A Better Understanding of Atrial-like and Ventricular-like Action Potentials in Stem Cell-Derived Cardiomyocytes: The Underestimated Role of the L-Type Ca2+ Current
Abstract
1. Introduction
2. Materials and Methods
2.1. hESC Maintenance, Differentiation, and Dissociation
2.1.1. hESC Maintenance and Differentiation to Cardiomyocytes
2.1.2. Single Cell Preparation
2.2. Patch Clamp Experiments
2.2.1. Data Acquisition
2.2.2. Action Potentials
2.2.3. Membrane Currents
2.3. Intracellular Ca2+ Measurements
2.4. Statistics
3. Results
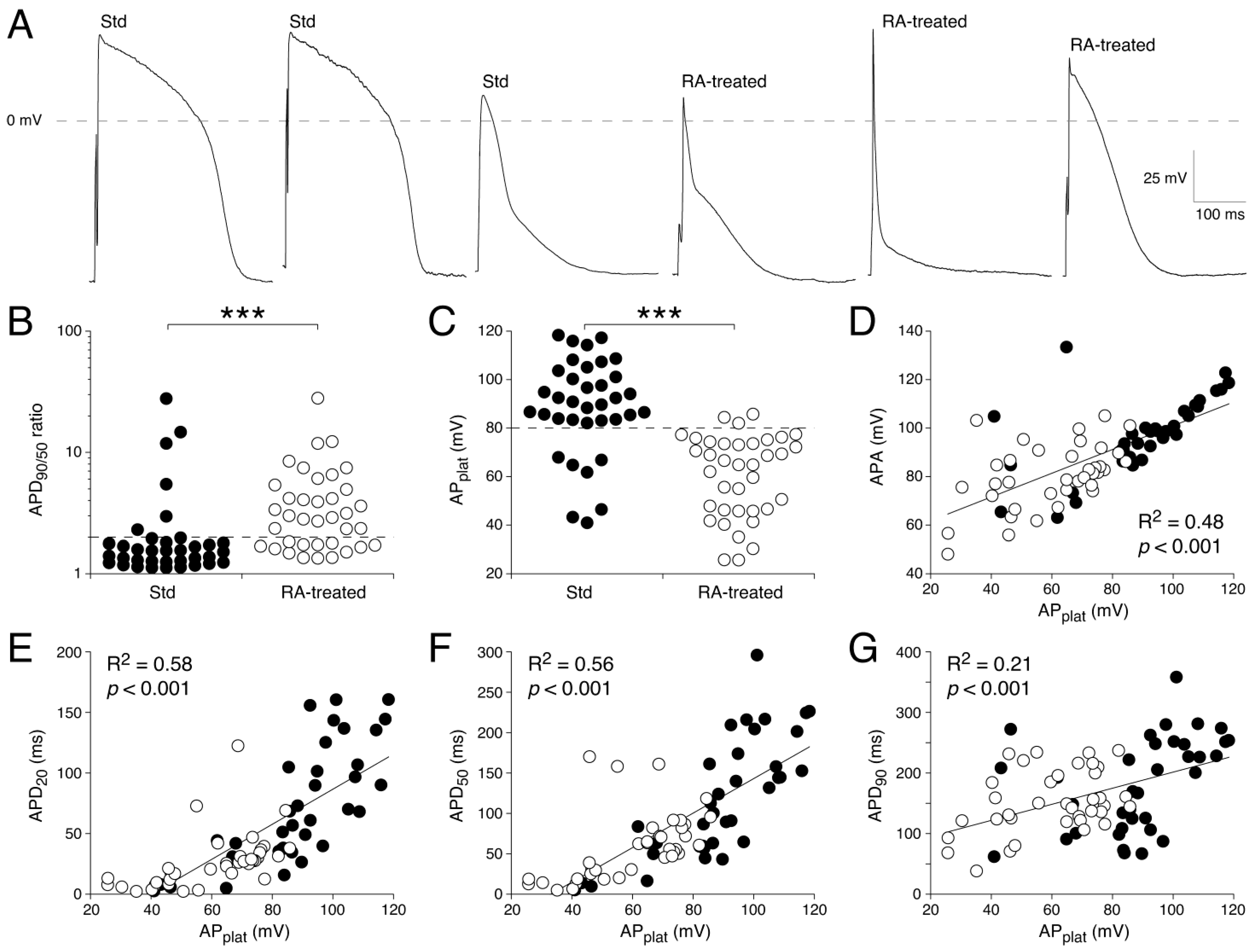
3.1. Action Potential Waveforms Explained by Ionic Currents
3.1.1. AP Parameters in Std and RA-Treated hESC-CM Populations
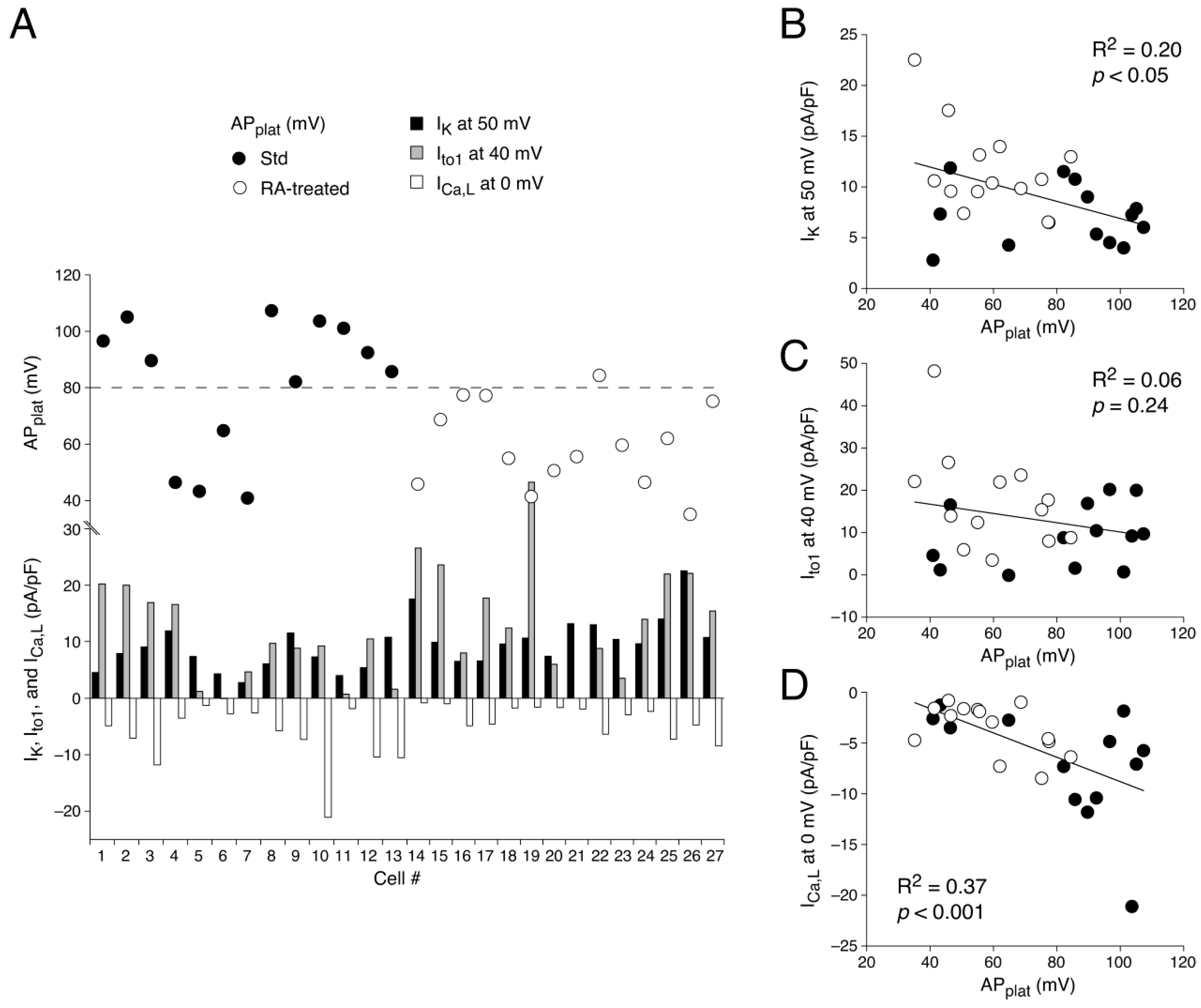
3.1.2. Net Membrane Currents in Std and RA-Treated hESC-CM Populations
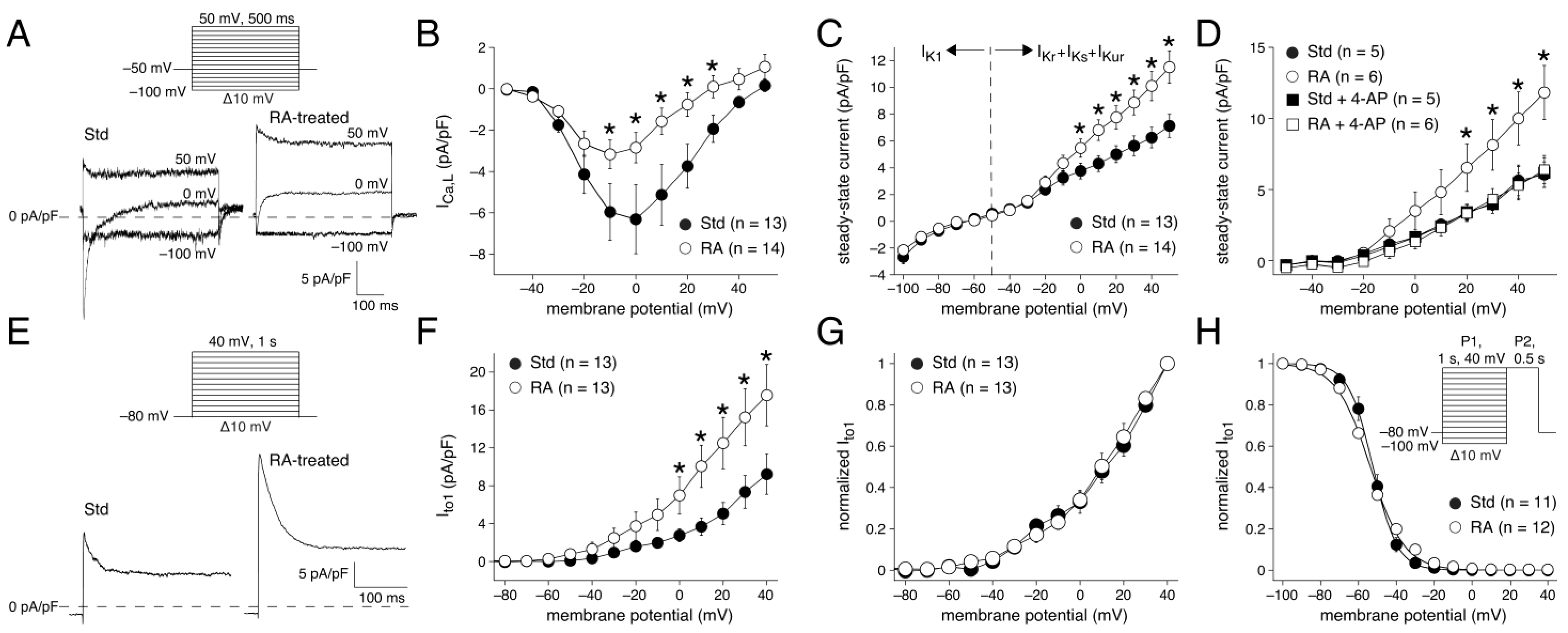
3.1.3. AP Waveforms Related to Individual Membrane Currents
3.2. Influx and Efflux of Ca2+ Contribute to Differences in AP Shape
3.2.1. ICa,L Determined by Square-Step Voltage Clamp Protocols
3.2.2. ICa,L Determined by AP Clamp Protocols
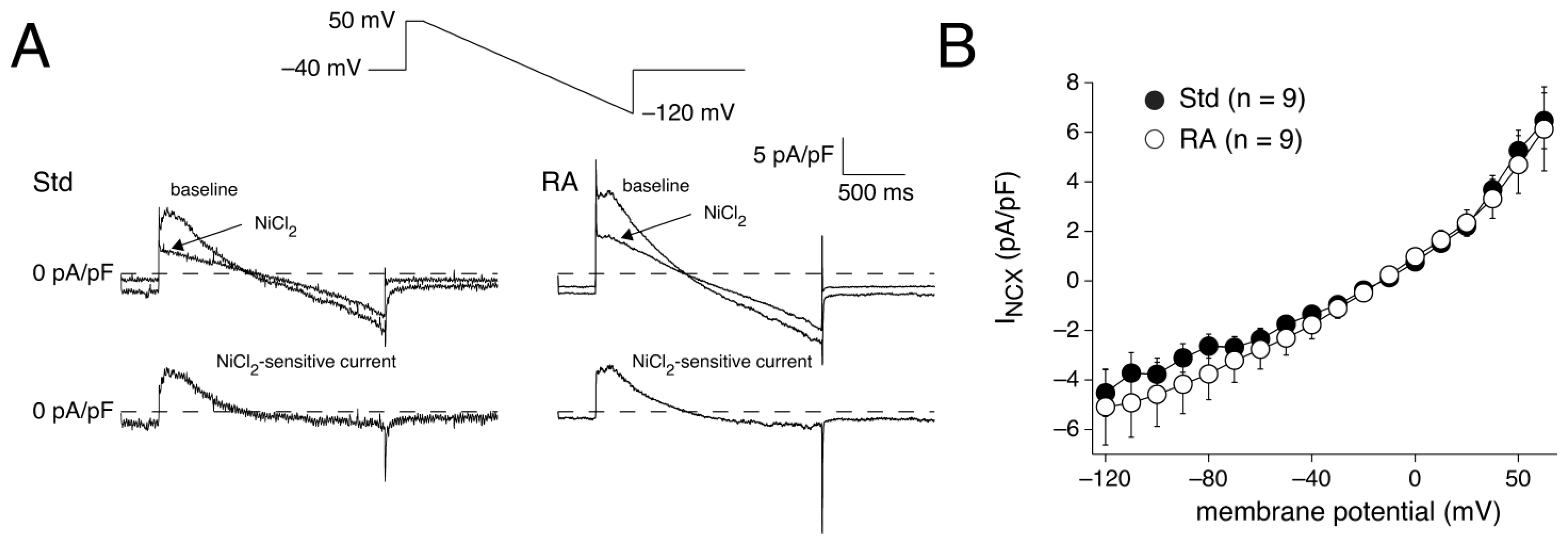
3.2.3. INCX Densities in Std and RA-Treated hESC-CMs
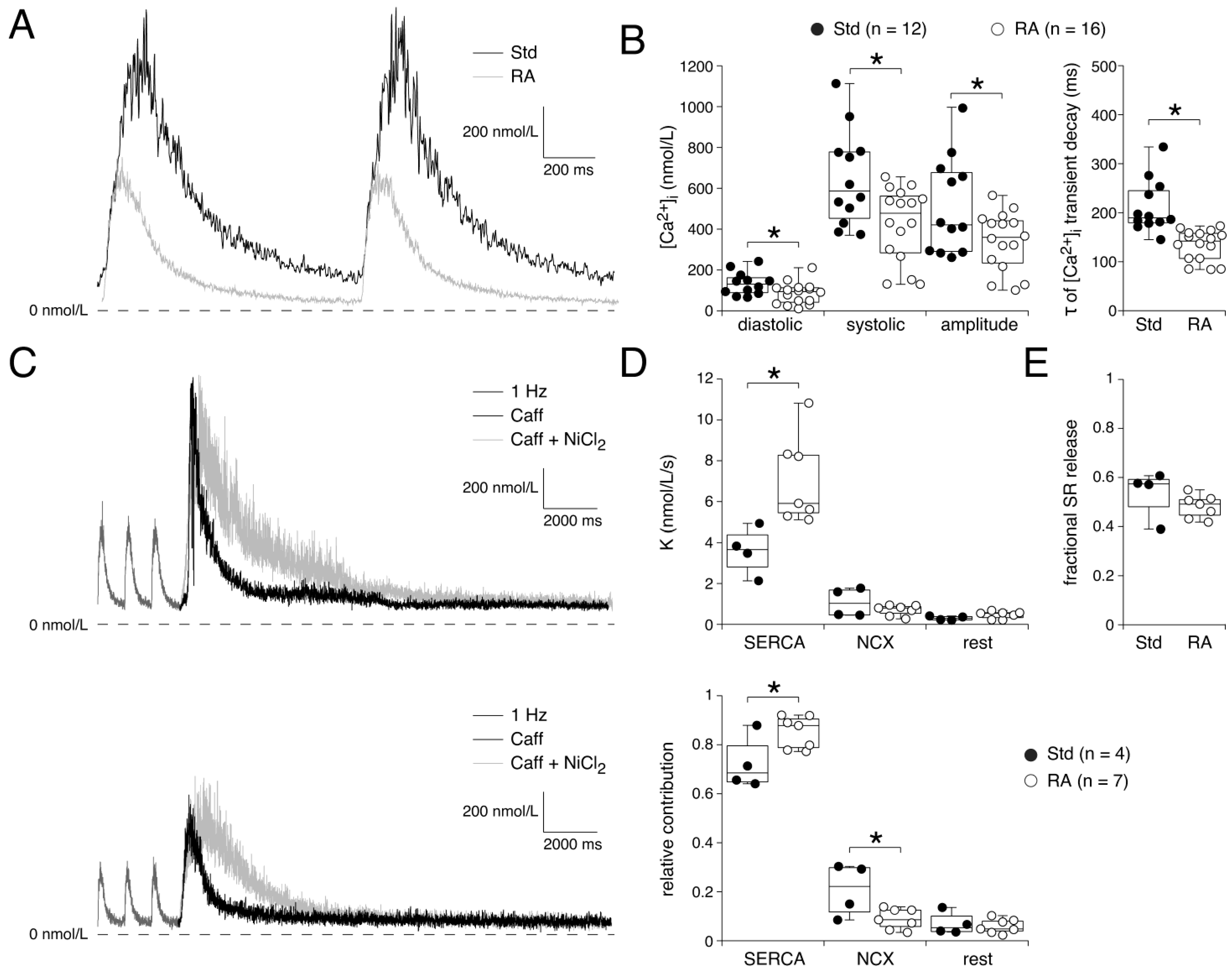
3.3. Ca2+ Transients Reflect Ionic Current Differences During A- and V-like AP Shapes
4. Discussion
4.1. Overview
4.2. RA Treatment to Increase the Amount of CMs with an Atrial Fate
4.3. A- and V-like APs Explained by Membrane Current Differences
4.3.1. MDP and IK1
4.3.2. dV/dtmax and INa
4.3.3. AP Repolarization and IKur
4.3.4. AP Repolarization and Ito1 Differences
4.3.5. AP Repolarization and ICa,L Differences
4.3.6. AP Repolarization and INCX
4.4. [Ca2+]i Transient Differences
4.5. Spontaneous Beating Rate
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef]
- Brandão, K.O.; Tabel, V.A.; Atsma, D.E.; Mummery, C.L.; Davis, R.P. Human pluripotent stem cell models of cardiac disease: From mechanisms to therapies. Dis. Model. Mech. 2017, 10, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, L.; Grandela, C.; Mummery, C.L.; Davis, R.P. Inherited cardiac diseases, pluripotent stem cells, and genome editing combined—The past, present, and future. Stem Cells 2020, 38, 174–186. [Google Scholar] [CrossRef]
- Parrotta, E.I.; Lucchino, V.; Scaramuzzino, L.; Scalise, S.; Cuda, G. Modeling cardiac disease mechanisms using induced pluripotent stem cell-derived cardiomyocytes: Progress, promises and challenges. Int. J. Mol. Sci. 2020, 21, 4354. [Google Scholar] [CrossRef]
- Correia, C.D.; Ferreira, A.; Fernandes, M.T.; Silva, B.M.; Esteves, F.; Leitão, H.S.; Bragança, J.; Calado, S.M. Human stem cells for cardiac disease modeling and preclinical and clinical applications—Are we on the road to success? Cells 2023, 12, 1727. [Google Scholar] [CrossRef]
- Joukar, S. A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: Extrapolation of experimental insights to clinic. Lab. Anim. Res. 2021, 37, 25. [Google Scholar] [CrossRef]
- Ripplinger, C.M.; Glukhov, A.V.; Kay, M.W.; Boukens, B.J.; Chiamvimonvat, N.; Delisle, B.P.; Fabritz, L.; Hund, T.J.; Knollmann, B.C.; Li, N.; et al. Guidelines for assessment of cardiac electrophysiology and arrhythmias in small animals. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1137–H1166. [Google Scholar] [CrossRef] [PubMed]
- Kamga, M.V.K.; Reppel, M.; Hescheler, J.; Nguemo, F. Modeling genetic cardiac channelopathies using induced pluripotent stem cells—Status quo from an electrophysiological perspective. Biochem. Pharmacol. 2021, 192, 114746. [Google Scholar] [CrossRef] [PubMed]
- Laurita, K.R.; Vasireddi, S.K.; Mackall, J.A. Elucidating arrhythmogenic right ventricular cardiomyopathy with stem cells. Birth Defects Res. 2022, 114, 948–958. [Google Scholar] [CrossRef]
- Reisqs, J.B.; Moreau, A.; Sleiman, Y.; Boutjdir, M.; Richard, S.; Chevalier, P. Arrhythmogenic cardiomyopathy as a myogenic disease: Highlights from cardiomyocytes derived from human induced pluripotent stem cells. Front. Physiol. 2023, 14, 1191965. [Google Scholar] [CrossRef]
- Velichkova, G.; Dobreva, G. Human pluripotent stem cell-based models of heart development and disease. Cells Dev. 2023, 175, 203857. [Google Scholar] [CrossRef]
- Ovics, P.; Regev, D.; Baskin, P.; Davidor, M.; Shemer, Y.; Neeman, S.; Ben-Haim, Y.; Binah, O. Drug development and the use of induced pluripotent stem cell-derived cardiomyocytes for disease modeling and drug toxicity screening. Int. J. Mol. Sci. 2020, 21, 7320. [Google Scholar] [CrossRef]
- Garg, P.; Garg, V.; Shrestha, R.; Sanguinetti, M.C.; Kamp, T.J.; Wu, J.C. Human induced pluripotent stem cell-derived cardiomyocytes as models for cardiac channelopathies: A primer for non-electrophysiologists. Circ. Res. 2018, 123, 224–243. [Google Scholar] [CrossRef]
- Sala, L.; Van Meer, B.J.; Tertoolen, L.G.J.; Bakkers, J.; Bellin, M.; Davis, R.P.; Denning, C.; Dieben, M.A.E.; Eschenhagen, T.; Giacomelli, E.; et al. MUSCLEMOTION: A versatile open software tool to quantify cardiomyocyte and cardiac muscle contraction in vitro and in vivo. Circ. Res. 2018, 122, e5–e16. [Google Scholar] [CrossRef]
- Lodrini, A.M.; Barile, L.; Rocchetti, M.; Altomare, C. Human induced pluripotent stem cells derived from a cardiac somatic source: Insights for an in-vitro cardiomyocyte platform. Int. J. Mol. Sci. 2020, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Takasuna, K.; Kazusa, K.; Hayakawa, T. Comprehensive cardiac safety assessment using hiPS-cardiomyocytes (Consortium for Safety Assessment using Human iPS cells: CSAHi). Curr. Pharm. Biotechnol. 2020, 21, 829–841. [Google Scholar] [CrossRef]
- Djemai, M.; Cupelli, M.; Boutjdir, M.; Chahine, M. Optical mapping of cardiomyocytes in monolayer derived from induced pluripotent stem cells. Cells 2023, 12, 2168. [Google Scholar] [CrossRef]
- Melgari, D.; Calamaio, S.; Frosio, A.; Prevostini, R.; Anastasia, L.; Pappone, C.; Rivolta, I. Automated patch-clamp and induced pluripotent stem cell-derived cardiomyocytes: A synergistic approach in the study of Brugada syndrome. Int. J. Mol. Sci. 2023, 24, 6687. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.L.; Testa, G. Taming human genetic variability: Transcriptomic meta-analysis guides the experimental design and interpretation of iPSC-based disease modeling. Stem Cell Rep. 2017, 8, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Millard, D.; Dang, Q.; Shi, H.; Zhang, X.; Strock, C.; Kraushaar, U.; Zeng, H.; Levesque, P.; Lu, H.R.; Guillon, J.M.; et al. Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: Results from a blinded CiPA pilot study. Toxicol. Sci. 2018, 164, 550–562. [Google Scholar] [CrossRef]
- Guerrelli, D.; Pressman, J.; Salameh, S.; Posnack, N. hiPSC-CM electrophysiology: Impact of temporal changes and study parameters on experimental reproducibility. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H12–H27. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.L.; Zhang, J.; Ng, E.S.; Elliott, D.A.; Elefanty, A.G.; Kamp, T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef]
- Devalla, H.D.; Passier, R. Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Sci. Transl. Med. 2018, 10, eaah5457. [Google Scholar] [CrossRef]
- Campostrini, G.; Windt, L.M.; Van Meer, B.J.; Bellin, M.; Mummery, C.L. Cardiac tissues from stem cells: New routes to maturation and cardiac regeneration. Circ. Res. 2021, 128, 775–801. [Google Scholar] [CrossRef]
- Streckfuss-Bömeke, K.; Wolf, F.; Azizian, A.; Stauske, M.; Tiburcy, M.; Wagner, S.; Hübscher, D.; Dressel, R.; Chen, S.; Jende, J.; et al. Comparative study of human-induced pluripotent stem cells derived from bone marrow cells, hair keratinocytes, and skin fibroblasts. Eur. Heart J. 2013, 34, 2618–2629. [Google Scholar] [CrossRef]
- Itzhaki, I.; Maizels, L.; Huber, I.; Zwi-Dantsis, L.; Caspi, O.; Winterstern, A.; Feldman, O.; Gepstein, A.; Arbel, G.; Hammerman, H.; et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011, 471, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, I.; Maizels, L.; Huber, I.; Gepstein, A.; Arbel, G.; Caspi, O.; Miller, L.; Belhassen, B.; Nof, E.; Glikson, M.; et al. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 2012, 60, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Stauske, M.; Luo, X.; Wagner, S.; Vollrath, M.; Mehnert, C.S.; Schubert, M.; Cyganek, L.; Chen, S.; Hasheminasab, S.M.; et al. Disease phenotypes and mechanisms of iPSC-derived cardiomyocytes from Brugada syndrome patients with a loss-of-function SCN5A mutation. Front. Cell Dev. Biol. 2020, 8, 592893. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Ng, K.-M.; Lai, W.-H.; Chan, Y.-C.; Lau, Y.-M.; Lian, Q.; Tse, H.-F.; Siu, C.-W. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev. Rep. 2011, 7, 976–986. [Google Scholar] [CrossRef]
- Lan, F.; Lee, A.S.; Liang, P.; Sanchez-Freire, V.; Nguyen, P.K.; Wang, L.; Han, L.; Yen, M.; Wang, Y.; Sun, N.; et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013, 12, 101–113. [Google Scholar] [CrossRef]
- Laksman, Z.; Wauchop, M.; Lin, E.; Protze, S.; Lee, J.; Yang, W.; Izaddoustdar, F.; Shafaattalab, S.; Gepstein, L.; Tibbits, G.F.; et al. Modeling atrial fibrillation using human embryonic stem cell-derived atrial tissue. Sci. Rep. 2017, 7, 5268. [Google Scholar] [CrossRef]
- De la Roche, J.; Angsutararux, P.; Kempf, H.; Janan, M.; Bolesani, E.; Thiemann, S.; Wojciechowski, D.; Coffee, M.; Franke, A.; Schwanke, K.; et al. Comparing human iPSC-cardiomyocytes versus HEK293T cells unveils disease-causing effects of Brugada mutation A735V of NaV1.5 sodium channels. Sci. Rep. 2019, 9, 11173. [Google Scholar] [CrossRef]
- Peng, S.; Lacerda, A.E.; Kirsch, G.E.; Brown, A.M.; Bruening-Wright, A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J. Pharmacol. Toxicol. Methods 2010, 61, 277–286. [Google Scholar] [CrossRef]
- Pei, F.; Jiang, J.; Bai, S.; Cao, H.; Tian, L.; Zhao, Y.; Yang, C.; Dong, H.; Ma, Y. Chemical-defined and albumin-free generation of human atrial and ventricular myocytes from human pluripotent stem cells. Stem Cell Res. 2017, 19, 94–103. [Google Scholar] [CrossRef]
- Chapotte-Baldacci, C.A.; Pierre, M.; Djemai, M.; Pouliot, V.; Chahine, M. Biophysical properties of NaV1.5 channels from atrial-like and ventricular-like cardiomyocytes derived from human induced pluripotent stem cells. Sci. Rep. 2023, 13, 20685. [Google Scholar] [CrossRef]
- Jara-Avaca, M.; Kempf, H.; Rückert, M.; Robles-Diaz, D.; Franke, A.; De la Roche, J.; Fischer, M.; Malan, D.; Sasse, P.; Solodenko, W.; et al. EBIO does not induce cardiomyogenesis in human pluripotent stem cells but modulates cardiac subtype enrichment by lineage-selective survival. Stem Cell Rep. 2017, 8, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Bett, G.C.L.; Kaplan, A.D.; Lis, A.; Cimato, T.R.; Tzanakakis, E.S.; Zhou, Q.; Morales, M.J.; Rasmusson, R.L. Electronic “expression” of the inward rectifier in cardiocytes derived from human-induced pluripotent stem cells. Heart Rhythm 2013, 10, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Yang, L.; Lin, B.; Zhu, X.; Sun, B.; Kaplan, A.D.; Bett, G.C.L.; Rasmusson, R.L.; London, B.; Salama, G. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2015, 81, 81–93. [Google Scholar] [CrossRef]
- Altomare, C.; Bartolucci, C.; Sala, L.; Balbi, C.; Burrello, J.; Pietrogiovanna, N.; Burrello, A.; Bolis, S.; Panella, S.; Arici, M.; et al. A dynamic clamping approach using in silico IK1 current for discrimination of chamber-specific hiPSC-derived cardiomyocytes. Commun. Biol. 2023, 6, 291. [Google Scholar] [CrossRef]
- Moretti, A.; Bellin, M.; Welling, A.; Jung, C.B.; Lam, J.T.; Bott-Flügel, L.; Dorn, T.; Goedel, A.; Höhnke, C.; Hofmann, F.; et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010, 363, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- El-Battrawy, I.; Lang, S.; Zhao, Z.; Akin, I.; Yücel, G.; Meister, S.; Patocskai, B.; Behnes, M.; Rudic, B.; Tülümen, E.; et al. Hyperthermia influences the effects of sodium channel blocking drugs in human-induced pluripotent stem cell-derived cardiomyocytes. PLoS ONE 2016, 11, e0166143. [Google Scholar] [CrossRef]
- Matsa, E.; Rajamohan, D.; Dick, E.; Young, L.; Mellor, I.; Staniforth, A.; Denning, C. Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. Eur. Heart J. 2011, 32, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Hayano, M.; Makiyama, T.; Kamakura, T.; Watanabe, H.; Sasaki, K.; Funakoshi, S.; Wuriyanghai, Y.; Nishiuchi, S.; Harita, T.; Yamamoto, Y.; et al. Development of a patient-derived induced pluripotent stem cell model for the investigation of SCN5A-D1275N-related cardiac sodium channelopathy. Circ. J. 2017, 81, 1783–1791. [Google Scholar] [CrossRef]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Lambers, E.; Hong, L.; Sridhar, A.; Zhang, M.; Chalazan, B.; Menon, A.; Savio-Galimberti, E.; Wu, J.C.; Rehman, J.; et al. Electrophysiologic characterization of calcium handling in human induced pluripotent stem cell-derived atrial cardiomyocytes. Stem Cell Rep. 2018, 10, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Nesterenko, V.V.; Sicouri, S.; Goodrow, R.J., Jr.; Treat, J.A.; Desai, M.; Wu, Y.; Doss, M.X.; Antzelevitch, C.; Di Diego, J.M. Identification and characterization of a transient outward K+ current in human induced pluripotent stem cell-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2013, 60, 36–46. [Google Scholar] [CrossRef]
- Zhang, M.; Schulte, J.S.; Heinick, A.; Piccini, I.; Rao, J.; Quaranta, R.; Zeuschner, D.; Malan, D.; Kim, K.-P.; Röpke, A.; et al. Universal cardiac induction of human pluripotent stem cells in two and three-dimensional formats: Implications for in vitro maturation. Stem Cells 2015, 33, 1456–1469. [Google Scholar] [CrossRef]
- Ma, D.; Wei, H.; Zhao, Y.; Lu, J.; Li, G.; Sahib, N.B.; Tan, T.H.; Wong, K.Y.; Shim, W.; Wong, P.; et al. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int. J. Cardiol. 2013, 168, 5277–5286. [Google Scholar] [CrossRef]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzi, K.; Elliot, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
- Gorospe, G.; Zhu, R.; Millrod, M.A.; Zambidis, E.T.; Tung, L.; Vidal, R. Automated grouping of action potentials of human embryonic stem cell-derived cardiomyocytes. IEEE Trans. Biomed. Eng. 2014, 61, 2389–2395. [Google Scholar] [CrossRef]
- Du, D.T.M.; Hellen, N.; Kane, C.; Terracciano, C.M.N. Action potential morphology of human induced pluripotent stem cell-derived cardiomyocytes does not predict cardiac chamber specificity and is dependent on cell density. Biophys. J. 2015, 108, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.; Du, D.T.M.; Hellen, N.; Terracino, C.M. The fallacy of assigning chamber specificity to iPSC cardiac myocytes from action potential morphology. Biophys. J. 2016, 110, 281–283. [Google Scholar] [CrossRef]
- Bett, G.C.L.; Kaplan, A.D.; Rasmusson, R.L. Action potential shape is a crucial measure of cell type of stem cell-derived cardiocytes. Biophys. J. 2016, 110, 284–286. [Google Scholar] [CrossRef]
- Choi, S.W.; Lee, H.-A.; Moon, S.-H.; Park, S.-J.; Kim, H.J.; Kim, K.-S.; Zhang, Y.H.; Youm, J.B.; Kim, S.J. Spontaneous inward currents reflecting oscillatory activation of Na+/Ca2+ exchangers in human embryonic stem cell-derived cardiomyocytes. Pflügers Arch. 2016, 468, 609–622. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Tertoolen, L.G.; Guadix, J.A.; Bellin, M.; Kosmidis, G.; D’Aniello, C.; Monshouwer-Kloots, J.; Goumans, M.J.; Wang, Y.L.; Feinberg, A.W.; et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro—Correlation between contraction force and electrophysiology. Biomaterials 2015, 51, 138–150. [Google Scholar] [CrossRef]
- Sala, L.; Bellin, M.; Mummery, C.L. Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: Has the time come? Br. J. Pharmacol. 2017, 174, 3749–3765. [Google Scholar] [CrossRef]
- Prajapati, C.; Ojala, M.; Lappi, H.; Aalto-Setälä, K.; Pekkanen-Mattila, M. Electrophysiological evaluation of human induced pluripotent stem cell-derived cardiomyocytes obtained by different methods. Stem Cell Res. 2021, 51, 102176. [Google Scholar] [CrossRef] [PubMed]
- West, T.C.; Amory, D.W. Single fiber recording of the effects of quinidine at atrial and pacemaker sites in the isolated right atrium of the rabbit. J. Pharmacol. Exp. Ther. 1960, 130, 183–193. [Google Scholar] [CrossRef]
- Grant, A.O. Cardiac ion channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Bueno-Orovio, A.; Wettwer, E.; Loose, S.; Simon, J.; Ravens, U.; Pueyo, E.; Rodriguez, B. Inter-subject variability in human atrial action potential in sinus rhythm versus chronic atrial fibrillation. PLoS ONE 2014, 9, e105897. [Google Scholar] [CrossRef] [PubMed]
- Schwach, V.; Verkerk, A.O.; Mol, M.; Monshouwer-Kloots, J.J.; Devalla, H.D.; Orlova, V.V.; Anastassiadis, K.; Mummery, C.L.; Davis, R.P.; Passier, R. A COUP-TFII human embryonic stem cell reporter line to identify and select atrial cardiomyocytes. Stem Cell Rep. 2017, 9, 1765–1779. [Google Scholar] [CrossRef]
- Hilderink, S.; Devalla, H.D.; Bosch, L.; Wilders, R.; Verkerk, A.O. Ultrarapid delayed rectifier K+ channelopathies in human induced pluripotent stem cell-derived cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 536. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Marchal, G.A.; Zegers, J.G.; Kawasaki, M.; Driessen, A.H.G.; Remme, C.A.; De Groot, J.R.; Wilders, R. Patch-clamp recordings of action potentials from human atrial myocytes: Optimization through dynamic clamp. Front. Pharmacol. 2021, 12, 649414. [Google Scholar] [CrossRef]
- Sheng, X.; Reppel, M.; Nguemo, F.; Mohammad, F.I.; Kuzmenkin, A.; Hescheler, J.; Pfannkuche, K. Human pluripotent stem cell-derived cardiomyocytes: Response to TTX and lidocain reveals strong cell to cell variability. PLoS ONE 2012, 7, e45963. [Google Scholar] [CrossRef]
- Seibertz, F.; Sutanto, H.; Dülk, R.; Pronto, J.R.D.; Springer, R.; Rapedius, M.; Liutkute, A.; Ritter, M.; Jung, P.; Stelzer, L.; et al. Electrophysiological and calcium-handling development during long-term culture of human-induced pluripotent stem cell-derived cardiomyocytes. Basic Res. Cardiol. 2023, 118, 14. [Google Scholar] [CrossRef]
- Cofiño-Fabres, C.; Passier, R.; Schwach, V. Towards improved human in vitro models for cardiac arrhythmia: Disease mechanisms, treatment, and models of atrial fibrillation. Biomedicines 2023, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Biendarra-Tiegs, S.M.; Li, X.; Ye, D.; Brandt, E.B.; Ackerman, M.J.; Nelson, T.J. Single-cell RNA-sequencing and optical electrophysiology of human induced pluripotent stem cell-derived cardiomyocytes reveal discordance between cardiac subtype-associated gene expression patterns and electrophysiological phenotypes. Stem Cells Dev. 2019, 28, 659–673. [Google Scholar] [CrossRef] [PubMed]
- Lieu, D.K.; Fu, J.D.; Chiamvimonvat, N.; Tung, K.C.; McNerney, G.P.; Huser, T.; Keller, G.; Kong, C.-W.; Li, R.A. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythm. Electrophysiol. 2013, 6, 191–201. [Google Scholar] [CrossRef]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2016, 35, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, P.A.; Darche, F.F.; Ullrich, N.D.; Geschwill, P.; Greber, B.; Rivinius, R.; Seyler, C.; Müller-Decker, K.; Draguhn, A.; Utikal, J.; et al. Subtype-specific differentiation of cardiac pacemaker cell clusters from human induced pluripotent stem cells. Stem Cell Res. Ther. 2017, 8, 229. [Google Scholar] [CrossRef]
- Wiesinger, A.; Li, J.; Fokkert, L.; Bakker, P.; Verkerk, A.O.; Christoffels, V.M.; Boink, G.J.J.; Devalla, H.D. A single cell transcriptional roadmap of human pacemaker cell differentiation. eLife 2022, 11, e76781. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wiesinger, A.; Fokkert, L.; Boukens, B.J.; Verkerk, A.O.; Christoffels, V.M.; Boink, G.J.J.; Devalla, H.D. Molecular and electrophysiological evaluation of human cardiomyocyte subtypes to facilitate generation of composite cardiac models. J. Tissue Eng. 2022, 13, 20417314221127908. [Google Scholar] [CrossRef]
- Wang, F.; Yin, L.; Zhang, W.; Tang, Y.; Wang, X.; Huang, C. The method of sinus node-like pacemaker cells from human induced pluripotent stem cells by BMP and Wnt signaling. Cell Biol. Toxicol. 2023, 39, 2725–2741. [Google Scholar] [CrossRef]
- Lee, J.H.; Protze, S.I.; Laksman, Z.; Backx, P.H.; Keller, G.M. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell 2017, 21, 179–194.e4. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Veerman, C.C.; Zegers, J.G.; Mengarelli, I.; Bezzina, C.R.; Wilders, R. Patch-clamp recording from human induced pluripotent stem cell-derived cardiomyocytes: Improving action potential characteristics through dynamic clamp. Int. J. Mol. Sci. 2017, 18, 1873. [Google Scholar] [CrossRef]
- Marczenke, M.; Piccini, I.; Mengarelli, I.; Fell, J.; Röpke, A.; Seebohm, G.; Verkerk, A.O.; Greber, B. Cardiac subtype-specific modeling of Kv1.5 ion channel deficiency using human pluripotent stem cells. Front. Physiol. 2017, 8, 469. [Google Scholar] [CrossRef]
- Cyganek, L.; Tiburcy, M.; Sekeres, K.; Gerstenberg, K.; Bohnenberger, H.; Lenz, C.; Henze, S.; Stauske, M.; Salinas, G.; Zimmermann, W.H.; et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight 2018, 3, e99941. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, A.; Boink, G.J.J.; Christoffels, V.M.; Devalla, H.D. Retinoic acid signaling in heart development: Application in the differentiation of cardiovascular lineages from human pluripotent stem cells. Stem Cell Rep. 2021, 16, 2589–2606. [Google Scholar] [CrossRef]
- Elliott, D.A.; Braam, S.R.; Koutsis, K.; Ng, E.S.; Jenny, R.; Lagerqvist, E.L.; Biben, C.; Hatzistavrou, T.; Hirst, C.E.; Yu, Q.C.; et al. NKX2-5eGFP/w hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods 2011, 8, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Bairoch, A. The Cellosaurus, a cell-line knowledge resource. J. Biomol. Tech. 2018, 29, 25–38. [Google Scholar] [CrossRef]
- Ng, E.S.; Davis, R.; Stanley, E.G.; Elefanty, A.G. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat. Protoc. 2008, 3, 768–776. [Google Scholar] [CrossRef]
- Van Ginneken, A.C.G.; Giles, W. Voltage clamp measurements of the hyperpolarization-activated inward current If in single cells from rabbit sino-atrial node. J. Physiol. 1991, 434, 57–83. [Google Scholar] [CrossRef]
- Davis, R.P.; Casini, S.; Van den Berg, C.W.; Hoekstra, M.; Remme, C.A.; Dambrot, C.; Salvatori, D.; Oostwaard, D.W.; Wilde, A.A.M.; Bezzina, C.R.; et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 2012, 125, 3079–3091. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R. Injection of IK1 through dynamic clamp can make all the difference in patch-clamp studies on hiPSC-derived cardiomyocytes. Front. Physiol. 2023, 14, 1326160. [Google Scholar] [CrossRef]
- Barry, P.H.; Lynch, J.W. Liquid junction potentials and small cell effects in patch-clamp analysis. J. Membr. Biol. 1991, 121, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Hobai, I.A.; Bates, J.A.; Howarth, F.C.; Levi, A.J. Inhibition by external Cd2+ of Na/Ca exchange and L-type Ca channel in rabbit ventricular myocytes. Am. J. Physiol. 1997, 272, H2164–H2172. [Google Scholar] [CrossRef]
- Sheets, M.F.; Hanck, D.A. Mechanisms of extracellular divalent and trivalent cation block of the sodium current in canine cardiac Purkinje cells. J. Physiol. 1992, 454, 299–320. [Google Scholar] [CrossRef]
- Eroglu, T.E.; Mohr, G.H.; Blom, M.T.; Verkerk, A.O.; Souverein, P.C.; Torp-Pedersen, C.; Folke, F.; Wissenberg, M.; Van den Brink, L.; Davis, R.P.; et al. Differential effects on out-of-hospital cardiac arrest of dihydropyridines: Real-world data from population-based cohorts across two European countries. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 347–355. [Google Scholar] [CrossRef]
- Kimura, J.; Noma, A.; Irisawa, H. Na-Ca exchange current in mammalian heart cells. Nature 1986, 319, 596–597. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; Coronel, R.; Ravesloot, J.H.; Verheijck, E.E. Ionic remodeling of sinoatrial node cells by heart failure. Circulation 2003, 108, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Devalla, H.D.; Gélinas, R.; Aburawi, E.H.; Beqqali, A.; Goyette, P.; Freund, C.; Chaix, M.-A.; Tadros, R.; Jiang, H.; Le Béchec, A.; et al. TECRL, a new life-threatening inherited arrhythmia gene associated with overlapping clinical features of both LQTS and CPVT. EMBO Mol. Med. 2016, 8, 1390–1408. [Google Scholar] [CrossRef] [PubMed]
- Bassani, R.A.; Bers, D.M. Rate of diastolic Ca release from the sarcoplasmic reticulum of intact rabbit and rat ventricular myocytes. Biophys. J. 1995, 68, 2015–2022. [Google Scholar] [CrossRef]
- Berecki, G.; Wilders, R.; De Jonge, B.; Van Ginneken, A.C.G.; Verkerk, A.O. Re-evaluation of the action potential upstroke velocity as a measure of the Na+ current in cardiac myocytes at physiological conditions. PLoS ONE 2010, 5, e15772. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.C.; Mengarelli, I.; Koopman, C.D.; Wilders, R.; Van Amersfoorth, S.C.; Bakker, D.; Wolswinkel, R.; Hababa, M.; De Boer, T.P.; Guan, K.; et al. Genetic variation in GNB5 causes bradycardia by augmenting the cholinergic response via increased acetylcholine-activated potassium current (IK,ACh). Dis. Model. Mech. 2019, 12, dmm037994. [Google Scholar] [CrossRef]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R. Dynamic clamp in electrophysiological studies on stem cell-derived cardiomyocytes—Why and how? J. Cardiovasc. Pharmacol. 2021, 77, 267–279. [Google Scholar] [CrossRef]
- Gunawan, M.G.; Sangha, S.S.; Shafaattalab, S.; Lin, E.; Heims-Waldron, D.A.; Bezzerides, V.J.; Laksman, Z.; Tibbits, G.F. Drug screening platform using human induced pluripotent stem cell-derived atrial cardiomyocytes and optical mapping. Stem Cells Transl. Med. 2021, 10, 68–82. [Google Scholar] [CrossRef]
- Kayser, A.; Dittmann, S.; Šarić, T.; Mearini, G.; Verkerk, A.O.; Schulze-Bahr, E. The W101C KCNJ5 mutation induces slower pacing by constitutively active GIRK channels in hiPSC-derived cardiomyocytes. Int. J. Mol. Sci. 2023, 24, 15290. [Google Scholar] [CrossRef]
- Schulz, C.; Sönmez, M.; Krause, J.; Schwedhelm, E.; Bangfen, P.; Alihodzic, D.; Hansen, A.; Eschenhagen, T.; Christ, T. A critical role of retinoic acid concentration for the induction of a fully human-like atrial action potential phenotype in hiPSC-CM. Stem Cell Rep. 2023, 18, 2096–2107. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Ortega, F.; Verkerk, A.O.; Mengarelli, I.; Krogh-Madsen, T.; Christini, D.J.; Coronel, R.; Vigmond, E.J. Validation of quantitative measure of repolarization reserve as a novel marker of drug induced proarrhythmia. J. Mol. Cell. Cardiol. 2020, 145, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Ernault, A.C.; Verkerk, A.O.; Bayer, J.D.; Aras, K.; Montañés-Agudo, P.; Mohan, R.A.; Veldkamp, M.; Rivaud, M.R.; De Winter, R.; Kawasaki, M.; et al. Secretome of atrial epicardial adipose tissue facilitates reentrant arrhythmias by myocardial remodeling. Heart Rhythm 2022, 19, 1461–1470. [Google Scholar] [CrossRef]
- Yuan, W.; Ginsburg, K.S.; Bers, D.M. Comparison of sarcolemmal calcium channel current in rabbit and rat ventricular myocytes. J. Physiol. 1996, 493, 733–746. [Google Scholar] [CrossRef]
- Fülöp, L.; Bányász, T.; Magyar, J.; Szentandrássy, N.; Varró, A.; Nánási, P.P. Reopening of L-type calcium channels in human ventricular myocytes during applied epicardial action potentials. Acta Physiol. Scand. 2004, 180, 39–47. [Google Scholar] [CrossRef]
- Linz, K.W.; Meyer, R. Profile and kinetics of L-type calcium current during the cardiac ventricular action potential compared in guinea-pigs, rats and rabbits. Pflügers Arch. 2000, 439, 588–599. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J.W. L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef]
- Neef, S.; Maier, L.S. Novel aspects of excitation-contraction coupling in heart failure. Basic Res. Cardiol. 2013, 108, 360. [Google Scholar] [CrossRef]
- Eisner, D.A. Ups and downs of calcium in the heart. J. Physiol. 2018, 596, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Seibertz, F.; Rubio, T.; Springer, R.; Popp, F.; Ritter, M.; Liutkute, A.; Bartelt, L.; Stelzer, L.; Haghighi, F.; Pietras, J.; et al. Atrial fibrillation-associated electrical remodelling in human induced pluripotent stem cell-derived atrial cardiomyocytes: A novel pathway for antiarrhythmic therapy development. Cardiovasc. Res. 2023, 119, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Mainardi, L.; Rodriguez Matas, J.F. Cellular heterogeneity and repolarisation across the atria: An in silico study. Med. Biol. Eng. Comput. 2022, 60, 3153–3168. [Google Scholar] [CrossRef]
- Veerman, C.C.; Mengarelli, I.; Guan, K.; Stauske, M.; Barc, J.; Tan, H.L.; Wilde, A.A.M.; Verkerk, A.O.; Bezzina, C.R. hiPSC-derived cardiomyocytes from Brugada Syndrome patients without identified mutations do not exhibit clear cellular electrophysiological abnormalities. Sci. Rep. 2016, 6, 30967. [Google Scholar] [CrossRef]
- Quaranta, R.; Fell, J.; Rühle, F.; Rao, J.; Piccini, I.; Araúzo-Bravo, M.J.; Verkerk, A.O.; Stoll, M.; Greber, B. Revised roles of ISL1 in a hES cell-based model of human heart chamber specification. eLife 2018, 7, e31706. [Google Scholar] [CrossRef]
- Amos, G.J.; Wettwer, E.; Metzger, F.; Li, Q.; Himmel, H.M.; Ravens, U. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J. Physiol. 1996, 491, 31–50. [Google Scholar] [CrossRef]
- Ford, J.; Milnes, J.; Wettwer, E.; Christ, T.; Rogers, M.; Sutton, K.; Madge, D.; Virag, L.; Jost, N.; Horvath, Z.; et al. Human electrophysiological and pharmacological properties of XEN-D0101: A novel atrial-selective Kv1.5/IKur inhibitor. J. Cardiovasc. Pharmacol. 2013, 61, 408–415. [Google Scholar] [CrossRef]
- Horváth, A.; Lemoine, M.D.; Löser, A.; Mannhardt, I.; Flenner, F.; Uzun, A.U.; Neuber, C.; Breckwoldt, K.; Hansen, A.; Girdauskas, E.; et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of hiPSC-derived cardiomyocytes. Stem Cell Rep. 2018, 10, 822–833. [Google Scholar] [CrossRef]
- Varró, A.; Nánási, P.P.; Lathrop, D.A. Potassium currents in isolated human atrial and ventricular cardiocytes. Acta Physiol. Scand. 1993, 149, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yue, L.; White, M.; Pelletier, G.; Nattel, S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation 1998, 98, 2422–2428. [Google Scholar] [CrossRef]
- Cohen, N.M.; Lederer, W.J. Calcium current in single human cardiac myocytes. J. Cardiovasc. Electrophysiol. 1993, 4, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.D.; Rasmusson, R.L.; Bett, G.C.L. Ionic basis of repolarization of atrial and ventricular specific cell types derived from human induced pluripotent stem cells. Biophys. J. 2016, 110 (Suppl. 1), 343a. [Google Scholar] [CrossRef]
- Lemme, M.; Ulmer, B.M.; Lemoine, M.D.; Zech, A.T.L.; Flenner, F.; Ravens, U.; Reichenspurner, H.; Rol-Garcia, M.; Smith, G.; Hansen, A.; et al. Atrial-like engineered heart tissue: An in vitro model of the human atrium. Stem Cell Rep. 2018, 11, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Rudy, Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ. Res. 1994, 74, 1071–1096. [Google Scholar] [CrossRef]
- Workman, A.J.; Marshall, G.E.; Rankin, A.C.; Smith, G.L.; Dempster, J. Transient outward K+ current reduction prolongs action potentials and promotes afterdepolarisations: A dynamic-clamp study in human and rabbit cardiac atrial myocytes. J. Physiol. 2012, 590, 4289–4305. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Tort, L. L-type Ca2+ current and excitation-contraction coupling in single atrial myocytes from rainbow trout. Am. J. Physiol. 1998, 275, R2061–R2069. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Pelzer, D.J.; Pelzer, S. Modulation of L-type Ca2+ current by fast and slow Ca2+ buffering in guinea pig ventricular cardiomyocytes. Biophys. J. 1997, 72, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Mewes, T.; Ravens, U. L-type calcium currents of human myocytes from ventricle of non-failing and failing hearts and from atrium. J. Mol. Cell. Cardiol. 1994, 26, 1307–1320. [Google Scholar] [CrossRef]
- Ouadid, H.; Albat, B.; Nargeot, J. Calcium currents in diseased human cardiac cells. J. Cardiovasc. Pharmacol. 1995, 25, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, T.; Arita, M.; Muramatsu, H.; Spindler, A.J.; Noble, D. Ionic mechanisms of action potential prolongation at low temperature in guinea-pig ventricular myocytes. J. Physiol. 1993, 468, 85–106. [Google Scholar] [CrossRef]
- Uzun, A.U.; Mannhardt, I.; Breckwoldt, K.; Horváth, A.; Johannsen, S.S.; Hansen, A.; Eschenhagen, T.; Christ, T. Ca2+-currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions. Front. Pharmacol. 2016, 7, 300. [Google Scholar] [CrossRef]
- Li, J.; Wiesinger, A.; Fokkert, L.; Bakker, P.; De Vries, D.K.; Tijsen, A.J.; Pinto, Y.M.; Verkerk, A.O.; Christoffels, V.M.; Boink, G.J.J.; et al. Modeling the atrioventricular conduction axis using human pluripotent stem cell-derived cardiac assembloids. Cell Stem Cell 2024, 31, 1667–1684.e6. [Google Scholar] [CrossRef]
- Filippini, L.; Ortner, N.J.; Kaserer, T.; Striessnig, J. Cav1.3-selective inhibitors of voltage-gated L-type Ca2+ channels: Fact or (still) fiction? Br. J. Pharmacol. 2023, 180, 1289–1303. [Google Scholar] [CrossRef]
- Qu, Y.; Baroudi, G.; Yue, Y.; El-Sherif, N.; Boutjdir, M. Localization and modulation of α1D (Cav1.3) L-type Ca channel by protein kinase A. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2123–H2130. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, N.; Le Bouter, S.; Szuts, V.; Varro, A.; Escande, D.; Nattel, S.; Demolombe, S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 2007, 582, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, S.; Srivastava, U.; Qu, Y.S.; Chahine, M.; Boutjdir, M. Pathophysiology of Cav1.3 L-type calcium channels in the heart. Front. Physiol. 2023, 14, 1144069. [Google Scholar] [CrossRef]
- Honda, Y.; Li, J.; Hino, A.; Tsujimoto, S.; Lee, J.-K. High-throughput drug screening system based on human induced pluripotent stem cell-derived atrial myocytes ∼ A novel platform to detect cardiac toxicity for atrial arrhythmias. Front. Pharmacol. 2021, 12, 680618. [Google Scholar] [CrossRef]
- Janvier, N.C.; Boyett, M.R. The role of Na-Ca exchange current in the cardiac action potential. Cardiovasc. Res. 1996, 32, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, D.; Gurr, K.; Horváth, A.; Yuan, L.; Lemoine, M.D.; Schulz, C.; Sani, J.; Petersen, J.; Reichenspurner, H.; Kirchhof, P.; et al. Regulation of APD and force by the Na+/Ca2+ exchanger in human-induced pluripotent stem cell-derived engineered heart tissue. Cells 2022, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Christ, T.; Kovács, P.P.; Acsai, K.; Knaut, M.; Eschenhagen, T.; Jost, N.; Varró, A.; Wettwer, E.; Ravens, U. Block of Na+/Ca2+ exchanger by SEA0400 in human right atrial preparations from patients in sinus rhythm and in atrial fibrillation. Eur. J. Pharmacol. 2016, 788, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Teos, L.Y.; Lederer, W.J. Unique atrial myocyte Ca2+ signaling. J. Mol. Cell. Cardiol. 2009, 46, 448–451. [Google Scholar] [CrossRef][Green Version]
- Bootman, M.D.; Smyrnias, I.; Thul, R.; Coombes, S.; Roderick, H.L. Atrial cardiomyocyte calcium signalling. Biochim. Biophys. Acta 2011, 1813, 922–934. [Google Scholar] [CrossRef]
- Greiser, M. Calcium signalling silencing in atrial fibrillation. J. Physiol. 2017, 595, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Denham, N.C.; Pearman, C.M.; Caldwell, J.L.; Madders, G.W.P.; Eisner, D.A.; Trafford, A.W.; Dibb, K.M. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front. Physiol. 2018, 9, 1380. [Google Scholar] [CrossRef] [PubMed]
- Tanaami, T.; Ishida, H.; Seguchi, H.; Hirota, Y.; Kadono, T.; Genka, C.; Nakazawa, H.; Barry, W.H. Difference in propagation of Ca2+ release in atrial and ventricular myocytes. Jpn. J. Physiol. 2005, 55, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Guatimosim, S.; Dilly, K.; Santana, L.F.; Jafri, M.S.; Sobie, E.A.; Lederer, W.J. Local Ca2+ signaling and EC coupling in heart: Ca2+ sparks and the regulation of the [Ca2+]i transient. J. Mol. Cell. Cardiol. 2002, 34, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Trafford, A.W.; Hutchings, D.C. The control of diastolic calcium in the heart: Basic mechanisms and functional implications. Circ. Res. 2020, 126, 395–412. [Google Scholar] [CrossRef]
- Bokník, P.; Unkel, C.; Kirchhefer, U.; Kleideiter, U.; Klein-Wiele, O.; Knapp, J.; Linck, B.; Lüss, H.; Müller, F.U.; Schmitz, W.; et al. Regional expression of phospholamban in the human heart. Cardiovasc. Res. 1999, 43, 67–76. [Google Scholar] [CrossRef]
- Maier, L.S.; Barckhausen, P.; Weisser, J.; Aleksic, I.; Baryalei, M.; Pieske, B. Ca2+ handling in isolated human atrial myocardium. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H952–H958. [Google Scholar] [CrossRef]
- Minajeva, A.; Kaasik, A.; Paju, K.; Seppet, E.; Lompré, A.M.; Veksler, V.; Ventura-Clapier, R. Sarcoplasmic reticulum function in determining atrioventricular contractile differences in rat heart. Am. J. Physiol. 1997, 273, H2498–H2507. [Google Scholar] [CrossRef]
- Lüss, I.; Boknik, P.; Jones, L.R.; Kirchhefer, U.; Knapp, J.; Linck, B.; Lüss, H.; Meissner, A.; Müller, F.U.; Schmitz, W.; et al. Expression of cardiac calcium regulatory proteins in atrium v ventricle in different species. J. Mol. Cell. Cardiol. 1999, 31, 1299–1314. [Google Scholar] [CrossRef]
- Walden, A.P.; Dibb, K.M.; Trafford, A.W. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J. Mol. Cell. Cardiol. 2009, 46, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Dibb, K.M.; Eisner, D.A.; Trafford, A.W. Regulation of systolic [Ca2+]i and cellular Ca2+ flux balance in rat ventricular myocytes by SR Ca2+, L-type Ca2+ current and diastolic [Ca2+]i. J. Physiol. 2007, 585, 579–592. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Nargeot, J. Genesis and regulation of the heart automaticity. Physiol. Rev. 2008, 88, 919–982. [Google Scholar] [CrossRef]
- Zhao, Z.; Lan, H.; El-Battrawy, I.; Li, X.; Buljubasic, F.; Sattler, K.; Yücel, G.; Lang, S.; Tiburcy, M.; Zimmermann, W.H.; et al. Ion channel expression and characterization in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cells Int. 2018, 2018, 6067096. [Google Scholar] [CrossRef]
- Horváth, A.; Christ, T.; Koivumäki, J.T.; Prondzynski, M.; Zech, A.T.L.; Spohn, M.; Saleem, U.; Mannhardt, I.; Ulmer, B.; Girdauskas, E.; et al. Case report on: Very early afterdepolarizations in hiPSC-cardiomyocytes—An artifact by big conductance calcium activated potassium current (Ibk,Ca). Cells 2020, 9, 253. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R.; Zegers, J.G.; Van Borren, M.M.G.J.; Ravesloot, J.H.; Verheijck, E.E. Ca2+-activated Cl− current in rabbit sinoatrial node cells. J. Physiol. 2002, 540, 105–117. [Google Scholar] [CrossRef]
- Lacinová, L.; Klugbauer, N.; Hofmann, F. Regulation of the calcium channel α1G subunit by divalent cations and organic blockers. Neuropharmacology 2000, 39, 1254–1266. [Google Scholar] [CrossRef]
- Bers, D.M.; Perez-Reyes, E. Ca channels in cardiac myocytes: Structure and function in Ca influx and intracellular Ca release. Cardiovasc. Res. 1999, 42, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.P.; MacQuaide, N.; Louch, W.E. Dyadic plasticity in cardiomyocytes. Front. Physiol. 2018, 9, 1773. [Google Scholar] [CrossRef]
- Lu, F.; Pu, W.T. The architecture and function of cardiac dyads. Biophys. Rev. 2020, 12, 1007–1017. [Google Scholar] [CrossRef]
- Satin, J.; Itzhaki, I.; Rapoport, S.; Schroder, E.A.; Izu, L.; Arbel, G.; Beyar, R.; Balke, C.W.; Schiller, J.; Gepstein, L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells 2008, 26, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Lieu, D.K.; Liu, J.; Siu, C.-W.; McNerney, G.P.; Tse, H.-F.; Abu-Khalil, A.; Huser, T.; Li, R.A. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009, 18, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Barad, L.; Novak, A.; Reiter, I.; Itskovitz-Eldor, J.; Binah, O.; Popescu, L.M. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: Comparative ultrastructure. J. Cell. Mol. Med. 2011, 15, 2539–2551. [Google Scholar] [CrossRef] [PubMed]
- Dark, N.; Cosson, M.V.; Tsansizi, L.I.; Owen, T.J.; Ferraro, E.; Francis, A.J.; Tsai, S.; Bouissou, C.; Weston, A.; Collinson, L.; et al. Generation of left ventricle-like cardiomyocytes with improved structural, functional, and metabolic maturity from human pluripotent stem cells. Cell Rep. Methods 2023, 3, 100456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef]
- Gupta, M.K.; Illich, D.J.; Gaarz, A.; Matzkies, M.; Nguemo, F.; Pfannkuche, K.; Liang, H.; Classen, S.; Reppel, M.; Schultze, J.L.; et al. Global transcriptional profiles of beating clusters derived from human induced pluripotent stem cells and embryonic stem cells are highly similar. BMC Dev. Biol. 2010, 10, 98. [Google Scholar] [CrossRef]
- Wang, K.; Terrenoire, C.; Sampson, K.J.; Iyer, V.; Osteen, J.D.; Lu, J.; Keller, G.; Kotton, D.N.; Kass, R.S. Biophysical properties of slow potassium channels in human embryonic stem cell derived cardiomyocytes implicate subunit stoichiometry. J. Physiol. 2011, 589, 6093–6104. [Google Scholar] [CrossRef]
- Mandel, Y.; Weissman, A.; Schick, R.; Barad, L.; Novak, A.; Meiry, G.; Goldberg, S.; Lorber, A.; Rosen, M.R.; Itskovitz-Eldor, J.; et al. Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation 2012, 125, 883–893. [Google Scholar] [CrossRef]
- Gharanei, M.; Shafaattalab, S.; Sangha, S.; Gunawan, M.; Laksman, Z.; Hove-Madsen, L.; Tibbits, G.F. Atrial-specific hiPSC-derived cardiomyocytes in drug discovery and disease modeling. Methods 2022, 203, 364–377. [Google Scholar] [CrossRef]
- Botti, S.; Bartolucci, C.; Altomare, C.; Paci, M.; Barile, L.; Krause, R.; Pavarino, L.F.; Severi, S. A novel ionic model for matured and paced atrial-like hiPSC-CMs integrating IKur and IKCa currents. Comput. Biol. Med. 2024, 180, 108899. [Google Scholar] [CrossRef]
- Zhao, Y.; Rafatian, N.; Feric, N.T.; Cox, B.J.; Aschar-Sobbi, R.; Wang, E.Y.; Aggarwal, P.; Zhang, B.; Conant, G.; Ronaldson-Bouchard, K.; et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019, 176, 913–927.e18. [Google Scholar] [CrossRef] [PubMed]

| Study | Diff. | Temp. (°C) | EGTA | Criterion for N-like | Criterion for A-like | Criterion for V-like | N-|A-|V-like (%) |
|---|---|---|---|---|---|---|---|
| Itzhaki et al. [26] | Std | 32 | + | N/A | N/A | N/A | 13|27|60 |
| Itzhaki et al. [27] | Std | 32 | + | N/A | N/A | N/A | 14|30|56 |
| Li et al. [28] | Std | Room | − | N/A | N/A | N/A | N/A|N/A|81 |
| Streckfuss-Bömeke et al. [25] | Std | Room | − | N/A | N/A | N/A | 29|17|54 †,A 20|20|60 †,B 29|19|52 †,C |
| Lee et al. [29] | Std | N/A | N/A | N/A | N/A | N/A | 45|17|38 |
| Lan et al. [30] | Std | 36–37 | + | N/A | N/A | N/A | ≈5|35|60 |
| Laksman et al. [31] | Std RA | 22–23 | − | N/A | N/A | N/A | 7|≈13|≈80 ≈7|≈87|≈7 |
| De la Roche et al. [32] | Std | Room | + | N/A | 20 ms < APD50 < 200 ms | APD50 > 200 ms | N/A|12|88 ‡ |
| Peng et al. [33] | Std | 35 | − | Slow upstrokes and fast pacing rate | APD90 < 150 ms | APD90 > 150 ms | <1|18|82 |
| Pei et al. [34] | Std RA | 35 | − | Slow upstrokes and fast pacing rate | APD90 < 150 ms | APD90 > 150 ms | N/A|N/A|93 N/A|90|N/A |
| Chapotte-Baldacci et al. [35] | Std RA | Room | + | APD90 < 250 ms, APD50 − APD20 ≤ 10 ms | APD90 < 250 ms, APD50 − APD20 > 10 ms | APD90 > 250 ms | 0|31|69 # 9|66|27 # |
| Jara-Avaca et al. [36] | Std | 37 | + | Bell-shaped APs, APD90/50 > 1.4 | APD50 < 100 ms, APD90/50 > 3 | APD50 > 100 ms, APD90/50 < 1.4 | 0|≈33|≈63 |
| Bett et al. [37] | Std | Room | + | N/A | APD30 < 300 ms, APD30/90 < 0.75 | APD30 > 300 ms, APD30/90 > 0.75 | N/A|≈64|≈28 # |
| Kim et al. [38] | Std | Room | + | N/A | APD30 < 300 ms, APD30/90 < 0.75 | APD30 > 300 ms, APD30/90 > 0.75 | <1|19|68 |
| Altomare et al. [39] | Std RA | 35 | − | N/A | APD20/90 < 0.44 | APD20/90 > 0.44 | N/A|34|66 N/A|74|26 |
| Moretti et al. [40] | Std | 35 | + | Depolarized; low dV/dt, low APA | 1.3 < APD90/50 < 1.6 | 1.1 < APD90/50 <1.3 | 18|20|62 |
| El-Battrawy et al. [41] | Std | 36 | + | 1.4 < APD90/50 < 1.7 | APD90/50 > 1.7 | APD90/50 < 1.4 | 5|22|73 |
| Matsa et al. [42] | Std | 37 | + | 1.4 < APD90/50 < 1.7 | APD90/50 > 1.7 | APD90/50 < 1.4 | N/A|N/A|N/A |
| Hayano et al. [43] | Std | 36–37 | + | APD90/50 > 1.2, APA < 60 mV | APD90/50 > 1.2, APA > 80 mV | APD90/50 ≤ 1.2, APA > 80 mV | 14|38|48 |
| Ma et al. [44] | Std | 35–37 | − | APD30–40/APD70–80 < 1.5, dV/dtmax < 10 V/s | APD30–40/APD70–80 < 1.5 | APD30–40/APD70–80 > 1.5 | 22|24|54 |
| Argenziano et al. [45] * | Std RA | N/A | − | APD30–40/APD70–80 < 1.5, dV/dtmax < 10 V/s | APD30–40/APD70–80 < 1.5 | APD30–40/APD70–80 > 1.5 | 7|7|86 6|85|9 |
| Cordeiro et al. [46] * | Std | 37 | − | N/A | APD30–40/APD70–80 < 1.5 | APD30–40/APD70–80 > 1.5 | N/A|54|46 D |
| Zhang et al. [47] | Std | Room | + | APD30–40/APD70–80 < 1.5, dV/dtmax < 10 V/s | APD30–40/APD70–80 < 1.5 | APD30–40/APD70–80 > 1.5 | 18|65|17 E 0|50|50 F |
| Ma et al. [48] | Std | 37 | − | APD30–40/APD70–80 < 1.5, dV/dtmax < 10 V/s | APD30–40/APD70–80 < 1.5 | APD30–40/APD70–80 > 1.5 | 11|15|74 |
| Devalla et al. [49] | Std RA | 36 | − | N/A | APplat < 80 mV | APplat > 80 mV | <1|≈19|≈80 <1|≈85|≈14 |
| Spontaneously Active | Quiescent—1 Hz Stimulation | |||
|---|---|---|---|---|
| Std (n = 15) | RA-Treated (n = 10) | Std (n = 36) | RA-Treated (n = 37) | |
| Cycle length (ms) | 814 ± 69 | 591 ± 56 * | N/A | N/A |
| MDP (mV) | −63.7 ± 1.9 | −62.0 ± 2.0 | −69.4 ± 0.8 # | −70.1 ± 0.8 # |
| APA (mV) | 90.1 ± 4.5 | 76.9 ± 3.7 * | 98.2 ± 2.4 | 80.3 ± 2.2 * |
| APplat (mV) | 87.5 ± 4.8 | 70.7 ± 5.6 * | 89.9 ± 3.3 | 60.3 ± 2.8 * |
| dV/dtmax (V/s) | 8.4 ± 1.2 | 7.9 ± 2.5 | 60.3 ± 11.3 # | 41.4 ± 6.8 # |
| APD20 (ms) | 107.1 ± 8.8 | 64.2 ± 13.6 * | 74.9 ± 8.3 # | 26.8 ± 3.9 *,# |
| APD50 (ms) | 191.1 ± 21.5 | 103.9 ± 20.3 * | 121.5 ± 12.6 # | 59.4 ± 7.1 * |
| APD90 (ms) | 267.7 ± 30.1 | 162.6 ± 21.2 * | 185.8 ± 8.5 # | 152.8 ± 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verkerk, A.O.; Veerman, C.C.; Hoekstra, M.; Devalla, H.D.; Wilders, R. A Better Understanding of Atrial-like and Ventricular-like Action Potentials in Stem Cell-Derived Cardiomyocytes: The Underestimated Role of the L-Type Ca2+ Current. Cells 2025, 14, 1226. https://doi.org/10.3390/cells14161226
Verkerk AO, Veerman CC, Hoekstra M, Devalla HD, Wilders R. A Better Understanding of Atrial-like and Ventricular-like Action Potentials in Stem Cell-Derived Cardiomyocytes: The Underestimated Role of the L-Type Ca2+ Current. Cells. 2025; 14(16):1226. https://doi.org/10.3390/cells14161226
Chicago/Turabian StyleVerkerk, Arie O., Christiaan C. Veerman, Maaike Hoekstra, Harsha D. Devalla, and Ronald Wilders. 2025. "A Better Understanding of Atrial-like and Ventricular-like Action Potentials in Stem Cell-Derived Cardiomyocytes: The Underestimated Role of the L-Type Ca2+ Current" Cells 14, no. 16: 1226. https://doi.org/10.3390/cells14161226
APA StyleVerkerk, A. O., Veerman, C. C., Hoekstra, M., Devalla, H. D., & Wilders, R. (2025). A Better Understanding of Atrial-like and Ventricular-like Action Potentials in Stem Cell-Derived Cardiomyocytes: The Underestimated Role of the L-Type Ca2+ Current. Cells, 14(16), 1226. https://doi.org/10.3390/cells14161226








