CREB5 Promotes the Proliferation of Neural Stem/Progenitor Cells in the Rat Subventricular Zone via the Regulation of NFIX Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Rats
2.2. Primary Culture of SVZ-Derived NSPCs
2.3. siRNA-Mediated CREB5 Knockdown
2.4. Cell Viability Assay
2.5. Sphere Formation Assay
2.6. Lentiviral Transfection
2.7. Immunostaining
2.8. Western Blot
2.9. qRT-PCR
2.10. Chromatin Immunoprecipitation (ChIP)-qPCR
2.11. Luciferase Reporter Assay
2.12. Statistical Analysis
3. Results
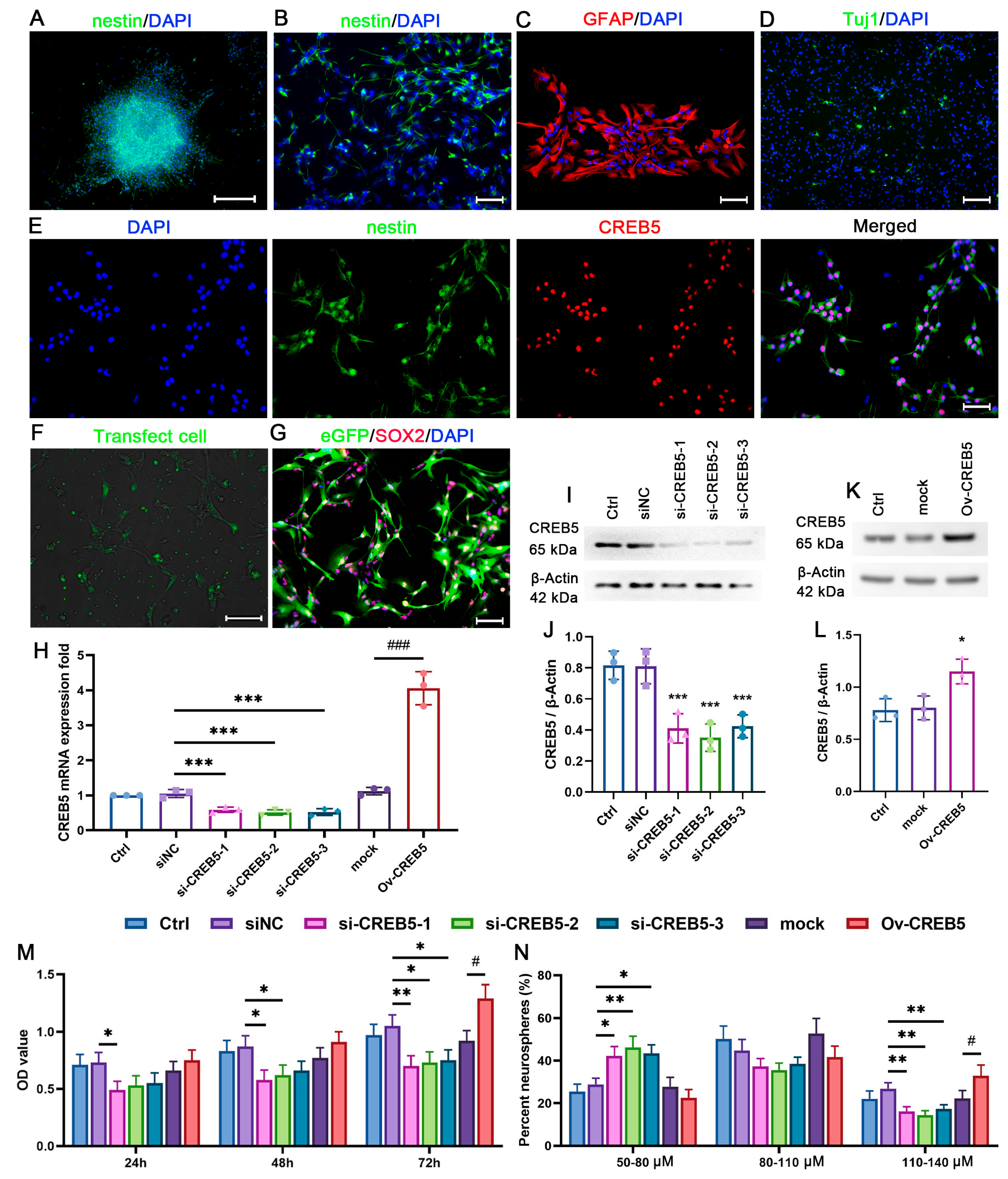
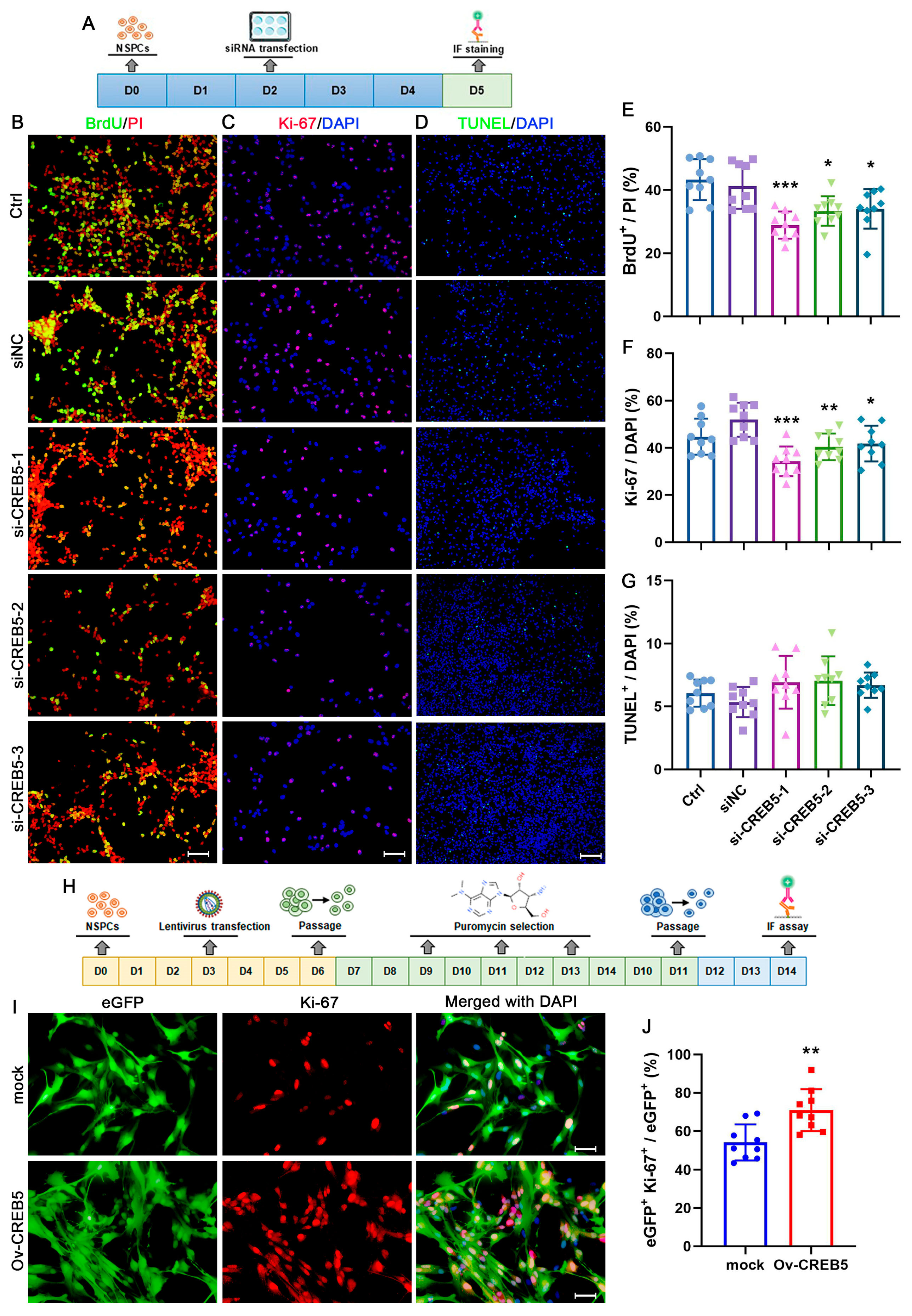
3.1. CREB5 Suppression Reduces Cell Viability and Sphere Formation Ability in Cultured Rat SVZ NSPCs
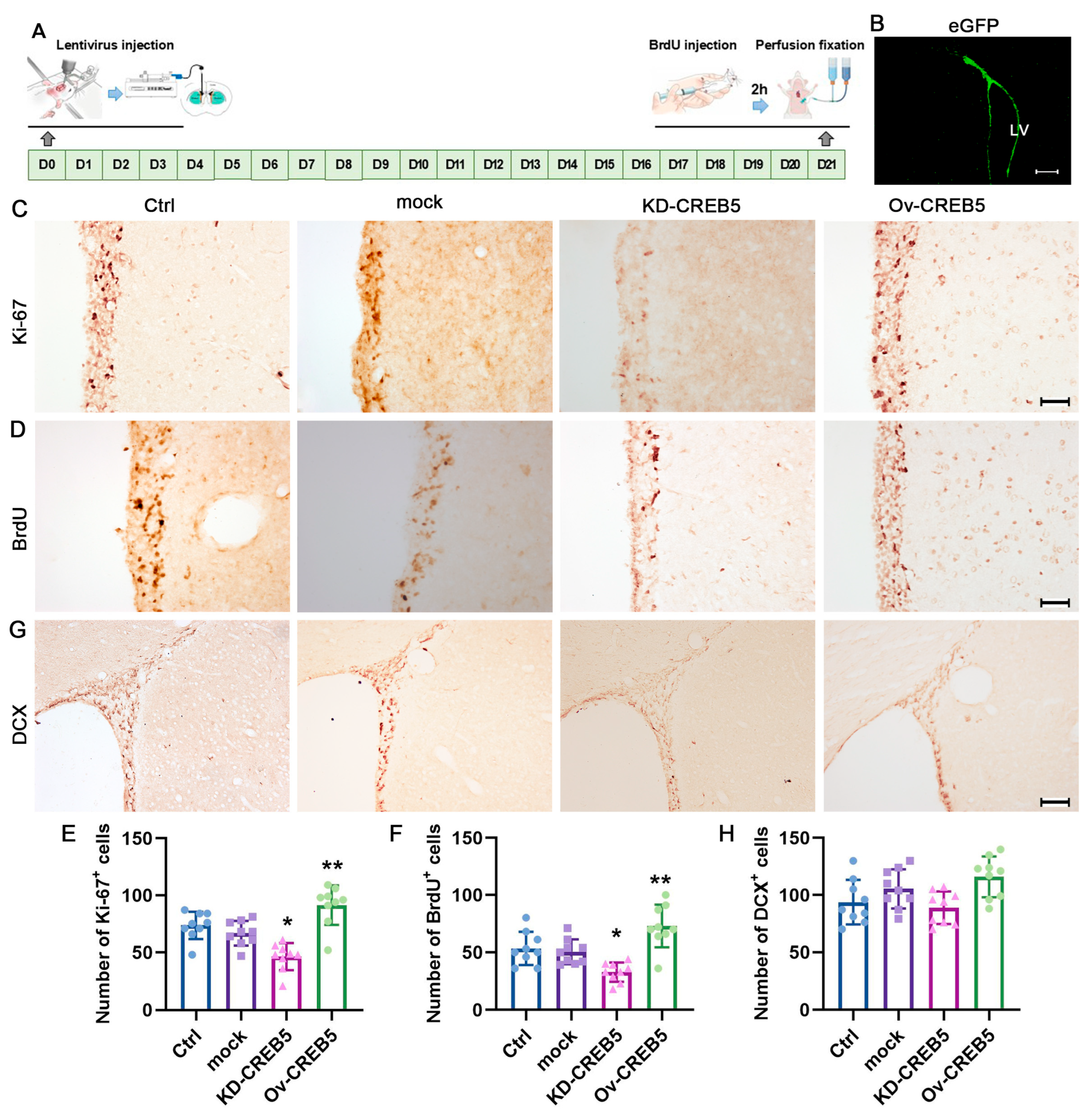
3.2. Modulation of CREB5 Expression Regulates NSPC Proliferation in the Rat SVZ
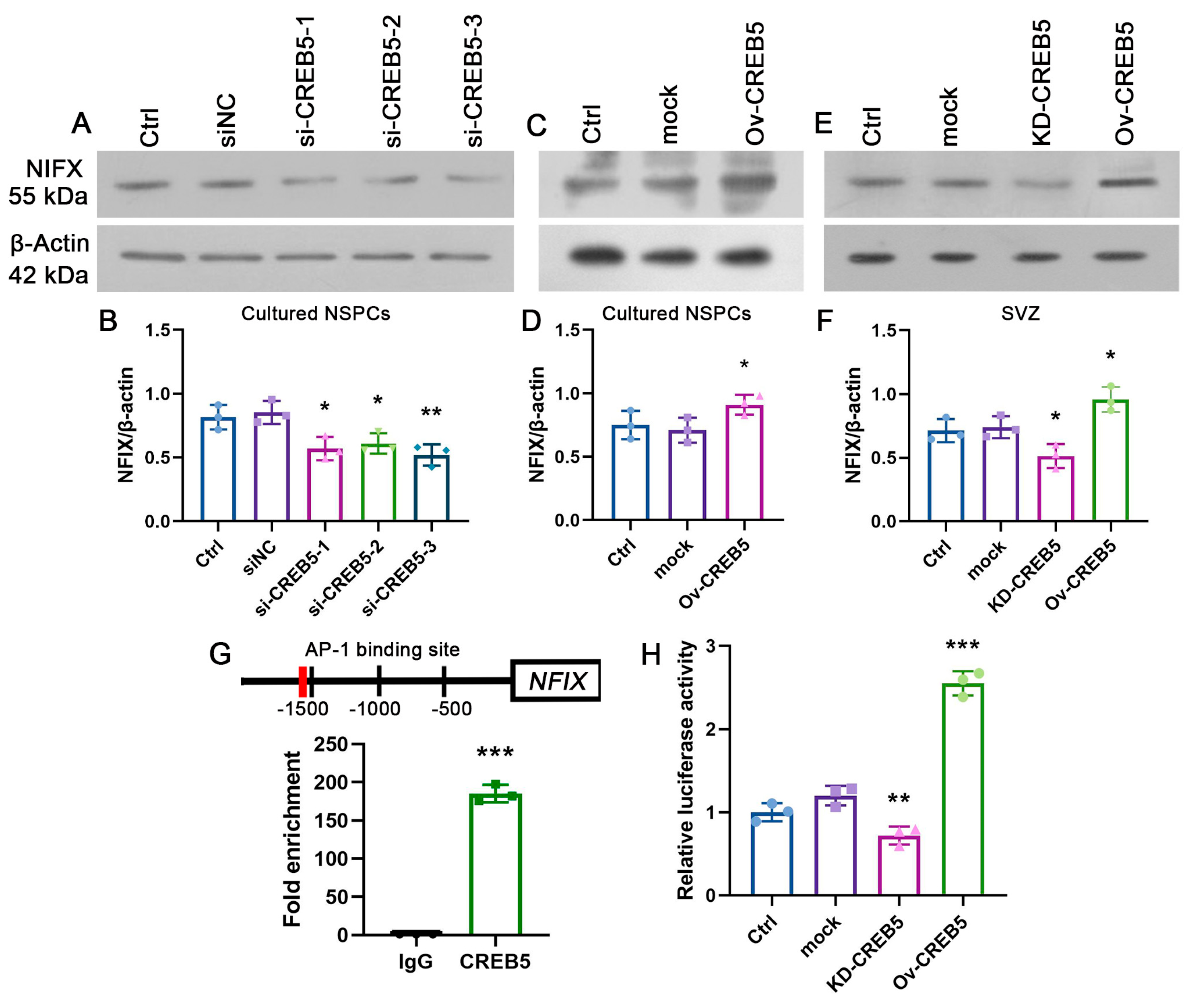
3.3. CREB5 Regulates NFIX Expression in Rat SVZ-Derived NSPCs
3.4. CREB5 Influences Proliferation of Rat SVZ-Derived NSPCs by Regulating NFIX Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSPCs | neural stem/progenitor cells |
| SVZ | subventricular zone |
| CNS | central nervous system |
| CREB5 | cAMP responsive element-binding protein 5 |
| NFIX | nuclear factor one X |
| SGZ | subgranular zone |
| mCRPCs | metastatic castration-resistant prostate cancers |
| ChIP | chromatin immunoprecipitation |
| PKA | protein kinase A |
| HDACs | histone deacetylases |
| HATs | histone acetyltransferases |
| PFA | paraformaldehyde |
References
- Bond, A.M.; Ming, G.-L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef]
- Bao, H.; Song, J. Treating Brain Disorders by Targeting Adult Neural Stem Cells. Trends Mol. Med. 2018, 24, 991–1006. [Google Scholar] [CrossRef]
- Cui, A.; Ding, D.; Li, Y. Regulation of Hepatic Metabolism and Cell Growth by the ATF/CREB Family of Transcription Factors. Diabetes 2021, 70, 653–664. [Google Scholar] [CrossRef]
- Zu, Y.L.; Maekawa, T.; Nomura, N.; Nakata, T.; Ishii, S. Regulation of trans-activating capacity of CRE-BPa by phorbol ester tumor promoter TPA. Oncogene 1993, 8, 2749–2758. [Google Scholar]
- Zhang, C.-H.; Gao, Y.; Hung, H.-H.; Zhuo, Z.; Grodzinsky, A.J.; Lassar, A.B. Creb5 coordinates synovial joint formation with the genesis of articular cartilage. Nat. Commun. 2022, 13, 7295. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Smith, C.L.; Blake, J.A.; Kadin, J.A.; Richardson, J.E.; Bult, C.J.; the Mouse Genome Database Group. Mouse Genome Database (MGD)-2018: Knowledgebase for the laboratory mouse. Nucleic Acids Res. 2017, 46, D836–D842. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiu, J.; Liu, L.; Su, C.; Qi, L.; Huang, C.; Chen, X.; Zhang, Y.; Ye, Y.; Ding, Y.; et al. CREB5 promotes invasiveness and metastasis in colorectal cancer by directly activating MET. J. Exp. Clin. Cancer Res. 2020, 39, 168. [Google Scholar] [CrossRef]
- Hwang, J.H.; Arafeh, R.; Seo, J.-H.; Baca, S.C.; Ludwig, M.; E Arnoff, T.; Sawyer, L.; Richter, C.; Tape, S.; E Bergom, H.; et al. CREB5 reprograms FOXA1 nuclear interactions to promote resistance to androgen receptor-targeting therapies. eLife 2022, 11, 73223. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.; Heath, J.K.; Dejong-Curtain, T.A.; Hogan, B.M.; Lieschke, G.J.; Malaterre, J.; Ramsay, R.G.; Mantamadiotis, T. CREB activity modulates neural cell proliferation, midbrain–hindbrain organization and patterning in zebrafish. Dev. Biol. 2007, 307, 127–141. [Google Scholar] [CrossRef]
- Dworkin, S.; Malaterre, J.; Hollande, F.; Darcy, P.K.; Ramsay, R.G.; Mantamadiotis, T. cAMP Response Element Binding Protein Is Required for Mouse Neural Progenitor Cell Survival and Expansion. STEM CELLS 2009, 27, 1347–1357. [Google Scholar] [CrossRef]
- Herold, S.; Jagasia, R.; Merz, K.; Wassmer, K.; Lie, D. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol. Cell. Neurosci. 2011, 46, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- Piper, M.; Gronostajski, R.; Messina, G. Nuclear Factor One X in Development and Disease. Trends Cell Biol. 2019, 29, 20–30. [Google Scholar] [CrossRef]
- Harris, L.; Zalucki, O.; Clément, O.; Fraser, J.; Matuzelski, E.; Oishi, S.; Harvey, T.J.; Burne, T.H.J.; Heng, J.I.-T.; Gronostajski, R.M.; et al. Neurogenic differentiation by hippocampal neural stem and progenitor cells is biased by NFIX expression. Development 2018, 145, dev155689. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeon, H.-M.; Batara, D.C.; Lee, S.; Lee, S.J.; Yin, J.; Park, S.-I.; Park, M.; Seo, J.B.; Hwang, J.; et al. CREB5 promotes the proliferation and self-renewal ability of glioma stem cells. Cell Death Discov. 2024, 10, 103. [Google Scholar] [CrossRef]
- Sanyal, S.; Sandstrom, D.J.; Hoeffer, C.A.; Ramaswami, M. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 2002, 416, 870–874. [Google Scholar] [CrossRef]
- Wang, L.-L.; He, N.; Mao, X.-J.; Ding, Y.-M.; Zuo, T.; Chen, Y.-Y. New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system. Neural Regen. Res. 2023, 18, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, C.; Wang, R.; Li, X.; Bao, X. The advantages of multi-level omics research on stem cell-based therapies for ischemic stroke. Neural Regen. Res. 2023, 19, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Bellenchi, G.C.; Volpicelli, F.; Piscopo, V.; Perrone-Capano, C.; di Porzio, U. Adult neural stem cells: An endogenous tool to repair brain injury? J. Neurochem. 2012, 124, 159–167. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, H.; Luan, Y.; Hu, B.; Shen, T.; Ma, B.; Zhang, Z.; Zheng, X. KDM4C promotes mouse hippocampal neural stem cell proliferation through modulating ApoE expression. FASEB J. 2024, 38, e23511. [Google Scholar] [CrossRef]
- Hwang, J.H.; Seo, J.-H.; Beshiri, M.L.; Wankowicz, S.; Liu, D.; Cheung, A.; Li, J.; Qiu, X.; Hong, A.L.; Botta, G.; et al. CREB5 Promotes Resistance to Androgen-Receptor Antagonists and Androgen Deprivation in Prostate Cancer. Cell Rep. 2019, 29, 2355–2370.e6. [Google Scholar] [CrossRef]
- Tong, T.; Qin, X.; Jiang, Y.; Guo, H.; Wang, X.; Li, Y.; Xie, F.; Lu, H.; Zhai, P.; Ma, H.; et al. A novel CREB5/TOP1MT axis confers cisplatin resistance through inhibiting mitochondrial apoptosis in head and neck squamous cell carcinoma. BMC Med. 2022, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, S.-T.; Zhang, Z.-j.; Zhou, Q.; Peng, B.-G. CREB5 promotes cell proliferation and correlates with poor prognosis in hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 4908–4916. [Google Scholar] [PubMed]
- Tirino, V.; Desiderio, V.; Paino, F.; De Rosa, A.; Papaccio, F.; La Noce, M.; Laino, L.; De Francesco, F.; Papaccio, G. Cancer stem cells in solid tumors: An overview and new approaches for their isolation and characterization. FASEB J. 2012, 27, 13–24. [Google Scholar] [CrossRef]
- Li, S.; Dong, L.; Pan, Z.; Yang, G. Targeting the neural stem cells in subventricular zone for the treatment of glioblastoma: An update from preclinical evidence to clinical interventions. Stem Cell Res. Ther. 2023, 14, 125. [Google Scholar] [CrossRef]
- Dolatabadi, S.; Oskuei, S.R.; Mehri, S.; Hosseinzadeh, H. A comprehensive review of medicinal plants and their beneficial roles in alleviating bisphenol A–induced organ toxicity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 7801–7876. [Google Scholar] [CrossRef] [PubMed]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Screaton, R.A.; Conkright, M.D.; Katoh, Y.; Best, J.L.; Canettieri, G.; Jeffries, S.; Guzman, E.; Niessen, S.; Yates, J.R.; Takemori, H.; et al. The CREB Coactivator TORC2 Functions as a Calcium- and cAMP-Sensitive Coincidence Detector. Cell 2004, 119, 61–74. [Google Scholar] [CrossRef]
- Hong, E.J.; McCord, A.E.; Greenberg, M.E. A Biological Function for the Neuronal Activity-Dependent Component of Bdnf Transcription in the Development of Cortical Inhibition. Neuron 2008, 60, 610–624. [Google Scholar] [CrossRef]
- Dinevska, M.; Widodo, S.S.; Cook, L.; Stylli, S.S.; Ramsay, R.G.; Mantamadiotis, T. CREB: A multifaceted transcriptional regulator of neural and immune function in CNS tumors. Brain Behav. Immun. 2023, 116, 140–149. [Google Scholar] [CrossRef]
- E Lonze, B.; Ginty, D.D. Function and Regulation of CREB Family Transcription Factors in the Nervous System. Neuron 2002, 35, 605–623. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; An, J.; Jeong, S. The Pleiotropic Face of CREB Family Transcription Factors. Mol. Cells 2023, 46, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Landles, C.; Bates, G.P. Huntingtin and the molecular pathogenesis of Huntington’s disease. Embo Rep. 2004, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Eggert, S.; Kins, S.; Endres, K.; Brigadski, T. Brothers in arms: proBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of Alzheimer’s disease. Biol. Chem. 2021, 403, 43–71. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Yuan, P.; Cheng, L.; Sun, H.; Gui, J.; Pan, Y.; Huang, D.; Chen, H.; Jiang, L. Role of PKA/CREB/BDNF signaling in PM2.5-induced neurodevelopmental damage to the hippocampal neurons of rats. Ecotoxicol. Environ. Saf. 2021, 214, 112005. [Google Scholar] [CrossRef]
- Mason, S.; Piper, M.; Gronostajski, R.M.; Richards, L.J. Nuclear Factor One Transcription Factors in CNS Development. Mol. Neurobiol. 2008, 39, 10–23. [Google Scholar] [CrossRef]
- E Campbell, C.; Piper, M.; Plachez, C.; Yeh, Y.-T.; Baizer, J.S.; Osinski, J.M.; Litwack, E.D.; Richards, L.J.; Gronostajski, R.M. The transcription factor Nfixis essential for normal brain development. BMC Dev. Biol. 2008, 8, 52. [Google Scholar] [CrossRef]
- Harris, L.; Genovesi, L.A.; Gronostajski, R.M.; Wainwright, B.J.; Piper, M. Nuclear factor one transcription factors: Divergent functions in developmental versus adult stem cell populations. Dev. Dyn. 2014, 244, 227–238. [Google Scholar] [CrossRef]
- Walker, M.; Li, Y.; Morales-Hernandez, A.; Qi, Q.; Parupalli, C.; Brown, S.; Christian, C.; Clements, W.K.; Cheng, Y.; McKinney-Freeman, S. An NFIX-mediated regulatory network governs the balance of hematopoietic stem and progenitor cells during hematopoiesis. Blood Adv. 2023, 7, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Ala, C.; Ramalingam, S.; Gowri, C.S.K.V.; Sankaranarayanan, M. A critique review of fetal hemoglobin modulators through targeting epigenetic regulators for the treatment of sickle cell disease. Life Sci. 2025, 369, 123536. [Google Scholar] [CrossRef]
- Holmfeldt, P.; Pardieck, J.; Saulsberry, A.C.; Nandakumar, S.K.; Finkelstein, D.; Gray, J.T.; Persons, D.A.; McKinney-Freeman, S. Nfix is a novel regulator of murine hematopoietic stem and progenitor cell survival. Blood 2013, 122, 2987–2996. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J.; Cannon, J.R.; Baltier, K.; Gebreab, F.; Gygi, M.P.; Thornock, A.; Zarraga, G.; Tam, S.; et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 2021, 184, 3022–3040.e28. [Google Scholar] [CrossRef]
- Cho, N.H.; Cheveralls, K.C.; Brunner, A.-D.; Kim, K.; Michaelis, A.C.; Raghavan, P.; Kobayashi, H.; Savy, L.; Li, J.Y.; Canaj, H.; et al. OpenCell: Endogenous tagging for the cartography of human cellular organization. Science 2022, 375, eabi6983. [Google Scholar] [CrossRef]
- Pagin, M.; Pernebrink, M.; Giubbolini, S.; Barone, C.; Sambruni, G.; Zhu, Y.; Chiara, M.; Ottolenghi, S.; Pavesi, G.; Wei, C.-L.; et al. Sox2 Controls Neural Stem Cell Self-Renewal Through a Fos-Centered Gene Regulatory Network. STEM CELLS 2021, 39, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Hata, R.; Ma, Y.; Tanaka, J.; Mitsuda, N.; Kumon, Y.; Hanakawa, Y.; Hashimoto, K.; Nakajima, K.; Sakanaka, M. Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J. Neurosci. Res. 2005, 81, 163–171. [Google Scholar] [CrossRef]
- Dias, J.M.; Ilkhanizadeh, S.; Karaca, E.; Duckworth, J.K.; Lundin, V.; Rosenfeld, M.G.; Ericson, J.; Hermanson, O.; Teixeira, A.I. CtBPs Sense Microenvironmental Oxygen Levels to Regulate Neural Stem Cell State. Cell Rep. 2014, 8, 665–670. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Zhang, H.; Zhang, C.; Ma, G.; Shen, T.; Luan, Y.; Zhang, Z. CREB5 Promotes the Proliferation of Neural Stem/Progenitor Cells in the Rat Subventricular Zone via the Regulation of NFIX Expression. Cells 2025, 14, 1240. https://doi.org/10.3390/cells14161240
Yu T, Zhang H, Zhang C, Ma G, Shen T, Luan Y, Zhang Z. CREB5 Promotes the Proliferation of Neural Stem/Progenitor Cells in the Rat Subventricular Zone via the Regulation of NFIX Expression. Cells. 2025; 14(16):1240. https://doi.org/10.3390/cells14161240
Chicago/Turabian StyleYu, Tao, Hanyue Zhang, Chuang Zhang, Guorui Ma, Tu Shen, Yan Luan, and Zhichao Zhang. 2025. "CREB5 Promotes the Proliferation of Neural Stem/Progenitor Cells in the Rat Subventricular Zone via the Regulation of NFIX Expression" Cells 14, no. 16: 1240. https://doi.org/10.3390/cells14161240
APA StyleYu, T., Zhang, H., Zhang, C., Ma, G., Shen, T., Luan, Y., & Zhang, Z. (2025). CREB5 Promotes the Proliferation of Neural Stem/Progenitor Cells in the Rat Subventricular Zone via the Regulation of NFIX Expression. Cells, 14(16), 1240. https://doi.org/10.3390/cells14161240






