Multi-Omic Characterization of Epithelial–Mesenchymal Transition: Lipidomic and Metabolomic Profiles as Key Markers of TGF-β-Induced Transition in Huh7 Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and EMT Induction
2.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4. Immunoblotting
2.5. Fluorescence Microscopy
2.6. Seahorse Glycolytic Activity Analysis
2.7. Lipid and Polar Metabolite Analyses by Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
2.8. Statistical Analysis
3. Results
3.1. EMT In Vitro Model Induction and Characterization
3.2. Lipidomic Profile of EMT Model
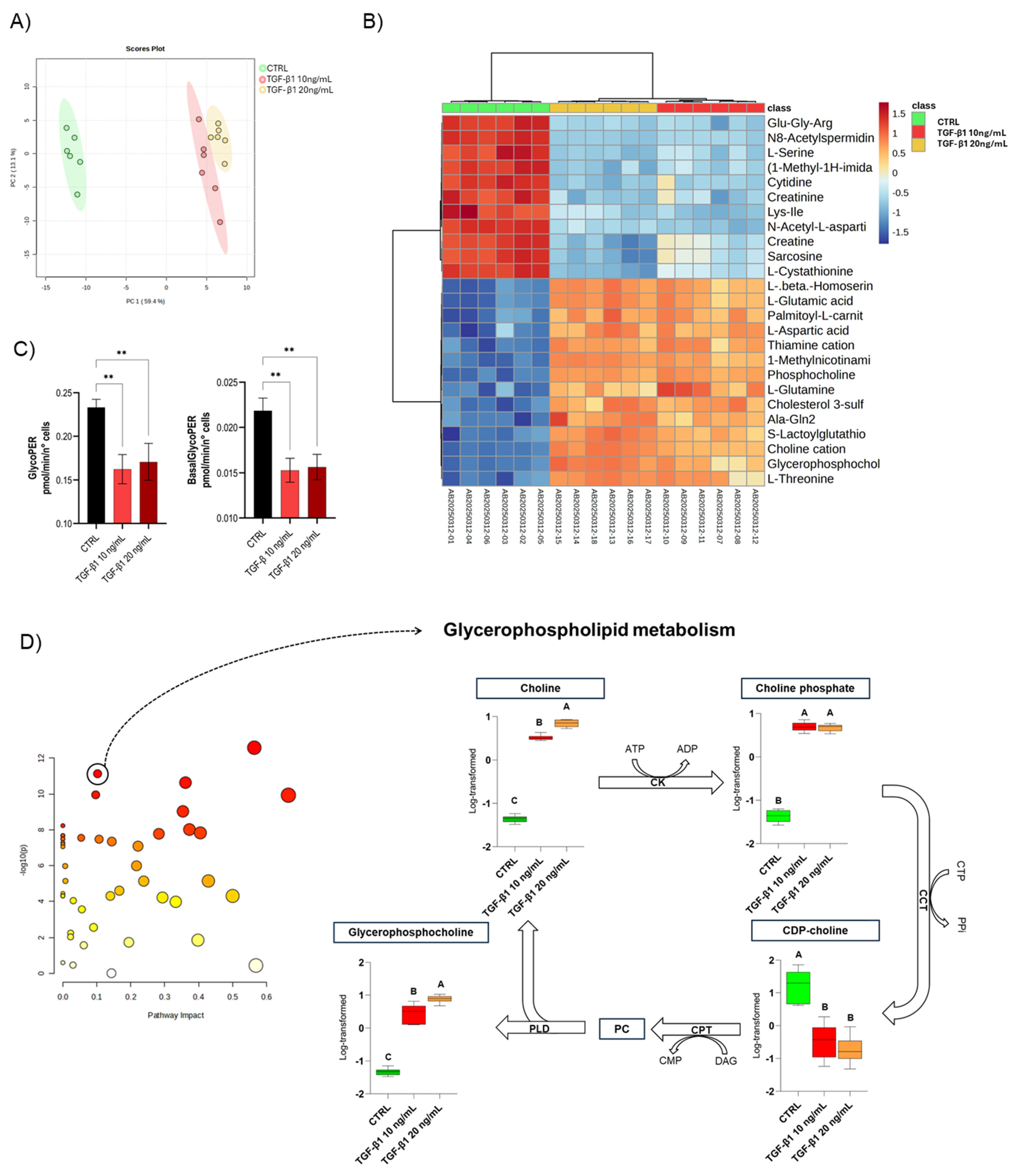
3.3. Metabolic Profile of the EMT Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-DG2 | 2-Deoxy-D-glucose |
| ADP | Adenosine Diphosphate |
| ATP | Adenosine Triphosphate |
| BasalGlycoPER | Basal Glycolytic Proton Efflux Rate |
| CCT | CTP:Phosphocholine Cytidylyltransferase |
| CDP-choline | Cytidine Diphosphate-Choline |
| CDP-DAG | Cytidine Diphosphate-Diacylglycerol |
| CDS2 | CDP-Diacylglycerol Synthase 2 |
| CE | Cholesteryl Ester |
| Cer | Ceramide |
| CK | Choline Kinase |
| CL | Cardiolipin |
| CMP | Cytidine Monophosphate |
| CPT | CDP-Choline:1,2-Diacylglycerol Cholinephosphotransferase |
| CTCF | Corrected Total Cell Fluorescence |
| CTP | Cytidine Triphosphate |
| DAG or DG | Diacylglycerol |
| DGAT1 | Diacylglycerol O-Acyltransferase 1 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| DTT | Dithiothreitol |
| ECL | Enhanced Chemiluminescence |
| EMT | Epithelial-to-Mesenchymal Transition |
| FBS | Fetal Bovine Serum |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GlycoPER | Glycolytic Proton Efflux Rate |
| GPE | Glycerophosphoethanolamine |
| GPS | Glycerophosphoserine |
| HCC | Hepatocellular Carcinoma |
| HexCer | Hexosylceramide |
| Huh7 | Human Hepatoma Cell Line HuH-7 |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| LPI | Lysophosphatidylinositol |
| MAPK | Mitogen-Activated Protein Kinase |
| MUFA | Monounsaturated Fatty Acids |
| PA | Phosphatidic Acid |
| PBS | Phosphate Buffered Saline |
| PC | Phosphatidylcholine |
| PCA | Principal Component Analysis |
| PE | Phosphatidylethanolamine |
| p-EMT | Partial Epithelial-to-Mesenchymal Transition |
| PEMT | Phosphatidylethanolamine N-Methyltransferase |
| PE-O | Ether-Linked Phosphatidylethanolamine |
| PE-P | Plasmalogen-type Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PGS1 | Phosphatidylglycerophosphate Synthase 1 |
| PI | Phosphatidylinositol |
| PI3K/AKT | Phosphoinositide 3-Kinase/Protein Kinase B Pathway |
| PLD | Phospholipase D |
| PPi | Inorganic Pyrophosphate |
| PS | Phosphatidylserine |
| PSD | Phosphatidylserine Decarboxylase |
| PTDSS1/2 | Phosphatidylserine Synthase 1 and 2 |
| PUFA | Polyunsaturated Fatty Acids |
| qRT-PCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| ROIs | Region of Interest |
| SCD1 | Stearoyl-CoA Desaturase 1 |
| SDS | Sodium Dodecyl Sulfate |
| Slug | Transcription Factor Slug (SNAI2) |
| SM | Sphingomyelin |
| SMADs | Signal Transducers and Transcriptional Modulators Activated by TGF-β Receptors |
| Snail | Transcription Factor Snail (SNAI1) |
| TAG or TG | Triacylglycerol |
| TGF-β | Transforming Growth Factor Beta |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| Twist | Transcription Factor Twist |
| VIP | Variable Importance in Projection (PLS/OPLS models) |
| Zeb1 | Zinc Finger E-box-Binding Homeobox 1 |
References
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Perelli, L.; Zhang, L.; Mangiameli, S.; Giannese, F.; Mahadevan, K.K.; Peng, F.; Citron, F.; Khan, H.; Le, C.; Gurreri, E.; et al. Evolutionary Fingerprints of Epithelial-to-Mesenchymal Transition. Nature 2025, 640, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. Epithelial–Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Wang, W.; Pu, N.; Liu, L. Epithelial-Mesenchymal Transition Orchestrates Tumor Microenvironment: Current Perceptions and Challenges. J. Transl. Med. 2025, 23, 386. [Google Scholar] [CrossRef]
- Zhai, X.; Zhu, H.; Wang, W.; Zhang, S.; Zhang, Y.; Mao, G. Abnormal Expression of EMT-Related Proteins, S100A4, Vimentin and E-Cadherin, Is Correlated with Clinicopathological Features and Prognosis in HCC. Med. Oncol. 2014, 31, 970. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Loh, C.-Y.; Chai, J.; Tang, T.; Wong, W.; Sethi, G.; Shanmugam, M.; Chong, P.; Looi, C. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, M.; Zheng, Y.; Wu, J.; Yu, B.; Zhang, H.; Kong, W.; Wu, H.; Yu, X. Epigenetic Suppression of E-Cadherin Expression by Snail2 during the Metastasis of Colorectal Cancer. Clin. Epigenetics 2018, 10, 154. [Google Scholar] [CrossRef]
- Ichikawa, M.K.; Endo, K.; Itoh, Y.; Osada, A.H.; Kimura, Y.; Ueki, K.; Yoshizawa, K.; Miyazawa, K.; Saitoh, M. Ets Family Proteins Regulate the EMT Transcription Factors Snail and ZEB in Cancer Cells. FEBS Open Bio 2022, 12, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Pawlicka, M.; Gumbarewicz, E.; Błaszczak, E.; Stepulak, A. Transcription Factors and Markers Related to Epithelial–Mesenchymal Transition and Their Role in Resistance to Therapies in Head and Neck Cancers. Cancers 2024, 16, 1354. [Google Scholar] [CrossRef]
- Garinet, S.; Didelot, A.; Denize, T.; Perrier, A.; Beinse, G.; Leclere, J.-B.; Oudart, J.-B.; Gibault, L.; Badoual, C.; Le Pimpec-Barthes, F.; et al. Clinical Assessment of the miR-34, miR-200, ZEB1 and SNAIL EMT Regulation Hub Underlines the Differential Prognostic Value of EMT miRs to Drive Mesenchymal Transition and Prognosis in Resected NSCLC. Br. J. Cancer 2021, 125, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, E.; Ortega-Hernández, V.; Zavala, V.A.; Gamboa, J.; Fernández, W.; Carvallo, P. Suppression of Breast Cancer Metastatic Behavior by microRNAs Targeting EMT Transcription Factors. A Relevant Participation of miR-196a-5p and miR-22-3p in ZEB1 Expression. Breast Cancer Res. Treat. 2025, 212, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Silvestris, N.; Mohammadi, A.; Khaze, V.; Baghbani, E.; Mokhtarzadeh, A.; Shanehbandi, D.; Derakhshani, A.; Duijf, P.H.G.; Baradaran, B. miR-34a and miR-200c Have an Additive Tumor-Suppressive Effect on Breast Cancer Cells and Patient Prognosis. Genes 2021, 12, 267. [Google Scholar] [CrossRef]
- Li, D.; Xia, L.; Huang, P.; Wang, Z.; Guo, Q.; Huang, C.; Leng, W.; Qin, S. Heterogeneity and Plasticity of Epithelial–Mesenchymal Transition (EMT) in Cancer Metastasis: Focusing on Partial EMT and Regulatory Mechanisms. Cell Prolif. 2023, 56, e13423. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Lau, H.S.-H.; Carter, L.M.; Lee, E.H.C.; Lam, H.Y.; Okina, E.; Tan, D.J.J.; Tan, W.; Ang, H.L.; Carbone, D.; et al. Harnessing the Tumor Microenvironment: Targeted Cancer Therapies through Modulation of Epithelial-Mesenchymal Transition. J. Hematol. Oncol. 2025, 18, 6. [Google Scholar] [CrossRef]
- Din, Z.U.; Cui, B.; Wang, C.; Zhang, X.; Mehmood, A.; Peng, F.; Liu, Q. Crosstalk between Lipid Metabolism and EMT: Emerging Mechanisms and Cancer Therapy. Mol. Cell Biochem. 2025, 480, 103–118. [Google Scholar] [CrossRef]
- Soukupova, J.; Malfettone, A.; Bertran, E.; Hernández-Alvarez, M.I.; Peñuelas-Haro, I.; Dituri, F.; Giannelli, G.; Zorzano, A.; Fabregat, I. Epithelial–Mesenchymal Transition (EMT) Induced by TGF-β in Hepatocellular Carcinoma Cells Reprograms Lipid Metabolism. IJMS 2021, 22, 5543. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic Reprogramming and Therapeutic Resistance in Primary and Metastatic Breast Cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef]
- Raeisi, M.; Hassanbeigi, L.; Khalili, F.; Kharrati-Shishavan, H.; Yousefi, M.; Mehdizadeh, A. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for Cancer: A Focus on Hepatocellular Carcinoma. Mol. Biol. Rep. 2022, 49, 8871–8882. [Google Scholar] [CrossRef]
- Man, S.; Cui, Y.; Shi, D.; Lv, P.; Ma, L.; Gao, W. Formosanin C Inhibits Pulmonary Metastasis by Targeting Stearyl CoA Desaturase-1. Phytomedicine 2024, 129, 155689. [Google Scholar] [CrossRef]
- Wang, L.; Ye, G.; Wang, Y.; Wang, C. Stearoyl-CoA Desaturase 1 Regulates Malignant Progression of Cervical Cancer Cells. Bioengineered 2022, 13, 12941–12954. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Kong, W.; Shen, X.; Han, C.; Zhao, Z.; Chen, S.; Zhou, C.; Bae-Jump, V. The Role of DGAT1 and DGAT2 in Regulating Tumor Cell Growth and Their Potential Clinical Implications. J. Transl. Med. 2024, 22, 290. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid Rafts as Signaling Hubs in Cancer Cell Survival/Death and Invasion: Implications in Tumor Progression and Therapy. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Zhong, X.; Zhang, Z.; Zhao, Y. Endothelial-to-Mesenchymal Transition in the Tumor Microenvironment: Roles of Transforming Growth Factor-β and Matrix Metalloproteins. Heliyon 2024, 10, e40118. [Google Scholar] [CrossRef]
- Stuelten, C.H.; Zhang, Y.E. Transforming Growth Factor-β: An Agent of Change in the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 764727. [Google Scholar] [CrossRef]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, W.-L.; Zhang, R.-N.; He, X.-S.; Wang, J.-R.; Liu, Y.-X.; Wang, Y.; Yang, X.-M.; Zhang, Y.-J.; Gan, W.-J. The Role of TGF-β Signaling Pathways in Cancer and Its Potential as a Therapeutic Target. Evid.-Based Complement. Altern. Med. 2021, 2021, 6675208. [Google Scholar] [CrossRef] [PubMed]
- Runa, F.; Ortiz-Soto, G.; De Barros, N.R.; Kelber, J.A. Targeting SMAD-Dependent Signaling: Considerations in Epithelial and Mesenchymal Solid Tumors. Pharmaceuticals 2024, 17, 326. [Google Scholar] [CrossRef] [PubMed]
- Melchionna, R.; Trono, P.; Tocci, A.; Nisticò, P. Actin Cytoskeleton and Regulation of TGFβ Signaling: Exploring Their Links. Biomolecules 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Bridle, K.R.; Crawford, D.H.G.; Jayachandran, A. Immune Checkpoint Molecules Are Regulated by Transforming Growth Factor (TGF)-Β1-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma. Int. J. Med. Sci. 2021, 18, 2466–2479. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, S.; Zhan, Y.; Ke, J.; Wang, K.; Liang, Q.; Hou, Y.; Zhu, P.; Ao, W.; Wei, X.; et al. Dioscin Inhibits the Invasion and Migration of Hepatocellular Carcinoma HepG2 Cells by Reversing TGF-Β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 2222. [Google Scholar] [CrossRef]
- Liu, Q.-Q.; Huo, H.-Y.; Ao, S.; Liu, T.; Yang, L.; Fei, Z.-Y.; Zhang, Z.-Q.; Ding, L.; Cui, Q.-H.; Lin, J.; et al. TGF-β1-induced Epithelial-mesenchymal Transition Increases Fatty Acid Oxidation and OXPHOS Activity via the p-AMPK Pathway in Breast Cancer Cells. Oncol. Rep. 2020, 44, 1206–1215. [Google Scholar] [CrossRef]
- Frey, P.; Devisme, A.; Rose, K.; Schrempp, M.; Freihen, V.; Andrieux, G.; Boerries, M.; Hecht, A. SMAD4 Mutations Do Not Preclude Epithelial–Mesenchymal Transition in Colorectal Cancer. Oncogene 2022, 41, 824–837. [Google Scholar] [CrossRef]
- Al-Azayzih, A.; Gao, F.; Somanath, P.R. P21 Activated Kinase-1 Mediates Transforming Growth Factor Β1-Induced Prostate Cancer Cell Epithelial to Mesenchymal Transition. Biochim. Biophys. Acta 2015, 1853, 1229–1239. [Google Scholar] [CrossRef]
- van Zijl, F.; Mair, M.; Csiszar, A.; Schneller, D.; Zulehner, G.; Huber, H.; Eferl, R.; Beug, H.; Dolznig, H.; Mikulits, W. Hepatic Tumor–Stroma Crosstalk Guides Epithelial to Mesenchymal Transition at the Tumor Edge. Oncogene 2009, 28, 4022–4033. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef]
- Alqurashi, Y.E.; Al-Hetty, H.R.A.K.; Ramaiah, P.; Fazaa, A.H.; Jalil, A.T.; Alsaikhan, F.; Gupta, J.; Ramírez-Coronel, A.A.; Tayyib, N.A.; Peng, H. Harnessing Function of EMT in Hepatocellular Carcinoma: From Biological View to Nanotechnological Standpoint. Environ. Res. 2023, 227, 115683. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, Y.; Wu, J.; Lin, S.; Chen, Y.; Zheng, J. Identification and Clinical Validation of EMT-Associated Prognostic Features Based on Hepatocellular Carcinoma. Cancer Cell Int. 2021, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, Z.C.; Battello, N.; Rothley, M.; Piorońska, W.; Sitek, B.; Ebert, M.P.; Hofmann, U.; Sleeman, J.; Wölfl, S.; Meyer, C.; et al. Liver Cancer Cell Lines Distinctly Mimic the Metabolic Gene Expression Pattern of the Corresponding Human Tumours. J. Exp. Clin. Cancer Res. 2018, 37, 211. [Google Scholar] [CrossRef]
- Calzoni, E.; Bertoldi, A.; Cesaretti, A.; Alabed, H.B.R.; Cerrotti, G.; Pellegrino, R.M.; Buratta, S.; Urbanelli, L.; Emiliani, C. Aloe Extracellular Vesicles as Carriers of Photoinducible Metabolites Exhibiting Cellular Phototoxicity. Cells 2024, 13, 1845. [Google Scholar] [CrossRef] [PubMed]
- Urbanelli, L.; Delo, F.; Cerrotti, G.; Albertini, E.; Lucci, J.; Buratta, S.; Calzoni, E.; Giovagnoli, S.; Lugini, L.; Federici, C.; et al. Cross-Kingdom Communication via Plant-Derived Extracellular Vesicle Nucleic Acids in Genetically Engineered Nicotiana Tabacum. Genes 2025, 16, 356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, Y.; Liu, J.; Wang, Y.; Yi, T.; Huang, B.; He, S.; Zheng, B.; Jiang, Y. Norepinephrine Induced Epithelial–Mesenchymal Transition in HT-29 and A549 Cells in Vitro. J. Cancer Res. Clin. Oncol. 2016, 142, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Kawami, M.; Harabayashi, R.; Miyamoto, M.; Harada, R.; Yumoto, R.; Takano, M. Methotrexate-Induced Epithelial-Mesenchymal Transition in the Alveolar Epithelial Cell Line A549. Lung 2016, 194, 923–930. [Google Scholar] [CrossRef]
- El Amrani, M.; Corfiotti, F.; Corvaisier, M.; Vasseur, R.; Fulbert, M.; Skrzypczyk, C.; Deshorgues, A.-C.; Gnemmi, V.; Tulasne, D.; Lahdaoui, F.; et al. Gemcitabine-Induced Epithelial-Mesenchymal Transition-like Changes Sustain Chemoresistance of Pancreatic Cancer Cells of Mesenchymal-like Phenotype. Mol. Carcinog. 2019, 58, 1985–1997. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wu, G. SDHB Deficiency Promotes TGFβ-Mediated Invasion and Metastasis of Colorectal Cancer through Transcriptional Repression Complex SNAIL1-SMAD3/4. Transl. Oncol. 2016, 9, 512–520. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Valicenti, M.L.; Morena, F.; Misra, R.; Carlotti, B.; Martino, S. Phenothiazine-Based Nanoaggregates: Dual Role in Bioimaging and Stem Cell-Driven Photodynamic Therapy. Nanomaterials 2025, 15, 894. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Di Veroli, A.; Valeri, A.; Goracci, L.; Cruciani, G. LC/MS Lipid Profiling from Human Serum: A New Method for Global Lipid Extraction. Anal. Bioanal. Chem. 2014, 406, 7937–7948. [Google Scholar] [CrossRef]
- Cajka, T.; Hricko, J.; Rudl Kulhava, L.; Paucova, M.; Novakova, M.; Kuda, O. Optimization of Mobile Phase Modifiers for Fast LC-MS-Based Untargeted Metabolomics and Lipidomics. IJMS 2023, 24, 1987. [Google Scholar] [CrossRef]
- Alabed, H.B.R.; Pellegrino, R.M.; Buratta, S.; Lema Fernandez, A.G.; La Starza, R.; Urbanelli, L.; Mecucci, C.; Emiliani, C.; Gorello, P. Metabolic Profiling as an Approach to Differentiate T-Cell Acute Lymphoblastic Leukemia Cell Lines Belonging to the Same Genetic Subgroup. Int. J. Mol. Sci. 2024, 25, 3921. [Google Scholar] [CrossRef]
- Alabed, H.B.R.; Mancini, D.F.; Buratta, S.; Calzoni, E.; Giacomo, D.D.; Emiliani, C.; Martino, S.; Urbanelli, L.; Pellegrino, R.M. LipidOne 2.0: A Web Tool for Discovering Biological Meanings Hidden in Lipidomic Data. Curr. Protoc. 2024, 4, e70009. [Google Scholar] [CrossRef]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. miR-9, a MYC/MYCN-Activated microRNA, Regulates E-Cadherin and Cancer Metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef]
- Guo, F.; Parker Kerrigan, B.C.; Yang, D.; Hu, L.; Shmulevich, I.; Sood, A.K.; Xue, F.; Zhang, W. Post-Transcriptional Regulatory Network of Epithelial-to-Mesenchymal and Mesenchymal-to-Epithelial Transitions. J. Hematol. Oncol. 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The Role of Lipids in Cancer Progression and Metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef]
- Li, J.; Gu, D.; Lee, S.S.-Y.; Song, B.; Bandyopadhyay, S.; Chen, S.; Konieczny, S.F.; Ratliff, T.L.; Liu, X.; Xie, J.; et al. Abrogating Cholesterol Esterification Suppresses Growth and Metastasis of Pancreatic Cancer. Oncogene 2016, 35, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, I.; Gianfanti, F.; Desbats, M.A.; Orso, G.; Berretta, M.; Prayer-Galetti, T.; Ragazzi, E.; Cocetta, V. Cholesterol Metabolic Reprogramming in Cancer and Its Pharmacological Modulation as Therapeutic Strategy. Front. Oncol. 2021, 116, 82911. [Google Scholar] [CrossRef]
- Edmond, V.; Dufour, F.; Poiroux, G.; Shoji, K.; Malleter, M.; Fouqué, A.; Tauzin, S.; Rimokh, R.; Sergent, O.; Penna, A.; et al. Downregulation of Ceramide Synthase-6 during Epithelial-to-Mesenchymal Transition Reduces Plasma Membrane Fluidity and Cancer Cell Motility. Oncogene 2015, 34, 996–1005. [Google Scholar] [CrossRef]
- Li, Z.; Guan, M.; Lin, Y.; Cui, X.; Zhang, Y.; Zhao, Z.; Zhu, J. Aberrant Lipid Metabolism in Hepatocellular Carcinoma Revealed by Liver Lipidomics. Int. J. Mol. Sci. 2017, 18, 2550. [Google Scholar] [CrossRef] [PubMed]
- Grammatikos, G.; Schoell, N.; Ferreirós, N.; Bon, D.; Herrmann, E.; Farnik, H.; Köberle, V.; Piiper, A.; Zeuzem, S.; Kronenberger, B.; et al. Serum Sphingolipidomic Analyses Reveal an Upregulation of C16-Ceramide and Sphingosine-1-Phosphate in Hepatocellular Carcinoma. Oncotarget 2016, 7, 18095–18105. [Google Scholar] [CrossRef]
- Piacentino, M.L.; Hutchins, E.J.; Andrews, C.J.; Bronner, M.E. Temporal Changes in Plasma Membrane Lipid Content Induce Endocytosis to Regulate Developmental Epithelial-to-Mesenchymal Transition. Proc. Natl. Acad. Sci. USA 2022, 119, e2212879119. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Hishikawa, D.; Harayama, T.; Eto, M.; Shimizu, T. Generation of Membrane Diversity by Lysophospholipid Acyltransferases. J. Biochem. 2013, 154, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. IJMS 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. IJMS 2023, 24, 1353. [Google Scholar] [CrossRef]
- Morita, S.; Terada, T. Enzymatic Measurement of Phosphatidylglycerol and Cardiolipin in Cultured Cells and Mitochondria. Sci. Rep. 2015, 5, 11737. [Google Scholar] [CrossRef]
- Kay, J.G.; Koivusalo, M.; Ma, X.; Wohland, T.; Grinstein, S. Phosphatidylserine Dynamics in Cellular Membranes. Mol. Biol. Cell 2012, 23, 2198–2212. [Google Scholar] [CrossRef]
- Vance, J.E. Phosphatidylserine and Phosphatidylethanolamine in Mammalian Cells: Two Metabolically Related Aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- Ramírez de Molina, A.; Gallego-Ortega, D.; Sarmentero-Estrada, J.; Lagares, D.; Gómez del Pulgar, T.; Bandrés, E.; García-Foncillas, J.; Lacal, J.C. Choline Kinase as a Link Connecting Phospholipid Metabolism and Cell Cycle Regulation: Implications in Cancer Therapy. Int. J. Biochem. Cell Biol. 2008, 40, 1753–1763. [Google Scholar] [CrossRef]
- Yalcin, A.; Clem, B.; Makoni, S.; Clem, A.; Nelson, K.; Thornburg, J.; Siow, D.; Lane, A.N.; Brock, S.E.; Goswami, U.; et al. Selective Inhibition of Choline Kinase Simultaneously Attenuates MAPK and PI3K/AKT Signaling. Oncogene 2010, 29, 139–149. [Google Scholar] [CrossRef]
- Abalsamo, L.; Spadaro, F.; Bozzuto, G.; Paris, L.; Cecchetti, S.; Lugini, L.; Iorio, E.; Molinari, A.; Ramoni, C.; Podo, F. Inhibition of Phosphatidylcholine-Specific Phospholipase C Results in Loss of Mesenchymal Traits in Metastatic Breast Cancer Cells. Breast Cancer Res. 2012, 14, R50. [Google Scholar] [CrossRef]
- Chang, H.; Meng, H.; Liu, S.; Wang, Y.; Yang, X.; Lu, F.; Wang, H. Identification of Key Metabolic Changes during Liver Fibrosis Progression in Rats Using a Urine and Serum Metabolomics Approach. Sci. Rep. 2017, 7, 11433. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Horibata, Y.; Domae, M.; Nakamura, Y.; Yokoyama, T.; Arai, R.; Shiobara, T.; Takemasa, A.; Koike, R.; Uchida, N.; et al. Dysregulated Metabolic Pathways of Pulmonary Fibrosis and the Lipids Associated with the Effects of Nintedanib Therapy. Respir. Res. 2025, 26, 166. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. Transforming Growth Factor Beta Signaling in Hepatocytes Participates in Steatohepatitis through Regulation of Cell Death and Lipid Metabolism in Mice. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Mou, K.; Peng, L.; Zhou, H.; Lei, Y.; Wang, H.; Zhao, Z.; Wang, J.; Wu, J.; et al. SDPR Inhibits TGF-β Induced Cancer Metastasis Through Fatty Acid Oxidation Regulation in Gastric Cancer. Int. J. Biol. Sci. 2023, 19, 2999–3014. [Google Scholar] [CrossRef]
- Ueda, N. A Rheostat of Ceramide and Sphingosine-1-Phosphate as a Determinant of Oxidative Stress-Mediated Kidney Injury. Int. J. Mol. Sci. 2022, 23, 4010. [Google Scholar] [CrossRef]
- Pascual, G.; Majem, B.; Benitah, S.A. Targeting Lipid Metabolism in Cancer Metastasis. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189051. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Spiegel, S. Sphingolipid Metabolites in Inflammatory Disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Fuentes, J.M.; Morcillo, P. The Role of Cardiolipin in Mitochondrial Function and Neurodegenerative Diseases. Cells 2024, 13, 609. [Google Scholar] [CrossRef]
- Joshi, A.; Richard, T.H.; Gohil, V.M. Mitochondrial Phospholipid Metabolism in Health and Disease. J. Cell Sci. 2023, 136, jcs260857. [Google Scholar] [CrossRef]
- Colpman, P.; Dasgupta, A.; Archer, S.L. The Role of Mitochondrial Dynamics and Mitotic Fission in Regulating the Cell Cycle in Cancer and Pulmonary Arterial Hypertension: Implications for Dynamin-Related Protein 1 and Mitofusin2 in Hyperproliferative Diseases. Cells 2023, 12, 1897. [Google Scholar] [CrossRef] [PubMed]
- Paidi, C.; Nuthalapati, Y.; Samudrala, A.S.; Bhamidipati, P.; Mangam, C.; Welch, D.R.; Nagaraju, G.P.; Malla, R. ER-Mitochondria Tethering and Its Signaling: A Novel Therapeutic Target in Breast Cancer. Mol. Ther. Oncol. 2025, 33, 200995. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; Hatch, G.M. Regulation of Cardiolipin Biosynthesis by Fatty Acid Transport Protein-1 IN HEK 293 Cells. Biochim. Biophys. Acta 2009, 1788, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Condello, S.; Thomes-Pepin, J.; Ma, X.; Xia, Y.; Hurley, T.D.; Matei, D.; Cheng, J.-X. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell 2017, 20, 303–314.e5. [Google Scholar] [CrossRef]
- Schwab, A.; Rao, Z.; Zhang, J.; Gollowitzer, A.; Siebenkäs, K.; Bindel, N.; D’Avanzo, E.; van Roey, R.; Hajjaj, Y.; Özel, E.; et al. Zeb1 Mediates EMT/Plasticity-Associated Ferroptosis Sensitivity in Cancer Cells by Regulating Lipogenic Enzyme Expression and Phospholipid Composition. Nat. Cell Biol. 2024, 26, 1470–1481. [Google Scholar] [CrossRef]
- Ward, A.V.; Anderson, S.M.; Sartorius, C.A. Advances in Analyzing the Breast Cancer Lipidome and Its Relevance to Disease Progression and Treatment. J. Mammary Gland. Biol. Neoplasia 2021, 26, 399–417. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Ader, N.R.; Hoffmann, P.C.; Ganeva, I.; Borgeaud, A.C.; Wang, C.; Youle, R.J.; Kukulski, W. Molecular and Topological Reorganizations in Mitochondrial Architecture Interplay during Bax-Mediated Steps of Apoptosis. eLife 2019, 8, e40712. [Google Scholar] [CrossRef]
- Wen, T.; Thapa, N.; Cryns, V.L.; Anderson, R.A. Regulation of Phosphoinositide Signaling by Scaffolds at Cytoplasmic Membranes. Biomolecules 2023, 13, 1297. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Chandel, N.S. Targeting Mitochondria Metabolism for Cancer Therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef]
- Markov, N.; Sabirova, S.; Sharapova, G.; Gomzikova, M.; Brichkina, A.; Barlev, N.A.; Egger, M.; Rizvanov, A.; Simon, H.-U. Mitochondrial, Metabolic and Bioenergetic Adaptations Drive Plasticity of Colorectal Cancer Cells and Shape Their Chemosensitivity. Cell Death Dis. 2025, 16, 253. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zhang, T.; Zhou, Q.; Liu, J.; Liu, Y.; Kong, D.; Yu, W.; Liu, R.; Hai, C. TGF-Β1 Induces Epithelial-to-Mesenchymal Transition via Inhibiting Mitochondrial Functions in A549 Cells. Free. Radic. Res. 2018, 52, 1432–1444. [Google Scholar] [CrossRef]
- Kang, H.; Kim, H.; Lee, S.; Youn, H.; Youn, B. Role of Metabolic Reprogramming in Epithelial–Mesenchymal Transition (EMT). IJMS 2019, 20, 2042. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β Signaling in the Tumor Metabolic Microenvironment and Targeted Therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, B.; Yang, G.; Wang, H.; Chen, G.; You, L.; Zhang, T.; Zhao, Y. The Enhancement of Glycolysis Regulates Pancreatic Cancer Metastasis. Cell Mol. Life Sci. 2019, 77, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Paul, B.; Lewinska, M.; Lafuente-Barquero, J.; De Gauna, M.R.; Buqué, X.; Mattanovich, M.; Geng, D.; Rodrigues, R.; Kjær, M.; et al. Hepatocellular Carcinoma Overcomes Lipid Depletion by Utilizing Serine for Phospholipid Synthesis and Enhanced Survival; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Han, X.; Burrows, M.; Kim, L.C.; Xu, J.P.; Vostrejs, W.; Van Le, T.N.; Poltorack, C.; Jiang, Y.; Cukierman, E.; Stanger, B.Z.; et al. Cancer-Associated Fibroblasts Maintain Critical Pancreatic Cancer Cell Lipid Homeostasis in the Tumor Microenvironment. Cell Rep. 2024, 43, 114972. [Google Scholar] [CrossRef] [PubMed]



| Sequence 5′-3′ | Name | Reference |
|---|---|---|
| TGCACCACCAACTGCTTAGC | GAPDH F | [43] |
| GGCATGGACTGTGGTCATGAG | GAPDH R | |
| AGCCTCAGGTCATAAACATCATTG | E-cadherin F | [44] |
| GATAGATTCTTGGGTTGGGTCG | E-cadherin R | |
| ATTGGACCATCACTCGGCTTA | N-cadherin F | [45] |
| CACACTGGCAAACCTTCACG | N-cadherin R | |
| AGTCCACTGAGTACCGGAGAC | Vimentin F | [46] |
| CATTTCACGCATCTGGCGTTC | Vimentin R | |
| TCGGAAGCCTAACTACAGCGA | Snail F | [47] |
| AGATGAGCATTGGCAGCGAG | Snail R |
| Class | Count | CTRL | TGF-β1 10 ng/mL | TGF-β1 20 ng/mL | p-Value | Sign. | |||
|---|---|---|---|---|---|---|---|---|---|
| CE | 5 | 2.53173 ± 0.15611 | b | 3.22515 ± 0.40402 | b | 5.49683 ± 0.41794 | a | 1.54 × 10−4 | *** |
| CL | 9 | 0.10918 ± 0.01037 | 0.08659 ± 0.00393 | 0.09885 ± 0.00695 | 1.49 × 10−1 | ||||
| Cer | 12 | 0.02440 ± 0.00110 | c | 0.03244 ± 0.00122 | b | 0.04826 ± 0.00152 | a | 6.49 × 10−8 | *** |
| DG | 9 | 1.00271 ± 0.03512 | 1.31249 ± 0.17875 | 1.56231 ± 0.20878 | 8.38 × 10−2 | ||||
| HexCer | 3 | 0.00230 ± 0.00006 | c | 0.00408 ± 0.00021 | a | 0.00338 ± 0.00014 | b | 8.47 × 10−6 | *** |
| LPC | 1 | 0.02879 ± 0.00059 | a | 0.01318 ± 0.00319 | b | 0.02019 ± 0.00466 | a | 1.83 × 10−2 | * |
| LPE | 1 | 0.00992 ± 0.00053 | c | 0.01316 ± 0.00063 | b | 0.02142 ± 0.00087 | a | 1.90 × 10−7 | *** |
| LPI | 1 | 0.00339 ± 0.00021 | c | 0.00538 ± 0.00021 | b | 0.00838 ± 0.00035 | a | 6.37 × 10−8 | *** |
| PA | 3 | 0.05569 ± 0.00248 | a | 0.02675 ± 0.00146 | b | 0.02398 ± 0.00247 | b | 3.86 × 10−7 | *** |
| PC | 23 | 6.93216 ± 1.30989 | 4.72462 ± 0.74767 | 5.72477 ± 1.31097 | 4.26 × 10−1 | ||||
| PE | 24 | 1.65486 ± 0.05482 | b | 1.89319 ± 0.14558 | b | 2.61706 ± 0.16727 | a | 6.40 × 10−4 | *** |
| PE-O | 2 | 0.00474 ± 0.00020 | c | 0.00648 ± 0.00051 | b | 0.00869 ± 0.00053 | a | 1.36 × 10−4 | *** |
| PE-P | 5 | 0.00957 ± 0.00026 | c | 0.01337 ± 0.00080 | b | 0.02592 ± 0.00124 | a | 3.57 × 10−8 | *** |
| PG | 12 | 0.13965 ± 0.00445 | b | 0.14419 ± 0.00732 | b | 0.18684 ± 0.01239 | a | 4.16 × 10−3 | ** |
| PI | 18 | 2.20432 ± 0.06209 | 2.01694 ± 0.08885 | 2.31428 ± 0.10065 | 8.22 × 10−2 | ||||
| PS | 13 | 1.92422 ± 0.05494 | a | 1.45601 ± 0.06127 | b | 1.59096 ± 0.08233 | b | 1.04 × 10−3 | ** |
| SM | 13 | 1.39350 ± 0.30270 | 1.15554 ± 0.22155 | 1.41455 ± 0.35496 | 7.96 × 10−1 | ||||
| TG | 29 | 7.63852 ± 0.77395 | 13.54987 ± 2.91798 | 19.20528 ± 8.68190 | 3.38 × 10−1 | ||||
| Total | 183 | 25.66967 ± 1.95333 | 29.67943 ± 3.13796 | 40.37196 ± 8.40289 | 1.71 × 10−1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertoldi, A.; Cusumano, G.; Calzoni, E.; Alabed, H.B.R.; Pellegrino, R.M.; Buratta, S.; Urbanelli, L.; Emiliani, C. Multi-Omic Characterization of Epithelial–Mesenchymal Transition: Lipidomic and Metabolomic Profiles as Key Markers of TGF-β-Induced Transition in Huh7 Hepatocellular Carcinoma. Cells 2025, 14, 1233. https://doi.org/10.3390/cells14161233
Bertoldi A, Cusumano G, Calzoni E, Alabed HBR, Pellegrino RM, Buratta S, Urbanelli L, Emiliani C. Multi-Omic Characterization of Epithelial–Mesenchymal Transition: Lipidomic and Metabolomic Profiles as Key Markers of TGF-β-Induced Transition in Huh7 Hepatocellular Carcinoma. Cells. 2025; 14(16):1233. https://doi.org/10.3390/cells14161233
Chicago/Turabian StyleBertoldi, Agnese, Gaia Cusumano, Eleonora Calzoni, Husam B. R. Alabed, Roberto Maria Pellegrino, Sandra Buratta, Lorena Urbanelli, and Carla Emiliani. 2025. "Multi-Omic Characterization of Epithelial–Mesenchymal Transition: Lipidomic and Metabolomic Profiles as Key Markers of TGF-β-Induced Transition in Huh7 Hepatocellular Carcinoma" Cells 14, no. 16: 1233. https://doi.org/10.3390/cells14161233
APA StyleBertoldi, A., Cusumano, G., Calzoni, E., Alabed, H. B. R., Pellegrino, R. M., Buratta, S., Urbanelli, L., & Emiliani, C. (2025). Multi-Omic Characterization of Epithelial–Mesenchymal Transition: Lipidomic and Metabolomic Profiles as Key Markers of TGF-β-Induced Transition in Huh7 Hepatocellular Carcinoma. Cells, 14(16), 1233. https://doi.org/10.3390/cells14161233










