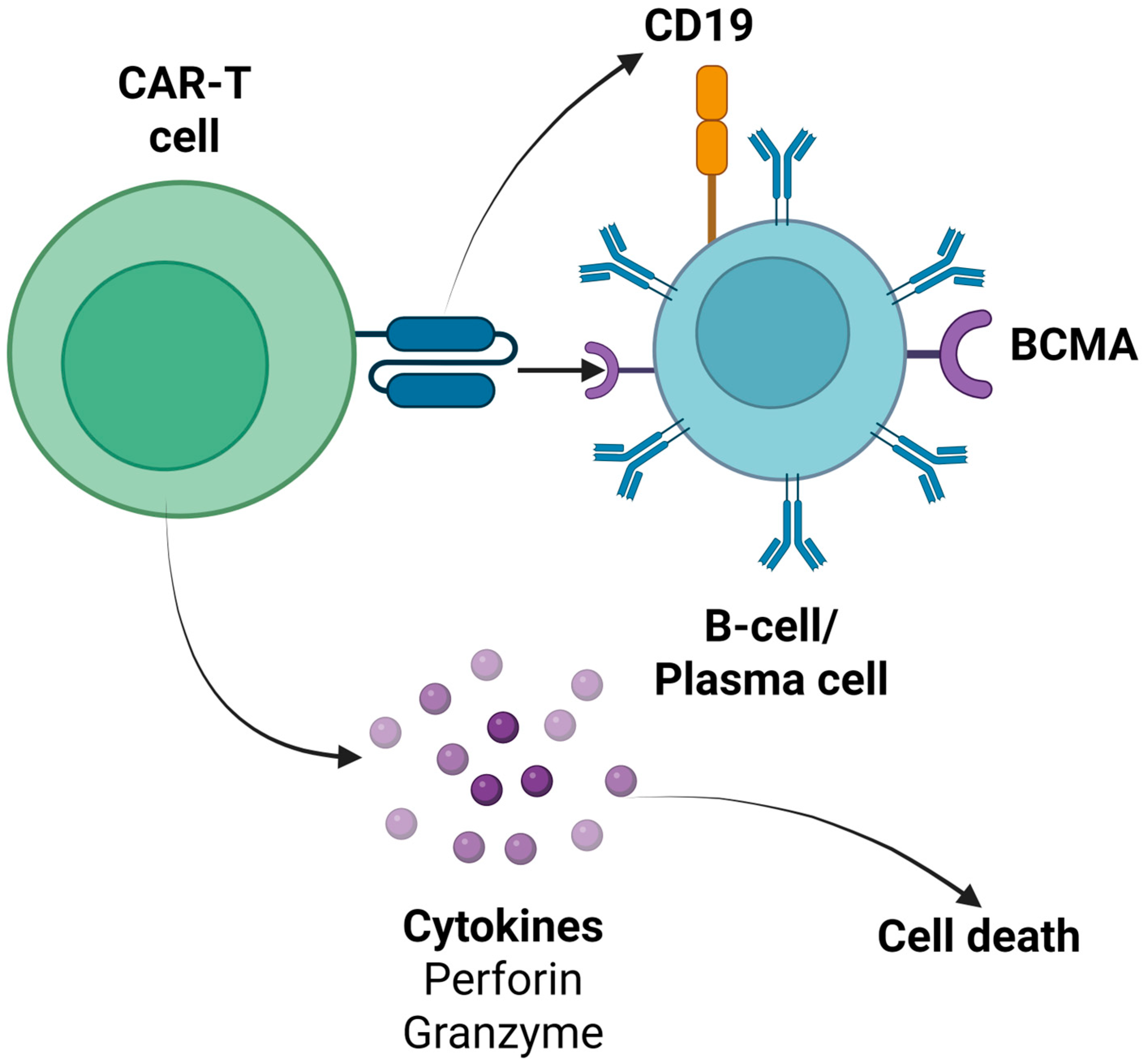
| First Author (Year) | Study Design-CAR-T Cell Product | Patient Characteristics | Outcomes | CRS (Grade) | ICANS (Grade) | Infections During Follow-Up | Prolonged Hematological Toxicity |
|---|---|---|---|---|---|---|---|
| [23] | Single-case report; refractory SLE → autologous CD19-CAR (1.1 × 106 cells/kg) | 20-y-old female with refractory SLE (class IIIA lupus nephritis, nephrotic syndrome, pericarditis, pleuritis, malar rash, arthritis, and prior Libman–Sacks endocarditis). Failed hydroxychloroquine, steroids, CYC, MMF, tacrolimus, belimumab, and rituximab. | SLEDAI ↓ 18 → 0 by day 44; complete B cell depletion (≥44 days); anti–dsDNA normalized; complement (C3/C4) normalized; proteinuria improved. | None reported (no CRS) | None reported | None reported | None reported |
| [24] | Prospective case series (five patients); refractory SLE → autologous CD19-CAR (1 × 106 cells/kg) | Adults (4 F/1 M; median 22 y; baseline SLEDAI 8–16) with refractory SLE despite multiple immunosuppressants (such as CYC, MMF, tacrolimus, belimumab, and rituximab). | 5/5 achieved SLEDAI 0 by month 3; anti-dsDNA and ANA normalized; proteinuria resolved; drug-free remission maintained (median follow-up: 8 months); reconstituted B cells naïve phenotype. | 3/5 had grade 1 (fever, hypotension); 2/5 received tocilizumab | None reported | No serious infections (≥12 months follow-up) | No prolonged cytopenias or hypogammaglobulinemia survived beyond 3 months |
| [26] | Single-case report; refractory SLE in pregnancy → autologous CD19-CAR (1 × 106 cells/kg) | 32-y-old female with refractory SLE (serositis, nephritis, cytopenias, hypocomplementemia, and suspected CNS involvement) diagnosed during pregnancy; off all immunosuppression except low-dose steroids before CAR-T. | Achieved LLDAS by month 3; proteinuria resolved; anti-dsDNA undetectable; B cell reappearance at ~month 2 without disease flare. | None reported | None reported | None reported | None reported |
| [27] | Single-case report; severe CNS SLE → autologous CD19-CAR (1 × 106 cells/kg) | 21-y-old male with transverse myelitis and vasculitis from CNS SLE (SLEDAI: 22), refractory to standard of care. | Achieved SLEDAI 0 by week 12; full neurologic recovery; MRI lesions resolved; anti-dsDNA and IFN-α undetectable. | None reported | None reported | None reported | None reported |
| [28] | Single-case report; refractory SLE + DLBCL → dual-target CD19/BCMA-CAR (5.3 × 106 cells/kg) | 41-y-old female with 20-y refractory SLE complicated by stage IV DLBCL; intolerant to R-CHOP. | CAR-T expansion to ~8% lymphocytes; sustained B cell aplasia to ~9 months; complement C3/C4 normalized; ANA titers undetectable by weeks 9–37; IgG/A/M ↓; PET-CT CR of DLBCL at 4 months; SLE in remission at 23 months post-CAR-T infusion. | Minimal (not specified; no severe CRS) | None reported | None reported | No prolonged hematologic toxicity reported |
| [29] | Two-case pediatric report; refractory pediatric SLE → low-dose CD19-CAR (1 × 105 cells/kg) |
| Patient 1: SLEDAI-2K 12 → 0 by month 4; C3 normalized by day 28; anti-dsDNA undetectable; cutaneous lesions resolved; proteinuria resolved. Patient 2: SLEDAI-2K 12 → 4; proteinuria 28 → 13.6 mg/kg/day; C3/C4 normalized; pleuritis and hematuria resolved; LN activity improved on biopsy. Both off immunosuppression at 4–5 months. | 2/2 grade 1 CRS (fever); managed supportively | 1/2 transient grade 1 (mild encephalopathy) | Transient hypogammaglobulinemia managed with IVIG | No prolonged cytopenias beyond month 2 |
| [32] | Single-case report; refractory dcSSc → autologous CD19-CAR (1 × 106 cells/kg) | 60-y-old male with dcSSc: skin, pulmonary, and cardiac fibrosis; anti-RNA Pol III +; prior MTX (3 mo) and MMF (23 mo) failed; P-AH/ILD/digital ulcers. Renal impairment → reduced lymphodepletion (Flu 12.5 mg/m2 × 3 d, CYC 500 mg/m2 × 1 d). | mRSS ↓ progressively; Joint inflammation resolved; FVC ↑; pulmonary hemodynamics improved; troponin T stabilized; anti-RNA Pol III undetectable by month 3. | 1/1 grade 1 (fever) | None reported | None reported | No persistent cytopenias (resolved by month 2) |
| [33] | Prospective case series (six patients); severe diffuse SSc → autologous CD19-CAR (1 × 106 cells/kg) | Adults (median age: 45 y; 4 F/2 M) with severe diffuse SSc: skin (mRSS ≥ 20), ILD (HRCT > 15% lung), P21+AH, and joint involvement; refractory to ≥1 DMARD (e.g., MMF, CYC, nintedanib). | At 6 months: median ACR-CRISS improvement probability 100%; mRSS ↓ median 8 points (~31%); ILD extent ↓ 4% on HRCT; FVC ↑ 195 mL at the last follow-up. | 1/6 grade 0; 3/6 grade 1; 2/6 grade 2 | 0/6 | 1/6 hospitalized for influenza + bacterial superinfection | No prolonged cytopenias beyond month 2 |
| [34] | Single-case report; anti-Scl-70 + diffuse SSc → autologous CD19-CAR (third gen) (5 × 106 cells/kg) | 38-y-old female with anti-Scl-70 + diffuse SSc; progressive nonspecific ILD; prior MMF and nintedanib. | mRSS ↓ steadily over 11 months; FVC and DLCO ↑; CT → regression of GGO/fibrosis; ^68 Ga-FAPI PET uptake ↓; anti-Scl-70, CRP, hs-troponin T normalized; Fcγ-R-activating immune complexes disappeared. | 1/1 grade 1 (fever) | None reported | None reported | No persistent cytopenias |
| [25] | Prospective cohort (15 patients); refractory SLE (n = 8), IIMs (n = 3), SSc (n = 4) → autologous CD19-CAR (1 × 106 cells/kg) |
| SLE (8/8): SLEDAI ↓ to LLDAS/remission by month 3; complement and anti-dsDNA normalized. IIM (3/3): CK ↓ normalized; muscle strength ↑; ACR-EULAR major response. SSc (4/4): EUSTAR ↓; mRSS ↓; ILD ↓ 4–6%; FVC ↑; steroid/DMARD discontinuation. All remissions sustained 12–29 months. | 11/15 (73%) grade 1–2 (fever, mild hypotension); 1/15 grade 1 ICANS | 1/15 grade 1 | Mostly mild (upper respiratory, CMV reactivation—all resolved) | Transient cytopenias (grade 3–4) in 100%; resolved by month 2; transient hypogammaglobulinemia (IVIG support) |
| [36] | Single-case report; refractory RA + DLBCL → bispecific CD20/CD19-CAR (zamtocabtagene) | 73-y-old male with long-standing RA refractory to methotrexate and tocilizumab; developed DLBCL. RA meds discontinued pre-lymphodepletion. | Rheumatoid factor ↓ 1200 → 13 IU/mL by month 1; drug-free RA remission by week 4 (persisted 12 months); DLBCL CR by week 48. | 1/1 grade 1 (fever) | None reported | No serious infections | No prolonged cytopenias beyond month 2 (neutropenia managed with G-CSF) |
| [47] | Single-case report; pediatric JDM (MDA5+) → autologous CD19-CAR (ARI-0001) | 12-y-old female with MDA5+ dermatomyositis complicated by rapidly progressive ILD; refractory to high-dose steroids, tacrolimus, and IVIG. Complete peripheral B cell aplasia at infusion. | Over 11 months: cutaneous lesions resolved; muscle strength ↑; respiratory function ↑; neurologic symptoms improved; remained off immunosuppressives. | None reported | None reported | None reported | No prolonged cytopenias |
| [38] | Single-case report; refractory anti-Jo-1 antisynthetase syndrome → CD19-CAR (1 × 106 cells/kg) | 41-y-old male with severe anti-Jo-1 antisynthetase syndrome: CK > 9000 U/L, ILD, high anti-Jo-1 titers; refractory to steroids, rituximab, tacrolimus, IVIG, and CYC. | CK ↓ 13,600 → 102 U/L by day 180; anti-Jo-1 331 → 5 U/mL; muscle strength normalized; ILD resolved on CT; functional tests normalized; sustained remission at 6 months; B cells reappeared naïve. | 1/1 grade 1 (fever, myalgia, CK spikes); managed with tocilizumab | None reported | Received monthly IVIG for hypogammaglobulinemia; no serious infections | Transient cytopenias; resolved by month 2 |
| [39] | Single-case report; anti-Jo-1 syndrome → CD19-CAR (1.23 × 106/kg) + mycophenolate | 41-y-old male with refractory anti-Jo-1 antisynthetase syndrome (7 y): severe ILD, muscle weakness and high anti–Jo-1; prior rituximab and AZA failed. MMF started on day 35 to co-target CD8+ T cells. | Muscle enzymes and inflammatory cytokines normalized by week 4; anti-Jo-1 ↓; IgA/IgG/IgM partially recovered; muscle strength and pulmonary function improved (MRI and PFTs); remission persisted at 8 months; steroid/DMARD discontinued. | None reported | None reported | None reported | No prolonged cytopenias beyond month 2 |
| [40] | Phase I trial (first subject); anti-SRP IMNM → fully human CD19-CAR (CABA-201) (1 × 106 cells/kg) | 39-y-old male with refractory anti-SRP-positive IMNM: CK elevated, anti-SRP, anti-Ro-52; refractory to steroids, rituximab, tacrolimus, IVIG, and CYC. | CK normalized by week 8; muscle strength and endurance improved; anti-SRP-9/-54/-72 and anti-Ro-52 titers ↓; vaccine/pathogen Ab titers unchanged; B cells depleted and reappeared naïve. | None reported | None reported | None reported | No prolonged cytopenias |
| [41] | Single-case report; APS + SLE + relapsed aggressive B cell lymphoma → CD19-CAR (axi-cel) | 65-y-old female with SLE (longstanding) and triple-positive APS (recurrent DVT) complicated by relapsed B cell lymphoma. On warfarin throughout. | At day 79: anticardiolipin IgG/IgM and anti-β2-GPI IgG/IgM undetectable; lupus anticoagulant and ANA negative; sustained B cell aplasia (<1 cell/μL); no new thromboses; lymphoma remission at 12 months; continued anticoagulation. | 1/1 grade 1 (fever, hypotension); tocilizumab + dexamethasone | 1/1 grade 4 (severe encephalopathy); resolved with high-dose steroids | Transient neutropenia; no serious infections | None beyond month 2 |
| [42] | Single-case report; 29-y APS + relapsed DLBCL → CD19-CAR (axi-cel) | 67-y-old female with 29-y APS (recurrent thromboses on warfarin); relapsed/refractory DLBCL after multiple chemo regimens. | DLBCL CR by day 30; anticardiolipin IgM normalized; warfarin discontinued; no thrombotic events to 12 months; sustained B cell aplasia; APS serology remained negative at 12 months. | 1/1 grade 1 (fever) | None reported | No infections reported | No prolonged cytopenias beyond month 2 |
| [44] | Single-case report; pSS + relapsed DLBCL → CD19-CAR (axi-cel) (2 × 106 cells/kg) | 76-y-old female with 10-y active primary Sjögren’s (ANA +/anti-Ro-52 +; ESSDAI 5) and relapsed DLBCL; prior R-CHOP, lenalidomide, and ICE + zanubrutinib. | DLBCL: CR by day 28 (Deauville 2); sustained to 6 months. pSS: anti-Ro-52 + → negative by day 90; cytokines (IL-6, IL-10, TNF-α, IFN-γ) normalized by month 1; ESSDAI 5 → 2 by month 3; xerostomia/xerophthalmia improved; drug-free at 3 months. | 1/1 grade 2 (fever, hypotension, heart failure on day 6; tocilizumab × 3) | 1/1 grade 1 (tremor, dysgraphia; steroids + levetiracetam) | None serious; transient cytopenias resolved by week 6; no infections reported (except one pneumonia in MG-2). | None beyond week 6 |
| Clinical Trial Registration Number, Reference | Country | Design Phase | CAR-T Cell Product | Autoimmune Rheumatic Disease | Primary Study Endpoints | Status |
|---|---|---|---|---|---|---|
| NCT06375993 [84] | United States | Open label, multiple-arm, phase I | ADI-001 (allogeneic anti-CD20) |
|
| Recruiting |
| NCT06946485 [91] | China | Open label, single-arm, early phase I | Allogeneic universal CHT101 (anti-CD70) | Relapsed/refractory SLE |
| Not yet recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyfanti, P.; Evangelidis, P.; Kotsiou, N.; Papakonstantinou, A.; Eftychidis, I.; Sakellari, I.; Dimitroulas, T.; Gavriilaki, E. Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now? Cells 2025, 14, 1242. https://doi.org/10.3390/cells14161242
Anyfanti P, Evangelidis P, Kotsiou N, Papakonstantinou A, Eftychidis I, Sakellari I, Dimitroulas T, Gavriilaki E. Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now? Cells. 2025; 14(16):1242. https://doi.org/10.3390/cells14161242
Chicago/Turabian StyleAnyfanti, Panagiota, Paschalis Evangelidis, Nikolaos Kotsiou, Anna Papakonstantinou, Ioannis Eftychidis, Ioanna Sakellari, Theodoros Dimitroulas, and Eleni Gavriilaki. 2025. "Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now?" Cells 14, no. 16: 1242. https://doi.org/10.3390/cells14161242
APA StyleAnyfanti, P., Evangelidis, P., Kotsiou, N., Papakonstantinou, A., Eftychidis, I., Sakellari, I., Dimitroulas, T., & Gavriilaki, E. (2025). Chimeric Antigen Receptor T Cell Immunotherapy for Autoimmune Rheumatic Disorders: Where Are We Now? Cells, 14(16), 1242. https://doi.org/10.3390/cells14161242







