The Influence of Irisin on Selected Organs—The Liver, Kidneys, and Lungs: The Role of Physical Exercise
Abstract
1. Introduction
2. Irisin
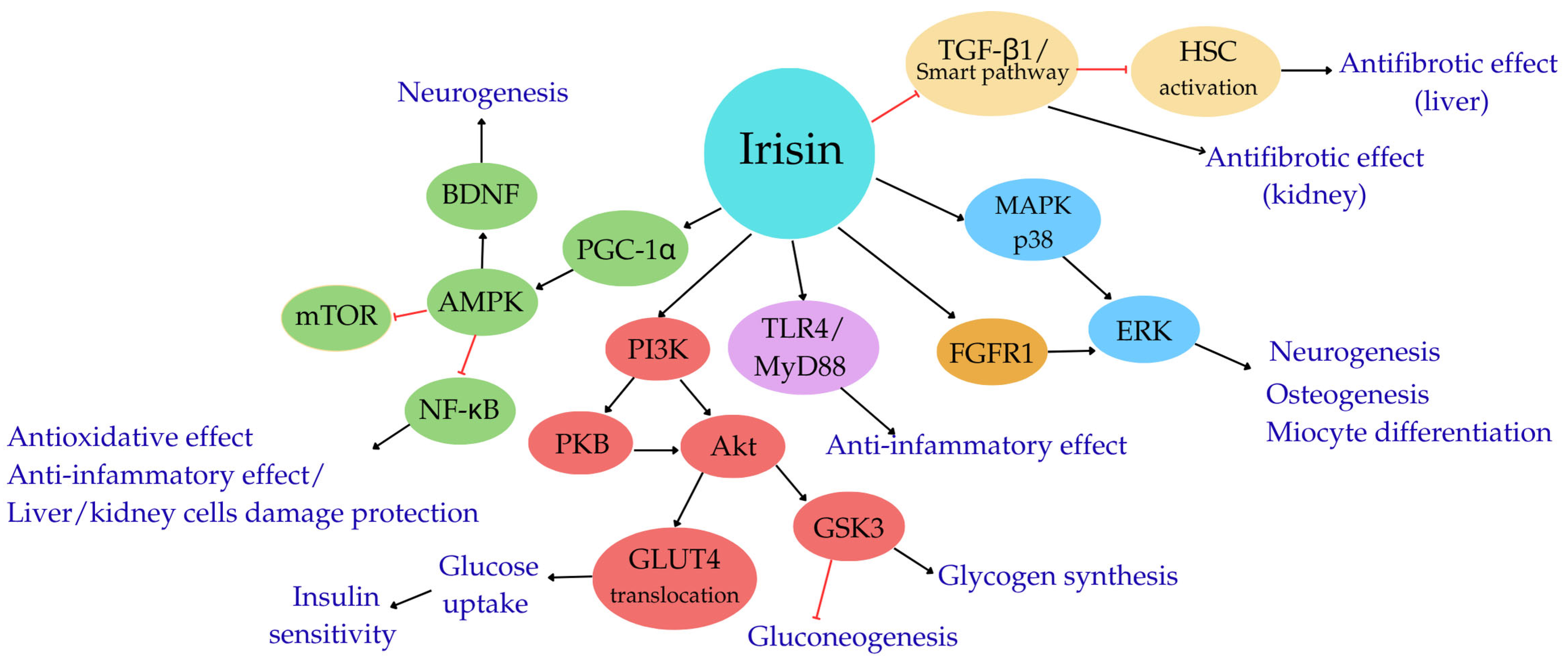
Irisin—Molecular Mechanisms of Action
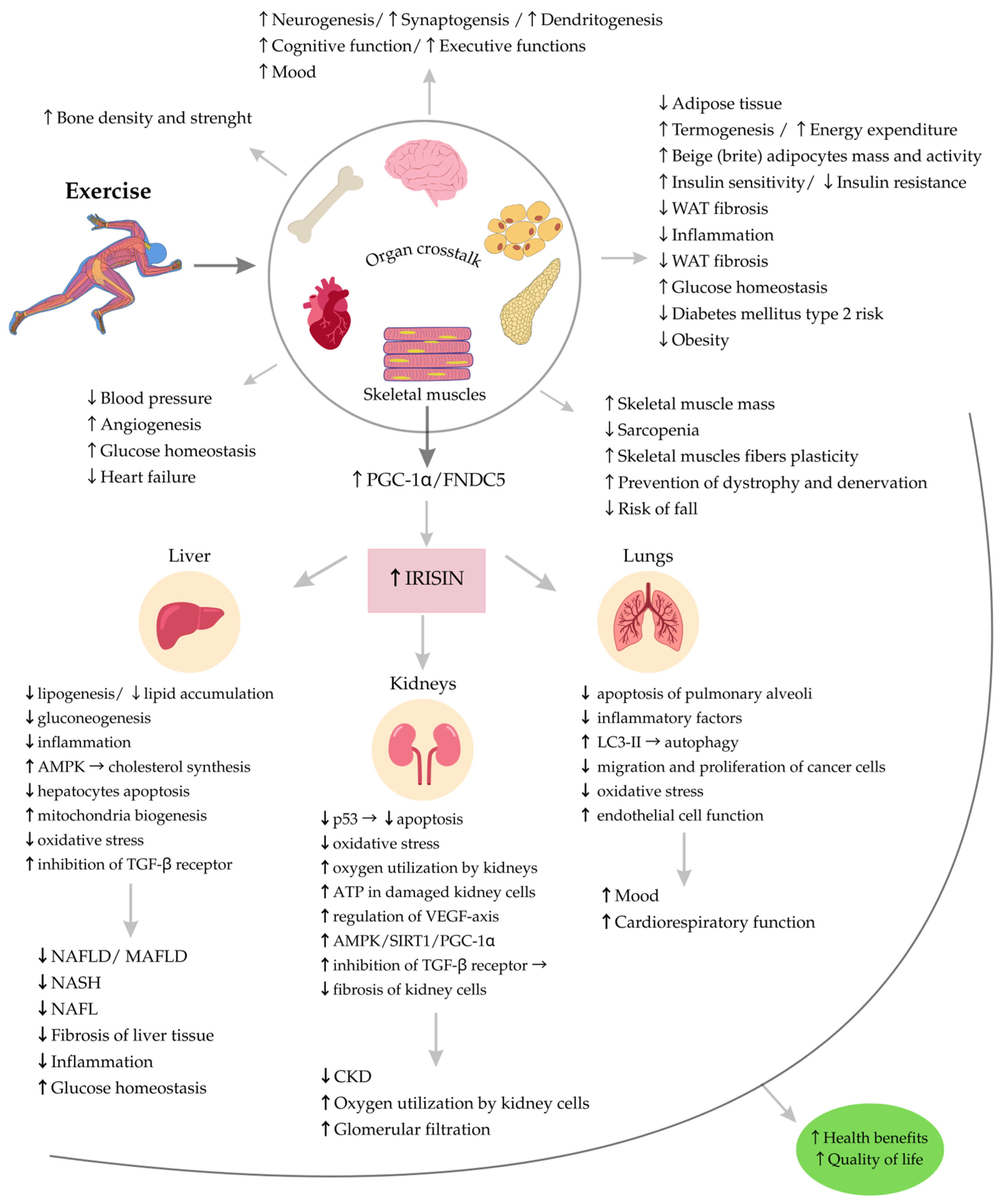
3. Irisin and Physical Exercise
4. Liver and Physical Exercise
5. Irisin–Liver–Physical Exercise
6. Kidneys and Physical Exercise
7. Irisin–Kidneys–Physical Exercise
8. Irisin–Lungs–Physical Exercise
9. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; So, K.-F.; Xiao, J.; Wang, H. Organ-Organ Communication: The Liver’s Perspective. Theranostics 2021, 11, 3317–3330. [Google Scholar] [CrossRef]
- Ciołkiewicz, M.; Kuryliszyn-Moskal, A.; Hryniewicz, A.; Kamiński, K. Sarcopenia and myokines profile as risk factors in cardiovascular diseases? Postepy Hig. Med. Dosw. 2019, 73, 550–562. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Lőrincz, H.; Somodi, S.; Ratku, B.; Harangi, M.; Paragh, G. Crucial Regulatory Role of Organokines in Relation to Metabolic Changes in Non-Diabetic Obesity. Metabolites 2023, 13, 270. [Google Scholar] [CrossRef]
- Inyushkin, A.N.; Poletaev, V.S.; Inyushkina, E.M.; Kalberdin, I.S.; Inyushkin, A.A. Irisin/BDNF Signaling in the Muscle-Brain Axis and Circadian System: A Review. J. Biomed. Res. 2024, 38, 1–16. [Google Scholar] [CrossRef]
- Armutcu, F. Organ Crosstalk: The Potent Roles of Inflammation and Fibrotic Changes in the Course of Organ Interactions. Inflamm. Res. 2019, 68, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y.; Shi, Y.; Shi, Y.; Su, X.; Chen, P.; Wu, D.; Shi, H. Exercise and Exerkines: Mechanisms and Roles in Anti-Aging and Disease Prevention. Exp. Gerontol. 2025, 200, 112685. [Google Scholar] [CrossRef] [PubMed]
- Samant, V.; Prabhu, A. Exercise, Exerkines and Exercise Mimetic Drugs: Molecular Mechanisms and Therapeutics. Life Sci. 2024, 359, 123225. [Google Scholar] [CrossRef] [PubMed]
- Leitner, L.M.; Wilson, R.J.; Yan, Z.; Gödecke, A. Reactive Oxygen Species/Nitric Oxide Mediated Inter-Organ Communication in Skeletal Muscle Wasting Diseases. Antioxid. Redox Signal. 2017, 26, 700. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1α-Dependent Myokine That Drives Browning of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Aydin, S. Three New Players in Energy Regulation: Preptin, Adropin and Irisin. Peptides 2014, 56, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.L. Irisin-Immunoreactivity in Neural and Non-Neural Cells of the Rodent—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3637839/ (accessed on 24 October 2024).
- Korek, E.; Krauss, H. Novel adipokines: Their potential role in the pathogenesis of obesity and metabolic disorders. Postepy Hig. Med. Dosw. 2015, 69, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Pukajło, K.; Kolackov, K.; Łaczmański, Ł.; Daroszewski, J. Irisin—A new mediator of energy homeostasis. Postepy Hig. Med. Dosw. 2015, 69, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E. Jak Mięśnie Komunikują Się z Innymi Narządami i Co z Tego Wynika? Kosmos 2021, 69, 673–687. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association with Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Panati, K.; Suneetha, Y.; Narala, V.R. Irisin/FNDC5—An Updated Review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 689–697. [Google Scholar]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Crujeiras, A.B.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Grześkiewicz, M.; Kobak, J.E.; Drewniak, H.; Terlecki, P.; Przywara, S. Irisin—The Future of Ischemic Stroke Therapy? Acta Angiol. 2023, 29, 19–24. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef]
- Zhao, R. Irisin at the Crossroads of Inter-Organ Communications: Challenge and Implications. Front. Endocrinol. 2022, 13, 989135. [Google Scholar] [CrossRef]
- Norman, D.; Drott, C.J.; Carlsson, P.-O.; Espes, D. Irisin—A Pancreatic Islet Hormone. Biomedicines 2022, 10, 258. [Google Scholar] [CrossRef]
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M.; et al. The Identification of Irisin in Human Cerebrospinal Fluid: Influence of Adiposity, Metabolic Markers, and Gestational Diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E512–E518. [Google Scholar] [CrossRef]
- Bakal, U.; Aydin, S.; Sarac, M.; Kuloglu, T.; Kalayci, M.; Artas, G.; Yardim, M.; Kazez, A. Serum, Saliva, and Urine Irisin with and Without Acute Appendicitis and Abdominal Pain. Biochem. Insights 2016, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Contreras, C.; Romero-Picó, A.; López, M.; Diéguez, C. Irisin, Two Years Later. Int. J. Endocrinol. 2013, 2013, 746281. [Google Scholar] [CrossRef]
- Arias-Loste, M.T.; Ranchal, I.; Romero-Gómez, M.; Crespo, J. Irisin, a Link among Fatty Liver Disease, Physical Inactivity and Insulin Resistance. Int. J. Mol. Sci. 2014, 15, 23163–23178. [Google Scholar] [CrossRef]
- Wojtaszewski, J.F.P.; Nielsen, P.; Hansen, B.F.; Richter, E.A.; Kiens, B. Isoform-Specific and Exercise Intensity-Dependent Activation of 5′-AMP-Activated Protein Kinase in Human Skeletal Muscle. J. Physiol. 2000, 528, 221–226. [Google Scholar] [CrossRef]
- Witczak, C.A.; Sharoff, C.G.; Goodyear, L.J. AMP-Activated Protein Kinase in Skeletal Muscle: From Structure and Localization to Its Role as a Master Regulator of Cellular Metabolism. Cell. Mol. Life Sci. 2008, 65, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.; Goodyear, L.J. Contraction Signaling to Glucose Transport in Skeletal Muscle. J. Appl. Physiol. 2005, 99, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of Irisin in Physiology and Pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1α: A Key Regulator of Energy Metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Bao, J.-F.; She, Q.-Y.; Hu, P.-P.; Jia, N.; Li, A. Irisin, a Fascinating Field in Our Times. Trends Endocrinol. Metab. 2022, 33, 601–613. [Google Scholar] [CrossRef]
- McDonald, M.E.; Li, C.; Bian, H.; Smith, B.D.; Layne, M.D.; Farmer, S.R. Myocardin-Related Transcription Factor A Regulates Conversion of Progenitors to Beige Adipocytes. Cell 2015, 160, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Harms, M.; Boucher, J. The Colorful Versatility of Adipocytes: White-to-Brown Transdifferentiation and Its Therapeutic Potential in Humans. FEBS J. 2021, 288, 3628–3646. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Svensson, K.J.; Tsai, L.; Zeng, X.; Roh, H.C.; Kong, X.; Rao, R.R.; Lou, J.; Lokurkar, I.; Baur, W.; et al. Ribosomal Profiling Provides Evidence for a Smooth Muscle-Like Origin of Beige Adipocytes. Cell Metab. 2014, 19, 810–820. [Google Scholar] [CrossRef]
- Raajendiran, A.; Ooi, G.; Bayliss, J.; O’Brien, P.E.; Schittenhelm, R.B.; Clark, A.K.; Taylor, R.A.; Rodeheffer, M.S.; Burton, P.R.; Watt, M.J. Identification of Metabolically Distinct Adipocyte Progenitor Cells in Human Adipose Tissues. Cell Rep. 2019, 27, 1528–1540.e7. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ikeda, K.; Chen, Y.; Alba, D.L.; Stifler, D.; Shinoda, K.; Hosono, T.; Maretich, P.; Yang, Y.; Ishigaki, Y.; et al. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018, 27, 180–194.e6. [Google Scholar] [CrossRef]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-Independent Signaling Involving SERCA2b-Mediated Calcium Cycling Regulates Beige Fat Thermogenesis and Systemic Glucose Homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise Hormone Irisin Is a Critical Regulator of Cognitive Function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Ito, A.; Appiah, M.G.; Obeng, G.; Darkwah, S.; Kawamoto, E.; Gaowa, A.; Park, E.J.; Shimaoka, M. Irisin Supports Integrin-Mediated Cell Adhesion of Lymphocytes. Biochem. Biophys. Rep. 2021, 26, 100977. [Google Scholar] [CrossRef]
- Mu, A.; Wales, T.E.; Zhou, H.; Draga-Coletă, S.-V.; Gorgulla, C.; Blackmore, K.A.; Mittenbühler, M.J.; Kim, C.R.; Bogoslavski, D.; Zhang, Q.; et al. Irisin Acts through Its Integrin Receptor in a Two-Step Process Involving Extracellular Hsp90α. Mol. Cell 2023, 83, 1903–1920.e12. [Google Scholar] [CrossRef]
- Oguri, Y.; Shinoda, K.; Kim, H.; Alba, D.L.; Bolus, W.R.; Wang, Q.; Brown, Z.; Pradhan, R.N.; Tajima, K.; Yoneshiro, T.; et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell 2020, 182, 563–577.e20. [Google Scholar] [CrossRef]
- Paoletti, I.; Coccurello, R. Irisin: A Multifaceted Hormone Bridging Exercise and Disease Pathophysiology. Int. J. Mol. Sci. 2024, 25, 13480. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-Induced Irisin Secretion Is Independent of Age or Fitness Level and Increased Irisin May Directly Modulate Muscle Metabolism Through AMPK Activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Gruhn, K.; Siteneski, A.; Camargo, A.; Freitas, A.E.; Olescowicz, G.; Brocardo, P.S.; Rodrigues, A. Lúcia.S. Physical Exercise Stimulates Hippocampal mTORC1 and FNDC5/Irisin Signaling Pathway in Mice: Possible Implication for Its Antidepressant Effect. Behav. Brain Res. 2021, 400, 113040. [Google Scholar] [CrossRef]
- Albensi, B.C.; Mattson, M.P. Evidence for the Involvement of TNF and NF-kappaB in Hippocampal Synaptic Plasticity. Synapse 2000, 35, 151–159. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Lee, P.; Linderman, J.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S.; et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab. 2014, 19, 302–309. [Google Scholar] [CrossRef]
- Kyosseva, S.V. Mitogen-Activated Protein Kinase Signaling. Int. Rev. Neurobiol. 2004, 59, 201–220. [Google Scholar]
- Lin, J.; Liu, X.; Zhou, Y.; Zhu, B.; Wang, Y.; Cui, W.; Peng, Y.; Wang, B.; Zhao, C.; Zhao, R. Molecular Basis of Irisin Regulating the Effects of Exercise on Insulin Resistance. Appl. Sci. 2022, 12, 5837. [Google Scholar] [CrossRef]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New Insights into the Cellular Activities of Fndc5/Irisin and Its Signaling Pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Algul, S.; Ozdenk, C.; Ozcelik, O. Variations in Leptin, Nesfatin-1 and Irisin Levels Induced by Aerobic Exercise in Young Trained and Untrained Male Subjects. Biol. Sport 2017, 34, 339–344. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Landis-Piwowar, K.; Byrd, G.; Seimer, M.; Seigneurie, N.; Byrd, B.; Muzik, O. Plasma Irisin in Runners and Nonrunners: No Favorable Metabolic Associations in Humans. Physiol. Rep. 2015, 3, e12262. [Google Scholar] [CrossRef]
- Nygaard, H.; Slettaløkken, G.; Vegge, G.; Hollan, I.; Whist, J.E.; Strand, T.; Rønnestad, B.R.; Ellefsen, S. Irisin in Blood Increases Transiently after Single Sessions of Intense Endurance Exercise and Heavy Strength Training. PLoS ONE 2015, 10, e0121367. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-del-Valle, M.; Short, M.J.; Chung, E.; McComb, J.; Kloiber, S.; Naclerio, F.; Larumbe-Zabala, E. Effects of High-Intensity Resistance Training on Circulating Levels of Irisin in Healthy Adults: A Randomized Controlled Trial. Asian J. Sports Med. 2018, 9, 13025. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589. [Google Scholar] [CrossRef] [PubMed]
- Shabani, R.; Izaddoust, F. Effects of Aerobic Training, Resistance Training, or Both on Circulating Irisin and Myostatin in Untrained Women. Acta Gymnica 2018, 48, 47–55. [Google Scholar] [CrossRef]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A Myth Rather than an Exercise-Inducible Myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef]
- Sanchis-Gomar, F.; Alis, R.; Lippi, G. Circulating Irisin Detection: Does It Really Work? Trends Endocrinol. Metab. 2015, 26, 335–336. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Pardo, M.; Casanueva, F.F. Irisin: ‘Fat’ or Artefact. Clin. Endocrinol. 2015, 82, 467–474. [Google Scholar] [CrossRef]
- Fatouros, I.G. Is Irisin the New Player in Exercise-Induced Adaptations or Not? A 2017 Update. Clin. Chem. Lab. Med. 2018, 56, 525–548. [Google Scholar] [CrossRef]
- Löffler, D.; Müller, U.; Scheuermann, K.; Friebe, D.; Gesing, J.; Bielitz, J.; Erbs, S.; Landgraf, K.; Wagner, I.V.; Kiess, W.; et al. Serum Irisin Levels Are Regulated by Acute Strenuous Exercise. J. Clin. Endocrinol. Metab. 2015, 100, 1289–1299. [Google Scholar] [CrossRef]
- Lagzdina, R.; Rumaka, M.; Gersone, G.; Tretjakovs, P. Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina 2020, 56, 274. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating Irisin in Healthy, Young Individuals: Day-Night Rhythm, Effects of Food Intake and Exercise, and Associations With Gender, Physical Activity, Diet, and Body Composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Messner, M.; Brissot, P. Traditional Management of Liver Disorders. Drugs 1990, 40, 45–57. [Google Scholar] [CrossRef]
- Koya, S.; Kawaguchi, T.; Hashida, R.; Goto, E.; Matsuse, H.; Saito, H.; Hirota, K.; Taira, R.; Matsushita, Y.; Imanaga, M.; et al. Effects of In-Hospital Exercise on Liver Function, Physical Ability, and Muscle Mass during Treatment of Hepatoma in Patients with Chronic Liver Disease. Hepatol. Res. 2017, 47, E22–E34. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, C.R.A.; Inggriani, S.; Cahyadinata, L.; Lesmana, L.A.L. Deep Vein Thrombosis in Patients with Advanced Liver Cirrhosis: A Rare Condition? Hepatol. Int. 2010, 4, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, C.; Li, C.; Witt, R.G.; Huang, H.; Tsung, A.; Zhang, H. Physical exercise in liver diseases. Hepatology 2024. [Google Scholar] [CrossRef]
- Ghamarchehreh, M.E.; Shamsoddini, A.; Alavian, S.M. Investigating the Impact of Eight Weeks of Aerobic and Resistance Training on Blood Lipid Profile in Elderly with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Gastroenterol. Hepatol. Bed Bench 2019, 12, 190. [Google Scholar]
- Bianco, A.; Franco, I.; Curci, R.; Bonfiglio, C.; Campanella, A.; Mirizzi, A.; Fucilli, F.; Di Giovanni, G.; Giampaolo, N.; Pesole, P.L.; et al. Diet and Exercise Exert a Differential Effect on Glucose Metabolism Markers According to the Degree of NAFLD Severity. Nutrients 2023, 15, 2252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; He, J.; Pan, L.-L.; Ma, Z.-M.; Han, C.-K.; Chen, C.-S.; Chen, Z.; Han, H.-W.; Chen, S.; Sun, Q.; et al. Effects of Moderate and Vigorous Exercise on Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 1074–1082. [Google Scholar] [CrossRef]
- Trefts, E.; Williams, A.S.; Wasserman, D.H. Exercise and the Regulation of Hepatic Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 203. [Google Scholar] [CrossRef]
- Hughey, C.C.; Bracy, D.; Lantier, L.; Foretz, M.; Viollet, B.; Wasserman, D. 1556-P: Hepatic AMP-Activated Protein Kinase Governs Adaptations in Liver Glucose Fluxes and Lipid Homeostasis in Response to Exercise Training in Mice. Diabetes 2023, 72, 1556-P. [Google Scholar] [CrossRef]
- Hughey, C.C.; Bracy, D.P.; Rome, F.I.; Goelzer, M.; Donahue, E.P.; Viollet, B.; Foretz, M.; Wasserman, D.H. Exercise Training Adaptations in Liver Glycogen and Glycerolipids Require Hepatic AMP-Activated Protein Kinase in Mice. Am. J. Physiol.-Endocrinol. Metab. 2024, 326, E14–E28. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Shi, C.-X.; Gao, R.; Sun, H.-J.; Xiong, X.-Q.; Ding, L.; Chen, Q.; Li, Y.-H.; Wang, J.-J.; Kang, Y.-M.; et al. Irisin Inhibits Hepatic Gluconeogenesis and Increases Glycogen Synthesis via the PI3K/Akt Pathway in Type 2 Diabetic Mice and Hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef]
- Tang, H.; Yu, R.; Liu, S.; Huwatibieke, B.; Li, Z.; Zhang, W. Irisin Inhibits Hepatic Cholesterol Synthesis via AMPK-SREBP2 Signaling. EBioMedicine 2016, 6, 139–148. [Google Scholar] [CrossRef]
- Mo, L.; Shen, J.; Liu, Q.; Zhang, Y.; Kuang, J.; Pu, S.; Cheng, S.; Zou, M.; Jiang, W.; Jiang, C.; et al. Irisin Is Regulated by CAR in Liver and Is a Mediator of Hepatic Glucose and Lipid Metabolism. Mol. Endocrinol. 2016, 30, 533–542. [Google Scholar] [CrossRef]
- Zhao, J.; Qiao, L.; Dong, J.; Wu, R. Antioxidant Effects of Irisin in Liver Diseases: Mechanistic Insights. Oxid. Med. Cell Longev. 2022, 2022, 3563518. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, D.-I.; Choi, J.-H.; Heo, Y.-R.; Park, S.-H. Nowa Rola Iryzyny w Hepatocytach: Efekt Ochronny Stłuszczenia Wątroby in Vitro. Cell. Signal. 2015, 27, 1831–1839. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin Alleviates Liver Ischemia-Reperfusion Injury by Inhibiting Excessive Mitochondrial Fission, Promoting Mitochondrial Biogenesis and Decreasing Oxidative Stress. Redox Biol. 2018, 20, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. 2014, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-F.; Wang, M.-Z.; Bi, J.-B.; Zhang, J.; Zhang, L.; Liu, W.-M.; Wei, S.-S.; Lv, Y.; Wu, Z.; Wu, R.-Q. Irisin Attenuates Intestinal Injury, Oxidative and Endoplasmic Reticulum Stress in Mice with L-Arginine-Induced Acute Pancreatitis. World J. Gastroenterol. 2019, 25, 6653–6667. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Bilski, J.; Pochec, E.; Brzozowski, T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. implication for exercise in obesity. J. Physiol. Pharmacol. 2017, 68, 243–251. [Google Scholar]
- Canivet, C.M.; Bonnafous, S.; Rousseau, D.; Leclere, P.S.; Lacas-Gervais, S.; Patouraux, S.; Sans, A.; Luci, C.; Bailly-Maitre, B.; Iannelli, A.; et al. Hepatic FNDC5 Is a Potential Local Protective Factor against Non-Alcoholic Fatty Liver. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165705. [Google Scholar] [CrossRef]
- Garbuzenko, D.V. Pathophysiological Mechanisms of Hepatic Stellate Cells Activation in Liver Fibrosis. World J. Clin. Cases 2022, 10, 3662–3676. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic Stellate Cells as Key Target in Liver Fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27. [Google Scholar] [CrossRef]
- Kasztelan-Szczerbińska, B.; Słomka, M.; Daniluk, J.; Celiński, K.; Cichoż-Lach, H.; Szczerbiński, M.; Zwolak, A.; Jastrzębska, I. Stellate cells as a central regulator of intracellular signaling in the process of liver fibrosis. Postępy Nauk. Med. 2009, 1, 69–74. [Google Scholar]
- Henderson, N.C.; Forbes, S.J. Hepatic Fibrogenesis: From within and Outwith. Toxicology 2008, 254, 130–135. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.N.; Park, S.Y.; Le, C.T.; Choi, D.-H.; Cho, E.-H. Irisin Regulates the Functions of Hepatic Stellate Cells. Endocrinol. Metab. 2020, 35, 647–655. [Google Scholar] [CrossRef]
- Chen, C.; Li, R.; Ross, R.S.; Manso, A.M. Integrins and Integrin-Related Proteins in Cardiac Fibrosis. J. Mol. Cell Cardiol. 2016, 93, 162–174. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The Role of Integrins in Inflammation and Angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Ławkowska, K.; Bonowicz, K.; Jerka, D.; Bai, Y.; Gagat, M. Integrins in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Opportunities. Biomolecules 2025, 15, 233. [Google Scholar] [CrossRef] [PubMed]
- Danen, E.H.; Sonnenberg, A. Erratum: Integrins in Regulation of Tissue Development and Function. J Pathol; 200: 471–480. J. Pathol. 2003, 201, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.R.; Roper, J.A.; Grove, J.I.; Aithal, G.P.; Pun, K.T.; Bennett, A.J. Integrins as a Drug Target in Liver Fibrosis. Liver Int. 2022, 42, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.L.; Park, S.Y.; Nguyen, G.; Pham, P.T.M.; Kang, S.M.; Hong, J.; Lee, J.-H.; Im, S.-S.; Choi, D.-H.; Cho, E.-H. Irisin Attenuates Hepatic Stellate Cell Activation and Liver Fibrosis in Bile Duct Ligation Mice Model and Improves Mitochondrial Dysfunction. Endocrinol. Metab. 2024, 39, 908–920. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Henry, L.; Bush, H.; Mishra, A. Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and Nonalcoholic Fatty Liver Disease: Biochemical, Metabolic and Clinical Implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Croci, I.; Coombes, J.S.; Bucher Sandbakk, S.; Keating, S.E.; Nauman, J.; Macdonald, G.A.; Wisloff, U. Non-Alcoholic Fatty Liver Disease: Prevalence and All-Cause Mortality According to Sedentary Behaviour and Cardiorespiratory Fitness. The HUNT Study. Prog. Cardiovasc. Dis. 2019, 62, 127–134. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328. [Google Scholar] [CrossRef]
- Kistler, K.D.; Brunt, E.M.; Clark, J.M.; Diehl, A.M.; Sallis, J.F.; Schwimmer, J.B. Physical Activity Recommendations, Exercise Intensity, and Histological Severity of Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2011, 106, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Albillos, A.; Villanueva, C.; Genescá, J.; Ardevol, A.; Augustín, S.; Calleja, J.L.; Bañares, R.; García-Pagán, J.C.; Mesonero, F.; et al. Effects of an Intensive Lifestyle Intervention Program on Portal Hypertension in Patients with Cirrhosis and Obesity: The SportDiet Study. Hepatology 2017, 65, 1293. [Google Scholar] [CrossRef]
- Pang, Y.; Lv, J.; Kartsonaki, C.; Yu, C.; Guo, Y.; Du, H.; Bennett, D.; Bian, Z.; Chen, Y.; Yang, L.; et al. Association of Physical Activity with Risk of Hepatobiliary Diseases in China: A Prospective Cohort Study of 0.5 Million People. Br. J. Sports Med. 2021, 55, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.; Stine, J.G. Exercise as Medicine: The Impact of Exercise Training on Nonalcoholic Fatty Liver Disease. Curr Hepatol. Rep. 2020, 19, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Glass, O.; Filozof, C.; Noureddin, M.; Berner-Hansen, M.; Schabel, E.; Omokaro, S.O.; Schattenberg, J.M.; Barradas, K.; Miller, V.; Francque, S.; et al. Standardisation of Diet and Exercise in Clinical Trials of NAFLD-NASH: Recommendations from the Liver Forum. J. Hepatol. 2020, 73, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; DiJoseph, K.; Pattison, Z.; Harrington, A.; Chinchilli, V.M.; Schmitz, K.H.; Loomba, R. Exercise Training Is Associated With Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 1204–1213. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef]
- Yazdani, H.O.; Kaltenmeier, C.; Morder, K.; Moon, J.; Traczek, M.; Loughran, P.; Zamora, R.; Vodovotz, Y.; Li, F.; Wang, J.H.-C.; et al. Exercise Training Decreases Hepatic Injury via Changes in Immune Response to Liver Ischemia/Reperfusion in Mice. Hepatology 2021, 73, 2494–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Sahar, N.E.; Javaid, H.M.A.; Pak, E.S.; Liang, G.; Wang, Y.; Ha, H.; Huh, J.Y. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 2021, 10, 3306. [Google Scholar] [CrossRef]
- Tine Kartinah, N.; Rosalyn Sianipar, I.; Nafi’ah; Rabia. The Effects of Exercise Regimens on Irisin Levels in Obese Rats Model: Comparing High-Intensity Intermittent with Continuous Moderate-Intensity Training. Biomed. Res. Int. 2018, 2018, 4708287. [Google Scholar] [CrossRef]
- Wang, T.; Yu, M.; Li, H.; Qin, S.; Ren, W.; Ma, Y.; Bo, W.; Xi, Y.; Cai, M.; Tian, Z. FNDC5/Irisin Inhibits the Inflammatory Response and Mediates the Aerobic Exercise-Induced Improvement of Liver Injury after Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 4159. [Google Scholar] [CrossRef] [PubMed]
- Belviranlı, M.; Okudan, N. Exercise Training Increases Cardiac, Hepatic and Circulating Levels of Brain-Derived Neurotrophic Factor and Irisin in Young and Aged Rats. Horm. Mol. Biol. Clin. Investig. 2018, 36, 20180053. [Google Scholar] [CrossRef]
- Kosmalski, M.; Drzewoski, J.; Szymczak-Pajor, I.; Zieleniak, A.; Mikołajczyk-Solińska, M.; Kasznicki, J.; Śliwińska, A. Irisin Is Related to Non-Alcoholic Fatty Liver Disease (NAFLD). Biomedicines 2022, 10, 2253. [Google Scholar] [CrossRef]
- Nayak, S.; Sahu, P.K.; Rattan, R.; Mahapatra, S.; Mohapatra, D. Irisin Levels in Type 2 Diabetes Mellitus with and without Non-Alcoholic Fatty Liver Disease: A Case-Control Study. Prospect. Pharm. Sci. 2024, 22, 210–218. [Google Scholar] [CrossRef]
- Ulualan, G.; Kiraz, Z.K.; Kırel, B. Relation of Serum Irisin Levels to Obesity and Non-Alcoholic Fatty Liver Disease. Turk. J. Pediatr. 2022, 64, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Shanaki, M.; Moradi, N.; Emamgholipour, S.; Fadaei, R.; Poustchi, H. Lower Circulating Irisin Is Associated with Nonalcoholic Fatty Liver Disease and Type 2 Diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S467–S472. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Anastasilakis, A.D.; Geladari, E.V.; Mantzoros, C.S. Irisin in Patients with Nonalcoholic Fatty Liver Disease. Metab. Clin. Exp. 2014, 63, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhang, Y.-Y.; Cai, X.-M.; Chen, X. Low Circulating Levels of Omentin-1 and Irisin in Type 2 Diabetes Mellitus Patients with Metabolic-Associated Fatty Liver Disease. Turk. J. Gastroenterol. 2025, 36, 450–458. [Google Scholar] [CrossRef]
- So, R.; Oh, S.; Shida, T.; Tanaka, K.; Shoda, J. Irisin Is Associated with Physical Activity Level and Hepatic Steatosis Grade in Non-Alcoholic Fatty Liver Disease Subjects: 841. Hepatology 2014, 60, 606A. [Google Scholar]
- Hu, J.; Ke, Y.; Wu, F.; Liu, S.; Ji, C.; Zhu, X.; Zhang, Y. Circulating Irisin Levels in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2020, 2020, 8818191. [Google Scholar] [CrossRef]
- Shen, C.; Wu, K.; Ke, Y.; Zhang, Q.; Chen, S.; Li, Q.; Ruan, Y.; Yang, X.; Liu, S.; Hu, J. Circulating Irisin Levels in Patients with MAFLD: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2024, 15, 1464951. [Google Scholar] [CrossRef]
- Poortmans, J.R. Exercise and Renal Function. Sports Med. 1984, 1, 125–153. [Google Scholar] [CrossRef]
- Poortmans, J.R. Postexercise Proteinuria in Humans. Facts and Mechanisms. JAMA 1985, 253, 236–240. [Google Scholar] [CrossRef]
- Suzuki, M.; Sudoh, M.; Matsubara, S.; Kawakami, K.; Shiota, M.; Ikawa, S. Changes in Renal Blood Flow Measured by Radionuclide Angiography Following Exhausting Exercise in Humans. Europ. J. Appl. Physiol. 1996, 74, 1–7. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Haggenmacher, C.; Vanderstraeten, J. Postexercise Proteinuria in Humans and Its Adrenergic Component. J. Sports Med. Phys. Fit. 2001, 41, 95–100. [Google Scholar]
- Bakońska-Pacoń, E.; Borkowski, J. The Effect of the Physical Effort on the Activity of Brush Border Enzymes and Lysosomal Enzymes of Nephron Excreted in the Urine. Biol. Sport 2003, 20, 69–78. [Google Scholar]
- Podhorska-Okolow, M.; Dziegiel, P.; Murawska-Cialowicz, E.; Saczko, J.; Kulbacka, J.; Gomulkiewicz, A.; Rossini, K.; Jethon, Z.; Carraro, U.; Zabel, M. Effects of Adaptive Exercise on Apoptosis in Cells of Rat Renal Tubuli. Eur. J. Appl. Physiol. 2007, 99, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R.; Vanderstraeten, J. Kidney Function during Exercise in Healthy and Diseased Humans. An Update. Sports Med. 1994, 18, 419–437. [Google Scholar] [CrossRef]
- Garibotto, G.; Esposito, P.; Picciotto, D.; Verzola, D.; Costigliolo, F.; Zanetti, V.; Saio, M.; Russo, E.; Viazzi, F. Targeting Kidney Cell Senescence: A New Paradigm for the Treatment of Chronic Kidney Disease? Top Ital. Sci. J. 2024, 1. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Battaglia, Y.; Straudi, S.; Belvederi Murri, M.; Donadi, M.; Piva, G.; Fabbian, F.; López-Soto, P.J.; Grassi, L.; et al. A Personalized Patient-Centered Intervention to Empower through Physical Activity the Patient in the Dialysis Center: Study Protocol for a Pragmatic Nonrandomized Clinical Trial. Methods Protoc. 2020, 3, 83. [Google Scholar] [CrossRef]
- Suzuki, M. Physical Exercise and Renal Function. J. Phys. Fit. Sports Med. 2015, 4, 17–29. [Google Scholar] [CrossRef]
- Fukuta, Y.; Arizono, S.; Tanaka, S.; Kawaguchi, T.; Tsugita, N.; Fuseya, T.; Magata, J.; Tawara, Y.; Segawa, T. Effects of Exercise Around the Ventilation Threshold on Renal Blood Flow in Healthy Individuals. J. Sci. Sport Exerc. 2024, 6, 44–51. [Google Scholar] [CrossRef]
- Souza, M.K.; Neves, R.V.P.; Rosa, T.S.; Cenedeze, M.A.; Arias, S.C.A.; Fujihara, C.K.; Bacurau, R.F.P.; Câmara, N.O.S.; Moraes, M.R.; Filho, A.P.E.S. Resistance Training Attenuates Inflammation and the Progression of Renal Fibrosis in Chronic Renal Disease. Life Sci. 2018, 206, 93–97. [Google Scholar] [CrossRef]
- Peng, C.-C.; Chen, K.-C.; Hsieh, C.-L.; Peng, R.Y. Swimming Exercise Prevents Fibrogenesis in Chronic Kidney Disease by Inhibiting the Myofibroblast Transdifferentiation. PLoS ONE 2012, 7, e37388. [Google Scholar] [CrossRef]
- Wang, X.H.; Price, S.R. Organ Crosstalk Contributes to Muscle Wasting in Chronic Kidney Disease. Semin. Nephrol. 2023, 43, 151409. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.-S.; Kim, N.; Kong, I.D. Circulating Irisin Levels as a Predictive Biomarker for Sarcopenia: A Cross-Sectional Community-Based Study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Jenkin, K.A.; Perry, B.D. Skeletal Muscle and Kidney Crosstalk in Chronic Kidney Disease. Cell. Physiol. Biochem. 2022, 56, 587–601. [Google Scholar] [CrossRef]
- Leal, D.V.; Ferreira, A.; Watson, E.L.; Wilund, K.R.; Viana, J.L. Muscle-Bone Crosstalk in Chronic Kidney Disease: The Potential Modulatory Effects of Exercise. Calcif. Tissue Int. 2021, 108, 461–475. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in Chronic Kidney Disease: What Have We Learned so Far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef] [PubMed]
- Kawao, N.; Kaji, H. Interactions between Muscle Tissues and Bone Metabolism. J. Cell Biochem. 2015, 116, 687–695. [Google Scholar] [CrossRef]
- Bataille, S.; Chauveau, P.; Fouque, D.; Aparicio, M.; Koppe, L. Myostatin and Muscle Atrophy during Chronic Kidney Disease. Nephrol. Dial. Transplant. 2021, 36, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of Muscle Wasting—The Role of the Ubiquitin–Proteasome Pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E. Mechanisms of Muscle Wasting in Chronic Kidney Disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef]
- Ewendt, F.; Feger, M.; Föller, M. Myostatin Regulates the Production of Fibroblast Growth Factor 23 (FGF23) in UMR106 Osteoblast–like Cells. Pflug. Arch. 2021, 473, 969–976. [Google Scholar] [CrossRef]
- Lara-Castillo, N.; Johnson, M.L. Bone-Muscle Mutual Interactions. Curr. Osteoporos. Rep. 2020, 18, 408–421. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Kaczmarek, A.; Kaczmarek, M.; Ciałowicz, M.; Arslan, E.; Silva, A.F.; Clemente, F.M.; Murawska-Ciałowicz, E. Sclerostin as a Biomarker of Physical Exercise in Osteoporosis: A Narrative Review. Front. Endocrinol. 2022, 13, 954895. [Google Scholar] [CrossRef]
- Hittel, D.S.; Axelson, M.; Sarna, N.; Shearer, J.; Huffman, K.M.; Kraus, W.E. Myostatin Decreases with Aerobic Exercise and Associates with Insulin Resistance. Med. Sci. Sports Exerc. 2010, 42, 2023–2029. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of Muscle Mass by Follistatin and Activins. Mol. Endocrinol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef]
- Li, W.; Yin, R.; Xia, X.; Xing, Q.; Xu, S. Correlations of Serum Myostatin and Irisin with Sarcopenia and Osteoporosis in Rheumatoid Arthritis Patients: A Cross-Sectional Study. Sci. Rep. 2025, 15, 23068. [Google Scholar] [CrossRef]
- Planella-Farrugia, C.; Comas, F.; Sabater-Masdeu, M.; Moreno, M.; Moreno-Navarrete, J.M.; Rovira, O.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin and Myostatin as Markers of Muscle Strength and Physical Condition in Elderly Subjects. Front. Physiol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.-H. The Regulation of TGFβ Signal Transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.M.; Isakova, T.; Cai, X.; Bayes, L.Y.; Faul, C.; Scialla, J.J.; Lash, J.P.; Chen, J.; He, J.; Navaneethan, S.; et al. Inflammation and Elevated Levels of Fibroblast Growth Factor 23 Are Independent Risk Factors for Death in Chronic Kidney Disease. Kidney Int. 2017, 91, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Haffner, D. How FGF23 Shapes Multiple Organs in Chronic Kidney Disease. Mol. Cell Pediatr. 2021, 8, 12. [Google Scholar] [CrossRef]
- De Jong, M.A.; Eisenga, M.F.; van Ballegooijen, A.J.; Beulens, J.W.J.; Vervloet, M.G.; Navis, G.; Gansevoort, R.T.; Bakker, S.J.L.; De Borst, M.H. Fibroblast Growth Factor 23 and New-Onset Chronic Kidney Disease in the General Population: The Prevention of Renal and Vascular Endstage Disease (PREVEND) Study. Nephrol. Dial Transplant. 2020, 36, 121–128. [Google Scholar] [CrossRef]
- Oris, C.; Lautrette, A.; Dougé, A.; Bouraima, F.; Kahouadji, S.; Pickering, M.-E.; Garrouste, C.; Gagnière, J.; Guièze, R.; D’Ostrevy, N.; et al. Prevalence of FGF23 Elevation in Patients with Hypophosphatemia. Clin. Chim. Acta 2024, 554, 117782. [Google Scholar] [CrossRef]
- Kraen, M.; Frantz, S.; Nihlén, U.; Engström, G.; Löfdahl, C.G.; Wollmer, P.; Dencker, M. Fibroblast Growth Factor 23 Is an Independent Marker of COPD and Is Associated with Impairment of Pulmonary Function and Diffusing Capacity. Respir. Med. 2021, 182. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone. Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Liu, S.; Wong, M.D.S.; Tan, C.S.H.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Relationship between Circulating Irisin, Renal Function and Body Composition in Type 2 Diabetes. J. Diabetes Complicat. 2014, 28, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carmona, A.; Pérez Fontán, M.; Sangiao Alvarellos, S.; García Falcón, T.; Pena Bello, M.L.; López Muñiz, A.; Cordido, F. Serum Levels of the Adipomyokine Irisin in Patients with Chronic Kidney Disease. Nefrologia 2016, 36, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Shad, J.; Akbari, R.; Qujeq, D.; Hajian-Tilaki, K. Measurement of Serum Irisin in the Different Stages of Chronic Kidney Disease. Casp. J. Intern. Med. 2019, 10, 314–319. [Google Scholar] [CrossRef]
- Shelbaya, S.; Shady, M.M.A.; Nasr, M.S.; Bekhet, M.M.; Mageed, Y.A.-A.; Abbas, M. Study of Irisin Hormone Level in Type 2 Diabetic Patients and Patients with Diabetic Nephropathy. Curr. Diabetes Rev. 2018, 14, 481–486. [Google Scholar] [CrossRef]
- Kawao, N.; Kawaguchi, M.; Ohira, T.; Ehara, H.; Mizukami, Y.; Takafuji, Y.; Kaji, H. Renal Failure Suppresses Muscle Irisin Expression, and Irisin Blunts Cortical Bone Loss in Mice. J. Cachexia Sarcopenia Muscle 2022, 13, 758–771. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Bergamaschi, C.T.; Fernandes, M.J.S.; Paredes-Gamero, E.J.; Curi, M.V.; Ferreira, A.T.; Araujo, S.R.R.; Punaro, G.R.; Maciel, F.R.; Nogueira, G.B.; et al. P2×7 Receptor in the Kidneys of Diabetic Rats Submitted to Aerobic Training or to N-Acetylcysteine Supplementation. PLoS ONE 2014, 9, e97452. [Google Scholar] [CrossRef]
- Formigari, G.P.; Dátilo, M.N.; Vareda, B.; Bonfante, I.L.P.; Cavaglieri, C.R.; Lopes de Faria, J.M.; Lopes de Faria, J.B. Renal Protection Induced by Physical Exercise May Be Mediated by the Irisin/AMPK Axis in Diabetic Nephropathy. Sci. Rep. 2022, 12, 9062. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, Y.; Liu, Z.; Shu, S.; Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Irisin Is Induced in Renal Ischemia-Reperfusion to Protect against Tubular Cell Injury via Suppressing P53. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ji, J.; Zhou, X.; Li, R. Irisin Pretreatment Protects Kidneys against Acute Kidney Injury Induced by Ischemia/Reperfusion via Upregulating the Expression of Uncoupling Protein 2. Biomed. Res. Int. 2020, 2020, 6537371. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yu, L.; Cong, W.; Jiang, S.; Qiu, X.; Wei, C.; Zheng, G.; Mao, J.; Liu, R.; Patzak, A.; et al. Irisin Preserves Mitochondrial Integrity and Function in Tubular Epithelial Cells after Ischemia-Reperfusion-Induced Acute Kidney Injury. Acta Physiol. 2024, 240, e14211. [Google Scholar] [CrossRef]
- Peng, H.; Wang, Q.; Lou, T.; Qin, J.; Jung, S.; Shetty, V.; Li, F.; Wang, Y.; Feng, X.-H.; Mitch, W.E.; et al. Myokine Mediated Muscle-Kidney Crosstalk Suppresses Metabolic Reprogramming and Fibrosis in Damaged Kidneys. Nat. Commun. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Zhou, F.; Guo, Y.; Chen, N. Exercise promotes irisin expression to ameliorate renal injury in type 2 diabetic rats. Nan Fang Yi Ke Da Xue Xue Bao 2024, 44, 675–681. [Google Scholar] [CrossRef]
- Liu, H.-W.; Kao, H.-H.; Wu, C.-H. Exercise Training Upregulates SIRT1 to Attenuate Inflammation and Metabolic Dysfunction in Kidney and Liver of Diabetic Db/Db Mice. Nutr. Metab. 2019, 16, 22. [Google Scholar] [CrossRef]
- Han, F.; Kan, C.; Wu, D.; Kuang, Z.; Song, H.; Luo, Y.; Zhang, L.; Hou, N.; Sun, X. Irisin Protects against Obesity-Related Chronic Kidney Disease by Regulating Perirenal Adipose Tissue Function in Obese Mice. Lipids Health Dis. 2022, 21, 115. [Google Scholar] [CrossRef]
- Dong, H.; Lv, X.; Gao, P.; Hao, Y. Potential role of irisin in lung diseases and advances in research. Front. Pharmacol. 2023, 14, 1307651. [Google Scholar] [CrossRef]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Zhang, Y.; Liu, C.; Wang, Y.; Zhang, L.; Shi, Z.; Wu, Z.; et al. Exercise Hormone Irisin Mitigates Endothelial Barrier Dysfunction and Microvascular Leakage-Related Diseases. JCI Insight 2020, 5, e136277. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Aydin, S.; Ogeturk, M.; Kuloglu, T.; Kavakli, A.; Aydin, S. Effect of Carnosine Supplementation on Apoptosis and Irisin, Total Oxidant and Antioxidants Levels in the Serum, Liver and Lung Tissues in Rats Exposed to Formaldehyde Inhalation. Peptides 2015, 64, 14–23. [Google Scholar] [CrossRef]
- Gür, F.; Timurkaan, S.; Yalçın, M.H.; Tarakci, B. Immunohistochemical Distribution of Irisin in the Lung and Tongue of Porcupine (Hystrix Cristata). Indian J. Anim. Res. 2017, 51, 537–540. [Google Scholar] [CrossRef]
- Bernardes-Ribeiro, M.; Patrone, L.G.A.; Cristina-Silva, C.; Bícego, K.C.; Gargaglioni, L.H. Exercise Derived Myokine Irisin as Mediator of Cardiorespiratory, Metabolic and Thermal Adjustments during Central and Peripheral Chemoreflex Activation. Sci. Rep. 2024, 14, 12262. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, Z.; Liu, Y.; Wang, Z.; Li, Y.; Xu, X.; Chen, C.; Xia, T.; Liao, Q.; Yao, Y.; et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci. Transl. Med. 2017, 9, eaao6298. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jamal, M.; Guo, P.; Jin, Z.; Zheng, F.; Song, X.; Zhan, J.; Wu, H. Irisin Alleviates Pulmonary Epithelial Barrier Dysfunction in Sepsis-Induced Acute Lung Injury via Activation of AMPK/SIRT1 Pathways. Biomed. Pharmacother. 2019, 118, 109363. [Google Scholar] [CrossRef]
- Kubo, H.; Asai, K.; Kojima, K.; Sugitani, A.; Kyomoto, Y.; Okamoto, A.; Yamada, K.; Ijiri, N.; Watanabe, T.; Hirata, K.; et al. Exercise Ameliorates Emphysema Of Cigarette Smoke-Induced COPD In Mice Through The Exercise-Irisin-Nrf2 Axis. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2507–2516. [Google Scholar] [CrossRef]
- Shao, L.; Meng, D.; Yang, F.; Song, H.; Tang, D. Irisin-Mediated Protective Effect on LPS-Induced Acute Lung Injury via Suppressing Inflammation and Apoptosis of Alveolar Epithelial Cells. Biochem. Biophys. Res. Commun. 2017, 487, 194–200. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Asai, K.; Yamada, K.; Kureya, Y.; Ijiri, N.; Watanabe, T.; Kanazawa, H.; Hirata, K. Decreased Levels of Irisin, a Skeletal Muscle Cell-Derived Myokine, Are Related to Emphysema Associated with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 765–772. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Alyami, R.M.; Alhwaikan, A.M.; Alharbi, A.R.; AL-Nafisah, G. Impact of Supervised Exercise Training on Pulmonary Function Parameters, Exercise Capacity and Irisin Biomarker in Interstitial Lung Disease Patients. Pak. J. Med. Sci. 2020, 36, 1089. [Google Scholar] [CrossRef]
- Ding, B.; DiBonaventura, M.; Karlsson, N.; Bergström, G.; Holmgren, U. A Cross-Sectional Assessment of the Burden of COPD Symptoms in the US and Europe Using the National Health and Wellness Survey. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 529–539. [Google Scholar] [CrossRef]
- Papp, C.; Pak, K.; Erdei, T.; Juhasz, B.; Seres, I.; Szentpéteri, A.; Kardos, L.; Szilasi, M.; Gesztelyi, R.; Zsuga, J. Alteration of the Irisin–Brain-Derived Neurotrophic Factor Axis Contributes to Disturbance of Mood in COPD Patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Ghanei, M.; Shirvani, H.; Roshani Koosha, M.S.; Shakibaee, A.; Arabzadeh, E. Exercise Training and Muscle–Lung Crosstalk: The Emerging Roles of Irisin and Semaphorin-3A in Pulmonary Diseases. A Narrative Review. J. Exerc. Organ Cross Talk 2021, 1, 24–28. [Google Scholar] [CrossRef]
- Vina, J.; Sanchis-Gomar, F.; Martinez-Bello, V.; Gomez-Cabrera, M. Exercise Acts as a Drug; the Pharmacological Benefits of Exercise. Br. J. Pharmacol. 2012, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Sallis, R.; Joy, E.; Jaworski, C.A.; Stuhr, R.M.; Trilk, J.L. Exercise Is Medicine. Am. J. Lifestyle Med. 2020, 14, 511–523. [Google Scholar] [CrossRef]
- Villamil-Parra, W.; Moscoso-Loaiza, L. Effects of Physical Exercise on Irisin and BDNF Concentrations, and Their Relationship with Cardiometabolic and Mental Health of Individuals with Metabolic Syndrome: A Systematic Review. Exp. Gerontol. 2024, 198, 112640. [Google Scholar] [CrossRef]
- Łężak, W.; Mokros, Ł.; Kaźmierski, J.M.; Strzelecki, D.; Jerczyńska, H.; Kowalczyk, E.; Pietras, T. Can Irisin Become a Biomarker of Physical Activity, or Another Metabolic Risk Assessment Parameter, in Psychiatric Care Patients? Adv. Psychiatry Neurol. 2021, 29, 205–214. [Google Scholar] [CrossRef]
- Rahi, M.S.; Thilagar, B.; Balaji, S.; Prabhakaran, S.Y.; Mudgal, M.; Rajoo, S.; Yella, P.R.; Satija, P.; Zagorulko, A.; Gunasekaran, K. The Impact of Anxiety and Depression in Chronic Obstructive Pulmonary Disease. Adv. Respir. Med. 2023, 91, 123–134. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting Cellular Senescence as a New Approach to Chronic Obstructive Pulmonary Disease Therapy. Curr. Opin. Pharmacol. 2021, 56, 68–73. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Ijiri, N.; Kanazawa, H.; Asai, K.; Watanabe, T.; Hirata, K. Irisin, a Newly Discovered Myokine, Is a Novel Biomarker Associated with Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Respirology 2015, 20, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cuttitta, G.; Ferraro, M.; Cibella, F.; Alfano, P.; Bucchieri, S.; Patti, A.M.; Muratori, R.; Pace, E.; Bruno, A. Relationship among Body Composition, Adipocytokines, and Irisin on Exercise Capacity and Quality of Life in COPD: A Pilot Study. Biomolecules 2023, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Han, Z.; Yan, J.; Liu, Z.; Meng, A. Expression of Irisin in Pulmonary Rehabilitation of COPD Patients and Its Prognostic Value. Eur. Respir. J. 2024, 64 (Suppl. 68), PA533. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I. Irisin Acts as a Regulator of Macrophages Host Defense. Life Sci. 2017, 176, 21–25. [Google Scholar] [CrossRef]
| References | Group Characteristic | Type of Exercise/Training Characteristic | Irisin | Biological Material/Analytical Methods |
|---|---|---|---|---|
| Boström et al. [11] | Non-diabetic Individuals (n = 8 ♂) | Endurance training (cycloergometer) S: 20–30 min/day 4–5x/week/10 weeks I: ~65% VO2max | ↑ Irisin 2x | Plasma samples Western blot antibodies against Fndc5 were from Abcam, Cambridge, United Kingdom |
| Jędrychowski et al. [17] | Exercised (n = 6 ♂) Age: 25 ± 5 yrs BMI = 24.3 ± 2.5 kg/m2 Sedentary (n = 4 ♂) Age: 26 ± 3 yrs BMI = 26.1 ± 3.4 kg/m2 |
HIAT
S: (4 × 4 min/3x/week/12 weeks; I: >90% VO2peak 3 min rest) separated by 2x/week treadmill; S: 45 min; I: 70% peak aerobic capacity | ↑ Irisin Sedentary: ~3.6 ng/mL Exercised: ~4.3% | Plasma samples Mass spectrometry with control peptides enriched with stable isotopes as internal standards |
| Algul et al. [59] | Trained (n = 60 ♂) Age: 19.2 ± 0.7 yrs BMI: 21.3 ± 0.4 kg/m2 Untrained (n = 30) Age: 19.5 ± 0.6 yrs BMI: 21.7 ± 0.4 kg/m2 |
All the individuals randomly performed two 30-min bouts aerobic/running
I: 64–76% of their predicted maximal. | ↑ Irisin In both groups after the morning exercise and night-time exercise |
Serum samples
ELISA kit; Phoenix Pharmaceuticals Inc., Burlingame, CA, USA) |
| Hew-Butler et al. [60] | Runners (n = 16) 8 ♂ and 8 ♀; (>50 km/week) Nonrunners (n = 17) 8 ♂ and 9 ♀; (<60 min endurance activity) | 10-week supervised run/walk 5 km training program | = Irisin (statistically no significant differences) | Plasma samples ELISA kit; Phoenix Pharmaceuticals, Burlingame, CA, USA |
| Nygaard et al. [61] | Healthy individuals Moderately trained n = 9 (7♂ 2 ♀) Age: 32 ± 9 yrs BMI: 4.5 ± 2.4 kg/m2 FFM: 61 ± 10 kg FAT: 24 ± 6% VO2max = 50 ± 7 mL/kg/min | 60 min endurance (END) 60 min strength (STR) Without exercise (CON) END: One session of HIIT S: 6 × 5 min/2 min of rest; I: ≥18 on the Borg 6–20 scale; STR: Many exercises performed as three sets of 10–12RM | ↑ Irisin Effect of intervention and time; CON: no change in irisin levels from pre to the other points; END: irisin increased at 0 h, 1 h then decreased until 24 h. STR: irisin increased at 1 h and tended to at 2 h 4 h then decreased, until 24 h. STR |
Plasma samples
Measurements- 15 min prior to exercise, 0 h post-exercise, and 1 h, 2 h, 4 h, 6 h and 24 h post-exercise (END, STR); ELISA kits (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) |
| Fernandez-del-Valle et al. [62] | Healthy young adults (n = 26) 14 ♂ and 12 ♀ Age: ♂ 21.18 ± 1.93/ ♀ 21.35 ± 2.52 yrs | HIRT S: 55 min 9x/3 weeks I: 70–80% 1RM | = Irisin compared to baseline | Serum samples ELISA kit; Adipogen Life Sciences, Liestal, Switzerland |
| Murawska—Ciałowicz et al. [63] | Trained (n = 15) Sedentary (Healthy subjects) (n = 10) Age: 32.39 ± 6.63 yrs | HIIT following Tabata protocol 60 min/2x/week/8 weeks; Before training period and after Wingate and GXT were made and level of irisin measured | ↑ Irisin (29.7%) p < 0.05 | Serum samples ELISA kit by BioVendor Laboratorni medicina (Brno, Czech Republic) |
| Shabani et al. [64] | Non-obese women Control (n = 6) Age: 25.50 ± 4.80 yrs RET (n = 10) Age 24.60 ± 2.45 yrs AET (n = 9) Age: 24.66 ± 2.29 yrs CET (n = 10) Age: 26.60 ± 4.00 yrs | RET: 65 min 3x/week/8 week I: 55–75% 1RM AET: step training and running; I: 60–75% age-predicted HRmax; CET: 25 min resistance training + 25 min aerobic training | ↓ Irisin | Serum samples ELISA kit (ZellBio GmbH, Ulm, Germany) |
| Study | Disease | Group | Irisin/ Analytical Methods/ Biological Material | Effects/ Comments |
|---|---|---|---|---|
| Kosmalski et al. [121] | NAFLD+ T2DM | 138 patients—70♂/68 ♀ Age: 65.61 ± 10.44 yrs; (n = 72) In NAFLD group (n = 72), 46 patients with T2DM In without NAFLD group (n = 66), 31 patients with T2DM | ↑ Irisin in NAFLD patients/ ELISA Kit (BioVendor—Laboratorní medicína a.s. Brno, Czech Republic)/ Serum samples | Irisin corelated with BMI, HbA1c, AST, Cr, urea NAFLD risk with irisin level above 3.235 μg/mL was 4.57 times higher than in patients with lower level of irisin |
| Nayak et al. [122] | T2DM with NAFLD | T2DM patients (n = 90) Healthy controls (n = 90) Age: 30–55 yrs Patients divided into 4 groups: T2DM with NAFL T2DM without NAFLD Controls with NAFLD Controls without NAFLD | ↓ Irisin in patients with T2DM compared to health controls. Patients with T2DM with NAFLD had ↓ irisin levels than those without NAFLD/ ELISA kit (Biocodon Technologies, Mission, KS, USA)/ Serum samples | Significant correlations of irisin levels with insulin sensitivity markers such as HOMA-IR and QUICKI across different groups |
| Ulualan et al. [123] | NAFLD | 60 pubertal obese children (31 ♀, 29 ♂), Age: 11–18 yrs 30 of them had NAFLD Control group—healthy children (n = 28; 28 (14 ♀, 14 ♂)) similar in age and sex to the obese group | ↓ Irisin (median) in the obese than in control group; ↓ Irisin (median) in patients with and without NAFLD in comparison to control. No differences between irisin level in patients with and without NAFLD/ ELISA kits (BioVendor Inc., Candler, NC, USA)/ Serum samples | Irisin negatively correlated with BMI, waist, hip and arm circumferences, waist/hip ratio, skinfold thickness, and AST and ALT levels |
| Shanaki et al. [124] | NAFLD T2DM | NAFLD (n = 41) T2DM (n = 41) NAFLD + T2DM (n = 40) Control (n = 40) | ↓ Irisin in NAFLD, T2DM and NAFLD+T2DM compared to control; ↑ Irisin correlated with reduce risk of T2DM, NAFLD and NAFLD+T2DM; ↓ levels of irisin cannot be associated with T2DM and NAFLD; ELISA kit (BioVendor, Brno, Czech Republic)/Plasma samples | Irisin levels negatively correlated with BMI, WHR, visceral fat, HOMA-IR, FBG, insulin, liver stiffness, and liver enzymes; based on multiple stepwise linear regression, ALT and irisin level were independent predictors for liver stiffness |
| Polyzos et al. [125] | NAFL NASH | NAFL (n = 15) NASH (n = 16) Control lean (n = 24) Control obese without NAFLD (n = 28) | ↓ Irisin in obese controls and patients with NAFL and NASH compared with lean controls; there were no differences between irisin level in patients with NAFL, NASH, and obese controls/ELISA kit (Phoenix Pharmaceuticals, CA, USA)/Serum samples | Difference remained significant after adjustment for BMI (or waist circumference), gender, age, insulin resistance (assessed by HOMA-IR or QUICKI), exercise, and time since blood collection |
| Li et al. [126] | T2DM concomitant with MAFLD | T2DM without MAFLD (n = 80) MAFLD without T2DM (n = 62) T2DM with MAFLD (n = 50) Healthy (n = 80) | ↓ Irisin in patients with T2DM with MAFLD compared to T2DM and MAFLD alone ELISA kit R&D Systems, USA) Serum samples | Irisin’s level ↓ as the levels of FPG and FINS increased |
| So et al. [127] | NAFLD | NAFLD (n = 274) Healthy volunteers (n = 37) Subjects with NAFLD divided into 4 groups according to physical activity levels and body adiposity (by BMI = 30): Inactive and Non-obese (n = 99) Inactive and Obese (n = 51) Active and Non-obese (n = 85) Active and Obese (n = 39) 124 active subjects took part in intervention study with a 12-weeks weight loss program | ↓ Irisin in NAFLD than in healthy volunteers ↑ Irisin in active groups than in inactive ELISA kit Serum samples | Hepatic steatosis level inversely correlated with irisin levels In the weight loss program, subjects with ↑ irisin levels had a great reduction in fatty mass, subcutaneous adipose area, γGTP, leptin, and TNFα |
| Study | Disease | Group | Irisin/ Analytical Methods/ Biological Material | Effects/ Comments |
|---|---|---|---|---|
| Liu et al. [167] | T2DM with and without kidney insufficiency | Patients with T2DM across a wide range of renal function (n = 365) | ↓ Irisin in T2DM with renal insufficiency (77.4 ± 13.7 ng/mL in T2DM with eGFR ≥ 60 mL/min/1.73 m(2) versus 72.5 ± 14.9 ng/mL in those with eGFR < 60 mL/min/1.73 m(2), p = 0.001) ELISA kit Phoenix Pharmaceuticals Inc (Burlingame, CA) |
Reduction in irisin level was most pronounced in stage 5 CKD patients.
Irisin in T2DM with preserved renal function correlated with age, and pulse pressure. In patients with renal insufficiency, irisin was correlated with BMI, fat mass, percentage of fat mass, and eGFR. |
| Rodríguez-Carmona et al. [168] | CKD | CKD with peritoneal dialysis or with hemodialysis (n = 95) Healthy control (n = 40) | ↓ Irisin in all CKD groups in comparison to control. Furthermore, patients with peritoneal dialysis had higher serum level of irisin than those on hemodialysis and those managed conservatively ELISA kits Adipogen International, San Diego, USA) | Limited correlations between irisin, on the one hand, and fat (but not lean) mass, GFR, and plasma albumin and bicarbonate. Plasma bicarbonate and GFR identified as independent predictors of irisin levels. |
| Sadeghi Shad et al. [169] | CKD | CKD patients in stage 2 and stage 4 (n = 90) Stage 2 (n = 45) Stage 4 (n = 45) | ↓ Irisin in patients in stage 4 compared with patients at stage 2 (ELISA) kit (Zell BioGmbH, Germany | Serum irisin, GFR, Alb, HDL and Hb levels significantly ↓ Inversely, Cr, TG, LDL, FBS, BUN, and urea significantly ↑ from stage 2 to stage 4. These findings suggest that irisin may be involved in the regulation of biochemical factor levels in CKD. |
| Shelbaya et al. [170] | T2DM |
T2DM (n = 60)
Healthy subjects (n = 30) T2DM patients divided: Without diabetic complications (n = 30) With diabetic nephropathy (DN) (n = 30) | ↓ Irisin in diabetic patients compared to controls ↓ Irisin in diabetic patients with DN compared to those without complications |
There was a statistically significant negative correlation between irisin and serum Cr, SBP, DBP duration of diabetes, BMI albumin/creatinine ratio, and HbA1c in all T2DM patients.
Duration of diabetes was the only independent determinant of irisin level. |
| Study | Group | Irisin/Analytical Methods/ Biological Material | Effects/Comments |
|---|---|---|---|
| Papp et al. [196] | COPD (n = 74) Quality of life was evaluated by Saint George’s Respiratory Questionnaire (SGRQ) | ↓ Irisin in COPD patients with mood disturbances (ELISA) kit (Phoenix Pharmaceuticals, Burlingame, CA, USA)/Serum samples | Association between low irisin level and mood disturbances was stronger among patients with the lower level of BDNF and weaker in the higher level of BDNF. |
| Ijiri et al. [205] | COPD patients (n = 72) Healthy control (n = 27) 8 weeks of physical training | ↓ Irisin in COPD patients than in healthy subjects (ELISA) kit (Phoenix Pharmaceuticals, Burlingame, CA, USA)/Serum | Irisin not correlated with pulmonary function parameters. Irisin level associated with physical activity level in all subjects. 8-week training was linked to increase in irisin level. |
| Sugiyama et al. [192] | COPD patients (n = 40) | ↓ Irisin in COPD patients ELISA kit Phoenix Pharmaceuticals, Burlingame, CA, USA/Serum | Decreased serum irisin levels are related to emphysema in patients with COPD and involved. |
| Cuttitta et al. [206] | COPD patients (n = 25) Smokers and nonsmokers Healthy subjects (n = 26) | ↓ Irisin in COPD patients who are smokers than in nonsmokers ELISA kit (Cusabio, Houston, TX, 77054, USA) | Irisin/muscle mass ratio higher in non-smokers vs. smokers and in females vs. males. |
| Ma et al. [207] | Patients with COPD Before and after pulmonary rehabilitation (n = 68) Healthy control (n = 35) | ↓ Irisin in COPD patients than in healthy control group ELISA/serum | ↑ Irisin after physiotherapy rehabilitation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciałowicz, M.; Woźniewski, M.; Murawska-Ciałowicz, E.; Dzięgiel, P. The Influence of Irisin on Selected Organs—The Liver, Kidneys, and Lungs: The Role of Physical Exercise. Cells 2025, 14, 1228. https://doi.org/10.3390/cells14161228
Ciałowicz M, Woźniewski M, Murawska-Ciałowicz E, Dzięgiel P. The Influence of Irisin on Selected Organs—The Liver, Kidneys, and Lungs: The Role of Physical Exercise. Cells. 2025; 14(16):1228. https://doi.org/10.3390/cells14161228
Chicago/Turabian StyleCiałowicz, Maria, Marek Woźniewski, Eugenia Murawska-Ciałowicz, and Piotr Dzięgiel. 2025. "The Influence of Irisin on Selected Organs—The Liver, Kidneys, and Lungs: The Role of Physical Exercise" Cells 14, no. 16: 1228. https://doi.org/10.3390/cells14161228
APA StyleCiałowicz, M., Woźniewski, M., Murawska-Ciałowicz, E., & Dzięgiel, P. (2025). The Influence of Irisin on Selected Organs—The Liver, Kidneys, and Lungs: The Role of Physical Exercise. Cells, 14(16), 1228. https://doi.org/10.3390/cells14161228







