Reversing Preeclampsia Pathology: AXL Inhibition Restores Mitochondrial Function and ECM Balance
Abstract
1. Introduction
2. Materials and Methods
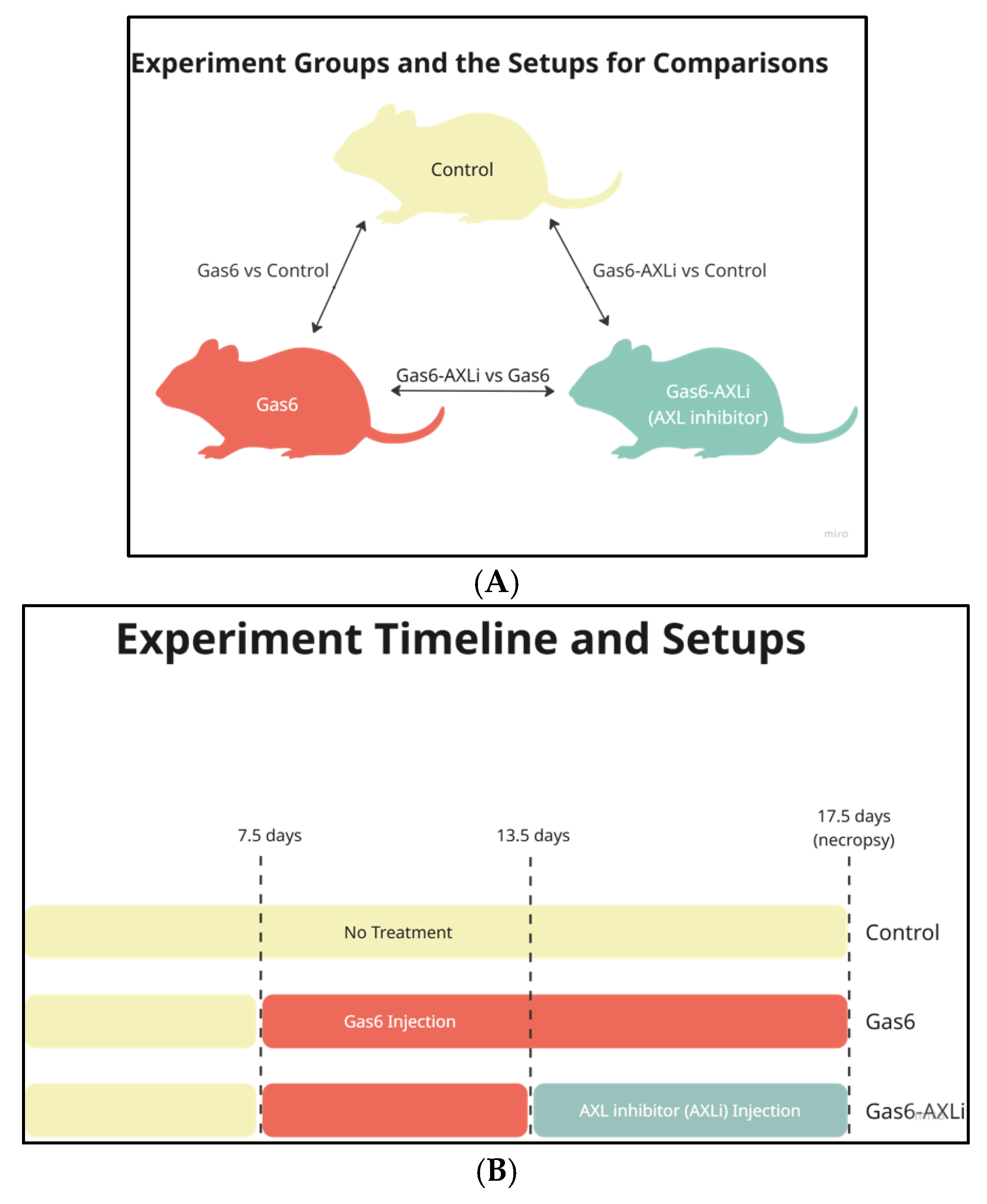
2.1. Animals and Tissue Collection
2.2. Animal Treatment Protocols
2.3. RNA Isolation and Extraction
2.4. RNA Sequencing Data Analysis
2.5. Statistical Analysis
3. Results
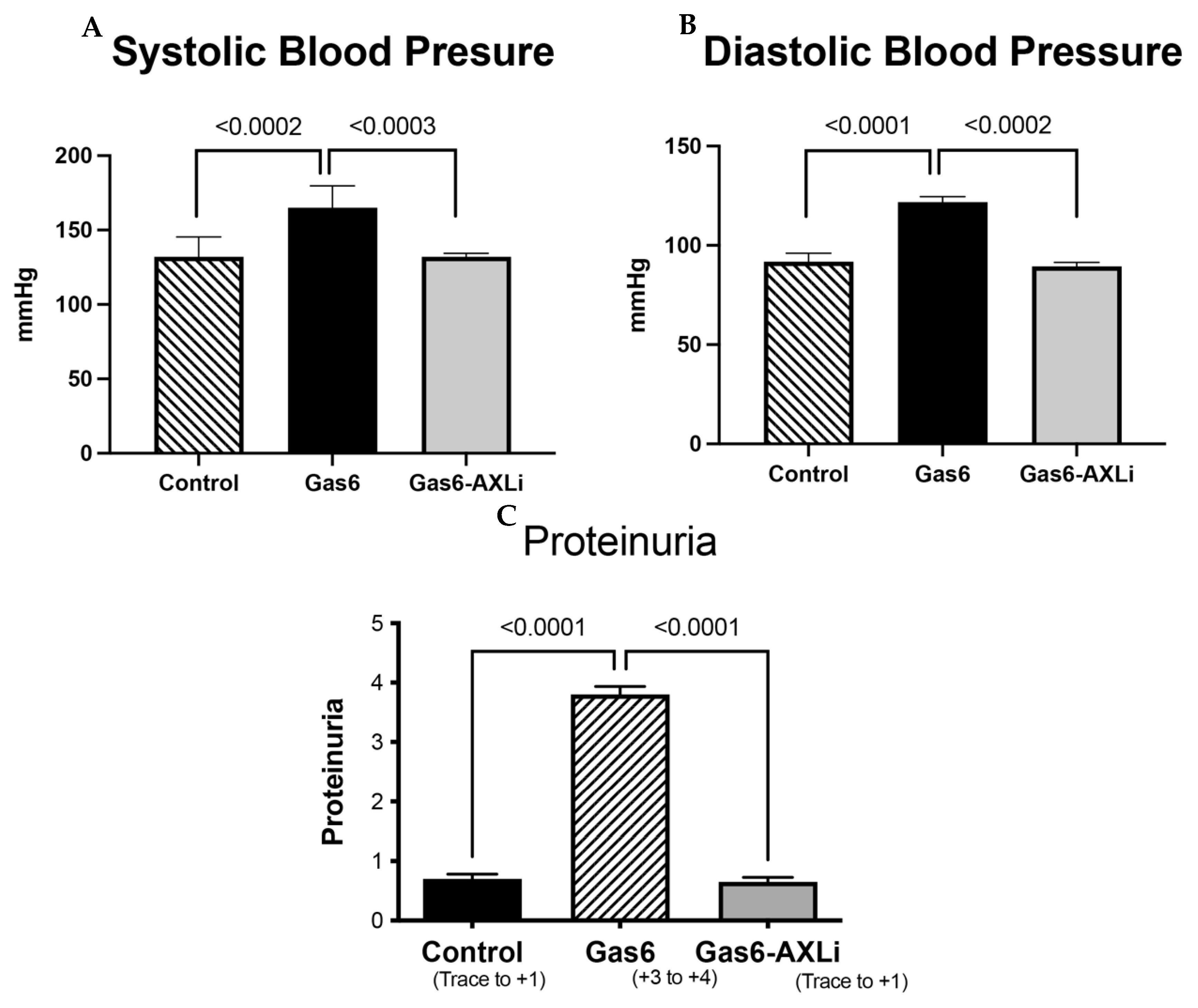
3.1. Gas6 Preeclampsia and AXL Inhibition
3.2. Differentially Expressed Genes (DEGs) Analysis
3.3. Network and Gene Ontology (GO) Analysis of Gas6-AXLi vs. Gas6
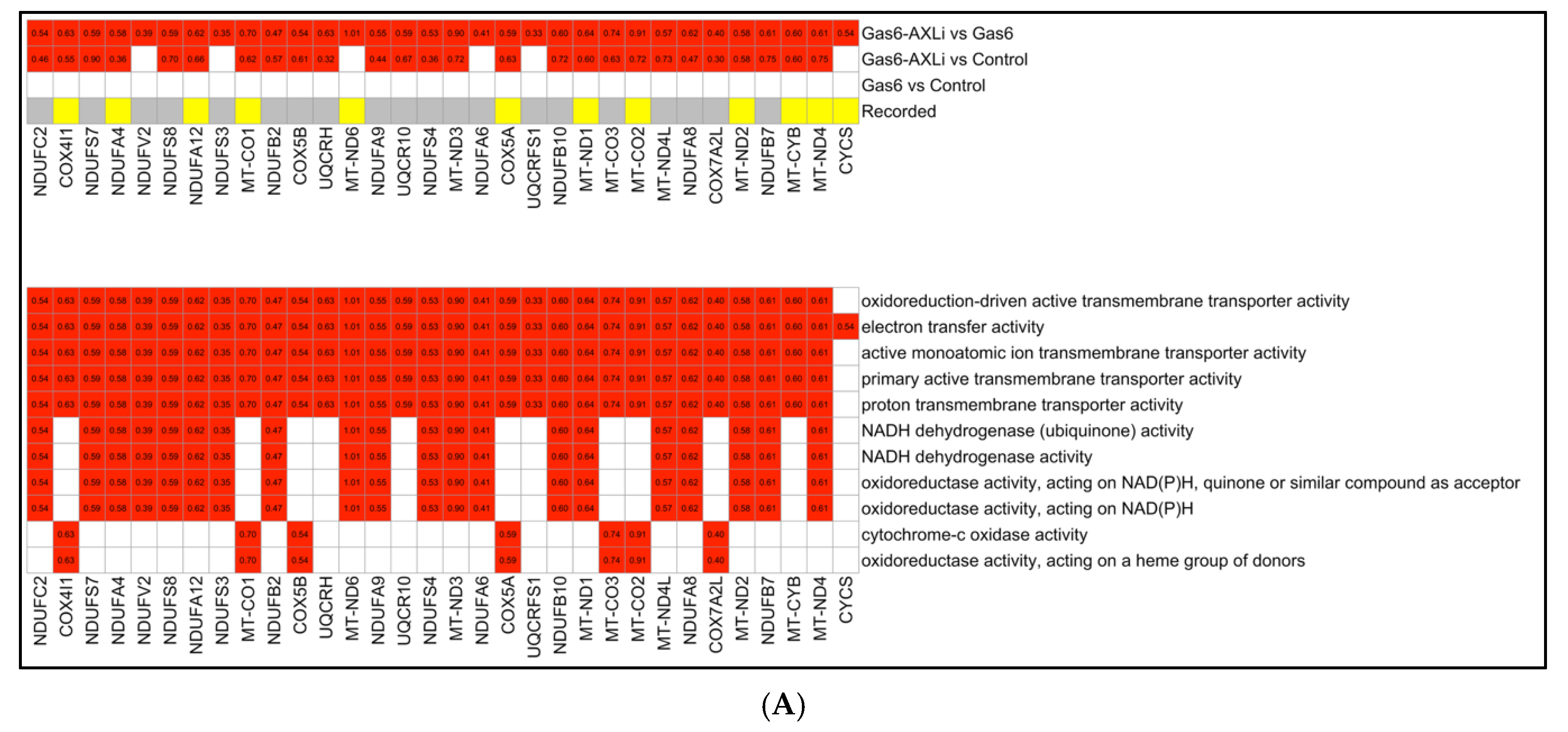
3.4. Mitochondrial Functions (Gas6-AXLi vs. Gas6)
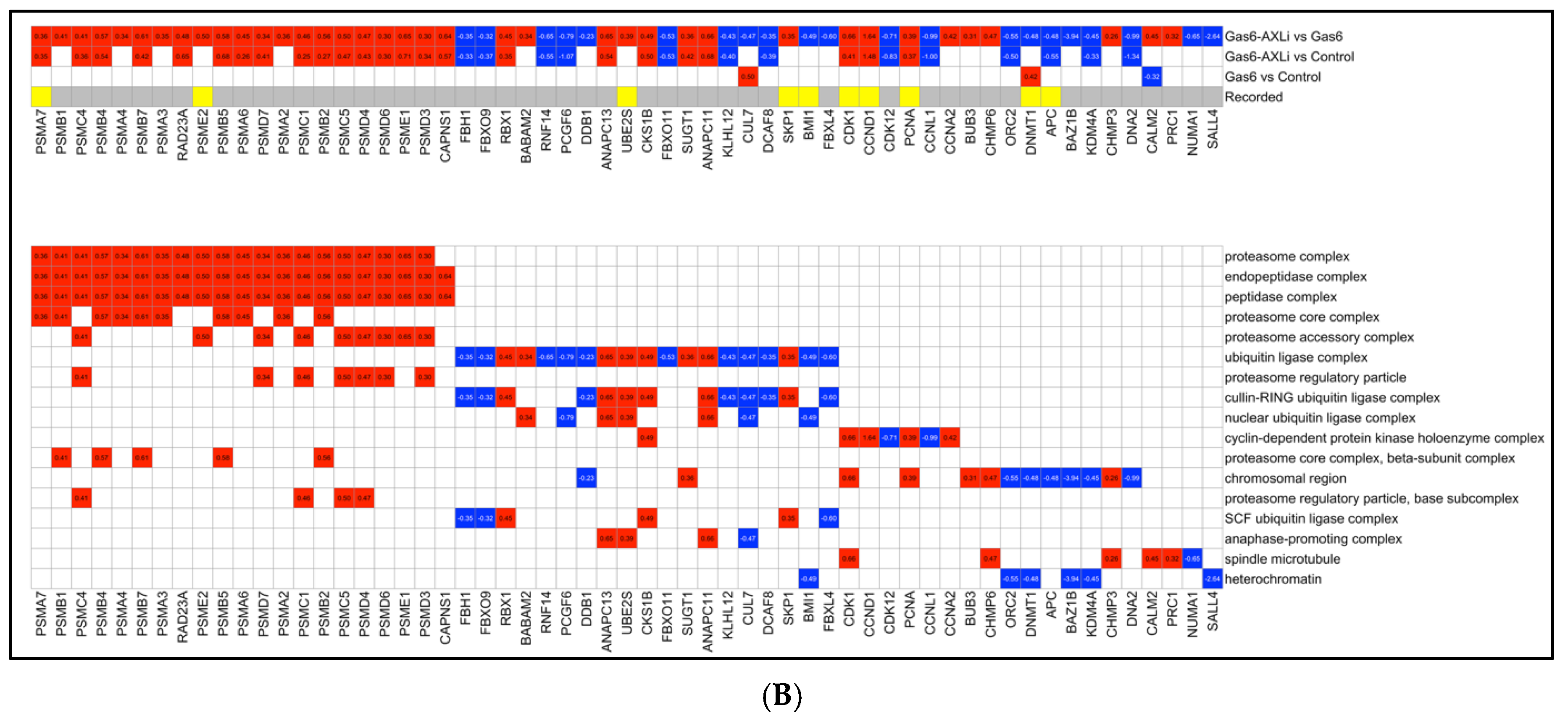
3.5. Upregulation of Proteasome and Ubiquitin Ligase Complex (Gas6-AXLi vs. Gas6)
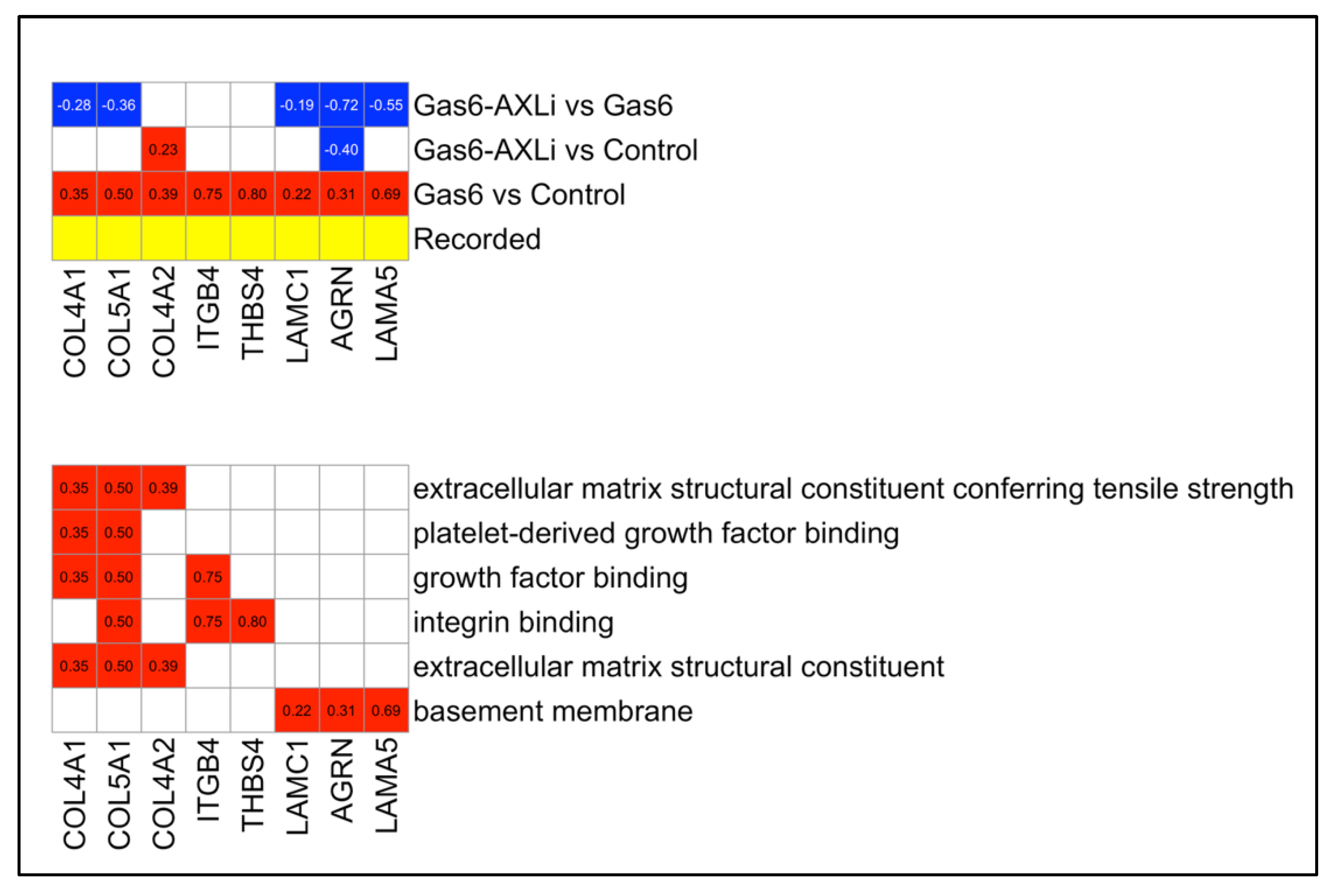
3.6. Gas6 vs. Control Secondary Analysis: Reversal of Collagen Expression
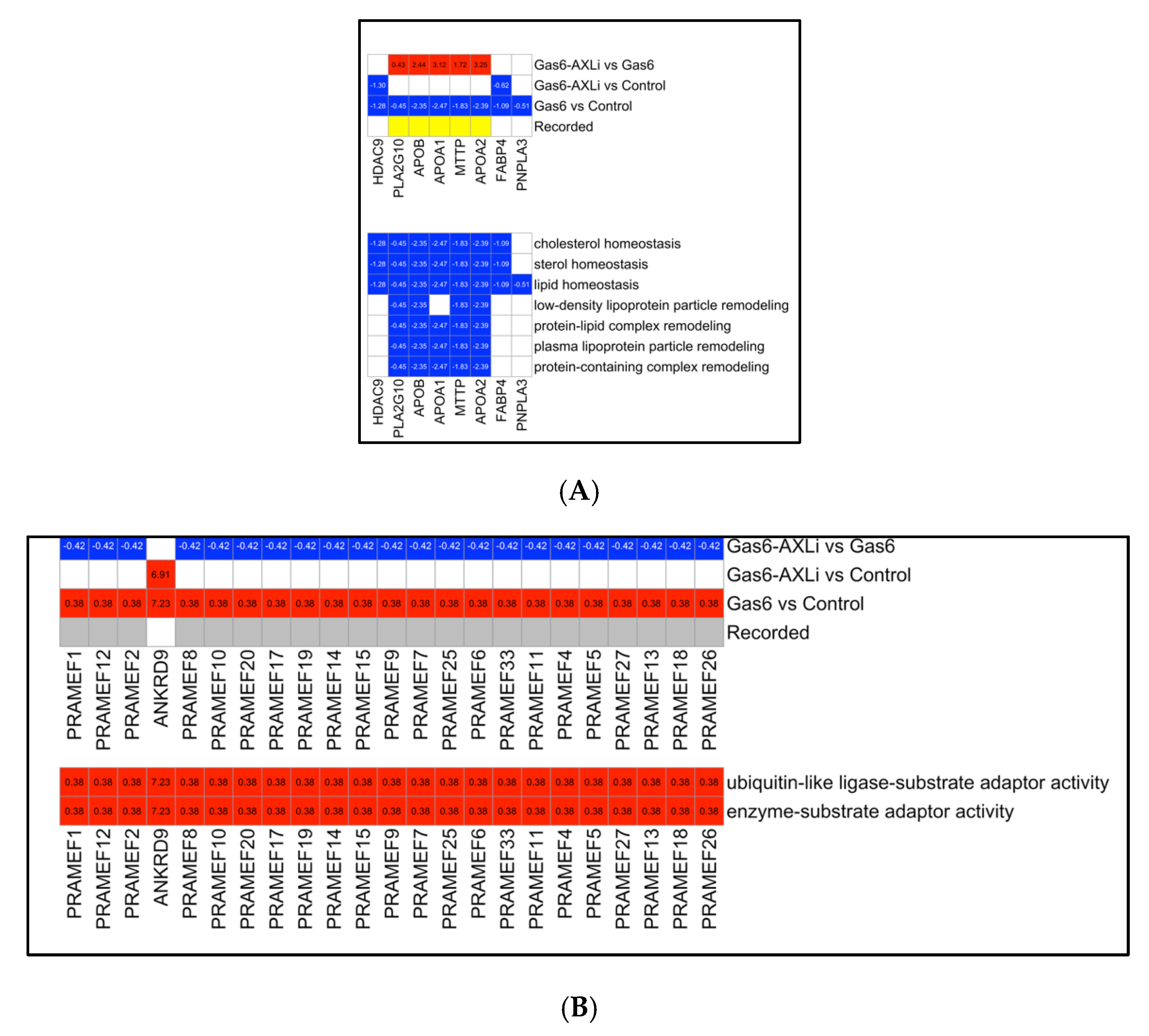
3.7. Gas6 vs. Control Secondary Analysis: Lipid Metabolism and Ubiquitin-Associated Functions
3.8. Reversal of Gas6-Induced Upregulation
3.9. Reversal of Gas6-Induced Downregulation
4. Discussion
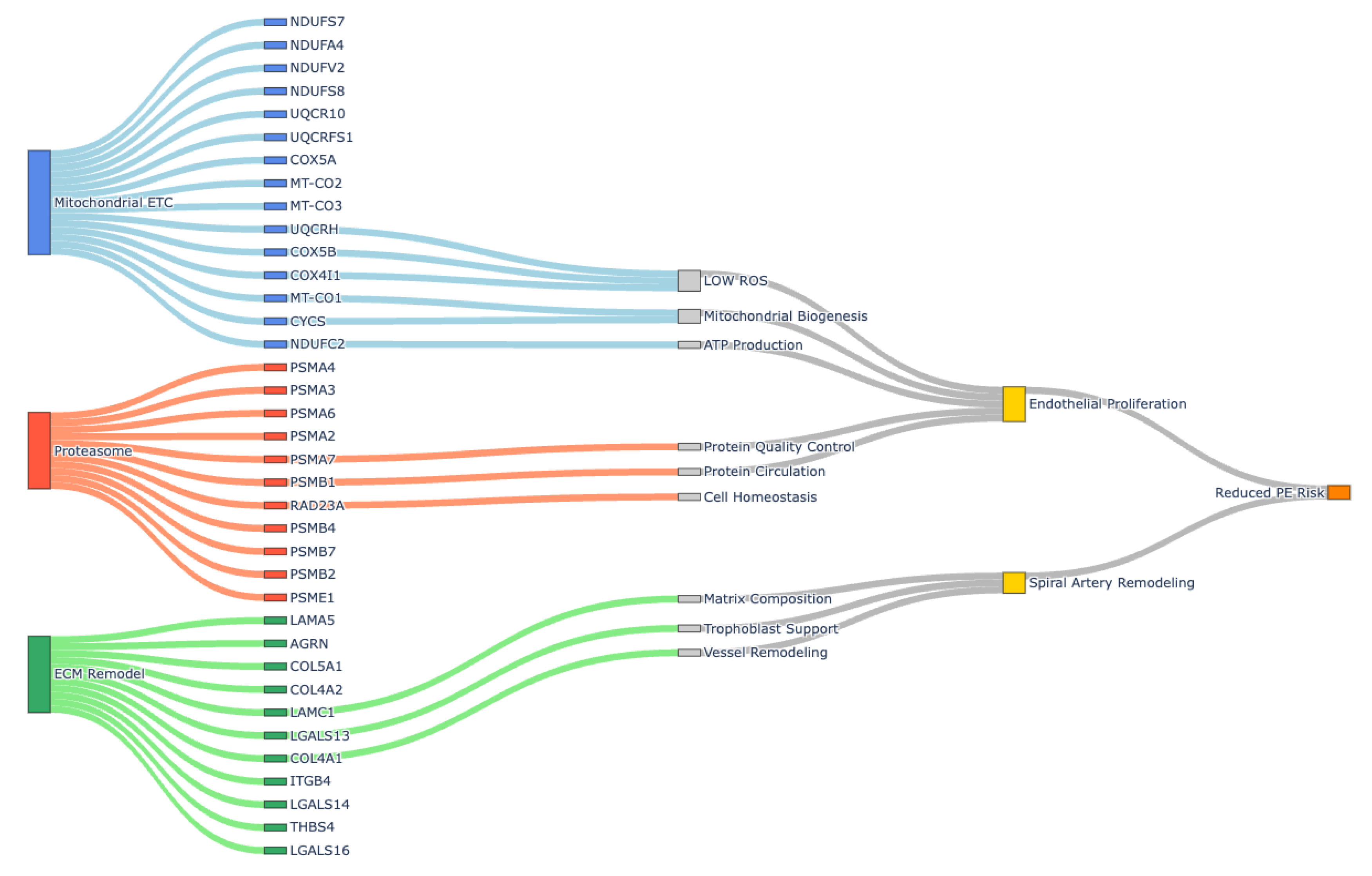
4.1. Restoration of Mitochondrial Function by AXL Inhibition
4.2. Reduction in ECM Deposition and Fibrosis Reversal
4.3. Modulation of Proteasome-Ubiquitin Pathways
4.4. Gene Expression Patterns and Reversal Categories
4.5. Implications of Gas6-AXLi Signaling
4.6. Limitations and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Say, L.; Chou, D.; Gemmill, A.; Tuncalp, O.; Moller, A.B.; Daniels, J.; Gulmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Bokslag, A.; van Weissenbruch, M.; Mol, B.W.; de Groot, C.J. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Staff, A.C.; Fjeldstad, H.E.; Fosheim, I.K.; Moe, K.; Turowski, G.; Johnsen, G.M.; Alnaes-Katjavivi, P.; Sugulle, M. Failure of physiological transformation and spiral artery atherosis: Their roles in preeclampsia. Am. J. Obs. Obstet. Gynecol. 2022, 226, S895–S906. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obs. Obstet. Gynecol. 2022, 226, S907–S927. [Google Scholar] [CrossRef]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef]
- The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Publications and Reports of the Surgeon General; U.S. Department of Health and Human Services: Washington, DC, USA, 2006.
- Li, K.W.; Ganz, A.B.; Smit, A.B. Proteomics of neurodegenerative diseases: Analysis of human post-mortem brain. J. Neurochem. 2019, 151, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakova, P.A.; Volodina, M.A.; Tarasova, N.V.; Marey, M.V.; Tsvirkun, D.V.; Vavina, O.V.; Khodzhaeva, Z.S.; Kan, N.E.; Menon, R.; Vysokikh, M.Y.; et al. Mitochondrial role in adaptive response to stress conditions in preeclampsia. Sci. Rep. 2016, 6, 32410. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Carnicer-Lombarte, A.; Gardner, L.; Thomas, J.; Brosens, J.J.; Moffett, A.; Sharkey, A.M.; Franze, K.; Burton, G.J.; Oyen, M.L. Tissue stiffness at the human maternal-fetal interface. Hum. Reprod. 2019, 34, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Oefner, C.M.; Sharkey, A.; Gardner, L.; Critchley, H.; Oyen, M.; Moffett, A. Collagen type IV at the fetal-maternal interface. Placenta 2015, 36, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rukosuev, V.S.; Nanaev, A.K.; Milovanov, A.P. Participation of collagen types I, III, IV, V, and fibronectin in the formation of villi fibrosis in human term placenta. Acta Histochem. 1990, 89, 11–16. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Wang, H.; Chen, X.; Lan, Z.; Li, P.; Cao, Y.; Liu, M.; Lv, J.; Chen, Y.; et al. Collagen I Induces Preeclampsia-Like Symptoms by Suppressing Proliferation and Invasion of Trophoblasts. Front. Endocrinol. 2021, 12, 664766. [Google Scholar] [CrossRef]
- Jiang, R.; Teng, Y.; Huang, Y.; Gu, J.; Ma, L.; Li, M.; Zhou, Y. Preeclampsia serum-induced collagen I expression and intracellular calcium levels in arterial smooth muscle cells are mediated by the PLC-gamma1 pathway. Exp. Mol. Med. 2014, 46, e115. [Google Scholar] [CrossRef]
- Hafizi, S.; Dahlback, B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.M.; Tsai, K.Y.; Davis, T.; Clark, J.C.; Knowlton, M.N.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J.A. Growth arrest-specific protein-6/AXL signaling induces preeclampsia in rats. Biol. Reprod. 2020, 102, 199–210. [Google Scholar] [CrossRef]
- Peng, S.; Sun, M.; Sun, X.; Wang, X.; Jin, T.; Wang, H.; Han, C.; Meng, T.; Li, C. Plasma levels of TAM receptors and ligands in severe preeclampsia. Pregnancy Hypertens. 2018, 13, 116–120. [Google Scholar] [CrossRef]
- Liu, X.; Gong, Y.; Jia, J.; Bai, Y.; Gui, S.; Wang, T.; Zhou, R. Plasma concentrations of sAxl are associated with severe preeclampsia. Clin. Biochem. 2014, 47, 173–176. [Google Scholar] [CrossRef]
- Gui, S.; Zhou, S.; Liu, M.; Zhang, Y.; Gao, L.; Wang, T.; Zhou, R. Elevated Levels of Soluble Axl (sAxl) Regulates Key Angiogenic Molecules to Induce Placental Endothelial Dysfunction and a Preeclampsia-Like Phenotype. Front. Physiol. 2021, 12, 619137. [Google Scholar] [CrossRef]
- Sober, S.; Reiman, M.; Kikas, T.; Rull, K.; Inno, R.; Vaas, P.; Teesalu, P.; Marti, J.M.L.; Mattila, P.; Laan, M. Extensive shift in placental transcriptome profile in preeclampsia and placental origin of adverse pregnancy outcomes. Sci. Rep. 2015, 5, 13336. [Google Scholar] [CrossRef] [PubMed]
- Munchel, S.; Rohrback, S.; Randise-Hinchliff, C.; Kinnings, S.; Deshmukh, S.; Alla, N.; Tan, C.; Kia, A.; Greene, G.; Leety, L.; et al. Circulating transcripts in maternal blood reflect a molecular signature of early-onset preeclampsia. Sci. Transl. Med. 2020, 12, eaaz0131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jonassen, I. RASflow: An RNA-Seq analysis workflow with Snakemake. BMC Bioinform. 2020, 21, 110. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Clauset, A.; Newman, M.E.; Moore, C. Finding community structure in very large networks. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2004, 70, 066111. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.P.; Mi, H. PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Zhang, L. Mitochondrial Dysfunction in the Pathogenesis of Preeclampsia. Curr. Hypertens. Rep. 2022, 24, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Holland, O.J.; Hickey, A.J.R.; Alvsaker, A.; Moran, S.; Hedges, C.; Chamley, L.W.; Perkins, A.V. Changes in mitochondrial respiration in the human placenta over gestation. Placenta 2017, 57, 102–112. [Google Scholar] [CrossRef]
- Jahan, F.; Vasam, G.; Green, A.E.; Bainbridge, S.A.; Menzies, K.J. Placental Mitochondrial Function and Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2023, 24, 4177. [Google Scholar] [CrossRef] [PubMed]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31, S66–S69. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; Scharnagl, H.; Wadsack, C.; Marsche, G. Preeclampsia Affects Lipid Metabolism and HDL Function in Mothers and Their Offspring. Antioxidants 2023, 12, 795. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Maloyan, A.; Myatt, L. Mitochondrial function and glucose metabolism in the placenta with gestational diabetes mellitus: Role of miR-143. Clin. Sci. 2016, 130, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Parameshwar, P.K.; Sagrillo-Fagundes, L.; Fournier, C.; Girard, S.; Vaillancourt, C.; Moraes, C. Disease-specific extracellular matrix composition regulates placental trophoblast fusion efficiency. Biomater. Sci. 2021, 9, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, H.; Wu, R.; Wang, Q.; Guan, Q.; Chen, Y.; Cao, S.; Tang, L.; Lin, Z.; Li, L.; et al. Downregulated PSME3 Contributes to Severe Preeclampsia by Promoting Trophoblast Cell Apoptosis. Hypertension 2025, 82, 690–703. [Google Scholar] [CrossRef]
- Nakashima, A.; Shima, T.; Tsuda, S.; Aoki, A.; Kawaguchi, M.; Furuta, A.; Yasuda, I.; Yoneda, S.; Yamaki-Ushijima, A.; Cheng, S.B.; et al. Aggrephagy Deficiency in the Placenta: A New Pathogenesis of Preeclampsia. Int. J. Mol. Sci. 2021, 22, 2432. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Abumaree, M.H.; Perkins, A.V.; Brennecke, S.P.; Kalionis, B. Reduced aldehyde dehydrogenase expression in preeclamptic decidual mesenchymal stem/stromal cells is restored by aldehyde dehydrogenase agonists. Sci. Rep. 2017, 7, 42397. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Liu, C.; Deng, H.; Zhang, N.; Wang, S.; Zhang, Z. Transthyretin as a novel candidate biomarker for preeclampsia. Exp. Ther. Med. 2014, 7, 1332–1336. [Google Scholar] [CrossRef][Green Version]
- Mayer-Pickel, K.; Nanda, M.; Gajic, M.; Cervar-Zivkovic, M. Preeclampsia and the Antiphospholipid Syndrome. Biomedicines 2023, 11, 2298. [Google Scholar] [CrossRef]
- Liu, Z.; Pei, J.; Zhang, X.; Wang, C.; Tang, Y.; Liu, H.; Yu, Y.; Luo, S.; Gu, W. APOA1 Is a Novel Marker for Preeclampsia. Int. J. Mol. Sci. 2023, 24, 16363. [Google Scholar] [CrossRef] [PubMed]
- Waller, D.K.; Lustig, L.S.; Cunningham, G.C.; Feuchtbaum, L.B.; Hook, E.B. The association between maternal serum alpha-fetoprotein and preterm birth, small for gestational age infants, preeclampsia, and placental complications. Obs. Obstet. Gynecol. 1996, 88, 816–822. [Google Scholar] [CrossRef]
- Serrano, N.C.; Guio-Mahecha, E.; Quintero-Lesmes, D.C.; Becerra-Bayona, S.; Paez, M.C.; Beltran, M.; Herrera, V.M.; Leon, L.J.; Williams, D.; Casas, J.P. Lipid profile, plasma apolipoproteins, and pre-eclampsia risk in the GenPE case-control study. Atherosclerosis 2018, 276, 189–194. [Google Scholar] [CrossRef]
- Buhl, K.B.; Friis, U.G.; Svenningsen, P.; Gulaveerasingam, A.; Ovesen, P.; Frederiksen-Moller, B.; Jespersen, B.; Bistrup, C.; Jensen, B.L. Urinary plasmin activates collecting duct ENaC current in preeclampsia. Hypertension 2012, 60, 1346–1351. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Wang, P.; Xu, Y.; Jin, P.; Wu, Z.; Qian, Y.; Bai, L.; Dong, M. Galectin-14 Promotes Trophoblast Migration and Invasion by Upregulating the Expression of MMP-9 and N-Cadherin. Front. Cell Dev. Biol. 2021, 9, 645658. [Google Scholar] [CrossRef]
- Than, N.G.; Romero, R.; Xu, Y.; Erez, O.; Xu, Z.; Bhatti, G.; Leavitt, R.; Chung, T.H.; El-Azzamy, H.; LaJeunesse, C.; et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta 2014, 35, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hou, F.; Cheng, D.; Gao, F.; Wang, J.; Cui, B. GATA1-mediated macrophage polarization via TrkB/cGMP-PKG signaling pathway to promote the development of preeclampsia. Eur. J. Med. Res. 2025, 30, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Lv, X.F.; Huang, C.C.; Sun, L.; Ma, M.M.; Liu, C.; Guan, Y.Y. LRRC8A is essential for volume-regulated anion channel in smooth muscle cells contributing to cerebrovascular remodeling during hypertension. Cell Prolif. 2021, 54, e13146. [Google Scholar] [CrossRef]
- Jackson, M.; Gibson, T.M.; Frank, E.; Hill, G.; Davidson, B.; Reynolds, P.R.; Bikman, B.T.; Pickett, B.E.; Arroyo, J.A. Transcriptomic Insights into Gas6-Induced Placental Dysfunction: Gene Targets for Preeclampsia Therapy. Cells 2025, 14, 278. [Google Scholar] [CrossRef]
- Banu, S.K.; Stanley, J.A.; Sivakumar, K.K.; Taylor, R.J.; Arosh, J.A.; Burghardt, R.C. Editor’s Highlight: Exposure to CrVI during Early Pregnancy Increases Oxidative Stress and Disrupts the Expression of Antioxidant Proteins in Placental Compartments. Toxicol. Sci. 2017, 155, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Moliterno, A.R.; Resar, L.M. AKNA: Another AT-hook transcription factor “hooking-up” with inflammation. Cell Res. 2011, 21, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Oshiumi, H.; Matsumoto, M.; Seya, T. DDX60, a DEXD/H box helicase, is a novel antiviral factor promoting RIG-I-like receptor-mediated signaling. Mol. Cell Biol. 2011, 31, 3802–3819. [Google Scholar] [CrossRef] [PubMed]

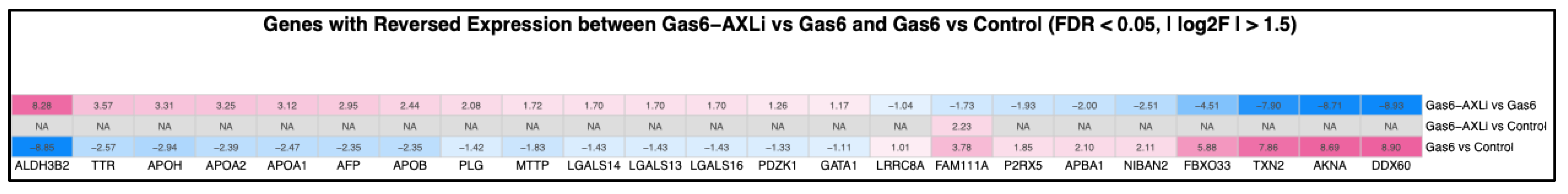
| Gene | Log2FC Gas6 | FDR Gas6 | Log2F AXLi | FDR AXLi |
|---|---|---|---|---|
| ALDH3B2 | −8.854 | 8.991 × 10−4 | 8.285 | 0.007 |
| TTR | −2.573 | 0.006 | 3.567 | 1.751 × 10−4 |
| APOA1 | −2.471 | 7.537 × 10−4 | 3.119 | 5.955 × 10−5 |
| APOH | −2.940 | 0.003 | 3.312 | 0.004 |
| APOA2 | −2.392 | 0.019 | 3.246 | 0.003 |
| APOB | −2.355 | 0.038 | 2.441 | 0.011 |
| AFP | −2.351 | 0.006 | 2.947 | 9.469 × 10−4 |
| PLG | −1.422 | 0.024 | 2.078 | 0.010 |
| MTTP | −1.828 | 0.007 | 1.718 | 0.043 |
| LGALS14 | −1.427 | 2.510 × 10−6 | 1.700 | 3.517 × 10−7 |
| LGALS13 | −1.427 | 2.510 × 10−6 | 1.700 | 3.517 × 10−7 |
| LGALS16 | −1.427 | 2.510 × 10−6 | 1.700 | 3.517 × 10−7 |
| PDZK1 | −1.327 | 0.006 | 1.260 | 0.003 |
| GATA1 | −1.115 | 0.022 | 1.174 | 0.004 |
| LRRC8A | 1.000 | 0.006 | −1.040 | 4.410 × 10−5 |
| FAM111A | 3.780 | 1.910 × 10−23 | 1.730 | 0.004 |
| P2RX5 | 1.850 | 0.032 | 1.930 | 0.004 |
| APBA1 | 2.100 | 0.014 | −2.000 | 0.004 |
| NIBAN2 | 2.110 | 0.040 | 2.510 | 2.420 × 10−5 |
| FBXO33 | 5.880 | 0.220 | −4.510 | 0.006 |
| TXN2 | 7.860 | 0.026 | −0.400 | 0.001 |
| AKNA | 8.690 | 7.790 × 10−8 | −8.710 | 6.930 × 10−9 |
| DDX60 | 8.900 | 1.500 × 10−4 | −8.930 | 1.550 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, A.; Davidson, B.; Reynolds, P.R.; Pickett, B.E.; Arroyo, J.A. Reversing Preeclampsia Pathology: AXL Inhibition Restores Mitochondrial Function and ECM Balance. Cells 2025, 14, 1229. https://doi.org/10.3390/cells14161229
Chou A, Davidson B, Reynolds PR, Pickett BE, Arroyo JA. Reversing Preeclampsia Pathology: AXL Inhibition Restores Mitochondrial Function and ECM Balance. Cells. 2025; 14(16):1229. https://doi.org/10.3390/cells14161229
Chicago/Turabian StyleChou, Archarlie, Benjamin Davidson, Paul R. Reynolds, Brett E. Pickett, and Juan A. Arroyo. 2025. "Reversing Preeclampsia Pathology: AXL Inhibition Restores Mitochondrial Function and ECM Balance" Cells 14, no. 16: 1229. https://doi.org/10.3390/cells14161229
APA StyleChou, A., Davidson, B., Reynolds, P. R., Pickett, B. E., & Arroyo, J. A. (2025). Reversing Preeclampsia Pathology: AXL Inhibition Restores Mitochondrial Function and ECM Balance. Cells, 14(16), 1229. https://doi.org/10.3390/cells14161229








