Mycn Is Essential for Pubertal Mammary Gland Development and Promotes the Activation of Bcl11b-Maintained Quiescent Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
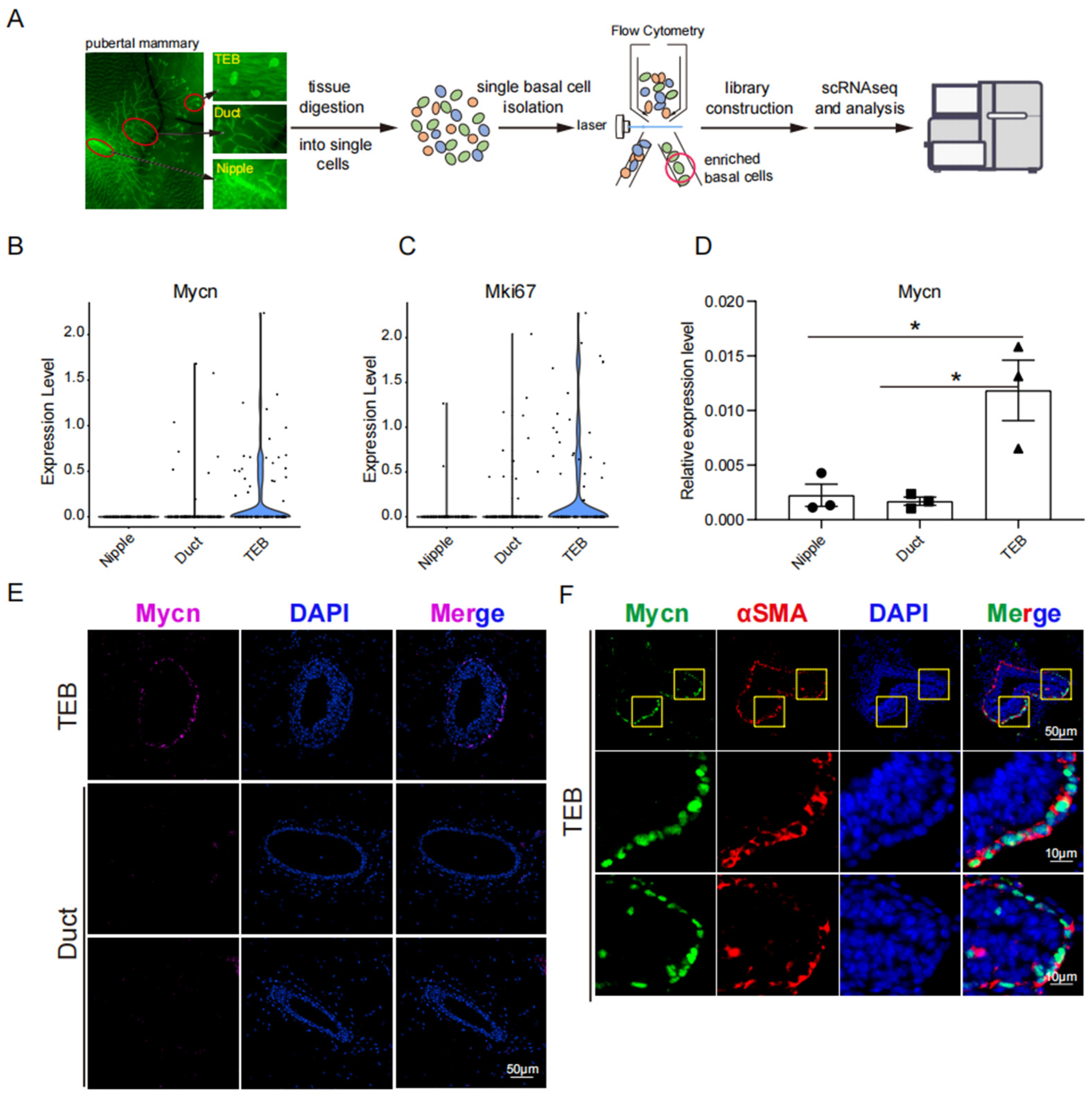
2.2. Tissue Processing and Flow Cytometry
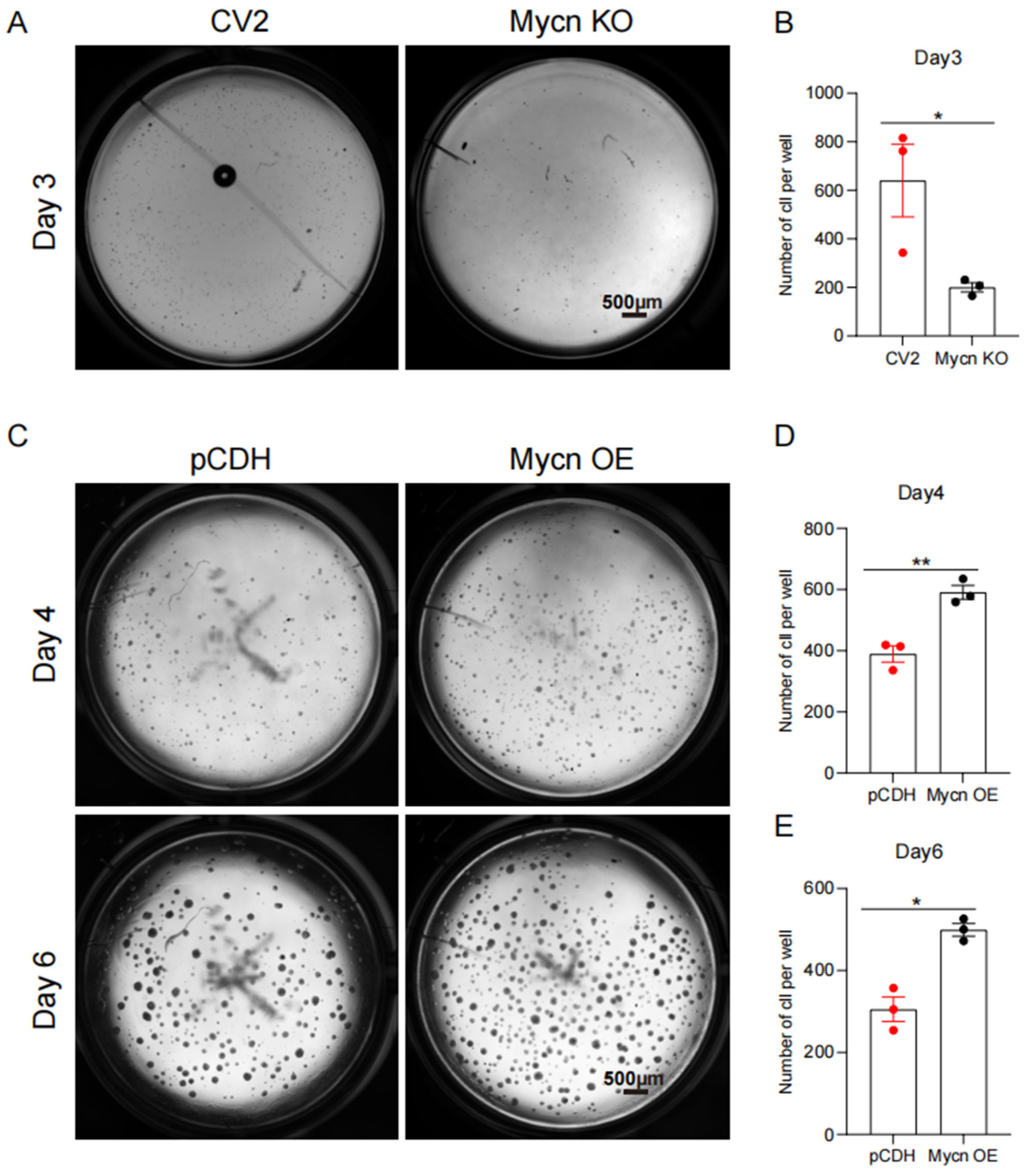
2.3. Colony Formation Assay
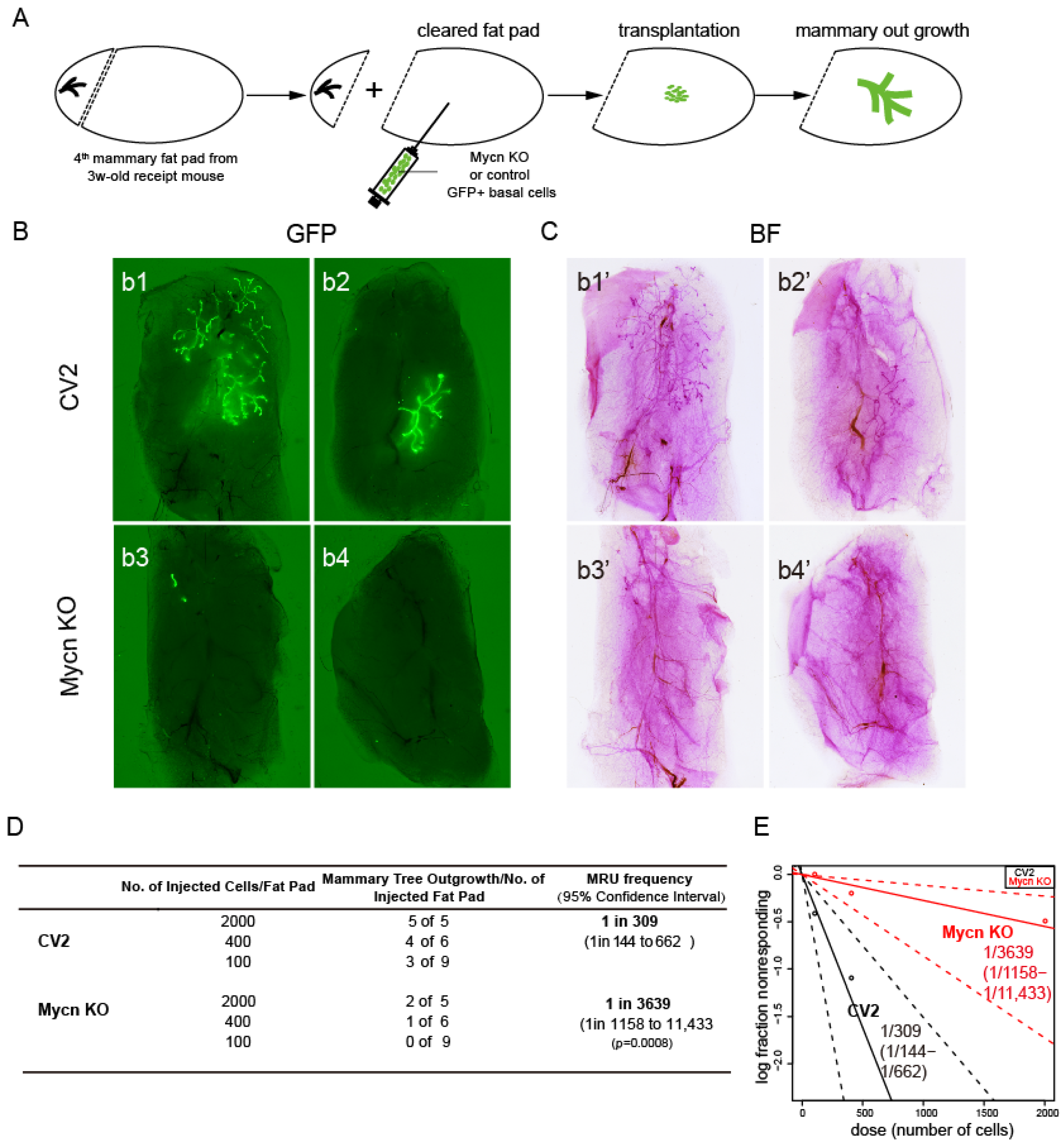
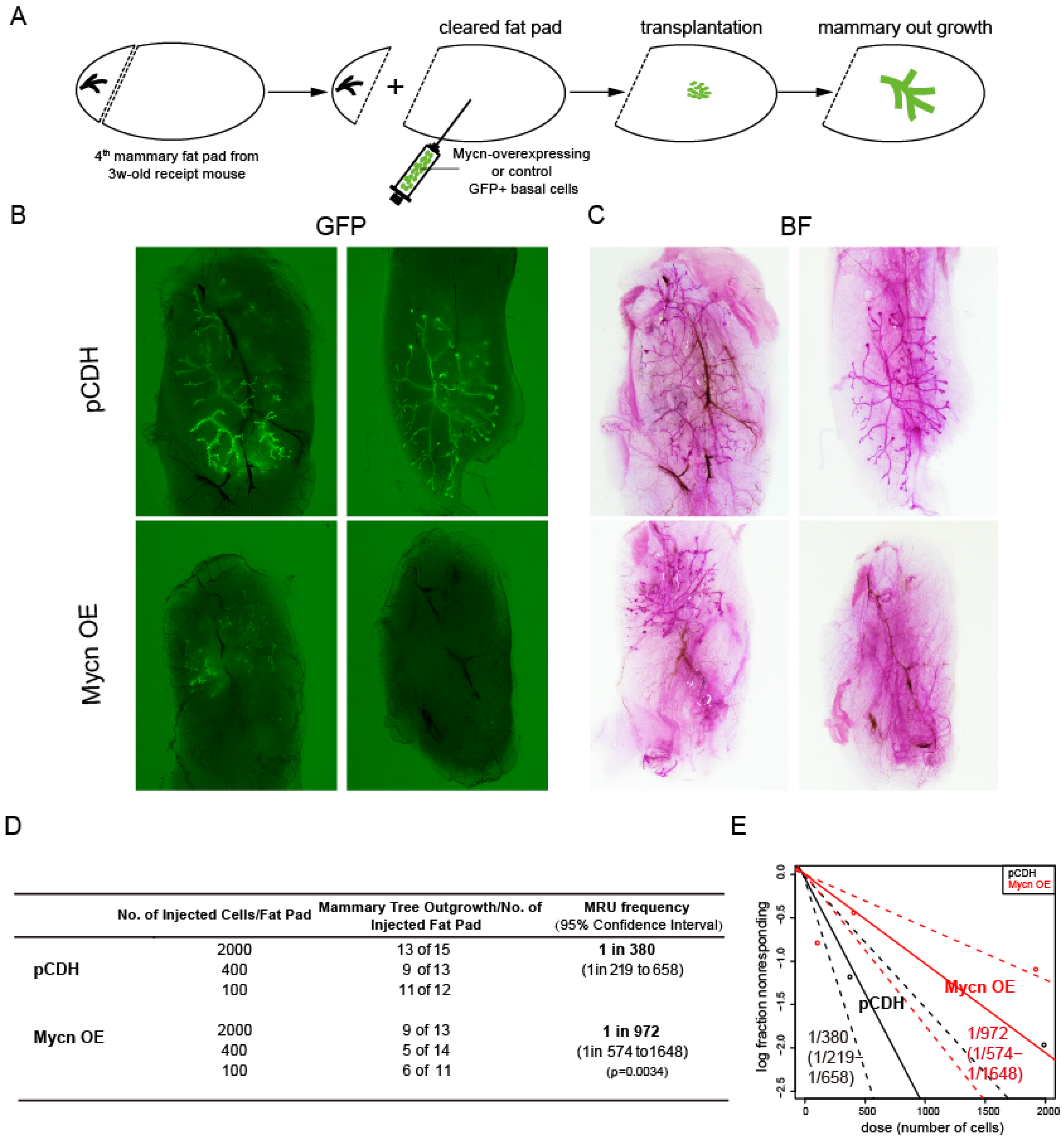
2.4. Transplantation Assay
2.5. Real-Time PCR
- β-Actin: Forward ACCTTCTACAATGAGCTGCG, Reverse CTGGATGGCTACGTACATGG;
- Bcl11b: Forward AGGAGAGTATCTGAGCCAGTG, Reverse GTTGTGCAAATGTAGCTGGAAG;
- Tspan8: Forward AGTTCCGTTTACCCAAAGACC, Reverse GCACCATAGAAAACACCAAACC;
- Mycn: Forward GTCTGTTCCAGCTACTGCC, Reverse TCCTCTTCATCTTCCTCCTCG.
2.6. Paraffin Sections and Immunofluorescence
2.7. Carmine Stain (Whole Mount)
2.8. Mycn-Overexpressing Cell Line
2.9. CRISPR-Mediated Mycn Knockout Primary Basal Cells
2.10. Single-Cell RNA Sequencing (scRNA-seq) and Data Analysis
2.11. D-CUT&RUN
2.12. Data Analysis and Related Software
3. Results
3.1. Mycn Was Spatially Enriched in TEB
3.2. Mycn Was Indispensable for Mammary Ductal Development
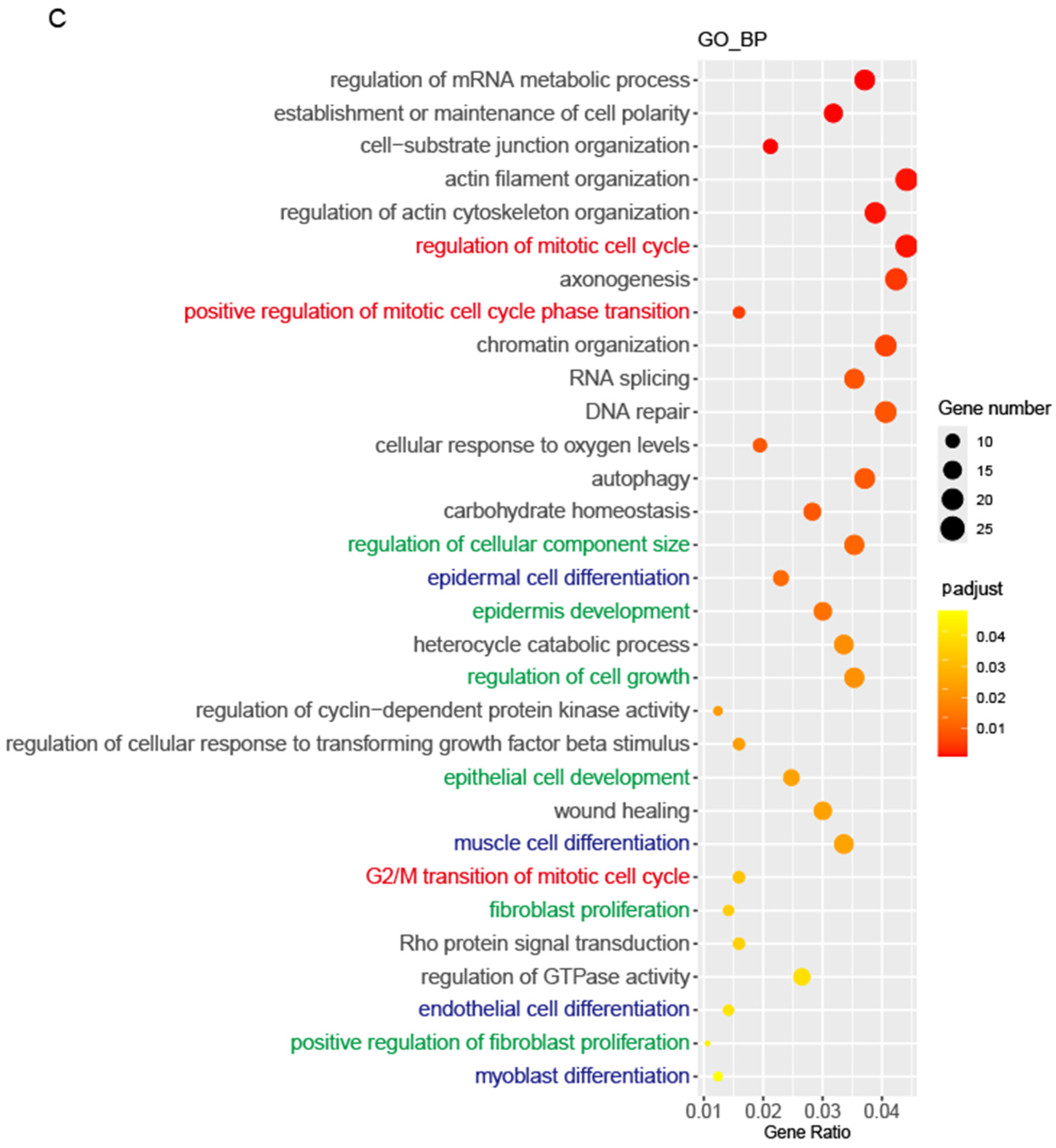
3.3. Mycn Promoted Cell Proliferation
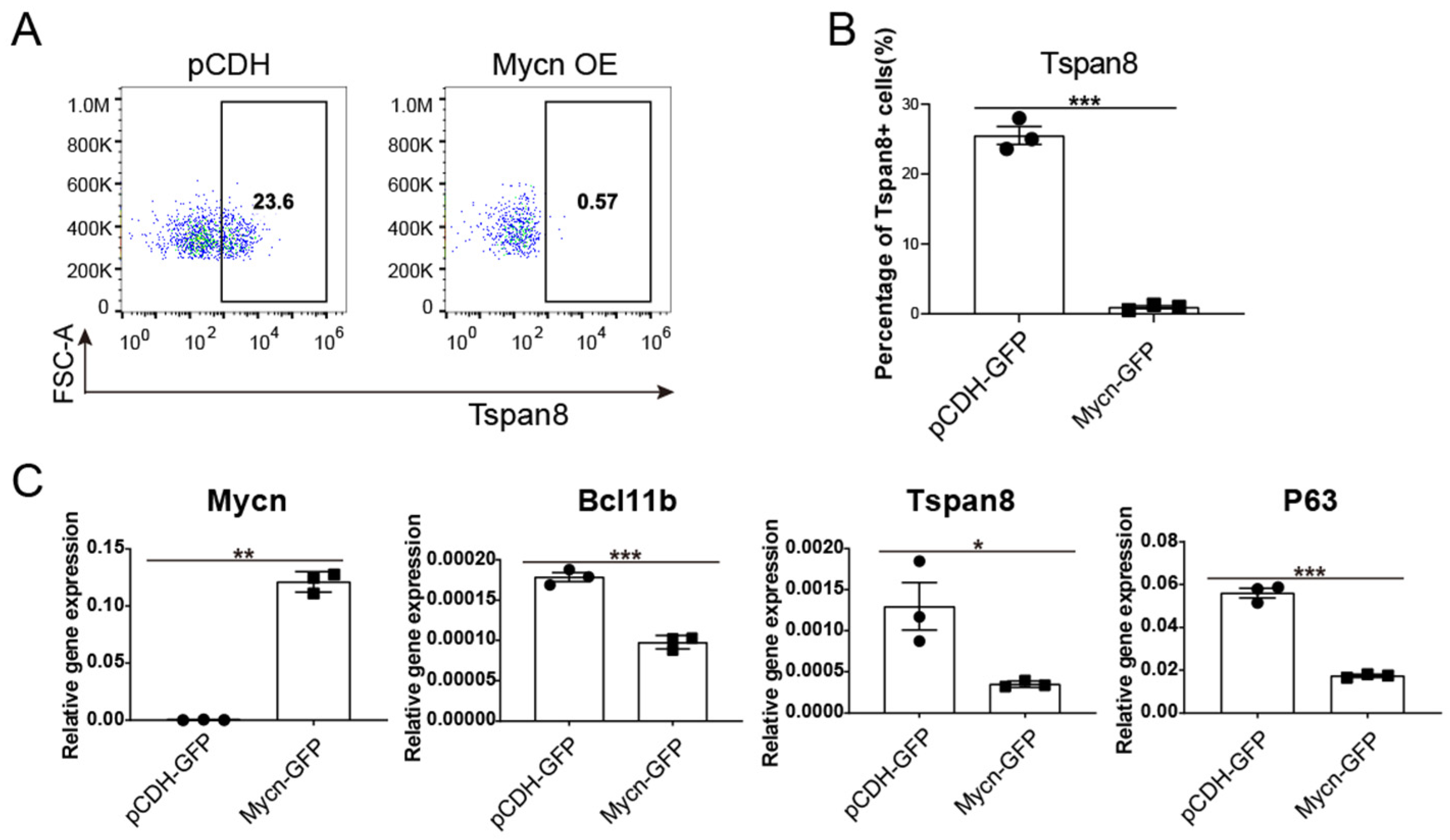
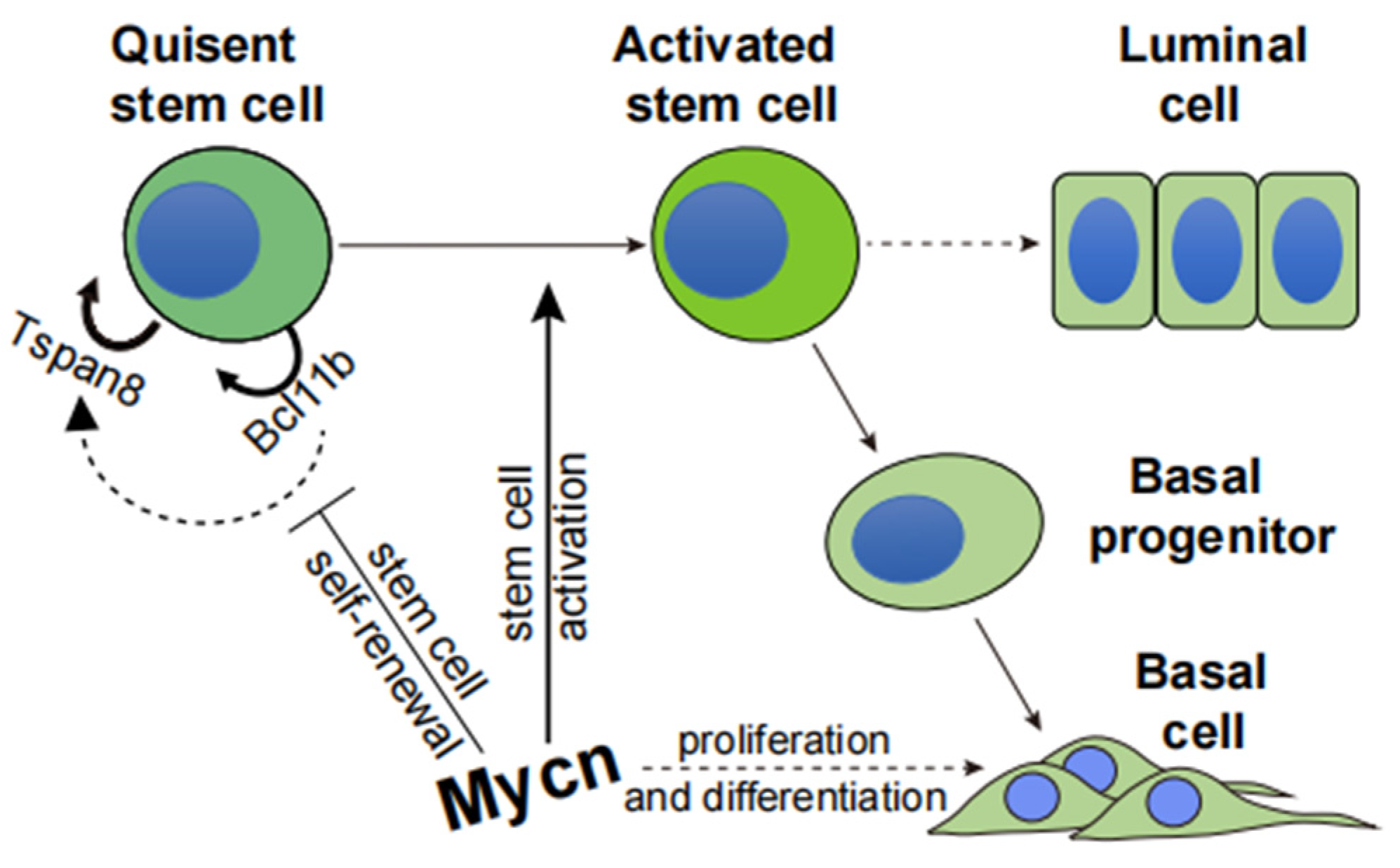
3.4. Mycn Downregulated the Quiescent Stem Cell Regulators Bcl11b and Tspan8
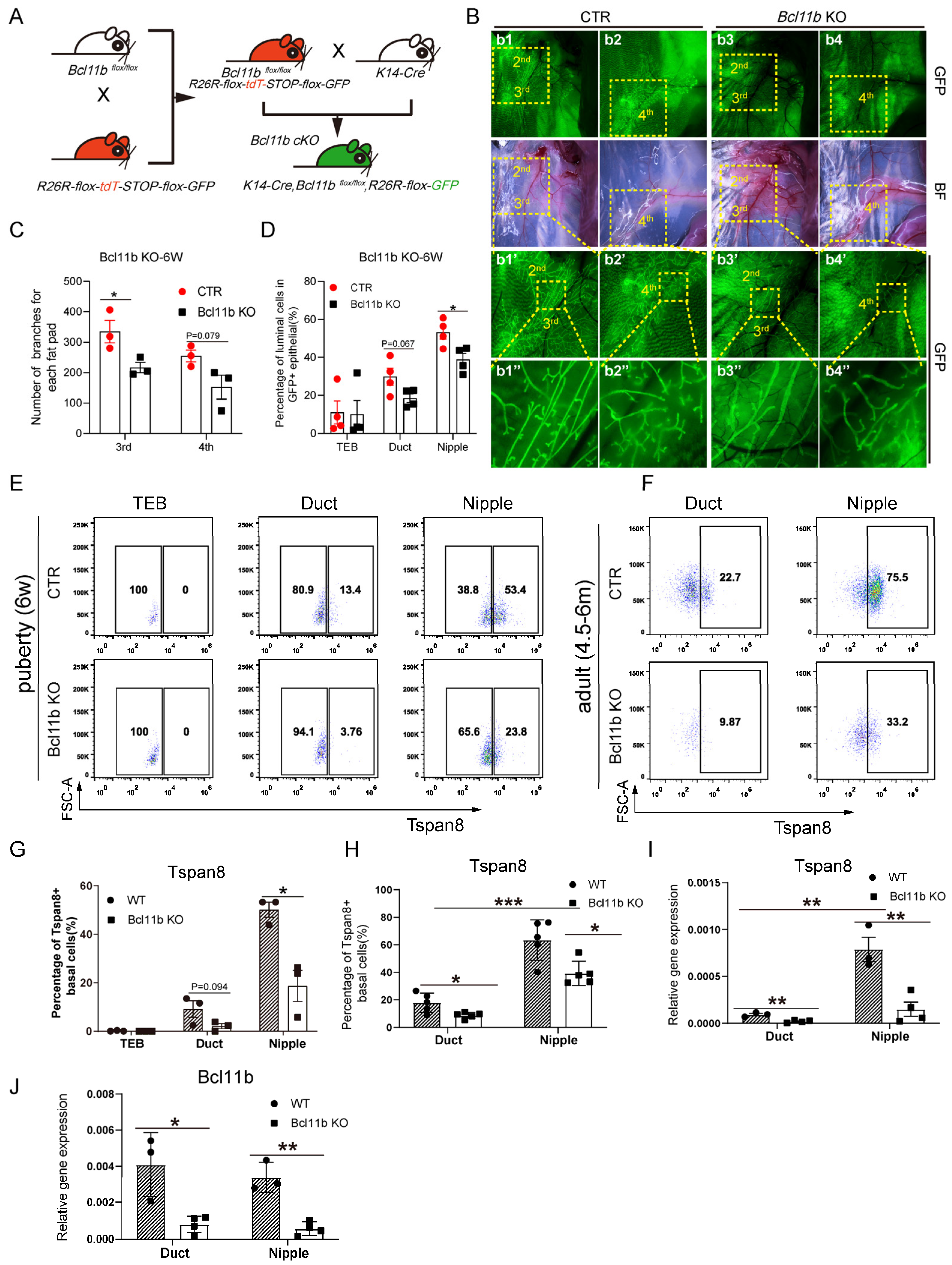
3.5. Bcl11b KO Decreased the Expression Level of Tspan8 and Activated Cell Proliferation
3.6. Mycn Coordinated Cell Proliferation and Differentiation
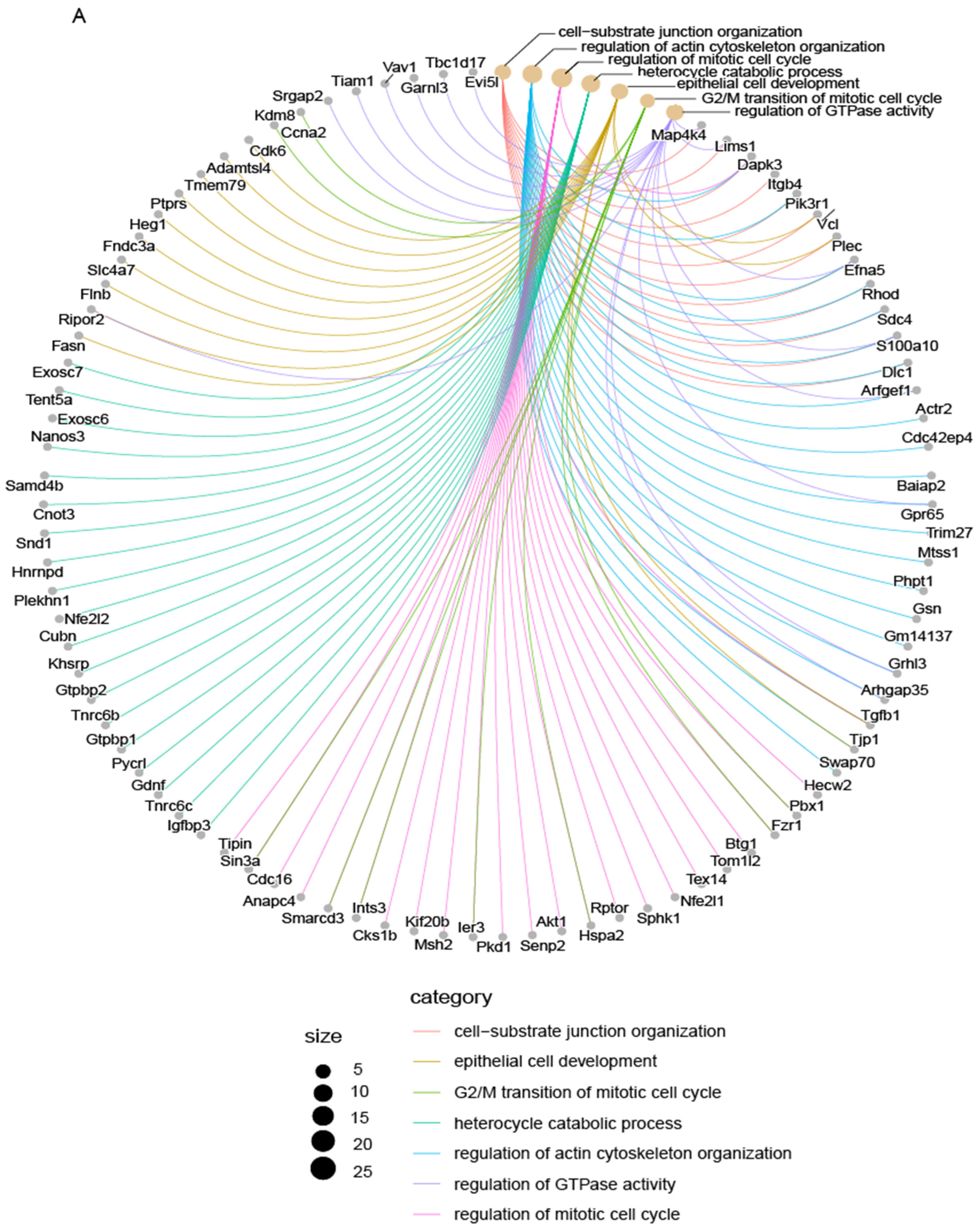
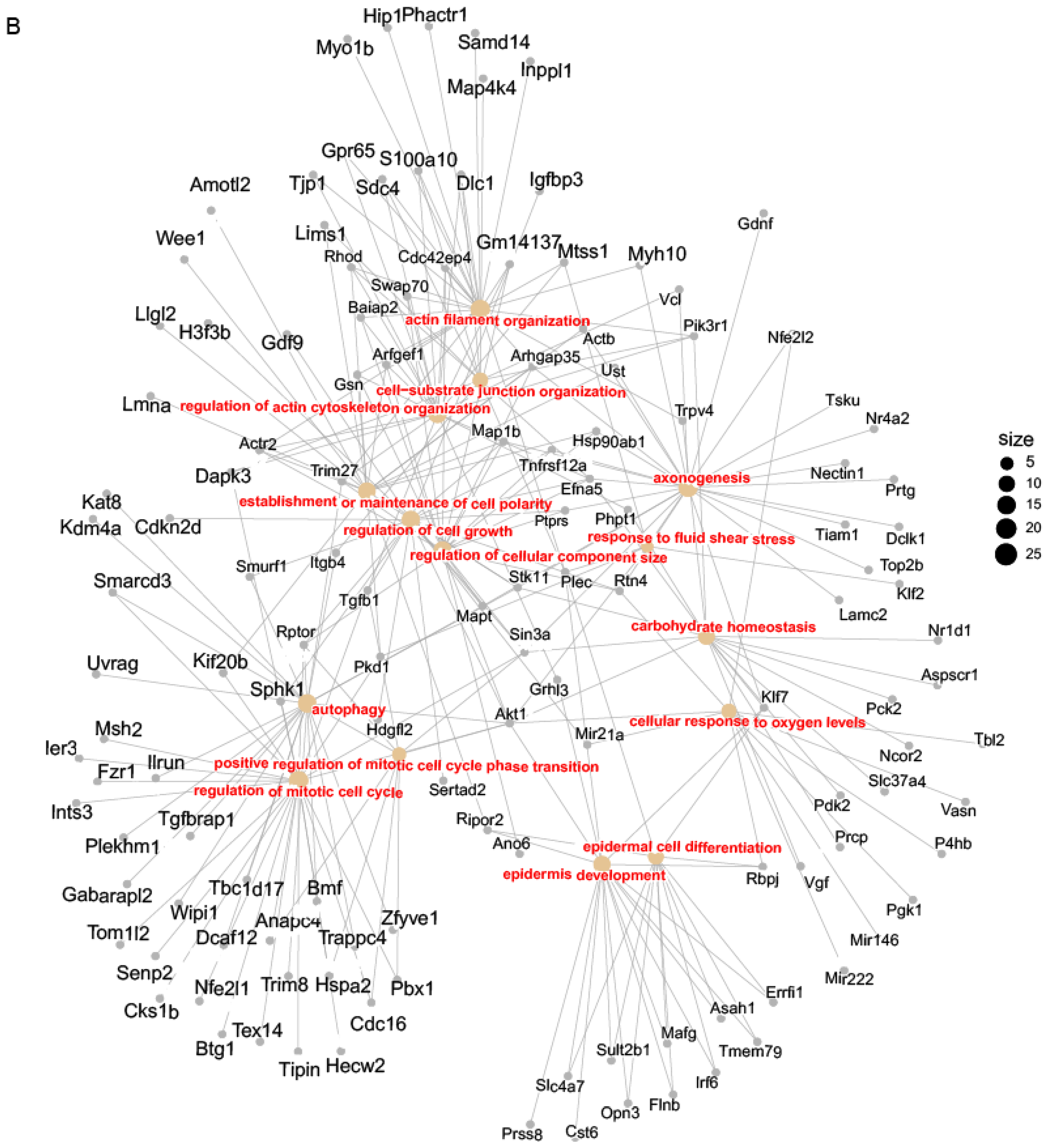
3.7. Mycn Indirectly Targeted Bcl11b and Tspan8
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonkin, D.; Thomas, A.; Teicher, B.A. Cancer treatments: Past, present, and future. Cancer Genet. 2024, 286–287, 18–24. [Google Scholar] [CrossRef]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Wang, X.; Venet, D.; Lifrange, F.; Larsimont, D.; Rediti, M.; Stenbeck, L.; Dupont, F.; Rouas, G.; Garcia, A.J.; Craciun, L.; et al. Spatial transcriptomics reveals substantial heterogeneity in triple-negative breast cancer with potential clinical implications. Nat. Commun. 2024, 15, 10232. [Google Scholar] [CrossRef]
- Chen, Q.S.; Cai, R.Z.; Wang, Y.; Liang, G.H.; Zhang, K.M.; Yang, X.Y.; Yang, D.; Zhao, D.C.; Zhu, X.F.; Deng, R.; et al. Profiling triple-negative breast cancer-specific super-enhancers identifies high-risk mesenchymal development subtype and BETi-Targetable vulnerabilities. Mol. Cancer 2025, 24, 141. [Google Scholar] [CrossRef]
- Asleh, K.; Riaz, N.; Nielsen, T.O. Heterogeneity of triple negative breast cancer: Current advances in subtyping and treatment implications. J. Exp. Clin. Cancer Res. 2022, 41, 265. [Google Scholar] [CrossRef] [PubMed]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Takebe, N.; Hinoue, T.; Hoadley, K.A.; Cardenas, M.F.; Hamilton, A.M.; Laird, P.W.; Wang, L.; Johnson, A.; Dewal, N.; et al. Molecular Features of Cancers Exhibiting Exceptional Responses to Treatment. Cancer Cell 2021, 39, 38–53.e7. [Google Scholar] [CrossRef]
- Bowling, E.A.; Wang, J.H.; Gong, F.; Wu, W.; Neill, N.J.; Kim, I.S.; Tyagi, S.; Orellana, M.; Kurley, S.J.; Dominguez-Vidaña, R.; et al. Spliceosome-targeted therapies trigger an antiviral immune response in triple-negative breast cancer. Cell 2021, 184, 384–403.e21. [Google Scholar] [CrossRef]
- Corces, M.R.; Granja, J.M.; Shams, S.; Louie, B.H.; Seoane, J.A.; Zhou, W.; Silva, T.C.; Groeneveld, C.; Wong, C.K.; Cho, S.W.; et al. The chromatin accessibility landscape of primary human cancers. Science 2018, 362, eaav1898. [Google Scholar] [CrossRef]
- Cossa, G.; Roeschert, I.; Prinz, F.; Baluapuri, A.; Vidal, R.S.; Schülein-Völk, C.; Chang, Y.C.; Ade, C.P.; Mastrobuoni, G.; Girard, C.; et al. Localized inhibition of protein phosphatase 1 by NUAK1 promotes spliceosome activity and reveals a MYC-sensitive feedback control of transcription. Mol. Cell 2021, 81, 2495. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.Y.; Nolan, E.; Lindeman, G.J.; Visvader, J.E. Stem Cells and the Differentiation Hierarchy in Mammary Gland Development. Physiol. Rev. 2020, 100, 489–523. [Google Scholar] [CrossRef]
- Cai, S.; Kalisky, T.; Sahoo, D.; Dalerba, P.; Feng, W.; Lin, Y.; Qian, D.; Kong, A.; Yu, J.; Wang, F.; et al. A Quiescent Bcl11b High Stem Cell Population Is Required for Maintenance of the Mammary Gland. Cell Stem Cell 2017, 20, 247–260.e5. [Google Scholar] [CrossRef]
- Fu, N.Y.; Rios, A.C.; Pal, B.; Law, C.W.; Jamieson, P.; Liu, R.; Vaillant, F.; Jackling, F.; Liu, K.H.; Smyth, G.K.; et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat. Cell Biol. 2017, 19, 164–176. [Google Scholar] [CrossRef]
- Van Keymeulen, A.; Rocha, A.S.; Ousset, M.; Beck, B.; Bouvencourt, G.; Rock, J.; Sharma, N.; Dekoninck, S.; Blanpain, C. Distinct stem cells contribute to mammary gland development and maintenance. Nature 2011, 479, 189–193. [Google Scholar] [CrossRef]
- Wang, C.; Christin, J.R.; Oktay, M.H.; Guo, W. Lineage-Biased Stem Cells Maintain Estrogen-Receptor-Positive and -Negative Mouse Mammary Luminal Lineages. Cell Rep. 2017, 18, 2825–2835. [Google Scholar] [CrossRef]
- Van Keymeulen, A.; Fioramonti, M.; Centonze, A.; Bouvencourt, G.; Achouri, Y.; Blanpain, C. Lineage-Restricted Mammary Stem Cells Sustain the Development, Homeostasis, and Regeneration of the Estrogen Receptor Positive Lineage. Cell Rep. 2017, 20, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Van Keymeulen, A.; Blanpain, C. Tracing epithelial stem cells during development, homeostasis, and repair. J. Cell Biol. 2012, 197, 575–584. [Google Scholar] [CrossRef]
- Wuidart, A.; Sifrim, A.; Fioramonti, M.; Matsumura, S.; Brisebarre, A.; Brown, D.; Centonze, A.; Dannau, A.; Dubois, C.; Van Keymeulen, A.; et al. Early lineage segregation of multipotent embryonic mammary gland progenitors. Nat. Cell Biol. 2018, 20, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Lilja, A.M.; Rodilla, V.; Huyghe, M.; Hannezo, E.; Landragin, C.; Renaud, O.; Leroy, O.; Rulands, S.; Simons, B.D.; Fre, S. Clonal analysis of Notch1-expressing cells reveals the existence of unipotent stem cells that retain long-term plasticity in the embryonic mammary gland. Nat. Cell Biol. 2018, 20, 677–687. [Google Scholar] [CrossRef]
- McBryan, J.; Howlin, J. Pubertal Mammary Gland Development: Elucidation of In Vivo Morphogenesis Using Murine Models. Methods Mol. Biol. 2017, 1501, 77–114. [Google Scholar] [PubMed]
- Paine, I.S.; Lewis, M.T. The Terminal End Bud: The Little Engine that Could. J. Mammary Gland. Biol. Neoplasia 2017, 22, 93–108. [Google Scholar] [CrossRef]
- Slepicka, P.F.; Somasundara, A.V.H.; Dos Santos, C.O. The molecular basis of mammary gland development and epithelial differentiation. Semin. Cell Dev. Biol. 2021, 114, 93–112. [Google Scholar] [CrossRef]
- Williams, J.M.; Daniel, C.W. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev. Biol. 1983, 97, 274–290. [Google Scholar] [CrossRef]
- Smalley, M.; Ashworth, A. Stem cells and breast cancer: A field in transit. Nat. Rev. Cancer 2003, 3, 832–844. [Google Scholar] [CrossRef]
- Lewis, M.T.; Porter, W.W. Methods in mammary gland biology and breast cancer research: An update. J. Mammary Gland. Biol. Neoplasia 2009, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Kouros-Mehr, H.; Slorach, E.M.; Sternlicht, M.D.; Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 2006, 127, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Strickland, P.; Valdes, A.; Shin, G.C.; Hinck, L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev. Cell 2003, 4, 371–382. [Google Scholar] [CrossRef]
- Bai, L.; Rohrschneider, L.R. s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev. 2010, 24, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, A.; Roarty, K.; Rosen, J.M. The mammary stem cell hierarchy: A looking glass into heterogeneous breast cancer landscapes. Endocr. Relat. Cancer 2015, 22, T161–T176. [Google Scholar] [CrossRef]
- Sahu, S.; Albaugh, M.E.; Martin, B.K.; Patel, N.L.; Riffle, L.; Mackem, S.; Kalen, J.D.; Sharan, S.K. Growth factor dependency in mammary organoids regulates ductal morphogenesis during organ regeneration. Sci. Rep. 2022, 12, 7200. [Google Scholar] [CrossRef]
- Lawson, D.A.; Werb, Z.; Zong, Y.; Goldstein, A.S. The Cleared Mammary Fat Pad Transplantation Assay for Mammary Epithelial Organogenesis. Cold Spring Harb. Protoc. 2015, 2015, pdb.prot078071. [Google Scholar] [CrossRef]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Stingl, J.; Eirew, P.; Ricketson, I.; Shackleton, M.; Vaillant, F.; Choi, D.; Li, H.I.; Eaves, C.J. Purification and unique properties of mammary epithelial stem cells. Nature 2006, 439, 993–997. [Google Scholar] [CrossRef]
- van Amerongen, R.; Bowman, A.N.; Nusse, R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 2012, 11, 387–400. [Google Scholar] [CrossRef]
- dos Santos, C.O.; Rebbeck, C.; Rozhkova, E.; Valentine, A.; Samuels, A.; Kadiri, L.R.; Osten, P.; Harris, E.Y.; Uren, P.J.; Smith, A.D.; et al. Molecular hierarchy of mammary differentiation yields refined markers of mammary stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7123–7130. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cai, C.; Dong, X.; Yu, Q.C.; Zhang, X.O.; Yang, L.; Zeng, Y.A. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 2015, 517, 81–84. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Celia-Terrassa, T.; Kumar, S.; Hang, X.; Wei, Y.; Choudhury, A.; Hwang, J.; Peng, J.; Nixon, B.; Grady, J.J.; et al. Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science 2018, 360, eaan4153. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, L.; Brand, A.H. Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science 2018, 360, 99–102. [Google Scholar] [CrossRef]
- Urban, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Bai, H.; Liu, X.; Lin, M.; Meng, Y.; Tang, R.; Guo, Y.; Li, N.; Clarke, M.F.; Cai, S. Progressive senescence programs induce intrinsic vulnerability to aging-related female breast cancer. Nat. Commun. 2024, 15, 5154. [Google Scholar] [CrossRef]
- Johnstone, M.E.; Leck, A.L.; Lange, T.E.; Wilcher, K.E.; Shephard, M.S.; Paranjpe, A.; Schutte, S.; Wells, S.I.; Kappes, F.; Salomonis, N.; et al. DEK promotes mammary hyperplasia and is associated with H3K27me3 epigenetic modifications. Life Sci. Alliance 2025, 8, e202503230. [Google Scholar] [CrossRef]
- Marescal, O.; Cheeseman, I.M. Cellular Mechanisms and Regulation of Quiescence. Dev. Cell 2020, 55, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hu, H.; McNeil, M.; Xu, J.; Bi, X.; Lou, P.; Guerrero-Juarez, C.F.; Dai, X.; Plikus, M.V.; Shuai, J.; et al. Dormant Nfatc1 reporter-marked basal stem/progenitor cells contribute to mammary lobuloalveoli formation. iScience 2022, 25, 103982. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.Y.; Pal, B.; Chen, Y.; Jackling, F.C.; Milevskiy, M.; Vaillant, F.; Capaldo, B.D.; Guo, F.; Liu, K.H.; Rios, A.C.; et al. Foxp1 Is Indispensable for Ductal Morphogenesis and Controls the Exit of Mammary Stem Cells from Quiescence. Dev. Cell 2018, 47, 629–644.e8. [Google Scholar] [CrossRef]
- Han, Y.; Villarreal-Ponce, A.; Gutierrez, G., Jr.; Nguyen, Q.; Sun, P.; Wu, T.; Sui, B.; Berx, G.; Brabletz, T.; Kessenbrock, K.; et al. Coordinate control of basal epithelial cell fate and stem cell maintenance by core EMT transcription factor Zeb1. Cell Rep. 2022, 38, 110240. [Google Scholar] [CrossRef]
- Kohl, N.E.; Kanda, N.; Schreck, R.R.; Bruns, G.; Latt, S.A.; Gilbert, F.; Alt, F.W. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell 1983, 35 Pt 1, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kohl, N.E.; Gee, C.E.; Alt, F.W. Activated expression of the N-myc gene in human neuroblastomas and related tumors. Science 1984, 226, 1335–1337. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.; Varmus, H.E.; Bishop, J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Nesbit, C.E.; Tersak, J.M.; Prochownik, E.V. MYC oncogenes and human neoplastic disease. Oncogene 1999, 18, 3004–3016. [Google Scholar] [CrossRef]
- Miranda Peralta, E.I.; Valles Ayoub, Y.; Hernandez Mendoza, L.; Rangel Ramirez, L.M.; Castrejon Rojas, A.; Collazo-Jaloma, J.; Gutierrez Romero, M.; Gonzalez Constance, R.; Gariglio Vidal, P. MYC protein and proteins antigenically related with MYC in acute lymphoblastic leukemia. Rev. Investig. Clin. 1991, 43, 139–145. [Google Scholar]
- Burmeister, T.; Schwartz, S.; Horst, H.A.; Rieder, H.; Gokbuget, N.; Hoelzer, D.; Thiel, E. Molecular heterogeneity of sporadic adult Burkitt-type leukemia/lymphoma as revealed by PCR and cytogenetics: Correlation with morphology, immunology and clinical features. Leukemia 2005, 19, 1391–1398. [Google Scholar] [CrossRef]
- Herms, J.W.; von Loewenich, F.D.; Behnke, J.; Markakis, E.; Kretzschmar, H.A. c-myc oncogene family expression in glioblastoma and survival. Surg. Neurol. 1999, 51, 536–542. [Google Scholar] [CrossRef]
- Sardi, I.; Dal Canto, M.; Bartoletti, R.; Guazzelli, R.; Travaglini, F.; Montali, E. Molecular genetic alterations of c-myc oncogene in superficial and locally advanced bladder cancer. Eur. Urol. 1998, 33, 424–430. [Google Scholar] [CrossRef]
- Mizukami, Y.; Nonomura, A.; Takizawa, T.; Noguchi, M.; Michigishi, T.; Nakamura, S.; Ishizaki, T. N-myc protein expression in human breast carcinoma: Prognostic implications. Anticancer Res. 1995, 15, 2899–2905. [Google Scholar]
- Ioannidis, P.; Mahaira, L.; Papadopoulou, A.; Teixeira, M.R.; Heim, S.; Andersen, J.A.; Evangelou, E.; Dafni, U.; Pandis, N.; Trangas, T. 8q24 Copy number gains and expression of the c-myc mRNA stabilizing protein CRD-BP in primary breast carcinomas. Int. J. Cancer 2003, 104, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Wahab, N.A.; Yadav, M.; Kutty, M.K. Protein expression and molecular analysis of c-myc gene in primary breast carcinomas using immunohistochemistry and differential polymerase chain reaction. Int. J. Mol. Med. 2002, 9, 189–196. [Google Scholar] [CrossRef]
- Han, S.; Kim, H.Y.; Park, K.; Cho, H.J.; Lee, M.S.; Kim, H.J.; Kim, Y.D. c-Myc expression is related with cell proliferation and associated with poor clinical outcome in human gastric cancer. J. Korean Med. Sci. 1999, 14, 526–530. [Google Scholar] [CrossRef]
- Kawate, S.; Fukusato, T.; Ohwada, S.; Watanuki, A.; Morishita, Y. Amplification of c-myc in hepatocellular carcinoma: Correlation with clinicopathologic features, proliferative activity and p53 overexpression. Oncology 1999, 57, 157–163. [Google Scholar] [CrossRef]
- Treszl, A.; Adany, R.; Rakosy, Z.; Kardos, L.; Begany, A.; Gilde, K.; Balazs, M. Extra copies of c-myc are more pronounced in nodular melanomas than in superficial spreading melanomas as revealed by fluorescence in situ hybridisation. Cytom. B Clin. Cytom. 2004, 60, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Baker, V.V.; Borst, M.P.; Dixon, D.; Hatch, K.D.; Shingleton, H.M.; Miller, D. c-myc amplification in ovarian cancer. Gynecol. Oncol. 1990, 38, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Lui, W.O.; Tanenbaum, D.M.; Larsson, C. High level amplification of 1p32-33 and 2p22-24 in small cell lung carcinomas. Int. J. Oncol. 2001, 19, 451–457. [Google Scholar] [CrossRef]
- Gugger, M.; Burckhardt, E.; Kappeler, A.; Hirsiger, H.; Laissue, J.A.; Mazzucchelli, L. Quantitative expansion of structural genomic alterations in the spectrum of neuroendocrine lung carcinomas. J. Pathol. 2002, 196, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef]
- Malynn, B.A.; de Alboran, I.M.; O’Hagan, R.C.; Bronson, R.; Davidson, L.; DePinho, R.A.; Alt, F.W. N-myc can functionally replace c-myc in murine development, cellular growth, and differentiation. Genes Dev. 2000, 14, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, E.; Varnum-Finney, B.; Wilson, A.; Ferrero, I.; Blanco-Bose, W.E.; Ehninger, A.; Knoepfler, P.S.; Cheng, P.F.; MacDonald, H.R.; Eisenman, R.N.; et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell 2008, 3, 611–624. [Google Scholar] [CrossRef]
- Peramangalam, P.S.; Surapally, S.; Veltri, A.J.; Zheng, S.; Burns, R.; Zhu, N.; Rao, S.; Muller-Tidow, C.; Bushweller, J.H.; Pulikkan, J.A. N-MYC regulates cell survival via eIF4G1 in inv(16) acute myeloid leukemia. Sci. Adv. 2024, 10, eadh8493. [Google Scholar] [CrossRef]
- King, B.; Boccalatte, F.; Moran-Crusio, K.; Wolf, E.; Wang, J.; Kayembe, C.; Lazaris, C.; Yu, X.; Aranda-Orgilles, B.; Lasorella, A.; et al. The ubiquitin ligase Huwe1 regulates the maintenance and lymphoid commitment of hematopoietic stem cells. Nat. Immunol. 2016, 17, 1312–1321. [Google Scholar] [CrossRef]
- Kerosuo, L.; Neppala, P.; Hsin, J.; Mohlin, S.; Vieceli, F.M.; Torok, Z.; Laine, A.; Westermarck, J.; Bronner, M.E. Enhanced expression of MycN/CIP2A drives neural crest toward a neural stem cell-like fate: Implications for priming of neuroblastoma. Proc. Natl. Acad. Sci. USA 2018, 115, E7351–E7360. [Google Scholar] [CrossRef]
- Zhang, J.T.; Weng, Z.H.; Tsang, K.S.; Tsang, L.L.; Chan, H.C.; Jiang, X.H. MycN Is Critical for the Maintenance of Human Embryonic Stem Cell-Derived Neural Crest Stem Cells. PLoS ONE 2016, 11, e0148062. [Google Scholar]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef] [PubMed]
- Hatton, B.A.; Knoepfler, P.S.; Kenney, A.M.; Rowitch, D.H.; de Alboran, I.M.; Olson, J.M.; Eisenman, R.N. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006, 66, 8655–8661. [Google Scholar] [CrossRef]
- Wey, A.; Knoepfler, P.S. c-myc and N-myc promote active stem cell metabolism and cycling as architects of the developing brain. Oncotarget 2010, 1, 120–130. [Google Scholar] [CrossRef]
- Wilson, A.; Murphy, M.J.; Oskarsson, T.; Kaloulis, K.; Bettess, M.D.; Oser, G.M.; Pasche, A.C.; Knabenhans, C.; Macdonald, H.R.; Trumpp, A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004, 18, 2747–2763. [Google Scholar] [CrossRef] [PubMed]
- Satoh, Y.; Matsumura, I.; Tanaka, H.; Ezoe, S.; Sugahara, H.; Mizuki, M.; Shibayama, H.; Ishiko, E.; Ishiko, J.; Nakajima, K.; et al. Roles for c-Myc in self-renewal of hematopoietic stem cells. J. Biol. Chem. 2004, 279, 24986–24993. [Google Scholar] [CrossRef] [PubMed]
- Deming, S.L.; Nass, S.J.; Dickson, R.B.; Trock, B.J. C-myc amplification in breast cancer: A meta-analysis of its occurrence and prognostic relevance. Br. J. Cancer 2000, 83, 1688–1695. [Google Scholar] [CrossRef]
- Shajahan-Haq, A.N.; Cook, K.L.; Schwartz-Roberts, J.L.; Eltayeb, A.E.; Demas, D.M.; Warri, A.M.; Facey, C.O.; Hilakivi-Clarke, L.A.; Clarke, R. MYC regulates the unfolded protein response and glucose and glutamine uptake in endocrine resistant breast cancer. Mol. Cancer 2014, 13, 239. [Google Scholar] [CrossRef]
- McNeil, C.M.; Sergio, C.M.; Anderson, L.R.; Inman, C.K.; Eggleton, S.A.; Murphy, N.C.; Millar, E.K.; Crea, P.; Kench, J.G.; Alles, M.C.; et al. c-Myc overexpression and endocrine resistance in breast cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 147–155. [Google Scholar] [CrossRef]
- Fallah, Y.; Brundage, J.; Allegakoen, P.; Shajahan-Haq, A.N. MYC-Driven Pathways in Breast Cancer Subtypes. Biomolecules 2017, 7, 53. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, D.; Kusdra, L.; Huskey, N.E.; Chandriani, S.; Lenburg, M.E.; Gonzalez-Angulo, A.M.; Creasman, K.J.; Bazarov, A.V.; Smyth, J.W.; Davis, S.E.; et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp. Med. 2012, 209, 679–696. [Google Scholar] [CrossRef]
- Knudsen, E.S.; McClendon, A.K.; Franco, J.; Ertel, A.; Fortina, P.; Witkiewicz, A.K. RB loss contributes to aggressive tumor phenotypes in MYC-driven triple negative breast cancer. Cell Cycle 2015, 14, 109–122. [Google Scholar] [CrossRef]
- Zimmerli, D.; Brambillasca, C.S.; Talens, F.; Bhin, J.; Linstra, R.; Romanens, L.; Bhattacharya, A.; Joosten, S.E.P.; Da Silva, A.M.; Padrao, N.; et al. MYC promotes immune-suppression in triple-negative breast cancer via inhibition of interferon signaling. Nat. Commun. 2022, 13, 6579. [Google Scholar] [CrossRef]
- Tang, M.; O’Grady, S.; Crown, J.; Duffy, M.J. MYC as a therapeutic target for the treatment of triple-negative breast cancer: Preclinical investigations with the novel MYC inhibitor, MYCi975. Breast Cancer Res. Treat. 2022, 195, 105–115. [Google Scholar] [CrossRef]
- Schafer, J.M.; Lehmann, B.D.; Gonzalez-Ericsson, P.I.; Marshall, C.B.; Beeler, J.S.; Redman, L.N.; Jin, H.; Sanchez, V.; Stubbs, M.C.; Scherle, P.; et al. Targeting MYCN-expressing triple-negative breast cancer with BET and MEK inhibitors. Sci. Transl. Med. 2020, 12, eaaw8275. [Google Scholar] [CrossRef]
- Golonzhka, O.; Liang, X.; Messaddeq, N.; Bornert, J.M.; Campbell, A.L.; Metzger, D.; Chambon, P.; Ganguli-Indra, G.; Leid, M.; Indra, A.K. Dual role of COUP-TF-interacting protein 2 in epidermal homeostasis and permeability barrier formation. J. Investig. Dermatol. 2009, 129, 1459–1470. [Google Scholar] [CrossRef]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Lin, M.; Meng, Y.; Bai, H.; Cai, S. An improved CUT&RUN method for regulation network reconstruction of low abundance transcription factor. Cell. Signal. 2022, 96, 110361. [Google Scholar] [CrossRef] [PubMed]
- Deome, K.B.; Faulkin, L.J., Jr.; Bern, H.A.; Blair, P.B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959, 19, 515–520. [Google Scholar]
- Faulkin, L.J., Jr.; Deome, K.B. Regulation of growth and spacing of gland elements in the mammary fat pad of the C3H mouse. J. Natl. Cancer Inst. 1960, 24, 953–969. [Google Scholar]
- Papadopoulos, D.; Solvie, D.; Baluapuri, A.; Endres, T.; Ha, S.A.; Herold, S.; Kalb, J.; Giansanti, C.; Schulein-Volk, C.; Ade, C.P.; et al. MYCN recruits the nuclear exosome complex to RNA polymerase II to prevent transcription-replication conflicts. Mol. Cell 2022, 82, 159–176.e12. [Google Scholar] [CrossRef]
- Papadopoulos, D.; Ha, S.A.; Fleischhauer, D.; Uhl, L.; Russell, T.J.; Mikicic, I.; Schneider, K.; Brem, A.; Valanju, O.R.; Cossa, G.; et al. The MYCN oncoprotein is an RNA-binding accessory factor of the nuclear exosome targeting complex. Mol. Cell 2024, 84, 2070–2086.e20. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, S.; Liu, Y.; Gu, J.; Gu, S.; Xu, Z.; Zhang, R.; Wang, Z.; Ma, H.; Chen, Y.; et al. Overexpression of MYCN promotes proliferation of non-small cell lung cancer. Tumour Biol. 2016, 37, 12855–12866. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Wan, W. MYCN-induced E2F5 promotes neuroblastoma cell proliferation through regulating cell cycle progression. Biochem. Biophys. Res. Commun. 2019, 511, 35–40. [Google Scholar] [CrossRef]
- Huang, R.; Cheung, N.K.; Vider, J.; Cheung, I.Y.; Gerald, W.L.; Tickoo, S.K.; Holland, E.C.; Blasberg, R.G. MYCN and MYC regulate tumor proliferation and tumorigenesis directly through BMI1 in human neuroblastomas. FASEB J. 2011, 25, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.H.; Jin, D.X.; Sokol, E.S.; Cabrera, J.R.; Superville, D.A.; Gorelov, R.A.; Kuperwasser, C.; Gupta, P.B. BCL11B Drives Human Mammary Stem Cell Self-Renewal In Vitro by Inhibiting Basal Differentiation. Stem Cell Rep. 2018, 10, 1131–1145. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Wei, Y.; Hwang, J.; Hang, X.; Andres Blanco, M.; Choudhury, A.; Tiede, B.; Romano, R.A.; DeCoste, C.; Mercatali, L.; et al. ΔNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol. 2014, 16, 1004–1015. [Google Scholar] [CrossRef]
- Cismasiu, V.B.; Adamo, K.; Gecewicz, J.; Duque, J.; Lin, Q.; Avram, D. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene 2005, 24, 6753–6764. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Guo, R.; Yang, L.; Liu, G.; Zhang, Z.; Xu, Z.; Tian, Y.; Yang, Z.; Li, X.; et al. Transcription factors Bcl11a and Bcl11b are required for the production and differentiation of cortical projection neurons. Cereb. Cortex 2022, 32, 3611–3632. [Google Scholar] [CrossRef]
- Li, H.L.; Dong, L.L.; Jin, M.J.; Li, Q.Y.; Wang, X.; Jia, M.Q.; Song, J.; Zhang, S.Y.; Yuan, S. A Review of the Regulatory Mechanisms of N-Myc on Cell Cycle. Molecules 2023, 28, 1141. [Google Scholar] [CrossRef]
- Ma, M.; Wu, W.; Li, Q.; Li, J.; Sheng, Z.; Shi, J.; Zhang, M.; Yang, H.; Wang, Z.; Sun, R.; et al. N-myc is a key switch regulating the proliferation cycle of postnatal cerebellar granule cell progenitors. Sci. Rep. 2015, 5, 12740. [Google Scholar] [CrossRef]
- Cowling, V.H.; Cole, M.D. An N-Myc truncation analogous to c-Myc-S induces cell proliferation independently of transactivation but dependent on Myc homology box II. Oncogene 2008, 27, 1327–1332. [Google Scholar] [CrossRef]
- Dominguez-Frutos, E.; Lopez-Hernandez, I.; Vendrell, V.; Neves, J.; Gallozzi, M.; Gutsche, K.; Quintana, L.; Sharpe, J.; Knoepfler, P.S.; Eisenman, R.N.; et al. N-myc controls proliferation, morphogenesis, and patterning of the inner ear. J. Neurosci. 2011, 31, 7178–7189. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, G.R.; Matos-Rodrigues, G.E.; Zhao, Y.; Gomes, A.L.; Anand, D.; Predes, D.; de Lima, S.; Abreu, J.G.; Zheng, D.; Lachke, S.A.; et al. N-myc regulates growth and fiber cell differentiation in lens development. Dev. Biol. 2017, 429, 105–117. [Google Scholar] [CrossRef]
- Streeter, S.A.; Williams, A.G.; Evans, J.R.; Wang, J.; Guarnaccia, A.D.; Florian, A.C.; Al-Tobasei, R.; Liu, Q.; Tansey, W.P.; Weissmiller, A.M. Mitotic gene regulation by the N-MYC-WDR5-PDPK1 nexus. BMC Genom. 2024, 25, 360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Gires, O.; Zhu, L.; Liu, J.; Li, J.; Yang, H.; Ju, G.; Huang, J.; Ge, W.; Chen, Y.; et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 2019, 10, 2863. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Lam, J.S.W.; Fan, H.; Fu, N.Y. Molecular Regulation and Oncogenic Functions of TSPAN8. Cells 2024, 13, 193. [Google Scholar] [CrossRef]
- Lu, X.; An, L.; Fan, G.; Zang, L.; Huang, W.; Li, J.; Liu, J.; Ge, W.; Huang, Y.; Xu, J.; et al. EGFR signaling promotes nuclear translocation of plasma membrane protein TSPAN8 to enhance tumor progression via STAT3-mediated transcription. Cell Res. 2022, 32, 359–374. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Wang, P. Genetic expression in cancer research: Challenges and complexity. Gene Rep. 2024, 37, 102042. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Karsidag, M.; Tu, T.; Wang, P. Technical and Biological Biases in Bulk Transcriptomic Data Mining for Cancer Research. J. Cancer 2025, 16, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, A.; Rasteh, A.M.; Wang, P.; Weng, J. Identification of the novel exhausted T cell CD8+ markers in breast cancer. Sci. Rep. 2024, 14, 19142. [Google Scholar] [CrossRef]
- Yang, W.J.; Sun, Y.F.; Jin, A.L.; Lv, L.H.; Zhu, J.; Wang, B.L.; Zhou, Y.; Zhang, C.Y.; Wang, H.; Hu, B.; et al. BCL11B suppresses tumor progression and stem cell traits in hepatocellular carcinoma by restoring p53 signaling activity. Cell Death Dis. 2020, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Kominami, R. Role of the transcription factor Bcl11b in development and lymphomagenesis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 72–87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Wang, C.; Bai, H.; Zhang, Y.; Lin, M.; Liu, X.; Hu, T.; Meng, Y. Mycn Is Essential for Pubertal Mammary Gland Development and Promotes the Activation of Bcl11b-Maintained Quiescent Stem Cells. Cells 2025, 14, 1239. https://doi.org/10.3390/cells14161239
Lin Z, Wang C, Bai H, Zhang Y, Lin M, Liu X, Hu T, Meng Y. Mycn Is Essential for Pubertal Mammary Gland Development and Promotes the Activation of Bcl11b-Maintained Quiescent Stem Cells. Cells. 2025; 14(16):1239. https://doi.org/10.3390/cells14161239
Chicago/Turabian StyleLin, Zuobao, Chunhui Wang, Huiru Bai, Yue Zhang, Meizhen Lin, Xiaoqin Liu, Tian’en Hu, and Yuan Meng. 2025. "Mycn Is Essential for Pubertal Mammary Gland Development and Promotes the Activation of Bcl11b-Maintained Quiescent Stem Cells" Cells 14, no. 16: 1239. https://doi.org/10.3390/cells14161239
APA StyleLin, Z., Wang, C., Bai, H., Zhang, Y., Lin, M., Liu, X., Hu, T., & Meng, Y. (2025). Mycn Is Essential for Pubertal Mammary Gland Development and Promotes the Activation of Bcl11b-Maintained Quiescent Stem Cells. Cells, 14(16), 1239. https://doi.org/10.3390/cells14161239






